Abstract
Synopsis All organisms must anticipate and balance energetic demands and available resources in order to maximize fitness. As hormones coordinate many interactions between an organism’s internal condition and the external environment, they may be key in mediating the allocation of resources to meet these demands. However, given that individuals differ considerably in how they react to changes in energetic demand, we asked whether variations in endocrine traits also correspond with life history variation. We tested whether natural variation in glucocorticoid hormone levels, oxidative stress measurements, and condition related to reproductive effort in a free-living songbird, the tree swallow, Tachycineta bicolor. We then tested whether any of these traits predicted the probability of a particular individual’s return to the local population in the following two years, an indicator of survival in this philopatric species. We found that males and females with longer telomeres had lighter nestlings. Moreover, individuals with lower plasma antioxidant capacity and higher reactive oxygen metabolites (i.e., greater oxidative stress) were less likely to return to the population. However, none of these traits were related to glucocorticoid levels. Our findings suggest a trade-off between reproduction and survival, with individuals with shorter telomeres having heavier nestlings but potentially paying a cost in terms of higher oxidative stress and lower survival. Interestingly, the evidence of this trade-off was unrelated to natural variation in glucocorticoids.
Introduction
Understanding the mechanisms underlying life history variation is a major goal in evolutionary biology. Every organism attempts to track current and anticipate future energetic demands, allocating available resources in a way that maximizes fitness in unpredictable environments. These energetic demands can be broadly grouped into two categories, the costs of self-maintenance and the costs of reproduction, resulting in a classic survival–reproduction life history trade-off (Stearns 1989). An individual’s life history strategy, reflected in the relative amount of resources that the individual allocates toward each function, can also depend on its survival probability, age, reproductive value, and current environmental conditions. Therefore, a central question remains: How is an individual able to make these allocation decisions in changing environments?
The answer to this question might lie in a system that is capable of integrating information about internal and external conditions and transmitting that information to induce a response. Hormones participate in organizing life stage transitions and can provide the mechanistic basis of individual differences in behavior. There is substantial individual variation in endocrine traits, based upon each individual’s facultative decisions, given their current energetic state, and local environmental conditions. Glucocorticoid hormones (GCs), released in response to stimulation of the hypothalamic–pituitary–adrenal (HPA) axis, are central in regulating energy balance. GCs (corticosterone in birds) at baseline versus stress-induced concentrations have very different functions (Landys et al. 2006). Baseline corticosterone mobilizes energy stores with clear diel and seasonal rhythms in birds; it peaks with increased activity at daybreak and peaks seasonally during the energetically demanding breeding season (Astheimer et al. 1992; Romero 2002). In contrast, stress-induced corticosterone is responsible for mediating an emergency set of activities to help the animal survive and recover from an immediate challenge. Long-term GC exposure can lead to a number of deleterious effects (Sapolsky et al. 2000; Wingfield and Silverin 2002). Some of the deleterious impacts of GCs may arise through their effect on the oxidative balance of the organism (Costantini et al. 2011).
Oxidative stress (OxS) occurs when damaging reactive oxygen species (ROS) overcome defense mechanisms, such as antioxidants and repair enzymes (Sies 1991; Monaghan et al. 2009). Managing oxidative stress is important for regulating life history trade-offs, and, given that virtually all metabolic activities generate ROS, oxidative stress is also a major component of animal performance (Monaghan et al. 2009). OxS can impair DNA function, degrade proteins and lipids, and decrease fitness-relevant traits including reproduction and longevity (Costantini 2008; Haussmann and Mauck 2008; Sorce and Krause 2009; Metcalfe and Alonso-Alvarez 2010). There is also a bi-directional link between GCs and oxidative stress because elevated GC concentrations increase metabolic production of ROS, decrease antioxidant protection, and disrupt repair mechanisms (Cohen et al. 2009; Haussmann and Marchetto 2010; Costantini et al. 2011). Experimental increases in GCs in both embryos and adults may have transgenerational effects on increased OxS (Lin et al. 2004a; Lin et al. 2004b; Haussmann and Heidinger 2015). Thus, an endocrine stress response is often associated with OxS, both in the short and long terms (Haussmann et al. 2012). In turn, pro-oxidant molecules can affect the functioning of the HPA axis, such as when only moderate levels of OxS inhibit the expression of the genes encoding GC receptors (Allen and Tresini 2000). Because free-living organisms experience a wide range of stressors, increasing oxidative damage may act as an underlying mechanism connecting the glucocorticoid stress response and survival in natural populations.
Telomeres are protective structures at the ends of chromosomes, and regulation of their length is a dynamic process, lengthening and shortening due to various internal and external changes (Greider 1996). They appear particularly vulnerable to ROS attack, which can result in their shortening (Haussmann and Marchetto 2010). The shortening of telomeres through cell division and oxidative stress is related to cellular aging and organismal growth. In this way, telomeres can link organismal processes with molecular and cellular mechanisms, and may explain variation in a number of life history traits (Haussmann and Marchetto 2010). For example, older, diseased, or individuals in poor health have shorter telomeres (Monaghan 2010) and longer telomere length has been shown to be associated with increased survival rates in wild vertebrates (Haussmann et al. 2005; Pauliny et al. 2006; Angelier et al. 2013). There is a link between elevated GCs and telomere dynamics in natural populations because GCs can increase ROS and thus induce telomere shortening (Costantini et al. 2011). Such a link provides a mechanism whereby environmental perturbation might impact life history trajectories.
Our goal in this study was to determine whether point measures of an individual’s GC levels and oxidative stress status can describe its life history phenotype. We measured corticosterone concentrations, OxS, telomere lengths, and hematocrit in adult breeding tree swallows and investigated correlations between these physiological variables and body condition and reproductive investment. We then determined whether these correlations predict the likelihood that the individual will return to the same population the following year. We predicted that we would see evidence of the classic life history trade-off reflected in opposing measures in self-maintenance strategies (e.g., elevated antioxidant capacity, higher return rates, and decreased ROS) versus reproductive effort (e.g., increased nestling mass, GCs, and oxidative stress, and shorter telomeres). Alternatively, we predicted that we might instead find positive correlations among some metrics, reflecting individual variation in quality, access to resources, or exposure to challenges (van Noordwijk and de Jong 1986). We also predicted that individuals with longer telomeres would have higher survival rates, as has been found in previous studies (Haussmann et al. 2005).
Materials and methods
Study area and general field procedures
We conducted this study from May 1–July 30, 2013, at the Queen’s University Biological Station in Elgin, Ontario, Canada (44.6 ºN, 76.3 ºW, ∼140 m elevation). The study site consists of 229 nest boxes set up in nine grid networks in open fields. We monitored nests daily toward the end of the incubation period to determine the date of hatching (Day 0 of the nestling period). On Day 4 of the nestling period (May 31–June 8), we captured both parents in their nest box and collected a blood sample from a brachial vein (200 µl) within 3 min of capture (mean ± standard error: 2.08 ± 0.07 min). We excluded one sample from our analyses in which the sampling time was 3.5 min. After collecting the blood sample, we measured the bird’s skull length (head + bill) to the nearest 0.1 mm, and body mass to the nearest 0.25 g. Blood was kept cool in heparinized capillary tubes on ice until centrifuging for 10 min within 3 h of collection. Hematocrit was measured with a ruler to the nearest mm. We separated red blood cells and plasma, placing the red blood cells into 1 mL of buffer solution (10% dimethyl sulfoxide, 90% newborn bovine serum) for telomere analysis, and all samples were then stored at −20 °C.
We measured body mass of each nestling (±0.01 g) on Day 4, immediately after catching both parents at 35 nests, and, at 20 other nests, we measured nestling mass on Day 6 (in a separate experiment that had no influence on this study). We calculated average nestling mass as a measure of reproductive effort of the parents, and we used the residuals from a linear model of average nestling mass controlling for age in all analyses. We investigated whether focal individuals from 2013 returned to the study population in either of the following two years (2014, 2015) as a measure of adult survival (Winkler et al. 2004).
All procedures followed guidelines for animal care outlined by the Canadian Council on Animal Care and were approved by the Virginia Tech’s Institutional Animal Care and Use Committee (protocol no. 12-020) and Queen’s University Animal Care Committee (protocol no. 2013-019).
Hormone analysis
Total corticosterone from plasma samples was quantified through direct radioimmunoassay, as described by Bonier et al. (2009b). Briefly, plasma samples were spiked with 2000 cpm radiolabeled hormone to quantify extraction efficiency, then corticosterone was extracted from plasma using dichloromethane, and extracts were reconstituted in phosphate-buffered saline with gelatin. Mean extraction efficiency was 73%, and final concentrations were corrected for individual extraction efficiencies. We used a commercial antiserum (Esoterix Endocrinology, Calabasas Hills, CA 91301, product number: B3–163). The extracts were incubated overnight at 4 °C with ∼10K cpm of 3H-Cort (Perkin Elmer, product number: NET399250UC) and antiserum. Dextran-coated charcoal was added to separate corticosterone bound to antibodies. After centrifugation, the radioactivity of the bound fraction was counted in a liquid scintillation counter. Within-assay variation among replicate known-concentration standard samples was 3.12%. Minimal detectable corticosterone levels were ∼1.16 ng/mL, and no samples fell below this detection limit.
Oxidative stress analysis
We measured reactive oxygen metabolites (ROMs) using the d-ROMs test (Diacron International, Grosseto, Italy), which measures the level of hydroperoxides, compounds that signal lipid and protein oxidative damage (Costantini 2008). We diluted 5 µL of plasma in 200 µL of the provided acidic buffer solution, and then read the plate kinetically (once per minute) for the next 20 minutes. Absorbance was measured at 490 nm (BioTek ELx800, VT, USA), and we calculated area under the curve over the 20-min reaction and expressed this in ROMs concentrations (in mmol of H2O2 equivalents) from these absorbencies over the 20-min reaction. All samples were run in a single assay plate.
We measured total plasma antioxidant capacity (TAC) using the OXY-Adsorbent test (Diacron International, Grosseto, Italy), which measures the effectiveness of the blood antioxidant barrier by quantifying its ability to cope with oxidant action of hypochlorous acid (HClO). We diluted 10 µL plasma in 990 µL of distilled water; we then mixed 5 µL of this diluted plasma with 195 µl of the provided HClO solution and continued by following the manufacturer’s instructions. We measured absorbance at 490 nm (BioTek ELx800) for 20 minutes and calculated the area under the curve over the 20-min reaction and expressed this as TAC (in mmol of HClO neutralized). All samples were run in a single assay plate.
Telomere analysis
Briefly, we extracted DNA using Gentra Purgene Blood Kit (Qiagen), followed by restriction digestion with 15 U of HinfI, 75 U of HaeIII, and 40 U of RsaI at 37 °C. Ten µg of digested DNA were loaded into a 0.8% non-denaturing agarose gel. DNA was separated using pulsed field gel electrophoresis (3 V/cm, 0.5–7.0 s switch times, 14 °C) for 19 h, followed by in-gel hybridization at 37 °C overnight with a radioactive-labeled telomere-specific oligo (CCCTAA)4. We placed hybridized gels on a phosphorscreen (Amersham Biosciences, Buckinghamshire, UK), which was scanned on a Storm 540 Variable Mode Imager (Amersham Biosciences). We used densitometry (ImageQuant 5.03v and ImageJ 1.42q) to determine the position and strength of the radioactive signal in each of the lanes compared to the molecular marker (1 kb DNA extension ladder; Invitrogen, CA, USA). The background was fixed as the nadir of the low molecular weight region on the gel (<1 kb). Genome-wide mean telomere length was calculated in the range of 1–40 kb (the limits of our molecular marker). Intra- and inter-gel coefficients of variation were, respectively: 3.3% and 2.0%.
Statistical analysis
We did statistical tests using R, version 3.2.3 (R Core Team 2015). Body condition was estimated as a scaled mass index (a body mass index corrected for body size sensu Peig and Green 2009), using each individual’s body mass and skull length. We used a principle component analysis (PCA) to combine measurements of telomere length that included genome-wide mean telomere length and percentile telomere lengths calculated every 10th percentile between 10 and 90 for the entire telomere distribution. PC1 explained 91% of the variance, so we used this component as the dependent variable in our analyses (loadings: mean: 0.28, 90% = 0.57, 80% = 0.44, 70% = 0.36, 60% = 0.30, 50% = 0.26, 40% = 0.22, 30% = 0.18, 20% = 0.14). We also used a PCA to combine measures of oxidative stress (loadings: plasma antioxidant capacity = −0.77 and concentration of ROS = 0.64). High values of the first principle component (explaining 63% of the variance) for OxS is equivalent to lower antioxidant capacity and higher oxidative damage, in other words, relatively higher oxidative stress.
We calculated partial correlation coefficients for physiological trait measurements (corticosterone, oxidative stress PC1, telomeres PC1, and hematocrit), body condition, and nestling mass separately for males and females to investigate correlations between two traits, while controlling for their correlations with other parameters. We used a general linear model with a binomial link function to determine whether individuals that returned to breed in the population within two years after the current study were different from those that did not return. Independent variables included corticosterone levels, oxidative stress PC1, telomeres PC1, hematocrit, body condition, and sex. We then used the second order Akaike’s Information Criterion (AICc) to select from all possible candidate models (Burnham and Anderson 2002).
Results
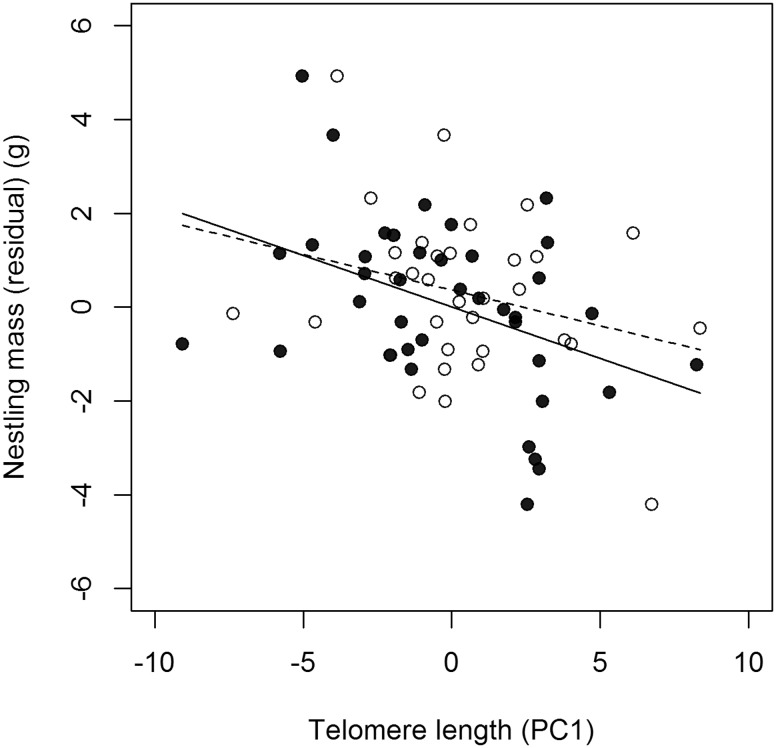
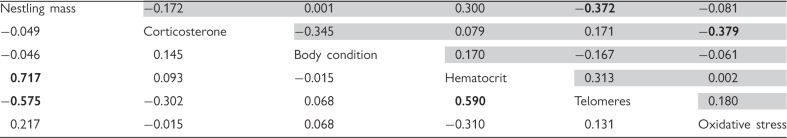
We obtained data from 46 females and 36 males in our study population. Both males and females with longer telomeres had lighter nestlings (Table 1, Fig. 1). Males with higher hematocrit values had longer telomeres and heavier nestlings (Table 1). Females with higher corticosterone levels had higher antioxidant capacity and lower oxidative damage (i.e., lower oxidative stress, reflected in lower oxidative PC1 values; Table 1).
Table 1.
Partial correlation coefficients for physiological traits and residual nestling mass in adult tree swallows during the breeding season for males (below, open squares) and females (above, shaded squares)
|
Telomere lengths and oxidative stress are the first components resulting from a PCA. High values of the oxidative PC1 indicate relatively higher oxidative stress (i.e., low antioxidant capacity and high ROMs). Numbers in bold indicate a significant relationship.
Fig. 1.
Adult tree swallows with longer telomeres (estimated as a principle component of average and the distribution of telomere lengths at each 10% percentile) had lighter offspring (residuals controlling for age). Females are represented by solid circles and solid regression line. Males are represented by open circles and dashed regression line.
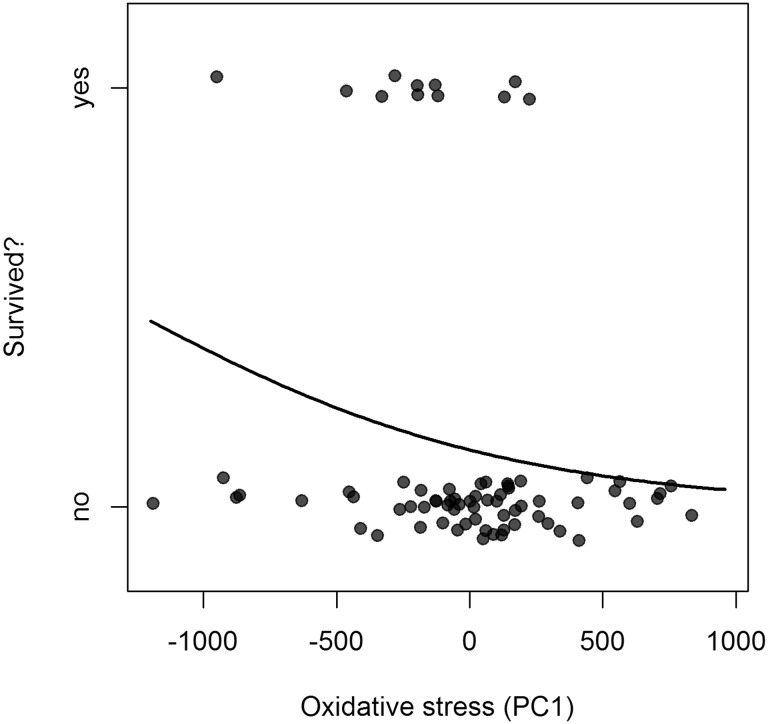
After model comparison, four models were within 2 AICc of the best-performing model (Table 2). The best-performing (i.e., lowest AICc) models all included the principle component of oxidative stress and sex. We show the parameter estimates as a result of the model averaging of the top four candidate models (Table 3). There was a tendency for males to return at a higher rate than females and a significant effect in which individuals with lower oxidative stress were more likely to return (Fig. 2).
Table 2.
Top candidate model set (within 2 AICc of the best-performing model) for the traits that best predicted return rates in adult tree swallows
| Model ID | K | AICc | Effects in the model | ΔAICc |
|---|---|---|---|---|
| 1 | 3 | 50.98 | Sex + OxsPC1 | 0.00 |
| 2 | 4 | 52.44 | Sex + OxsPC1 + teloPC1 | 1.46 |
| 3 | 4 | 52.49 | Sex + OxsPC1 + cort | 1.51 |
| 4 | 4 | 52.74 | Sex + OxsPC1 + cond | 1.76 |
K denotes the number of parameters in the model, AICc refers to the second order Akaike Information Criterion, ΔAICc shows the difference between the AICc value of the actual model and the best model in the model set. Variable abbreviations: OxsPC1 = principle component of oxidative stress, teloPC1 = principle component of telomere lengths, cort = corticosterone levels, cond = body condition.
Table 3.
Parameter estimates from model averages of the top four candidate models predicting return rates in adult tree swallows in relation to predictor variables.
| Variable | Coef | se(coef) | P value |
|---|---|---|---|
| Principle component of oxidative stress | −2.107 | 1.041 | 0.047 |
| Sex | 1.832 | 0.933 | 0.054 |
| Principle component of telomere lengths | 0.023 | 0.072 | 0.756 |
| Corticosterone | −0.011 | 0.038 | 0.771 |
| Body condition | 0.037 | 0.148 | 0.805 |
‘Coef’ indicates the coefficient and se(coef) the standard error of the estimate of the given parameter in the model.
Fig. 2.
Whether adult tree swallows returned to breed in the population is predicted by the magnitude of oxidative stress they experience. Oxidative stress (OxS) was expressed as the first axis of a principal component analysis of antioxidant status and oxidative damage. There is a typo here. It should read: Higher principle component values indicate low antioxidant capacity and higher ROMs. Raw data are represented with the predicted mean of the logistic model presented in the text. Values are jittered along the y-axis for better visibility.
Discussion
We found significant relationships between some physiological variables and life history traits in one-point samples of adult tree swallows during the breeding season. Following the predictions of life history theory, we found that individuals with high reproductive investment (reflected in heavy nestlings) had shorter telomeres. Moreover, the individuals that returned to breed within two years following initial sampling had lower oxidative stress. Additionally, the same pattern can reflect life-history strategy change across lifespan: Older individuals with more experience have greater reproductive investment and lower chance of survival.
Telomere lengths can be representative of an individual’s biological age as wear and tear on an organism’s cells will be recorded to a degree in the length of their telomeres (Pauliny et al. 2006; Angelier et al. 2013). While we cannot determine causality of the relationships we found, we speculate that females that invested into a larger clutch and males that fed their chicks more might have resulted in them having shorter telomeres than their counterparts. Longitudinal sampling for telomeres could have shed light on this hypothesis, but we were unable to collect repeat samples. However, in previous work, we found that male investment is a better predictor of nestling mass than female investment (Dakin et al. 2016). Indeed, the alternative might also be true, in which individuals with shorter telomeres were toward the end of their biological lifespan and having fewer chances to reproduce, invested more into the current reproductive attempt (Heidinger et al. 2006). Finally, if individuals with shorter telomeres were older and more experienced, those individuals might simply have been more efficient parents, and therefore able to raise larger nestlings. Our study corroborates a long-term cross-sectional study in common terns (Sterna hirundo) showing that individuals with higher reproductive success also had shorter telomeres, suggesting that reproductive success is achieved at the expense of telomere loss (Bauch et al. 2013). While manipulations of telomere lengths are not currently possible (although it is possible to block telomerase; Yegorov et al. 1996), a study has shown that manipulations of reproductive effort lead to increases in antioxidant defense but no change in telomere lengths (Beaulieu et al. 2011). Experimental studies have also shown that increased GC levels can cause increased telomere shortening (Haussmann et al. 2011; Beery et al. 2012). However, we did not find any relationship between telomere lengths and GCs, so the role of GCs in mediating these life-history trade-offs may be more complicated or context-dependent.
Counter to our predictions, we found that in females, relatively lower oxidative stress was associated with high corticosterone levels. On the one hand, GCs may increase oxidative damage, but on the other, they may initiate up-regulation of antioxidant defenses and thus reduce free radical production. Thus, GCs may protect against oxidative stress (Costantini et al. 2011). For example, GCs can induce synthesis of superoxide dismutase through modulation of gene expression in bovine cells (Yoshioka et al. 1994). Alternatively, higher GCs might have been reflective of individual quality, with higher quality females able to maintain lower oxidative stress. Moreover, GCs are notoriously labile, so it could be that GCs measured earlier or later may have a different relationship with OxS (Bonier et al. 2009). Given that we sampled the females during the nestling stage, in which a previous study also found that females during this stage with high GCs had higher reproductive investment, it could be another indicator of female quality (Bonier et al. 2009). We did not find this relationship in males, which might be driven by sex specific differences in energetic investment in reproduction. In contrast, males with low hematocrit values had shorter telomeres and heavier nestlings. This pattern could indicate that males with greater levels of investment in their offspring suffer reduced blood oxygen capacity. Additionally, this pattern could reflect male age and life history strategy, with older males having shorter telomeres, and investing more in reproduction (nestling mass) at the cost of self-maintenance (hematocrit). Having known age for all individuals might help explain some of these patterns.
We also found that individuals with higher oxidative stress (relatively higher ROMs and lower antioxidant capacity) were less likely to return to breed in the population in subsequent years. Individual survival may be dependent on oxidative damage, or simply correlate with it. As antioxidant capacity decreases, individuals may not be able to recover and thus develop pathology that results in higher mortality (Angelier et al. 2013; Barrett et al. 2013; Herborn et al. 2015). Alternatively, higher oxidative stress might be found in older birds that are investing more in reproduction or other energetic demands at the cost of less investment in self-maintenance. However, we did not find a direct link between oxidative stress and telomere lengths, or telomere lengths and survival. It could be that we needed to measure changes in telomere length to detect this relationship, or control for individual age. For example, in a study of another population of tree swallows, Haussmann et al. (2005) found that one-year old birds with shorter telomeres had lower survival than birds of the same age with longer telomeres. If survival rates are predicted by oxidative stress measurements in other free-living species, this might prove to be a better predictor of annual survival than corticosterone levels, which can be reflective of energetic demands, reproductive investment, and/or healthy individuals mounting a normal stress response.
In summary, with one-point samples, we found that some physiological traits are correlated to each other and to life history parameters, potentially elucidating costs of reproduction and resource allocation strategies. However, corticosterone levels were not related to reproductive investment or survival in this study, unlike previous studies in this same population (Bonier et al. 2009; Bonier et al. 2011). Therefore, we must be cautious in interpreting single point samples for endocrine traits, as variation measured can be a result of within individual rather than being informative about among individual differences (Lendvai et al. 2014). Indeed, in our previous work in tree swallows, we have found that within-individual changes in corticosterone can be more informative than single point measures (Bonier et al. 2011). On the other hand, oxidative stress measurements and telomere length were related to survival and reproduction, respectively. These findings could reflect a cost of reproduction, and demonstrate that individuals with high investment may pay a cost in decreasing chances of future reproduction.
Acknowledgments
The authors would like to thank the Queen’s University Biological Station. We acknowledge financial support from SICB (Divisions of Animal Behavior, Comparative Endocrinology, Ecology and Evolution, and Evolutionary Developmental Biology) and NSF (IOS 1539936) that facilitated the symposium and discussion. We thank B. Cox, B. Pinshow, and two anonymous reviewers for their comments.
Funding
This work was supported by a U.S. National Science Foundation grant (NSF; FB, ITM, and MH; IOS-1145625), a Natural Sciences and Engineering Research Council of Canada (NSERC) Banting Postdoctoral Fellowship (FB), a NSERC Discovery Grant (FB), a NSF postdoctoral research fellowship in biology (JQO; DBI-1306025), and a Hungarian Research Fund Grant (OTKA K113108 to ÁZL).
References
- Allen RG, Tresini M. 2000. Oxidative stress and gene regulation. Fr Rad Biol Med 28:463–99. [DOI] [PubMed] [Google Scholar]
- Angelier F, Vleck CM, Holberton RL, Marra PP. 2013. Telomere length, non-breeding habitat and return rate in male American redstarts. Func Ecol 27:342–50. [Google Scholar]
- Astheimer LB, Buttemer WA, Wingfield JC. 1992. Interactions of corticosterone with feeding, activity and metabolism in passerine birds. Ornis Scand 23:355–65. [Google Scholar]
- Barrett ELB, Burke TA, Hammers M, Komdeur J, Richardson DS. 2013. Telomere length and dynamics predict mortality in a wild longitudinal study. Mol Ecol 22:249–59. [DOI] [PubMed] [Google Scholar]
- Bauch C, Becker PH, Verhulst S. 2013. Telomere length reflects phenotypic quality and costs of reproduction in a long-lived seabird. Proc R Soc Lond B Biol Sci 280:20122540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu M, Reichert S, Le Maho Y, Ancel A, Criscuolo F. 2011. Oxidative status and telomere length in a long-lived bird facing a costly reproductive event. Func Ecol 25:577–85. [Google Scholar]
- Beery AK, Lin J, Biddle JS, Francis DD, Blackburn EH, Epel ES. 2012. Chronic stress elevates telomerase activity in rats. Biol Lett 8:1063–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonier F, Moore I, Martin P, Robertson R. 2009. The relationship between fitness and baseline glucocorticoids in a passerine bird. Gen Comp Endocrinol 163:208–13. [DOI] [PubMed] [Google Scholar]
- Bonier F, Moore IT, Robertson RJ. 2011. The stress of parenthood? Increased glucocorticoids in birds with experimentally enlarged broods. Biol Lett 7:944–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. 2002. Model Selection and Multi-Model Inference: A Practical Information-Theoretical Approach. New York: Springer-Verlag. [Google Scholar]
- Cohen AA, Mauck RA, Wheelwright NT, Huntington CE, McGraw KJ. 2009. Complexity in relationships between antioxidants and individual life-history parameters in a seabird and a songbird. Oikos 118:1854–61. [Google Scholar]
- Costantini D. 2008. Oxidative stress in ecology and evolution: lessons from avian studies. Ecol Lett 11:1238–51. [DOI] [PubMed] [Google Scholar]
- Costantini D, Marasco V, Moller AP. 2011. A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. J Comp Physiol B Biochem Syst Environ Physiol 181:447–56. [DOI] [PubMed] [Google Scholar]
- Dakin R, Lendvai ÁZ, Ouyang JQ, Moore IT, Bonier F. 2016. Plumage colour is associated with partner parental care in mutually ornamented tree swallows. Anim Behav 111:111–8. [Google Scholar]
- Greider CW. 1996. Telomere length regulation. Ann Rev Biochem 65:337–65. [DOI] [PubMed] [Google Scholar]
- Haussmann MF, Heidinger BJ. 2015. Telomere dynamics may link stress exposure and ageing across generations. Biol Lett 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussmann MF, Longenecker AS, Marchetto NM, Juliano SA, Bowden RM. 2011. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc R Soc Lond B Biol Sci. 279:1447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussmann MF, Longenecker AS, Marchetto NM, Juliano SA, Bowden RM. 2012. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc R Soc Lond B [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussmann MF, Marchetto NM. 2010. Telomeres: Linking stress and survival, ecology and evolution. Curr Zool 56:714–27. [Google Scholar]
- Haussmann MF, Mauck RA. 2008. Telomeres and longevity: testing an evolutionary hypothesis. Mol Biol Evol 25:220–8. [DOI] [PubMed] [Google Scholar]
- Haussmann MF, Winkler DW, Vleck CM. 2005. Longer telomeres associated with higher survival in birds. Biol Lett 1:212–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidinger BJ, Nisbet ICT, Ketterson ED. 2006. Older parents are less responsive to a stressor in a long-lived seabird: a mechanism for increased reproductive performance with age? Proc R Soc Lond B Biol Sci 273:2227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herborn KA, Daunt F, Heidinger BJ, Granroth-Wilding HMV, Burthe SJ, Newell MA, Monaghan P. 2015. Age, oxidative stress exposure and fitness in a long-lived seabird. Func Ecol. DOI: 10.1111/1365-2435.12578. [Google Scholar]
- Landys MM, Ramenofsky M, Wingfield JC. 2006. Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen Comp Endocrinol 148:132–49. [DOI] [PubMed] [Google Scholar]
- Lendvai ÁZ, Ouyang JQ, Schoenle LA, Fasanello V, Haussmann MF, Bonier F, Moore IT. 2014. Experimental food restriction reveals individual differences in corticosterone reaction norms with no oxidative costs. PLoS ONE 9:e110564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Deculypere E, Buyse J. 2004a. Oxidative stress induced by corticosterone administration in broiler chickens (Gallus gallus domesticus) - 2. Short-term effect. Comp Biochem Physiol B Biochem Mol Biol 139:745–51. [DOI] [PubMed] [Google Scholar]
- Lin H, Decuypere E, Buyse J. 2004b. Oxidative stress induced by corticosterone administration in broiler chickens (Gallus gallus domesticus) - 1. Chronic exposure. Comp Biochem Physiol B Biochem Mol Biol 139:737–44. [DOI] [PubMed] [Google Scholar]
- Metcalfe NB, Alonso-Alvarez C. 2010. Oxidative stress as a life-history constraint: the role of reactive oxygen species in shaping phenotypes from conception to death. Func Ecol 24:984–96. [Google Scholar]
- Monaghan P. 2010. Telomeres and life histories: the long and the short of it Annals of the New York Academy of Sciences 1206:130–42. [DOI] [PubMed] [Google Scholar]
- Monaghan P, Metcalfe NB, Torres R. 2009. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol Lett 12:75–92. [DOI] [PubMed] [Google Scholar]
- Pauliny A, Wagner RH, Augustin J, Szép T, Blomqvist D. 2006. Age-independent telomere length predicts fitness in two bird species. Mol Ecol 15:1681–7. [DOI] [PubMed] [Google Scholar]
- Peig J, Green AJ. 2009. New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118. [Google Scholar]
- Romero LM. 2002. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen Comp Endocrinol 128:1–24. [DOI] [PubMed] [Google Scholar]
- R Core Team 2015. R: A Language and Environment for Statistical Computing. Vienna, Austria: http://www.R-project.org. [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endoc Rev 21:55–89. [DOI] [PubMed] [Google Scholar]
- Sies H. 1991. Oxidative stress: From basic research to clinical application. Am J Med 91:S31–8. [DOI] [PubMed] [Google Scholar]
- Sorce S, Krause K-H. 2009. NOX Enzymes in the central nervous system: from signaling to disease. Antiox Red Sig 11:2481–504. [DOI] [PubMed] [Google Scholar]
- Stearns SC. 1989. Trade-offs in life-history evolution. Func Ecol 3:259–68. [Google Scholar]
- van Noordwijk AJ, de Jong G. 1986. Acquisition and allocation of resources: their influence on variation in life history tactics. Am Nat 128:137–42. [Google Scholar]
- Wingfield J, Silverin B. 2002. Ecophysiological studies of hormone-behavior relations in birds. In: Hormones, Brain and Behavior. p. 587–647. [Google Scholar]
- Winkler DW, Wrege PH, Allen PE, Kast TL, Senesac P, Wasson MF, Llambías PE, Ferretti V, Sullivan PJ. 2004. Breeding dispersal and philopatry in the tree swallow. The Condor 106:768–76. [Google Scholar]
- Yegorov YE, Chernov DN, Akimov SS, Bolsheva NL, Krayevsky AA, Zelenin AV. 1996. Reverse transcriptase inhibitors suppress telomerase function and induce senescence-like processes in cultured mouse fibroblasts. FEBS Lett 389:115–8. [DOI] [PubMed] [Google Scholar]
- Yoshioka T, Homma T, Meyrick B, Takeda M, Moore-Jarrett T, Kon V, Ichikawa I. 1994. Oxidants induce transcriptional activation of manganese superoxide dismutase in glomerular cells. Kid Intern 46:405–13. [DOI] [PubMed] [Google Scholar]