Abstract
OBJECTIVE:
To examine the association between the 2014 U.S. Food and Drug Administration (FDA) safety communication on power morcellation and surgical approach and morbidity after myomectomy.
METHODS:
In this retrospective cohort study, data were abstracted from the American College of Surgeons National Surgical Quality Improvement Program database on 3,160 myomectomies between April 2012 and December 2013 (pre-FDA) and 4,378 between April 2014 and December 2015 (post-FDA). Aims were to 1) compare rates of abdominal and laparoscopic myomectomy pre-FDA and post-FDA (primary outcome), 2) directly compare the morbidity of abdominal and laparoscopic myomectomy during each time period (secondary outcome 1), and 3) compare the morbidity after all myomectomies performed pre-FDA and post-FDA (secondary outcome 2). Adjusted means, odds ratios, and rate ratios with 95% confidence intervals were calculated using linear, logistic, and Poisson regression, respectively, adjusting for age, race, ethnicity, body mass index, and myoma burden.
RESULTS:
Myomectomies performed post-FDA were more likely to be abdominal (60.0%, 95% confidence interval [CI] 58.6–61.5%) than laparoscopic (40.0%, 95% CI 38.5–41.4%) as compared with myomectomies pre-FDA, which were equally divided between surgical approaches (49.1% abdominal, 95% CI 47.4–50.9% compared with 50.9% laparoscopic, 95% CI 49.1–52.6%; P<.001). When directly compared with laparoscopic myomectomy, abdominal myomectomy was associated with longer hospitalizations, higher readmission rates, and greater morbidity both pre-FDA and post-FDA (P<.05, all comparisons). Adjusted models demonstrated shorter operative times post-FDA for all myomectomies (P<001), although composite morbidity was similar between myomectomies performed pre-FDA and post-FDA (P=.809).
CONCLUSIONS:
The FDA safety communication on power morcellation was associated with an 11% absolute increase in the use of abdominal myomectomy. Although morbidity is consistently higher after abdominal as compared with laparoscopic myomectomy, the increased reliance on abdominal myomectomy post-FDA did not result in clinically significant changes in morbidity in this cohort.
Myomectomy is the surgical standard of care for reproductive-aged women with symptomatic uterine fibroids. A recent meta-analysis suggests that laparoscopic myomectomy has half the complication rate of abdominal myomectomy while offering a minimally invasive approach with diminished blood loss and faster recovery.1 Traditionally, laparoscopic myomectomy is aided by power morcellation, allowing for efficient specimen retrieval through laparoscopic port sites. However, safety concerns regarding the potential dissemination of occult leiomyosarcoma with power morcellation were raised in the lay press in December 2013.2 The U.S. Food and Drug Administration (FDA) published its initial safety communication in April 2014 discouraging the use of power morcellation for the treatment of uterine fibroids based on concern for the inadvertent spread of occult uterine malignancy.3 Although alternatives such as hand-based morcellation exist, fewer minimally invasive hysterectomies have been performed since the FDA communication with an associated increase in major surgical complications, blood transfusion, and 30-day hospital readmission.4 It is plausible that similar trends in surgical approach and morbidity have occurred for myomectomy, although this question has not been evaluated to date.
We hypothesize that an increased proportion of myomectomies will be performed abdominally after the FDA safety communication. Assuming a higher relative morbidity of abdominal as compared with laparoscopic myomectomy, we hypothesize that this change in practice will result in an overall increase in surgical morbidity for all myomectomies performed after the FDA safety communication.
MATERIALS AND METHODS
This retrospective cohort study aims to 1) compare rates of abdominal and laparoscopic myomectomy in the 21 months before and after the FDA safety communication regarding power morcellation (primary outcome), 2) directly compare the morbidity of abdominal and laparoscopic myomectomies during each time period (secondary outcome 1), and 3) compare the morbidity after all myomectomies performed in the 21 months before and after the FDA safety communication regarding power morcellation (secondary outcome 2) using data abstracted from the American College of Surgeons (ACS) National Quality Improvement Program database.
The ACS National Surgical Quality Improvement Program was established in 2004 as a nationally validated database intended to measure and improve surgical outcomes across specialties. Preoperative, intraoperative, and 30-day postoperative data are abstracted directly from patient medical records by specially trained personnel at each of the greater than 600 participating hospitals nationwide. Data collection methods for the ACS National Surgical Quality Improvement Program are publically available5 and have been described in detail in other publications.6–8 In brief, trained surgical clinical reviewers at each site prospectively collect preoperative and postoperative morbidity and mortality data from each patient’s medical record during the course of hospital admission and for up to 30 days after surgery. Regular audits ensure the quality of the data set, which has been shown to have an overall interrater variability of less than 1.6% in recent years.9
Data were abstracted on 3,160 women undergoing myomectomy between April 2012 and December 2013 (before FDA communication=pre-FDA) and 4,378 women undergoing myomectomy between April 2014 and December 2015 (after FDA communication=post-FDA). Time points were selected based on the increased scrutiny of morcellation by the lay press and professional societies starting in mid-December 2013. Cases performed between January and March 2014 were excluded from analysis as a washout period to allow for changing practices and were retained to create the complete timeline included in Figure 1. Data were limited to premenopausal women younger than 55 years of age based on the nationally reported mean age of menopause at age 51 years10 with a standard deviation of 4.15 years.11 Emergent cases, myomectomies performed by a nongynecologist, vaginal myomectomy, and myomectomy completed at the time of cesarean delivery or hysterectomy were excluded from analysis. Patients with underlying malignancy, recent chemotherapy, or radiation were also excluded. The study of existing publically available data was granted category four exemption by the institutional review board of the University of Pennsylvania.
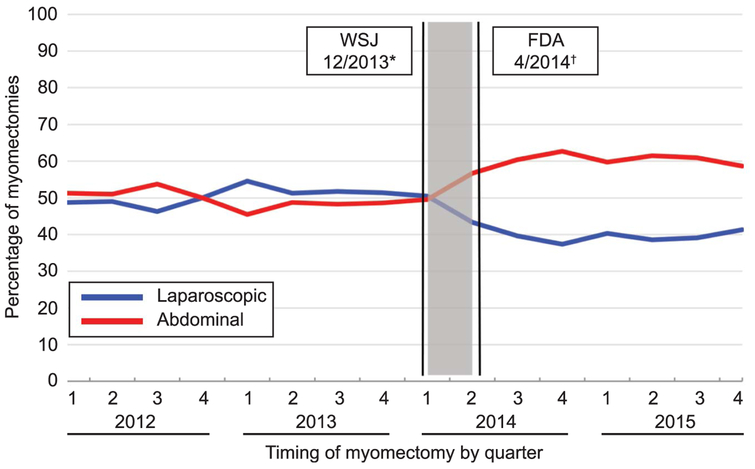
Fig. 1.
Surgical approach by percentages of all myomectomies by annual quarter, 2012–2015. Laparoscopic (blue line) and abdominal (red line) myomectomy were used comparably until quarter 2 of 2014 after which significantly more myomectomies were performed using the abdominal approach (P<.001). Gray box corresponds to washout period for analysis, January to March 2014. *Wall Street Journal (WSJ) communication December 18, 2013.2 †U.S. Food and Drug Administration (FDA) safety communication April 17, 2014.3
Current Procedural Terminology codes were used to classify abdominal and laparoscopic myomectomy. Current Procedural Terminology codes 58545 and 58140 were used to correspond to low myoma burden (one to four myomas or total weight 250 g or less), and 58146 and 58546 correspond to high myoma burden (greater than five myomas or myoma weight greater than 250 g). Analyses focused on a subset of outcome variables within the ACS National Surgical Quality Improvement Program database thought to capture the majority of patient morbidity after myomectomy. Assessed outcomes included medical variables (deep venous thrombosis [DVT], pulmonary embolus [PE], urinary tract infection, and sepsis), surgical variables (blood transfusion, wound infection, and wound dehiscence) as well as operative time in minutes (from incision to closure), length of stay in days, and rates of reoperation and readmission within 30 days of the initial surgery. A composite for wound infection was created combining superficial, deep, and organ space surgical site infections; a composite for thromboembolism was created combining DVT and PE; and a composite for sepsis was created combining sepsis and septic shock. A composite score of morbidity was created combining blood transfusion, wound dehiscence, wound infection, urinary tract infection, sepsis, and DVT and PE. Assuming 3,000 myomectomies both pre-FDA and post-FDA with a baseline morbidity rate of 15%, a power calculation was performed to confirm we would have 80% power to detect a 2.7% absolute increase in morbidity after the FDA, a number we found to be clinically significant. Power would be greater with baseline morbidity rates less than 15%.
Unadjusted comparisons of categorical baseline characteristics, surgical approach, and perioperative morbidity pre-FDA and post-FDA were performed with χ2 tests. Age, body mass index (BMI, calculated as weight (kg)/[height (m)]2), and operative time were normally distributed and compared using two-sample t tests. Length of stay was right-skewed and evaluated with Wilcoxon rank-sum. Multivariable analysis was preformed adjusting for age, race, ethnicity, BMI, and myoma burden. Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for approach, readmission, transfusion, and composite morbidity were calculated using multivariable logistic regression. Adjusted mean operative times and CIs were calculated using multivariable linear regression, and adjusted incidence rate ratios and CIs for total length of stay were calculated using an overdispersed Poisson regression model. Given the limited number of events, adjusted analysis was unable to be completed for rates of reoperation, DVT and PE, urinary tract infection, sepsis, surgical site infections, and wound dehiscence. Analysis was completed using STATA 13 and two-sided P values of ≤.05 were considered statistically significant.
RESULTS
A total of 8,286 women underwent laparoscopic or abdominal myomectomy between April 2012 and December 2015. Excluded cases were comprised of: 564 cases completed during the washout period of January to March 2014, 114 women older than 55 years of age, 56 emergent cases, three cases completed at the time of cesarean delivery, and 11 cases completed at the time of hysterectomy. All demographic variables were complete with the exception of 10.1% missing data for Hispanic race and 13.2% missing data on race. Demographic characteristics of the study population are shown in Table 1. A larger proportion of black patients (48.9%, 95% CI 47.3–50.5%) and a smaller proportion of white patients (40.8%, 95% CI 39.2–42.4%) underwent myomectomy post-FDA as compared with pre-FDA (41.9%, 95% CI 40.0–43.7% compared with 48.6%, 95% CI 46.7–50.4%, respectively, P<.001). Age and BMI were statistically different (P<.001) but clinically similar (0.9 years and 0.7, respectively) between groups. Remaining demographic characteristics were comparable between groups.
Table 1.
Demographic Characteristics of all Myomectomies From 2012 to 2015 and Those Examined Before and After the U.S. Food and Drug Administration Communication
| Demographic Characteristic | All Myomectomies (N=8,438)* | Pre-FDA (n=3,160)* | Post-FDA (n=4,378)* | P |
|---|---|---|---|---|
| Age (y) | 36.9±6.0 | 37.4±6.0 | 36.5±5.9 | <.001 |
| Race† | ||||
| White | 3,207 (43.8) | 1,325 (48.5) | 1,552 (40.8) | <.001 |
| Black | 3,383 (46.2) | 1,143 (41.9) | 1,861 (48.9) | |
| Asian | 666 (9.0) | 236 (8.7) | 352 (9.3) | |
| Other | 72 (1.0) | 25 (0.9) | 38 (1.0) | |
| Ethnicity | ||||
| Hispanic† | 844 (11.1) | 331 (11.4) | 448 (11.5) | .888 |
| Smoking status | 838 (9.9) | 314 (9.9) | 423 (9.7) | .692 |
| Comorbidity | ||||
| BMI (kg/m2) | 28.8±6.8 | 28.4±6.9 | 29.1±6.8 | <.001 |
| Diabetes | 200 (2.4) | 79 (2.5) | 107 (2.4) | .877 |
| HTN | 776 (9.2) | 305 (9.7) | 380 (8.7) | .147 |
FDA, U.S. Food and Drug Administration; BMI, body mass index;HTN, hypertension.
Data are mean±standard deviation or n (%) unless otherwise specified.
Includes all participants who had myomectomies from January 2012 to December 2015; pre-FDA, April 2012 to December 2013; post-FDA, April 2014 to December 2015.
Demographic data are complete, with the exception of 10.1% missing data for Hispanic ethnicity and 13.2% missing data for race.
When comparing surgical approach with myomectomy in the 21 months before and after the FDA safety communication regarding power morcellation, the percentage of abdominal myomectomies increased from 49.1% (95% CI 47.4–50.9%) pre-FDA to 60.0% (95% CI 58.6–61.5%) post-FDA with an associated decrease in the percentage of laparoscopic myomectomies from 50.9% (95% CI 49.1–52.6%) to 40.0% (95% CI 38.5–41.4%; P<.001; Fig. 1; Table 2). Disease burden was similar in myomectomies performed pre-FDA and post-FDA (P=.070).
Table 2.
Unadjusted Changes in Operative Approach and Morbidity Before and After the U.S. Food and Drug Administration Communication
| Operative Variable |
Pre-FDA (n=3,160)* |
Post-FDA (n=4,378)* |
P |
|---|---|---|---|
| Surgical approach | |||
| Abdominal | 1,552 (49.1) | 2,628 (60.0) | <.001 |
| Laparoscopic | 1,608 (50.9) | 1,750 (40.0) | |
| Burden of disease | |||
| Low | 1,932 (61.1) | 2,586 (59.1) | .070 |
| High | 1,228 (38.9) | 1,792 (40.9) | |
| Abdominal | |||
| Low burden | 843 (54.3) | 1,397 (53.2) | .468 |
| High burden | 709 (45.7) | 1,231 (46.8) | |
| Laparoscopic | |||
| Low burden | 108 (67.7) | 1,189 (67.9) | .892 |
| High burden | 519 (32.3) | 561 (32.1) | |
| OR time (min) | 152.4±82.1 | 147.0±76.4 | .003 |
| Length of stay (d) | 1 (0–2) | 1 (0–2) | <.001 |
| Reoperation within 30 d | 23 (0.7) | 37 (0.9) | .572 |
| Readmission within 30 d | 54 (1.7) | 74 (1.7) | .966 |
| Medical morbidity | |||
| DVT or PE | 11 (0.4) | 16 (0.4) | .901 |
| UTI | 32 (1.0) | 45 (1.0) | .948 |
| Sepsis | 9 (0.3) | 23 (0.5) | .113 |
| Surgical morbidity | |||
| Blood transfusion | 298 (9.4) | 399 (9.1) | .640 |
| Surgical site infections | 37 (1.2) | 66 (1.5) | .214 |
| Wound dehiscence | 4 (0.1) | 6 (0.1) | .902 |
| Composite morbidity† | 363 (11.5) | 512 (11.7) | .790 |
FDA, U.S. Food and Drug Administration;OR time, operative time; DVT, deep venous thrombosis;PE, pulmonary embolism; UTI, urinary tract infection.
Data are n (%), mean±standard deviation, or median (interquartile range) unless otherwise specified.
Pre-FDA April 2012 to December 2013; Post-FDA April 2014 to December 2015.
Morbidity represents a composite of blood transfusion, wound dehiscence, wound infection, UTI, sepsis or septic shock, and DVT or PE.
When compared directly with laparoscopic myomectomy, abdominal myomectomy was associated with significantly longer hospitalizations and higher rates of readmission, sepsis, and blood transfusion in both pre-FDA and post-FDA time periods (P<.05 for all comparisons; Tables 3 and 4). Disease burden was similar in myomectomies pre-FDA and post-FDA examined separately by surgical approach (P=.468 abdominal, P=.892 laparoscopic) in unadjusted analyses (Table 2). Composite morbidity remained constant over time (time by procedure approach interaction P=.781). Composite morbidity remained threefold higher after abdominal as compared with laparoscopic myomectomy both pre-FDA (adjusted OR 3.1, 95% CI 2.3–4.0) and post-FDA (adjusted OR 3.0, 95% CI 2.3–3.9) when adjusting for age, race, ethnicity, BMI, and myoma burden.
Table 3.
Unadjusted Changes in Operative Approach and Morbidity Before the U.S. Food and Drug Administration Communication, by Myomectomy Approach*
| Operative Approach |
Laparoscopic (n=1,608) |
Abdominal (n=1,552) |
P |
|---|---|---|---|
| Burden of disease | |||
| Low | 1,089 (67.7) | 843 (54.3) | <.001 |
| High | 519 (32.3) | 709 (45.7) | |
| OR time (min) | 169.9±89.2 | 134.4±69.6 | <.001 |
| Length of stay (d) | 0 (0–1) | 2 (2–3) | <.001 |
| Reoperation within 30 d | 11 (0.7) | 12 (0.8) | .768 |
| Readmission within 30 d | 18 (1.1) | 36 (2.3) | .009 |
| Medical morbidity | |||
| DVT or PE | 2 (0.1) | 9 (0.6) | .030 |
| UTI | 11 (0.7) | 21 (1.4) | .060 |
| Sepsis | 2 (0.1) | 7 (0.5) | .085 |
| Surgical morbidity | |||
| Blood transfusion | 66 (4.1) | 232 (15.0) | <.001 |
| Surgical site infections | 15 (0.9) | 22 (1.4) | .205 |
| Wound dehiscence | 0 (0.0) | 4 (0.3) | .042 |
| Composite Morbidity† | 90 (5.6) | 273 (17.6) | <.001 |
OR time, operative time;DVT, deep venous thrombosis; PE, pulmonary embolism; UTI, urinary tract infection.
Data are n (%), mean±standard deviation, or median (interquartile range) unless otherwise specified.
Time period April 2012 to December 2013.
Morbidity represents a composite of blood transfusion, wound dehiscence, wound infection, UTI, sepsis or septic shock, and DVT or PE.
Table 4.
Unadjusted Changes in Operative Approach and Morbidity After the U.S. Food and Drug Administration Communication, by Myomectomy Approach*
| Operative Variable |
Laparoscopic (n=1,750) |
Abdominal (n=2,628) |
P |
|---|---|---|---|
| Burden of disease | |||
| Low | 1,189 (67.9) | 1,397 (53.2) | <.001 |
| High | 561 (32.1) | 1,231 (46.8) | |
| OR time (min) | 169.8±85.3 | 131.8±65.6 | <.001 |
| Length of stay (d) | 0 (0–1) | 2 (2–3) | <.001 |
| Reoperation within 30 d | 8 (0.5) | 29 (1.1) | .022 |
| Readmission within 30 d | 21 (1.2) | 53 (2.1) | .034 |
| Medical morbidity | |||
| DVT or PE | 5 (0.3) | 11 (0.4) | .475 |
| UTI | 13 (0.7) | 32 (1.2) | .127 |
| Sepsis | 3 (0.2) | 20 (0.8) | .008 |
| Surgical morbidity | |||
| Blood transfusion | 49 (2.8) | 350 (13.3) | <.001 |
| Surgical site infections | 22 (1.3) | 44 (1.7) | .267 |
| Wound dehiscence | 1 (0.1) | 5 (0.2) | .244 |
| Composite morbidity† | 88 (5.0) | 424 (16.1) | <.001 |
OR time, operative time;DVT, deep venous thrombosis; PE, pulmonary embolism; UTI, urinary tract infection.
Data are n (%), mean±standard deviation, or median (interquartile range) unless otherwise specified.
Time period April 2014 to December 2015.
Morbidity represents a composite of blood transfusion, wound dehiscence, wound infection, UTI, sepsis or septic shock, and DVT or PE.
When comparing operative times and morbidity after all myomectomies performed in the 21 months before and after the FDA safety communication regarding power morcellation, unadjusted analysis revealed shorter operative times (P=.003) but no differences in rates of reoperation, readmission, or morbidity (P>.05 for all comparisons; Table 2). These trends were confirmed in multivariate analyses adjusting for age, race, ethnicity, BMI, and myoma burden (Table 5). Adjusted models demonstrated shorter operative times post-FDA for all myomectomies (116.5 minutes, 95% CI 97.6–135.4 compared with 125.2 minutes, 95% CI 110.1–140.2; P<.001) and abdominal myomectomies (80.3 minutes, 95% CI 58.1–102.4 compared with 102.4 minutes, 95% CI 68.3–103.4; P=.017) with no change in the length of laparoscopic myomectomy (P=.230). Patients undergoing myomectomy after the FDA communication had a 50% increased odds of having abdominal as opposed to laparoscopic surgery (adjusted OR 1.5, 95% CI 1.3–1.6) with no change in length of stay, readmission rates, blood transfusion, or composite morbidity scores (P>.143 for all comparisons).
Table 5.
Adjusted Analysis of Operative Time, Length of Hospital Stay, and Morbidity After the U.S. Food and Drug Administration Communication
| Operative Variable | Pre-FDA* | Post-FDA* | P |
|---|---|---|---|
| Abdominal approach† | 1.0 (referent) | 1.5 (1.3–1.6) | <.001 |
| All OR time (min)‡ | 125.2 (110.1–140.2) | 116.5 (97.6–135.4) | <.001 |
| Abdominal OR time‡ | 85.8 (68.3–103.4) | 80.3 (58.1–102.4) | .017 |
| Laparoscopic OR time‡ | 157.0 (133.4–180.5) | 153.2 (123.5–182.9) | .230 |
| Length of stay§ | 1.0 (referent) | 1.0 (1.0–1.1) | .143 |
| Readmission† | 1.0 (referent) | 1.0 (0.7–1.5) | .897 |
| Transfusion† | 1.0 (referent) | 1.0 (0.8–1.1) | .628 |
| Composite morbidit†‖ | 1.0 (referent) | 1.0 (0.9–1.2) | .809 |
FDA, U.S. Food and Drug Administration; OR time, operative time.
All analyses adjusted for age, race, ethnicity, body mass index, and myoma burden. Given the limited number of events, adjusted analysis was unable to be completed for reoperation, deep vein thrombosis or pulmonary embolism, urinary tract infection, sepsis, surgical site infections, and wound dehiscence.
Pre-FDA, April 2012 to December 2013; post-FDA, April 2014 to December 2015.
Results from logistic regression models, expressed as adjusted means with 95% confidence intervals (CIs).
Results from linear regression models, expressed as adjusted odds ratios with 95% CIs.
Results from an overdispersed Poisson regression model, expressed as adjusted incidence rate ratios with 95% CIs.
Morbidity represents a composite of blood transfusion, wound dehiscence, wound infection, urinary tract infection, sepsis or septic shock, and deep vein thrombosis or pulmonary embolism.
DISCUSSION
The FDA safety communication on power morcellation in April 2014 was associated with changes in the practice of myomectomy. Although laparoscopic and abdominal approaches were used comparably before the FDA communication, significantly more myomectomies are performed abdominally after the FDA communication. Although abdominal myomectomy is consistently more morbid than laparoscopic myomectomy, the increased reliance on abdominal myomectomy after the FDA communication did not result in detectable changes in overall morbidity in this cohort.
The 11% increase in the rate of abdominal myomectomy after the FDA communication identified in the present study is lower than the 19% increase previously reported by a study examining myomectomy rates at a single institution in the 16 months surrounding the FDA communication.12 This may be attributable to the lower rate of abdominal myomectomy performed at this institution before the FDA communication (37.2%) compared with the 49.1% rate in the national ACS National Quality Improvement Program cohort. Absolute rates of abdominal myomectomy after the FDA communication were similar between studies (49/87 [56.3%] in the single-institution study compared with 2,628/ 4,388 [60.0%] in the current study).
It is notable that the 11% absolute increase in abdominal myomectomy identified in the current study did not result in a corresponding change in morbidity. In contrast, an examination of hysterectomy trends after the FDA communication demonstrated increased morbidity after a much smaller 1.5–5.8% absolute increase in the rate of abdominal hysterectomy.4 This contrast is likely the result of differences in the patient population undergoing myomectomy compared with hysterectomy; myomectomy is generally performed in younger women whose lower preoperative risk profile results in a lower morbidity rate. The relative increase in abdominal compared with laparoscopic myomectomy may have to be larger before significant change in morbidity is seen.
A primary strength of this study is its ability to assess changes in practice and morbidity of myomectomy at a national level in private and academic settings. This high-volume data set is particularly suited to study rare outcomes as a result of its size and associated power. As with any study using large aggregate data, our study has limitations. The ACS National Surgical Quality Improvement Program data are inherently limited in scope in that only 30-day postoperative outcomes are available. Prior studies1,13–15 have demonstrated longer time off work and delayed return to daily functioning after abdominal as compared with laparoscopic myomectomy. Because these variables are not captured by the present database, our findings likely understate the true morbidity associated with the increased use of abdominal myomectomy. Although excluded from multivariate analysis, limited amounts of missing data for ethnicity (10.1%) and race (13.2%) may contribute to biased estimates. Additionally, a greater proportion of black women underwent myomectomy post-FDA. Although multivariate models adjusted for race, the complex interaction between race and morbidity after myomectomy warrants future investigation. Furthermore, although multiple techniques exist for specimen extraction (electronic power morcellation, scalpel morcellation, intra- and extracorporeal, contained and uncontained), the present study lacks information on morcellation technique. Finally, the ACS National Quality Improvement Program database does not collect data on final pathologic diagnosis and the present study cannot comment on leiomyosarcoma in this cohort.
The FDA safety communication discouraged the use of electronic power morcellation based on concern for the inadvertent spread of occult uterine malignancy and included a limited literature review estimating the prevalence of unsuspected uterine sarcoma to be 1 in 350.3 A recent meta-analysis of prospective studies on surgery for presumed fibroids has estimated the prevalence of unsuspected leiomyosarcoma to be much lower at 1 in 2,000–8,300,16 as have studies examining the risk of uterine malignancy after myomectomy with electronic power morcellation (1/1,073).17 Both authors noted that age is among the most significant risk factors for undiagnosed leiomyosarcoma. These findings are significant when considering the clinical implications of the current study. As opposed to hysterectomy, myomectomy is typically performed in younger women, a population with a lower risk of undiagnosed uterine malignancy. Moreover, although abdominal hysterectomy confers the ability to resect an undiagnosed uterine malignancy en bloc, the same cannot be said of any myomectomy. Indeed, recent studies have demonstrated dissemination of leiomyoma cells even at the time of abdominal myomectomy.18 Regardless, the findings of the present study demonstrate a new reliance on abdominal myomectomy after the FDA safety communication. Although no discernable differences in short-term postoperative morbidity were noted, the outstanding clinical effect of this practice change on hospitalization costs, extended postoperative morbidity, and delayed return to work remains critical to understand in future study.
Footnotes
Presented at the annual meeting of the American Society for Reproductive Medicine, October 16–20, 2016, Salt Lake City, Utah.
The American College of Surgeons National Surgical Quality Improvement Program and its participating hospitals are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Each author has indicated that he or she has met the journal’s requirements for authorship.
Financial Disclosure
The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Jin C, Hu Y, Chen X, Zheng FY, Lin F, Zhou K, et al. Laparoscopic versus open myomectomy–a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol 2009; 145:14–21. [DOI] [PubMed] [Google Scholar]
- 2.Levitz J. Doctors eye cancer risk in uterine procedure. Wall Street J December 18, 2013. [Google Scholar]
- 3.U.S. Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. Washington, DC: U.S. Food and Drug Administration; 2014. [Google Scholar]
- 4.Harris JA, Swenson CW, Uppal S, Kamdar N, Mahnert N, As-Sanie S, Morgan DM, et al. Practice patterns and postoperative complications before and after US Food and Drug Administration safety communication on power morcellation. Am J Obstet Gynecol 2016;214:98.e1–98.e13. [DOI] [PubMed] [Google Scholar]
- 5.American College of Surgeons. American College of Surgeons National Surgical Quality Improvement Program user guide. Chicago (IL): American College of Surgeons; 2012. [Google Scholar]
- 6.Birkmeyer JD, Shahian DM, Dimick JB, Finlayson SR, Flum DR, Ko CY, et al. Blueprint for a new American College of Surgeons: National Surgical Quality Improvement Program. J Am Coll Surg 2008;207:777–82. [DOI] [PubMed] [Google Scholar]
- 7.Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg 2010;44:251–67. [DOI] [PubMed] [Google Scholar]
- 8.Khuri SF, Henderson WG, Daley J, Jonasson O, Jones RS, Campbell DA Jr, et al. Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: the Patient Safety in Surgery study. Ann Surg 2008;248:329–36. [DOI] [PubMed] [Google Scholar]
- 9.Shiloach M, Frencher SK Jr, Steeger JE, Rowell KS, Bartzokis K, Tomeh MG, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg 2010;210:6–16. [DOI] [PubMed] [Google Scholar]
- 10.McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas 2008;61:4–16. [DOI] [PubMed] [Google Scholar]
- 11.van Noord PA, Dubas JS, Dorland M, Boersma H, te Velde E. Age at natural menopause in a population-based screening cohort: the role of menarche, fecundity, and lifestyle factors. Fertil Steril 1997;68:95–102. [DOI] [PubMed] [Google Scholar]
- 12.Barron KI, Richard T, Robinson PS, Lamvu G. Association of the U.S. Food and Drug Administration morcellation warning with rates of minimally invasive hysterectomy and myomectomy. Obstet Gynecol 2015;126:1174–80. [DOI] [PubMed] [Google Scholar]
- 13.Herrmann A, De Wilde RL. Laparoscopic myomectomy–the gold standard. Gynecol Minim Invasive Ther 2014;3:31–8. [Google Scholar]
- 14.Mais V, Ajossa S, Guerriero S, Mascia M, Solla E, Melis GB. Laparoscopic versus abdominal myomectomy: a prospective, randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol 1996;174:654–8. [DOI] [PubMed] [Google Scholar]
- 15.Pundri J, Pundri V, Walavalkar R, Omanwa K, Lancaster G, Kayani S. Robotic-assisted laparoscopic vs abdominal and laparoscopic myomectomy: systematic review and meta-analysis. J Minim Invasive Gynecol 2013;20:335–45. [DOI] [PubMed] [Google Scholar]
- 16.Pritts EA, Vanness DJ, Berek JS, Parker W, Feinberg R, Feinberg J, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg 2015;12:165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright JD, Tergas AI, Cui R, Burke WM, Hou JY, Ananth CV, et al. Use of electric power morcellation and prevalence of underlying cancer in women who undergo myomectomy. JAMA Oncol 2015;1:69–77. [DOI] [PubMed] [Google Scholar]
- 18.Sandberg EM, van den Haak L, Bosse T, Jansen FW. Disseminated leiomyoma cells can be identified following conventional myomectomy. BJOG 2016;123:2183–7. [DOI] [PubMed] [Google Scholar]