Abstract
Obesity-related diseases are an important part of public health; and obesity is related with colorectal cancer. Adipocyte hypertrophy and visceral adipose tissue accumulation can cause adipocitis-related diseases and pathogenic adipocyte formation. Adipose tissue has a very important and active role in immune response formation. Cytokines/adipokines, which are secreted from adipose tissue, have an active role in communication between adipocytes and macrophages. Thus, visceral adipocyte is related with low-grade chronic systemic inflammation. Adipocytes have an important role in colorectal cancer pathogenesis because of proinflammatory cytokines, growth factors, and hormones secretion. Most highlighted cytokines are adiponectin, resistin, and ghrelin. Also, insulin resistance, glucose intolerance, increased plasma insulin levels, body mass index, insulin-like growth factor (IGF-1), glucose, and serum free fatty acids levels are considered to be related with colorectal cancer pathogenesis. Thus, in this review, we focus on the role of adipokines and insulin in colorectal cancer.
Keywords: Colorectal cancer, resistin, insulin, adipokines, adiponectin, obesity
Introduction
The term cancer describes various malignant tumors that may influence almost all organs and tissues. With changed life styles, increased interaction with various carcinogenic agents, and unfavorable changes in quality of life, incidence of cancer has gradually increased [1]. According to the data obtained from Global cancer statistics database (GLOBOCAN), it is estimated that 14.1 million people were diagnosed with cancer in 2012. Worldwide, deaths from cancers were 8.2 million; and an estimated 32.6 million people were five-year cancer survivors [2]. In Turkey, cancers account for 19.7% of deaths [3]. According to the data of “Turkish Cancer Statistics” published by Turkish Ministry of Health in 2016, colorectal cancers are the third most common type of cancer in both females and males [4]. Similarly, while colorectal cancers are the third most common type of cancer among men worldwide (10% of all cancers), it ranks as the second among women (9.2% of all cancers). Industrialization and urbanization have led to increase in the rate of this type of cancer; and although it is more prevalent in high-income countries, it has been increasing in low- and moderate-income countries [2].
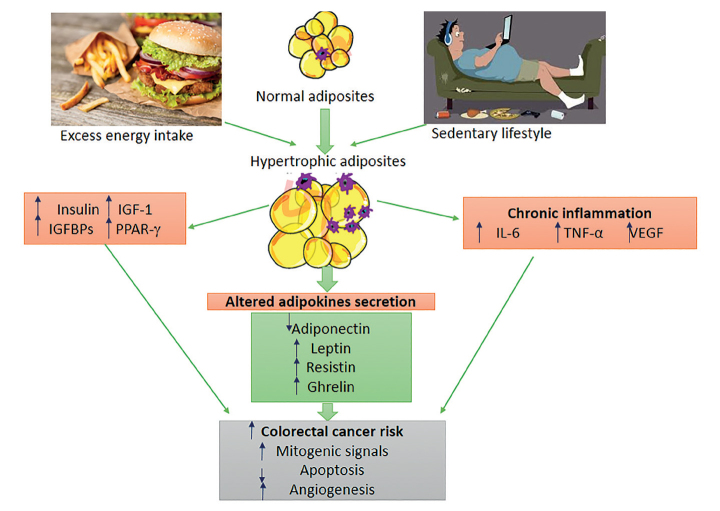
Obesity is associated with increased risk of several cancer types with colon, endometrial, postmenopausal breast cancer, kidney, esophageal, pancreatic, gallbladder, liver and hematological cancers [5]. The underlying mechanism of the correlation between colorectal cancers and obesity has not been understood; adipokines and the insulin are considered to play a key role in this relationship. The first mechanism among these is that obesity leads to insulin resistance and hyperinsulinism, causing a reduction in insulin-binding protein 1 (IGFBP-1) levels. This reduction, in turn, leads to an increase in levels of insulin-like growth factors (IGF-1), promoting cell proliferation and inhibiting cell apoptosis. The second mechanism is that obesity alters levels of adipokines secreted from adipose tissue (resistin, leptin, adiponectin, and proinflammatory cytokines such as interleukin-6 and tumor necrosis factor-α). Increased level of these adipokines other than adiponectin may lead to tumor formation, progression, and metastasis [6]. Summary of obesity and potential factors considered to be associated with colorectal cancers is given in Figure 1 [7]. There is obesity-associated insulin resistance, inflammation, and adipokine production in mechanism of correlation of carcinogenesis of colorectal cancers and adiposity. Adipose tissue macrophages that infiltrate into visceral fat tissue exhibit a phenotypic switch from an anti-inflammatory and adipostatic to proinflammatory, and pro-adipogenic properties. Thus, they contribute to obesity-associated inflammation and insulin resistance [7].
Figure 1.
Obesity and potential factors though to be associated with colorectal cancers
IL-6: Interleukin-6; IGF-1: Insulin-like growth factor-1; IGFBPs: Insulin-like growth factor binding protein; TNF-α: Tumor necrosis factor-α; VEGF: Vascular endothelial growth factor; PPAR-γ: Peroxisome proliferator-activated receptor-γ. [Adapted from 7]
An imbalance between food intake and energy expenditure leads to obesity, which leads to an excessive accumulation of adipose tissue. The patterns of altered levels of adipokine among obese subjects may be considered as important predictors for risk of colorectal cancer. Hence, the arrangements to prevent obesity will be protective against both chronic diseases and cancer.
Insulin
Although insulin is known for its metabolic effects, it also plays an important role in colorectal carcinogenesis and cancer progression. It increases the number of receptors of IGF-1and growth hormone, which stimulates synthesis of ovarian androgens and inhibits synthesis of sex hormone binding globulin and IGFBP-1 [8]. Excessive energy intake, physical inactivity, and obesity lead to insulin resistance. Consequently, plasma insulin concentrations tend to increase [9]. Insulin resistance has been associated with increased plasma insulin levels, glucose intolerance, increased IGF-I, glucose and free fatty acids, body mass index, and increased risk for colorectal cancer [8, 10].
In the literature, the studies do not explain clearly association between colorectal cancer and insulin level [11–14]. While some studies reported that hyperinsulinemia and insulin resistance may increase colorectal cancer risk [11, 12], some studies reported no association [13, 14]. In a meta-analysis study, hyperinsulinism and insulin resistance were associated with an increased risk of colorectal cancer [11]. In another meta-analysis study, it was reported that in non-Asian societies, high levels of c-peptide and insulin were significantly associated with risk of colorectal cancer, and there was no association in Asians [12]. No statistical significance was determined in another study in which insulin levels of the patients with colorectal cancer were evaluated based on tumor stage [15]. Meta-analysis studies conducted also suggest that hyperinsulinism, high c-peptide levels, and insulin resistance (HOMA-IR) are associated with increased risk of colorectal cancer [11, 12]. As a result, insulin resistance is mainly caused by excessive energy intake leading to adiposity. Obesity-related colorectal cancer is a growing health concern that has been tied to the dramatic change in low physical activity level, dietary habits, and the adoption of the Western diet (red and processed meats, high-fat dairy products refined grains, desserts, low intakes of vegetables, fruits, whole grains, and fish). For example, a higher fiber diet may be beneficial in hyperinsulinemia, as dietary fiber reduces postprandial hyperglycemia by delaying the digestion and absorption of carbohydrates, and increasing satiety with the effects of a resultant weight loss. With this weight loss and balanced/healthy food patterns as Mediterranean diet, the development of colorectal cancer can be consequently prevented.
Adiponectin
Adiponectin, a large adipokine secreted from adipose tissue, attracts attention with its anti-inflammatory, anti-atherogenic, anti-angiogenic, and insulinsensitizing properties, as well as its beneficial role in glucose metabolism [6, 7]. Adiponectin suppresses secretion of inflammatory cytokines like TNF-α and initiates release of anti-inflammatory cytokines like IL-10 during atherogenic process [16]. Low adiponectin levels may be a risk factor for obesity-associated types of cancer such as colorectal and prostatic cancers [17]. Adiponectin provides suppression over cancer via various pathways. In this pathway, adenosine monophosphate-activated protein kinase (AMPK) has a central role. Adiponectin activates AMPK and inhibits phosphaditylinositol-3-kinase/protein kinase B (PI3K/AKT), mTOR, glycogen synthase kinase 3-β, Janus kinase /signal transducer, and transcription pathway activator (JAK/STAT) pathways. AMPK influences cell growth signaling via mTOR; and, thus inhibits development of carcinogenesis, it also prevents promotion of tumor cell adhesion and migration [18].
The association between circulating adiponectin levels and risk of colorectal cancer has been evaluated in many studies [19–23]. Nevertheless, there are discrepancies between results of epidemiological studies. In a study conducted by Kumor et al. [19] on patients with colorectal cancers with adenomatous polyps, adiponectin levels of the patients with colorectal cancer were determined to be significantly decreased compared with the those in both adenoma and control groups. Nakajima and et al. [23] determined that adiponectin levels were lower in multivariate analysis in patients with adenoma compared with those in the control group, and it was inversely related with number of adenomas; but there was no association with size of adenoma and stage of cancer. In studies conducted in a similar way, adiponectin levels were determined to be lower in individuals with colorectal cancer [20, 22]. Also in another study conducted in Turkey, adiponectin levels of individuals diagnosed with colon cancer and the control group were found to be similar [21]. Genetic, environmental, and ethnic factors are considered effective in serum adiponectin levels. Although regulatory ways for these levels remain unclear, it has been emphasized that some genetic variations may also be effective [24]. Lifestyle habits such as alcohol consumption, eating habits, exercising, smoking may influence serum adiponectin levels [24]. Dietary intakes have a major role in controlling the inflammation and weight management. For example, the consumption of fruits, vegetables, whole grain, and nuts produces several nutraceuticals that have been beneficial in modifying carcinogenesis via multiple pathways. Adherence to diet in fiber and complex carbohydrate leads to improved concentration of adiponectin stimulating insulin sensitivity. It is possible that these dietary pattern decrease risk for carcinogenesis through the elevation of adiponectin.
Leptin
Leptin is one of the most commonly found adipokines that has a key role in control of hunger and satiety by being involved in regulation of food intake, energy balance, and body weight [25]. Leptin is a product of the obese (ob) gene mapped to chromosome 7, and it shows its activity via OB-R receptor. Stomach is the major source of leptin in gastrointestinal tract. Although leptin is produced in both endocrine and exocrine cells in gastric mucosa, exocrine cells have a greater role [26]. Leptin receptors are more predominant particularly in proximal of intestines, and exist in basolateral margins and lumen of intestinal cells [27]. It was demonstrated that in ob-ob mice with leptin deficiency, cell proliferation decreased and apoptosis in intestinal cells increased after small intestinal resection [28]. Leptin levels may vary among women and men because leptin levels are higher in women due to high amount of fat tissue; and an increase in leptin levels may be possible in obesity-associated cancers [29]. It was reported that as leptin stimulates cell migration and proliferation in colorectal cancers and normal intestinal epithelial cells, leptin expression could be used as a marker for characteristics of tumor cells and prognosis [30]. In a previous study, it was determined that a significant increase occurred in proliferative activity of normal colonic epithelial cells in obese models, whereas tumor cell proliferation was significantly reduced in those with leptin deficiency, and tumor growth was inhibited in mice with leptin deficiency or leptin receptor deficiency [31]. Nevertheless, in a meta-analysis involving 13 studies, it was emphasized that leptin levels and risk of colorectal cancer were not correlated [32]. More studies are required for evaluate leptin signalization and determine potential mechanisms on prognosis in tumor tissues [33]. However, diet has an important role on change the concentration of leptin. Not only the total energy intake on the concentration of leptin, but also the type and amount of fatty acid, carbohydrate consumption, carbohydrate type, and glycemic load in the diet play the key roles caused by insulin resistance and leptin resistance.
Ghrelin
Ghrelin is a 28-aminoacid hormone that is principally produced in stomach, has growth hormone-secreting effect, and has a role in energy balance and regulation of food intake. Approximately 60%–70% of ghrelin is secreted from stomach and more than 30% is secreted from small intestines. Ghrelin has two important forms in stomach and plasma: acyl and deacyl forms [34]. Effects of ghrelin are provided by ghrelin receptor, also known as growth hormone secretagogue receptor (GHSR) [35]. Ghrelin has various physiological effects in gastrointestinal, cardiovascular, pulmonary, and immune systems. Additionally, it promotes food intake (orexigenic effect) and influences cell proliferation. It stimulates differentiation of pre-adipocytes; and as it inhibits lipolysis, it has a principal role in process of adipogenesis [35]. It has an anti-apoptotic or pro-apoptotic role in different cancer cells [7]. Effect of ghrelin on cancer cell proliferation among various cancers remains controversial. Recently, it was reported to induce proliferation of colon cancer cells via GHSR, Ras, phosphatidylinositol 3 kinase (PI3K), Akt, and mTOR pathways [36].
In literature, the association between serum ghrelin levels and colorectal cancer have not clearly demonstrated, and results vary. No difference was determined between plasma ghrelin levels of 78 patients who were diagnosed with gastric or colorectal cancer and those in the control group. Furthermore, ghrelin levels were reported not to be influenced by tumor localization and levels of other hormones (growth hormone, glucagon, and cortisol) [37]. In another study involving individuals with gastrointestinal tract cancers, it was determined that while ghrelin and adiponectin levels of individuals both with colon and rectum cancers were lower and leptin levels were higher compared with those in the control group, there was no difference in levels of these hormones by gender [38]. Nevertheless, in another study, when 95 individuals with colon cancer and 39 health controls were compared by age, BMI, and gender, total serum ghrelin levels were determined to be higher compared with those in the healthy control group. Additionally, while it was determined to show a positive correlation with tumor size and advanced stage tumors (compared with initial stage), it exhibited an inverse relationship with tumor differentiation; and it was not associated with tumor localization, demographical/clinical characteristics, and survival [39].
Large-scale prospective clinical studies are required to clarify the effects of ghrelin on general activity in tumors and various types of cancer including colorectal cancers, and to examine reliability and benefits of treatment with ghrelin/ghrelin receptor agonist in patients with cancer [40].
Resistin
Resistin is a recently discovered member of cysteine-rich protein family, so-called resistin-like molecule. It is produced from peripheral blood monocytes and stromavascular portion of adipose tissue [41]. Leptin, adiponectin, and resistin have physiological roles in reduction of food intake, energy balance, and regulation of body weight [19]. Insulin resistance as well as alterations in hormones secreted by adipose tissue such as adiponectin, leptin, and resistin are directly associated with colorectal cancers and inflammatory bowel diseases [42].
Role of resistin in colorectal carcinogenesis has not been explained. However, serum resistin concentration might be a factor that contributes to increased risk of colorectal cancer. Activation of monocytes, as a part of the inflammatory process, has been associated with increased resistin levels in patients with colorectal cancer. Chronic inflammation plays an important role in pathogenesis of cancer, and there is a close relationship between resistine and inflammatory markers [43]. In a study, resistin levels of individuals with colon cancer were determined to be higher compared with those in the control group; and high resistin levels were reported to be associated with the chronic inflammation-associated cancer [44]. Although the role of resistin in colorectal cancers has not been completely clarified, various mechanisms are considered to be effective on these results. In-vitro studies report that resistin induces a proinflammatory effect via TLR4 receptor stimulation and NF-κB pathway [45, 46]. Furthermore, resistin shows its effects by execution of release of vascular endothelial growth factor, which supports tumor invasion, and of production of matrix metalloproteins [47].
In a study, resistin levels of individuals with colorectal cancer were determined to be higher compared with those in the control group [20]. Sălăgeanu et al. [44] determined that resistin levels were higher in patients with colon cancer compared with those in the control group, but there was no correlation between tumor grade, stage and tumor localization, and resistin levels. Nakajima et al. [23] concluded that resistin levels were higher in patients compared with those in the control group, independently of BMI, but resistin levels increased proportionally to tumor stage. However, in another study, no difference was determined in plasma resistin levels when patients with colorectal cancer were compared with those in the control group [48].
In a meta-analysis study, it was emphasized that resistin levels were higher in individuals with colorectal cancer compared with those in the healthy control group, but more randomized and experimental studies that may confirm effect of resistin on development of colorectal cancers are required [49].
In conclusion, obesity is a well-defined risk factor for various diseases including metabolic, cardiovascular, and several types of cancer. Especially, the epidemiological evidence clearly indicates common factors linking obesity and colon cancer. This can be explained by several changes in hormonal and cytokine profiles that stimulate cell growth, inhibit apoptosis, and promote angioneogenesis. Cancer and obesity can be associated with consumption of high-energy diets, a sedentary lifestyle, increased age, processed red meat, and reduced consumption of fruit, vegetables, and fiber. All these factors influence adipose tissue. These factors have the potential to influence the production of adipose-derived hormones as adiponectin, leptin, resistin, and ghrelin. Alterations in adipokine levels are closely related with colorectal cancers. In terms of weight management and control of inflammation, it is necessary to take into account that dietary intake of individuals has an important role.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - G.O.U., N.S.; Design - G.O.U., N.S.; Resource - G.O.U., N.S.; Materials - G.O.U., N.S.; Analysis and /or Interpretation - G.O.U., N.S.; Literature Search - G.O.U.; Writing - G.O.U.; Critical Reviews - G.O.U., N.S.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.von Meyenfeldt M. Cancer-associated malnutrition: An introduction. Eur J Oncol Nurs. 2005;9(Supplement 2):S35–8. doi: 10.1016/j.ejon.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008: Cancer incidence and mortality worldwide: IARC CancerBase No. 10. Vol. 2010. Lyon, France: International Agency for Research on Cancer; 2010. p. 29. [Google Scholar]
- 3.TÜİK. Death Cause Statistics 2016. Available from: http://www.tuik.gov.tr/PreHaberBultenleri.do?id=24572.
- 4.Şencan İ, İnce GN. Turkey Cancer Statistics. Ministry of Health, Turkish Public Health Institution; 2016. [Google Scholar]
- 5.World Cancer Research Fund, American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Amer Inst for Cancer Research; 2007. [Google Scholar]
- 6.Joshi RK, Kim WJ, Lee SA. Association between obesity-related adipokines and colorectal cancer: a case-control study and meta-analysis. World J Gastroenterol. 2014;20:7941–9. doi: 10.3748/wjg.v20.i24.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riondino S, Roselli M, Palmirotta R, Della-Morte D, Ferroni P, Guadagni F. Obesity and colorectal cancer: role of adipokines in tumor initiation and progression. World J Gastroenterol. 2014;20:5177–90. doi: 10.3748/wjg.v20.i18.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan AT, Giovannucci EL. Primary Prevention of Colorectal Cancer. Gastroenterology. 2010;138:2029–43.e10. doi: 10.1053/j.gastro.2010.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaaks R, Toniolo P, Akhmedkhanov A, et al. Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst. 2000;92:1592–600. doi: 10.1093/jnci/92.19.1592. [DOI] [PubMed] [Google Scholar]
- 10.Yuan C, Bao Y, Sato K, et al. Influence of dietary insulin scores on survival in colorectal cancer patients. Br J Cancer. 2017;117:1079–87. doi: 10.1038/bjc.2017.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chi F, Wu R, Zeng YC, Xing R, Liu Y. Circulation insulin-like growth factor peptides and colorectal cancer risk: an updated systematic review and meta-analysis. Mol Biol Rep. 2013;40:3583–90. doi: 10.1007/s11033-012-2432-z. [DOI] [PubMed] [Google Scholar]
- 12.Yoon YS, Keum N, Zhang X, Cho E, Giovannucci EL. Hyperinsulinemia, insulin resistance and colorectal adenomas: A meta-analysis. Metabolism. 2015;64:1324–33. doi: 10.1016/j.metabol.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Keku TO, Lund PK, Galanko J, Simmons JG, Woosley JT, Sandler RS. Insulin resistance, apoptosis, and colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev. 2005;14:2076–81. doi: 10.1158/1055-9965.EPI-05-0239. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto S, Nakagawa T, Matsushita Y, et al. Visceral fat area and markers of insulin resistance in relation to colorectal neoplasia. Diabetes Care. 2010;33:184–9. doi: 10.2337/dc09-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nowakowska-Zajdel E, Muc-Wierzgon M, Kokot T, et al. Serum insulin levels in patients with colorectal cancer. Pol Arch Med Wewn. 2008;118:273–9. doi: 10.20452/pamw.386. [DOI] [PubMed] [Google Scholar]
- 16.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–9. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 17.Muppala S, Konduru SKP, Merchant N, et al. Adiponectin: Its role in obesity-associated colon and prostate cancers. Crit Rev Oncol Hematol. 2017;116:125–33. doi: 10.1016/j.critrevonc.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Otani K, Ishihara S, Yamaguchi H, et al. Adiponectin and colorectal cancer. Surg Today. 2017;47:151–8. doi: 10.1007/s00595-016-1334-4. [DOI] [PubMed] [Google Scholar]
- 19.Kumor A, Daniel P, Pietruczuk M, Malecka-Panas E. Serum leptin, adiponectin, and resistin concentration in colorectal adenoma and carcinoma (CC) patients. Int J Colorectal Dis. 2009;24:275–81. doi: 10.1007/s00384-008-0605-y. [DOI] [PubMed] [Google Scholar]
- 20.Gonullu G, Kahraman H, Bedir A, Bektas A, Yucel I. Association between adiponectin, resistin, insulin resistance, and colorectal tumors. Int J Colorectal Dis. 2010;25:205–12. doi: 10.1007/s00384-009-0828-6. [DOI] [PubMed] [Google Scholar]
- 21.Erdogan S, Yilmaz FM, Yazici O, et al. Inflammation and chemerin in colorectal cancer. Tumour Biol. 2016;37:6337–42. doi: 10.1007/s13277-015-4483-y. [DOI] [PubMed] [Google Scholar]
- 22.Erarslan E, Turkay C, Koktener A, Koca C, Uz B, Bavbek N. Association of visceral fat accumulation and adiponectin levels with colorectal neoplasia. Dig Dis Sci. 2009;54:862–8. doi: 10.1007/s10620-008-0440-6. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima TE, Yamada Y, Hamano T, et al. Adipocytokines as new promising markers of colorectal tumors: adiponectin for colorectal adenoma, and resistin and visfatin for colorectal cancer. Cancer Sci. 2010;101:1286–91. doi: 10.1111/j.1349-7006.2010.01518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotani K, Sakane N, Saiga K, et al. Serum adiponectin levels and lifestyle factors in Japanese men. Heart Vessels. 2007;22:291–6. doi: 10.1007/s00380-006-0969-2. [DOI] [PubMed] [Google Scholar]
- 25.Boguszewski CL, Paz-Filho G, Velloso LA. Neuroendocrine body weight regulation: integration between fat tissue, gastrointestinal tract, and the brain. Endokrynologia Polska. 2010;61:194–206. [PubMed] [Google Scholar]
- 26.Cammisotto PG, Gingras D, Renaud C, Levy E, Bendayan M. Secretion of soluble leptin receptors by exocrine and endocrine cells of the gastric mucosa. Am J Physiol Gastrointest Liver Physiol. 2006;290:G242–G9. doi: 10.1152/ajpgi.00334.2005. [DOI] [PubMed] [Google Scholar]
- 27.Barrenetxe J, Villaro AC, Guembe L, et al. Distribution of the long leptin receptor isoform in brush border, basolateral membrane, and cytoplasm of enterocytes. Gut. 2002;50:797–802. doi: 10.1136/gut.50.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiely JM, Noh JH, Pitt HA, Swartz-Basile DA. Impaired intestinal cell proliferation and cell death in leptin-deficient obese mice. JPEN J Parenter Enteral Nutr. 2005;29:30–5. doi: 10.1177/014860710502900130. [DOI] [PubMed] [Google Scholar]
- 29.Gupta A, Herman Y, Ayers C, et al. Plasma leptin levels and risk of incident cancer: results from the Dallas Heart Study. PloS One. 2016;11:e0162845. doi: 10.1371/journal.pone.0162845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koda M, Sulkowska M, Kanczuga-Koda L, Surmacz E, Sulkowski S. Overexpression of the obesity hormone leptin in human colorectal cancer. J Clin Pathol. 2007;60:902–6. doi: 10.1136/jcp.2006.041004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Endo H, Hosono K, Uchiyama T, et al. Leptin acts as a growth factor for colorectal tumours at stages subsequent to tumour initiation in murine colon carcinogenesis. Gut. 2011;60:1363–71. doi: 10.1136/gut.2010.235754. [DOI] [PubMed] [Google Scholar]
- 32.Joshi RK, Lee SA. Obesity related adipokines and colorectal cancer: a review and meta-analysis. Asian Pac J Cancer Prev. 2014;15:397–405. doi: 10.7314/APJCP.2014.15.1.397. [DOI] [PubMed] [Google Scholar]
- 33.Drew JE. Molecular mechanisms linking adipokines to obesity-related colon cancer: focus on leptin. Proc Nutr Soc. 2012;71:175–80. doi: 10.1017/S0029665111003259. [DOI] [PubMed] [Google Scholar]
- 34.Sheikhpour R. Role of ghrelin in cancer. Iranian Journal of Blood and Cancer. 2016;8:1–4. [Google Scholar]
- 35.Nikolopoulos D, Theocharis S, Kouraklis G. Ghrelin: A potential therapeutic target for cancer. Regul Pept. 2010;163:7–17. doi: 10.1016/j.regpep.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 36.Lien GS, Lin CH, Yang YL, Wu MS, Chen BC. Ghrelin induces colon cancer cell proliferation through the GHS-R, Ras, PI3K, Akt, and mTOR signaling pathways. Eur J Pharmacol. 2016;776:124–31. doi: 10.1016/j.ejphar.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 37.Huang Q, Fan YZ, Ge BJ, Zhu Q, Tu ZY. Circulating ghrelin in patients with gastric or colorectal cancer. Dig Dis Sci. 2007;52:803–9. doi: 10.1007/s10620-006-9508-3. [DOI] [PubMed] [Google Scholar]
- 38.Kemik O, Kemik A, Begenik H, et al. The relationship among acute-phase responce proteins, cytokines, and hormones in various gastrointestinal cancer types patients with cachectic. Hum Exp Toxicol. 2012;31:117–25. doi: 10.1177/0960327111417271. [DOI] [PubMed] [Google Scholar]
- 39.Nikolopoulos D, Theocharis S, Moutsios-Rentzos A, Kouraklis G, Kostakis A. The role of serum total ghrelin level elevation in colon cancer patients. J Buon. 2014;19:388–93. [PubMed] [Google Scholar]
- 40.Sever S, White DL, Garcia JM. Is there an effect of ghrelin/ghrelin analogs on cancer? A systematic review. Endocr Relat Cancer. 2016;23:R393–R409. doi: 10.1530/ERC-16-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun CA, Wu MH, Chu CH, et al. Adipocytokine resistin and breast cancer risk. Breast Cancer Res Treat. 2010;123:869–76. doi: 10.1007/s10549-010-0792-4. [DOI] [PubMed] [Google Scholar]
- 42.Farquharson AJ, Steele RJ, Carey FA, Drew JE. Novel multiplex method to assess insulin, leptin and adiponectin regulation of inflammatory cytokines associated with colon cancer. Mol Biol Rep. 2012;39:5727–36. doi: 10.1007/s11033-011-1382-1. [DOI] [PubMed] [Google Scholar]
- 43.Tulubas F, Mete R, Oznur M, Topcu B. The Role of Adipocytokines in Colon Cancer and Adenomas/Uloga adipocitokina u kanceru i adenomima debelog creva. J Med Biochem. 2013;33:135–42. doi: 10.2478/jomb-2013-0001. [DOI] [Google Scholar]
- 44.Salageanu A, Tucureanu C, Lerescu L, et al. Serum levels of adipokines resistin and leptin in patients with colon cancer. J Med Life. 2010;3:416–20. [PMC free article] [PubMed] [Google Scholar]
- 45.Tarkowski A, Bjersing J, Shestakov A, Bokarewa MI. Resistin competes with lipopolysaccharide for binding to toll-like receptor 4. J Cell Mol Med. 2010;14:1419–31. doi: 10.1111/j.1582-4934.2009.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howe LR, Subbaramaiah K, Hudis CA, Dannenberg AJ. Molecular pathways: adipose inflammation as a mediator of obesity-associated cancer. Clin Cancer Res. 2013;19:6074–83. doi: 10.1158/1078-0432.CCR-12-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mu H, Ohashi R, Yan S, et al. Adipokine resistin promotes in vitro angiogenesis of human endothelial cells. Cardiovasc Res. 2006;70:146–57. doi: 10.1016/j.cardiores.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 48.Wagsater D, Mumtaz M, Lofgren S, Hugander A, Dimberg J. Resistin in human colorectal cancer: increased expression independently of resistin promoter −420C > G genotype. Cancer Invest. 2008;26:1008–14. doi: 10.1080/07357900802087267. [DOI] [PubMed] [Google Scholar]
- 49.Joshi RK, Lee SA. Obesity related adipokines and colorectal cancer: a review and meta-analysis. Asian Pac J Cancer Prev. 2014;15:397–405. doi: 10.7314/APJCP.2014.15.1.397. [DOI] [PubMed] [Google Scholar]