Abstract
Hypervariable T cell receptors (TCRs) play a key role in adaptive immunity, recognizing a vast diversity of pathogen-derived antigens. Our ability to extract clinically relevant information from large high-throughput sequencing of TCR repertoires (RepSeq) data is limited, because little is known about TCR–disease associations. We present Antigen-specific Lymphocyte Identification by Clustering of Expanded sequences (ALICE), a statistical approach that identifies TCR sequences actively involved in current immune responses from a single RepSeq sample and apply it to repertoires of patients with a variety of disorders — patients with autoimmune disease (ankylosing spondylitis [AS]), under cancer immunotherapy, or subject to an acute infection (live yellow fever [YF] vaccine). We validate the method with independent assays. ALICE requires no longitudinal data collection nor large cohorts, and it is directly applicable to most RepSeq datasets. Its results facilitate the identification of TCR variants associated with diseases and conditions, which can be used for diagnostics and rational vaccine design.
Hypervariable T-cell receptors (TCRs) recognise a vast diversity of pathogen-derived antigens; this study shows that TCRs responding to ongoing immune challenges can be identified using a statistical null model based on sequence similarity.
Introduction
A major goal of quantitative immunology is to be able to detect and predict T cell receptor (TCR) specificity from high-throughput sequencing of TCR repertoires (RepSeq) data. Current methods that rely on epitope-specific in vitro experiments such as MHC multimer assays [1–4] require knowledge of the individual’s HLA type as well as the presented peptide and do not capture the context of the immune response in vivo. Alternatives based on mining public TCRs from large cohorts of patients with a common condition [5–9] are very costly and only capture TCRs specific to widely shared HLA/epitope pairs, ignoring the private response. Another approach is to use longitudinal data to identify responding clonotypes [10], but this requires carefully planned experimental setups with time points taken before the infection, which is not always possible. Antigen-specific Lymphocyte Identification by Clustering of Expanded sequences (ALICE) overcomes these issues by predicting TCR involved in the immune response from single repertoire snapshots of single individuals, using sequence similarity.
Recent work has shown that TCRs recognizing the same epitopes often have similar sequences [2–4, 11, 12]. However, highly similar TCRs may also arise regardless of their binding properties, by virtue of their high generation probability by V(D)J recombination [13, 14], with clusters of similar TCRs found even in naive repertoires [2, 15]. To correct for those naive clusters, ALICE evaluates the number of similar sequences relative to the baseline expectation from V(D)J recombination statistics, allowing it to identify clusters of TCRs responding to the same antigen (as schematized in Fig 1a).
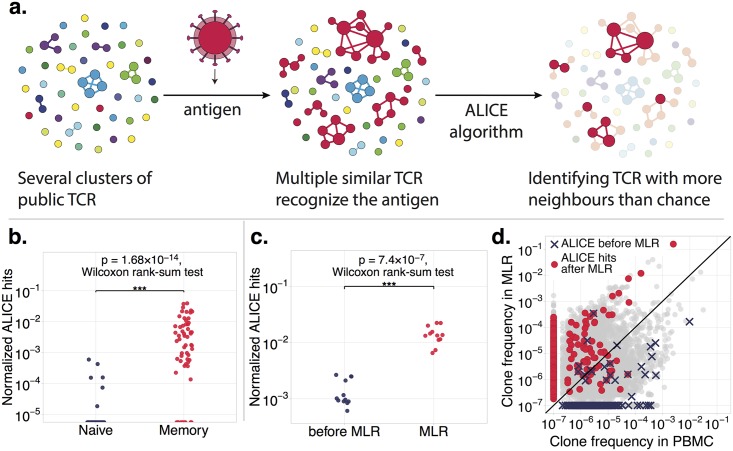
Fig 1. Identification of antigen-responding clonotypes using their recombination-based frequency.
(a) ALICE identifies locally enriched regions of the TCR sequence space, represented here as a graph. Vertices are TCR clonotypes observed in the repertoire, and edges connect sequences differing by at most 1 CDR3 amino acid. Antigen exposure induces the proliferation of multiple clonotypes with similar sequences recognizing a few immunodominant epitopes. ALICE identifies clonotypes with a higher numbers of neighbors than expected by a null model of recombination, separating clusters of antigen-responding clonotypes (in red) from clusters arising from recombination statistics (blue, green, and purple clusters). (b) The number of significant results (normalized by the total number of unique nucleotide sequences) found in naive- versus memory-published TCR beta repertoires from Thome and colleagues [16] demonstrates ALICE’s ability to selectively detect immune response signatures in the memory subset only. (c) Normalized number of significant hits found in published repertoires of MLR cultures compared to an unstimulated control [17]. The algorithm finds many more hits in the MLR repertoire. (d) Most clonotypes identified as antigen-responding in MLR culture expanded during the assay, as evidenced by their higher frequency in MLR culture than in the control (red dots). By contrast, clonotypes identified in the unstimulated repertoire (blue crosses) mostly remain unexpanded after the assay, as they probably are signatures of previous immune responses. The individual numerical values could be found in S1 Data. ALICE, Antigen-specific Lymphocyte Identification by Clustering of Expanded sequences; CDR3, Complementarity Determining Region 3; MLR, mixed lymphocyte reaction; PBMC, peripheral blood mononuclear cell; TCR, T cell receptor.
For each TCR amino acid sequence in the data, ALICE uses a stochastic TCR recombination model [18, 19] to estimate the fraction of the repertoire composed of TCR variants, called ‘neighbors’, differing by at most 1 amino acid in their Complementarity Determining Region 3 (CDR3). This allows us to predict theoretically the number of neighboring clonotypes (nucleotide sequences) for each TCR under the null hypothesis of no antigen-driven TCR selection and identify TCRs with a significantly higher number of neighbors in the data than the null expectation (see Materials and methods). We refer to such significant results as ALICE signatures or hits. Although the basic version of the algorithm discards clonotype abundances and should thus be sensitive to sequencing depth, we also implemented an advanced (but much slower) version that includes read counts and shuffles them among clonotypes in the null (see Materials and methods).
Results
As a minimal requirement for its validity, we applied our algorithm to published naive (CD45RA+CCR7+) and effector memory (CD45RA−CCR7−) TCR beta repertoires from Thome and colleagues [16]. Our algorithm identified multiple signatures in the memory subsets and virtually no significant hits in the naive subsets (Fig 1b and S1 Fig), in agreement with the definition that naive cells have never responded to antigen stimulation.
To further validate the method’s ability to detect clonal expansion during an ongoing immune response, we applied it to published TCR beta repertoires from mixed lymphocyte reaction (MLR) assay [17]. In this assay, peripheral blood mononuclear cells (PBMCs) from 2 individuals (a responder and a stimulator) are mixed, and reactive T-cell clones from the responder’s repertoire proliferate in response to the antigens presented by the stimulator’s cells. ALICE identified many more hits in the responder’s repertoire in the MLR culture than in unstimulated cells (Fig 1c). Furthermore, the clonotypes identified by ALICE are enriched in MLR culture compared to bulk PBMCs (Fig 1d), clearly demonstrating that these hits correspond to antigen-specific clonal expansions.
We then asked whether our method could identify TCRs specific to a particular target using an in vivo acute viral infection model. In a previous study, peripheral blood of 6 donors was collected, and their TCR beta repertoire was sequenced at several time points before and after immunization with live yellow fever (YF) vaccine (YF-17D) [10]. Clonotypes that significantly expanded following vaccination were identified by temporal comparisons. Notably, even the most strongly expanded clonotypes after YF immunization are not the most abundant clones in the repertoire even at the peak of the response (day 15 time point), and the overall clone size distribution on day 15 is similar to the one observed before vaccination. Thus, it is not possible to identify expanded clonotypes using only their frequencies on day 15. Here, we applied ALICE to each time point to identify responding clonotypes independently, using only single repertoire snapshots.
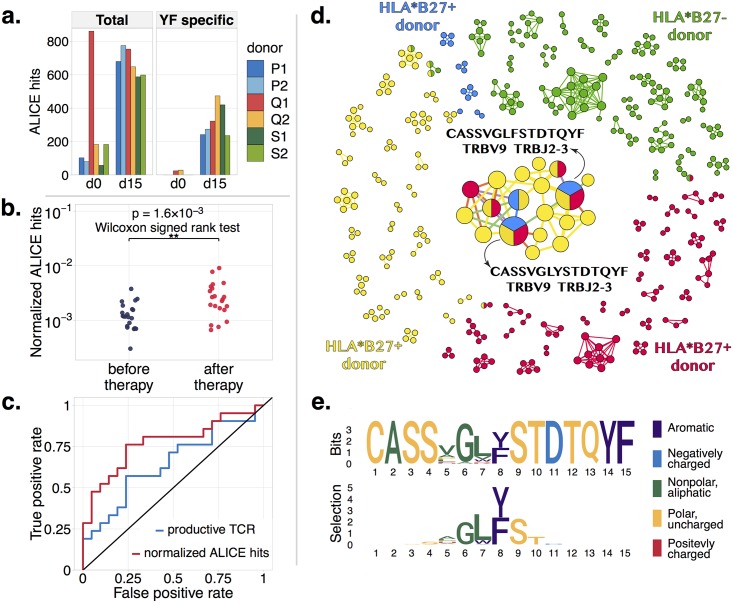
ALICE identified more immune response signatures on the peak of response (day 15) than before immunization (day 0) in all donors (Fig 2a, left) except one who was probably undergoing another immune response at the moment of immunization (see S2 Fig). Applying the advanced version of ALICE with read counts yielded almost identical results (see S3 Fig). To validate ALICE’s expanded clonotypes, we compared its predictions to known YF-17D–reactive sequences obtained from longitudinal data in the source study. We found that 35% to 73% of ALICE hits on day 15 were highly similar (same VJ combination and up to one CDR3 amino acid mismatch) to previously identified YF-specific clonotypes (Fig 2a, right).
Fig 2. ALICE hits are found in various conditions.
(a) Identification of reactive clonotypes following immunization. Left panel shows the total number of ALICE immune response signatures before (day 0) and on the peak of the response to YF vaccine (day 15). Right panel shows the number of clonotypes identified by the algorithm that have high similarity to clonotypes significantly expanded after YF immunization from Pogorelyy and colleagues [10]. (b) Analysis of peripheral blood repertoires before and after checkpoint blockade cancer immunotherapy [20]. The larger number of ALICE signatures after (red) compared with before (blue) therapy represent clones triggered by the therapy. (c) ROC curves for distinguishing pre- and post-immunotherapy repertoires. The number of ALICE hits (red, AUROC = 0.77) is a better discriminant than the number of unique clonotypes (blue, AUROC = 0.65). The individual numerical values can be found in S1 Data. (d) Graph of expanded clonotypes in synovial fluid of 4 AS patients. Vertices represent significant clonotypes identified by the algorithm, and edges connect clonotypes with at most 1 amino acid mismatch. Zero-degree vertices are not shown. Vertices are colored according to the patients, and split vertices represent public sequences identified in several donors. The 2 sequences shared among all 3 HLA-B27+ patients were previously associated with AS and HLA-B27. (e) While the classical sequence logo of the central cluster in panel D is dominated by germline-encoded positions (top), selection factors highlight position-specific pressures acting on the expanded sequences (bottom). ALICE, Antigen-specific Lymphocyte Identification by Clustering of Expanded sequences; AS, ankylosing spondylitis; AUROC, area under the ROC; ROC, receiver operating characteristic; YF, yellow fever.
Next, we applied our approach to peripheral blood TCR beta repertoire samples from 2 cytotoxic T-lymphocyte-associated protein 4 (CTLA4) checkpoint blockade cancer immunotherapy studies [20, 21]. We found more ALICE immune response signatures after the treatment than before (Fig 2b and S4 Fig). The number of these signatures is a better measure than previously proposed summary statistics of peripheral TCR repertoires aimed at detecting the effect of immunotherapy (richness and Shannon entropy): it discriminated pre- and post-treatment time points better than the number of unique clonotypes (richness) proposed in Robert and colleagues [20], as evidenced by the comparison of their receiver operating characteristic curves (Fig 2c). ALICE hits increased significantly after immunotherapy, both in the data from Robert and colleagues [20] (p = 0.0016 for ALICE versus p = 0.0005 for richness and p = 0.04 for entropy, Wilcoxon signed rank test) and in the data from Subudhi and colleagues [21] (p = 0.0003 for ALICE versus p = 0.06 for richness and p = 0.6 for entropy).
In contrast to these other measures, ALICE identifies particular clonotypes that are likely to be activated by the therapy. Tracking of such clonotypes in time and in the tumor tissue could provide insights into therapy efficiency and adverse effects.
Lastly, we asked whether ALICE was able to identify condition-associated clonotypes in patients with autoimmune diseases. We analyzed 4 TCR beta repertoires of CD8+ T cells from the synovial fluid of ankylosing spondylitis (AS) patients from Komech and colleagues [8]. Fig 2d shows clusters of ALICE-predicted clonotypes in 3 HLA-B27+ donors and 1 HLA-B27− donor. Although most predicted TCRs were patient-specific, 2 clonotypes were independently found in all 3 HLA-B27+ patients but not in the HLA-B27− patient. These 2 clonotypes exactly coincide with previously reported public clonotypes in a population of HLA-B27+ patients with AS [7, 8] and were also found in synovial fluid spectratyping of patients with AS and reactive arthritis [22, 23]. The independent identification of these sequences by ALICE demonstrates the relevance of its predictions, as well as suggests that these public clonotypes actively participate in the immune response in inflamed joints. ALICE also predicts previously unreported patient-specific expanded clonotypes, which population studies cannot detect by design.
To visualize CDR3 sequence motifs identified by the algorithm, we developed a novel approach to highlight differences in amino acid composition relative to the background recombination statistics (similar to [3]), based on a position-weight matrix selection model learned from the TCR sequence subset (as in [24], see Materials and methods). In a classical sequence logo derived from the central cluster of Fig 2d, positions encoded in the germline by V and J segments at the two ends of the CDR3 are very conserved and dominate the logo (Fig 2e, top). By contrast, our selection logo highlights amino acids that are enriched relative to that baseline (Fig 2e, bottom), showing a high enrichment in aromatic (Y and F) residues at CDR3 position 8. We speculate that these residues form contacts with the antigen that are crucial for TCR recognition.
Discussion
Our method can thus be applied to variety of conditions for which information about HLA type or epitope is not available, from autoimmune disease to infection models. While ALICE has different principles and scope than the computational method of Pogorelyy and colleagues [6], which was designed to analyze the public repertoire of cohorts, the two approaches could be combined to leverage the statistical power of large cohorts with the information from sequence similarity exploited by ALICE.
Our approach has several limitations. It can only identify responding TCR with high enough frequencies. A significant fraction of responding TCR are rare and individual specific [10, 12, 15] and are unlikely to have similar variants and thus to be detected by the algorithm. Extending our method to more refined distance measures (e.g., [3]) could help mitigate this issue. Another limitation is a natural consequence of its main advantage — antigen independence. In individuals with multiple conditions, the algorithm will identify clonotypes associated with all of them, and potentially with memory clones from previous immune responses as well, with no way of telling them apart. Repeating the analysis at different time points (e.g., after clearance of an infection) can help to distinguish TCR associated with each condition. In S2 Fig, we performed such an analysis for the outlying YF vaccinee Q1, who was probably undergoing another transient immune response, and identified ALICE signatures that were truly YF-specific. Finally, while ALICE can reliably detect expanded clonotypes from TCRβ repertoire, specificity is ultimately determined by the full αβ clonotype. ALICE could be extended to analyse αβ repertoires by estimating the joint probability of αβ recombinations using the OLGA algorithm [25], because the two chains are largely independent in their recombinations [26, 27].
As we have illustrated in our applications, the number of ALICE hits can be indicative of the immune activity. This new repertoire-wide metric could be used in combination or comparison with other metrics such as clonality, diversity, or maximum clone frequency [16, 20, 21] to predict immune status. However, ALICE’s main advance is to be able to identify particular sequences, which can be studied across patients that share a condition to identify publicly responding clones, as we did in the AS example. They could be tracked over time during and after the disease to help design biomarkers for diagnostics and to understand the persistence of immune memory. They could be searched in the repertoires of T-cell subpopulations to gain insight into their immunological function. As more repertoire sequence datasets associated with various conditions are being collected, ALICE could be used to rapidly grow databases of condition-specific TCR, with applications in the diagnostic and treatment of diseases.
Materials and methods
ALICE statistical model formulation
The algorithm operates on a dataset of n unique nucleotide TCR sequences (clonotypes) with a given VJ combination. The procedure is then applied to all VJ combinations present in the data. Unique nucleotide sequences have corresponding amino acid sequences. The goal is to find outlying sequences that have an abnormal number of nucleotide variants in the data that differ by at most 1 amino acid. The algorithm considers distinct nucleotide sequences as separate entities even if they have identical amino acid sequences, because they originate from different T-cell clones.
For each amino acid sequence σ, under the null hypothesis we expect the number of neighbors d to be Poisson distributed:
| (1) |
with mean λ = nΣσ' ~ σ QPgen(σ'). The sum is over all possible similar variants σ' of σ. Here, similarity σ' ~ σ is defined by having at most 1 amino acid mismatch, but other measures could be used instead. Pgen(σ') is the probability to generate a given amino acid sequence σ' by V(D)J recombination, and Q a rescaling factor accounting for thymic selection [6, 14] that eliminates a fraction 1/Q of generated sequences. Its value was set to Q = 9.41 as the average over all VJ combinations reported by Pogorelyy and colleagues [6]. There is an option in the algorithm to use separate selection factors for different CDR3 lengths L within each VJ class, QL|VJ. In this case, QL|VJ = Q × RL|VJ / ΣL'RL'|VJPdata (L'|VJ), where Q the same scaling factor as above, Pdata (L|VJ) is the probability of CDR3 length L given the VJ combination, and RL|VJ = Pdata (L|VJ)/Pgen (L|VJ), where Pdata (L|VJ) and Pgen (L|VJ) are the distribution of CDR3 lengths in each VJ class in the data and in the simulated sequences, respectively. We redid analysis for YF-vaccination datasets with this approach and got very similar results (see S5 Fig).
Estimating the generation probability of amino acid sequences
We estimated Pgen(σ) of amino acid sequences by Monte Carlo simulation, as described by Pogorelyy and colleagues [6]. We generated 100 million TCRs with fixed VJ choice in silico using the VDJ recombination model from Murugan and colleagues [18]. Sequences were then translated to amino acids, and the overall frequency of each distinct amino acid sequence was estimated by counting. The advantage of Monte Carlo simulations is that it can be done for all sequences of interest simultaneously. The exact computation of Pgen of each sequence of interest by OLGA [25] is available within the ALICE software and may be faster for datasets of moderate sizes. Another option would be to use large number of published datasets [5, 28] and treat the number of occurrences of each TCR sequence of interest in these datasets as a proxy for TCR recombination probability, as implemented into VDJtools [29]. An implementation of the corresponding routine for VDJtools software framework is described at http://vdjtools-doc.readthedocs.io/en/master/annotate.html#calcdegreestats. Note that VDJtools implementation allows setting an arbitrary Levenstein distance threshold for defining neighboring clonotypes. Forcing clonotypes to have the same VJ/V segments or allowing segment mismatches is also optional. The implementation relies on a precompiled control dataset instead of using a generative VDJ rearrangement model; control datasets can be obtained from https://zenodo.org/record/1318986.
ALICE pipeline
Nucleotide sequences with low numbers of reads may represent erroneous variants of high-frequency clonotypes and thus inflate their neighbor counts and lead to false positives. Here, we counted as neighbors only clonotypes with more than 1 read. To correct for sequencing errors in the germline regions, clonotypes with the same CDR3 nucleotide sequence and V- and J-segments were collapsed. To additionally filter sequencing errors, ALICE hits with Monte Carlo–estimated neighborhood size of 0 were also discarded. For each amino acid sequence σ present in the data, we count how many one-mismatch variants are also present in the data, and we denote that number d(σ). The neighborhood of σ includes σ, meaning that different nucleotide variants of the same amino acid sequence are counted as valid neighbors. For each σ such that d(σ) > 2, we generate all possible one-mismatch variants σ' in silico and calculate their Pgen(σ') using Monte Carlo simulations as described above. Finally, we calculate a p-value for each σ corresponding to the probability that σ has no less similar variants in null model than in the data, Σd' ≥ d(σ) P(d'|σ) using Eq 1. We correct p-values for multiple testing using Benjamini-Hochberg (BH) correction and select clonotypes with BH-adjusted p < 0.001 as significant results (ALICE hits).
Number of neighbors per se is not enough to reliably identify responding clonotypes
The main innovation of our approach is to use the probability of TCR sequence generation, Pgen, to get the null model for the expected number of neighbors for each sequence in the data. We wanted to quantify the improvement our null model provides on top of the initial clustering step. We expect the observed number of neighbors, d(σ), to grow after an immune challenge due to the expansion of many similar clonotypes. d(σ) scales with the total number of possible neighbors (equal to n, the number of unique clonotypes in a given VJ combination). As a simplest method, which does not use Pgen, one could simply select all clones with d(σ)/n above a certain threshold. We performed this analysis for the YF-vaccination dataset (see S1 Data). For each donor we picked a threshold d(σ)/n that selects the same amount of clones on day 15 in each donor as we identify with the ALICE approach, and the same threshold is used for the day 0 time point. For brevity, we call clones with d(σ)/n larger than the threshold d-hits. The number of d-hits is larger after vaccination than before (with the exception of Q1 donor). On day 0, there are 2 to 6 times more d-hits than ALICE hits (e.g., for donor P2, 478 d-hits versus 81 ALICE hits). On day 15, much (1.5–40 times) fewer d-hits are similar to known YF clones than ALICE hits. Notably, for one twin pair, P1 and P2, almost all d-hits are unrelated to the YF vaccination (e.g., for P2, there are 7 YF-like d-hits out of 775 on day 15 versus 274 YF-like ALICE hits). To summarize, the number of neighbors alone is not enough to identify interesting and potentially responding clonotypes, but the ALICE approach substantially improves on this, both decreasing the number of false positives (number of hits pre vaccination) and increasing the true positive rate (fraction of YF-like hits post vaccination).
Including abundance information
The basic pipeline only takes the occurrence of nucleotide sequences in the sample into account, and not their abundance (read count). To include that information, we replace in the pipeline the number of similar sequences, d, by a sum of transformed abundances over these sequences, , where ci is the abundance of the ith nucleotide variant with similar amino acid sequence. There exist several choices for the transformation f. f(c) = 1 − δc,0 gives back the basic method, s = d. f(c) = c corresponds to summing the abundances of all similar variants, whereas f(c) = log(c) corresponds to summing their logarithms. To define the null model, we assume that the abundance of each sequence is sampled at random from the distribution of empirical frequencies. Because this distribution followed a power law, we worked on the logarithmic scale, and we picked f(c) = log(c). To calculate P(s|σ), under null hypothesis, we use the identity P(s|σ) = Σd P(s|d)P(d|σ), where P(d|σ) was computed as described in the basic pipeline (Eq 1). Then, P(s|d) is obtained as a d-fold convolution of Pf(f), the probability distribution of the transformed abundances f (ci). For instance, P(s|d = 1) = Pf(f), P(s|d = 2) = ΣfPf(f)Pf(s − f) = (Pf * Pf) (s), etc., so that . These quantities do not depend on σ and are computed just once at the beginning of the procedure from the clonotype abundance distribution. Applying this advanced version of ALICE to the YF data of Fig 2a [10] yielded very similar results (S3 Fig) as the basic method. Although it is slower to implement, the advanced method could still be useful because it is expected to be robust to wide ranges of repertoire sampling depths, while the basic version implicitly relies on many sequences not being captured by the sample.
Statistics
To compare the normalized number of ALICE hits and maximum frequency of productive rearrangement between memory and naive repertoires (Fig 1b), we used Wilcoxon rank-sum two-tailed test (N = 52 naive subsets and N = 60 memory repertoires, p = 2.3 × 10−14 for maximum frequency of productive rearrangement, and p = 1.7 × 10−14 for normalized number of ALICE hits). To compare the normalized number of ALICE hits in repertoires before and after MLR (Fig 1c), we used Wilcoxon rank-sum two-tailed test (N = 12 pre-MLR PBMC samples, N = 12 MLR cultures, p = 7.4 × 10−7). To compare number of ALICE hits with other statistics between pre- and post-treatment time points for immunotherapy patients, we used Wilcoxon signed-rank two-tailed test: In Fig 2c (N = 21 before and N = 21 after therapy), the total number of clonotypes gave p = 0.0005, Shannon entropy gave p = 0.042, and normalized ALICE hits gave p = 0.0016. In S4 Fig (N = 40 before and N = 40 after first dose of therapy), the total number of clonotypes gave p = 0.6, Shannon entropy gave p = 0.6, and normalized ALICE hits gave p = 0.0003.
Estimating enrichment in certain amino acid position of the TCR motif
To estimate the enrichment of amino acids at specific positions in the set of expanded TCR, we used a position weight matrix model of TCR selection [24]. The sequence enrichment ratio takes a factorized form over amino acid positions, parametrized by selection coefficient si(σi), where σi denotes the amino acid of sequence σ at position i. The predicted frequency in the expanded set is then
| (2) |
where Z a normalization factor.
The si parameters were learned by gradient ascent of the likelihood function, to which an L2 regularization term, −λ || s ||2, was added. Specifically, selection coefficients are updated according to si(σi) ← si(σi) + ε[Pdata(σi) − Psel(σi) − 2λsi(σi)], where Psel(σi) is the predicted frequency of given amino acid at position i and Pdata(σi) is its observed frequency in the data. After each update, all si(σi) are shifted by a common additive constant to satisfy following normalization constraint: .
We applied this inference procedure on the 26 sequences forming the central cluster of sequences from AS patients in Fig 2d. ε was set to 0.5, and λ was set to 0.02. The algorithm was initialized with si(σi) = 0. The iterative procedure was repeated until the sum of the squared update difference was lower than 10−6. The bottom logo of Fig 2e shows values of si(σi) weighted by amino acid frequencies, so that the height of each letter is Pdata(σi)si(σi).
Supporting information
The classifier based on ALICE hits has a much higher true positive rate for low (up to 20%) false positive levels, but it could not distinguish naive and memory subpopulations both having 0 hits. To break these ties, we ranked memory and naive subsets with 0 ALICE hits by the maximum frequency of productive rearrangements (combined classifier, purple curve). The AUROCs for these classifiers are 0.89 (ALICE hits-based), 0.92 (maximum productive frequency-based), and 0.95 (combined classifier). ALICE, Antigen-specific Lymphocyte Identification by Clustering of Expanded sequences; AUROC, area under the ROC curve; ROC, receiver operating characteristic.
(TIFF)
One of the limitations of ALICE is inability to distinguish clonotypes specific for multiple conditions happening simultaneously, for instance, between a response to vaccination and a mild viral infection. Neither the signatures identified on day 0 (blue curve) nor the signatures identified on day 15 (red curve) are able to recapitulate the dynamics of the YF vaccine response. However, the subset of day 15 signatures that are absent on day 0 (purple curve) shows a clear YF-specific response with a peak on day 15. The 122 clonotypes found as significant on both day 0 and day 15 are not similar (defined as 1 amino acid mismatch) to any of the responding clonotypes identified by temporal differences [10], further suggesting that they are not YF-specific but instead correspond to another immune response that is already contracting at day 0. ALICE, Antigen-specific Lymphocyte Identification by Clustering of Expanded sequences; YF, yellow fever.
(TIFF)
The results of this analysis are almost identical to the results of Fig 2a, with many more signatures identified after immunization than before (with the exception of donor Q1) and a large fraction of ALICE hits identified on day 15 having similar sequences to previously identified YF-specific clonotypes from Pogorelyy and colleagues [10]. ALICE, Antigen-specific Lymphocyte Identification by Clustering of Expanded sequences; YF, yellow fever.
(TIFF)
(a) The number of ALICE hits is significantly higher after immunotherapy than before. (b) Scatterplot of the normalized number of ALICE hits before and after therapy in each patient; most points are concentrated above the equality line, showing an increase in the number of hits after therapy in most patients. ALICE, Antigen-specific Lymphocyte Identification by Clustering of Expanded sequences.
(TIFF)
The results of this analysis are almost identical to the results of Fig 2a, with many more signatures identified after immunization than before (with the exception of donor Q1) and a large fraction of ALICE hits identified on day 15 having similar sequences to previously identified YF-specific clonotypes from Pogorelyy and colleagues [10]. Antigen-specific Lymphocyte Identification by Clustering of Expanded sequences; CDR3, Complementarity Determining Region 3; YF, yellow fever.
(TIFF)
For each donor we set a threshold on normalized number of neighbors for each clone d/n, so the selected number of clonotypes on day 15 is the same as identified by ALICE, see Fig 2a. Here, we plot the absolute number of clones exceeding this threshold (d-hits). Notably, on day 0 the number of d-hits is larger than the number of ALICE hits. On the other hand, the fraction of YF-related d-hits is lower (reaching almost 0 for donors P1–P2) on day 15 than the same fraction for ALICE hits. ALICE, Antigen-specific Lymphocyte Identification by Clustering of Expanded sequences; YF, yellow fever.
(TIFF)
(XLSX)
Acknowledgments
We thank Y. Elhanati for his valuable help in running TCRdist.
Abbreviations
- ALICE
Antigen-specific Lymphocyte Identification by Clustering of Expanded sequences
- AS
ankylosing spondylitis
- BH
Benjamini-Hochberg
- CDR3
Complementarity Determining Region 3
- CTLA4
cytotoxic T-lymphocyte-associated protein 4
- MLR
mixed lymphocyte reaction
- PBMC
peripheral blood mononuclear cell
- RepSeq
high-throughput sequencing of TCR repertoires
- TCR
T cell receptor
- YF
yellow fever
Data Availability
The following references refer to the references in the manuscript. We used processed data provided by the authors of the following papers: mixed lymphocyte reaction data from [19]; sorted naive and effector memory repertoires from [18]; repertoires of patients before and after anti-CTLA4 immunotherapy from [20,21]; repertoires before and after immunization with live yellow fever vaccine from [10]; and ankylosing spondylitis data from [8]. For [10], only the first replicate of each time point (day 0 and day 15) was used. For [21], we used paired samples of patients before and after the first dose of therapy. Links to all data sets produced by Adaptive biotechnogies, data sets from [8,10] used in this study, and all ALICE hits for each data set along with their p-values and theoretical and observed neighborhood sizes are available at at: https://github.com/pogorely/ALICE_sample_data. All code for the ALICE pipeline is available at https://github.com/pogorely/ALICE. The numerical values presented in all the plots can be found in the Supporting Information data file (S1_Data.xlsx) as indicated in the manuscript.
Funding Statement
This work was supported by the Russian Science Foundation Grant Number 15-15-00178. MVP is supported by the Skoltech Systems biology fellowship. This work was partially supported by the European Research Council Consolidator Grant Number 724208. DMC and MS were supported by a grant from the Ministry of Education and Science of the Russian Federation Number 14.W03.31.0005 (in part of cancer immunotherapy data analysis). MS is supported by Russian Science Foundation Grant Number 17-15-01495.
References
- 1.Davis MM, Altman JD, Newell EW. Interrogating the repertoire: broadening the scope of peptide–MHC multimer analysis. Nature Reviews Immunology. 2011;11(8):551–558. 10.1038/nri3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glanville J, Huang H, Nau A, Hatton O, Wagar LE, Rubelt F, et al. Identifying specificity groups in the T cell receptor repertoire. Nature. 2017;547(7661):94–98. 10.1038/nature22976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dash P, Fiore-Gartland AJ, Hertz T, Wang GC, Sharma S, Souquette A, et al. Quantifiable predictive features define epitope-specific T cell receptor repertoires. Nature. 2017;547(7661):89–93. 10.1038/nature22383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shugay M, Bagaev DV, Zvyagin IV, Vroomans RM, Crawford JC, Dolton G, et al. VDJdb: a curated database of T-cell receptor sequences with known antigen specificity. Nucleic Acids Research. 2017;46(September 2017):419–427. 10.1093/nar/gkx760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emerson RO, DeWitt WS, Vignali M, Gravley J, Hu JK, Osborne EJ, et al. Immunosequencing identifies signatures of cytomegalovirus exposure history and HLA-mediated effects on the T cell repertoire. Nature Genetics. 2017;49(5):659–665. 10.1038/ng.3822 [DOI] [PubMed] [Google Scholar]
- 6.Pogorelyy MV, Minervina AA, Chudakov DM, Mamedov IZ, Lebedev YB, Mora T, et al. Method for identification of condition-associated public antigen receptor sequences. eLife. 2018;7:1–12. 10.7554/eLife.33050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faham M, Carlton V, Moorhead M, Zheng J, Klinger M, Pepin F, et al. Discovery of T cell receptor β motifs specific to HLA-B27-positive ankylosing spondylitis by deep repertoire sequence analysis. Arthritis & Rheumatology. 2017;69(4):774–784. 10.1002/art.40028 [DOI] [PubMed] [Google Scholar]
- 8.Komech EA, Pogorelyy MV, Egorov ES, Britanova OV, Rebrikov DV, Bochkova AG, et al. CD8+ T cells with characteristic T cell receptor beta motif are detected in blood and expanded in synovial fluid of ankylosing spondylitis patients. Rheumatology. 2018;36(8):878–883. 10.1093/rheumatology/kex517 [DOI] [PubMed] [Google Scholar]
- 9.DeWitt WS, Smith A, Schoch G, Hansen JA, Matsen FA, Bradley PH. Human T cell receptor occurrence patterns encode immune history, genetic background, and receptor specificity. bioRxiv/313106. 2018; 10.1101/313106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pogorelyy MV, Minervina AA, Touzel MP, Sycheva AL, Komech EA, Kovalenko EI, et al. Precise tracking of vaccine-responding T cell clones reveals convergent and personalized response in identical twins. Proceedings of the National Academy of Sciences. 2018; 10.1073/pnas.1809642115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venturi V, Kedzierska K, Price DA, Doherty PC, Douek DC, Turner SJ, et al. Sharing of T cell receptors in antigen-specific responses is driven by convergent recombination. Proc Natl Acad Sci. 2006;103(49):18691–6. 10.1073/pnas.0608907103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi Q, Cavanagh MM, Le Saux S, NamKoong H, Kim C, Turgano E, et al. Diversification of the antigen-specific T cell receptor repertoire after varicella zoster vaccination. Science Translational Medicine. 2016;8(332):332ra46–332ra46. 10.1126/scitranslmed.aaf1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venturi V, Quigley MF, Greenaway HY, Ng PC, Ende ZS, McIntosh T, et al. A mechanism for TCR sharing between T cell subsets and individuals revealed by pyrosequencing. J Immunol. 2011;186(7):4285–4294. 10.4049/jimmunol.1003898 [DOI] [PubMed] [Google Scholar]
- 14.Elhanati Y, Sethna Z, Callan CG, Mora T, Walczak AM. Predicting the spectrum of TCR repertoire sharing with a data-driven model of recombination. Immunological reviews. 2018;284(1):167–179. 10.1111/imr.12665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madi A, Poran A, Shifrut E, Reich-Zeliger S, Greenstein E, Zaretsky I, et al. T cell receptor repertoires of mice and humans are clustered in similarity networks around conserved public CDR3 sequences. eLife. 2017;6 10.7554/eLife.22057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thome JJC, Grinshpun B, Kumar BV, Kubota M, Lerner H, Sempowski GD, et al. Longterm maintenance of human naive T cells through in situ homeostasis in lymphoid tissue sites. Sci Immunol. 2016;1(6):1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emerson RO, Mathew JM, Konieczna IM, Robins HS, Leventhal JR. Defining the alloreactive T cell repertoire using high-throughput sequencing of mixed lymphocyte reaction culture. PLoS ONE. 2014;9(11):1–7. 10.1371/journal.pone.0111943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murugan A, Mora T, Walczak AM, Callan CG. Statistical inference of the generation probability of T-cell receptors from sequence repertoires. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(40):16161–6. 10.1073/pnas.1212755109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcou Q, Mora T, Walczak AM. High-throughput immune repertoire analysis with IGoR. Nat Commun. 2018;9(1):561 10.1038/s41467-018-02832-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robert L, Tsoi J, Wang X, Emerson R, Homet B, Chodon T, et al. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clinical Cancer Research. 2014;20(9):2424–2432. 10.1158/1078-0432.CCR-13-2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subudhi SK, Aparicio A, Gao J, Zurita AJ, Araujo JC, Logothetis CJ, et al. Clonal expansion of CD8 T cells in the systemic circulation precedes development of ipilimumab-induced toxicities. Proceedings of the National Academy of Sciences. 2016;113(42):11919–11924. 10.1073/pnas.1611421113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dulphy N, Peyrat MA, Tieng V, Douay C, Rabian C, Tamouza R, et al. Common intra-articular T cell expansions in patients with reactive arthritis: identical beta-chain junctional sequences and cytotoxicity toward HLA-B27. Journal of immunology (Baltimore, MD: 1950). 1999;162(7):3830–9. [PubMed] [Google Scholar]
- 23.May E, Dulphy N, Frauendorf E, Duchmann R, Bowness P, Lopez de Castro JA, et al. Conserved TCR beta chain usage in reactive arthritis; evidence for selection by a putative HLA-B27-associated autoantigen. Tissue antigens. 2002;60(4):299–308. 10.1034/j.1399-0039.2002.600404.x [DOI] [PubMed] [Google Scholar]
- 24.Elhanati Y, Murugan A, Callan CG, Mora T, Walczak AM, Callan CG Jr, et al. Quantifying selection in immune receptor repertoires. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(27):9875–80. 10.1073/pnas.1409572111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sethna Z, Elhanati Y, Callan CG, Mora T, Walczak AM. OLGA: fast computation of generation probabilities of B- and T-cell receptor amino acid sequences and motifs. Bioinformatics, 2019, btz035, 10.1093/bioinformatics/btz035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grigaityte K, Carter JA, Goldfless SJ, Jeffery EW, Hause RJ, Jiang Y, et al. Single-Cell Sequencing Reveals Aβ Chain Pairing Shapes the T Cell Repertoire. bioRxiv. 2017; p. 213462. 10.1101/213462 [DOI] [Google Scholar]
- 27.Dupic T, Marcou Q, Walczak AM, Mora T. Genesis of the αβ T-cell receptor. PLoS Comput Biol. 2019;15(3):e1006874 10.1371/journal.pcbi.1006874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Britanova OV, Shugay M, Merzlyak EM, Staroverov DB, Putintseva EV, Turchaninova MA, et al. Dynamics of Individual T Cell Repertoires: From Cord Blood to Centenarians. The Journal of Immunology. 2016;196(12):5005–13. 10.4049/jimmunol.1600005 [DOI] [PubMed] [Google Scholar]
- 29.Shugay M, Bagaev DV, Turchaninova MA, Bolotin DA, Britanova OV, Putintseva EV, et al. VDJtools: Unifying Post-analysis of T Cell Receptor Repertoires. PLoS Comput Biol. 2015;11(11):e1004503 10.1371/journal.pcbi.1004503 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The classifier based on ALICE hits has a much higher true positive rate for low (up to 20%) false positive levels, but it could not distinguish naive and memory subpopulations both having 0 hits. To break these ties, we ranked memory and naive subsets with 0 ALICE hits by the maximum frequency of productive rearrangements (combined classifier, purple curve). The AUROCs for these classifiers are 0.89 (ALICE hits-based), 0.92 (maximum productive frequency-based), and 0.95 (combined classifier). ALICE, Antigen-specific Lymphocyte Identification by Clustering of Expanded sequences; AUROC, area under the ROC curve; ROC, receiver operating characteristic.
(TIFF)
One of the limitations of ALICE is inability to distinguish clonotypes specific for multiple conditions happening simultaneously, for instance, between a response to vaccination and a mild viral infection. Neither the signatures identified on day 0 (blue curve) nor the signatures identified on day 15 (red curve) are able to recapitulate the dynamics of the YF vaccine response. However, the subset of day 15 signatures that are absent on day 0 (purple curve) shows a clear YF-specific response with a peak on day 15. The 122 clonotypes found as significant on both day 0 and day 15 are not similar (defined as 1 amino acid mismatch) to any of the responding clonotypes identified by temporal differences [10], further suggesting that they are not YF-specific but instead correspond to another immune response that is already contracting at day 0. ALICE, Antigen-specific Lymphocyte Identification by Clustering of Expanded sequences; YF, yellow fever.
(TIFF)
The results of this analysis are almost identical to the results of Fig 2a, with many more signatures identified after immunization than before (with the exception of donor Q1) and a large fraction of ALICE hits identified on day 15 having similar sequences to previously identified YF-specific clonotypes from Pogorelyy and colleagues [10]. ALICE, Antigen-specific Lymphocyte Identification by Clustering of Expanded sequences; YF, yellow fever.
(TIFF)
(a) The number of ALICE hits is significantly higher after immunotherapy than before. (b) Scatterplot of the normalized number of ALICE hits before and after therapy in each patient; most points are concentrated above the equality line, showing an increase in the number of hits after therapy in most patients. ALICE, Antigen-specific Lymphocyte Identification by Clustering of Expanded sequences.
(TIFF)
The results of this analysis are almost identical to the results of Fig 2a, with many more signatures identified after immunization than before (with the exception of donor Q1) and a large fraction of ALICE hits identified on day 15 having similar sequences to previously identified YF-specific clonotypes from Pogorelyy and colleagues [10]. Antigen-specific Lymphocyte Identification by Clustering of Expanded sequences; CDR3, Complementarity Determining Region 3; YF, yellow fever.
(TIFF)
For each donor we set a threshold on normalized number of neighbors for each clone d/n, so the selected number of clonotypes on day 15 is the same as identified by ALICE, see Fig 2a. Here, we plot the absolute number of clones exceeding this threshold (d-hits). Notably, on day 0 the number of d-hits is larger than the number of ALICE hits. On the other hand, the fraction of YF-related d-hits is lower (reaching almost 0 for donors P1–P2) on day 15 than the same fraction for ALICE hits. ALICE, Antigen-specific Lymphocyte Identification by Clustering of Expanded sequences; YF, yellow fever.
(TIFF)
(XLSX)
Data Availability Statement
The following references refer to the references in the manuscript. We used processed data provided by the authors of the following papers: mixed lymphocyte reaction data from [19]; sorted naive and effector memory repertoires from [18]; repertoires of patients before and after anti-CTLA4 immunotherapy from [20,21]; repertoires before and after immunization with live yellow fever vaccine from [10]; and ankylosing spondylitis data from [8]. For [10], only the first replicate of each time point (day 0 and day 15) was used. For [21], we used paired samples of patients before and after the first dose of therapy. Links to all data sets produced by Adaptive biotechnogies, data sets from [8,10] used in this study, and all ALICE hits for each data set along with their p-values and theoretical and observed neighborhood sizes are available at at: https://github.com/pogorely/ALICE_sample_data. All code for the ALICE pipeline is available at https://github.com/pogorely/ALICE. The numerical values presented in all the plots can be found in the Supporting Information data file (S1_Data.xlsx) as indicated in the manuscript.