Abstract
Momilactones are allelochemicals in rice and moss defense. Momilactone-like compounds are therefore considered important secondary metabolites for plant defense. They may serve as promising lead compounds for crop-friendly herbicides, as well as anti-fungal and anti-bacterial agents. Many of these substances possess potent cytotoxicity property against cancer cell lines as well. The present paper is the first review on these versatile molecules, focusing on the structure, biological activity, chemical synthesis, and biosynthesis of the naturally occurring momilactone-like molecules reported from 1973 to 2017.
Keywords: momilactone, (9β-H)-pimarane, allelochemical, phytoalexin, germination inhibitor
Graphical Abstract
1. Introduction
Momilactones belong to a small family of naturally occurring diterpenes known as (9β-H)-pimaranes, which is characterized by a β-orientation of the proton on carbon-9 of the pimarane skeleton. Momilactones A (1) and B (2) were first isolated from the seed husks of rice (Oryza sativa L., cv. Koshihikari),1,2 bearing a 19,6β-lactone structure. The name “momi” refers to the origin of these compounds, the rice husk, as known in the Japanese language, thus “momilactone”. These compounds were soon found to exhibit germination-inhibitory,3 seedling growth-inhibitory,3 and phytoalexin2, 4, 5 activities. Later, the allelochemical property of these compounds were also established.6–14 Momilactone B exhibited potent antifungal activity against the rice blast pathogen Piricularia oryzae, which caused the devastating disease leading to 10–30% loss of the total rice harvest, as well as production loss of wheat, barley, and millet crops.2, 4 Momilactones were also found to be allelochemicals in the defense responses of the moss Hypnum plumaeforme.15–18
The roles of momilactones in rice defense and their biological significance are evident. Up till now, several momilactone-like compounds have been identified from rice plants and mosses.19–23 It is likely that they are stress-induced secondary metabolites with limited structural diversity. However, literature search revealed that a few plants in the Casimirella and Icacina genera (Icacinaceae) are also producers of (9β-H)-pimarane-19,6β-lactones and related metabolites. This review focuses on the structures, biological activities, chemical synthesis, and biosynthesis of naturally occurring momilactone-like molecules reported since 1973. A comprehensive overview is provided with the aim of inspiring researchers on these unique metabolites as potential agricultural tools.
2. Natural Distribution and Chemical Classification of (9β-H)-Pimaranes
Momilactone-like molecules have been reported from the bryophytes (Hypnum plumaeforme and Pseudoleskeella papillosa) and flowering plants, including monocots (Oryza sativa) and dicots (Icacina, Casimirella and Annona spp.).
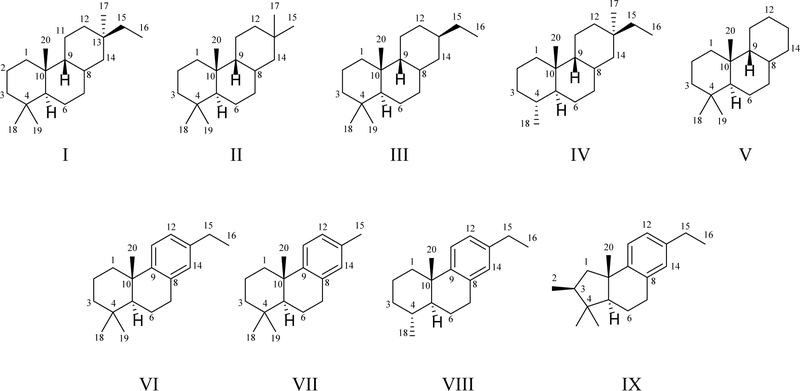
These (9β-H)-pimaranes and derivatives can be classified into the following structural types (Figure 1 and Table 1):
Figure 1.
Key structural skeletons of momilactone and related diterpenoids. (I) (9β-H)-pimarane; (II) 16-nor-(9β-H)-pimarane; (III) 17-nor-(9β-H)-pimarane; (IV) 19-nor-(9β-H)-pimarane; (V) 15,16,17-tri-nor-(9β-H)-pimarane; (VI) 17-nor-pimarane; (VII) 16,17-di-nor-pimarane; (VIII) 17,19-di-nor-pimarane; (IX) rearranged 17-nor-pimarane
Table 1.
Naturally occurring momilactone and related diterpenoids
| Name | Structural Type | Source |
|---|---|---|
| Momilactone A (1) | (9β-H)-pimarane | Oryza sativa;1, 2 Hypnum plumaeforme;15 Pseudoleskeella papillosa20 |
| Momilactone B (2) | (9β-H)-pimarane | O. sativa;1, 2 H. plumaeforme15 |
| Momilactone C (3)a Momilactone C (3-Oxo-pimara-7,9-dien-19,6β-olide, 4)a |
(9β-H)-pimarane (9β-H)-pimarane derivative |
O. sativa19 P. papillosa20 |
| Momilactone D (5) | (9β-OH)-pimarane | O. sativa23 |
| Momilactone E (6) | 19-nor-(9β-H)-pimarane | O. sativa23 |
| 6,19β-Epoxy-3β-hydroxy-5α,9β-pimara-7,15-diene (7) | (9β-H)-pimarane | O. sativa21 |
| (9βH)-Pimara-7,15-diene-3β,6β,19-triol (8) | (9β-H)-pimarane | O. sativa22 |
| Humirianthenolide A (9) | 15,16,17-tri-nor-(9β-H)-pimarane | Casimirella rupestris25 |
| Humirianthenolide B (10) | 15,16,17-tri-nor-(9β-H)-pimarane | C. rupestris25 |
| Humirianthenolide C (11) | 17-nor-(9β-H)-pimarane | C. rupestris;25 Icacina trichantha29 |
| 2β-Hydroxyhumiriantholide C (12) | 17-nor-(9β-H)-pimarane | I. trichantha39 |
| Humirianthenolide D (13) | 15,16,17-tri-nor-(9β-H)-pimarane | C. rupestris25 |
| Humirianthenolide E (14) | 15,16,17-tri-nor-(9β-H)-pimarane | C. rupestris25 |
| Humirianthenolide F (15) | 15,16,17-tri-nor-(9β-H)-pimarane | C. rupestris25 |
| Humirianthol (16) | (9β-H)-pimarane | C. spp.;26 C. ampla;27 I. trichantha29 |
| 15R-Humirianthol (17) | (9β-H)-pimarane | C. spp26 |
| 14α-Methoxyhumirianthol (18) | (9β-H)-pimarane | I. trichantha36 |
| Acrenol (19) | (9β-H)-pimarane | C. ampla27 |
| Humirianthone (20) | (9β-H)-pimarane | C. spp.26 |
| 1β-Hydroxyhumirianthone (21) | (9β-H)-pimarane | C. spp.26 |
| Annonalide (22) | (9β-H)-pimarane | C. spp.;26, 28 Annona coriacea30–32 |
| Oxidized annonalide (23) | 16-nor-(9β-H)-pimarane | C. spp.26 |
| Icacinol (24) | (9β-H)-pimarane | C. spp.;26 I. claessensis;33 I. trichantha29 |
| 17-Hydroxyicacinol (25) | (9β-H)-pimarane | I. trichantha29 |
| Icacenone (26) | 17-nor-(9β-H)-pimarane | I. mannii;34 I. trichantha29 |
| 7α-Hydroxyicacenone (27) | 17-nor-(9β-H)-pimarane | I. trichantha39 |
| Icacinlactone A (28) | 17-nor-pimarane | I. trichantha37 |
| 12-Hydroxyicacinlactone A (29) | 17-nor-pimarane | I. trichantha36 |
| Icacinlactone B (30) | 17-nor-pimarane | I. trichantha37 |
| 2β-Hydroxyicacinlactone B (Icacinlactone H, 31) |
17-nor-pimarane | I. trichantha38 |
| 7α-Hydroxyicacinlactone B (32) | 17-nor-pimarane | I. trichantha39 |
| 7β-Hydroxyicacinlactone B (33) | 17-nor-pimarane | I. trichantha36 |
| Icacinlactone C (34) | 17-nor-pimarane | I. trichantha37 |
| Icacinlactone D (35) | 17-nor-pimarane | I. trichantha37 |
| Icacinlactone E (2-Dehydroxyicacenone, 36) |
17-nor-(9β-H)-pimarane | I. trichantha37 |
| Icacinlactone F (8β-Hydroxyicacinlactone E, 37) |
17-nor-(9β-H)-pimarane | I. trichantha37 |
| Icacinlactone G (38) | 17-nor-(9β-H)-pimarane | I. trichantha37 |
| Icacinlactone I (39) | (9β-H)-pimarane | I. trichantha36 |
| Icacinlactone J (40) | 17-nor-(9β-H)-pimarane | I. trichantha37 |
| Icacinlactone K (41) | 16,17-di-nor-pimarane | I. trichantha39 |
| Icacinlactone L (42) | 17-nor-pimarane | I. trichantha39 |
| Icacintrichanone (43) | 17,19-di-nor-pimarane | I. trichantha39 |
| Icacintrichantholide (44) | rearranged 17-nor-pimarane | I. trichantha38 |
| Icacine (45) | (9β-H)-pimarane alkaloid | I. guesfeldtii40 |
| Icaceine (46) | (9β-H)-pimarane alkaloid | I. guesfeldtii41 |
| De-N-methylicaceine (47) | (9β-H)-pimarane alkaloid | I. guesfeldtii41 |
In the literature, two structurally distinct compounds have been named momilactone C.
(9β-H)-pimarane (1−3, 7, 8, 16−22, 24, 25, 39),
19-nor-(9β-H)-pimarane (6),
15,16,17-tri-nor-(9β-H)-pimarane (9, 10, 13−15),
17-nor-(9β-H)-pimarane (11, 12, 26, 27, 36−38, 40),
16-nor-(9β-H)-pimarane (23),
17-nor-pimarane (28−35, 42),
16,17-di-nor-pimarane (41),
17,19-di-nor-pimarane (43),
rearranged 17-nor-pimarane (44),
(9β-H)-pimarane alkaloid (45−47), and
other derivatives of (9β-H)-pimarane (4, 5).
Compounds 4, 5, 28−35, 41−47 were proposed to be biogenetically derived from (9β-H)-pimaranes in the related plant resources (Figures 1 and 2, and Table 1).
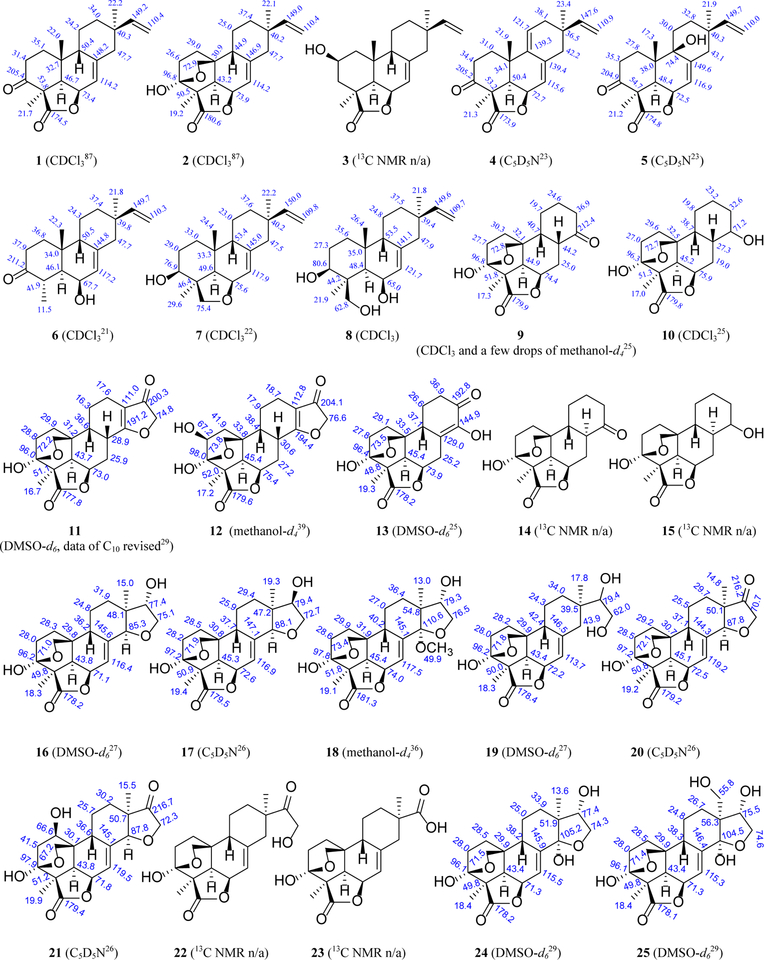
Figure 2.
Structures of naturally occurring momilactone and related diterpenoids (showing13C NMR spectroscopic data, solvents and references)
(9β-H)-Pimaranes from rice and moss. During the investigation of growth regulating substances in higher plants, two active components momilactones A (1) and B (2) were isolated from the seed husks of Oryza sativa cv. Koshihikari in 1973.1 The (9β-H)-configuration of 1 and 2 was initially erroneously assigned but later revised through X-ray single crystal diffraction analysis, when momilactone C (3) was identified from the same source.19 Momilactones A−C possess a 9β-H in the structure in contrast to the 9α-H in normal pimaranes. Subsequently, momilactones D (5) and E (6) were identified from the roots of O. sativa.23 Strictly speaking, momilactone E (6), which is a 19-nor-(9β-H)-pimarane, should have not been called ‘momilactone’ because it does not contain a lactone structure in the molecule. Two other non-lactone derivatives, 6,19β-epoxy-3β-hydroxy-5α,9β-pimara-7,15-diene (7)21 and (9β-H)-pimara-7,15-diene-3β,6β,19-triol (8),22 were subsequently isolated from the rice husks and from rice leaves after UV-irradiation, respectively.
Besides rice plants, momilactones A (1) and B (2), and 3-oxo-pimara-7,9-dien-19,6β-olide (4) (which was arbitrarily called momilactone C in a paper published in 2012,20 albeit the same trivial name had already been assigned to structure 3 in 1976)19 are present in two species of moss (Hypnum plumaeforme and Pseudoleskeella papillosa).15–17, 20
(9β-H)-Pimaranes from Casimirella and Icacina Plants (Icacinaceae). According to Howard, the genus name of Humirianthera is a synonym of Casimirella.24 The name Casimirella is therefore used in this review. In Amazon, the tubers of Casimirella rupestris are used to make into a kind of flour as a food ingredient by the natives;25 while those of C. ampla are used for the treatment of snakebites.26 Phytochemical investigation on the tubers of C. rupestris led to the identification of six degraded diterpenes, humirianthenolides A−F (9−11, 13−15).25 Among them, humirianthenolide C (11) is a 17-nor-(9β-H)-pimarane, while the others are 15,16,17-tri-nor-(9β-H)-pimaranes. On the other hand, humirianthol (16) and acrenol (19) were isolated from the tubers of C. ampla.27 The absolute configuration of humirianthol (16) was established later by X-ray analysis of its 15-O-aectate derivative based on the assumption of (15S)-configuration as suggested by the Horeau method,28 and further confirmed by single-crystal X-ray diffraction using CuKα X-ray source.29 The C-15 configuration of 19 remains undefined. In another study, bioassay-guided isolation of two Casimirella spp. led to the identification of 15(R)-humirianthol (17), humirianthone (20), 1β-hydroxyhumirianthone (21), and an oxidized derivative of annonalide (23). Compound 23 is a 16-nor-(9β-H)-pimarane previously reported as an oxidation product of annonalide (22).26 Interestingly, annonalide is a (9β-H)-pimarane first found in the bulbs of Annona coriacea,30–32 and later isolated from C. amper and other unidentified C. species.26, 27 The configurations of C-9 and C-13 were initially erroneously assigned30, 31 but later revised based on single-crystal X-ray diffraction data.32
Icacinol (24) was first isolated from Icacina claessensis,33 and its absolute configuration was established by single-crystal X-ray diffraction.29 From the root of I. mannii, a shrub endemic to tropical Africa as a popular folkloric medicine for the treatment of tumors, a 17-nor-(9β-H)-pimarane, icacenone (26), was identified by NMR and X-ray analyses.34 On the other hand, I. trichantha is a traditional herbal medicine in Nigeria and neighboring countries, whose tuber is often prescribed by the herbalists for the treatment of food poisoning, constipation, and malaria.35 Phytochemical investigations of this plant in our laboratories have resulted in the isolation of twenty-five (9β-H)-pimaranes and derivatives, including humirianthenolide C (11), 2β-hydroxyhumirianthenolide C (12), humirianthol (16), 14α-methoxyhumirianthol (18), icacinol (24), 17-hydroxyicacinol (25), icacenone (26), 7α-hydroxyicacenone (27), icacinlactones A−L (28, 30, 31, 34−42), 12-hydroxyicacinlactone A (29), 7α-hydroxyicacinlactone B (32), 7β-hydroxyicacinlactone B (33), icacintrichanone (43), and icacintrichantholide (44).29, 36–39 The absolute configurations of 18, 27, 30, 31, 40, and 41 were established as (3S,4R,5R,6S,9S,10S,13S,14S,15S) (18), (2S,3R,4R,5R,6S,7S,8S,9R) (27), (3S,4R,5R,6R,10R) (30), (2S,3R,4R,5R,6R,10R) (31), and (3S,4R,5R,6R,8R,9R,10S) (40), (3S,4R,5R,6R,10R) (41), and (3S,4R,5R,6R,10R) (44), respectively, by the single-crystal X-ray diffraction data. Compounds 28−35, and 41−44 are proposed to be biosynthesized via a (9β-H)-pimarane pathway. In addition, three diterpene alkaloids, icacine (45), icaceine (46), and de-N-methylicaceine (47), have been reported from the leaves and roots of I. guesfeldtii, whose root decoction is used as an anticonvulsant in traditional medicine in tropical Africa. The structure of 45 was confirmed by single-crystal X-ray diffraction,40 while the configurations of 46 and 47 were not fully assigned though they were thought to be structurally related to 45.41
3. Biological Activities
3.1. Seeds Germination Inhibition
Momilactones A (1) and B (2) inhibited the germination of Arabidopsis at concentrations above 30 μM and 10 μM, with IC50 values of 742 μM and 48.4 μM, respectively.42 Momilactone B (2) could also completely inhibit the germination of Leptochloa chinenesis (at 4 ppm), Amaranthus retroflexus (at 20 ppm), and Cyperus difformis (at 20 ppm).43
Our recent study revealed that icacinol (24) was able to reduce the germination of Arabidopsis seeds at 5.3 μM by 20%. Dose-dependent responses were observed at 26.5 μM (66%), 53 μM (36%), and 265 μM (<1%).44
3.2. Plant Growth Inhibition on Rice Field Weeds and Other Plants
Barnyard grass (Echinochloa crus-galli) is a major noxious weed associated with rice especially in southern China. Momilactone B (2) inhibited the growth of shoots and roots of barnyard grass at concentrations greater than 1 μM, displaying 59−82% inhibition,11 with an IC50 values of ca. 6.5 μM.45 The compound also inhibited the growth of the shoots and roots of other monocots and dicots in a concentration-dependent manner, including cress (Lepidium sativum), lettuce (Lactusa sativa cv. Santanasu), alfafa (Medicago sativa), ryegrass (Lodium multiflorum), timothy (Phleum pretense), Echinochloa colonum, crabgrass (Digitaria sanguinalis), Chinese cabbage (Brassica rapa cv. Harumaki-ichigou), and Arabidopsis thaliana.45, 46 On the other hand, the potency of momilactone A (1) was far less than that of 2.
The plant growth inhibitory mechanism for momilactones in barnyard grass has been associated with the altered expressions of miRNA relevant to plant hormone signal transduction, nucleotide excision repair, peroxisome proliferator-activated receptor pathway (PPAR pathway), and the p53 signaling pathway.47 When Arabidopsis was treated with momilactone A (1) or B (2), protein expressions of cruciferin 2, cruciferin 3 and cruciferina were up-regulated. These proteins are storage proteins playing an important role as source of nitrogen for seed germination. Momilactones may therefore inhibit the germination of Arabidopsis seeds through a process of inhibition of the degradation process of cruciferins and cruciferina.42 Momilactone B was also found to inhibit the growth of Arabidopsis seedlings by the accumulation of subtilisin-like serine protease, amyrin synthase LUP2, β-glucosidase and malate synthase.48 These proteins are involved in the metabolic turnover and the production of intermediates needed for cell structures during plant growth and development. In addition, momilactone B induced proteins associated with plant defense responses, such as glutathione S-transferase and 1-cysteine peroxiredoxin 1.
The inhibitory effects of momilactones A (1) and B (2) on the root and shoot growth of rice seedlings have been shown to be minimal; they displayed positive results only at concentrations greater than 100 and 300 μM, respectively.45 At concentration levels cytotoxic to the weeds, 1 and 2 exerted no visible damage to rice seedlings.49
Apart from the momilactones, icacinol (24) caused significant damages to Arabidopsis leaf expansion.44 Arabidopsis plant treated with 24 also showed indications of stress, where anthocyanins increased and chlorophyll was reduced. Such findings reinforce the hypothesis that (9β-H)-pimarane lactones may serve as a potential source of agrichemicals.
3.3. Antifungal and Antibacterial Activities
Rice blast, mainly caused by infection from Magnaporthe oryzae (syn. Magnaporthe grisea, Pyricularia grisea /oryzae), is the most devastating disease of rice crop all over the world. Momilactones A (1) and B (2) were reported to display anti-M. oryzae activity in 1977,4 and subsequently, many other components from the resistant strains of rice were tested against M. oryzae (Table 2). Among them, momilactone B (2) showed highest potency against both spore germination and germ tube growth of the fungus. Humirianthone (20) exhibited weak anti-fungal activity as well (IC50 > 69 μM).26
Table 2. Inhibitory activities against Magnaporthe oryzae of rice components (IC50, μM).
| Compound Class | Spore Germination88 | Germ Tube Growth | ||
|---|---|---|---|---|
| Momilactone A (1) | (9β-H)-pimarane | 47.8 | 7422, 15.988 | |
| Momilactone B (2) | (9β-H)-pimarane | 9.1 | 3.04 | |
| Oryzalexin A | ent-sandaracopimarane | 384.1 | - | |
| Oryzalexin B | ent-sandaracopimarane | 245.0 | - | |
| Oryzalexin C | ent-sandaracopimarane | 453.3 | - | |
| Oryzalexin D | ent-sandaracopimarane | 75.7 | - | |
| Oryzalexin E | ent-sandaracopimarane | 205.6 | - | |
| Oryzalexin F | ent-sandaracopimarane | 338.8 | - | |
| Oryzalexin S | stemarane | 65.8 | 12222 | |
| Phytocassane A | ent-cassane | 63.3 | 15.889 | |
| Phytocassane B | ent-cassane | 12.0 | 4.589 | |
| Phytocassane C | ent-cassane | 22.0 | 9.489 | |
| Phytocassane D | ent-cassane | 79.190 | 31.689 | |
| Phytocassane E | ent-cassane | 19.090 | 6.389 | |
| Phytocassane F | ent-cassane | *22 | 1622 | |
| Sakuranetin | flavanone | 52.490 | 3722 | |
Completely inhibited at 300 μM
Other anti-fungal assays demonstrated that momilactone B (2) possessed more potent antifungal activity than momilactone A (1), in test species such as Botrytis cinerea, Fusarium solani, and Colletrotrichum gloeosporioides.50 At the same time, humirianthone (17) displayed potent anti-fungal activity against Phytophthora infestans with an IC50 value of 1.1 μM.26
Momilactones A (1) and B (2) exhibited antibacterial activity against Pseudomonus ovalis, Bacillus cereus, Bacillus pumilus, and Escherichia coli.50 Momilactone A showed high selectivity toward E. coli with a minimal inhibitory concentration value of 5 μM.
3.4. Cytotoxicity against Cancer Cell Lines
Momilactones A (1) and B (2) exhibited cytotoxic activity against P388 murine leukemia cells with IC50 values of 2.71 μM and 0.21 μM, respectively.51 In addition, momilactone B (2) decreased viability of blood cancer cells, i.e. HL-60 (human myeloblastic leukemia cells), Jurkart (human leukemic T cells), RBL-2H3 (a basophilic leukemia cell line), and p815 (mouse mastocytoma cells), at concentrations below 6 μM. The cytotoxic activity on Jurkart cells was associated with an induction of apoptosis via caspase and mitochondria.52 Momilactone B (2) was also cytotoxic against human colon cancer cells lines HT-29 and SW620 (IC50 values < 1 μM),53 and the T47D breast cancer cell line. The effect was accompanied by a regulation of the expression of apoptosis-related genes and an induction of apoptosis through STAT5b and a caspase-3 dependent pathway.54 In human leukemia U937 cells, 2 caused G1 cell cycle arrest and apoptosis through the induction of p21 expression, inhibition of Cdk/cyclin-associated kinase activities, and a down-regulation of phosphorylation of pRB.55
Several other cytotoxic momilactone-like compounds are summarized in Table 3. Among them, humirianthenolide C (11) displayed the highest potencies.26, 29, 36–38
Table 3.
Cytotoxic activities of momilactone-like molecules (IC50, μM)*
| Compound | MDA-MB-435 | HT-29 | MDA-MB-231 | OVCAR3 | A2780 |
|---|---|---|---|---|---|
| Humirianthenolide C (11) | 0.66 | 3.00 | 0.67 | 1.05 | - |
| 2β-Humirianthenolide C (12) | 1.48 | - | 2.85 | 3.23 | - |
| Humirianthol (16) | 1.65 | 4.94 | 3.74 | 4.12 | 3.0 |
| 15R-Humirianthol (17) | - | - | - | - | 2.2 |
| 14α-Methoxyhumirianthol (18) | 7.04 | - | 16.91 | 15.23 | - |
| Acrenol (19) | - | - | - | - | 1.8 |
| Humirianthone (20) | - | - | - | - | 6.1 |
| Annonalide (22) | - | - | - | - | 3.9 |
| Icacinol (24) | 1.25 | 4.23 | 7.30 | 7.55 | 1.7 |
| 17-Hydroxyicacinol (25) | 5.61 | 18.27 | - | - | - |
| Icacenone (26) | 6.44 | 13.25 | 10.85 | 18.71 | - |
| 7α-Hydroxyicacenone (27) | 2.91 | - | 7.60 | 7.53 | - |
| Icacinlactone F (37) | 6.16 | - | 8.94 | 10.5 | - |
MDA-MB-435: Human melanoma cell line; HT-29: Human colorectal adenocarcinoma epithelial cell line; MDA-MB-231: Human breast adenocarcinoma epithelial cell line; OVCAR3: Human ovarian adenocarcinoma epithelial cell line; A2780: Human ovarian cancer epithelial cell line. Compounds 25−29, 31−37 were inactive against MDA-MB-231, MDA-MB-435, and OVCAR3 (IC50 > 20 μM);
4. Biogenesis of Momilactone-Like Molecules
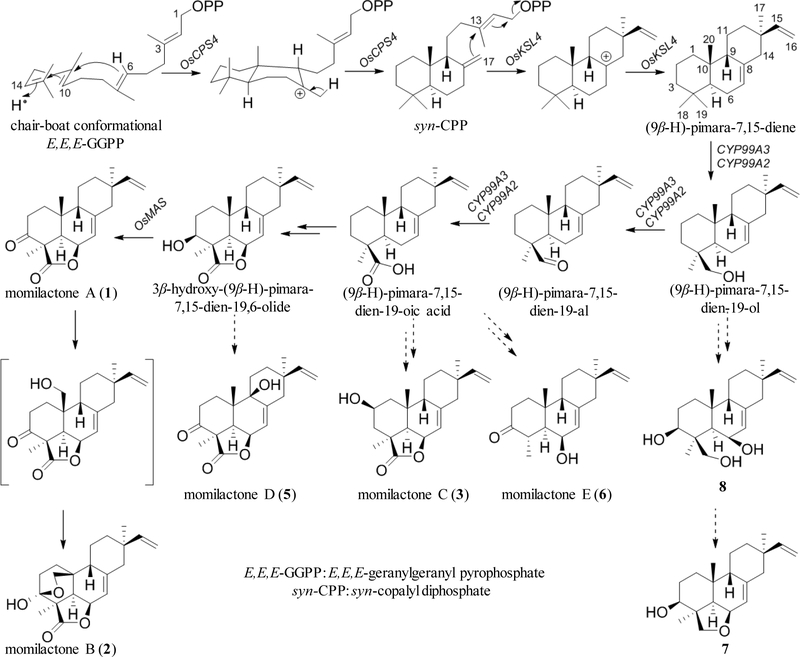
In plants, the C5 unit of isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) are biosynthesized through the mevalonate (MVA) and/or methylerythritol 4-phosphate (MEP) pathways.56 During the process, E,E,E-geranylgeranyl pyrophosphate (GGPP) is formed in the plastids,57, 58 and subsequent enzyme-catalyzed E,E,E-GGPP cyclization reactions lead to the formation of a diversity of diterpene skeletons.
For (9β-H)-pimaranes, syn-copalyl diphosphate (syn-CPP) serves as a key intermediate during their biosynthesis (Scheme 1). Thus, initiated by protonation at the terminal double bond, the chair-boat conformational E,E,E-GGPP gives rise to syn-CPP via the 8-carbonium ion. The syn-CPP is then cyclized to (9β-H)-pimarane-7,15-diene by a si-face attack of C-17. Type A and type B cyclases are involved in the process.59 In rice, type B cyclase OsCPS4 takes part in the formation of syn-CPP, and type A cyclase OsKSL4 functions as (9β-H)-pimarane-7,15-diene synthase, which could be induced in rice leaves by UV-irradiation.60 The microsomal cytochrome P450 monooxygenases (P450s) are also involved in the downstream oxidation of the (9β-H)-pimarane-7,15-diene leading to diverse structures of momilactone-like molecules.
Scheme 1.
Biogenetic pathway for momilactone and related diterpenoids in rice plant
The genes for momilactone biosynthesis in rice have been identified in a 168-kb region on chromosome-4, on which both OsKSL4 and OsCPS4 genes are located. The OsKSL4 gene also locates in the proximity of P450 CYP99A2 and CYP99A3 genes, as well as a putative dehydrogenase gene (AK103462), which are chitin- and UV-inducible.61, 62 The involvements of CYP99A2 and CYP99A3 in momilactone biosynthesis have been confirmed by gene-knockdown experiments.62 CYP99A3 was found to catalyze consecutive oxidations of the C19 methyl group of the momilactone precursor, (9β-H)-pimara-7,15-diene, to form, sequentially, (9β-H)-pimara-7,15-dien-19-ol, (9β-H)-pimara-7,15-dien-19-al, and (9β-H)-pimara-7,15-dien-19-oic acid.63 On the other hand, CYP99A2 is involved to a much lesser extent.
The (9β-H)-pimara-7,15-dien-19-oic acid is presumed to give rise to the (9β-H)-pimara-7,15-dien-19,6β-olide via 3β- and 6β-hydroxylation through the participation of P450 genes CYP701A864 and CYP76M865, respectively. The 3β-hydroxy-(9β-H)-pimara-7,15-dien-19,6β-olide is then converted to momilactone A (1) by AK103462 (OsMAS). The same reaction has also been observed in UV-irradiated rice leaves, in which the conversion was accomplished by a soluble protein fraction in the presence of NAD+.66
Momilactone B (2) was proposed to form from 1 through C20-hydroxylation and hemi-ketal ring closure. The possible biosynthesis of momilactone C (3), momilactones D and E (5 and 6), 6,19β-epoxy-3β-hydroxy-5α,9β-pimara-7,15-diene (7), and (9β-H)-pimara-7,15-diene-3β,6β,19-triol (8) in rice have also been proposed as shown in Scheme 1.
The OsTGAP1 is an elicitor-inducible rice basic leucine zipper (bZIP) transcription factor, which is essential for momilactone biosynthesis and play an essential role on the expression of all five genes in the gene cluster (OsCPS4, OsKSL4, CYP99A2, CYP99A3, and OsMAS). OsTGAP1 is also involved in the transcriptional regulation of OsDXS3 in the MEP pathway. It serves as a crucial master regulator that controls the inducible expression of biosynthetic genes and upstream pathway genes required for diterpenoid phytoalexin production as part of the rice defensive response.67 In addition, a rice transcription factor named diterpenoid phytoalexin factor (DPF), a basic helix-loop-helix transcription factor, was also reported.68 DPF plays a central and positive role in the biosynthesis of diterpenoid phytoalexin in rice, and it activates the promoters of CPS2 (copalyl diphosphate synthase 2) and CYP99A2, whose products are implicated in the biosynthesis of momilactones. The gene encoding DPF is expressed mainly in roots and panicles, and inducible in leaves by blast infection, copper chloride, or UV light.
For the biosynthesis of momilactone-like molecules in Icacina and Casimirella, little information is available up till now. (9β-H)-Pimara-7,15-diene was proposed to be the common precursor for most of these molecules.
5. Synthetic Studies toward Momilactone-Like Molecules
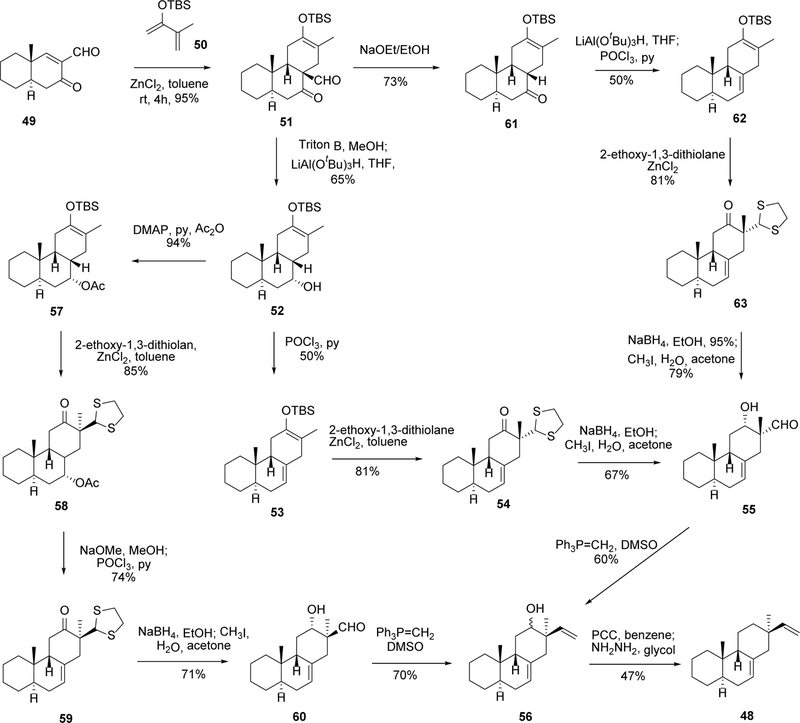
5.1. Synthesis of model compound (±)-4,4-dinor-(9β-H)-pimara-7,15-diene (48)
The unusual trans-syn tricyclic skeleton of momilactones, as well as the high functionalization of rings A and B, makes the chemical synthesis of this family of diterpenes challenging. Early efforts towards the total synthesis of momilactones focused on the construction of the trans-syn tricyclic skeleton, exemplified by the model compound (±)-4,4-dinor-(9βH)-pimara-7,15-diene (48, Scheme 2). Compound 48 possesses all main features of the BC-ring system of momilactones, including the trans-syn ring arrangement, the △7,8-double bond, and the α-methyl and β-vinyl substitutions at C-13.
Scheme 2.
Synthesis of model compound (±)-4,4-dinor-(9β-H)-pimara-7,15-diene (48).
As shown in Scheme 2, the syntheses of compound 48 through three different approaches were reported by de Groot’s group.69, 70 Starting from alkene 49, Diels-Alder annulation using 2-t-butyldimethylsilyloxybutadiene (50) catalyzed by dry ZnCl2 provides the trans-syn-cis adduct 51 in high yield. Deformylation of 51 followed by hydride reduction affords the alcohol 52. The conversion of 52 to the desired product 48 was accomplished through two different approaches. In the first route, the dehydration of 52 using POCl3/pyridine produced enolate 53. Then an electrophilic attacked by 2-ethoxy-1,3-dithiolane provides intermediate 54, which could be reduced and deprotected to give an aldehyde 55. The aldehyde group of 55 was then converted to the 13-vinyl substitution through a Wittig reaction with methylenetriphenylphosphorane. Interestingly, considerable epimerization at C-12 and C-13 occured in the Wittig conditions and only a very small amount of α-vinyl product was obtained. Oxidation of the racemic alcohol followed by Wolff-Kishner reduction afforded compound 48 as optically pure product.
The second approach started by protecting the alcohol group of 52 as an acetyl ester 57.69 The α-ester group was used as a directing group for the introduction of 2-ethoxy-1,3-dithiolane to give 58. The ester was then hydrolyzed and dehydrated to intermediate 59, followed by the reduction of the ketone to make 60. Similarly, the Wittig reaction of 60 led to the epimerization of the C-12 alcohol, but no α-vinyl product could be detected, and the intermediate 56 was converted to 48 using the same conditions as described for the first route.
The third synthetic route of 48 was initiated by first deformylating intermediate 51, followed by reduction and dehydration to provide enolate 62.70 Electrophilic attack of 62 by 2-ethoxy-1,3-dithiolane, followed by reduction with NaBH4, afforded intermediate 55, which could be converted to 48 using the same procedures as described for the previous two routes.
Taken together, the key step for the above synthetic routes is the Diels-Alder annulation, which provides the trans-syn-cis skeleton of the diterpene. Subsequent conversion of this skeleton to the desired product could thus be accomplished through different functionalization methods.
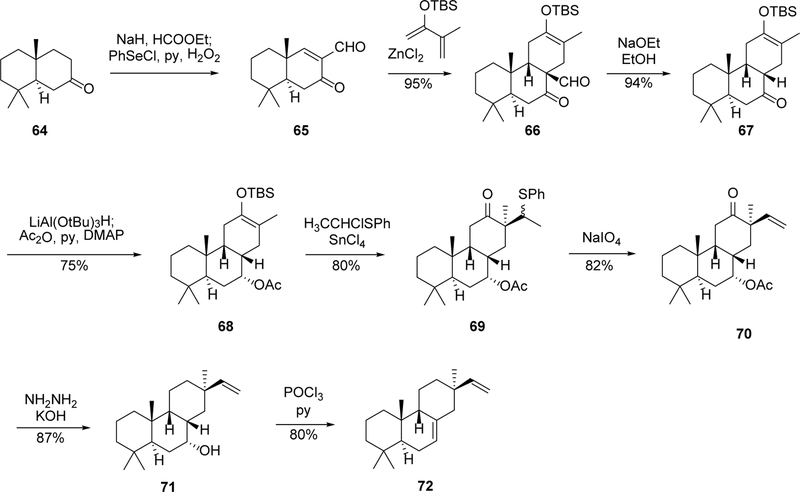
5.2. Synthesis of model compound (9β-H)-pimara-7,15-diene (72) and 3β-hydroxy-(9β-H)-pimara-7,15-diene (86)
The Diels-Alder annulation approach was used by de Groot’s group for the synthesis of (9β-H)-pimara-7,15-diene (72),71 another model compound which differs from 48 by a dimethyl substitution on C-14. As shown in Scheme 3, the dimethyl Diels-Alder substrate 65 was prepared from compound 64, and the annulation of 65 with 2-t-butyldimethylsilyloxybutadiene afforded compound 66, which was the counterpart of 51 in the above routes. A sequential conversion including deformylation, reduction, and acetylation provides the enolate 68, which was substituted with [(1-chloroethyl)thio]benzene to give intermediate 69. The 1β-phenylethylsulfane group was introduced as a vinyl precursor, which was subjected to an oxidative de-sulfur reaction to afford compound 70. The ketone group was reduced with Wolff-Kishner conditions and the △7,8-double bond was introduced by the dehydration of C-8 hydroxyl, affording the target product 72.71
Scheme 3.
Synthesis of (9β-H)-pimara-7,15-diene (72).
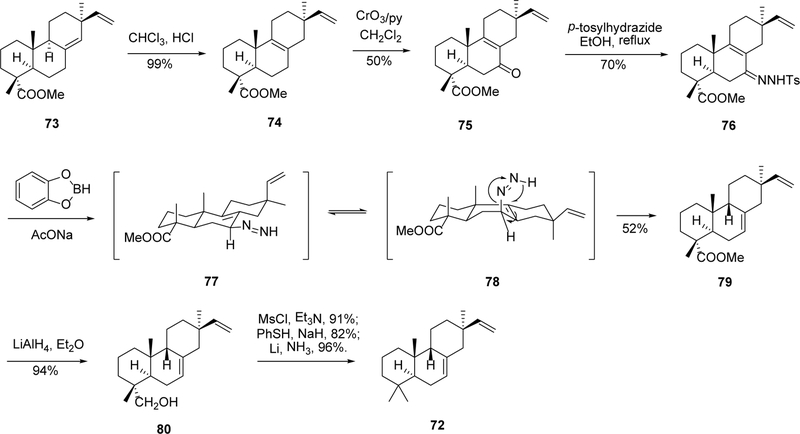
Another synthesis of compound 72 was reported by Chu and Coates.72 To introduce the 9β-H, a methodology employing catecholborane reduction of tosylhydrazones was used to construct the trans-syn tricyclic system. As shown in Scheme 4, the isolated natural product 73 was treated with HCl to afford its △8 isomer, followed by a regioselective allylic oxidation at C-7 with CrO3•2pyr to afford intermediate 75. Condensation of this intermediate with p-tosylhydrazine provides the isopimaradienone tosylhydrazone 76. Reaction of 76 with catecholborane in CHC13 followed by allylic diazene rearrangement73, 74 promoted by sodium acetate leads to compound 79 in 52% yield (along with the 15, l6-dihydro byproduct). The stereochemistry of the tosylhydrazone reductions was rationalized assuming initial ψ-axial (7α) delivery of hydride from catecholborane to the C=N group, followed by boro sulfinate elimination and β-facial transfer of hydrogen to C-9. Conformational inversion of ring B to a half-boat form (intermediate 77 to 78) was presumably necessary for a concerted fragmentation to occur. The 4α ester substitution is then converted to a methyl group through a sequence of reduction, mesylation, sulfur substitution, and reductive de-sulfur reactions, affording the desired product 72.
Scheme 4.
Another synthesis of (9β-H)-pimara-7,15-diene (72).
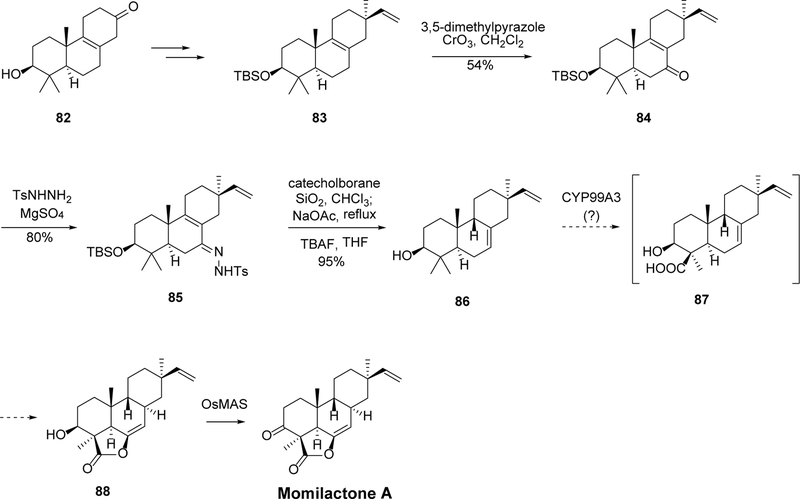
The aforementioned methodology has been applied in the synthesis of 3β-hydroxy-(9β-H)-pimara-7,15-diene (86) by Yajima et al,75 as depicted in Scheme 5. The starting material 82 was first converted to C-13 substituted intermediate 83, the allylic oxidation of which afforded ketone 84. Condensation of this ketone with p-tosylhydrazine provided the tosylhydrazone precursor 85, which was then subjected to catecholborane reduction and allylic diazene rearrangement to afford the desired product 86. This compound was proposed to be a possible biosynthetic precursor of momilactone A, through compound 87 as a possible intermediate.75
Scheme 5.
Synthesis of 3β-hydroxy-(9β-H)-pimara-7,15-diene (86) as a possible intermediate for the biosynthesis of momilactone A.
5.3. Total synthesis of (±)-momilactone A (1)
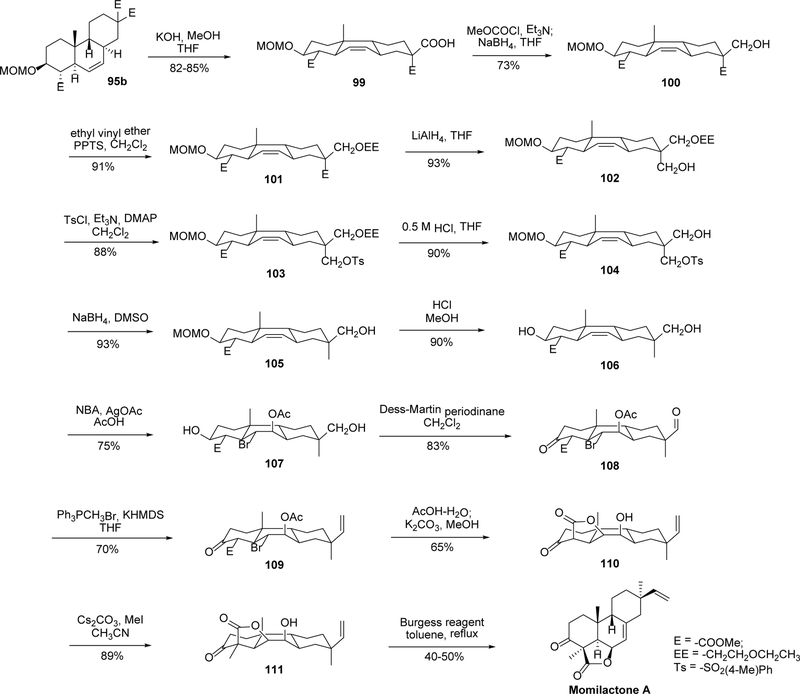
In 2002, Germain and Deslongchamps reported the total synthesis of (±)-momilactone A in more than 20 steps.76 A diastereoselective transannular Diels-Alder reaction on a trans-trans-cis macrocyclic triene, which had been developed by the same group,77, 78 was used as the key step. As shown in Scheme 6, an Aldol reaction between compounds 89 and 90 affords diastereomers 91a and 91b in 88% yield. The hydroxyl group was protected as MOM ether, and selective desilylation of the primary hydroxyl ether yielded compounds 93a and 93b. The terminal hydroxy was then converted to a chlorine using hexachloroacetone/Ph3P. The key step of the synthesis could be accomplished by the slow addition of these allylic chlorides into a suspension of cesium carbonate in refluxing acetonitrile under high dilution. The macrocyclization-cycloaddition took place in a single step to afford the trans-syn-trans tricycles 95a and 95b in 40% and 60% yields, respectively. A sterically favored endo transition state having a chair-boat-chair conformation was proposed to explain the specific formation of the trans-syn-trans tricycle. Furthermore, the isomerization of 95a to 95b was accomplished by a series of reactions including MOM removal (96), oxidation (97), reduction (98), and the re-protection of the hydroxyl group with MOMCl, through similar chair-boat-chair conformations of the intermediates.
Scheme 6.
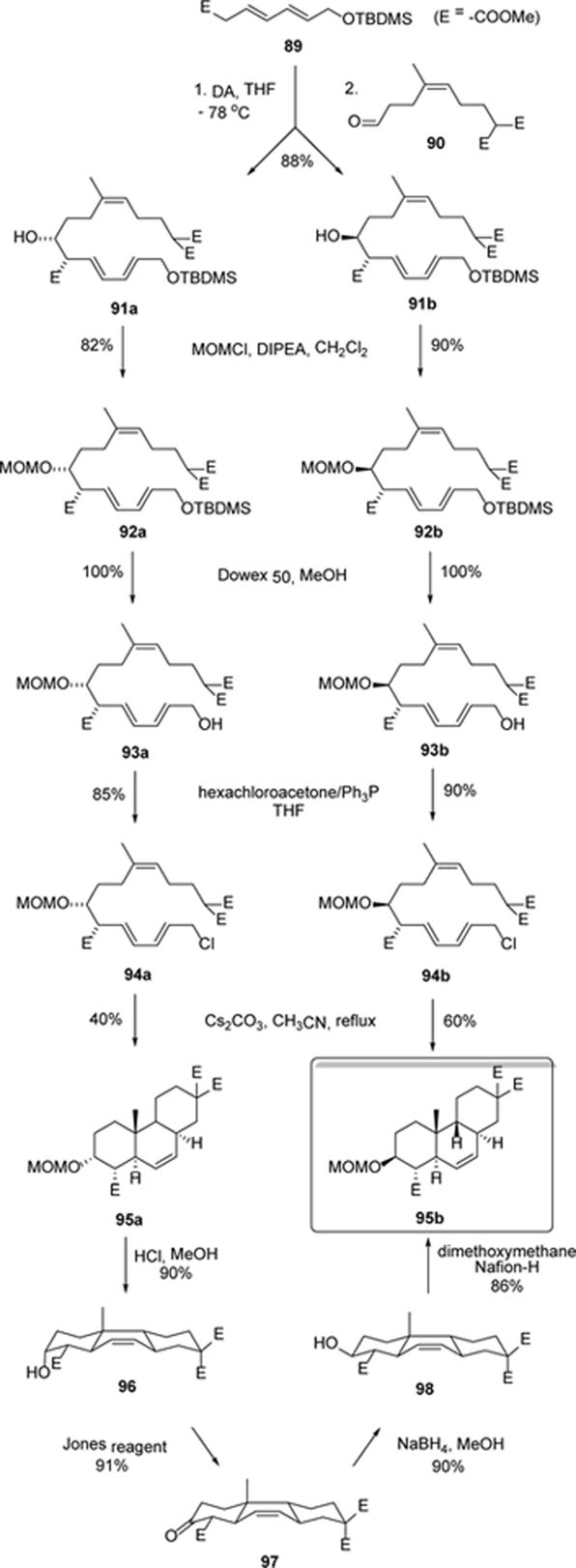
Synthesis of intermediate 95b.
Starting from intermediate 95b, a linear sequence of conversions was accomplished (Scheme 7).76 Thus, partial hydrolysis of the malonate 95b produced the equatorial β monoacid 99, which was then reduced to an alcohol and protected as an ethoxyethyl ether 101. Then the axial ester was reduced with LiAlH4 to give alcohol 102, which was converted to tosylate 103. The equatorial ether was de-protected, followed by the reduction of the axial tosylate to a methyl (105). Then the MOM ether on ring A was cleaved (106), and treatment of 106 with N-bromoacetamide (NBA) and silver acetate in acetic acid gave rise to bromoacetate 107 with excellent stereoselectivity. Simultaneous oxidation of both hydroxy groups using Dess-Martin periodinane yielded the corresponding keto aldehyde 108, and 108 was treated with Wittig conditions to convert the equatorial aldehyde to a terminal olefin (109). Subsequent displacement of the bromide was realized using AcOH-H2O followed by methanolysis of the acetate to yield the lactone 110. It was proposed that under basic conditions, epimerization at C-4 must have occurred and the axial ester is trapped by the C6-alcohol to form the γ-lactone. Treatment of 110 with Cs2CO3 and methyl iodide in acetonitrile afforded 111 in good yield. The final step of the synthesis was achieved using Burgess reagent in toluene at reflux,79 and momilactone A was obtained in a moderate yield. Interestingly, the Burgess reagent is normally used for syn-elimination, thus the success of it in this reaction is probably due to the formation of the highly reactive sulfamate ester intermediate (by addition of the Burgess reagent to the secondary alcohol) that decomposes at high temperature to give the alkene. Normal dehydration conditions (SOCl2, POCl3, Mitsunobu conditions, etc.) do not work in this case, likely due to the steric hindrance of the secondary alcohol.
Scheme 7.
Total synthesis of (±)-momilactone A from intermediate 95b.
6. Further Prospects
Momilactones, exemplified by the versatile momilactone B, are thought to be the natural choice of evolution for rice and moss defense. Momilactone-like molecules are therefore believed to be of high applicability to the biological systems and potential sources for promising lead compounds for crop-friendly pesticides to support the development of green agriculture. Indeed, momilactones A and B have been patented as herbicides and anticancer agents as well.80–82 However, the commercial supply is always a challenge for practical utilization of momilactones. Total synthesis remains a possible solution, but likely economically impractical and also not environment-friendly, despite the total synthesis of momilactone A has been reported many years ago.83 The attempt on bioengineering is another possible approach, but limited investigation has been reported so far.84–86 Natural supplies of momilactone-like molecules is another option, for which Icacina, Casimirella and their affinitive genera may be worthy to be further investigated. Unlike rice and moss in which these compounds are formed as stress-induced metabolites with limited quantities, momilactone-like molecules in Icacina and Casimirella display high structural diversity and high contents, providing opportunities for the discovery of promising lead compounds as well as precursors for semi-synthesis. Furthermore, in addition to their potentials for development into crop-friendly herbicides, antifungal, antibacterial, and antitumor agents, momilactone-like molecules are believed to possess other properties. Our group has demonstrated in vitro anti-herpes activity in some members of (9β-H)-pimarane lactones (unpublished), revealing their antiviral potential. It is our wish that this comprehensive overview will inspire further research interests on these unique molecules.
Footnotes
Conflict of Interest
The authors declare no competing financial interest.
References
- 1.Kato T; Kabuto C; Sasaki N; Tsunagawa M; Aizawa H; Fujita K; Kato Y; Kitahara Y, Momilactones, growth inhibitors from rice, Oryza sativa L. Tetrahedron Lett. 1973, 39, 3861–3864. [Google Scholar]
- 2.Cartwright DW; langcake P; Pryce RJ; Leworthy DP; Ride JP, Isolation and characterization of two phytoalexins from rice as momilactiones A and B. Phytochemistry 1981, 20, 535–537. [Google Scholar]
- 3.Takahashi N; Kato T; Tsunagawa M; Sasaki N; Kitahara Y, Mechanisms of dormancy in rice seeds. II. New growth inhibitors, momilactone-A and -B isolated from the hulls of rice seeds. Japan J. Breed 1976, 26, 91–98. [Google Scholar]
- 4.Cartwright D; Langcake P; Pryce RJ; Leworthy DP, Chemical activation of host defence mechanisms as a basis for crop protection. Nature 1977, 267, 511–513. [Google Scholar]
- 5.Langcake P; Cartwright D; Leworthy DP; Pryce RJ; Ride JP, The dichlorocyclopropanes, fungicides with an indirect mode of action? Neth. J. Pl. Path 1978, 83 (Suppl. 1), 153–155. [Google Scholar]
- 6.Kato-Noguchi H; Ino T; Sata N; Yamamura S, Isolation and identification of a potent allelopathic substance in rice rot exudates. Physiol. Plantarum 2002, 115, 401–405. [DOI] [PubMed] [Google Scholar]
- 7.Kato-Noguchi H, Isolation of allelopathic substances in rice seedlings. Plant Prod. Sci 2002, 5, 8–10. [Google Scholar]
- 8.Kato-Noguchi H; Ino T, Rice seedlings release momilactone B into the environment. Phytochemistry 2003, 63, 551–554. [DOI] [PubMed] [Google Scholar]
- 9.Kato-Noguchi H, Allelopathic substance in rice root exudates: Rediscovery of momilactone B as an allelochemical. J. Plant Physiol. 2004, 161, 271–276. [DOI] [PubMed] [Google Scholar]
- 10.Kato-Noguchi H; Ino T, Possible involvement of momilactone B in rice allelopathy. J. Plant Physiol. 2005, 162, 718–721. [DOI] [PubMed] [Google Scholar]
- 11.Kato-Noguchi H; Hasegawa M; Ino T; Ota K; Kujime H, Contribution of momilactone A and B to rice allelopathy. J. Plant Physiol. 2010, 167, 787–791. [DOI] [PubMed] [Google Scholar]
- 12.Kato-Noguchi H, Barnyard grass-induced rice allelopathy and momilactone B. J. Plant Physiol. 2011, 168, 1016–1020. [DOI] [PubMed] [Google Scholar]
- 13.Kato-Noguchi H, Rice allelopathy and momilactone. Pak. J. Weed Sci. Res 2012, 18, 289–296. [Google Scholar]
- 14.Kato-Noguchi H; Peters RJ, The role of momilactones in rice allelopathy. J. Chem. Ecol 2013, 39, 175–185. [DOI] [PubMed] [Google Scholar]
- 15.Nozaki H; Hayashi K; Nishimura N; Kawaide H; Matsuo A; Takaoka D, Momilactone A and B as allelochemicals from Moss Hypnum plumaeforme: First occurrence in Bryophytes. Biosci. Biotechnol. Biochem 2007, 71, 3127–3130. [DOI] [PubMed] [Google Scholar]
- 16.Kato-Noguchi H; Kobayashi K, Jasmonic acid, protein phosphatase inhibitor, metals and UV-irradiation increased momilactone A and B concentrations in the moss Hypnum plumaeforme. J. Plant Physiol. 2009, 166, 1118–1122. [DOI] [PubMed] [Google Scholar]
- 17.Kato-Noguchi H, Secretion of momilactone A and B by the moss Hypnum plumaeforme. Plant Signal. Behav. 2009, 4, 737–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato-Noguchi H; Kobayashi K; Shigemori H, Allelopathy of the moss Hypnum plumaeforme by the production of momilactone A and B. Weed Res. 2009, 49, 621–627. [Google Scholar]
- 19.Tsunakawa M; Ohba A; Sasaki N; Kabuto C; Kato T; Kitahara Y; Takahashi N, Momilactone-C, a minor constituent of growth inhibitors in rice husk. Chem. Lett 1976, 1157–1158. [Google Scholar]
- 20.Liu N; Wang S; Lou H, A new pimarane-type diterpenoid from moss Pseudoleskeella papillosa (Lindb.) Kindb. Acta Pharm. Sin. B 2012, 2, 256–259. [Google Scholar]
- 21.Li G; Xu Q-L; He C-M; Zeng L; Wang H-F, Two new anti-fungal diterpenoids from the husks of Oryza sativa. Phytochem. Lett 2014, 10, 309–312. [Google Scholar]
- 22.Horie K; Inoue Y; Sakai M; Yao Q; Tanimoto Y; Koga J; Toshima H; Hasegawa M, Identification of UV-induced diterpenes including a new diterpene phytoalexin, phytocassane F, from rice leaves by complementary GC/MS and LC/MS approaches. J. Agric. Food Chem. 2015, 63, 4050–4059. [DOI] [PubMed] [Google Scholar]
- 23.Cho J-G; Cha B-J; Lee SM; Shrestha S; Jeong R-H; Lee DS; Kim Y-C; Lee D-G; Kang H-C; Kim J; Baek N-I, Diterpenes from the roots of Oryza sativa L. and their inhibition activity on NO production in LPS-stimulated RAW264.7 macrophages. Chem. Biodivers 2015, 12, 1356–1364. [DOI] [PubMed] [Google Scholar]
- 24.Howard RA, A revision of Casimirella, including Humirianthera (Icacinaceae). Brittonia 1992, 44, 166–172. [Google Scholar]
- 25.Zoghbi MDGB; Roque NF; Gottlieb HE, Humirianthenolides, new degraded diterpenoids from Humirianthera rupestri. Phytochemistry 1981, 20, 1669–1673. [Google Scholar]
- 26.Adou E; Williams RB; Schilling JK; Malone S; Meyer J; Wisse JH; Frederik D; Koese D; Werkhoven MCM; Snipes CE; Werk TL; Kingston DGI, Cytotoxic diterpenoids from two lianas from the Suriname rainforest. Bioorg. Med. Chem 2005, 13, 6009–6014. [DOI] [PubMed] [Google Scholar]
- 27.Graebner IB; Mostardeiro MA; Ethur EM; Burrow RA; Dessoy ECS; Morel AF, Diterpenoids from Humirianthera ampla. Phytochemistry 2000, 53, 955–959. [DOI] [PubMed] [Google Scholar]
- 28.Burrow RA; Morel AF; Graebner IB; Farrar DH; Lough AJ, The acetyl derivative of humirianthol. Acta Crystallogr., Sect. E Struct. Rep. Online 2003, 59, o347–o349. [Google Scholar]
- 29.Monday Onakpa M.; Zhao M; Goedecke T; Chen W-L; Che C-T; Santarsiero BD; Swanson SM; Uzoma Asuzu I., Cytotoxic (9βH)-pimarane and (9βH)-17-norpimarane diterpenes from the tuber of Icacina trichantha. Chem. Biodivers 2014, 11, 1914–1922. [DOI] [PubMed] [Google Scholar]
- 30.Mussini P; Orsini F; Pelizzoni F; Ferrari G, Constituents of Annona coriacea. Structure of a new diterpenoid. J. Chem. Soc., Perkin Trans. 1 1973, 2551–2557. [Google Scholar]
- 31.Mussini P; Orsini F; Pelizzoni F; Buckwalter BL; Wenkert E, Carbon-13 nuclear magnetic resonance spectroscopy of naturally occurring substances.. XXII. Carbon-13 configuration of annonalide. Tetrahedron Lett. 1973, 14, 4849–4851. [Google Scholar]
- 32.Orsini F; Pellizoni F; McPhail AT; Onan KD; Wenkert E, The structure of annonalide. Tetrahedron Lett. 1977, 18, 1085–1088. [Google Scholar]
- 33.On’okoko P; Vanhaelen M; Vanhaelen-Fastre R; Declercq JP; Van Meerssche M, The constitution of icacinol, a new diterpene with a pimarane skeleton from Icacina claessensis. Tetrahedron 1985, 41, 745–748. [Google Scholar]
- 34.On’Okoko P; Vanhaelen M; Vanhaelen-Fastre R; Declercq JP; Van Meerssche M, Icacenone, a furanoditerpene with a pimarane skeleton from Icacina mannii. Phytochemistry 1985, 24, 2452–2453. [Google Scholar]
- 35.Asuzu IU; Abubakar II, The effects of Icacina trichantha tuber extract on the nervous system. Phytother. Res 1995, 9, 21–25. [Google Scholar]
- 36.Zhao M; Onakpa MM; Santarsiero BD; Chen W-L; Szymulanska-Ramamurthy KM; Swanson SM; Burdette JE; Che C-T, (9βH)-Pimaranes and derivatives from the tuber of Icacina trichantha. J. Nat. Prod 2015, 78, 2731–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao M; Onakpa MM; Chen W-L; Santarsiero BD; Swanson SM; Burdette JE; Asuzu IU; Che C-T, 17-Norpimaranes and (9βH)-17-norpimaranes from the tuber of Icacina trichantha. J. Nat. Prod 2015, 78, 789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao M; Onakpa MM; Santarsiero BD; Huang X-J; Zhang X-Q; Chen J; Cheng J-J; Longnecker R; Che C-T, Icacinlactone H and icacintrichantholide from the tuber of Icacina trichantha. Org. Lett 2015, 17, 3834–3837. [DOI] [PubMed] [Google Scholar]
- 39.Guo B; Onakpa MM; Huang X-J; Santarsiero BD; Chen W-L; Zhao M; Zhang X-Q; Swanson SM; Burdette JE; Che C-T, Di-nor- and 17-nor-pimaranes from Icacina trichantha. J. Nat. Prod 2016, 79, 1815–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.On’Okoko P; Hans M; Colau B; Hootele C; Declercq JP; Germain G; Van Meerssche M, Icacine, a new diterpene alkaloid from Icacina guessfeldtii. Bull. Soc. Chim. Belg 1977, 86, 655–661. [Google Scholar]
- 41.On’okoto P; Vanhaelen M, Two new diterpene-based alkaloids from Icacina guesfeldtii. Phytochemistry 1980, 19, 303–305. [Google Scholar]
- 42.Kato-Noguchi H; Ota K; Kujime H; Ogawa M, Effects of momilactone on the protein expression in Arabidopsis germination. Weed Biol. Manag 2013, 13, 19–23. [Google Scholar]
- 43.Chung I-M; Hahn S-J; Ahmad A, Confirmation of potential herbicidal agents in hulls of rice, Oryza sativa. J. Chem. Ecol 2005, 31, 1339–1352. [DOI] [PubMed] [Google Scholar]
- 44.Zhao M; Guo B; Onakpa MM; Wong T; Wakasa K; Che CT; Warpeha KM, Activity of icacinol from Icacina trichantha on seedling growth of Oryza sativa and Arabidopsis thaliana. J. Nat. Prod 2017, 80, 3314–3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kato-Noguchi H; Ota K, Biological activities of rice allelochemicals momilactone A and B. J. Rice Res. 2013, 1, 108. [Google Scholar]
- 46.Kato-Noguchi H; Ota K; Kujime H, Absorption of momilactone A and B by Arabidopsis thaliana L. and the growth inhibitory effects. J. Plant Physiol. 2012, 169, 1471–1476. [DOI] [PubMed] [Google Scholar]
- 47.Fang C; Li Y; Li C; Li B; Ren Y; Zheng H; Zeng X; Shen L; Lin W, Identification and comparative analysis of microRNAs in barnyardgrass (Echinochloa crus-galli) in response to rice allelopathy. Plant Cell Environ. 2015, 38, 1368–1381. [DOI] [PubMed] [Google Scholar]
- 48.Kato-Noguchi H; Kitajima S, Momilactone sensitive proteins in Arabidopsis thaliana. Nat. Prod. Commun 2015, 10, 729–732. [PubMed] [Google Scholar]
- 49.Kato-Noguchi H; Ota K; Ino T, Release of momilactone A and B from rice plants into the rhizosphere and its bioactivities. Allelopathy J. 2008, 22, 321–328. [Google Scholar]
- 50.Fukuta M; Xuan TD; Deba F; Tawata S; Khanh TD; Chung IM, Comparative efficacies in vitro of antibacterial, fungicidal, antioxidant, and herbicidal activities of momilatones A and B. J. Plant Interact. 2007, 2, 245–251. [Google Scholar]
- 51.Chung IM; Ali M; Hahn SJ; Siddiqui NA; Lim YH; Ahmad A, Chemical constituents from the hulls of Oryza sativa with cytotoxic activity. Chem. Nat. Compd 2005, 41, 182–189. [Google Scholar]
- 52.Lee SC; Chung I-M; Jin YJ; Song YS; Seo SY; Park BS; Cho KH; Yoo KS; Kim T-H; Yee S-B; Bae Y-S; Yoo YH, Momilactone B, an allelochemical of rice hulls, induces apoptosis on human lymphoma cells (Jurkat) in a micromolar concentration. Nutr. Cancer 2008, 60, 542–551. [DOI] [PubMed] [Google Scholar]
- 53.Kim S-J; Park H-R; Park E; Lee S-C, Cytotoxic and antitumor activity of momilactone B from rice hulls. J. Agric. Food Chem. 2007, 55, 1702–1706. [DOI] [PubMed] [Google Scholar]
- 54.Joung Y-H; Lim E-J; Kim M-S; Lim SD; Yoon S-Y; Lim YC; Yoo YB; Ye S-K; Park T; Chung I-M; Bae K-Y; Yang YM, Enhancement of hypoxia-induced apoptosis of human breast cancer cells via STAT5b by momilactone B. Int. J. Oncol 2008, 33, 477–484. [PubMed] [Google Scholar]
- 55.Park C; Jeong NY; Kim G-Y; Han MH; Chung I-M; Kim W-J; Yoo YH; Choi YH, Momilactone B induces apoptosis and G1 arrest of the cell cycle in human monocytic leukemia U937 cells through downregulation of pRB phosphorylation and induction of the cyclin-dependent kinase inhibitor p21Waf1/Cip1. Oncol. Rep 2014, 31, 1653–1660. [DOI] [PubMed] [Google Scholar]
- 56.Lichtenthaler HK; Schwender J; Disch A; Rohmer M, Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate-independent pathway. FEBS Lett. 1997, 400, 271–274. [DOI] [PubMed] [Google Scholar]
- 57.Lichtenthaler HK, The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol 1999, 50, 47–65. [DOI] [PubMed] [Google Scholar]
- 58.Rohmer M, The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat. Prod. Rep 1999, 16, 565–574. [DOI] [PubMed] [Google Scholar]
- 59.MacMillan J; Beale MH, Diterpene biosynthesis. In Comprehensive Natural Products Chemistry, Cane DE, Ed. Pergamon Press.: Oxford, 2000; Vol. 2, 217–241. [Google Scholar]
- 60.Yamane H, Biosynthesis of phytoalexins and regulatory mechanisms of it in rice. Biosci. Biotechnol. Biochem 2013, 77, 1141–1148. [DOI] [PubMed] [Google Scholar]
- 61.Otomo K; Kanno Y; Motegi A; Kenmoku H; Yamane H; Mitsuhashi W; Oikawa H; Toshima H; Itoh H; Matsuoka M; Sassa T; Toyomasu T, Diterpene cyclases responsible for the biosynthesis of phytoalexins, momilactones A, B, and oryzalexins A-F in rice. Biosci. Biotechnol. Biochem 2004, 68, 2001–2006. [DOI] [PubMed] [Google Scholar]
- 62.Shimura K; Okada A; Okada K; Jikumaru Y; Ko K-W; Toyomasu T; Sassa T; Hasegawa M; Kodama O; Shibuya N; Koga J; Nojiri H; Yamane H, Identification of a biosynthetic gene cluster in rice for momilactones. J. Biol. Chem 2007, 282, 34013–34018. [DOI] [PubMed] [Google Scholar]
- 63.Wang Q; Hillwig ML; Peters RJ, CYP99A3: functional identification of a diterpene oxidase from the momilactone biosynthetic gene cluster in rice. Plant J. 2011, 65, 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kitaoka N; Wu Y; Xu M; Peters RJ, Optimization of recombinant expression enables discovery of novel cytochrome P450 activity in rice diterpenoid biosynthesis. Appl. Microbiol. Biotechnol 2015, 99, 7549–7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Q; Hillwig ML; Okada K; Yamazaki K; Wu Y; Swaminathan S; Yamane H; Peters RJ, Characterization of CYP76M5–8 indicates metabolic plasticity within a plant biosynthetic gene cluster. J. Biol. Chem 2012, 287, 6159–6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Atawong A; Hasegawa M; Kodama O, Biosynthesis of rice phytoalexin: enzymatic conversion of 3β-hydroxy-9β-pimara-7,15-dien-19,6β-olide to momilactone A. Biosci. Biotechnol. Biochem 2002, 66, 566–570. [DOI] [PubMed] [Google Scholar]
- 67.Okada A; Okada K; Miyamoto K; Koga J; Shibuya N; Nojiri H; Yamane H, OsTGAP1, a bZIP transcription factor, coordinately regulates the inductive production of diterpenoid phytoalexins in Rice. J. Biol. Chem 2009, 284, 26510–26518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamamura C; Mizutani E; Okada K; Nakagawa H; Fukushima S; Tanaka A; Maeda S; Kamakura T; Yamane H; Takatsuji H; Mori M, Diterpenoid phytoalexin factor, a bHLH transcription factor, plays a central role in the biosynthesis of diterpenoid phytoalexins in rice. Plant J. 2015, 84, 1100–1113. [DOI] [PubMed] [Google Scholar]
- 69.Sichererroetman A; Jansen BJM; de Groot A, Stereospecific preparation of (+/−)-4,4-dinor-9-beta-H-pimara-7,15-diene, a model for the total synthesis of momilactone type diterpenes. Tetrahedron Lett. 1984, 25, 2593–2596. [Google Scholar]
- 70.Sicherer-Roetman A; Jansen BJM; de Groot A, Investigation into the total synthesis of momilactones. Stereoselective preparation of (±)-4,4-dinor-9βH-pimara-7,19-diene. Recl. Trav. Chim. Pays-B. 1985, 104, 193–202. [Google Scholar]
- 71.Jansen BJM; Schepers GC; de Groot A, The stereoselective synthesis of (±)-9βH-pimara-7,19-diene. Tetrahedron Lett. 1989, 45, 2773–2776. [Google Scholar]
- 72.Chu M; Coates RM, Partial synthesis of 9,10-syn diterpenes via tosylhydrazone reduction - (−)-(9-beta)-primara-7,15-diene and (−)-(9-beta)-isopimaradiene. J. Org. Chem 1992, 57, 4590–4597. [Google Scholar]
- 73.Kabalka GW; Yang DTC; Baker JD, Deoxygenation of alpha,beta-unsaturated aldehydes and ketones via catecholborane reduction of corresponding tosylhydrazones. J. Org. Chem 1976, 41, 574–575. [Google Scholar]
- 74.Wood JL; Porco JA; Taunton J; Lee AY; Clardy J; Schreiber SL, Application of the Allylic Diazene Rearrangement - Synthesis of the enediyne-bridged tricyclic core of dynemicin-A. J. Am. Chem. Soc 1992, 114, 5898–5900. [Google Scholar]
- 75.Yajima A; Toda K; Okada K; Yamane H; Yamamoto M; Hasegawa M; Katsuta R; Nukada T, Stereocontrolled total synthesis of (+/−)-3 beta-hydroxy-9 beta-pimara-7,15-diene, a putative biosynthetic intermediate of momilactones. Tetrahedron Lett. 2011, 52, 3212–3215. [Google Scholar]
- 76.Germain J; Deslongchamps P, Total synthesis of (+/−)-momilactone A. J. Org. Chem 2002, 67, 5269–5278. [DOI] [PubMed] [Google Scholar]
- 77.Marsault E; Toro A; Nowak P; Deslongchamps P, The transannular Diels-Alder strategy: applications to total synthesis. Tetrahedron 2001, 57, 4243–4260. [Google Scholar]
- 78.Germain J; Deslongchamps P, Transannular Diels-Alder approach to the synthesis of momilactone A. Tetrahedron Lett. 1999, 40, 4051–4054. [Google Scholar]
- 79.Burgess EM; Penton HR; Taylor EA, Thermal reactions of alkyl N-carbomethoxysulfamate esters. J. Org. Chem 1973, 38, 26–31. [Google Scholar]
- 80.Chung IM; Ateeque A; Kim JS Momilactones A and B from rice hulls as herbicides. 2005–12810220060160701, 20050512, 2006. [Google Scholar]
- 81.Yang YM; Jung IM; Jung YH; Bae GY Momilactone B for preventing and treating solid neoplasm. 2007–1018552009036688, 20071010, 2009. [Google Scholar]
- 82.You YH; Chung IM Pharmaceutical composition comprising momilactone B as major component for treating leukemia, preferably acute lymphocytic leukemia, and drug thereof. 2005–305632006108373, 20050413, 2006. [Google Scholar]
- 83.Germain J; Deslongchamps P, Total synthesis of (±)-momilactone A. J. Org. Chem 2002, 67, 5269–5278. [DOI] [PubMed] [Google Scholar]
- 84.Mohan RS; Yee NKN; Coates RM; Ren Y-Y; Stamenkovic P; Mendez I; West CA, Biosynthesis of cyclic diterpene hydrocarbons in rice cell suspensions: conversion of 9,10-syn-labda-8(17), 13-dienyl diphosphate to 9β-pimara-7,15-diene and stemar-13-ene. Arch. Biochem. Biophys 1996, 330, 33–47. [DOI] [PubMed] [Google Scholar]
- 85.Nojiri H; Sugimori M; Yamane H; Nishimura Y; Yamada A; Shibuya N; Kodama O; Murofushi N; Omori T, Involvement of jasmonic acid in elicitor-induced phytoalexin production in suspension-cultured rice cells. Plant Physiol. 1996, 110, 387–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shimizu T; Jikumaru Y; Okada A; Okada K; Koga J; Umemura K; Minami E; Shibuya N; Hasegawa M; Kodama O; Nojiri H; Yamane H, Effects of a bile acid elicitor, cholic acid, on the biosynthesis of diterpenoid phytoalexins in suspension-cultured rice cells. Phytochemistry 2008, 69, 973–981. [DOI] [PubMed] [Google Scholar]
- 87.Chung I-M; Ali M; Ahmad A, Oryzasesterpenolide from the hulls of Oryza sativa and complete NMR assignments of momilactones A, B and tricin. Asian J. Chem 2005, 17, 2467–2478. [Google Scholar]
- 88.Koga J, Structure, function, and biological activity of rice phytoalexins and elicitors In Plant-derived antimycotics: Current trends and future prospects, Rai M; Mares D, Eds. Haworth Press: New York, 2003; pp 497–524. [Google Scholar]
- 89.Toyomasu T; Kagahara T; Okada K; Koga J; Hasegawa M; Mitsuhashi W; Sassa T; Yamane H, Diterpene phytoalexins are biosynthesized in and exuded from the roots of rice seedlings. Biosci. Biotechnol. Biochem 2008, 72, 562–567. [DOI] [PubMed] [Google Scholar]
- 90.Koga J, Structure, function, and biological activity of rice phytoalexins and elicitors In Plant-Derived Antimycotics:Trends Current and Prospects Future, Rai M; D. M, Eds. Haworth Press: New York, 2003; 497–524. [Google Scholar]