Abstract
Background
Simple methods for the accurate triaging and screening of HIV-associated tuberculosis (TB) are urgently needed. We hypothesized that combining serum antibody with urine lipoarabinomannan (U-LAM) detection can improve the detection of HIV-associated TB.
Methods
We performed a case-control study with sampling from a prospective study of South African HIV-infected subjects who were screened for TB prior to initiating antiretroviral therapy. Sera from all available TB cases (n = 74) and randomly selected non-TB controls (n = 30), all tested for U-LAM, sputum microscopy, GeneXpert, and cultures, were evaluated for antibodies to LAM and arabinomannan (AM). Diagnostic logistic regression models for TB were developed based on the primary test results and the additive effect of antibodies with leave-one-out cross-validation.
Results
Antibody responses to LAM and AM correlated strongly (p<0.0001), and IgG and IgM reactivities were significantly higher in TB than non-TB patients (p<0.0001). At 80% specificity, the target specificity for a non-sputum-based simple triage/screening test determined by major TB stakeholders, combining U-LAM with IgG detection significantly increased the sensitivity for HIV-associated TB to 92% compared to 30% for U-LAM alone (p<0.001). Sputum microscopy combined with IgG detection increased sensitivity to 88% compared to 31% for microscopy alone, and Xpert with IgG increased sensitivity to 96% and 99% compared to 57% for testing one, and 70% for testing two sputa with Xpert alone, respectively.
Conclusion
Combining U-LAM with serum antibody detection could provide a simple low-cost method that meets the requirements for a non-sputum-based test for the screening of HIV-associated TB.
Introduction
Active tuberculosis (TB) is the leading cause of both death from a single pathogen and death in HIV co-infected individuals [1]. Of the estimated 10.4 million people who developed TB in 2016, 10% were HIV co-infected; and of the close to 1.7 million who died of the disease that year, nearly 0.4 million had HIV-associated TB [1]. Difficulty in the rapid identification of HIV-associated TB often delays treatment and negatively affects patient outcomes, particularly in resource poor settings [2]. Evidence for this is supported by post-mortem data showing that 40% of HIV-related deaths in resource-limited settings are due to TB—almost half of which were undiagnosed at the time of death [3]. The current gold standards for TB diagnosis are mycobacterial sputum cultures and nucleic acid amplification tests (NAATs) such as the GeneXpert® (commonly referred to as "Xpert"; Cepheid Inc., Sunnyvale, California) [4]. However, cultures take weeks to become positive, and both cultures and NAATs can have limited sensitivity in HIV-infected individuals who often have disseminated or extrapulmonary manifestation [2, 5–7]. Although the ability to obtain rapid results with NAATs is a major benefit, these tests require technology not available in many resource poor settings or at the community healthcare level [8]. Other methods currently used include sputum microscopy, which, although rapid and low-cost, has a limited sensitivity of typically below 50% in HIV-infected individuals [2, 5, 8, 9]. Thus, establishing a diagnosis of HIV-associated TB can be challenging and is further complicated by the myriad of other HIV-associated opportunistic diseases that can present with similar signs and symptoms. Furthermore, up to over 30% of HIV-infected individuals living in high TB incidence settings have undiagnosed TB when screened prior to initiation of antiretroviral therapy (ART) [9]. Therefore, as recently emphasized at a major TB stakeholder meeting, both rapid, low-cost screening as well as triage tests for HIV-associated TB are urgently needed [8]. Ideally, such tests should be non-sputum-based, device-free, and usable at the community health care level.
Additional diagnostic tests for HIV-associated TB are based on the detection of the mycobacterial cell wall glycolipid lipoarabinomannan (LAM) in the patients’ urine, as reviewed by Shah et al. [10]. These urinary LAM (U-LAM) tests are available in form of an enzyme-linked immunosorbent assay (ELISA; Clearview TB-ELISA, Alere, Waltham, MA, USA) or a simple lateral flow (dipstick) format (Determine TB-LAM, Alere). It has long been known that LAM can be isolated from M. tuberculosis cultures [11, 12]. Therefore, its detection in the urine could be either due to the presence of mycobacteria in the urinary tract, or, because of its small size of approximately 19 kDa, due to glomerular filtration of non-antibody bound LAM from the blood into the urine of TB patients with high mycobacterial burden [13]. It is thus not surprising that U-LAM detection has been predominantly associated with both renal as well as disseminated TB, but less with locally confined pulmonary disease [13–15]. These test characteristics are clinically relevant because extrapulmonary/disseminated TB occurs more frequently in late stage HIV patients with low CD4 counts and is particularly challenging to diagnose [8]. Pooled data from several studies show an overall test sensitivity and specificity of 45–47% and 92–96%, respectively, which change to 56% and 90%, respectively, in patients with CD4 counts <100 cells/ul [10]. Thus, the use of the lateral flow format has been endorsed by the World Health Organization (WHO) for HIV-infected patients with suspected TB and CD4 counts under 100 cells/μl [16]. Given that HIV-infected individuals frequently develop TB at CD4 counts above 100 cells/ul, the clinical value of the U-LAM test is limited if used alone [10]. The combination of the U-LAM test with other diagnostics, such as sputum smear microscopy and Xpert, can significantly increase the detection of HIV-associated TB and reduce mortality in HIV-infected TB suspects with low CD4 counts [15, 17]. However, due to the required equipment, these combinations are of limited value in many remote and low resource settings and at the level of community healthcare workers, leaving a need for simple, rapid, and non-sputum-based triage as well as screening tests for HIV-associated TB [2, 8].
Immunological methods such as TB serodiagnostic assays are based on detecting antibodies (Abs) to mycobacterial antigens, as reviewed by Steingart et al. [18]. Similar to U-LAM-based tests, the benefits of serologic assays are their independence of sputum analysis, potential for detection of all forms of TB, and suitability for rapid, simple, low-cost format development (e.g. dipstick test). Thus far, commercially available TB serodiagnostic assays have shown limited and variable sensitivity and specificity (0 to 100% and 31 to 100%, respectively), largely dependent on study population, settings, antigens, and diagnostic cut-off values used [18]. We and others have previously shown that TB patients have significantly higher Abs to LAM and/or its polysaccharide component arabinomannan (AM) than controls [19–24]. While most groups focused on HIV uninfected patients, we found that IgG responses to AM and LAM were significantly higher in both HIV uninfected and HIV-infected patients with TB compared to respective controls [19]. Although HIV co-infected patients had lower Ab titers than HIV uninfected TB patients, the detection of such Abs could have adjunctive value in identifying HIV-associated TB. We hypothesized that combining U-LAM detection with that of serum Abs to LAM or its non-lipidated counterpart AM can significantly increase the sensitivity for HIV-associated TB compared to U-LAM detection alone. Our primary objective was the assessment of serum Abs to LAM/AM and its adjunctive value to U-LAM detection in HIV-infected subjects who were screened for TB [25]. As a secondary objective, we evaluated whether Ab detection has adjunctive value to other available point-of-care (POC) tests such as sputum smear microscopy and Xpert.
Materials and methods
Study design and subjects
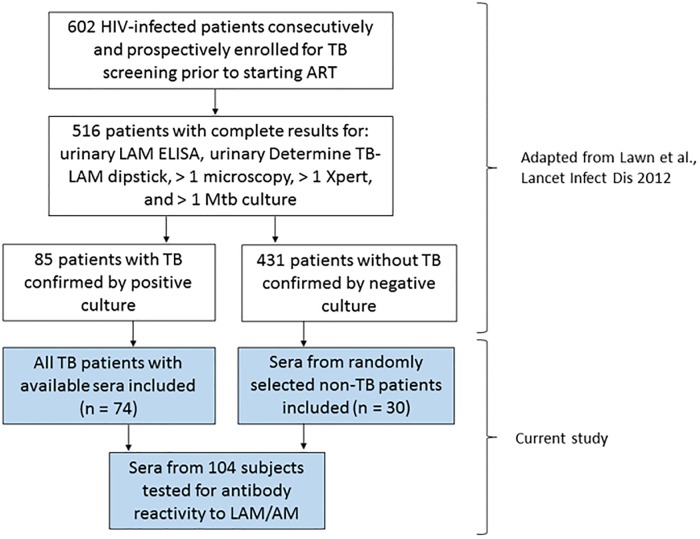
This was a case-control study in which sera were obtained from a study of HIV-infected patients who were previously prospectively and consecutively enrolled to assess the clinical utility of U-LAM detection for the detection of TB prior to initiation of antiretroviral treatment (ART; [25]). The parent study was in compliance with the standards for reporting of diagnostic accuracy (STARD; [26]) and enrolled 602 ART naive adult subjects, at least 18 years old and without a concurrent TB diagnosis, from March 2010 to April 2011 from the ART service in Gugulethu township, Cape Town, South Africa, a setting with a high TB incidence. Recruitment criteria, collection of urine and serum samples, and diagnostic tests were performed as described, and complete test results were available for 516 subjects [25]. In the parent study, 85 sputum culture-positive TB patients had results available for all diagnostic tests. Of these, sera were available for 74 subjects and all were included in our study (Fig 1). In the parent study, based on negative work-up, TB was ruled out in over 431 subjects. Because including of all of these non-TB subjects was not feasible for our study, 30 subjects without a history of TB were randomly chosen to be included as controls. This number was determined to be sufficient because in our prior US-based pilot study with biologically independent samples Ab response to AM were significantly higher in 14 HIV-infected TB patients compared to 38 HIV-infected controls (p < 0.01) [19]. Based on these results our power to detect differences in Ab reactivity with 74 TB+ and 30 non-TB subjects at a significance level < 0.05 exceeded 0.8. Sera were evaluated for Ab responses to LAM and AM to assess the additive diagnostic value of Ab to U-LAM detection and other POC tests as performed in the parent study. Approval for research on human subjects was obtained from the research ethics committees of the University of Cape Town, South Africa, the London School of Hygiene & Tropical Medicine, UK, and the institutional review board of the Albert Einstein College of Medicine, NY, USA. Written informed consent was obtained from all subjects prior to enrollment.
Fig 1. Study flow diagram for parent [25] and current study.
ART: antiretroviral therapy.
Mycobacterial antigen preparations
We used LAM and AM, components of the mycobacterial cell wall and capsule, respectively [27]. LAM isolated from the M. tuberculosis strain H37Rv was obtained from the Biodefense and Emerging Infections Research Resources Repository (BEI; Manassas, VA). Capsular AM was isolated and purified from M. tuberculosis strain H37Rv as described [19, 27]. Although Ab responses to AM and LAM correlated strongly and significantly among TB cases with or without HIV in our prior studies [19, 27], IgG responses to AM, in contrast to LAM, were significantly different between smear-positive versus smear-negative TB patients, suggesting that AM could be a more sensitive marker for mycobacterial burden [19]. For this reason, and also because i) LAM from BEI was only available in small quantities, and ii) the isolation of AM in larger quantities is less time-consuming than LAM, we planned to use AM in lieu of LAM for the assays after confirming the strong and significant correlation with 20 randomly chosen samples from subjects of this study.
U-LAM detection, microscopy and Xpert tests
The presence of LAM in the urine samples was determined in the parent study and quantified using the Determine TB-LAM dipstick test and the commercially available Clearview TB enzyme-linked immunosorbent assay (ELISA) as per manufacturer’s instructions [25]. Sputum samples were processed and evaluated for acid-fast bacilli by fluorescent microscopy and M. tuberculosis by Xpert according to standard procedures and protocols [25].
Ab detection assays
ELISAs were performed as described [19, 27]. Briefly, 96-well plates (Maxisorp nunc, Thermo Fisher Scientific) were coated with AM and initially also LAM (for comparisons between AM and LAM) at 10 μg/ml and blocked overnight with 3% BSA in 0.1% PBS-T. Serum samples, diluted 1:50, were added in duplicates to the antigen-coated wells, and the bound Abs detected with either alkaline phosphatase (AP)-conjugated protein A for the detection of IgG, goat anti-human IgM-AP or goat anti-human IgA-AP (1:1000; Sigma, St Louis, MO). Optical densities (OD) were measured at 405 nm. Negative and positive controls were processed in duplicates as described above, except that serum was replaced, respectively, with diluted blocking buffer for negative controls or the murine monoclonal Ab CS35 (1:200) known to recognize AM and LAM from various mycobacterial strains as a positive control, followed by detection with anti-mouse IgG3-AP (1:1000; Sigma). To assure reproducibility of data, each assay was repeated on two separate days. Although the person performing the assays was not blinded to the TB diagnosis of the subjects, she was at the time of performing the assays blinded to the diagnostic test results associated with each sample.
Statistical analysis
Statistical analysis was performed using GraphPad Prism, version 7 (GraphPad Inc., San Diego, CA) and R, version 3.3.2 (R Foundation, Vienna, Austria). Demographics, clinical characteristics and diagnostic test results were compared between patient groups using Fisher’s exact test for categorical variables and the Mann-Whitney test for continuous variables. Ab responses were compared between groups by Mann-Whitney and correlated within groups by Spearman rank correlation tests. Data analysis focused on U-LAM detection results with the POC dipstick format. Diagnostic models for TB+ were developed and their accuracy measured with M. tuberculosis positive culture as the gold standard. We first diagnosed patients to be TB+ if the primary test (e.g. U-LAM) result was positive. Then, for patients whose primary test results were negative, we built logistic regression models to diagnose TB status using Abs (IgG, IgM and IgA to AM/LAM) as covariates. In the Ab only models, we built a logistic regression model to diagnose all patients regardless of other diagnostic test results. The target product profile for a non-sputum-based simple low-cost triage test for TB was set at a specificity between 70 to 80% at a recent major TB stakeholders meeting, including the World Health Organization (WHO) and the Centers for Disease Control (CDC) [8]. We therefore evaluated sensitivity at 80% specificity. For each diagnostic model we considered clinically relevant (sensitivity >85% at a specificity of 80%), and because we considered multiple diagnostic models, we performed a final two-loop leave-one-out cross-validation to avoid overfitting of estimated receiver operating characteristic (ROC) curves [28, 29]. On each n-1 subject, we performed inner-loop cross-validation (with n-2 subjects) to estimate sensitivity at 80% specificity, and repeated the selection process n times to calculate the average mean sensitivity. While this method does not replace the validation on an independent sample set, it can provide an unbiased estimate of accuracy measures under mild assumptions. To compare the sensitivity of the diagnostic model, e.g. combining U-LAM with IgG detection and that with U-LAM alone, we used generalized linear mixed effects model taking into account the paired diagnoses for each sample.
Results
Demographics and clinical variables
No significant differences between TB patients and controls were observed for demographics as well as CD4 counts. Although many non-TB controls were symptomatic and the groups were not significantly different in the occurrence of fever, TB patients had a significantly more other TB-associated symptoms and other clinical characteristics such as cough, shortness of breath, sweats, weight loss and chest X-ray abnormalities (Table 1). Of the TB+ subjects, 22 (30%) were U-LAM+ by dipstick and 21 by ELISA as determined in the parent study [25]. U-LAM+ compared to U-LAM- TB+ subjects (as per U-LAM dipstick) were slightly but significantly younger and had significantly lower CD4 counts; otherwise the two groups were similar in demographics and clinical presentation (Table 2).
Table 1. Demographics and clinical characteristics of HIV+ subjects stratified by TB status.
| Characteristics | TB Patients (n = 74) |
Non-TB Patients (n = 30) |
P* |
|---|---|---|---|
| Male sex (%) | 27 (36) | 11 (37) | 1 |
| Age, median years [25%ile, 75%ile] |
33 [28, 38] |
31 [27, 36] |
0.19 |
| Current smoker (%) | 15 (20) | 6 (20) | 1 |
| Previous TB (%) | 14 (19) | 0 | 0.009 |
| CD4, median cells/mm3 [25%ile, 75%ile] |
138 [60, 204] |
135 [80, 201] |
0.94 |
| Abnormal CXRa (%) | 53 (75) | 9 (32) | 0.0002 |
| Initial sputum AFB smear positive (%) | 19 (26) | 0 | 0.001 |
| Two sputa combined AFBb smear positive (%) | 23 (31) | 0 | 0.0002 |
| Initial sputum GeneXpert positive (%) | 42 (57) | 0 | <0.0001 |
| Two sputa combined GeneXpert positive (%) | 52 (70) | 0 | <0.0001 |
| U-LAM dipstick+ (%) | 22 (30) | 0 | 0.0003 |
| U-LAM ELISA+ (%) | 21 (28) | 0 | 0.0004 |
| TB home contact | 1 (1) | 3 (10) | 0.07 |
| Symptoms present at evaluation: | |||
| Cough | 48 (65) | 10 (33) | 0.005 |
| Hemoptysis | 2 (3) | 1 (3) | 1 |
| Shortness of breath | 32 (43) | 3 (10) | 0.001 |
| Night sweats | 40 (54) | 9 (30) | 0.03 |
| Fever | 22 (30) | 7 (23) | 0.63 |
| Weight loss | 62 (84) | 18 (60) | 0.019 |
*P-values for categorical variables are given using the Fisher’s exact test and for continuous variables using the Mann-Whitney U test;
a: CXR: chest X-ray;
b: AFB: acid fast bacilli
Table 2. Demographics and clinical characteristics of HIV+ TB patients stratified by U-LAM dipstick result.
| Characteristics | U-LAM+ TB Patients (n = 22) |
U-LAM- TB Patients (n = 52) |
P* |
|---|---|---|---|
| Male sex (%) | 5 (23) | 22 (42) | 0.124 |
| Age, median years [25%ile, 75%ile] |
30 [26, 34] |
34 [29, 41] |
0.016 |
| Current smoker (%) | 2 (9) | 13 (25) | 0.205 |
| Previous TB (%) | 4 (18) | 10 (19) | 1 |
| CD4, median cells/mm3 [25%ile, 75%ile] |
36 [22, 131] |
176 [105, 216] |
0.0001 |
| Abnormal CXRa (%) | 15 (71) | 38 (76) | 0.768 |
| Initial sputum AFBb smear positive (%) | 11 (50) | 8 (15) | 0.003 |
| Two sputa combined AFB smear positive (%) | 11 (50) | 12 (23) | 0.030 |
| Initial sputum GeneXpert positive (%) | 19 (86) | 23 (44) | 0.0008 |
| Two sputa combined GeneXpert positive (%) | 20 (91) | 32 (62) | 0.013 |
*P-values for categorical variables are given using the Fisher’s exact test and for continuous variables using the Mann-Whitney U test;
a: CXR: chest X-ray;
b: AFB: acid fast bacilli
Correlation between Ab responses to LAM and AM
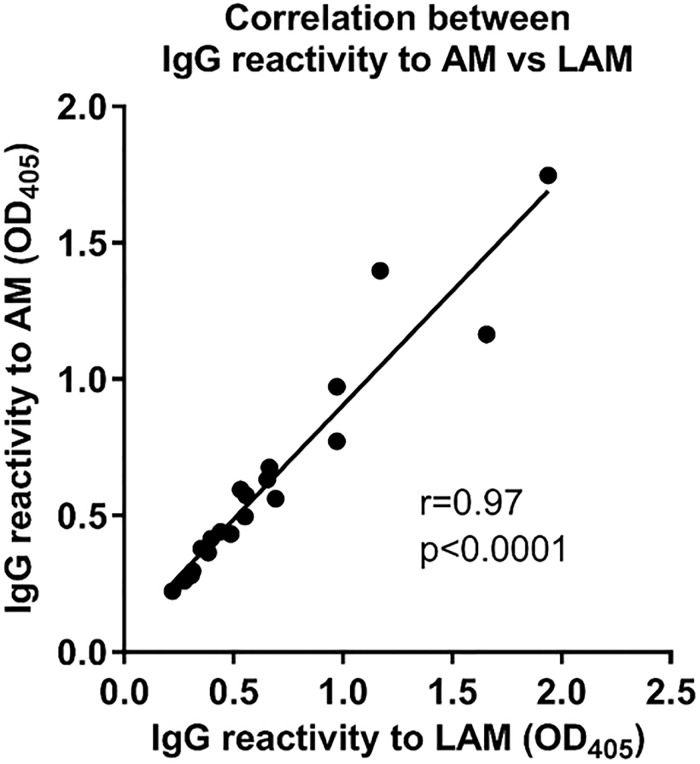
Consistent with our prior data [19, 27], IgG responses to LAM correlated strongly and significantly with those to AM (r = 0.97, p<0.0001; Fig 2). Thus, for practical reasons, we evaluated Ab responses using AM.
Fig 2. Correlation between IgG response to M. tuberculosis capsular arabinomannan (AM) and cell wall lipoarabinomannan (LAM) in HIV-infected TB patients.
Spearman rank correlation.
Ab responses to AM by TB and U-LAM status
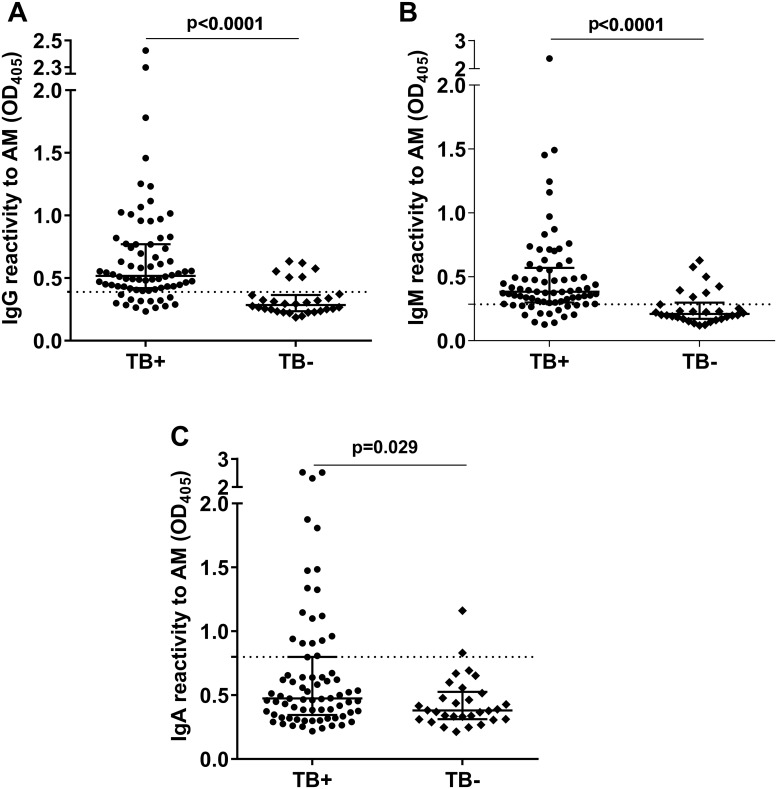
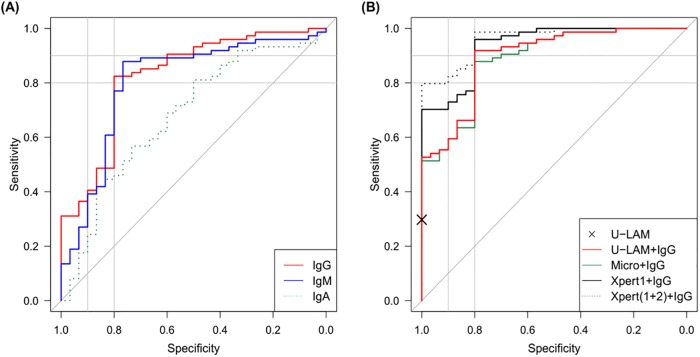
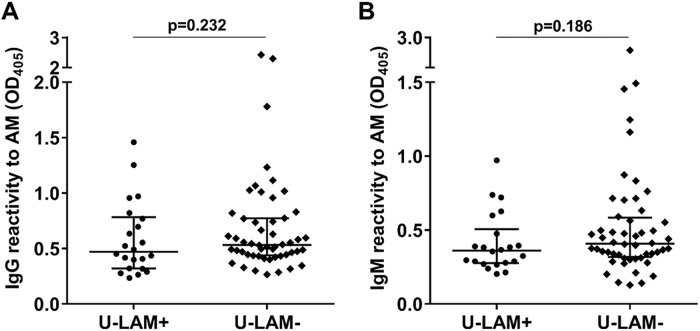
IgG, IgM, and IgA responses to AM were significantly higher in TB+ than non-TB subjects, with IgA discriminating the groups less (p = 0.029) than IgG or IgM (p<0.0001; Fig 3). Because our primary objective was the assessment of non-sputum-based methods for triage testing for HIV-associated TB, we set our target specificity at 80%, a number identified to be optimal for such a test [8]. At this specificity, the sensitivity for serum IgG to AM was 82%, for IgM 77%, and for IgA to AM 46% (Figs 3 and 4A). Combining the detection of Ab isotypes to AM did not increase sensitivity beyond that of IgG alone when adjusting cut-off values to maintain a specificity of 80%. Importantly, there was no significant correlation between CD4 counts and IgG (r = 0.06, p = 0.59), IgM (r = 0.06, p = 0.62), or IgA titers to AM (r = -0.05, p = 0.68), demonstrating that Ab detection was not influenced by the level of immune suppression. Similar to proportions reported for the larger parent study [25], the sensitivities of the U-LAM dipstick and ELISA for TB were 30% and 28%, respectively, with a specificity of 100% for both (Table 1). A non-significant trend towards higher Ab responses to AM was observed for IgG and IgM but not IgA in the U-LAM- compared to the U-LAM+ TB+ subjects (Fig 5), supporting the adjunctive value of IgG when combined with U-LAM detection.
Fig 3. Comparison of antibody responses to AM between TB+ and TB- subjects.
(A) IgG to AM; (B) IgM to AM; (C) IgA to AM. Lines and error bars represent medians with interquartile ranges. Mann-Whitney U test was used for statistical comparisons. Dotted lines represent Ab level cut-off determined by receiver operating curve (ROC) at specificity of 80% showing a sensitivity for TB of 82%, 77%, and 46% for IgG, IgM, and IgA, respectively.
Fig 4. Receiver operation characteristic (ROC) curves for detection of TB with M. tuberculosis culture as reference gold standard.
(A) IgG, IgM, and IgA to AM; (B) IgG to AM combined with other rapid diagnostic tests. ROC curves include cross-validation.
Fig 5. Comparison of IgG response to AM between U-LAM+ and U-LAM- TB+ subjects.
(A) IgG to AM; (B) IgM to AM. Lines and error bars represent medians with interquartile ranges. Mann-Whitney U test was used for statistical comparisons.
Diagnosis of TB by combining Ab with U-LAM detection and other POC tests
Next, we investigated the adjunctive value of Abs in detecting HIV-associated TB at a specificity of 80%. For patients whose primary test results (e.g. U-LAM dipstick) were negative, we built a logistic regression model using up to all three Ab isotypes to AM (always including IgG) as covariates. In this model, which included cross-validation, combining U-LAM with IgG detection significantly increased the sensitivity for HIV-associated TB to 92% compared to 30% for U-LAM alone (p<0.001; Table 3 & Figs 4 and 6). While sputum microscopy (two sputa) combined with IgG detection increased sensitivity to 88% compared to 31% for microscopy alone, combining Xpert with IgG increased sensitivity to 96% and 99% compared to 57% for testing one, or 70% for testing two sputum samples with Xpert alone, respectively (Table 3 & Figs 4 and 6). Combining two or more diagnostic methods with IgG detection did not lead to further meaningful increases in sensitivity.
Table 3. Performances of diagnostic models for HIV-associated TB.
| Diagnostic model | SS at SP = .8 |
|---|---|
| U-LAMa+ or IgGb+ | 0.92 |
| Micro(1+2)+ or IgGb+ | 0.88 |
| Xpert(1)+ or IgGb+ | 0.96 |
| Xpert(1+2)+ or IgGb+ | 0.99 |
| U-LAMa+ or Micro(1+2)+ or IgGb+ | 0.95 |
| U-LAMa+ or Xpert(1)+ or IgGb+ | 0.96 |
| U-LAMa+ or Xpert(1+2)+ or IgGb+ | 0.99 |
SS: Sensitivity; SP: Specificity;
a: urinary lipoarabinomannan dipstick test (Determine TB-LAM, Alere);
b: IgG to AM at cut-off OD > 0.39
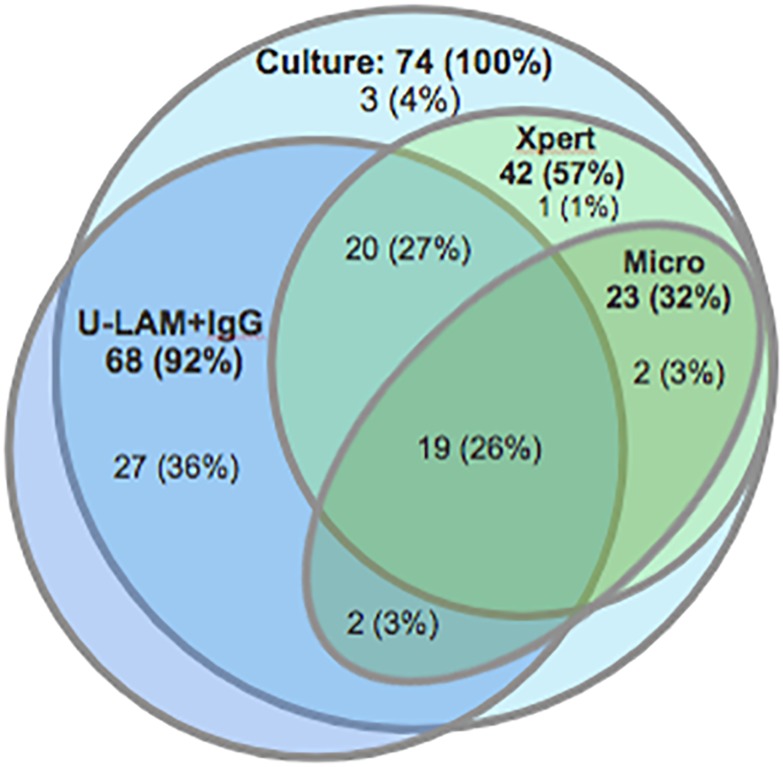
Fig 6. Venn diagram showing proportions of TB patients diagnosed by different test modalities relative to those diagnosed by mycobacterial culture (n = 74 (100%)).
Numbers in bold fonts represent patients testing positive by the respective test modalities while those in regular fonts show numbers and proportions of patients exclusively diagnosed by this/these compartment(s). Culture = M. tuberculosis culture positive; Xpert = initial sputum GeneXpert positive; Micro = two sputum smears for acid fast bacilli positive; and U-LAM+IgG = urinary LAM detection by urine dipstick test and/or IgG to AM positive. Of note, the dark blue fraction of the U-LAM+IgG circle extending outside the M. tuberculosis culture positive circle represents anti-AM IgG positive, culture negative subjects.
Discussion
A simple non-sputum-based screening POC test for HIV-associated TB, suitable for use in resource-limited settings, is a high priority diagnostic need [8]. Ideally such a test would be usable for both screening for TB in HIV clinics and triaging of symptomatic HIV-infected patients in community healthcare centers to identify those individuals that should undergo further confirmatory testing for HIV-associated TB. We here demonstrate that combining the detection of U-LAM with that of serum Abs to LAM or AM could provide such a method. With 92% sensitivity at a specificity of 80% for HIV-associated TB this combination, if validated in other studies, holds promise that it could meet the desired target product profile for a simple triage/screening test determined by major TB stakeholders including the WHO and CDC [8]. Since Abs can be detected with simple lateral flow (dipstick) formats, similar to the already available U-LAM POC test, a combination of these tests would be device-free and suitable for use of healthcare workers in settings without laboratory infrastructure. It could thus fill a gap in the urgent needs for additional TB diagnostics.
Improving the detection of TB is important in both symptomatic and asymptomatic HIV-infected individuals. TB preventative therapy is recommended by the WHO for all HIV-infected individuals living in TB endemic regions [30]. Ruling out TB prior to initiation of preventative therapy is critical because single or dual drug therapy could lead to drug-resistance when given to patients with disease. Furthermore, subclinical TB, an asymptomatic form of disease in HIV co-infected patients, occurs in up to over 10% of TB cases detected during screening prior to ART and can be associated with high mortality rates if left untreated for even a few months [31–36]. Because it is typically sputum microscopy and Xpert negative, its detection would require non-sputum-based tests. Given that our study population consisted of subjects being screened for TB prior to initiation of ART, our data hold promise that over 90% of HIV-associated TB cases could be identified by combining U-LAM with anti-AM/LAM IgG detection.
A simple triage or screening test with high sensitivity and moderate specificity would allow to focus on only the test positive subjects for confirmatory testing. In that respect, C-reactive protein (CRP), a non-specific inflammatory protein with POC potential for TB, has shown a high sensitivity of up to 98% albeit at a lower specificity of below 60% in the triaging for TB in symptomatic [37, 38], and a sensitivity of 89% with a specificity of 72%, respectively, in the screening of asymptomatic HIV-infected individuals [39]. Although detection of Abs to M. tuberculosis bear the risk of reduced specificity due to cross-reactivity with antigens of non-tuberculous mycobacteria, they have the potential to be more specific than inflammatory biomarkers. Consistent with this notion, sensitivities assessed at the 80% specificity level in our study demonstrate a more favorable profile than that of CRP. Due to limited available serum volumes, we focused on investigating Ab responses to LAM/AM in combination with U-LAM detection. It is conceivable that by either adding other easily and rapidly detectable and accurate serum biomarkers, some of which have been recently reported by us and others [40–46], and/or by enhancing the sensitivity of the current U-LAM detection via approaches recently reported [47–49], the sensitivity of combined methods for HIV-associated TB could be enhanced to over 95%.
The significant difference between anti-AM IgG reactivity in HIV-infected TB versus non-TB patients is in accordance with results from our previous studies with TB and non-TB patients living in the US [19]. This is an important observation because the US represents a region of low TB incidence while South Africa has one of the highest TB incidence rates globally [1]. The validation of our US with the South African data shown here supports the notion that despite the known limitations of Ab detection for TB diagnosis [18], it could, when used in combination with other simple tests, be a useful adjunctive biomarker for the screening or triaging of HIV-associated TB. Although less observed in this study, IgM is known for lower accuracy than other isotypes in TB serology studies [18, 19]. The high titers of IgM to AM in many of the South African HIV-infected TB patients in our current study could indicate high rates of new M. tuberculosis infection rather than reactivation of remote infection. Such hypothesis warrants further exploration but is supported by both significantly increased IgM responses to AM in Ugandan TB household contacts who convert their tuberculin skin-test versus those who do not (unpublished data by our group) and the known high rates of TB due to new M. tuberculosis infection in HIV-infected adults living in TB endemic regions [50–52]. The strong correlation between Ab responses to LAM and its non-lipidated counterpart AM in this study is also consistent with our prior human data [19, 27]. It confirms that LAM and AM can be used interchangeably and irrespective of HIV infection status in serology studies. It further provides the option to test for reactivity to synthetically generated AM oligosaccharide fragments in future studies. These are now available and, compared to native polysaccharide fragments, would have the advantage of greater product consistency [27].
Another important result was the lack of correlation between IgG responses and CD4 counts, assuring that the adjunctive diagnostic value of Ab detection was not limited to a subset of HIV-infected individuals. Although we believe that combining U-LAM with Ab detection would be the most suitable method for triaging or screening in resource-limited settings, a combination of other diagnostic tests with Ab detection showed also enhanced sensitivities for TB. Sputum microscopy combined with IgG detection increased sensitivity to 88% compared to 31% for microscopy alone, and Xpert with IgG increased sensitivity to 96% and 99% compared to 57% for testing one, and 70% for testing two sputum samples with Xpert alone, respectively. Depending on the setting, these results, if validated in larger studies, could have important clinical implications.
Limitations of our study include a moderate sample size and the lack of validation in an independent sample set. We thus applied leave-one-out cross-validation to avoid overfitting of our diagnostic models [28, 29]. Furthermore, our study limited selection bias by using sera from prospectively and consecutively enrolled HIV-infected subjects of a study that followed the STARD guidelines [25]. Comparison against the gold standard for TB, automated liquid culture of sputum done in an accredited laboratory [25], further support the accuracy of our results. Nevertheless, larger studies, using the by us determined cut-off values from positive Ab reactivity, are needed to validate our results. Because our subjects were HIV-infected outpatients being screened for TB prior to ART initiation regardless of symptoms, assessing the triage value of our method would also require the inclusion of symptomatic HIV-infected individuals with other respiratory diseases.
In conclusion, the combination of U-LAM and serum Ab detection could provide a simple non-sputum-based screening, and possibly also triage method, to identify those HIV-infected individuals who should undergo further confirmatory testing for HIV-associated TB. Larger studies are needed to validate our results. Ideally, such studies should include the evaluation of both symptomatic and asymptomatic subjects as well as additional biomarker and/or next generation U-LAM detection tests to further enhance the sensitivity of such a combination method to over 95%.
Data Availability
All relevant data are within the manuscript.
Funding Statement
This work was supported in part by institutional funds from the Global Health Center of the Albert Einstein College of Medicine and from funds by the National Institute of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID; AI117927 and AI127173 to JMA), the Einstein-Rockefeller-CUNY Center for AIDS Research (P30-AI124414), and the Einstein Clinical and Translational Science Award (NIH/NCATS; 1ULTR002556). No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. Global Tuberculosis Report 2017. Geneva, Switzerland: World Health Organization, 2017. [Google Scholar]
- 2.Reid MJ, Shah NS. Approaches to tuberculosis screening and diagnosis in people with HIV in resource-limited settings. Lancet Infect Dis. 2009;9(3):173–84. 10.1016/S1473-3099(09)70043-X . [DOI] [PubMed] [Google Scholar]
- 3.Gupta RK, Lucas SB, Fielding KL, Lawn SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS. 2015;29(15):1987–2002. 10.1097/QAD.0000000000000802 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin Infect Dis. 2017;64(2):111–5. 10.1093/cid/ciw778 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawn SD, Kerkhoff AD, Vogt M, Wood R. Clinical significance of lipoarabinomannan (LAM) detection in urine using a low-cost point-of-care diagnostic assay for HIV-associated tuberculosis. AIDS. 2012. Epub 2012/05/05. 10.1097/QAD.0b013e3283553685 . [DOI] [PubMed] [Google Scholar]
- 6.Heidebrecht CL, Podewils LJ, Pym AS, Cohen T, Mthiyane T, Wilson D. Assessing the utility of Xpert((R)) MTB/RIF as a screening tool for patients admitted to medical wards in South Africa. Sci Rep. 2016;6:19391 10.1038/srep19391 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gati S, Chetty R, Wilson D, Achkar JM. Utilization and Clinical Value of Diagnostic Modalities for Tuberculosis in a High HIV Prevalence Setting. Am J Trop Med Hyg. 2018;99(2):317–22. Epub 2018/06/13. 10.4269/ajtmh.17-0965 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denkinger CM, Kik SV, Cirillo DM, Casenghi M, Shinnick T, Weyer K, et al. Defining the needs for next generation assays for tuberculosis. J Infect Dis. 2015;211 Suppl 2:S29–38. 10.1093/infdis/jiu821 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bassett IV, Wang B, Chetty S, Giddy J, Losina E, Mazibuko M, et al. Intensive tuberculosis screening for HIV-infected patients starting antiretroviral therapy in Durban, South Africa. Clin Infect Dis. 2010;51(7):823–9. Epub 2010/08/26. 10.1086/656282 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah M, Hanrahan C, Wang ZY, Dendukuri N, Lawn SD, Denkinger CM, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in HIV-positive adults. The Cochrane database of systematic reviews. 2016;(5):CD011420 10.1002/14651858.CD011420.pub2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunter SW, Gaylord H, Brennan PJ. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem. 1986;261(26):12345–51. . [PubMed] [Google Scholar]
- 12.Lemassu A, Daffe M. Structural features of the exocellular polysaccharides of Mycobacterium tuberculosis. Biochem J. 1994;297 (Pt 2):351–7. Epub 1994/01/15. 10.1042/bj2970351 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood R, Racow K, Bekker LG, Middelkoop K, Vogt M, Kreiswirth BN, et al. Lipoarabinomannan in urine during tuberculosis treatment: association with host and pathogen factors and mycobacteriuria. BMC Infect Dis. 2012;12:47 10.1186/1471-2334-12-47 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox JA, Lukande RL, Kalungi S, Van Marck E, Van de Vijver K, Kambugu A, et al. Is Urinary Lipoarabinomannan the Result of Renal Tuberculosis? Assessment of the Renal Histology in an Autopsy Cohort of Ugandan HIV-Infected Adults. PLoS One. 2015;10(4):e0123323 10.1371/journal.pone.0123323 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerkhoff AD, Barr DA, Schutz C, Burton R, Nicol MP, Lawn SD, et al. Disseminated tuberculosis among hospitalised HIV patients in South Africa: a common condition that can be rapidly diagnosed using urine-based assays. Sci Rep. 2017;7(1):10931 10.1038/s41598-017-09895-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. The use of lateral flow urine lipoarabinomannan assay (LF-LAM) for the diagnosis and screening of active tuberculosis in people living with HIV. Geneva, Switzerland: World Health Organization, 2015. [Google Scholar]
- 17.Gupta-Wright A, Corbett EL, van Oosterhout JJ, Wilson D, Grint D, Alufandika-Moyo M, et al. Rapid urine-based screening for tuberculosis in HIV-positive patients admitted to hospital in Africa (STAMP): a pragmatic, multicentre, parallel-group, double-blind, randomised controlled trial. Lancet. 2018. Epub 2018/07/24. 10.1016/S0140-6736(18)31267-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steingart KR, Flores LL, Dendukuri N, Schiller I, Laal S, Ramsay A, et al. Commercial serological tests for the diagnosis of active pulmonary and extrapulmonary tuberculosis: an updated systematic review and meta-analysis. PLoS Med. 2011;8(8):e1001062 Epub 2011/08/23. 10.1371/journal.pmed.1001062 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu X, Prados-Rosales R, Jenny-Avital ER, Sosa K, Casadevall A, Achkar JM. Comparative evaluation of profiles of antibodies to mycobacterial capsular polysaccharides in tuberculosis patients and controls stratified by HIV status. Clinical and vaccine immunology: CVI. 2012;19(2):198–208. Epub 2011/12/16. 10.1128/CVI.05550-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tessema TA, Bjune G, Hamasur B, Svenson S, Syre H, Bjorvatn B. Circulating antibodies to lipoarabinomannan in relation to sputum microscopy, clinical features and urinary anti-lipoarabinomannan detection in pulmonary tuberculosis. Scand J Infect Dis. 2002;34(2):97–103. Epub 2002/04/04. . [DOI] [PubMed] [Google Scholar]
- 21.Baumann R, Kaempfer S, Chegou NN, Oehlmann W, Loxton AG, Kaufmann SH, et al. Serologic diagnosis of tuberculosis by combining Ig classes against selected mycobacterial targets. J Infect. 2014;69(6):581–9. 10.1016/j.jinf.2014.05.014 . [DOI] [PubMed] [Google Scholar]
- 22.Deng S, Yuan T, Xia J, Huang H, Cheng X, Chen M. Clinical utility of a combination of lipoarabinomannan, 38-kDa, and 16-kDa antigens as a diagnosis tool for tuberculosis. Diagn Microbiol Infect Dis. 2011;71(1):46–50. 10.1016/j.diagmicrobio.2011.04.015 . [DOI] [PubMed] [Google Scholar]
- 23.Senoputra MA, Shiratori B, Hasibuan FM, Koesoemadinata RC, Apriani L, Ashino Y, et al. Diagnostic value of antibody responses to multiple antigens from Mycobacterium tuberculosis in active and latent tuberculosis. Diagn Microbiol Infect Dis. 2015;83(3):278–85. 10.1016/j.diagmicrobio.2015.07.021 . [DOI] [PubMed] [Google Scholar]
- 24.Chan ED, Reves R, Belisle JT, Brennan PJ, Hahn WE. Diagnosis of tuberculosis by a visually detectable immunoassay for lipoarabinomannan. Am J Respir Crit Care Med. 2000;161(5):1713–9. 10.1164/ajrccm.161.5.9908125 . [DOI] [PubMed] [Google Scholar]
- 25.Lawn SD, Kerkhoff AD, Vogt M, Wood R. Diagnostic accuracy of a low-cost, urine antigen, point-of-care screening assay for HIV-associated pulmonary tuberculosis before antiretroviral therapy: a descriptive study. Lancet Infect Dis. 2012;12(3):201–9. Epub 2011/10/22. 10.1016/S1473-3099(11)70251-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med. 2003;138(1):W1–12. . [DOI] [PubMed] [Google Scholar]
- 27.Chen T, Blanc C, Eder AZ, Prados-Rosales R, Souza AC, Kim RS, et al. Association of Human Antibodies to Arabinomannan With Enhanced Mycobacterial Opsonophagocytosis and Intracellular Growth Reduction. J Infect Dis. 2016;214(2):300–10. 10.1093/infdis/jiw141 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stone M. Cross-validatory choice and assessment of statistical predictions. J Royal Stat Soc. 1974;36(2):111–47. [Google Scholar]
- 29.Efron B. Estimating the error rate of a prediction rule: improvement on cross-validation. J Am Stat Assoc. 1983;78(382):316–31. [Google Scholar]
- 30.World Health Organization. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Geneva, Switzerland; http://www.who.int/tb/challenges/hiv/ICF_IPTguidelines/en/: 2011 ISBN 978 92 4 150070 8. [Google Scholar]
- 31.Achkar JM, Jenny-Avital ER. Incipient and subclinical tuberculosis: defining early disease states in the context of host immune response. J Infect Dis. 2011;204 Suppl 4:S1179–86. Epub 2011/10/26. 10.1093/infdis/jir451 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawn SD, Wilkinson RJ, Lipman MC, Wood R. Immune reconstitution and "unmasking" of tuberculosis during antiretroviral therapy. Am J Respir Crit Care Med. 2008;177(7):680–5. Epub 2008/01/19. 10.1164/rccm.200709-1311PP . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manabe YC, Breen R, Perti T, Girardi E, Sterling TR. Unmasked tuberculosis and tuberculosis immune reconstitution inflammatory disease: a disease spectrum after initiation of antiretroviral therapy. J Infect Dis. 2009;199(3):437–44. Epub 2008/12/19. 10.1086/595985 . [DOI] [PubMed] [Google Scholar]
- 34.Mtei L, Matee M, Herfort O, Bakari M, Horsburgh CR, Waddell R, et al. High rates of clinical and subclinical tuberculosis among HIV-infected ambulatory subjects in Tanzania. Clin Infect Dis. 2005;40(10):1500–7. 10.1086/429825 [DOI] [PubMed] [Google Scholar]
- 35.Oni T, Burke R, Tsekela R, Bangani N, Seldon R, Gideon HP, et al. High prevalence of subclinical tuberculosis in HIV-1-infected persons without advanced immunodeficiency: implications for TB screening. Thorax. 2011;66(8):669–73. 10.1136/thx.2011.160168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lawn SD, Kerkhoff AD, Wood R. Progression of subclinical culture-positive tuberculosis to symptomatic disease in HIV-infected individuals. AIDS. 2011;25(17):2190–1. Epub 2011/10/25. 10.1097/QAD.0b013e32834cda4e . [DOI] [PubMed] [Google Scholar]
- 37.Wilson D, Badri M, Maartens G. Performance of serum C-reactive protein as a screening test for smear-negative tuberculosis in an ambulatory high HIV prevalence population. PLoS One. 2011;6(1):e15248 Epub 2011/01/21. 10.1371/journal.pone.0015248 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drain PK, Mayeza L, Bartman P, Hurtado R, Moodley P, Varghese S, et al. Diagnostic accuracy and clinical role of rapid C-reactive protein testing in HIV-infected individuals with presumed tuberculosis in South Africa. Int J Tuberc Lung Dis. 2014;18(1):20–6. 10.5588/ijtld.13.0519 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon C, Semitala FC, Atuhumuza E, Katende J, Mwebe S, Asege L, et al. Point-of-care C-reactive protein-based tuberculosis screening for people living with HIV: a diagnostic accuracy study. Lancet Infect Dis. 2017;17(12):1285–92. 10.1016/S1473-3099(17)30488-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kunnath-Velayudhan S, Salamon H, Wang HY, Davidow AL, Molina DM, Huynh VT, et al. Dynamic antibody responses to the Mycobacterium tuberculosis proteome. Proc Natl Acad Sci U S A. 2010;107(33):14703–8. Epub 2010/07/30. 10.1073/pnas.1009080107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Achkar JM, Cortes L, Croteau P, Yanofsky C, Mentinova M, Rajotte I, et al. Host Protein Biomarkers Identify Active Tuberculosis in HIV Uninfected and Co-infected Individuals. EBioMedicine. 2015;2(9):1160–8. 10.1016/j.ebiom.2015.07.039 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adu-Gyamfi CG, Snyman T, Hoffmann CJ, Martinson NA, Chaisson RE, George JA, et al. Plasma Indoleamine 2, 3-Dioxygenase, a Biomarker for Tuberculosis in Human Immunodeficiency Virus-Infected Patients. Clin Infect Dis. 2017;65(8):1356–8. 10.1093/cid/cix550 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shete PB, Ravindran R, Chang E, Worodria W, Chaisson LH, Andama A, et al. Evaluation of antibody responses to panels of M. tuberculosis antigens as a screening tool for active tuberculosis in Uganda. PLoS One. 2017;12(8):e0180122 10.1371/journal.pone.0180122 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song L, Wallstrom G, Yu X, Hopper M, Van Duine J, Steel J, et al. Identification of Antibody Targets for Tuberculosis Serology using High-Density Nucleic Acid Programmable Protein Arrays. Mol Cell Proteomics. 2017;16(4 suppl 1):S277–S89. 10.1074/mcp.M116.065953 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y, Ndumnego OC, Chen T, Kim RS, Jenny-Avital ER, Ndung’u T, et al. Soluble CD14 as a Diagnostic Biomarker for Smear-Negative HIV-Associated Tuberculosis. Pathogens. 2018;7(1). 10.3390/pathogens7010026 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walzl G, McNerney R, du Plessis N, Bates M, McHugh TD, Chegou NN, et al. Tuberculosis: advances and challenges in development of new diagnostics and biomarkers. Lancet Infect Dis. 2018;18(7):e199–e210. 10.1016/S1473-3099(18)30111-7 . [DOI] [PubMed] [Google Scholar]
- 47.Paris L, Magni R, Zaidi F, Araujo R, Saini N, Harpole M, et al. Urine lipoarabinomannan glycan in HIV-negative patients with pulmonary tuberculosis correlates with disease severity. Science translational medicine. 2017;9(420). 10.1126/scitranslmed.aal2807 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amin AG, De P, Spencer JS, Brennan PJ, Daum J, Andre BG, et al. Detection of lipoarabinomannan in urine and serum of HIV-positive and HIV-negative TB suspects using an improved capture-enzyme linked immuno absorbent assay and gas chromatography/mass spectrometry. Tuberculosis (Edinb). 2018;111:178–87. 10.1016/j.tube.2018.06.004 . [DOI] [PubMed] [Google Scholar]
- 49.Sigal GB, Pinter A, Lowary TL, Kawasaki M, Li A, Mathew A, et al. A novel sensitive immunoassay targeting the MTX-Lipoarabinomannan epitope meets the WHO’s performance target for Tuberculosis diagnosis. J Clin Microbiol. 2018. 10.1128/JCM.01338-18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sonnenberg P, Murray J, Glynn JR, Shearer S, Kambashi B, Godfrey-Faussett P. HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: a cohort study in South African mineworkers. Lancet. 2001;358(9294):1687–93. 10.1016/S0140-6736(01)06712-5 . [DOI] [PubMed] [Google Scholar]
- 51.Marx FM, Dunbar R, Enarson DA, Williams BG, Warren RM, van der Spuy GD, et al. The temporal dynamics of relapse and reinfection tuberculosis after successful treatment: a retrospective cohort study. Clin Infect Dis. 2014;58(12):1676–83. 10.1093/cid/ciu186 . [DOI] [PubMed] [Google Scholar]
- 52.Guerra-Assuncao JA, Houben RM, Crampin AC, Mzembe T, Mallard K, Coll F, et al. Recurrence due to relapse or reinfection with Mycobacterium tuberculosis: a whole-genome sequencing approach in a large, population-based cohort with a high HIV infection prevalence and active follow-up. J Infect Dis. 2015;211(7):1154–63. 10.1093/infdis/jiu574 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.