Abstract
Objectives
Cell therapies using mesenchymal stromal cells (MSCs) have been proposed as a promising new tool for the treatment of vocal fold scarring. However, the mechanisms by which MSC promote healing as well as their duration of survival within the host vocal fold have yet to be defined. The aim of this work was to assess the persistence of embedded MSCs within a tissue-engineered vocal fold mucosal replacement in a rabbit model of vocal fold injury.
Methods
Male rabbit adipose-derived mesenchymal stromal cells (ASC) were embedded within a three-dimensional fibrin gel, forming the cell-based outer vocal fold replacement (COVR). Four female rabbits underwent unilateral resection of vocal fold epithelium and lamina propria and reconstruction with COVR implantation. Polymerase chain reaction and fluorescent in situ hybridization for the sex-determining region of the Y chromosome (SRY-II) in the sex-mismatched donor-recipient pairs sought persistent cells after 4 weeks.
Results
A subset of implanted male cells was detected in the implant site at 4 weeks. Many SRY-II negative cells were also detected at the implant site, presumably representing native female cells that migrated to the area. No SRY-II signal was detected in contralateral control vocal folds.
Conclusions
The emergent tissue after implantation of a tissue-engineered outer vocal fold replacement is derived both from initially embedded adipose-derived stromal cells and infiltrating native cells. Our results suggest this tissue-engineering approach can provide a well-integrated tissue graft with prolonged cell activity for repair of severe vocal fold scars.
Keywords: Adipose-derived stromal cell, tissue engineering, vocal fold scarring, vocal fold replacement
INTRODUCTION
Vocal fold scarring, which can be caused by inflammation, radiation, trauma, or surgery, is a disabling voice disorder. Scar tissue alters the extracellular matrix (ECM) of the vocal fold, making it stiff and inelastic, leading to altered voice quality. The composition and organization of the ECM establish the vocal fold’s biomechanical properties which in turn determine voice quality. Thus, treatment of the voice disturbance requires restoration of the unique microstructure of the ECM.
Cell-based approaches have received recent attention as potential therapies for vocal fold scarring. Among various cell types, mesenchymal stromal cells (MSCs) are particularly attractive given their ease of expansion in culture and extensive safety profile in humans.1,2 MSCs can be administered alone or combined with a scaffold. Scaffolds provide an instructive microenvironment that may enhance stem cell survival at the site of injury. We previously showed that adipose-derived mesenchymal stromal cells (ASCs) embedded within a three-dimensional fibrin gel scaffold improve vocal fold histology and vibration in rabbits.3,4
Although MSC have been proposed for vocal fold scarring, the therapeutic mechanisms by which MSC promote healing have yet to be defined. Reasons for benefit may include direct replacement of damaged resident cells, release of immunomodulatory or trophic factors, or a combination thereof. It is known that MSC secrete several anti-fibrotic and prosurvival factors that likely play a role,5–7 but some evidence for differentiation and engraftment has also been reported.8 The identification of MSC’s powerful paracrine actions in other tissues has de-emphasized long-term cell engraftment as a therapeutic requirement.9–11 Nonetheless, implanted cells must survive for some unknown duration to produce any paracrine effect. For vocal fold replacement, in which organized ECM remodeling is the therapeutic action, implanted cell influence should continue until remodeling is stabilized.
Understanding the fate of transplanted stem cells requires reliable techniques for identifying cells after implantation. Traditional methods using stem cells labeled with transgenic constructs (e.g. green fluorescent protein) or membrane dyes (e.g. CM-DiI) have been used in the vocal fold with widely varying findings regarding the timeline of cell survival.12–14 These methods are limited by loss of gene expression in unstable transfections and reduction in the concentration of membrane dye through cell divisions.15,16 An alternative approach implants sex-mismatched ASCs and seeks signal for Y chromosome DNA.17 Such an approach capitalizing on donor-specific antigens may overcome some of the limitations of cell labeling.17
The aim of the present study is to assess the engraftment of ASCs within the female rabbit vocal fold implants that were previously reported.[3,4] Cell-based Outer Vocal fold Replacements (COVR) were formed with male rabbit ASC in three-dimensional fibrin gel scaffolds. COVRs were implanted in four female rabbits after removing the native vocal fold epithelium and lamina propria. Excised larynx phonation and histology results at 4 weeks were previously described. This study uses Y chromosome DNA to identify persistent donor cells four weeks after implantation, and TUNEL labeling to identify apoptotic cells.
METHODS
In Vitro Development of Cell-Based Outer Vocal Fold Replacement
Rabbit adipose-derived multipotent stromal cells (rASCs) were isolated from male rabbits and tissue-engineered constructs were created as previously described (Figure 1A).1,18,19 Briefly, rASCs were harvested from inguinal fat by collagenase digestion and expanded in culture. Cell multipotency was confirmed by differentiation to osteogenic and adipogenic phenotypes.18 Rabbit fibrinogen was mixed with bovine thrombin and rASC cell suspension to form fibrin gels with embedded ASC within 12mm Transwell culture inserts. The tissue constructs were cultured with an air interface and supplied culture medium through the insert base. Medium contained 10% fetal bovine serum and 100 ng/mL of epidermal growth factor. After 2 weeks, the cell-based outer vocal fold replacements (COVR) were harvested for implantation or baseline histology. Histologic stains included standard hematoxylin and eosin (H&E), Masson’s trichrome to stain collagen fibers blue, and phosphotungstic acid hematoxylin (PTAH) to stain fibrin fibers blue. The mature COVR in vitro demonstrated homogeneous fibrin fibers, without detectable collagen.
Figure 1.
(A) Donor male rASCs were seeded in a COVR that was surgically implanted to female rabbit recipients. (B) Hematoxylin and eosin (H & E) stain of harvested larynx, where left vocal fold received COVR implant (L) and right vocal fold served as unoperated control (R). 10x. (Rabbit 4). Thick black line at the anterior commissure indicates the dividing line for extracting DNA from individual vocal folds.
COVR Implant Surgery
The local Institutional Animal Care and Use Committee approved this study which was conducted in accordance with all local and federal guidelines. Four female New Zealand white rabbits, weighing 3 to 3.5 kg, underwent survival surgery for outer vocal fold mucosa removal and implantation with COVR which was described previously.3 Briefly, rabbits were anesthetized, and the larynx exposed through a neck incision. After laryngofissure, the entire membranous cover layer was resected from the left inferior vocal fold. Immediately following resection, a mature COVR containing male rASC was placed to fill the defect and secured with sutures and fibrin glue. All right vocal folds remained as untreated controls. The laryngofissure, tracheotomy, and neck were closed and the animals recovered. Intramuscular dexamethasone, analgesics, and antibiotics were administered for three days to prevent laryngeal edema, pain, and infection. All four animals recovered without complication or apparent distress, maintained oral diet, and gained weight. After 4 weeks, animals were euthanized and larynges harvested. The 4-week time point was chosen for best comparison with previous work in this laboratory and because it lies within the range of other studies assessing MSC persistence.3,4,8,13,20 All four larynges produced phonation in an excised larynx setup, the same day as harvest as described previously.3 Larynges were then formalin-fixed and paraffin-embedded (FFPE) for sectioning and DNA extraction.
PCR Amplification of Rabbit Y Chromosome DNA
Genomic DNA from male New Zealand white rabbit thymuses was isolated and purified using the Qiagen genomic DNA kit (Germantown, MD). Primers were constructed for the second sex-determining region of the Y chromosome (SRY-II) of the European brown hare.21 The isolated DNA was amplified by PCR using the SRY-II primers with AmpliTaq Gold™ 360 Master Mix (Applied Biosystems, Foster City, CA) under the following conditions: 95°C for 2 minutes followed by 35 cycles of 95°C for 45 seconds, 55°C for 30 seconds, 72°C for 45 seconds with a final extension at 72°C for 5 minutes. PCR products were run on 1.5% agarose gel and images were captured using G:BOX chemi XRQ (Syngene, Frederick, MD) to confirm expected SRY-II size of 670 base pairs.
After validating the primer sequence with male rabbit DNA, the primers were used to amplify DNA from rabbit larynx tissues. Implanted female rabbit vocal fold tissues were dissected out of the paraffin blocks, using a scalpel to sharply divide the implanted (left) and control (right) vocal folds at the anterior commissure (as indicated in Figure 1B). DNA was isolated and purified using the QIAamp DNA FFPE tissue kit (Qiagen, Germantown, MD). PCR amplification proceeded as above using SRY-II primers. PCR products were separated on 1.5% agarose gel and imaged.
Fluorescent In Situ Hybridization (FISH)
DNA was isolated from normal male rabbit thymus, and Y chromosome-specific DNA was then amplified as described above. PCR products were separated on 0.8% agarose gel. The amplified 670 bp SRY-II product was excised from the gel and purified using QIAquick Gel Extraction kit (Qiagen, Germantown, MD). Purified DNA was labeled with Alexa Fluor 647 dye using Fish Tag DNA multicolor kit (Molecular Probes, Eugene, OR) following the protocol provided with the kit.
Five-micron FFPE tissue sections of implanted rabbit larynges were used for FISH. Y chromosome-specific rabbit DNA labeled with Alexa Fluor 647 was used as FISH probe. Tissue sections fixed on glass slides were de-paraffinized in Xylene (with three changes of 5 minutes each), then gradually rehydrated using 100%, 85%, and 50% ethanol (one minute each) followed by incubation in 0.2 N HCl for 20 minutes, distilled water for 10 minutes, 2X sodium-saline citrate for two minutes, 10mM sodium citrate buffer pH 6.0 at 80°C for 30 minutes, 20 ng proteinase K/ml in 10 mM HCl at 37°C for 20 minutes and finally in distilled water for three minutes. Slides were then dehydrated gradually in 50%, 70%, 85% and 100% ethanol (one minute each) and air dried for 30 minutes. For the rest of the procedures for pre-hybridization, hybridization, and post-hybridization, washes were carried out as described in the Fish Tag multicolor kit user guide (Invitrogen, Carlsbad, CA). Slides were coverslipped and imaged with a 647 nm fluorescence microscopy filter.
Apoptosis Assay
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay for apoptotic cells was performed on paraffin-embedded, formalin-fixed slides from implanted rabbit larynges (Trevigen TACS 2TdT DAB labeling kit, Gaitersburg, MD). The terminal deoxynucleotidyl transferase (TdT) enzyme incorporated biotin label into fragmented DNA of apoptotic cells. Positive controls were treated with TACS-Nuclease enzyme to induce DNA strand nicks. Biotin was detected with diaminobenzidine (DAB) substrate, and nuclei counterstained with methyl green. Images were taken at 40x.
RESULTS
Rabbit Y chromosome PCR
Thymic DNA from normal male New Zealand white rabbits confirmed amplification with the SRY-II primer sequences, producing a 670 bp PCR product.
DNA was isolated from implanted and control rabbit vocal folds, amplified with SRY-II primers, and separated on agarose. All four implanted vocal folds (with male cells applied to female larynx) showed an amplified DNA band that corresponded with the 670 bp PCR product from male thymus. All four contralateral control vocal folds (female larynx without implant) lacked any amplification with the SRY-II primer (Figure 2A).
Figure 2.
Presence of implanted cell DNA after 1 month. (A) PCR amplification of SRY-II DNA sequence. “SRY control”: normal male rabbit tissue. Lanes are shown for each rabbit’s implanted vocal fold (“Implant”) and contralateral non-implanted vocal fold (“Contra”). (B) Fluorescent in-situ hybridization for Y chromosome DNA (SRY-II probe, appearing red) in female rabbit vocal folds 4 weeks after implantation of a COVR seeded with male ASCs. Signal appears in the operated left vocal folds (L), but not in the contralateral unoperated right vocal folds (R), suggesting engraftment of implanted ASCs. Nuclei are stained with DAPI (blue). Rabbit 1 at 4X magnification, Rabbit 2 at 10X magnification.
Fluorescent In Situ Hybridization (FISH)
In situ hybridization with a tagged SRY-II probe confirmed the findings of PCR. In all four female larynges, scattered positive (male) cells were detected on the operated side. The area of implantation also contained many cells negative for the Y-chromosome signal that presumably represented native female cells that had migrated into the implanted site (Figure 2B). Implanted ASCs were primarily localized to the lamina propria, with relatively fewer in the epithelial layer and occasional pockets detected in deep tissue near the thyroarytenoid muscle. No SRY-positive cells were detected in contralateral control vocal folds.
Histological Examination
Figure 1B shows H&E staining, with implanted left vocal fold appearing similar to unoperated right vocal fold. Collagen fibers were previously demonstrated in the region of implantation on Masson’s trichrome stain, indicating remodeling of the provisional scaffold.3 Phosphotungstic acid hematoxylin (PTAH) staining was attempted to determine persistence of the fibrin matrix, but the non-specific nature of the staining was judged to be inconclusive.
Apoptosis assay
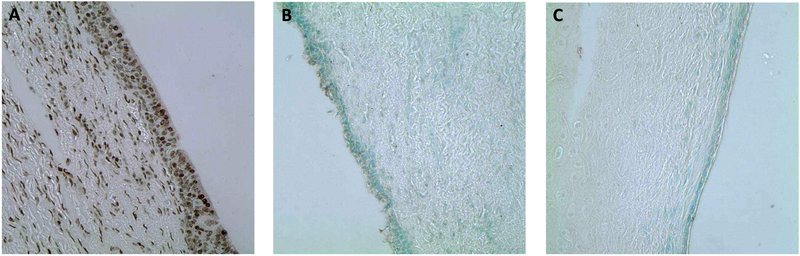
TUNEL labeling for apoptotic cells was negative within the area of COVR implant, consistent with its negative labeling in contralateral unoperated vocal folds. Positive controls of nuclease enzyme-treated implant slides did demonstrate positive TUNEL staining, confirming the labeling technique (Figure 3).
Figure 3.
TUNEL labeling for apoptotic cells. (A) Positive control for TUNEL staining, enzymatically treated to induce DNA nicks. R4, 40x (B) Harvested left vocal fold (COVR implant side) with negative TUNEL staining confirmed that the cells were not undergoing apoptosis. Rabbit 3, 40x. (C) Harvested right vocal fold (unoperated control) also with negative TUNEL staining, as expected. Rabbit 3, 40x.
DISCUSSION
In this study, we investigated the engraftment of rabbit ASCs within a tissue-engineered implant in the rabbit vocal fold. To distinguish transplanted cells from native cells, male rASCs were seeded into female hosts to permit DNA analysis for genes on the Y-chromosome. Four weeks after implantation, scattered male cells were localized within the implant site, along with many cells that were negative for the fluorescence hybridization signal. This finding suggests that the emergent tissue derives both from infiltrating native cells and implanted ASCs that were initially seeded onto the scaffold.
Simple injections of mesenchymal stromal cells have prevented scar formation in previous animal models.20,22,23 Postulated mechanisms include secretion of growth factors active in vocal fold tissue repair, including hepatocyte growth factor (HGF) and fibroblast growth factor 2 (FGF2).7,24,25 In addition, Hiwatashi et al reported that MSC treatment led to increased host expression of hyaluronan synthase, which is thought to maintain viscoelastic function of the vocal folds.12 Transient cell activity may be adequate to prevent scarring when the cells are applied concordant with injury. However, accumulating data have revealed that the survival rate of cells injected into previously scarred vocal folds is disappointingly low. Svensson et al did not detect any GFP-positive MSC three months after direct administration in scarred rabbit vocal folds.26 de Bonnecaze studied an earlier 3-week time point and also were unable to detect GFP-labeled cells after MSC injection.13 Survival of transplanted cells is limited by apoptosis, lack of trophic factors, pathologic conditions, and the inflammatory response.
Scaffolds, including hyaluronic acid (HA) based hydrogels27,28 and atelocollagen,22 have been used as delivery carriers and led to increased cell survival in a few prior studies. These scaffolds can be engineered to mimic the native extracellular matrix which may create a microenvironment more suitable to MSC survival. HA scaffolds have been most widely employed, but are limited by challenges in preventing HA degradation and clearance in vivo that allows cells to migrate rapidly away from the site of implantation.29 In the largest pre-clinical trial for vocal fold scar, Bartlett et al investigated cell engraftment after administration of MSC with and without a hyaluronic acid gel carrier in rabbits and detected no transplanted cells in either group at 2 weeks.30 In contrast, our study utilized fibrin as the scaffold matrix and detected implanted cells at 4 weeks in all four female rabbits. Protein-based scaffolds, such as fibrin, may be superior in promoting cell-to-surface binding compared to polysaccharide-based scaffolds such as HA, potentially enhancing cell survival.31 We hypothesize that the fibrin scaffold provides a supportive niche which improves cell survival relative to cell injection alone. Whether the surviving ASCs establish a long-term, stable sub-population residing within the scaffold cannot be adequately determined by our 4-week study, but this is a question worthy of further investigation. In this study, host cells did significantly infiltrate the implant, which by 4 weeks was more host-derived than graft-derived.
Characterizing the fate of transplanted cells in regenerative medicine applications depends on having reliable techniques for cell tracking or detection. To date, methods to identify implanted stem cells in the vocal fold have primarily relied on transgene construct labeling (e.g. green fluorescent protein) or membrane dye approaches. These methods are attractive given their ease of use, but suffer potential limitations in the long-term evaluation of stem cell persistence. The GFP transgene may become silenced as cells proliferate and differentiate in vivo, potentially masking cell detection.15,16,30 Alternatively, lipophilic membrane labels such as carbocyanine derivatives may be detected even after macrophage ingestion of dead cells, potentially leading to overestimation of surviving cell populations.32 Here, we target sex-specific antigens existing exclusively in the injected cells by immunofluorescence in a sex-mismatched animal model. Such a technique may overcome the limitations of cell labeling methods without a significant increase in complexity or cost and may be a desirable approach for future studies. The limitation of this approach is that it only applies to male cells in female hosts.
Because this methodology of Y-chromosome detection is invalid in the male-male transplant, the cell persistence in male hosts remains unknown. Inflammatory cell infiltrate did occur in the female hosts but not in male hosts receiving allogeneic male cells.3 This study transplanted allogeneic ASCs in the absence of immunosuppression, and the persistence of some implanted cells after 4 weeks indicates the inflammatory response did not reach the level of complete transplant rejection. ASCs themselves have significant immunomodulatory activity, which could assist implanted cells in evading the host immune system.33,34 Why the females exhibited greater leukocytosis is of interest for further investigation. It is conceivable that the female host has inherently greater immune activity than the males. Or, the greater female response may simply be due to greater antigen mismatch with male cells. In studies of solid organ transplant in humans, it has been observed that sex mismatch in donor-recipient pairs, especially cases of male donor to female recipient, does lead to higher incidence of acute rejection.35,36 The mechanism has been linked to the production of alloantibodies against the male H-Y minor histocompatibility complex antigen.37 Very limited basic work in cell therapies has explored sex as a biological variable, which is essential to distinguish these hypotheses in future research.38 For eventual clinical applications, the use of autologous or bank-matched stem cells would be expected to further reduce immune response which could prolong cell survival.
CONCLUSION
This study furthers the pre-clinical development of a tissue-engineered outer vocal fold replacement constructed from adipose-derived stromal cells. A subset of the implanted cells persisted at least 4 weeks in rabbits, in contrast to other studies with different implant designs. Our results suggest this tissue-engineering approach can provide a well-integrated tissue graft with prolonged cell activity for repair of severe vocal fold scars.
FINANCIAL SUPPORT:
This material is based upon work supported by the Department of Veteran Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development (Jennifer Long), VA Career Development Award IK2BX001944 (Jennifer Long); American Academy of Otolaryngology-Head and Neck Surgery Foundation Resident Research Grant (Travis Shiba); Dean’s Leadership in Health and Science Scholarship at David Geffen School of Medicine (Alexander Goel).
Footnotes
CONFLICT OF INTEREST: None
FINANCIAL DISCLOSURES: None
STATUS: New submission. This work has not been previously published in any language anywhere and is not under simultaneous consideration or in press by another journal
REFERENCES
- 1.Zuk P, Zhu M, Mizuno H, et al. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Engineering. 2001;7(2):211–228. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science. 1999;284(5411):143–147. [DOI] [PubMed] [Google Scholar]
- 3.Shiba TL, Hardy J, Luegmair G, Zhang Z, Long JL. Tissue-Engineered Vocal Fold Mucosa Implantation in Rabbits. Otolaryngol Head Neck Surg. 2016;154(4):679–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Long JL. Repairing the vibratory vocal fold. Laryngoscope. 2018;128(1):153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirano S, Bless D, Rousseau B, et al. Prevention of Vocal Fold Scarring by Topical Injection of Hepatocyte Growth Factor in a Rabbit Model. Laryngoscope. 2004;114(3):548–556. [DOI] [PubMed] [Google Scholar]
- 6.Cheng SL, Zhang SF, Mohan S, et al. Regulation of insulin-like growth factors I and II and their binding proteins in human bone marrow stromal cells by dexamethasone. J Cell Biochem. 1998;71(3):449–458. [DOI] [PubMed] [Google Scholar]
- 7.Weimar IS, Voremans C, Bourhis JH, et al. Hepatocyte growth factor/scatter factor (HGF/SF) affects proliferation and migration of myeloid leukemic cells. Leukemia. 1998;12(8):1195–1203. [DOI] [PubMed] [Google Scholar]
- 8.Cedervall J, Ahrlund-Richter L, Svensson B, et al. Injection of embryonic stem cells into scarred rabbit vocal folds enhances healing and improves viscoelasticity: short-term results. Laryngoscope. 2007;117(11):2075–2081. [DOI] [PubMed] [Google Scholar]
- 9.Chimenti I, Smith RR, Li TS, et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106(5):971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li TS, Cheng K, Malliaras K, et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol. 2012;59(10):942–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furuta T, Miyaki S, Ishitobi H, et al. Mesenchymal Stem Cell-Derived Exosomes Promote Fracture Healing in a Mouse Model. Stem Cells Transl Med. 2016;5(12):1620–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiwatashi N, Hirano S, Mizuta M, et al. Adipose-derived stem cells versus bone marrow-derived stem cells for vocal fold regeneration. Laryngoscope. 2014;124(12):E461–469. [DOI] [PubMed] [Google Scholar]
- 13.de Bonnecaze G, Chaput B, Woisard V, et al. Adipose stromal cells improve healing of vocal fold scar: Morphological and functional evidences. Laryngoscope. 2016;126(8):E278–285. [DOI] [PubMed] [Google Scholar]
- 14.Liang Q, Liu S, Han P, et al. Micronized acellular dermal matrix as an efficient expansion substrate and delivery vehicle of adipose-derived stem cells for vocal fold regeneration. Laryngoscope. 2012;122(8):1815–1825. [DOI] [PubMed] [Google Scholar]
- 15.Brazelton TR, Blau HM. Optimizing Techniques for Tracking Transplanted Stem Cells In Vivo. Stem Cells. 2005;23(9):1251–1265. [DOI] [PubMed] [Google Scholar]
- 16.Toth ZE, Shahar T, Leker R, et al. Sensitive detection of GFP utilizing tyramide signal amplification to overcome gene silencing. Exp Cell Res. 2007;313(9):1943–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terrovitis JV, Smith RR, Marban E. Assessment and optimization of cell engraftment after transplantation into the heart. Circ Res. 2010;106(3):479–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiba T, Hardy J, Long J, Chatzistefanou I. Laryngeal Tissue Engineering Using Rabbit Adipose Derived Stem Cells In Fibrin: A Pre-Clinical Model. Journal of Otolaryngology Advances. 2015;1(1):27–39. [Google Scholar]
- 19.Long JL, Zuk P, Berke GS, Chhetri DK. Epithelial differentiation of adipose-derived stem cells for laryngeal tissue engineering. Laryngoscope. 2010; 120(1): 125–131. [DOI] [PubMed] [Google Scholar]
- 20.Kim YM, Oh SH, Choi JS, et al. Adipose-derived stem cell-containing hyaluronic acid/alginate hydrogel improves vocal fold wound healing. Laryngoscope. 2014;124(3):E64–72. [DOI] [PubMed] [Google Scholar]
- 21.Putze M, Nürnberg S, Fickel J. Y-chromosomal markers for the European brown hare (Lepus europaeus, Pallas 1778). European Journal of Wildlife Research. 2007;53(4):257–264. [Google Scholar]
- 22.Hiwatashi N, Hirano S, Suzuki R, et al. Comparison of ASCs and BMSCs combined with atelocollagen for vocal fold scar regeneration. Laryngoscope. 2016;126(5):1143–1150. [DOI] [PubMed] [Google Scholar]
- 23.Kanemaru S, Nakamura T, Omori K, et al. Regeneration ofthe vocal fold using autologous mesenchymal stem cells. Ann Otol Rhinol Laryngol. 2003;112(11):915–920. [DOI] [PubMed] [Google Scholar]
- 24.Kishimoto Y, Hirano S, Kitani Y, et al. Chronic vocal fold scar restoration with hepatocyte growth factor hydrogel. Laryngoscope. 2010;120(1):108–113. [DOI] [PubMed] [Google Scholar]
- 25.Suehiro A, Hirano S, Kishimoto Y, Tateya I, Rouzzeau B, Ito J. Effects of basic fibroblast growth factor on rat vocal fold fibroblasts. .Ann ltol Rhinol Laryngol. 2010; 119(10):690–696. [DOI] [PubMed] [Google Scholar]
- 26.Svensson B, Nagubothu RS, Cedervall J, et al. Injection of human mesenchymal stem cells improves healing of scarred vocal folds: analysis using a xenograft model. Laryng oscope. 2010; 120(7): 1370–1375. [DOI] [PubMed] [Google Scholar]
- 27.Thibeault SL, Kiemuk SA, Smith ME, Leugers C, Prestwich G. In Vivo Comparison of Biomimetic Approaches for Tissue Regeneration of the Scarred Vocal Fold. Tissue Engineering: Part A. 2009;15(7):1481–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu W, Hu R, Fan E, Han D. Adipose-Derived Mesenchymal Stem Cells in Collagen Hyaluronic Acid Gel Composite Scaffolds for Vocal Fold Regeneration. Ann Otol Rhinol Laryngol. 2011;120(2):123–130. [DOI] [PubMed] [Google Scholar]
- 29.Lim JY, Kim HS, Kim YH, Kim KM, Choi HS. PMMA (polymethylmetacrylate) microspheres and stabilized hyaluronic acid as an injection laryngoplasty material for the treatment of glottal insufficiency: in vivo canine study. Eur Arch Otorhinolaryngol. 2008;265(3):321–326. [DOI] [PubMed] [Google Scholar]
- 30.Bartlett RS, Guille JT, Chen X, Christensen MB, Wang SF, Thibeault SL. Mesenchymal stromal cell injection promotes vocal fold scar repair without long-term engraftment. Cytotherapy. 2016;18(10):1284–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahearne M, Kelly DJ. A comparison of fibrin, agarose and gellan gum hydrogels as carriers of stem cells and growth factor delivery microspheres for cartilage regeneration. Biomed Mater. 2013;8(3):035004. [DOI] [PubMed] [Google Scholar]
- 32.Cocco R, Ucker DS. Distinct modes of macrophage recognition for apoptotic and necrotic cells are not specified exclusively by phosphatidylserine exposure. Molecular Biology of the Cell. 2001;12(4):919–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melief SM, Zwaginga JJ, Fibbe WE, Roelofs H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl Med. 2013;2(6):455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niemeyer P, Kornacker M, Mehlhorn A, et al. Comparison of immunological properties of bone marrow stromal cells and adipose tissue-derived stem cells before and after osteogenic differentiation in vitro. Tissue Eng. 2007;13(1): 111–121. [DOI] [PubMed] [Google Scholar]
- 35.Candinas D, Gunson B, Nightingale P, Hubscher S, McMaster P, Neuberger JM. Sex mismatch as a risk factor for chronic rejection of liver allografts. The Lancet. 1995;346(8983): 1117–1121. [DOI] [PubMed] [Google Scholar]
- 36.Li Z, Mei S, Xiang J, et al. Influence of donor-recipient sex mismatch on long-term survival of pancreatic grafts. Sci Rep. 2016;6:29298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan JC, Wadia PP, Coram M, et al. H-Y antibody development associates with acute rejection in female patients with male kidney transplants. Transplantation. 2008;86(1):75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. [DOI] [PubMed] [Google Scholar]