Abstract
The bacteria Helicobacter pylori (H. pylori) have been identified in the extragastric tissues in the head and neck. The origin and pathogenicity of these bacteria in the head and neck are not known. Gastric reflux and nasal or oral routes are the possible modes of spread. In many sinonasal, pharyngeal, laryngeal, and middle ear disorders, laryngopharyngeal reflux has been identified as a contributing or causative factor. One possible mode by which laryngopharyngeal reflux may contribute is by seeding of the extragastric mucosa with H. pylori. The clinical significance of the discovery of H. pylori in extragastric tissues in the head and neck is unclear. There is no evidence of a pathologic or active role of H. pylori in otorhinolaryngological disorders. The suggestion that the sinonasal cavities and pharynx may serve as a reservoir for H. pylori and that reinfection of the stomach occurs after eradication therapy awaits further studies for confirmation. No connection was observed between H. pylori found in the stomach and H. pylori found in the head and neck. Also, these bacteria, found in the head and neck tissues, may be accidental or innocent bystanders that do not affect the pathways of otolaryngological and gastroduodenal diseases. This review examines the evidence for a possible relationship of H. pylori with otorhinolaryngological diseases.
Keywords: Helicobacter pylori, otorhinolaryngology, laryngopharyngeal reflux, nasopharyngeal reflux
Introduction
Although the role of the bacteria Helicobacter pylori (H. pylori) in gastroduodenal disease is well established, the clinical significance of the discovery of H. pylori in extragastric tissues in the head and neck is unclear. This review examines the evidence for a possible relationship of H. pylori with otorhinolaryngological diseases.
Bacteria H. pylori in the Nose and Paranasal Sinuses
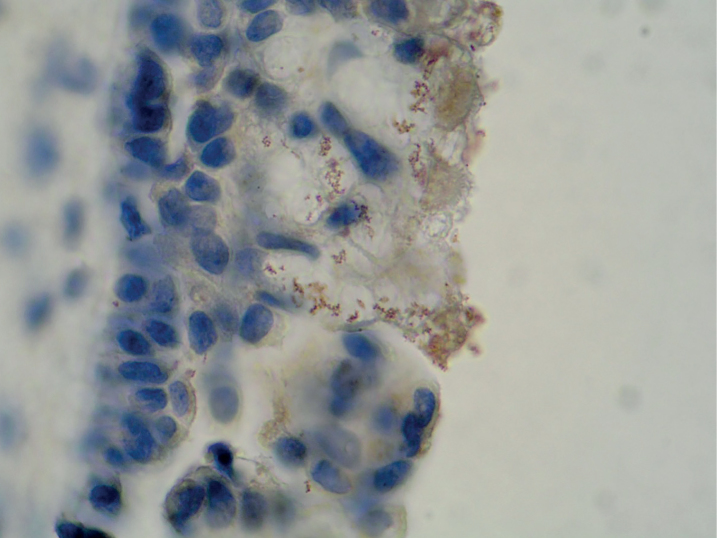
Chronic rhinosinusitis (CRS) is a clinical syndrome with many extrinsic and intrinsic etiological factors. It is considered that, among other potential factors, bacterial infection, colonization, allergy, and superantigen may play a causative or contributory role. Gastroesophageal reflux, or more precisely nasopharyngeal reflux, is thought to be a contributing factor in refractory CRS [1]. The exact mechanism by which nasopharyngeal reflux may contribute is unknown. One possibility is the sowing of the nasal mucosa with H. pylori, bacterium prevalent in gastric contents. Helicobacter pylori was found in the nasal cavity and paranasal sinuses (Figure 1).
Figure 1.
The nasal polyp: Brown stained immunoreactive structures above the epithelium represent the bacteria Helicobacter pylori. Immunohistochemistry with polyclonal rabbit anti-H. pylori antibody (peroxidase-antiperoxidase), light micrograph ×1000
Özdek et al. [2] were the first to report the presence of H. pylori in the sinus mucosa. Using nested polymerase chain reaction (PCR), H. pylori was detected in the ethmoidal mucosa in 4 of 12 patients with CRS, but it was not detected in 13 CRS-free patients with the concha bullosa. Interestingly, using real-time PCR, Ozyrt et al. [3] detected H. pylori ureA gene more often in the normal nasal mucosa than in the nasal polyps samples (70% vs. 59%, respectively). Helicobacter pylori cagA was identified in both H. pylori-positive normal nasal mucosa and nasal polyp samples (90% and 79%, respectively). Conversely, in the study of Burduk et al. [4], all 30 nasal polyps and concha bullosa specimens were H. pylori cagA negative, but H. pylori ureaA was found by PCR in all 30 nasal specimens. In contrast to aforementioned studies, Ozcan et al. [5] reported that all nasal polyps from 25 patients were H. pylori negative by immunohistochemistry (IHC). Using PCR and Giemsa stain, Cedeno et al. evaluated H. pylori in 28 children with CRS without polyps. Highly sensitive and specific primers (i.e., ureC, vacA, cagA, and babA) were used, but H. pylori was not detected in samples from the antral lavages or adenoids [6].
The following studies have raised a question about an active role of H. pylori in CRS. Compared with rhinologic patients without CRS, a statistically significant higher prevalence of sinonasal H. pylori in patients with CRS was found [7, 8]. Koc et al. [7] reported that nasal polyps were positive in 6 of 30 patients with CRS, whereas none of the control group samples were positive for H. pylori using IHC. In the study of Kim et al. [8], nasal specimens in 12 (out of 48) patients with CRS and in 1 (out of 29) patient without CRS were H. pylori positive by both rapid urease test (RUT) and IHC analysis. There were no significant differences between H. pylori positive and H. pylori negative patients with CRS when comparing their preoperative rhinosinusitis symptom scores and the preoperative disease extent assessed by sinus computed tomography scoring system. A great proportion of degenerative coccoid shapes were found by IHC [8]. Kariya et al. [9] demonstrated that the whole-cell proteins of H. pylori in a viable but not culturable state, not exclusively live bacteria, can induce immunological inflammation in the extragastric epithelium. It has been suggested that these coccoid forms constitute a dormant resistant form of the bacterium that may revert into an infectious spiral form in appropriate conditions and result in recrudescence of infection [10]. These findings imply that the sinonasal cavities may be a reservoir for H. pylori and possible gastric re-colonization rather than that H. pylori having an active role in CRS. It is not clear why H. pylori has been presented in the coccoid form. The use of antibiotics may be one possible explanation [10]. Long-term antibiotic therapy (e.g., clarithromycin for ~12 weeks) is included in the CRS treatment scheme and is performed by a number of rhinologists. If there is a failure of maximal medical therapy after 3 months, sinus surgery is considered in medically refractory CRS, as it was performed in aforementioned studies. Furthermore, the nasal cavities with good ventilation can provide an unfavorable oxygen excess. On the other hand, diseased ethmoids and massive polyposis can result in many poorly ventilated and drained narrow spaces. Such spaces can be a favorable environment for microaerophilic H. pylori.
The results of Dinis et al. [11] regarding H. pylori and pepsin/pepsinogen I status in the ethmoidal and sphenoidal mucosa did not support the notion that H. pylori and laryngopharyngeal reflux (LPR) had an important role in the etiopathogenesis of CRS. No significant differences were found between patients with CRS and without CRS controls neither in the blood and mucosa pepsin/pepsinogen I values nor in the H. pylori sinonasal colonization. In both groups, the sinonasal tissue pepsin/pepsinogen never rose above blood levels. This finding implies that H. pylori colonizes the sinus mucosa via a nasal or oral route rather than via a gastric reflux. The authors state that when in comorbidity, the pathogenic mechanisms of H. pylori infection and reflux disease probably run in a parallel fashion, independent from each other [11]. Also, it is possible that H. pylori colonizes the vulnerable damaged sinonasal mucosa, as a favorable harbor, after CRS is already developed. In that case, H. pylori colonization is the consequence, but not the cause, of CRS.
A possible predictive value of H. pylori sinonasal colonization for efficacy of endoscopic sinus surgery in patients suffering from CRS with nasal polyps has been studied [12]. Nasal polyps in 28 of 40 (70%) patients were positive for H. pylori by IHC. There were no significant differences between the H. pylori positive and H. pylori negative group comparing the nasal polyp eosinophils and the postoperative improvement of the CRS symptom scores. There was a prognostic value for the endonasal findings: compared with CRS patients with H. pylori negative nasal polyps, patients with H. pylori had statistically significant greater improvement in postoperative endoscopic scores [12]. Physicians who oppose “total war” against H. pylori might be delighted with this result. There is no consensus about Blaser’s suggestion that in addition to “bad” and “very bad” H. pylori, “neutral” or “good” ones also exist [13]. A possible positive influence of H. pylori on asthma has been reported and explained by the ability of some H. pylori compounds to drive T helper-1 polarization and to display a powerful inhibition of allergic T helper-2 response [14]. CRS is one of the most frequently encountered comorbid conditions associated with asthma. The current findings regarding a prognostic role of H. pylori in CRS must be interpreted with caution. The status of the most important H. pylori virulence factors was not determined [12]. Theoretically, there may have been a state of preponderance of VacA negative and CagA negative strains that may have influenced histological and clinical findings. Further studies, including a larger sample size, and stronger validated tools for assessment of CRS severity and determination of H. pylori virulence factors are needed. The most relevant studies presenting the current state of knowledge on the relationship between H. pylori and CRS are shown in Table 1. A pathological or active role of H. pylori in CRS was not established.
Table 1.
Studies presenting the current state of knowledge on the relationship between Helicobacter pylori and chronic rhinosinusitis (CRS)
| Author, Year | Method for detection of H. pylori | Specimen | Study group (total number/H. pylori positive) | Control group (total number/H. pylori positive) | Study group vs. Control group | Conclusion |
|---|---|---|---|---|---|---|
| Koc et al., 2004 [7] | IHC | Nasal polyp, middle concha | 30/6 | 20/0 | S | A possible involvement in the CRS pathogenesis |
| Dinis et al., 2006 [11] | PCR | CRS mucosa, normal sinus mucosa | 15/6 | 5/1 | NS | No significance of H. pylori for the CRS pathogenesis |
| Kim et al., 2007 [8] | IHC | Nasal polyps, nasal mucosa | 48/12 | 29/1 | S | A possible involvement in the CRS pathogenesis |
| Ozcan et al., 2009 [5] | IHC | Nasal polyp, nasal mucosa | 25/0 | 14/0 | NS | No presence of H. pylori in nasal polyps and normal nasal mucosa |
| Ozyrt et al., 2009 [3] | PCR | Nasal polyp, nasal mucosa | 32/19 | 27/19 | NS | Helicobacter pylori encountered more often in normal nasal mucosa than in CRS mucosa |
| Jelavic et al., 2012 [12] | IHC | Nasal polyp | 28/28 | 12/0 | S | A possible prognostic value of H. pylori for the ESS outcome |
IHC: immunohistochemistry; PCR: polymerase chain reaction; ESS: endoscopic sinus surgery; S: significant difference; NS: no significant difference
Bacteria H. pylori in the Pharynx
The study of Minocha et al. [15] has stimulated research on tonsils as a possible natural reservoir—extragastric site at which H. pylori evades treatment. They found a decreased prevalence of H. pylori gastric colonization in subjects with a history of tonsillectomy. The suggestion that tonsillectomy may protect the host against H. pylori infestation of the stomach has arisen, although contrary reports exist [16]. Fibrotic tonsils with debris trapped in crypts are a good environment, and they may be a permanent reservoir for microorganisms [17]. Tonsils, a component of mucosa-associated lymphoid tissue, are the first line of mucosal defense against invading pathogens. In the study of Skinner et al. [18], H. pylori-seropositive patients exhibited a higher expression of inducible nitric oxide synthase in the tonsillar macrophages than H. pylori-seronegative patients. It can be hypothesized that H. pylori may be an initiating trigger or factor resulting in an exaggerated inflammatory tonsillar response to otherwise commensal organisms [18].
Using PCR, Cirak et al. [19] found H. pylori DNA in tonsillar core or adenoid samples in 7 of 23 (30%) patients. The CagA gene was detected in adenotonsillar samples in 5 patients. Zhang et al. [20] detected H. pylori in the pharyngeal mucosa in 19 (out of 50) patients with chronic pharyngitis, and in zero of 20 healthy controls. Helicobacter pylori may be a cause of inflammation or a marker for reflux and chemical inflammation of the pharyngeal mucosa caused by gastric acid [20]. Using immunofluorescence and immunoelectron microscopy, the study of Kusano et al. [21] on 55 patients with recurrent pharyngotonsillitis or IgA nephropathy (IgAN) demonstrated the coccoid form of H. pylori in the palatine tonsils of 43 patients. CagA was expressed in 38 of the 43 strains of tonsillar H. pylori. No tonsillar H. pylori could be cultured. All 15 patients with gastric H. pylori had H. pylori in their tonsils. The prevalence of tonsillar H. pylori in patients with IgAN (100%) was significantly higher than that in patients with recurrent pharyngotonsillitis (71%). Helicobacter pylori may be a candidate for IgAN-pathogenic antigen [21]. It is unclear if the detection of adenotonsillar H. pylori represents transient or persistent colonization. Also, the origin of adenotonsillar H. pylori is not known. H. pylori may have reached the pharynx via a nasal or an oral route or by refluxed gastric content. Lukeš et al. [22] detected different strains in the stomach and oropharynx in the same individuals. The oral presence of H. pylori without concurrent stomach infection was found. According to these findings, the H. pylori oropharyngeal colonization seems to be independent to the gastric infection.
This fastidious bacterium is difficult to culture from oropharyngeal specimens because of more robust flora competing for a growth. In the study on 62 palatine tonsils and 77 pharyngeal tonsils in 77 children [23], 17 palatine tonsils in 14 children were RUT positive and H. pylori negative. Eight children were positive for both RUT in tonsils and 13Carbon-urea breath test. No significant difference was observed between children with tonsillar hypertrophy and those with recurrent tonsillitis in H. pylori seropositivity [23]. Siupsinskiene et al. found significantly higher prevalence of tonsillar H. pylori in patients with chronic tonsillitis in comparison to patients with tonsillar hypertrophy. They also reported significant correlation of H. pylori colonization with laryngeal signs of LPR [24].
Yilmaz et al. [25] were the first to isolate H. pylori from the pharyngeal tissue culture. Out of 22 children, there was a growth of H. pylori in the cultures of adenoids and tonsils in 11 and 12 patients, respectively. These findings were supported by positive PCR. In contrast, in the study of Bitar et al., all 25 adenoids tested were H. pylori negative by nested PCR [26]. Contradictory results exist regarding the presence of pharyngeal H. pylori in the studies performed on populations from the same country [25, 26]. It is not known if cognition of patchy distribution of H. pylori in the stomach may be applied to the pharynx. In that case, the number of samples taken from one site can affect the results.
In the study of Vilarinho et al. [27] on 46 palatine tonsils and 55 adenoids in 62 children, 3 tonsils in 2 H. pylori-seronegative children were IHC positive, but all the specimens studied were negative by PCR, directed to the vacA gene, and by fluorescence in situ hybridization with a specific H. pylori peptide nucleic acid probe. The authors did not consider the adenotonsillar tissue in children as a permanent reservoir for H. pylori infection [27]. There is no evidence of a pathological or active role of pharyngeal H. pylori. The suggestion that the pharynx may serve as a reservoir for H. pylori and that reinfection of the stomach occurs after eradication therapy awaits further studies for confirmation. This attitude is consistent with the results of other studies [6, 18, 28]. The most relevant studies presenting the current state of knowledge on the relationship between H. pylori and adenotonsillar pathology are shown in Table 2.
Table 2.
The studies presenting the current state of knowledge on the relationship between the bacteria Helicobacter pylori and adenotonsillar pathology
| Author, Year | Method for H. pylori detecting | Specimen | Study group (total number/H. pylori positive) | Control group (total number/H. pylori positive) | Study group vs. Control group | Conclusion |
|---|---|---|---|---|---|---|
| Cirak et al., 2003 [19] | Tonsil, adenoid | PCR | 23/7 | - | - | H. pylori may colonize tonsil and adenoid |
| Bitar et al., 2005 [26] | Adenoid | PCR | 25/0 | - | - | No presence of H. pylori in adenoids |
| Zhang et al., 2006 [20] | Pharyngeal mucosa | TDI-FP | 50/19 | 20/0 | S | A possible involvement in the chronic pharyngitis pathogenesis |
| Yilmaz et al., 2006 [25] | Tonsil, adenoid | PCR, culture | 22/16 | 20/9 | NS | Culturable H. pylori may exist in tonsil and adenoid |
| Kusano et al., 2010 [21] | Tonsil | IF and IE microscopy | 14/14 | 41/29 | S | Tonsillar H. pylori may be a candidate for IgA nephropathy-pathogenic antigen |
| Vilarinho et al., 2010 [27] | Tonsil, adenoid | IHC | 62/2 | - | - | Adenotonsillar tissue is not PCR-DEIA 62/0 an extragastric reservoir PNA-FISH 62/0 for H. pylori |
| Siupsinskiene et al., 2017 [24] | Tonsil | HC | 62/35 | 35/11 | S | A possible association with chronic tonsillitis and laryngopharyngeal reflux |
PCR: polymerase chain reaction; TDI-FP: template-directed dye-terminator incorporated with fluorescence polarization detection; IF: immunofluorescence; IE: immunoelectron; IHC: immunohistochemistry; HC: histochemistry; DEIA: DNA enzyme immunoassay; PNA-FISH: peptide nucleic acid-fluorescent in situ hybridization; S: significant difference; NS: no significant difference
Bacteria H. pylori in the Larynx
LPR is a form of extraesophageal reflux characterized by laryngopharyngeal symptoms, such as hoarseness, globus pharyngeus, throat clearing, and cough. The role of H. pylori toxins in gastric contents that reflux to the larynx and hypopharynx in laryngopharyngeal manifestations is uncertain. No relationship between gastric H. pylori infection and LPR was reported [29]. Conversely, Oridate et al. [30] demonstrated a lower laryngopharyngeal symptom-improvement rate influenced by acid-suppression therapy among H. pylori-seronegative patients than among H. pylori-seropositive patients with gastroesophageal reflux disease. In the study of Çekin et al. [31] using real-time PCR, laryngeal H. pylori and LPR were found in 56% and 70% of 43 patients with laryngeal lesions, respectively. Helicobacter pylori was detected in 18 of the 30 LPR-positive patients and in 6 of 13 LPR-negative patients. A statistically significant relationship of LPR positivity with malignant or premalignant laryngeal lesions and no association between laryngeal H. pylori and laryngeal lesions were found.
The bacterium H. pylori is the first formally recognized bacterial carcinogen [32]. A possibility that H. pylori may increase susceptibility to head and neck carcinoma, and not just to gastric carcinoma, is an important subject of research. The laryngeal epithelium has its origin in the respiratory diverticulum of the ventral wall of the foregut, and it embryologically develops from the same endodermal cells that line the gastric mucosa [33]. Compared with cancer-free controls, a significantly higher prevalence of H. pylori seropositivity was found in patients with laryngohypopharyngeal carcinoma [34]. Contrary reports also exist [35].
In the study of Titiz et al. [36], an association between laryngeal H. pylori and laryngeal carcinoma was found. All benign laryngeal lesions were H. pylori negative by PCR, but laryngeal H. pylori was found in 17 of 21 patients with laryngeal carcinoma. Of those 17 H. pylori-positive patients, H. pylori was detected in the normal laryngeal tissue only, tumor tissue only, and in both normal and tumor tissues in 8, 1, and 8 patients, respectively. All tissue cultures were H. pylori negative [36]. In the study conducted in Egypt [37], PCR was used to detect H. pylori ureaA and cagA genes in 49 patients with laryngeal carcinoma and 15 patients with laryngeal benign polyps. A significantly higher incidence of H. pylori was found in carcinoma specimens (65%) in comparison to benign polyps specimens (20%). These findings supported a positive association between H. pylori laryngeal colonization and susceptibility to develop laryngeal carcinoma [37]. Amizadeh et al. reported contradictory results suggesting the protective effect of laryngeal H. pylori against laryngeal carcinoma [38]. Iranian patients with laryngeal carcinoma had lower incidence of laryngeal H. pylori and statistically significant lower incidence of cagA gene than those with benign laryngeal lesions [38]. Using PCR, Yilmaz et al. detected laryngeal H. pylori in only 1 case out of 74 Turkish patients with laryngeal carcinoma [39]. The presence of laryngeal H. pylori DNA in all 30 patients with benign laryngeal lesions [4] and in 59% of 29 patients with benign or malignant laryngeal diseases [3] was reported. Helicobacter pylori cagA was found in 23% and 82% of these H. pylori-positive patients, respectively [4, 3]. Like in neighboring organs, the character and origin of such H. pylori colonization are not determined. A descending or ascending approach from the nose, mouth, and pharynx or stomach is possible. A pathological or active role of H. pylori in laryngeal disorders was not proved. Improved knowledge on the mechanisms responsible for H. pylori-associated gastric carcinogenesis may enhance research on a relationship between H. pylori and laryngeal malignancies. The most relevant studies presenting the current state of knowledge on the relationship between H. pylori and laryngeal disorders are shown in Table 3.
Table 3.
The studies presenting the current state of knowledge on the relationship between the bacteria Helicobacter pylori and the laryngeal disorders
| Author, Year | Method for H. pylori detecting | Specimen | Study group (total number/H. pylori positive) | Control group (total number/H. pylori positive) | Study group vs. Control group | Conclusion |
|---|---|---|---|---|---|---|
| Titiz et al., 2008 [36] | MLL, BLL | PCR | 21/17 | 19/0 | S | A possible involvement in the laryngeal carcinogenesis |
| Culture | 21/0 | - | - | |||
| Ozyurt et al., 2009 [3] | MLL, BLL | PCR | 29/17 | - | - | H. pylori may colonize MLL and BLL |
| Burduk et al., 2011 [4] | BLL | PCR | 30/30 | - | - | H. pylori may colonize BLL |
| Cekin et al., 2012 [31] | MLL, BLL | PCR | 21/9 | 22/15 | NS | No involvement in the laryngeal carcinogenesis |
| Amizadeh et al., 2015 [38] | MLL, BLL | PCR | 72/24 | 72/33 | S | A possible association with protection to development of laryngeal carcinoma |
| Barakat et al., 2016 [37] | MLL, BLL | PCR | 49/31 | 15/3 | S | A possible involvement in the laryngeal carcinogenesis |
MLL: malignant laryngeal lesion; BLL: benign laryngeal lesion; PCR: polymerase chain reaction; S: significant difference; NS: no significant difference
Bacteria H. pylori in the Middle Ear
Otitis media with effusion (OME) is a chronic inflammatory disease characterized by conductive hearing loss and the persistence of a middle ear effusion for at least 3 months without clinical signs and symptoms of active infection. It is not clear why OME develops. In the study of Tasker et al. [40], a possible role of gastroesophageal reflux in the disease pathogenesis has been studied. In 91% (out of 65) samples from children undergoing myringotomy for OME, pepsin/pepsinogen levels in the middle ear effusion samples were up to 1000 times higher than serum levels, indicating that pepsin in the middle ear was almost certainly due to reflux of gastric contents. Immunohistochemical analysis of biopsy samples from the middle ear showed no evidence of pepsin production by the middle ear [40]. Reflux of gastric acid and pepsin into the nasopharynx and eustachian tubes may have led to chemical inflammation and edema of the nasopharyngeal mucosa and dysfunction of the eustachian tube. These findings suggest that anti-reflux therapy may play a part inOME treatment.
Morinaka et al. [41] have reported that immunostained smears revealed H. pylori in 12 (80%) middle ear effusion specimens from 15 patients with OME, mostly adults. Since the adenoid tissue has been incriminated as a source of microorganisms causing the middle ear disease, adenoidectomy is a common procedure as a part of OME treatment. Although the study of Fancy et al. [42] demonstrated the H. pylori gene by PCR in 23 of 73 (32%) middle ear fluid samples from children with OME, the H. pylori prevalence rate in the adenoids of children with OME did not differ from those in the adenoids of OME free-children. Conversely, Bitar et al. [43] failed to detect H. pylori in all 28 middle ear effusion samples from 18 children with otitis media using culture and PCR. The study of Pitkäranta et al. [28] has shown H. pylori negative cultures of 12 middle ear fluid samples in all 8 examined children with recurrent otitis media.
Yilmaz et al. [25] were the first to show the growth of H. pylori in cultures of the middle ear fluid and tympanic mucosa. Compared with the healthy ears of controls, a statistically significant higher prevalence of H. pylori in the middle ear of children with OME was found using culture and PCR. The presence of viable and culturable H. pylori indicates that the middle ear with insufficient ventilation can be a favorable environment for H. pylori. In a study on rabbits [44], after inoculation of H. pylori in the tympanic cavity, H. pylori was cultured in 6 of 8 ears with histamine-induced inflammation, but not in any of the 8 normal middle ears. According to the previously reported findings [25, 40], transmission of H. pylori from the stomach to the middle ear by reflux might occur in humans. Also, approach via a nasal or an oral route is possible. Association, not causation, of OME with reflux and H. pylori was established. It seems plausible that refluxed acid and pepsin or H. pylori or both together have a role in the pathogenesis of OME. Helicobacter pylori may be a cause of inflammation, a marker for reflux and chemical inflammation caused by gastric acid, or an innocent bystander. The histopathological findings of the rabbit middle ears with histamine-induced inflammation, inoculated by H. pylori, suggest that H. pylori cannot initiate otitis media alone, but can contribute to the inflammation process in the presence of an effusion [44]. The in vivo study on mice [9] indicated that H. pylori might cause immunological inflammation in the middle ear epithelium. Helicobacter pylori whole-cell proteins directly injected into the tympanic cavity stimulated release of inflammatory mediators and induced inflammatory cell infiltration into the middle ear epithelium in mice.
Saki et al. [45] investigated a possible relationship between H. pylori and tympanosclerosis. Using PCR, they detected tympanal H. pylori in 34 of 56 (61%) Iranian patients with chronic otitis media. Their results suggested an association between H pylori and tympanosclerosis development. A total of 19 patients with tympanosclerosis had statistically significant higher prevalence of tympanal H. pylori in comparison with 37 patients suffering from other forms of chronic otitis media (84% and 45%, respectively) [45]. Contradictory results suggesting a lack of association between H. pylori and tympanosclerosis were reported by Dinç et al. The tympanosclerotic plaques in all of their 35 patients with tympanosclerosis were PCR negative for H. pylori [46]. The most relevant studies presenting the current state of knowledge on the relationship between H. pylori and OME are shown in Table 4. A pathological role of H. pylori in the middle ear disorders in humans was not established.
Table 4.
The studies presenting the current state of knowledge on the relationship between the bacteria Helicobacter pylori and the middle ear disorders
| Author, Year | Method for H. pylori detecting | Specimen | Study group (total number/H. pylori positive) | Control group (total number/H. pylori positive) | Study group vs. Control group | Conclusion |
|---|---|---|---|---|---|---|
| Pitkäranta et al., 2005 [28] | Culture | MEE | 12/0 | - | - | No presence of H. pylori in MEE |
| Morinaka et al., 2005 [41] | IHC | MEE | 15/12 | - | - | Helicobacter pylori may exist in MEE |
| Bitar at al., 2006 [43] | Culture, PCR | MEE | 28/0 | - | - | No presence of H. pylori in MEE |
| Yilmaz et al., 2006 [25] | Culture, PCR | MEM, MEE | 22/10 | 20/2 | S | A possible involvement in the OME pathogenesis |
| Fancy et al., 2009 [42] | PCR | MEE | 73/23 | - | - | Helicobacter pylori may exist in MEE |
| Saki et al., 2015 [45] | PCR | MEM | 19/16 | 37/15 | S | A possible involvement in the Tympanosclerotic tympanosclerosis pathogenesis plaques |
| Dinç et al., 2016 [46] | PCR | MEM | 35/0 | 53/0 | NS | No involvement in the Tympanosclerotic tympanosclerosis pathogenesis plaques |
MEE: middle ear effusion; MEM: middle ear mucosa; OME: otitis media with effusion; IHC: immunohistochemistry; PCR: polymerase chain reaction; S: significant difference
Conclusion
The bacteria H. pylori have been identified in the extragastric tissues in the head and neck. The origin and pathogenicity of these bacteria are not known. Gastric reflux or nasal or oral routes are the possible modes of spread. The clinical significance of such H. pylori colonization is debatable. No connection between H. pylori found in the stomach and H. pylori found in the head and neck was established. The suggestion that the sinonasal cavities and pharynx may serve as a reservoir for H. pylori and that reinfection of the stomach occurs after eradication therapy awaits further studies for confirmation. There is no evidence of an active role of H. pylori in otorhinolaryngological disorders, and a cause-effect correlation was not established. These bacteria, found in the head and neck tissues, may be accidental or innocent bystanders that do not affect the pathways of otolaryngological and gastroduodenal diseases. There is a lack of evidence to perform H. pylori testing for otorhinolaryngological diseases. Previous studies on this topic are conflicting. The role of H. pylori in some otorhinolaryngological disorders is likely to receive greater research attention.
Clinical and Research Consequences
According to the current state of knowledge, H. pylori testing for otorhinolaryngological diseases is not recommended. Also, there is no need for specific anti-H. pylori therapy in otorhinolaryngological diseases.
Recommendations for Future Research
Since the discovery of H. pylori has fundamentally changed the paradigms regarding causation of gastric diseases, investigators should be open-minded about the possibility that H. pylori contributes to some head and neck diseases. More research on the bacterium’s infectious and immunological properties should test the hypothesis that H. pylori may cause head and neck diseases even distal from the primary site of infection in the stomach by interfering with different biological processes. Further studies on this issue will need to include a larger sample size, stronger validated tools for assessment of disease extent and severity, and determine the status of the most important H. pylori virulence factors. While respecting ethical standards, invasive determination of the stomach H. pylori status and typing of H. pylori isolates are needed to show if H. pylori found in the head and neck is the same as that found in the stomach. Continuous advancements in analytical technology and molecular techniques for bacterial identification should be used for accurate identification of H. pylori. Future studies should determine the modes of spread of the bacteria into the head and neck and whether colonization is occasional or persistent. More information about H. pylori activities in patients with oral, pharyngeal, and laryngeal carcinogenesis is warranted.
Footnotes
Presented in: Part of this work was presented in the oral presentation „Bacterium Helicobacter pylori in chronic rhinosinusitis: cause or bystander” at the 2nd Balkan Rhino-Forum April 4–6, 2019, Belgrade, Serbia.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – B.J., J.P., I.M., M.B.; Design - B.J., J.P., I.M., M.B.; Supervision - B.J., J.P., I.M., M.B.; Analysis and/or Interpretation - B.J., J.P., I.M., M.B.; Literature Search - B.J., J.P., I.M., M.B.; Writing Manuscript – B.J., M.B.; Critical Review - B.J., J.P., I.M., M.B.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.DelGaudio JM. Direct nasopharyngeal reflux of gastric acid is a contributing factor in refractory chronic rhinosinusitis. Laryngoscope. 2005;115:946–57. doi: 10.1097/01.MLG.0000163751.00885.63. [DOI] [PubMed] [Google Scholar]
- 2.Ozdek A, Cirak MY, Samim E, Bayiz U, Safak MA, Turet S. A possible role of Helicobacter pylori in chronic rhinosinusitis: a preliminary report. Laryngoscope. 2003;113:679–82. doi: 10.1097/00005537-200304000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Ozyurt M, Gungor A, Ergunay K, Cekin E, Erkul E, Haznedaroglu T. Real-time PCR detection of Helicobacter pylori and virulence-associated cagA in nasal polyps and laryngeal disorders. Otolaryngol Head Neck Surg. 2009;141:131–5. doi: 10.1016/j.otohns.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Burduk PK, Kaczmarek A, Budzynska A, Kazmierczak W, Gospodarek E. Detection of Helicobacter pylori and cagA gene in nasal polyps and benign laryngeal diseases. Arch Med Res. 2011;42:686–9. doi: 10.1016/j.arcmed.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Ozcan C, Polat A, Otağ F, Görür K. Does Helicobacter pylori play a role in etiology of nasal polyposis? Auris Nasus Larynx. 2009;36:427–30. doi: 10.1016/j.anl.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Cedeño EE, Ortiz-Princz D, Figueredo SA, Porro ME. Adenoid hypertrophy and chronic rhinosinusitis: Helicobacter pylori on antral lavages, adenoid tissue and salival inmunoglobuline A on paediatric patients. Int J Pediatr Otorhinolaryngol. 2016;80:82–7. doi: 10.1016/j.ijporl.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Koc C, Arikan OK, Atasoy P, Aksoy A. Prevalence of Helicobacter pylori in patients with nasal polyps: a preliminary report. Laryngoscope. 2004;114:1941–4. doi: 10.1097/01.mlg.0000147924.96980.34. [DOI] [PubMed] [Google Scholar]
- 8.Kim HY, Dhong HJ, Chung SK, Chung KW, Chung YJ, Jang KT. Intranasal Helicobacter pylori colonization does not correlate with the severity of chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2007;136:390–5. doi: 10.1016/j.otohns.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Kariya S, Okano M, Fukushima K, et al. Expression of inflammatory mediators in the otitis media induced by Helicobacter pylori antigen in mice. Clin Exp Immunol. 2008;154:134–40. doi: 10.1111/j.1365-2249.2008.03740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.She FF, Su DH, Lin JY, Zhou LY. Virulence and potential pathogenicity of coccoid Helicobacter pylori induced by antibiotics. World J Gastroenterol. 2001;7:254–8. doi: 10.3748/wjg.v7.i2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinis PB, Subtil J. Helicobacter pylori and laryngopharyngeal reflux in chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2006;134:67–72. doi: 10.1016/j.otohns.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Jelavic B, Grgić M, Čupić H, Kordić M, Vasilj M, Baudoin T. Prognostic value of Helicobacter pylori sinonasal colonization for efficacy of endoscopic sinus surgery. Eur Arch Otorhinolaryngol. 2012;269:2197–202. doi: 10.1007/s00405-012-1923-9. [DOI] [PubMed] [Google Scholar]
- 13.Lee A. In: Helicobacter pylori is pathogenic flora. Helicobacter pylori basic mechanisms to clinical cure 2000. Hunt RH, Tytgat GNJ, editors. Dordrecht (Netherlands): Kluwer; 2000. pp. 31–5. [DOI] [Google Scholar]
- 14.Amedei A, Codolo G, Del Prete G, de Bernard M, D’Elios MM. The effect of Helicobacter pylori on asthma and allergy. J Asthma Allergy. 2010;3:139–47. doi: 10.2147/JAA.S8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minocha A, Raczkowski CA, Richards RJ. Is a history of tonsillectomy associated with a decreased risk of Helicobacter pylori infection? J Clin Gastroenterol. 1997;25:580–2. doi: 10.1097/00004836-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Uygur-Bayramiçli O, Kiliç D, Yavuzer D, Telatar B, Kavakli B. Helicobacter pylori colonization and immunological disease. Eur J Gastroenterol Hepatol. 2001;13:301–2. doi: 10.1097/00042737-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Brook I, Shah K. Bacteriology of adenoids and tonsils in children with recurrent adenotonsillitis. Ann Otol Rhinol Laryngol. 2001;110:844–8. doi: 10.1177/000348940111000908. [DOI] [PubMed] [Google Scholar]
- 18.Skinner LJ, Winter DC, Curran AJ, et al. Helicobacter pylori and tonsillectomy. Clin Otolaryngol. 2001;26:505–9. doi: 10.1046/j.1365-2273.2001.00513.x. [DOI] [PubMed] [Google Scholar]
- 19.Cirak MY, Ozdek A, Yilmaz D, Bayiz U, Samim E, Turet S. Detection of Helicobacter pylori and its CagA gene in tonsil and adenoid tissues by PCR. Arch Otolaryngol Head Neck Surg. 2003;129:1225–29. doi: 10.1001/archotol.129.11.1225. [DOI] [PubMed] [Google Scholar]
- 20.Zhang JP, Peng ZH, Zhang J, Zhang XH, Zheng QY. Helicobacter pylori infection in the pharynx of patients with chronic pharyngitis detected with TDI-FP and modified Giemsa stain. World J Gastroenterol. 2006;12:468–72. doi: 10.3748/wjg.v12.i3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kusano K, Inokuchi A, Fujimoto K, et al. Coccoid Helicobacter pylori exists in the palatine tonsils of patients with IgA nephropathy. J Gastroenterol. 2010;45:406–12. doi: 10.1007/s00535-009-0169-9. [DOI] [PubMed] [Google Scholar]
- 22.Lukeš P, Pavlik E, Potužnikova B, et al. Comparison of Helicobacter pylori genotypes obtained from the oropharynx and stomach of the same individuals - a pilot study. Prague Med Rep. 2012;113:231–9. doi: 10.14712/23362936.2015.21. [DOI] [PubMed] [Google Scholar]
- 23.Jelavic B, Bevanda M, Ostojic M, Leventic M, Vasilj M, Knezevic E. Tonsillar colonization is unlikely to play important role in Helicobacter pylori infection in children. Int J Pediatr Otorhinolaryngol. 2007;71:585–90. doi: 10.1016/j.ijporl.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Siupsinskiene N, Katutiene I, Jonikiene V, Janciauskas D, Vaitkus S. Helicobacter pylori in the tonsillar tissue: a possible association with chronic tonsillitis and laryngopharyngeal reflux. J Laryngol Otol. 2017;131:549–56. doi: 10.1017/S0022215117000597. [DOI] [PubMed] [Google Scholar]
- 25.Yilmaz T, Ceylan M, Akyön Y, Özçakýr O, Gürsel B. Helicobacter pylori: a possible association with otitis media with effusion. Otolaryngol Head Neck Surg. 2006;134:772–7. doi: 10.1016/j.otohns.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Bitar MA, Soweid A, Mahfouz R, Zaatari G, Fuleihan N. Is Helicobacter pylori really present in the adenoids of children? Eur Arch Otorhinolaryngol. 2005;262:987–92. doi: 10.1007/s00405-005-0926-1. [DOI] [PubMed] [Google Scholar]
- 27.Vilarinho S, Guimarães NM, Ferreira RM, et al. Helicobacter pylori colonization of the adenotonsillar tissue: fact or fiction? Int J Pediatr Otorhinolaryngol. 2010;74:807–11. doi: 10.1016/j.ijporl.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Pitkäranta A, Kolho KL, Rautelin H. Helicobacter pylori in children who are prone to upper respiratory tract infections. Arch Otolaryngol Head Neck Surg. 2005;131:256–8. doi: 10.1001/archotol.131.3.256. [DOI] [PubMed] [Google Scholar]
- 29.Ercan I, Çakır BO, Uzel TS, Sakız D, Karaca C, Turgut S. The role of gastric Helicobacter pylori infection in laryngopharyngeal reflux disease. Otolaryngol Head Neck Surg. 2006;135:52–5. doi: 10.1016/j.otohns.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 30.Oridate N, Takeda H, Yamamoto J, et al. Helicobacter pylori seropositivity predicts outcomes of acid suppression therapy for laryngopharyngeal reflux symptoms. Laryngoscope. 2006;116:547–53. doi: 10.1097/01.MLG.0000201907.24514.6A. [DOI] [PubMed] [Google Scholar]
- 31.Çekin E, Ozyurt M, Erkul E, et al. The association between Helicobacter pylori and laryngopharyngeal reflux in laryngeal pathologies. Ear Nose Throat J. 2012;91:E6–9. doi: 10.1177/014556131209100314. [DOI] [PubMed] [Google Scholar]
- 32.International Agency for Research on Cancer Working Group. Shistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the evaluation of carcinogenic risk to humans. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–124. [PMC free article] [PubMed] [Google Scholar]
- 33.Sadler TW. Langman’s Medical Embriology. 12th ed. Philadelphia (PA): Lippincot; 2012. [Google Scholar]
- 34.Rezaii J, Tavakoli H, Esfandiari K, et al. Association between Helicobacter pylori infection and laryngohypopharyngeal carcinoma: a case-control study and review of the literature. Head Neck. 2008;30:1624–7. doi: 10.1002/hed.20918. [DOI] [PubMed] [Google Scholar]
- 35.Nurgalieva ZZ, Graham DY, Dahlstrom KR, Wei Q, Sturgis EM. A pilot study of Helicobacter pylori infection and risk of laryngopharyngeal cancer. Head Neck. 2005;27:22–7. doi: 10.1002/hed.20108. [DOI] [PubMed] [Google Scholar]
- 36.Titiz A, Ozcakir O, Ceyhan S, Yilmaz YF, Unal A, Akyon Y. The presence of Helicobacter pylori in the larynx pathologies. Auris Nasus Larynx. 2008;35:534–8. doi: 10.1016/j.anl.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Barakat G, Nabiel Y, Ali O, El-Nady G, Musaad A, El-Sharkawy A. UreA and cagA genes of Helicobacter pylori in Egyptian patients with laryngeal squamous cell carcinoma and benign laryngeal polyps: a cohort study. Eur Arch Otorhinolaryngol. 2016;273:3243–8. doi: 10.1007/s00405-016-4114-2. [DOI] [PubMed] [Google Scholar]
- 38.Amizadeh M, Shamsadini A, Arabzadeh A, Jazayeri S. Association of cagA positive Helicobacter pylori infection and laryngeal squamous cell carcinoma: a PCR approach. Indian J Otolaryngol Head Neck Surg. 2015;67:51–5. doi: 10.1007/s12070-014-0750-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yilmaz I, Erkul E, Berber U, et al. The presence of Helicobacter pylori in laryngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2016;273:761–5. doi: 10.1007/s00405-015-3566-0. [DOI] [PubMed] [Google Scholar]
- 40.Tasker A, Dettmar PW, Panetti M, Koufman JA, Birchall J, Pearson JP. Is gastric reflux a cause of otitis media with effusion in children? Laryngoscope. 2002;112:1930–4. doi: 10.1097/00005537-200211000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Morinaka S, Tominaga M, Nakamura H. Detection of Helicobacter pylori in the middle ear fluid of patients with otitis media with effusion. Otolaryngol Head Neck Surg. 2005;133:791–4. doi: 10.1016/j.otohns.2005.05.050. [DOI] [PubMed] [Google Scholar]
- 42.Fancy T, Mathers PH, Ramadan HH. Otitis media with effusion: a possible role for Helicobacter pylori? Otolaryngol Head Neck Surg. 2009;140:256–8. doi: 10.1016/j.otohns.2008.11.023>. [DOI] [PubMed] [Google Scholar]
- 43.Bitar M, Mahfouz R, Soweid A, et al. Does Helicobacter pylori colonize the nasopharynx of children and contribute to their middle ear disease? Acta Otolaryngol. 2006;126:154–9. doi: 10.1080/00016480500312679. [DOI] [PubMed] [Google Scholar]
- 44.Aycicek A, Çetinkaya Z, Kıyıcı H, Bukulmez A, Yucedag F. Can Helicobacter pylori cause inflammation in the middle ear? Int J Pediatr Otorhinolaryngol. 2012;76:1087–90. doi: 10.1016/j.ijporl.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 45.Saki N, Jahani M, Samarbaf A, et al. Correlation between tympanosclerosis and Helicobacter pylori. Jundishapur J Microbiol. 2015;8:e16069. doi: 10.5812/jjm.16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dinç AE, Cömert F, Damar M, Şevik Eliçora S, Erdem D, Işık H. Role of Chlamydia pneumoniae and Helicobacteria pylori in the development of tympanosclerosis. Eur Arch Otorhinolaryngol. 2016;273:889–92. doi: 10.1007/s00405-015-3645-2. [DOI] [PubMed] [Google Scholar]