Abstract
The tomato leafminer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae), is a destructive pest of tomato that can cause up to 100% yield loss. The predatory bug Nabis pseudoferus (Remane) (Hemiptera: Nabidae) and the parasitoid Trichogramma brassicae (Bezdenko) (Hymenoptera: Trichogrammatidae) are natural enemies of this pest. Since the interaction between predators and parasitoids in different trophic levels including intraguild predation (IGP) can decrease or increase the efficiency of natural enemies, the effects of age-dependent parasitism of host eggs on IGP between these two species were investigated under laboratory conditions. In no-choice and choice preference tests, the predatory bug was exposed to 40 parasitized and nonparasitized eggs of different ages (24, 48, and 72 h old). Investigation of switching behavior was conducted using various combinations of tomato leafminer eggs (30:90, 45:75, 60:60, 75:45, and 90:30 nonparasitized:parasitized eggs) using eggs of different ages (24, 48, and 72 h old). In no-choice tests, the highest feeding rate of the predatory bug was 39.21 ± 0.36 eggs on 24-h-old nonparasitized eggs and the lowest feeding rate was 1.4 ± 0.80 eggs on 72-h-old parasitized eggs. In choice tests, comparison of the Manly’s β indices indicated that the predatory bug preferred to feed on nonparasitized eggs with 48- and 72-h-old eggs, but there was no significant preference for the 24-h-old eggs. Results of switching test showed that the linear regression between Manly’s β index and different ratios of nonparasitized eggs to parasitized and nonparasitized eggs was not significant in 72-h-old eggs. However, this regression was significant with 24- and 48-h-old eggs and the predator’s preference was dependent upon the ratio of nonparasitized and parasitized tomato leafminer eggs. Results of the current study showed that the increasing age of parasitized egg decreased intensity of IGP between N. pseudoferus and T. brassicae.
Keywords: biological control, preference, switching, Manly’s β
Intraguild predation (IGP) is the killing and consuming of prey by a predator that uses similar resources as the prey and is therefore also a potential competitor (Polis and Holt 1992). The aggressor is referred to as the intraguild predator (IG predator), the victim is the intraguild prey (IG prey), and the common resource is an extraguild prey (Lucas et al. 2009). IGP between predators and parasitoids is typically asymmetrical and favors the predator, with the parasitoid, the hunted natural enemy; and the predator, the true predator that preys on the parasitoid (Polis et al. 1989, Brodeur and Rosenheim 2000). Intensity of IGP depends on the rates of predation on parasitized and nonparasitized prey (Rosenheim 1998). Some egg parasitoids including Trichogramma spp. induce modifications in the host, such as changes in the texture, shape or coloration, and the composition or quantity of external semiochemical cues that can be used to avoid IGP (Vinson 1994). These changes with increasing age of parasitized egg become more clear. In some studies, where given a choice between parasitized and nonparasitized eggs in younger stages, the two egg types were consumed in the same proportion, but eggs containing older stages of parasitoids were less consumed. Some authors argued that the increasing age in eggs parasitized by Trichogramma spp. caused the preference of generalist predators like Podisus maculiventris (Say) (Hem.: Pentatomidae), Nesidiocoris tenuis (Reuter), and Macrolophus pygmaeus (Wagner) (Hem.: Miridae) for nonparasitized eggs over older parasitized eggs (Oliveira et al. 2004, Chailleux et al. 2013, Cabello et al. 2015).
Optimal foraging theory predicts that prey age can affect the number of each prey type consumed, depending on the prey nutrient quality and energy content (Pyke 1984). In parasitized eggs or nonparasitized eggs with increasing age, embryonic tissues become more complex and the quality of the egg resource decreases. These changes caused by ageing in parasitized eggs is important to avoid or minimize IGP. As a parasitoid larva develops in the host egg, it uses most of the resources and hardens the egg chorion.
Some IG predators are able to discriminate these morphological and physiological changes caused by the developing parasitoid immature. For example, some authors observed that predators have a lower predation rate on parasitoid pupae compared with parasitoids in the larval stages (Hoelmer et al. 1994, Heinz and Nelson 1996, Al-Zyoud and Sengonca 2004, Fazal and Xiang 2004, Kutuk et al. 2011). However, in some systems the predation rate is independent of parasitoid age (Bilu and Coll 2009), or may be highest on old parasitized prey (Naranjo 2007, Sohrabi et al. 2013). As Venzon et al. (2002) suggested, prey density is a factor that influences IGP. Some IG predators are able to switch their prey preference as prey relative densities change and aggregate in areas of high prey density. For example, M. pygmaeus have strong preference for the prey with the higher relative density regardless of its nutritional value (Enkegaard et al. 2001). In all cases the effect of host density on IGP has been examined when two predator species coexist (Chow et al. 2008), but there are no studies demonstrating the effect of host density on IGP between immature stages of parasitoids and predator. In addition, most studies have focused on either the effect of the age of prey eggs or the effect of prey density, but testing for interactions between these two factors has not been carried out. Hence, in systems involving IGP, it is vital to know the capacity of a predator to discriminate between parasitized and nonparasitized prey at different ages, as well as its preference in different combinations of prey densities (Malo et al. 2012). We used the generalist predator Nabis pseudoferus (Remane) (Hem.: Nabidae) to test the influence of parasitism by Trichogramma brassicae (Bezdenko) (Hym.: Trichogrammatidae) on its preference and switching behaviors. Nabis pseudoferus attacks a diverse range of pest insects, including aphids, lepidopterans, hemipterans, and mites, all of which cause yield losses and are difficult to control in agricultural and horticulture crops (Urbaneja et al. 2012). Among the pests, the tomato leafminer moth Tuta absoluta (Meyrick) (Lep.: Gelechiidae) is perhaps the most difficult to control (Desneux et al. 2011). Nabis pseudoferus was studied to provide solutions for T. absoluta under greenhouse conditions (Cabello et al. 2009). Although nabids are known to be generalist predators, studies indicate that they are selective in their prey choice (Ma et al. 2005) and have distinct foraging behavior (Wade et al. 2005). In this study, we planned a series of experiments to determine the IGP between N. pseudoferus and T. brassicae using T. absoluta, which is now the most serious economic pest of tomato in Iran. Our objectives in this study are to determine: 1) whether N. pseudoferus would attack different developmental ages of Trichogramma-parasitized and nonparasitized eggs in a no-choice test, 2) whether N. pseudoferus would show a preference for younger and nonparasitized eggs (two-choice test), and 3) if N. pseudoferus is engaging in prey switching behavior between Trichogramma-parasitized and nonparasitized eggs when encountering both prey at various densities.
Materials and Methods
Biological Materials
Tomato plants Solanum lycopersicum L. cv. Mobil, were grown in the greenhouse (25 ± 5°C, 70 ± 5% RH, and 12-h photoperiod) from seed in a compost/peat-moss/perlite mixture and watered daily. After 10 d, seedlings were transplanted into pots (10 cm × 12 cm) filled with soil. Fertilizer (15-15-20 N-P-K) was added weekly, and plants were watered daily. Tomato plants were placed individually inside plastic ventilated cages (45 × 85 cm diameter). The T. absoluta and N. pseudoferus used in the trials were obtained from natural populations collected from tomato fields in Mashhad, Iran. Tuta absoluta was reared on tomato plants according to the methodology devised by Cabello et al. (2015). Nabis pseudoferus predators were identified by Matocq (Paris, France). The nabids were reared on eggs of the factitious host Ephestia kuehniella (Zeller) (Lep.: Pyralidae) following the methodology described by Fernandez-Maldonado et al. (2017).
The colony of T. brassicae was supplied by the Department of Plant Protection at the University of Tehran, Iran. Trichogramma brassicae were reared on 1-d-old E. kuehniella eggs, in growth chambers (23 ± 1°C, 65 ± 5% RH, 16:8 [L:D] h). The eggs of E. kuehniella were glued with 10% honey-water solution to paper strips (1 × 6 cm). The paper strips were placed in glass tubes (2.5 × 16 cm) containing 20 parasitoid pairs. The paper strips were replaced every day. The parasitoids used for all experiments were 24 h old because it is known that they can mate and oviposit when they are 1 d old (Babendreier et al. 2003).
To obtain nonparasitized eggs, insect-free tomato plants were placed inside a T. absoluta rearing cage to obtain a uniform cohort of eggs. Plants were removed after 2 h. Eggs were collected under a stereoscopic binocular microscope with a small brush and placed on tomato leaflets kept inside an Eppendorf tube filled with water, to maintain leaf turgor during the experiments. Cohorts of eggs were held for 24, 48, or 72 h to obtain unparasitized eggs of the appropriate age for the experiments. To obtain parasitized eggs, we first followed the same procedure as described above for nonparasitized eggs. Then, after obtaining 2-h-old eggs, tomato leaves containing these eggs were isolated and placed inside a 50-ml falcon tube. Twenty pairs couples of the parasitoid T. brassicae were introduced into each falcon tube. Parasitoids were allowed to oviposit for 12 h, and then removed from the tube. The exposed eggs were held for 24, 48, and 72 h to obtain parasitized eggs of the appropriate age for the experiments.
All the experiments were conducted inside a 50-ml falcon tube (3 × 11.5 cm) with a mesh-covered ventilation hole (3 cm in diameter) in the screw top. Each tube contained a fresh cut broad tomato leaf with the stalk inside an Eppendorf tube filled with water and sealed with parafilm to prevent desiccation.
No-Choice Bioassay
No-choice tests were conducted in a completely randomized design, with two factors (egg age and egg type) with at least 15 replications per treatment. Forty nonparasitized eggs of three different age groups (24, 48, and 72 h old) were placed on separate leaves. Each leaf was kept separately in a falcon tube. Mated N. pseudoferus adult females (less than 1 wk after the last nymphal ecdysis) were introduced at the rate of one per falcon following the methodology of Cabello et al. (2015). The same experimental design was used with parasitized eggs. After 24 h in contact with the eggs, the predator was removed. The eggs were observed using 35× magnification, and the number of eggs consumed (i.e., empty egg with a ruptured chorion), recorded. All experiments were conducted in an incubator at 23°C, 65% RH under a 16:8 (L:D) h photoperiod.
Statistical Analysis
Data were subjected to two-way ANOVA and pairwise multiple comparisons were performed using a Tukey’s post hoc test. Data sets were first tested for normality and homogeneity of variance using Kolmogorov–Smirnov D test and Cochran’s test, respectively, and transformed if needed. This test was carried out also for all data sets from other experiments (below), and the SAS software was used for all analyses (SAS Institute 2000).
Choice Bioassay
Choice tests were conducted to assess preference for nonparasitized and parasitized T. absoluta eggs by N. pseudoferus. Individual N. pseudoferus were caged as above, except that we set up the arena containing 40 parasitized and 40 nonparasitized eggs together (for a total of 80 eggs). Thus predators were given a choice of parasitized or nonparasitized eggs for 24 h. Three different egg combinations were used in these experiments: 1) 40 nonparasitized eggs versus 40 parasitized eggs (24 h old); 2) 40 nonparasitized eggs versus 40 parasitized eggs (48 h old); and 3) 40 parasitized eggs versus 40 parasitized eggs (72 h old). To discriminate between parasitized and nonparasitized eggs, we marked one of the treatments using a nontoxic, point marker on the back of the leaflet. The choice test was replicated 15 times. In cases where the predator did not eat any eggs during the test period, the data were excluded from analysis.
Prey Preference by N. pseudoferus
Predator’s preference for the prey’s parasitized and nonparasitized eggs was evaluated by calculating a preference index that takes into account the depletion of prey over time (Manly 1974, Chesson 1983). This index can be expressed as follows:
where β1 is the preference for type 1, e1 and e2 are the numbers of prey type 1 and 2 remaining after the experiment; A1 and A2 are the number of prey types 1 and 2 offered, respectively. This index provides a value between 0 and 1. In a two-prey combination, the value 0.5 indicates that the predator shows no preference for a given prey type, values larger than 0.5 indicate a preference for prey 1, and smaller values indicate a preference for prey 2. This method applies to experimental situations where killed prey is not replaced (Manly 1974, Meyling et al. 2004). We tested whether our estimates deviated from 0.5 using a two-tailed t-test (P < 0.05).
Prey Switching by N. pseudoferus
In three assays, we evaluated prey switching by female N. pseudoferus offered two prey combinations: parasitized and nonparasitized eggs. Pilot feeding trials (N = 15) were conducted to determine the size range of prey that N. pseudoferus could successfully consume. For each assay, an adult N. pseudoferus female was confined in the falcon tube with different combinations of the two prey species at one of the five ratios of nonparasitized eggs to parasitized eggs: 30:90, 45:75, 60:60, 75:45, and 90:30. After 24 h, the predator was removed and the number of eggs eaten was counted. The experiment was replicated 15 times per ratio.
We used Manly’s modeling works (Manly 1974) to evaluate the capacity of N. pseudoferus to switch between the two prey types. The nonlinear model below is designed to qualify preference (Enkegaard et al. 2001):
where q and w are constants and ρ is the proportion of nonparasitized eggs to all prey available A1/(A1 + A2). The parameter w is an index of the predator’s capacity to switch for prey species. When w = 0, there is no switch. When w > 0, the preference of predator for prey type 1 increases as the proportion of prey type 1 increases, i.e., the predator exhibits frequency-dependent selection or switching behavior, and vice versa.
Results
No-Choice Test
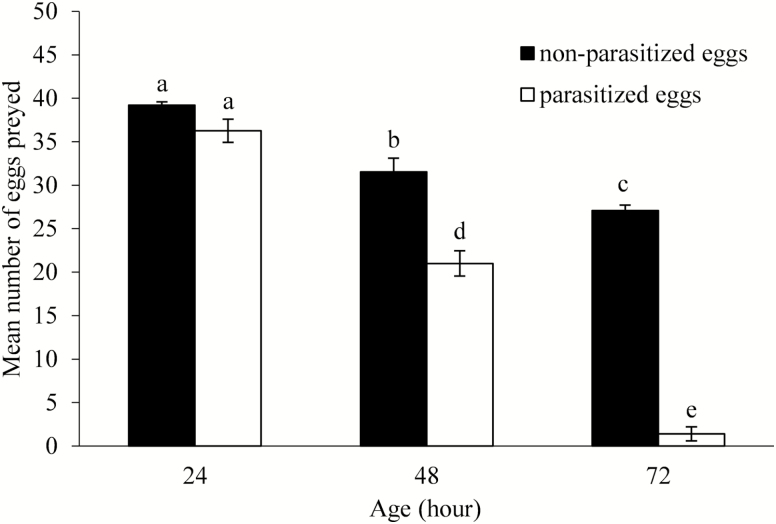
Prey consumption by N. pseudoferus is presented in Fig. 1. All six egg types were preyed by N. pseudoferus. There was a significant interaction between egg age (24, 48, and 72 h) and egg type (parasitized and nonparasitized) on predation of N. pseudoferus (F1,86 = 99.08, P < 0.0001). Also, two-way ANOVA indicates that the predation rate was significantly affected by egg type (parasitized and nonparasitized) (F1,86 = 8.62, P = 0.004) and egg age (F1,86 = 15.43, P = 0.0002). When T. absoluta eggs were parasitized, they were consumed less by N. pseudoferus than nonparasitized eggs, and the consumption rate was related to the age of the eggs (Fig. 1).
Fig. 1.
Mean number (±SEM) of 24-, 48-, 72-h-old nonparasitized and parasitized eggs of T. absoluta eaten in 24 h by a female N. pseudoferus in no-choice experiments. Bars followed by different letters indicate significant differences at P = 0.05.
Choice Test
Comparison of the Manly’s β index indicated that the predatory bug did not show any preference for 24-h-old nonparasitized and parasitized T. absoluta eggs (Fig. 2A). Furthermore, N. pseudoferus preferred nonparasitized T. absoluta eggs over parasitized eggs in the 48- and 72-h-old eggs (Fig. 2B and C). The least preferred egg age was 72-h-old parasitized eggs. The predator preferred 72-h-old nonparasitized eggs (t = 10.98, df = 22, P < 0.0001) and 48-h-old nonparasitized eggs (t = 7.11, df = 24, P < 0.0001) to similar aged parasitized eggs. The preference index was 0.9 ± 0.009 for the 72-h-old eggs, and 0.59 ± 0.01 for the 48-h-old eggs. The predator did not show any preference with the 24-h-old eggs (t = 0.90, df = 24, P = 0.37) and had a preference index of 0.48 ± 0.01).
Fig. 2.
Mean values (±SE) of Manly’s preference index for the choice experiments. Prey items were offered simultaneously to a female N. pseudoferus, either 24-h-old eggs (A), or 48-h-old eggs (B), or 72-h-old eggs (C). The broken line (a = 0.5) indicates random choice (different letters indicate significant differences between means).
Switching
When exposed to the various nonparasitized and parasitized prey ratios using eggs that were 24 and 48 h old, N. pseudoferus switched from nonparasitized T. absoluta eggs when they became rare to parasitized eggs which were more abundant. The Manly’s indices indicated that the proportion on nonparasitized eggs attacked was smaller when nonparasitized eggs were rare, and attack rates increased with the abundance of the nonparasitized eggs (Fig. 3). For 24- and 48-h-old eggs, the preference for nonparasitized T. absoluta eggs was described by the formula β = −1.247 + 2.85ρ3 (r2 = 0.60) (Fig. 3A) and β = −0.472 + 0.757ρ3 (r2 = 0.39) (Fig. 3B), respectively. The slope was significantly positive for both 24- and 48-h-old eggs (w = 2.852 ± 0.29 and w = 0.757 ± 0.18), which indicated that the predator N. pseudoferus was capable of switching between nonparasitized and parasitized T. absoluta eggs. For 72-h-old eggs, the preference for nonparasitized T. absoluta eggs was described by the formula β = –0.03 – 0.205ρ3 (r2 = 0.33). The slope was significantly negative (w = −0.205 ± 0.093), indicating no switching.
Fig. 3.
Observed (dots) and predicted (solid line) variation of the preference index for N. pseudoferus females with changing proportions of nonparasitized and parasitized eggs in the diet. Vertical lines indicate the SEs associated with the indices A: 24-h-old eggs; B: 48-h-old eggs.
Discussion
Even though N. pseudoferus has been detected actively preying on T. absoluta eggs in greenhouses (Cabello et al. 2009), studies of its efficacy and it use in biological control programs are lacking. This study revealed that the differently aged eggs of T. absoluta were all accepted by N. pseudoferus with a clear preference for younger and nonparasitized eggs in both choice and no-choice experiments. The risk of IGP was greatest when pest eggs contained eggs of the parasitoid (24-h-old eggs), which suffered similar levels of predation as nonparasitized eggs. These findings partially agree with the results of Cabello et al. (2015) who evaluated N. tenuis as the predator of T. absoluta. This predator was able to eat T. absoluta eggs of all ages, but predation significantly decreased on old parasitized eggs. Chailleux et al. (2013) also tested the preference of M. pygmaeus on Trichogramma-parasitized and nonparasitized eggs of T. absoluta in no-choice and choice assays. In no-choice assays, the predator fed at the same rate on younger stages of Trichogramma and nonparasitized eggs and at a lower rate on old parasitized eggs. In choice assays, no preference between nonparasitized and the newly parasitized eggs was observed, but fewer eggs were consumed when the parasitized eggs contained parasite pupae. Oliveira et al. (2004) reported also that the predator P. maculiventris preferred nonparasitized eggs of E. kuehniella rather than those containing pupae of the parasitoid T. brassicae. By contrast, some studies have reported predation on eggs parasitized by Trichogramma spp. (Brower and Press 1988, Smith 1996, Kuhar et al. 2002, Philip et al. 2005). Other studies have reported that chrysopids (Meyhöfer and Klug 2002), syrphids (Brodeur and Rosenheim 2000), and coccinellids (Bilu and Coll 2009) show no preference for either parasitized or nonparasitized prey items, regardless of prey age.
Our choice tests confirm that N. pseudoferus females have a capacity to identify the most suitable hosts but are unable to recognize 24-h-old parasitized eggs. Discrimination ability could enable these foraging predators to reduce loss of time and energy on parasitized eggs and to exploit the encountered preys according to their relative value. Thus the risk of IGP by N. pseudoferus was greatest when T. brassicae parasitoids were at the early developmental stage (egg), which suffered similar levels of predation as nonparasitized eggs.
Little is known of the mechanisms underlying prey discrimination by nabids. The reasons for the observed preference of adult of N. pseudoferus for nonparasitized and younger eggs over parasitized and older eggs of T. absoluta could be explained by the following factors: 1) prey appearance/visual cues (Malo et al. 2012), 2) mechanical aspects (Pak et al. 1986, Vinson 1994) (including the hardness of T. absoluta eggshells), 3) physiological/chemical changes in the host caused by adult female Trichogramma, including tissue necrosis (Takada et al. 2000, Jarjees and Merritt 2003, Naranjo 2007, Pehlivan et al. 2017), and 4) substances produced by parasitoid larvae (Jarjees et al. 1998, Wu et al. 2000).
Predation preference depended strongly on the relative abundances of the prey species; as the predator consistently showed disproportionately high and low predation on the most abundant and the rarest prey, respectively. The absence of a clear preference of N. pseudoferus between parasitized and nonparasitized 24-h-old eggs highlighted the importance of prey switching in the predation behavior of this predator. Results of the switching experiment indicate that the N. pseudoferus can exhibit prey switching when foraging in areas where both parasitized and nonparasitized 24-h-old and 48-h-old eggs are present in varying proportion. We found that N. pseudoferus predator switches to the most abundant prey on tomato plants whether foraging on 24-h-old eggs or 48-h-old eggs. Many predators exhibit switching behaviors, including M. pygmaeus that is capable of switching between eggs of Bemisia tabaci (Gennadius) (Hem.: Aleyrodidae) and spider mites when these are offered simultaneously (Enkegaard et al. 2001). Jaworski et al. (2013) showed that predation by M. pygmaeus on B. tabaci and/or T. absoluta depends on the types of available prey. The predator tended to switch to the most abundant type of prey.
Our data indicate that N. pseudoferus does not switch to preying more on 72-h-old parasitized eggs when these become dominant. Indeed, a strong preference of N. pseudoferus for 72-h-old nonparasitized eggs prevents the predator from switching to the most abundant type of prey (72-h-old parasitized eggs). This finding is in accordance with that of Murdoch (1969) who reported that when the predator has a intensive preference for one of two prey, it does not have a chance to switch to whichever prey is abundant.
Based on N. pseudoferus switching between different life stages of nonparasitized and parasitized eggs of T. absoluta, a scheme is being developed to prevent the predator from consuming parasitized eggs at an early stage of parasitoid development. It appears that the first release of predator insects should be made about 3 d after the parasitoid release. Studying how prey age and different combinations of parasitized and nonparasitized eggs can influence the numbers of T. absoluta egg consumed by N. pseudoferus is important in determining whether it is likely to be an effective biological control agent of T. absoluta. This information is also relevant to integration of N. pseudoferus into biological control programs with other natural enemies such as T. brassicae against T. absoluta.
Acknowledgments
The authors are grateful to the Ferdowsi University of Mashhad for Grant No. 3/45035 which provided financial support for this research. The authors declare no conflicts of interest.
References Cited
- Al-Zyoud F., and Sengonca C.. . 2004. Prey consumption preferences of Serangium parcesetosum Sicard (Col., Coccinellidae) for different prey stages, species and parasitized prey. J. Pest. Sci. 77: 197–204. [Google Scholar]
- Babendreier D., Rostás M., Höfte M., Kuske S., and Bigler F.. . 2003. Effects of mass releases of Trichogramma brassicae on predatory insects in maize. Entomol. Exp. Appl. 108: 115–124. [Google Scholar]
- Bilu E., and Coll M.. . 2009. Parasitized aphids are inferior prey for a coccinellid predator: implications for intraguild predation. Environ. Entomol. 38: 153–158. [DOI] [PubMed] [Google Scholar]
- Brodeur J., and Rosenheim J. A.. . 2000. Intraguild interactions in aphid parasitoids. Entomol. Exp. Appl. 97: 93–108. [Google Scholar]
- Brower J. H., and Press J. W.. . 1988. Interactions between the egg parasite Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) and a predator, Xylocoris flavipes, (Hemiptera: Anthocoridae) of the almond moth, Cadra cautella (Lepidoptera: Pyralidae). J. Entomol. Sci. 23: 342–349. [Google Scholar]
- Cabello T., Gallego J., Fernandez-Maldonado F., Soler A., Beltran D., Parra A., and Vila E.. . 2009. The damsel bug N. pseudoferus (Hem.: Nabidae) as a new biological control agent of the South American Tomato Pinworm, Tuta absoluta (Lep.: Gelechiidae), in tomato crops of Spain. IOBC/WPRS Bull. 49: 219–223. [Google Scholar]
- Cabello T., Bonfil F., Gallego J. R., Fernandez F. J., Gamez M., and Garay J.. . 2015. Can interactions between an omnivorous hemipteran and an egg parasitoid limit the level of biological control for the tomato pinworm? Environ. Entomol. 44: 12–26. [DOI] [PubMed] [Google Scholar]
- Chailleux A., Bearez P., Pizzol J., Amiens-Desneux E., Ramirez-Romero R., and Desneux N.. . 2013. Potential for combined use of parasitoids and generalist predators for biological control of the key invasive tomato pest Tuta absoluta. J. Pest. Sci. 86: 533–541. [Google Scholar]
- Chesson J. 1983. The estimation and analysis of preference and its relationship to foraging models. Ecology. 64: 1297–1304. [Google Scholar]
- Chow A., Chau A., and Heinz K. M.. . 2008. Compatibility of Orius insidiosus (Hemiptera: Anthocoridae) with Amblyseius (Iphiseius) degenerans (Acari: Phytoseiidae) for control of Frankliniella occidentalis (Thysanoptera: Thripidae) on greenhouse roses. Biol. Control. 44: 259–270. [Google Scholar]
- Desneux N., Luna M. G., Guillemaud T., and Urbaneja A.. . 2011. The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: the new threat to tomato world production. J. Pest. Sci. 84: 403–408. [Google Scholar]
- Enkegaard A., Brødsgaard H. F., and Hansen D. L.. . 2001. Macrolophus caliginosus: functional response to whiteflies and preference and switching capacity between whiteflies and spider mites. Entomol. Exp. Appl. 101: 81–88. [Google Scholar]
- Fazal S., and Xiang R. S.. . 2004. Interaction of Serangium japonicum (Coleoptera: Coccinellidae), an obligate predator of whitefly with immature stages of Eretmocerus sp. (Hymenoptera: Aphelinidae) within whitefly host (Homoptera: Aleyrodidae). Asian. J. Plant. Sci. 3: 243–246. [Google Scholar]
- Fernandez-Maldonado F., Gallego J., Valencia A., Gamez M., Varga Z., Garay J., Cabello T.. . 2017. Cannibalism: do risks of fighting and reprisal reduce predatory rates? Community Ecol. 18: 87–96. [Google Scholar]
- Heinz K. M., and Nelson J. M.. . 1996. Interspecific interactions among natural enemies of Bemisia in an inundative biological control program. Biol. Control. 6: 384–393. [Google Scholar]
- Hoelmer K., Osborne L., and Yokomi R.. . 1994. Interactions of the whitefly predator Delphastus pusillus (Coleoptera: Coccinellidae) with parasitized sweetpotato whitefly (Homoptera: Aleyrodidae). Environ. Entomol. 23: 136–139. [Google Scholar]
- Jarjees E. A., and Merritt D. J.. . 2003. Structure of the gut contents of Trichogramma australicum Girault (Hymenoptera: Trichogrammatidae) larvae fixed in situ in eggs of its host Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Austral. Entomol. 42: 203–209. [Google Scholar]
- Jarjees E., Merritt D. J., and Gordh G.. . 1998. Anatomy of the mouthparts and digestive tract during feeding in larvae of the parasitoid wasp Trichogramma australicum Girault (Hymenoptera: Trichogrammatidae). Int. J. Insect Morphol. Embryol. 27: 103–110. [Google Scholar]
- Jaworski C. C., Bompard A., Genies L., Amiens-Desneux E., and Desneux N.. . 2013. Preference and prey switching in a generalist predator attacking local and invasive alien pests. PLoS One. 8: e82231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhar T. P., Wright M. G., Hoffmann M. P., and Chenus S. A.. . 2002. Life table studies of European corn borer (Lepidoptera: Crambidae) with and without inoculative releases of Trichogramma ostriniae (Hymenoptera: Trichogrammatidae). Environ. Entomol. 31: 482–489. [Google Scholar]
- Kutuk H., Yigit A., and Alaoglu O.. . 2011. Intraguild predation of Serangium parcesetosum Sicard (Coleoptera: Coccinellidae), on whitefly Bemisia tabaci (Homoptera: Aleyrodidae) parasitized by Eretmocerus mundus (Hymenoptera: Aphelinidae). Biocontrol Sci. Technol. 21: 985–989. [Google Scholar]
- Lucas E., Frechetteand B., and Alomar O.. . 2009. Resource quality, resource availability, and intraguild predation among omnivorous mirids. Biocontrol Sci. Technol. 19: 555–572. [Google Scholar]
- Ma J., Li Y.Z., Keller M., and Ren S.X.. . 2005. Functional response and predation of Nabis kinbergii (Hemiptera: Nabidae) to Plutella xylostella (Lepidoptera: Plutellidae). Insect Sci. 12: 281–286. [Google Scholar]
- Malo S., Arnó J., and Gabarra R.. . 2012. Intraguild interactions between the predator Macrolophus pygmaeus and the parasitoid Eretmocerus mundus, natural enemies of Bemisia tabaci. Biocontrol Sci. Technol. 22: 1059–1073. [Google Scholar]
- Manly B. 1974. A model for certain types of selection experiments. Biometrics. 30: 281–294. [Google Scholar]
- Meyhöfer R., and Klug T.. . 2002. Intraguild predation on the aphid parasitoid Lysiphlebus fabarum (Marshall) (Hymenoptera: Aphidiidae): mortality risks and behavioral decisions made under the threats of predation. Biol. Control. 25: 239–248. [Google Scholar]
- Meyling N. V., Enkegaard A., and Brødsgaard H.. . 2004. Intraguild predation by Anthocoris nemorum (Heteroptera: Anthocoridae) on the aphid parasitoid Aphidius colemani (Hhymenoptera: Braconidae). Biocontrol Sci. Technol. 14: 627–630. [Google Scholar]
- Murdoch W. W. 1969. Switching in general predators: experiments on predator specificity and stability of prey populations. Ecol. Monogr. 39: 335–354. [Google Scholar]
- Naranjo S. E. 2007. Intraguild predation on Eretmocerus sp. nr. emiratus, a parasitoid of Bemisia tabaci, by three generalist predators with implications for estimating the level and impact of parasitism. Biocontrol Sci. Technol. 17: 605–622. [Google Scholar]
- Oliveira H. N., De Clercq P., Zanuncio J. C., Pratissoli D., and Pedruzzi E. P.. . 2004. Nymphal development and feeding preference of Podisus maculiventris (Heteroptera: Pentatomidae) on eggs of Ephestia kuehniella (Lepidoptera: Pyralidae) parasitised or not by Trichogramma brassicae (Hymenoptera: Trichogrammatidae). Braz. J. Biol. 64: 459–463. [DOI] [PubMed] [Google Scholar]
- Pak G., Buis H., Heck I., and Hermans M.. . 1986. Behavioural variations among strains of Trichogramma spp.: host‐age selection. Entomol. Exp. Appl. 40: 247–258. [Google Scholar]
- Pehlivan S., Kurtuluş A., Alinç T., and Atakan E.. . 2017. Intraguild predation of Orius niger (Hemiptera: Anthocoridae) on Trichogramma evanescens (Hymenoptera: Trichogrammatidae). Eur. J. Entomol. 114: 609–613. [Google Scholar]
- Philip M. M., Orr D. B., and Hain F. P.. . 2005. Evaluation of biological and biorational control tactics for suppression of Nantucket pine tip moth damage in Virginia pine Christmas trees. J. Econ. Entomol. 98: 409–414. [DOI] [PubMed] [Google Scholar]
- Polis G. A., and Holt R. D.. . 1992. Intraguild predation: the dynamics of complex trophic interactions. Trends Ecol. Evol. 7: 151–154. [DOI] [PubMed] [Google Scholar]
- Polis G. A., Myers C. A., and Holt R. D.. . 1989. The ecology and evolution of intraguild predation: potential competitors that eat each other. Annu. Rev. Ecol. Evol. Syst. 20: 297–330. [Google Scholar]
- Pyke G. H. 1984. Optimal foraging theory: a critical review. Annu. Rev. Ecol. Evol. Syst. 15: 523–575. [Google Scholar]
- Rosenheim J. A. 1998. Higher-order predators and the regulation of insect herbivore populations. Annu. Rev. Entomol. 43: 421–447. [DOI] [PubMed] [Google Scholar]
- SAS Institute 2000. SAS on-line docs, version 8. SAS Institute, Carey, NC. [Google Scholar]
- Smith S. M. 1996. Biological control with Trichogramma: advances, successes, and potential of their use. Annu. Rev. Entomol. 41: 375–406. [DOI] [PubMed] [Google Scholar]
- Sohrabi F., Enkegaard A., Shishehbor P., Saber M., and Mosaddegh M. S.. . 2013. Intraguild predation by the generalist predator Orius majusculus on the parasitoid Encarsia formosa. BioControl. 58: 65–72. [Google Scholar]
- Takada Y., Kawamura S., and Tanaka T.. . 2000. Biological characteristics: growth and development of the egg parasitoid Trichogramma dendrolimi (Hymenoptera: Trichogrammatidae) on the cabbage armyworm Mamestra brassicae (Lepidoptera: Noctuidae). Appl. Entomol. Zool. 35: 369–379. [Google Scholar]
- Urbaneja A., González-Cabrera J., Arnó J., and Gabarra R.. . 2012. Prospects for the biological control of Tuta absoluta in tomatoes of the Mediterranean basin. Pest Manag. Sci. 68: 1215–1222. [DOI] [PubMed] [Google Scholar]
- Venzon M., Janssen A., and Sabelis M. W.. . 2002. Prey preference and reproductive success of the generalist predator Orius laevigatus. Oikos. 97: 116–124. [Google Scholar]
- Vinson S. B. 1994. Physiological interactions between egg parasitoids and their hosts, pp. 245–271. InWajnberg E. and Hassan S. A. (eds.), Biological control with egg parasitoids. CAB International, Oxford, United Kingdom. [Google Scholar]
- Wade M. R., Zalucki M. P., and Franzmann B. A.. . 2005. Influence of observer presence on Pacific damsel bug behavior: who is watching whom? J. Insect. Behav. 18: 651–667. [Google Scholar]
- Wu Z., Cohen A., and Nordlund D. A.. . 2000. The feeding behavior of Trichogramma brassicae: new evidence for selective ingestion of solid food. Entomol. Exp. Appl. 96: 1–8. [Google Scholar]