Abstract
Objective
This study investigated acoustic and perceptual characteristics of the voice of patients with thyroid gland disorders such as hypothyroidism and hyperthyroidism immediately after the diagnosis was made and six months later, after using drug therapy.
Materials and Methods
The study includes 20 female outpatients with hypothyroidism and 27 female outpatients with hyperthyroidism. The criterion for the selection of the patients was a thyroid gland disorder medical diagnosis, no history of voice disorders and absence of other possible causes of voice changes. Acoustic, perceptual and aerodynamic parameters were assessed. Acoustic analysis was performed by specific software. Experienced speech and language pathologists made perceptual voice assessment by using grade, roughness, breathiness, asthenia, and strain (GRBAS) scale.
Results
Significant differences in patients with hypothyroidism were established on parameter amplitude perturbation, jitter and noise-to-harmonics ratio between pretreatment and posttreatment period, in which patients took drug therapy. In group of patients with hyperthyroidism significant difference was noted only on aerodynamic parameter maximum phonation time. There were a significant differences in all perceptual parameters in both groups of patients (p<0.05) in pre and posttreatment, except on grade and asthenia parameter in the group of patients with hypothyroidism and parameter grade was borderline insignificant in the group of patients with hyperthyroidism
Conclusion
Voice quality is affected by thyroid disease. Thyroid gland disorders cause minor changes in acoustic voice parameters of patients with hypothyroidism and hyperthyroidism, but perceptual deviations in these patients are especially noticeable.
Keywords: Hypothyroidism, hyperthyroidism, voice
Introduction
Hormones are known to have a major impact on the voice quality. Although several hormones are produced in the body, evidence has shown that the sex hormones and thyroid hormones directly affect particularly the voice [1]. Thyroid gland diseases and disruptions in the function of parathyroid glands are the most common endocrine disorders that can cause phonation disturbances [2]. Hypothyroidism is the most common thyroid gland disorder caused by an underactive thyroid not producing sufficient hormones [3]. Endocrine imbalances, such as hypothyroidism, are one of the common causes of tissue irritation [4]. The symptoms of hypothyroidism are even more subtle than those of hyperthyroidism, and changes in the voice are one of the well-known symptoms of hypothyroidism [5]. Voice changes may occur even in the cases of mild thyroid failure since thyroid hormone receptors have been found in the larynx, which proves that the thyroid hormone acts on the laryngeal tissue [6]. Hypothyroidism can cause notable voice changes, such as low voice, roughness, reduced range, and vocal fatigue [7].
Dysphonia can be caused by excessive thyroid hormone production or hyperthyroidism. The most commonly occurring change is the reduction of the fundamental frequency (F0) of the voice. Other changes may not only include hoarseness, roughness, and loss of voice [1, 8] but also trembling voice, reduced intensity of voice, and audible breathing [7, 9]. Follow-up studies have shown that voice changes are present in 27% of patients with hyperthyroidism and in 2%–98% of patients with hypothyroidism [10].
Patients with untreated thyroid gland diseases experience a wide range of symptoms that have a very high impact on the quality of life [10]. Currently, few studies are available regarding the impact of thyroid diseases on voice production, and thyroid gland dysfunction is mainly reported after surgery with regard to the paralysis of the recurrent laryngeal nerve or the superior laryngeal nerve and its impact on patients’ voice [11].
The aim of this study was to determine the acoustic and perceptual characteristics of the voice of patients with thyroid gland disorders, such as hypothyroidism and hyperthyroidism, immediately after the diagnosis was made and 6 months after drug therapy.
Materials and Methods
Patients
The participants included female patients with thyroid gland disorders, such as hypothyroidism and hyperthyroidism, who were treated at the clinic center. The patient selection criteria were medical diagnosis of a thyroid gland disorder, no history of voice disorders, absence of other known causes of voice changes, and patients with no history of a previous surgery or trauma in the head and neck. The diagnosis was made by a specialist in nuclear medicine based on a typical clinical examination, determination of hormones in serum (thyroid-stimulating hormone [TSH], tri-iodothyronine, thyroxine), and ultrasound and palpatory examinations. After the medical diagnosis of a thyroid disease, the patients underwent an ear, nose, and throat (ENT) examination to exclude the existence of other possible causes of voice changes. Patients were then instructed by a speech and language pathologist to record their voice. Only 47 followed the instructions and completed the protocol. The patients were divided into two groups: the first group included 20 patients (mean age, 45 years) with hypothyroidism (age range, 26–71 years), while the second group included 27 patients (mean age, 50 years) with hyperthyroidism (age range, 26–74 years).
Research Tools and Data Collection
The study was conducted at the ENT clinic of the university clinical center. A speech and language pathologist performed an acoustic and perceptual evaluation of the patients’ voice. The first evaluation was conducted after diagnosing the condition and an examination by an ENT specialist (pretreatment), and the second evaluation was done after 6 months (posttreatment). During this period, patients received drug therapy coordinated by a specialist in nuclear medicine.
The patient’s voice was recorded using the AKG 190 ES microphone. It was placed at a distance of 30 cm and at 45° according to the recommendations of Union of European Phoniatricians [12]. The recording was conducted in a sound isolation booth with noise level less than 40 dB. An average of three trials of prolongation of vowel “a” was used for the acoustic analysis, i.e., its middle part of the acoustic waveformsfor at least 2 seconds. Acoustic voice analysiswas performed using the computer software Speech Training for Windows, Version 4.00 - Dr. Speech and EZ Voice Plus™ Version 2.0. Theacoustic vocal parameters assessed were average F0, frequency perturbations (jitter), amplitude perturbations (shimmer), and harmonics-to-noise ratio (HNR).
The perceptual voice quality assessment was conducted using the Grade, Roughness, Breathiness, Asthenia, Strain (GRBAS) scale [13]. The patients read a standard text for 2 minutes and their voices were recorded. Voice recordings were assessed by three experienced speech and language pathologists familiar with the GRBAS scale in a double-blind randomized manner. The scale evaluates five vocal characteristics assigned on a score of 0–3, where 0 is normal or absence of deviance, 1 is slight deviance, 2 is moderate deviance, and 3 is severe deviance. The five characteristics are grade (G), a description of the degree of hoarseness, which relates to the overall voice quality, integrating all deviant components; roughness (R), the perceptual irregularity of vocal fold vibrations, abnormal fluctuations in F0 or amplitude of vibration; breathiness (B), an auditive impression of air leakage through the insufficient glottic closure; asthenia (A), the voice denotes weakness and lack of power; and strain (S), reflects a perception of vocal hyperfunction. The parameters of aerodynamic measurements were also evaluated: maximum phonation time (MPT) of vowel sound “a.”
The present study was approved by the ethics committee of university clinical center. All the participants provided signed informed consent before they were subjected to the research procedures.
Statistical Analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS®) software package version 24.0 (IBM Corp., Armonk, NY, USA). Descriptive statistics parameters were calculated. The normality of data distribution was tested using the Shapiro–Wilk test. Paired-samples t-test was used to determine differences in the acoustic parameters of voice in two related samples of patients in the pre- and post-treatment phases. The Wilcoxon signed rank test was used to calculate the differences of repeated measurements for perceptual parameters of two related samples. A statistical level of 95% (p<0.05) was considered the significance limit for all statistical tests.
Results
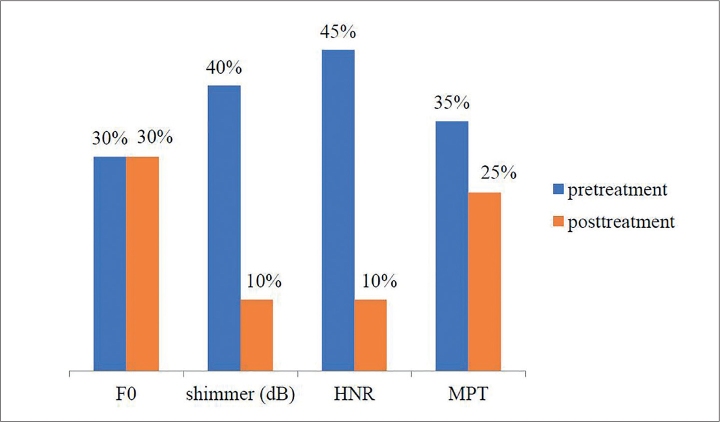
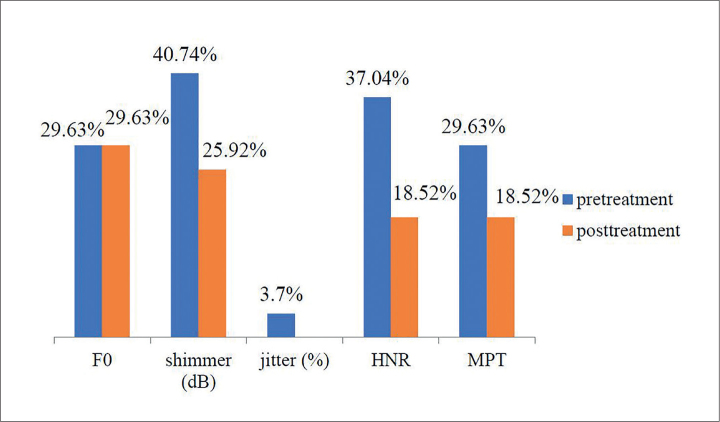
Figures 1 and 2 show the percentage of patients with hypo and hyperthyroidism and abnormal acoustic and aerodynamic parameters.
Figure 1.
F0-average fundamental frequency; shimmer-amplitude perturbations; HNR-harmonics-to-noise ratio; MPT-maximum phonation time of the vowel sound “a”
Figure 2.
F0-average fundamental frequency; shimmer-amplitude perturbations; jitter-frequency perturbations; HNR-harmonics-to-noise ratio; MPT-maximum phonation time of the vowel sound “a”
The percentage of patients with hypothyroidism and abnormal F0 was the same in the pretreatment period and in the post-treatment period wherein patients received drug therapy (30% of patients). Jitter was within the normal values. Only 10% of the patients with hypothyroidism had abnormal shimmer and HNR in the post-treatment period, and the MPT was abnormal in 25% of the patients compared to 35% in the initial evaluation.
The percentage of the patients with hyperthyroidism and abnormal F0 in the pretreatment and post-treatment phases was not changed. Also, no change in the percentage of the patients with hypothyroidism and abnormal F0 was observed, but the percentage of patients with abnormal values of the other acoustic and aerodynamic parameters measured had decreased in the post-treatment period, wherein the patients received drug therapy.
Furthermore, tests were conducted to determine whether there are significant differences in acoustic and aerodynamic parameters, in general, between the pretreatment and posttreatment periods.
Table 1 shows that a significant difference was found in the variable describing the amplitude perturbations (shimmer) in patients with hypothyroidism (p=0.032), wherein it was clear that this parameter had significantly decreased after treatment. In addition, there was a significant difference in the variable describing the HNR (p=0.017); the mean value improved in this voice parameter posttreatment. Although the values of the jitter parameter were in the normal range in patients with hypothyroidism in the pretreatment phase, there was a significant difference between pretreatment and posttreatment phases (p=0.03). There were no significant changes in pre- and post-treatment periods on variables describing the voice frequency. F0 was even lower posttreatment. Although insignificant, the mean value of the MPT improved in the posttreatment period.
Table 1.
Results of the t-test on differences in acoustic voice parameters in patients with hypo and hyperthyroidism in pretreatment and posttreatment periods
| Variable | Hypothyroidism | Hyperthyroidism | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Pretreatment | Posttreatment | Pretreatment | Posttreatment | |||||||
| Mean | SD | Mean | SD | p | Mean | SD | Mean | SD | p | |
| F0 | 192.35 | 25.85 | 189.41 | 20.5 | 0.566 | 195.39 | 33.65 | 192.04 | 24.58 | 0.575 |
| Shimmer (dB) | 0.51 | 0.46 | 0.27 | 0.18 | 0.032 | 0.44 | 0.36 | 0.31 | 0.2 | 0.12 |
| Jitter (%) | 0.31 | 0.2 | 0.2 | 0.06 | 0.03 | 0.31 | 0.26 | 0.24 | 0.09 | 0.209 |
| HNR | 8.45 | 5.04 | 11.53 | 3.6 | 0.017 | 8.21 | 4.11 | 9.89 | 3.82 | 0.1 |
| MPT (sec.) | 14 | 5.69 | 15.15 | 6.51 | 0.353 | 13.88 | 5.44 | 16 | 4.74 | 0.004 |
HNR: harmonics-to-noise ratio; MPT: maximum phonation time; SD: standard deviation
F0: average fundamental frequency; shimmer: amplitude perturbations; jitter: frequency perturbations.
Paired-samples t-test, p<0.05 was considered significant.
When the acoustic voice parameters in patients with hyperthyroidism in the pre and post-treatment phases were compared, significant differences were observed only in the MPT (p=0.004). As in the patients with hypothyroidism, the F0 showed a slight decrease in the voice pitch. Although insignificant, the patients with hyperthyroidism did have better results for jitter, shimmer, and HNR in the posttreatment period.
The perceptual voice assessments were also compared before and after treatment in patients with hypo and hyperthyroidism (Table 2). Significant differences were found in all the parameters in patients with hyperthyroidism, except for the grade parameter, but only in the parameters roughness, breathiness, and strain in the patients with hypothyroidism.
Table 2.
Results of the Wilcoxon signed rank test on the differences in perceptual voice parameters in patients with hypo and hyperthyroidism in the pretreatment and post treatment periods
| Variable | Hypothyroidism | Hyperthyroidism | ||
|---|---|---|---|---|
|
|
|
|||
| Z | p | Z | p | |
| Grade | −1.823 | 0.068 | −1.897 | 0.058 |
| Roughness | −2.373 | 0.018 | −2.956 | 0.003 |
| Breathiness | −1.89 | 0.059 | −2.06 | 0.039 |
| Asthenia | −1.298 | 0.194 | −2.913 | 0.004 |
| Strain | −2.058 | 0.040 | −2.98 | 0.003 |
Wilcoxon signed rank test; Z: standardized test statistic; p<0.05 is significant
Discussion
Although the relationship between the thyroid hormone and the larynx remains insufficiently investigated [6], the position of the thyroid gland in the neck region and its direct connection with the larynx can justify the presence of voice changes in patients with thyroid gland disorders [14]. The results of this study showed that the values of the parameters describing the voice frequency ranged within the normal values, and there was no change in the voice frequency between pretreatment period and the post-treatment period, wherein the patients received thyroid replacement therapy. Colton [15] stated that women usually produce a basic frequency of voice between 180 HZ and 220 Hz, and at the age of 40–49 years, F0 is 214 Hz [15].
Although jitter was within the normal values, patients with hypothyroidism showed significant improvement posttreatment for this parameter. The minor glottic pulse irregularities were even less noticeable after the treatment period. Most researchers consider that the normal value of jitter in adults ranged between 0.5% and 1% [16].
The results also showed that the patients with hypothyroidism had the biggest deviation in the variables describing shimmer and HNR, and significant differences before and after treatment were established in these parameters. The overall average shimmer value for females was 0.25 dB and the critical value was 0.48 dB [17]. In the pretreatment period, 40% of patients with hypothyroidism had abnormal shimmer, but in the posttreatment period, only 10% had abnormal shimmer. The signal-to-noise ratio also improved after the treatment. The higher HNR indicates better voice quality [18]. The value of the aerodynamic variable before treatment was slightly below the normal values, and it was on the lower limit of normal values after treatment. The MPT was approximately 15–20 seconds for adults [4].
Regarding hypothyroidism, Birkent et al. [19] established that after thyroidectomy was performed and an appropriate substitution therapy was applied, a statistically significant increase in the F0 parameter was observed, although this parameter was abnormal even prior to the therapy. The MPT was reduced after therapy, although not statistically significant, while the other objective parameters (jitter, shimmer, amplitude perturbation quotient, pitch perturbation quotient, and NHR) showed no significant changes after hormone replacement. This was not the case in this study: the patients were treated only with drugs. In cases of primary hypothyroidism, before the substitution therapy was applied, F0, voice turbulence index, and soft phonation index differed significantly from the control values of the patients without hypothyroidism [20]. However, only a certain number of patients in this study had voice deviations. Jitter, shimmer, and HNR seem to determine the basic perceptual elements of the voice quality: grade, roughness, and breathiness [21]. This data support results of the perceptual analysis performed in the present study, except for the parameter grade that was borderline insignificant. Voice hoarseness and loss of voice range are the major features of hypothyroidism. Hoarseness has a gradual onset and a slow progression, thus limiting the patient to notice any voice changes [22]. Therefore, the voice quality may be affected by the thyroid gland disease, and some of the symptoms that are referred to in literature regarding hypothyroidism are loss of vocal range; reduced voice frequency, especially in women; vocal fatigue; hoarseness; low voice; and decreased voice intensity [23]. In some cases, mild dysphonia may occur as a result of mild thyroid deficiency. Hoarseness is a common symptom in patients with hypothyroidism. Kadakia, Carlson, and Sataloff [1] stated that according to Ritter, the mechanism of action of the thyroid hormone to the voice is unknown in patients with hypothyroidism, but it is believed to be related to the increased levels of polysaccharides and the fluid accumulation in the lamina propria in the vocal folds, paresis of the cords due to the thyroid gland enlargement, myxedema of the cricothyroid muscle, and neural edema of the vagus nerve [6]. The thickening leads to a decrease in the vibratory capacity. Singers with hypothyroidism may complain about the limitations associated with the higher vocal range and vocal fatigue. In a number of patients who are singers and experienced voice problems and hypothyroidism, there was an increase in the voice clarity after small doses of thyroid hormone replacement [24]. Speech disorders in most patients with thyroid hypofunction and voice changes correlate with serum TSH levels [21]. Birkent et al. [19] stated that dysphonia associated with hypothyroidism may vary in relation to the amount and duration of hormone deficiency. Certain degree of changes in the patient’s thyroid status will affect the voice regardless of the absolute value of TSH level at the start of hormone replacement. It is believed that the supplements of thyroid hormones are usually sufficient to control the symptoms of hypothyroidism [1].
The assessment results of the acoustic voice parameters in patients with hyperthyroidism showed that the parameters describing the frequency characteristics were within normal in most patients, but 29.63% of patients had abnormal F0 in pre- and posttreatment periods. There was significant improvement before and after treatment only in the variable MPT. MPT is an indirect measure of the laryngeal function widely used in the studies involving patients with voice problems [21] including those with a breathy and weak voice [25]. In contrast, the results of the perceptual evaluation showed significant differences in all observed variables, except in grade, before and after treatment. Hyperthyroidism is mainly the cause of hoarseness or roughness, which is often overlooked [8]. Voice hoarseness is one of the problems that patients often complain about when they undergo vocal therapy, although it can often be associated with infection or injuries [4]. Apart from hoarseness, a number of patients with a high degree of hyperthyroidism complained of vocal disruption [24]. Although some symptoms are very specific in hyperthyroidism, the sensitivity in these patients when it comes to certain symptoms is low (2.9%–28.3%). In addition to hoarseness, a frequent symptom of hyperthyroidism is reported to be a deep voice. However, the absence of symptoms does not indicate the absence of thyroid gland disorders [26].
During a study conducted on 96 patients with thyroid gland disorders, a high prevalence of deviant perceptual voice features was observed. Most of these abnormalities were mild to moderate deviations, and only 8% of the patients had clinically significant perceptual abnormalities [23]. The results of another study showed that there are voice changes in patients with thyroid gland disorders, which was especially evident in the perceptual evaluation. The voice changes in these patients were not negligible, considering that the presence of voice change symptoms in patients with thyroid pathology has been reported to range from 9% to 38% [14]. Patients with thyroid pathology, apart from other symptoms, may also complain about voice changes [27], the most common being hoarseness, breathiness, strain, and uncertainty about how the voice will sound [28]. Cases of low voice, rough voice, reduced vocal range, and vocal fatigue were also reported [7]. Although the present study found significant differences in the posttreatment period (wherein the patients were under drug therapy) in only a small number of acoustic parameters, which do not include parameters describing the F0, and not the same parameters in hypo and hyperthyroidism, Birkent et al. [19] stated that it is possible, with the exemption of the F0 parameter, that other acoustic estimation parameters cannot detect the subtle changes in the mass of the vocal fold, and it is unlikely to detect any change without a significant vibratory or epithelial disease. In any case, a patient experiences voice changes caused by the thyroid gland disorders, and these changes may disappear completely within 3–6 months after achieving euthyroidism [22].
The limitation of this study is the small number of patients. Moreover, even though we recorded all patients’ voices in the morning after the morning routine to diminish fluctuations of acoustic voice parameters during the day and enforced the controlled use of voice prior to recording, these factors could still affect the voice parameters. Further studies should focus on the correlation of changes in the patient’s thyroid status and voice with regard to the thyroid hormones.
Thyroid gland disorders cause changes in the voice. Changes in the perceptual characteristics of the voice of patients are particularly evident, while the acoustic characteristics are less affected by the changes, particularly variables describing the voice frequency. The highest deviations in the voice of these patients are seen in the acoustic parameters of shimmer and HNR. There was an improvement in the voice of patients with thyroid gland disorders after the treatment period, as they received therapy. The results indicate the importance of assessing and treating the symptoms of changes in the voice of patients with thyroid gland disorders and possible the effect of hormone replacement therapy, especially on the perceptual voice features. Since there are fewer studies on the effect of thyroid hormone supplements and the long-term follow-up of voice discrepancies after the application of therapy, further studies should aim at monitoring the effects of thyroid hormone replacement therapy on the voice of patients with hypo- and hyperthyroidism.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of University Clinical Center Tuzla (02-09/2-44/14).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – L.J.Ž, A.I., S.A.; Design - L.J.Ž, A.I., S.A.; Supervision – L.J.Ž.; Materials –S.A.; Data Collection and/or Processing - L.J.Ž, A.I.,S.A.; Analysis and/or Interpretation - L.J.Ž, A.I., S.A.;Literature Search – L.J.Ž.; Writing Manuscript – L.J.Ž.;Critical Review - L.J.Ž, A.I., S.A.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Kadakia S, Carlson D, Sataloff RT. The effect of hormones on the voice. J Sing. 2013;69:571–4. [Google Scholar]
- 2.Petrovic-Lazic M. Fonopedija. Belgrade: Naucna knjiga; 2001. [Google Scholar]
- 3.Dodig K, Kusic Z. Klinicka nuklearna medicina. Zagreb: Medicinska naklada; 2012. [Google Scholar]
- 4.Andrews ML. Pediatrics through geriatrics. Canada: Thomson Del-mar Learning; 2006. Manual of voice treatment. [Google Scholar]
- 5.Garber JR, Cobin RH, Gharib H, et al. for the American Association of Clinical Endocrinolo-gists and American Thyroid Association Task-force on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in Adults: Thyroid. 2012;22:1200–35. doi: 10.1089/thy.2012.0205. [DOI] [PubMed] [Google Scholar]
- 6.Altman KW, Haines GK, Vakkalanka SK, Keni SP, Kopp PA, Radosevich JA. Identification of thyroid hormone receptors in the human larynx. Laryn-goscope. 2003;113:1931–4. doi: 10.1097/00005537-200311000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Melnick LA. Master Thesis. 2011. Perceptual evaluation of voice in patients with thyroid disease. The school of graduate studies and research department of education and educational technology: Indiana University of Pennsylvania. [Google Scholar]
- 8.Kim JE, Rasgon B. The hoarse patient: Asking the right questions. Perm J. 2010;14:51–3. doi: 10.7812/TPP/09-091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solter M. Klinicka tireoidologija. Zagreb: Medicinska naklada; 2007. Bolesti stitnjace. [Google Scholar]
- 10.Watt T, Groenvold M, Rasmussen AK, et al. Quality of life in patients with benign thyroid disorders. A review. Eur J Endocrinol. 2006;154:501–10. doi: 10.1530/eje.1.02124. [DOI] [PubMed] [Google Scholar]
- 11.McIvor NP, Flint DJ, Gillibrand J, Morton RP. Thy-roid surgery and voice-related outcomes. The Aust N Z J Surg. 2000;70:179–83. doi: 10.1046/j.1440-1622.2000.01781.x. [DOI] [PubMed] [Google Scholar]
- 12.Schutte HK, Seidner W. Recommendation by the Union of European Phoniatricians (UEP): Standard-izing voice area measurement/phonetography. Folia Phoniatr (Basel) 1983;35:286–8. doi: 10.1159/000265703. [DOI] [PubMed] [Google Scholar]
- 13.Hirano M. Psycho-Acoustic Evaluation of Voice. In: Hirano M, editor. Clinical examination of the voice. New York: Springer-Verlag; 1981. pp. 81–4. [Google Scholar]
- 14.de Morais Costa EB, de Araújo Pernambuco L. Vocal self-assessment and auditory-perceptual assessment of voice in women with thyroid disease. Revista Cefac. 2014;16:967–72. doi: 10.1590/1982-021620145913. [DOI] [Google Scholar]
- 15.Roth FP, Worthington CK. Thomson Delmar Learning. 2005. Treatment resourcemanual for speech language pathology. [Google Scholar]
- 16.Teixeira JP, Oliviera C, Lopes C. Vocal acousticanalysis-jitter, shimmer and HNR parameters. Proc Technol. 2013;9:1112–22. doi: 10.1016/j.protcy.2013.12.124. [DOI] [Google Scholar]
- 17.Sorensen D, Horii Z. Frequency and amplitudeperturbation in voice of female speakers. J Commun Disord. 1983;16:57–61. doi: 10.1016/0021-9924(83)90027-8. [DOI] [PubMed] [Google Scholar]
- 18.Finger LS, Cielo CA, Schwartz K. Acoustic vocalmeasures in women without voice complaintsand with normal larynxes. Braz J Otorhinolaryngol. 2009;75:432–40. doi: 10.1016/S1808-8694(15)30663-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birkent H, Karacalioglu O, Merati AL, Akcam T, Gerek M. Prospective study of the impact of thyroid hormone replacement on objective voiceparameters. Ann Otol Rhinol Laryngol. 2008;117:523–27. doi: 10.1177/000348940811700710. [DOI] [PubMed] [Google Scholar]
- 20.Mohammadzadeh A, Heydari E, Azizi F. Speechimpairment in primary hypothyroidism. J Endocrinol Invest. 2011;34:431–33. doi: 10.1007/BF03346708. [DOI] [PubMed] [Google Scholar]
- 21.Dejonckere PH. Assessment of Voice and Respiratory Function. In: Remacle M, Eckel HE, editors. Surgery of larynx and trachea. Berlin Heidelberg: Springer-Verlag; 2010. pp. 11–26. [DOI] [Google Scholar]
- 22.Hari Kumar KVS, Garg A, Asjai Chandra NS, Singh SP, Datta R. Voice and endocrinology. Indian J Endocrinol Metab. 2016;20:590–4. doi: 10.4103/2230-8210.190523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bone SL, Vertigan AE, Eisenberg RL. Auditory-perceptual voice characteristics in pre-operativepatients undergoing thyroid or parathyroid surgery. Folia Phoniatr Logop. 2012;64:87–93. doi: 10.1159/000335779. [DOI] [PubMed] [Google Scholar]
- 24.Brodnitz FS. Hormones and the human voice. Bull N Y Acad Med. 1971;47:183–91. [PMC free article] [PubMed] [Google Scholar]
- 25.Van Riper C. Speech correction: principles andmethods. Englewood Cliffs, N J: Prentice-Hall; 1954. pp. 469–71. [Google Scholar]
- 26.Canaris GJ, Steiner JF, Ridgway EC. Do traditional symptoms of hypothyroidism correlate with biochemical disease? J Gen Intern Med. 1997;12:544–50. doi: 10.1046/j.1525-1497.1997.07109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banks CA, Ayers CM, Hornig JD, et al. Thyroiddisease and compressive symptoms. Laryngoscope. 2011;122:13–6. doi: 10.1002/lary.22366. [DOI] [PubMed] [Google Scholar]
- 28.Lombardi CP, Raffaelli M, D'Alatri L, et al. Voiceand swallowing changes after thyroidectomy inpatients without inferior laryngeal nerve injuries. Surgery. 2006;140:1026–32. doi: 10.1016/j.surg.2006.08.008. [DOI] [PubMed] [Google Scholar]