Abstract
Background
Behçet’s disease (BD) is a recurrent, multisystemic, inflammatory disorder that mainly affects blood vessels. Because recurrent inflammation of blood vessels in the brain plays a crucial role in the development of ischemic stroke, we hypothesized that patients with BD might have an elevated risk of ischemic stroke. This potential association has been suggested in a few case reports, but not epidemiological studies. Hence, the present study aimed to examine the relation between BD and subsequent ischemic stroke in Taiwan using a nationwide, population-based database.
Methods
To establish a study cohort, the longitudinal data of 306 patients newly diagnosed with BD during 2000–2010 were extracted from the National Health Insurance Research Database, Taiwan. For comparison of ischemic stroke incidence, a control cohort of 1224 subjects without BD was established using a frequency-matched ratio of 1:4 for age, sex, and pre-existing comorbidities.
Results
During the 10-year follow-up, 13 (4.2%) patients with BD and 20 (1.6%) control subjects experienced ischemic stroke. Kaplan–Meier analysis revealed the higher prevalence of ischemic stroke in the BD group (log-rank test, p = 0.001). After adjusting for comorbidities and demographic characteristics, Cox regression analysis revealed that patients with BD had a 2.77-fold risk of ischemic stroke (95% confidence interval, 1.38–5.57) compared to control subjects.
Conclusions
Patients with BD have an elevated risk of ischemic stroke. Hence, BD may affect the vascular system in the brain, resulting in a stroke event.
Introduction
Behçet’s disease (BD) is an autoimmune inflammatory disease characterized by recurrent mucosal aphthous ulcers (primarily oral but also genital) and a variety of systemic symptoms, including skin lesions as well as ocular, neurological, articular, and gastrointestinal manifestations [1, 2]. The epidemiological distribution of BD is intriguing, as it is most common in areas along the ancient Silk Road that ran from the Mediterranean Sea to Eastern Asia. The worldwide prevalence of BD is highest in Turkey and lowest in North America and Europe [3–5]. The incidence of BD in Taiwan is moderate (2.40 per 100 000 person-years), a rate that is between those of the Middle East and Europe, the Americas, and Africa [6].
Studies on the etiopathogenesis of BD currently suggest an autoimmune origin [7, 8]. Further influences thought to contribute to BD development include genetic factors, altered host–bacteria interactions, vascular endothelial activation, hypercoagulation status, aberrant immune activity, the presence of immune complexes and autoantibodies, and alterations in hematopoietic cell populations and their associated cytokines [9–11]. Clinically, BD symptoms are primarily caused by vasculitis that affects arteries and veins of various sizes. Subtle but diffusely distributed vasculitis can lead to endothelial damage; such damage can be complicated by aberrant vascular endothelial activation and hypercoagulability status in BD patients. Therefore, the likelihood of developing thrombus, a precursor to ischemic stroke, is higher in BD patients, demonstrated by the finding that BD patients often develop cerebral arterial thrombosis [12, 13]. Thus, we hypothesize that BD patients may be at elevated risk of ischemic stroke.
In Taiwan, stroke is the most prevalent cause of severe disabilities (incidence of 3.29 ‰; prevalence of 1.93%) [14, 15] and the third most prevalent cause of death [16]. Ischemic stroke is the most common stroke type, and the majority of ischemic strokes involve small vessel occlusion. Age, sex, family history of stroke, socioeconomic status, smoking, alcohol consumption, hyperlipidemia, diabetes mellitus, hypertension, obesity, and atrial fibrillation are important risk factors for stroke in the general population [14, 15, 17–25]. While, the prevalence and potential risk factors for ischemic stroke in BD patients in Taiwan remain unknown. Although multiple factors are believed to increase the likelihood of acute ischemic stroke in the general public population, the higher risk of ischemic stroke in BD patients with vasculitis warrants further assessment. To date, only few published epidemiological studies investigate the association between BD and acute ischemic stroke events [26, 27]. Therefore, the present study aimed to evaluate the risk of ischemic stroke in BD patients using a nationwide, population-based database.
Materials and methods
Data source and ethical consideration
Since its implementation in 1995, the National Health Insurance (NHI) program of Taiwan has provided comprehensive, unified, and universal health care services to over 99% of the Taiwanese population [28]. We used a subset of the NHI Research Data (NHIRD) that includes the claims data of one million NHI enrollees (approximately 5% of the total Taiwanese population) randomly sampled in 2000. The data cover the period from 1995 to 2010. The NHIRD contains information on patient demographics, diagnoses, medication categories and details, prescription dates, drug dosages, and treatment duration. The protocol of the present study was approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB-EXEMPT(I)-20160003). The requirement of patient informed consent was waived because all data were extracted from the NHIRD, which is encrypted.
Study population
The study cohort comprised patients with BD who were diagnosed according to the International Study Group criteria published in 1990 and the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic criteria (ICD-9-CM code 136.1) between January 1, 2000, and December 31, 2009. To increase the diagnostic validity, we specifically selected the patients who had inpatient diagnosis records with a primary or secondary diagnosis, outpatient diagnosis records with at least three consistent diagnoses, or a catastrophic illness registration card for BD. In Taiwan, BD patients can apply for a catastrophic illness registration card from the NHI Bureau, which exempts BD patients from copayments when seeking health care for BD. We defined the patient’s first visit for the diagnosis of BD as the index date. Because this study examined the risk of ischemic stroke in BD patients, those who had received a diagnosis of any type of ischemic stroke (ICD-9-CM codes 433–438) before their BD diagnosis were excluded. We also recorded the presence, before their index date, of the following potential risk factors for stroke: a past history of hypertension (ICD-9-CM codes 401–405), hyperlipidemia (272.4), diabetes mellitus (250), valvular heart disease (398.9 and 424.0–424.3), and congestive heart failure (428.xx) [15, 19, 22–25].
To establish a control cohort, control subjects were selected after frequency-matching at a ratio of 1:4 for the following characteristics: age; sex; and pre-existing comorbidities, including hypertension, hyperlipidemia, diabetes mellitus, valvular heart disease, and congestive heart failure [15, 19, 22–25].
Ischemic stroke event measurement
The NHIRD is extensively used in Taiwan by scholars conducting pharmacoepidemiological researches. One study confirmed the validity of using the NHIRD for identifying patients who have received a principal diagnosis of ischemic stroke [29]. An ischemic stroke event was identified when any of the following criteria were met: (1) hospitalization claims; (2) four or more consecutive outpatient hospital visits, accompanied by either claims regarding the use of various types of neurological imaging techniques (magnetic resonance imaging, computed tomography, and carotid or transcranial Doppler sonography) or ischemic-stroke–related long-term prescriptions; and (3) claims for ischemic-stroke-related rehabilitation and long-term prescriptions [30]. The specificity and sensitivity of identifying ischemic stroke were 95% and 100%, respectively [31]. Related studies have reported a similar definition of ischemic stroke and have provided additional details [31, 32]. Identifying ischemic stroke by using insurance claims has been confirmed to be valid and was applied in a similar study [29]. The main outcome of the present study was the incidence of ischemic stroke after the index date. All participants were followed until either ischemic stroke after the index date, death, the end of follow-up in medical records, or the end of 2010.
Statistical analysis
For descriptive analysis of the BD and control cohorts, continuous variables were presented as means and standard deviations, while categorical variables were reported as numbers and percentages. Intergroup differences in continuous data were determined using Student’s t-test. The chi-square or Fisher's exact test was employed for evaluating intergroup differences in categorical or proportional variables, as appropriate. Ischemic-stroke–free survival was analyzed using the Kaplan–Meier method, with significance calculated using the log-rank test. Cox proportional hazard regression was used for multiple regression analysis. Statistical significance was defined as two-sided p < 0.05. All data were analyzed using IBM SPSS version 24.0 (IBM Corp, Armonk, NY, USA).
Results
Demographic characteristics of the study population
The present study included 306 BD patients and 1224 control subjects without BD. Table 1 summaries the demographic characteristics and selected comorbidities for these two groups. The mean age of the BD patients was 40.31 ± 14.86 years, and 57.84% of the BD patients were women. The comorbidity prevalence (e.g., cardiovascular diseases) did not differ significantly between the groups, as expected. The median follow-up periods of the BD and control groups were 3.48 years (range, 1.01–5.95) and 3.57 years (range, 0.62–6.51), respectively. Of the total 1530 participants, 33 (2.15%) experienced stroke during the follow-up period (Table 1), with 13 (4.25%) in the BD cohort and 20 (1.63%) in the control cohort.
Table 1. Comparison of baseline characteristics between subjects with and without BD.
| Behçet’s disease (n = 306) |
Comparisons (n = 1224) |
p | |||
|---|---|---|---|---|---|
| Age, years | 40.31 ± 14.86 | 40.31 ± 14.84 | 1.000 | ||
| Male | 129 | 42.16% | 516 | 42.16% | 1.000 |
| Comorbidities | |||||
| Hypertension | 72 | 23.53% | 288 | 23.53% | 1.000 |
| Diabetes mellitus | 34 | 11.11% | 136 | 11.11% | 1.000 |
| Valvular heart disease | 14 | 4.58% | 56 | 4.58% | 1.000 |
| Congestive heart failure | 4 | 1.31% | 16 | 1.31% | 1.000 |
| Hyperlipidemia | 34 | 11.11% | 136 | 11.11% | 1.000 |
| Ischemic stroke | |||||
| Occurrence | 13 | 4.25% | 20 | 1.63% | 0.005 |
| Duration, days | 1269.00 ± 902.04 | 1302.75 ± 1074.65 | 0.506 | ||
BD, Behçet’s Disease.
Risk factors for ischemic stroke in patients with BD
Kaplan–Meier and log-rank analyses indicated a significantly higher prevalence of ischemic stroke in the BD group compared with the control group (p, 0.004) (Fig 1), indicating that BD patients had an increased ischemic stroke risk over the study period. Univariate Cox regression analysis revealed that compared with the control group, the BD group had a crude hazard ratio (HR) for ischemic stroke of 2.690 (95% confidence interval [CI], 1.338–5.409; p, 0.005) during the follow-up period. After adjustment for age, sex, and pre-existing comorbidities, multivariate Cox regression analysis revealed that the stroke risk during the follow-up period was 2.768 times higher (95% CI, 1.376–5.570; p, 0.004) in BD patients than control subjects (Table 2), suggesting that BD patients have an increased risk of having an ischemic stroke event. Univariate analysis also revealed an increased ischemic stroke risk in BD patients who were older (crude HR, 1.057; 95% CI, 1.035–1.080) and in those with diabetes mellitus (crude HR, 4.249; 95% CI, 2.084–8.664), hypertension (crude HR, 6.980; 95% CI, 3.319–14.682), or previous congestive heart failure (crude HR, 8.859; 95% CI, 3.368–23.304) (Table 2). Furthermore, multivariate analysis showed that after adjustment for potential confounders, older age (adjusted HR, 1.030; 95% CI, 1.003–1.058; p, 0.028) and hypertension (adjusted HR, 3.672; 95% CI, 1.474–9.148; p, 0.005) were independent risk factors for ischemic stroke in BD patients (Table 2).
Fig 1. Kaplan–Meier curves of freedom from ischemic stroke between patients with Behçet’s Disease (BD) and the comparisons without.
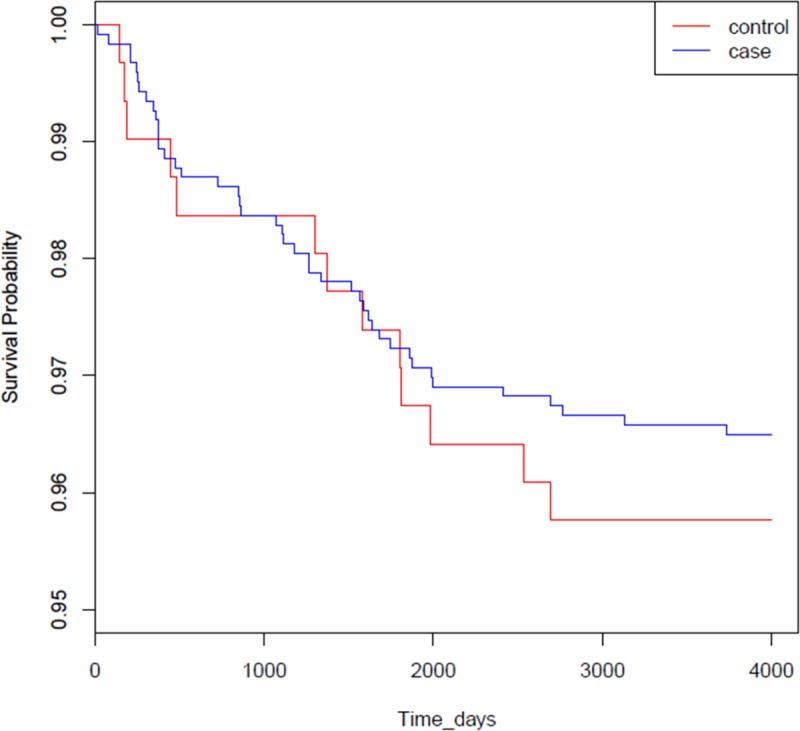
Group 1, patients with BD; group 2, control subjects without BD. The difference between the two curves is statistically significant (log-rank test, p < 0.01).
Table 2. Independent predictors of ischemic stroke in patients with BD.
| Variables | Crude HR (95% CI) | p | Adjusted HRa (95% CI) | p |
|---|---|---|---|---|
| Age | 1.057 (1.035–1.080) | < 0.001 | 1.030 (1.003–1.058) | 0.028 |
| Male | 1.021 (0.512–2.037) | 0.952 | 0.841 (0.408–1.736) | 0.640 |
| Hypertension | 6.980 (3.319–14.682) | < 0.001 | 3.672 (1.474–9.148) | 0.005 |
| Diabetes mellitus | 4.249 (2.084–8.664) | < 0.001 | 1.862 (0.873–3.975) | 0.108 |
| Valvular heart disease | 1.077 (0.257–4.509) | 0.919 | 0.511 (0.112–2.322) | 0.385 |
| Congestive heart failure | 8.859 (3.368–23.304) | < 0.001 | 2.476 (0.884–6.993) | 0.084 |
| Hyperlipidemia | 2.719 (1.262–5.858) | 0.011 | 0.898 (0.387–2.084) | 0.802 |
| Behçet’s disease | 2.690 (1.338–5.409) | 0.005 | 2.768 (1.376–5.570) | 0.004 |
BD, Behçet’s disease; HR, Hazards ratio; CI, Confidence interval
aAfter adjustment for age, male sex, hypertension, diabetes mellitus, valvular heart disease, congestive heart failure, hyperlipidemia, and Behçet’s disease
Discussion
Although the neurological deficits associated with BD are well recognized, the association between BD and ischemic stroke has been rarely explored [26, 33–36]. To the best of our knowledge, this study is the first nationwide population-based investigation of potential ischemic stroke risk factors in BD patients in Asia. The results revealed a significantly increased risk of ischemic stroke for BD patients compared to control subjects without BD. Moreover, old age and hypertension are independent risk factors for ischemic stroke in BD patients.
BD is a rare, chronic autoimmune disorder with a variety of etiologies [37]. The main etiologies include genetic factors; altered host responses to microbes, hematopoietic cells, and cytokines; autoantibody and immune complex formation; and altered vascular endothelial activation and hypercoagulability [9, 10]. The manifestations of BD are believed to be caused by vasculitis that results in damage to blood vessels throughout the body [38]. The pathogenesis of stroke in BD patients may be multifactorial, which remains to be a topic of debate and the focus of research. Although BD manifests as recurrent aphthous ulcers, uveitis, and genital ulcers, BD can be devastating and even fatal because of vascular aneurysm rupture or severe neurological complications [39–41].
In the present study, we observed a significantly increased risk of ischemic stroke among BD patients. The vasculitis–BD relationship may underlie this increased risk of acute ischemic stroke. Decreased endothelium-dependent flow-mediated dilatation has been observed in BD patients [42, 43]. In addition, BD patients exhibited increased intima-media thickness and decreased arterial distensibility compared with controls [44]. These factors all contribute to a tendency toward vascular stenosis in BD patients. Among stroke subtypes, ischemic stroke accounts for nearly 80% of stroke events [16]. Ischemic stroke is caused by decreased or completely blocked blood flow [45]; such reduction in the blood supply may be caused by severe stenosis, reduced systemic perfusion, or blood vessel occlusion. The primary causes of ischemia include thrombosis, embolization, and lacunar infarction resulting from small vessel disease. Therefore, thrombosis development is an important risk factor for ischemic stroke in BD patients.
Several BD-associated factors may contribute to thrombosis development to some degree in BD patients [28, 42]. Endothelial activation in the lumen of affected vessels may result in vascular inflammation and thrombosis in BD [46, 47]. Many BD patients appeared to be in a generalized hypercoagulable state, based on evidence of increased thrombin formation and decreased fibrinolysis [48, 49]. Arteritis that involves the vascular system in the brain parenchyma may lead to ischemic stroke, aneurysmal dilatation, dissection, and subarachnoid hemorrhage. In the present study, we observed a 2.77-fold higher ischemic stroke risk in BD patients.
In the present study, older age and hypertension were found to be independent factors contributing to ischemic stroke in BD patients. In addition, BD patients with older age and hypertension had a higher risk of stroke. The findings of this study show that BD patients had a considerably higher ischemic stroke risk than expected; therefore, the hazardous effects of vascular damage and hypercoagulative status on cerebral vessels should not be overlooked. A proactive and prompt preventive plan should be employed for BD patients, especially for those with concomitant risk factors for ischemic stroke. Our statistical analyses using information in a medical database do not provide sufficient information regarding the association between BD and stroke, especially regarding the mechanism of pathogenesis. However, our data show that BD patients have a significantly increased risk of ischemic stroke. Further research is necessary to confirm our findings and explore the mechanism underlying the association between BD and stroke.
Strength and limitation in the study
The main strength of this research is its data source, a nationwide population-based database that represents the general population of Taiwan. Most events could be traced and patients followed, and referral bias was kept to a minimum because Taiwan’s NHI program is a single-payer, mandatory program with low copayments and services that cover more than 99% of the Taiwanese population. Furthermore, ischemic stroke and comorbidity diagnoses were reliable; claims on the NHI are scrutinized and validated by medical reimbursement specialists and are subject to peer review.
Nevertheless, the present study also had some limitations. Firstly, the NHIRD does not contain detailed biochemical data, body weight or body mass index details, information indicating the clinical severity of diseases, family history, or lifestyle factors, each of which may be a risk factor or comorbidity for ischemic stroke. For example, the HLA-B51/B5 gene is recognized as a risk factor that substantially contributes to BD, but this information is not included in the NHIRD. In addition, the data regarding lifestyle-related risk factors for ischemic stroke such as dietary habits, cigarette smoking, alcohol consumption, and physical activity were unavailable. Second, this study had a retrospective design, and such studies tend to be more susceptible to bias than are those with a prospective design. However, this study avoided two possible major biases-related sampling problems by selecting patients randomly from a population-wide database and by avoiding recall problems via examining national health care records. Finally, as with the compilation of data in any administrative database, coding errors and undercoding are possible.
Conclusions
BD Patients in Taiwan are at increased risk of ischemic stroke. More aggressive primary and secondary prevention strategies are needed for this patient population. Further investigation is necessary to determine whether treating conditions that are stroke risk factors in these patients can decrease the likelihood of ischemic stroke.
Acknowledgments
The authors thank Taiwan’s NHRI and BNHI for the data. The interpretation and conclusions contained in this article do not represent the views of the NHRI and BNHI.
Data Availability
The data underlying this study is from the National Health Insurance Research Database (NHIRD), which has been transferred to the Health and Welfare Data Science Center (HWDC). Interested researchers can obtain the data through formal application to the HWDC, Department of Statistics, Ministry of Health and Welfare, Taiwan (http://dep.mohw.gov.tw/DOS/np-2497-113.html).
Funding Statement
The authors received no specific funding for this work.
References
- 1.Zeidan MJ, Saadoun D, Garrido M, Klatzmann D, Six A, Cacoub P. Behcet's disease physiopathology: a contemporary review. Auto Immun Highlights. 2016;7(1):4 10.1007/s13317-016-0074-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tursen U. Pathophysiology of the Behcet's Disease. Patholog Res Int. 2012;2012:493015 10.1155/2012/493015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azizlerli G, Kose AA, Sarica R, Gul A, Tutkun IT, Kulac M, et al. Prevalence of Behcet's disease in Istanbul, Turkey. Int J Dermatol. 2003;42(10):803–6. [DOI] [PubMed] [Google Scholar]
- 4.Davatchi F, Shahram F, Chams-Davatchi C, Shams H, Nadji A, Akhlaghi M, et al. Behcet's disease: from East to West. Clin Rheumatol. 2010;29(8):823–33. 10.1007/s10067-010-1430-6 [DOI] [PubMed] [Google Scholar]
- 5.Kappen JH, van Dijk EH, Baak-Dijkstra M, van Daele PL, Lam-Tse WK, van Hagen PM, et al. Behcet's disease, hospital-based prevalence and manifestations in the Rotterdam area. Neth J Med. 2015;73(10):471–7. [PubMed] [Google Scholar]
- 6.Lin YH, Tai TY, Pu CY, Hwang DK, Chung YM, Chou YJ. Epidemiology of Behcet's Disease in Taiwan: A Population-Based Study. Ophthalmic Epidemiol. 2018;25(4):323–9. 10.1080/09286586.2018.1469157 [DOI] [PubMed] [Google Scholar]
- 7.Hedayatfar A. Behcet's Disease: Autoimmune or Autoinflammatory? J Ophthalmic Vis Res. 2013;8(3):291–3. [PMC free article] [PubMed] [Google Scholar]
- 8.Galeone M, Colucci R, D'Erme AM, Moretti S, Lotti T. Potential Infectious Etiology of Behcet's Disease. Patholog Res Int. 2012;2012:595380 10.1155/2012/595380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gul A. Pathogenesis of Behcet's disease: autoinflammatory features and beyond. Semin Immunopathol. 2015;37(4):413–8. 10.1007/s00281-015-0502-8 [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi M, Kastner DL, Remmers EF. The immunogenetics of Behcet's disease: A comprehensive review. J Autoimmun. 2015;64:137–48. 10.1016/j.jaut.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berteau F, Rouviere B, Delluc A, Nau A, Le Berre R, Sarrabay G, et al. Autosomic dominant familial Behcet disease and haploinsufficiency A20: A review of the literature. Autoimmun Rev. 2018;17(8):809–15. 10.1016/j.autrev.2018.02.012 [DOI] [PubMed] [Google Scholar]
- 12.Akman-Demir G, Serdaroglu P, Tasci B. Clinical patterns of neurological involvement in Behcet's disease: evaluation of 200 patients. The Neuro-Behcet Study Group. Brain. 1999;122 (Pt 11):2171–82. 10.1093/brain/122.11.2171 [DOI] [PubMed] [Google Scholar]
- 13.Farah S, Al-Shubaili A, Montaser A, Hussein JM, Malaviya AN, Mukhtar M, et al. Behcet's syndrome: a report of 41 patients with emphasis on neurological manifestations. J Neurol Neurosurg Psychiatry. 1998;64(3):382–4. 10.1136/jnnp.64.3.382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin HC, Lin YJ, Liu TC, Chen CS, Chiu WT. Urbanization and stroke prevalence in Taiwan: analysis of a nationwide survey. J Urban Health. 2007;84(4):604–14. 10.1007/s11524-007-9195-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu HH, Sheng WY, Chu FL, Lan CF, Chiang BN. Incidence of stroke in Taiwan. Stroke. 1992;23(9):1237–41. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh FI, Chiou HY. Stroke: morbidity, risk factors, and care in taiwan. J Stroke. 2014;16(2):59–64. 10.5853/jos.2014.16.2.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart CL, Hole DJ, Smith GD. The contribution of risk factors to stroke differentials, by socioeconomic position in adulthood: the Renfrew/Paisley Study. Am J Public Health. 2000;90(11):1788–91. 10.2105/ajph.90.11.1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jakovljevic D, Sarti C, Sivenius J, Torppa J, Mahonen M, Immonen-Raiha P, et al. Socioeconomic status and ischemic stroke: The FINMONICA Stroke Register. Stroke. 2001;32(7):1492–8. [DOI] [PubMed] [Google Scholar]
- 19.Boysen G, Nyboe J, Appleyard M, Sorensen PS, Boas J, Somnier F, et al. Stroke incidence and risk factors for stroke in Copenhagen, Denmark. Stroke. 1988;19(11):1345–53. [DOI] [PubMed] [Google Scholar]
- 20.Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341–78. 10.1146/annurev.publhealth.18.1.341 [DOI] [PubMed] [Google Scholar]
- 21.Modan B, Wagener DK. Some epidemiological aspects of stroke: mortality/morbidity trends, age, sex, race, socioeconomic status. Stroke. 1992;23(9):1230–6. [DOI] [PubMed] [Google Scholar]
- 22.Caprio FZ, Sorond FA. Cerebrovascular Disease: Primary and Secondary Stroke Prevention. Med Clin North Am. 2019;103(2):295–308. 10.1016/j.mcna.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 23.O'Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388(10046):761–75. 10.1016/S0140-6736(16)30506-2 [DOI] [PubMed] [Google Scholar]
- 24.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383–93. 10.1056/NEJMoa021778 [DOI] [PubMed] [Google Scholar]
- 25.Cholesterol Treatment Trialists C, Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380(9841):581–90. 10.1016/S0140-6736(12)60367-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biniwale N, Kibe R, Biniwale A. Neuro-Behcet's Disease Presenting as a Young Stroke. J Assoc Physicians India. 2017;65(5):89–90. [PubMed] [Google Scholar]
- 27.Kaido T, Otsuki T, Ogawa M, Takahashi A, Kaneko Y, Yamamoto T, et al. Medullary ischemia due to vertebral arteritis associated with Behcet syndrome: a case report. Asian Pac J Allergy Immunol. 2012;30(3):239–42. [PubMed] [Google Scholar]
- 28.Wu X, Li G, Huang X, Wang L, Liu W, Zhao Y, et al. Behcet's disease complicated with thrombosis: a report of 93 Chinese cases. Medicine (Baltimore). 2014;93(28):e263 10.1097/MD.0000000000000263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20(3):236–42. 10.1002/pds.2087 [DOI] [PubMed] [Google Scholar]
- 30.Chiu CC, Huang CC, Chan WL, Chung CM, Huang PH, Lin SJ, et al. Increased risk of ischemic stroke in patients with systemic lupus erythematosus: a nationwide population-based study. Intern Med. 2012;51(1):17–21. 10.2169/internalmedicine.51.6154 [DOI] [PubMed] [Google Scholar]
- 31.Chen HJ, Bai CH, Yeh WT, Chiu HC, Pan WH. Influence of metabolic syndrome and general obesity on the risk of ischemic stroke. Stroke. 2006;37(4):1060–4. 10.1161/01.STR.0000206458.58142.f3 [DOI] [PubMed] [Google Scholar]
- 32.Leu HB, Chung CM, Chuang SY, Bai CH, Chen JR, Chen JW, et al. Genetic variants of connexin37 are associated with carotid intima-medial thickness and future onset of ischemic stroke. Atherosclerosis. 2011;214(1):101–6. 10.1016/j.atherosclerosis.2010.10.010 [DOI] [PubMed] [Google Scholar]
- 33.Yoshimura S, Ago T, Koga M, Kamouchi M, Kitazono T. Cerebral Small-Vessel Disease in Neuro-Behcet Disease. J Stroke Cerebrovasc Dis. 2015;24(8):e237–9. 10.1016/j.jstrokecerebrovasdis.2015.05.012 [DOI] [PubMed] [Google Scholar]
- 34.Koike Y, Sakai N, Umeda Y, Umeda M, Oyake M, Fujita N. [A case of Behcet disease developing recurrent ischemic stroke with fever and scrotal ulcers]. Rinsho Shinkeigaku. 2015;55(6):428–31. 10.5692/clinicalneurol.cn-000682 [DOI] [PubMed] [Google Scholar]
- 35.Ismail AM, Dubrey SW, Patel MC. Recurrent headaches: a case of neurological Behcet's disease. Br J Hosp Med (Lond). 2013;74(10):592–3. 10.12968/hmed.2013.74.10.592 [DOI] [PubMed] [Google Scholar]
- 36.Lee SK, Choi SJ, Kim SD, Lim DJ. Rapid Atypical Progression of Neuro-Behcet's Disease Involving Whole Brainstem and Bilateral Thalami. J Korean Neurosurg Soc. 2011;50(1):68–71. 10.3340/jkns.2011.50.1.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saadoun D, Wechsler B. Behcet's disease. Orphanet J Rare Dis. 2012;7:20 10.1186/1750-1172-7-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fei Y, Li X, Lin S, Song X, Wu Q, Zhu Y, et al. Major vascular involvement in Behcet's disease: a retrospective study of 796 patients. Clin Rheumatol. 2013;32(6):845–52. 10.1007/s10067-013-2205-7 [DOI] [PubMed] [Google Scholar]
- 39.Faezi ST, Chams-Davatchi C, Ghodsi SZ, Shahram F, Nadji A, Akhlaghi M, et al. Genital aphthosis in Behcet's disease: is it associated with less eye involvement? Rheumatol Int. 2014;34(11):1581–7. 10.1007/s00296-014-3011-5 [DOI] [PubMed] [Google Scholar]
- 40.Hosaka A, Miyata T, Hoshina K, Okamoto H, Shigematsu K, Oshima A. Prognosis of arterial aneurysm after surgery in patients with Behcet's disease. Int Angiol. 2014;33(5):419–25. [PubMed] [Google Scholar]
- 41.Li L, Gu Y, Qi L. Endovascular repair of a subclavian artery aneurysm in Behcet's disease. Interact Cardiovasc Thorac Surg. 2018;27(3):461–2. 10.1093/icvts/ivy098 [DOI] [PubMed] [Google Scholar]
- 42.Seyahi E, Yurdakul S. Behcet's Syndrome and Thrombosis. Mediterr J Hematol Infect Dis. 2011;3(1):e2011026 10.4084/MJHID.2011.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radenkovic M, Stojanovic M, Potpara T, Prostran M. Therapeutic approach in the improvement of endothelial dysfunction: the current state of the art. Biomed Res Int. 2013;2013:252158 10.1155/2013/252158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alan S, Ulgen MS, Akdeniz S, Alan B, Toprak N. Intima-media thickness and arterial distensibility in Behcet's disease. Angiology. 2004;55(4):413–9. 10.1177/000331970405500408 [DOI] [PubMed] [Google Scholar]
- 45.Xing C, Arai K, Lo EH, Hommel M. Pathophysiologic cascades in ischemic stroke. Int J Stroke. 2012;7(5):378–85. 10.1111/j.1747-4949.2012.00839.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Emmi G, Silvestri E, Squatrito D, Amedei A, Niccolai E, D'Elios MM, et al. Thrombosis in vasculitis: from pathogenesis to treatment. Thromb J. 2015;13:15 10.1186/s12959-015-0047-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Owlia MB, Mehrpoor G. Behcet's Disease: New Concepts in Cardiovascular Involvements and Future Direction for Treatment. ISRN Pharmacol. 2012;2012:760484 10.5402/2012/760484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.La Regina M, Gasparyan AY, Orlandini F, Prisco D. Behcet's Disease as a Model of Venous Thrombosis. Open Cardiovasc Med J. 2010;4:71–7. 10.2174/1874192401004020071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donmez A, Aksu K, Celik HA, Keser G, Cagirgan S, Omay SB, et al. Thrombin activatable fibrinolysis inhibitor in Behcet's disease. Thromb Res. 2005;115(4):287–92. 10.1016/j.thromres.2004.09.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this study is from the National Health Insurance Research Database (NHIRD), which has been transferred to the Health and Welfare Data Science Center (HWDC). Interested researchers can obtain the data through formal application to the HWDC, Department of Statistics, Ministry of Health and Welfare, Taiwan (http://dep.mohw.gov.tw/DOS/np-2497-113.html).


