Abstract
This protocol describes the 5-choice serial reaction time task, which is an operant based task used to study attention and impulse control in rodents. Test day challenges, modifications to the standard task, can be used to systematically tax the neural systems controlling either attention or impulse control. Importantly, these challenges have consistent effects on behavior across laboratories in intact animals and can reveal either enhancements or deficits in cognitive function that are not apparent when rats are only tested on the standard task. The variety of behavioral measures that are collected can be used to determine if other factors (i.e., sedation, motivation deficits, locomotor impairments) are contributing to changes in performance. The versatility of the 5CSRTT is further enhanced because it is amenable to combination with pharmacological, molecular, and genetic techniques.
Keywords: Neuroscience, Issue 90, attention, impulse control, neuroscience, cognition, rodent
Introduction
The 5-choice serial reaction time task (5CSRTT) was developed by Trevor Robbins and colleagues at the University of Cambridge in order to understand the behavioral deficits displayed by people diagnosed with attention deficit hyperactivity disorder (ADHD)1,2. It is based upon continuous performance tasks used to study attention in humans3; with attention being defined as the ability to allocate and sustain the focus of cognitive resources on specific stimuli or information while ignoring other information4. Although the task was originally designed for use with rats1,2, a mouse version has also been developed5,6.
The basic 5CSRTT requires rats to scan a horizontal array of five apertures for the presentation of a brief light stimulus (cue) in one of the apertures; once the rat detects the stimulus it must nose poke in the illuminated aperture to receive a sugar pellet reward. Thus, the task requires rats to both divide their attention across the 5 spatially distinct apertures and to sustain attention until the stimulus is presented in a given trial and across multiple trials in a session1,7. Attention is typically assessed by the accuracy of responses. Although the 5CSRTT was originally designed to assess attention, it is also used to assess impulsive behavior or response inhibition1,7,8: the ability to withhold pre-potent or inappropriate responding9. During the task, rats must withhold responding for the duration of the inter trial interval (ITI) and only respond once the stimulus is presented in one of the apertures1. Thus premature responses, those occurring during the ITI prior to stimulus presentation, provide a useful index of impulsive behavior.
The 5CSRTT is an incredibly flexible task—there are a number of modifications of the basic task (i.e., test day challenges) that can be implemented to more carefully examine how experimental manipulations affect behavior. For example, decreasing the stimulus duration or shortening the ITI are different mechanisms to increase the attentional load of the task and can be used to systematically assess subdomains of attention1,7,10-12. In contrast, increasing the stimulus duration minimizes the attentional demands of the task; this can be used to determine if a manipulation interferes with the ability to execute the basic response requirements of the task12. Increasing the duration of the ITI can be used to determine whether a particular manipulation affects impulsive responding1,7,8,13-15. Moreover, using test day challenges, such as those just described, can reveal deficits10 or enhancements16,17 of behavior that are not apparent in well trained rats tested using standard testing parameters.
Importantly, the 5CSRTT is amenable to combination with a number of different techniques; for example cognition has been investigated following lesions of discrete brain areas10,18-20, or selective neurotransmitter depletions2,21,22. Behavioral pharmacological investigations have used either systemic16,17,23-28 or discrete intracranial administration of drugs29-32. Moreover performance is easily assessed after acute12,16,17,29-32 and chronic drug administration13,14,23,33. The effects of task performance on neurotransmitter release34 and metabolic activity35 in discrete brain areas have also been assessed. In addition, performance on the task can be used to separate rats into groups based on baseline attentional performance30,31 or levels of impulsivity15,32. Finally, with the advent of a mouse version of the 5CSRTT5,6, the task has been used to investigate the genetic contributions to attention and impulse control5,36-39.
Because the 5CSRTT assesses multiple cognitive functions simultaneously and is amenable to use in combination of a variety of pharmacological, molecular and genetic approaches it has been routinely used to assess cognitive dysfunction in the context of animal models of psychiatric and neurological disorders. For example, the 5CSRTT has been used to investigate the neurobiology underlying the cognitive disruptions in attention deficit hyperactivity disorder (ADHD)37,40,41, schizophrenia23,33,42, drug addiction13,14,43-45, Alzheimer’s disease18,39, Parkinson’s disease36, and Huntington’s disease37.
This protocol provides guidelines for training rats on the 5CSRTT. Because a number of performance measures can be collected, we describe how common patterns of results should be interpreted. In addition several common modifications to the basic protocol, the test day challenges, are described.
Protocol
This procedure requires the use of animals; these procedures were approved by the Oberlin College Institutional Animal Care and Use committee and are in accordance with the Guide for the Care and Use of Laboratory Animals46.
1. 5CSRTT Apparatus
- A schematic of the 5CSRTT apparatus is provided in Figure 1.
- The 5CSRTT apparatus consists of an operant conditioning chamber (30.5 x 24.1 x 29.2 cm) with 2 Plexiglas sidewalls, and a stainless steel grid floor.
- The aluminum front wall is rounded and contains five nose poke apertures (2.5 x 2.2 x 2.2 cm each); each aperture is equipped with a light emitting diode (LED) and an infrared sensor capable of detecting the insertion of the rats’ nose.
- The aluminum back wall contains the food magazine; this is connected to a pellet dispenser and is equipped with an infrared sensor and a small incandescent light.
- An incandescent house light, capable of illuminating the entire chamber is affixed near the top of the back wall.
House the 5CSRTT apparatus within a ventilated, sound attenuated operant conditioning chamber.
Use a PC computer to control the operant chamber and to collect data.
2. Animal Housing and Preparation
The procedure requires use of experimental animals; obtain approval from the Institutional Animal Care and Use Committee (IACUC) prior to commencing any experiments.
- House rats in pairs.
- Maintain rats on a 14:10 light/dark cycle (lights on at 7:00 am) in a room with a constant temperature of 22 ± 1 °C.
- Allow rats free access to water while in their home cages, but food restrict them.
- Food restrict rats to 85-90% of their free feeding weight two to five days prior to the start of magazine training and throughout 5CSRTT training and testing. Feed rats after daily training sessions and weigh them daily to ensure that they are gaining weight (~5 g/week).
3. 5CSRTT Procedure
- General 5CSRTT Training Considerations
- Train rats at a standard time of day a minimum of 5 days per week; training occurs over many stages (see Table 1) and takes several weeks.
- Train and test rats in the same operant chamber (see sections 3.2-3.5 below); small deviations in the rats’ environment can affect their performance.
- At the end of each session clean all surfaces of the chamber with water containing the disinfectant sodium hypochlorite (or another disinfectant after consultation with your veterinarian).
- Magazine Training
- Introduce sugar pellets (45 mg) to rats in their home cage prior to the first session. This minimizes neophobia and ensures rats will retrieve sugar pellets from the food magazine.
- Place rats in operant chamber (fans on) with aperture holes occluded. Allow rats to habituate to chamber for 5 min.
- Deliver sixty sugar pellets to magazine on either a fixed interval 20 sec (FI-20, day 1) schedule or a FI-30 schedule (day 2). Note: The houselight can remain illuminated for the duration of the session and the magazine light should be illuminated upon pellet delivery and can remain illuminated until the pellet is retrieved.
- At the end of the session note whether sugar pellets are consumed; continue training for at least 2 days or until all of the pellets are consumed.
- 5CSRTT Training
- Figure 2 provides a schematic of a single 5CSRTT trial.
- Place each rat in an operant chamber with fans on. Allow rats to habituate to chamber for 5 min.
- At the end of the habituation period, illuminate the magazine light and deliver one sugar pellet. The first trial begins when this pellet is retrieved.
- Begin each trial with an inter trial interval (ITI) during which only the house light is illuminated. At the end of the ITI, pseudo randomly illuminate one of the aperture lights for the prescribed stimulus duration. Allow the rat time to respond to the stimulus presentation; the time the rat is allowed to respond is the limited hold (LH).
- Nose poke responses into the illuminated aperture result in the delivery of a sugar pellet to the magazine and illumination of the magazine light; such responses are considered correct responses. Extinguish the magazine light and initiate the next trial upon pellet retrieval.
- Nose poke responses into unlit apertures (incorrect responses) and failures to respond during the LH (omissions) result in a time out (TO). Extinguish the houselight during the TO. Signal the beginning of the next trial at the end of the TO by illuminating the houselight.
- Score nose poke responses during the ITI as premature responses; punish these responses with a TO period. Reinitiate the same trial after a TO caused by a premature response.
- End each session after 90 trials or 30 min, whichever comes first.
- For the first stage of training set the stimulus duration to 30 sec, the LH to 30 sec, the ITI to 2 sec and the TO to 2 sec. Adjust these parameters across training such that the stimulus duration is 1 sec and the LH, ITI and TO are all 5 sec (see Table 1). Move rats to the next training stage once they have reached the suggested training criteria. Test rats after they exhibit stable performance on the final stage. Note: Sprague-Dawley rats typically reach criterion performance (~65 % accuracy, < 20% omissions) within 4-8 weeks if trained 5 days per week. Different strains of rats however, have different abilities to perform the task. For example, Lister Hooded rats can attain high levels of accuracy (> 80%) with fewer omissions (< 20%) using a short stimulus duration (0.5 sec) (e.g., Bari et al., 2008; Mizra and Bright, 2001). It is recommended that researchers adjust stimulus parameters and criterion performance to reflect strain of rat (or mouse) used.
- 5CSRTT Testing
- Calculate baseline performance (% accuracy, % omissions, premature responses) by averaging performance on each measure across the last 3-5 sessions (see below). Use these data to divide rats into groups (if required) or as an individual rats’ baseline.
- Use test day challenges (Table 2) to further probe behavior.
- For within subjects designs, intersperse training sessions with test day challenge sessions. Note: Test day challenges can be used to complement examination of behavior on the standard task. These manipulations can reveal subtle changes in behavior that are not apparent after extensive training on the standard task and/or be used to gain a better understanding of the nature of a performance deficit.
- 5CSRTT Performance Measures
- Use a computer to calculate performance measures.
- Accuracy of responding (% accuracy): Divide the number of correct responses by the total of correct and incorrect responses. This is the primary measure of attention. [# correct responses/(# correct responses+ # incorrect response)] x 100
- Omissions: Divide the number of trials in which the rat did not respond by the total number of trials completed. % Omissions can reflect attention, but can also be influenced by sedation, motivation and motor ability, thus the interpretation of omissions depends on other performance measures. [# omissions/(# omissions + # correct responses + # incorrect responses)] x 100
- Correct Responses: Divide the number of correct responses by the total number of trials completed. [# correct responses /(# omissions + # correct responses + # incorrect responses)] x 100
- Incorrect Responses: Divide the number of incorrect responses by the total number of trials completed. [# incorrect responses /(# omissions + # correct responses + # incorrect responses)] x 100
- Premature Responses: Determine the number of responses made during the ITI. This is the primary measure of impulsive behavior.
- Perseverative Responses: Determine the number of nose poke responses into any aperture after the rat has made a correct response but before retrieval of the sugar pellet. This is a measure of compulsive behavior.
- Magazine Entries: Determine the number of nose poke responses into the magazine. This is a measure of motivation.
- Latency to Correct Response: Calculate the average time from the onset of the stimulus to a correct response; a measure of processing speed or decision-making.
- Latency to Incorrect Response: Calculate the average time from the onset of the stimulus to an incorrect response; a measure of processing speed or decision-making.
- Reward Retrieval Latency: Calculate the average time for a rat to retrieve the sugar pellet reward; can reflect motivation.
Representative Results
Manipulations of the 5CSRTT that Probe Visuospatial Attention
One approach to varying the attentional demands of the task is to alter the duration of the stimulus. As the stimulus duration decreases, % accuracy decreases (Figure 3A) and % omissions increase (Figure 3B; adapted from 12). Thus shorter stimulus durations increase the attentional demands of the task and longer stimulus durations decrease the attentional demands of the task. Changing the stimulus duration does not reliably affect premature responding and response latencies (data not shown)7,10,12.
Another approach to increasing the attentional demands of the 5CSRTT is to probe performance by shortening the ITI and thereby increasing the ‘event rate’. The ITI can either be decreased and of a fixed duration (i.e., shortened to 1.5 sec) or it can be both decreased and made unpredictable (i.e., shortened 0.5, 1.5, 3.0, or 4.5 sec with each ITI occurring in a pseudorandom fashion). Decreasing the ITI decreases % accuracy and increases % omissions. Because of the short duration of the ITI, the number of premature responses also tends to decrease (for hypothetical data see Figure 4A-C; based on Paine and Carlezon, unpublished observation)11.
There are other modifications to the standard 5CSRTT (introducing bursts of ‘distracting’ white noise, introducing a flashing light, or dimming the stimulus lights)1,2,7,10 that can be used to probe attention. Because these test day challenges required the use of additional equipment they weren’t discussed in detail here. That said, these challenges generally lead to reductions in the accuracy and/or increases in omissions1,2,7,10.
Manipulations of the 5CSRTT that Probe Impulse Control
As illustrated in Figure 4D-F, response inhibition can be systematically challenged in the 5CSRTT by increasing the duration of the ITI. The ITI can either be increased and of a fixed duration (i.e., increased to 7 sec) or it can be both increased and made unpredictable (i.e., increased to 4.5, 6.0, 7.5, or 9.0 sec with each ITI occurring pseudo randomly). Increasing the ITI reliably increases the number of premature responses committed without having major effects on other performance measures (based on Paine and Carlezon, unpublished observation)7,10,11,13,14.
Interpretation of Test Day Challenge Data
Importantly, it is possible to combine analysis of performance on the test day challenge tasks with performance on the standard task in order to gain a better understanding of the nature of the performance deficit observed following a particular manipulation. For example, Figures 5A and 5B shows that blocking cortical GABA synthesis with intra prefrontal cortex infusions of the glutamic acid decarboxylase (GAD) inhibitor L-allylglycine (LAG) increases omissions, but does not affect % accuracy, in the standard 5CSRTT12. Increased omissions on their own can be difficult to interpret as they can reflect attention deficits, sedation, motivation deficits or altered locomotor ability. Thus, in order to gain a better understanding of meaning of these deficits we used the test day challenges to determine whether the increase in omissions reflected an attention deficit or was the result of another factor. First we assessed performance on a more difficult version of the task in which the stimulus duration was reduced from 1.0 to 0.5 sec (Figures 5D-5F) and then we assessed performance on an easier version of the task in which the stimulus duration was increased from 1.0 to 5 sec (Figures 5G-5I). Because omissions were increased to the same degree (fold increase from 0.0 μg/μl dose) in all three versions of the task (Figures 5B, 5E, and 5H) and because accuracy was unaffected by LAG infusions in all three versions of the task (Figures 5A, 5D, and 5G), we concluded that the increase in omissions likely did not reflect an attention deficit. We excluded the possibility that the change in omissions reflected a decrease in motivation because neither the reward retrieval latency (Figures 5C, 5F, and 5I) nor the number of magazine entries (not shown) were systemically affected by LAG infusions. Using an open field we determined that LAG infusions likely increased omissions because they affected locomotor ability (data not shown)12.
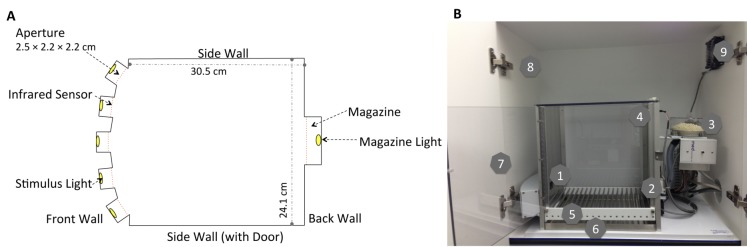 Figure 1.The 5CSRTT apparatus. A) Schematic of the 5CSRTT boxes showing the arrangement of apertures and the magazine from an aerial view. Each aperture and the food magazine are equipped with infrared sensors in order to detect nose pokes. B) A 5CSRTT operant conditioning box (Med-Associates, St. Albans VT). On the aluminum front wall of the chamber are the 5 nose poke apertures, each of which contains an LED stimulus light (1); the aluminum back wall contains the food magazine (2), which is connected to the pellet dispenser (3), and the house light (4). The floor is made of stainless steel bars (5); below the floor is a removable waste tray (6). The sidewalls (including the door) are constructed of clear polycarbonate (7). The chamber is housed in a sound-attenuating cubicle (8) and a fan (9) provides masking noise and circulates air.
Figure 1.The 5CSRTT apparatus. A) Schematic of the 5CSRTT boxes showing the arrangement of apertures and the magazine from an aerial view. Each aperture and the food magazine are equipped with infrared sensors in order to detect nose pokes. B) A 5CSRTT operant conditioning box (Med-Associates, St. Albans VT). On the aluminum front wall of the chamber are the 5 nose poke apertures, each of which contains an LED stimulus light (1); the aluminum back wall contains the food magazine (2), which is connected to the pellet dispenser (3), and the house light (4). The floor is made of stainless steel bars (5); below the floor is a removable waste tray (6). The sidewalls (including the door) are constructed of clear polycarbonate (7). The chamber is housed in a sound-attenuating cubicle (8) and a fan (9) provides masking noise and circulates air.
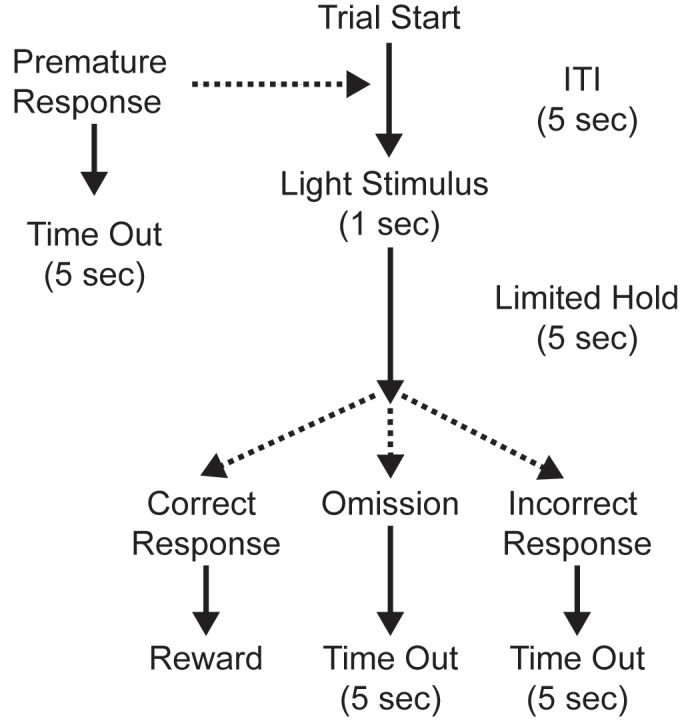 Figure 2.Structure of a trial in the 5CSRTT. Trials begin with an intertrial interval (ITI) during with the houselight is illuminated. At the end of the ITI a stimulus light is presented in one of the 5CSRTT apertures. If a rat nose pokes in the illuminated aperture, a food pellet is delivered to the magazine and the magazine light is illuminated. Pellet retrieval extinguishes the magazine light and starts the next trial. Incorrect responses and omissions (failure to respond) result in a time out (TO), during which the houselight is extinguished. The next trial begins at the end of the TO. Premature responses (occurring during the ITI) also result in a TO, but the same trial is reinitiated after a TO triggered by a premature response.
Figure 2.Structure of a trial in the 5CSRTT. Trials begin with an intertrial interval (ITI) during with the houselight is illuminated. At the end of the ITI a stimulus light is presented in one of the 5CSRTT apertures. If a rat nose pokes in the illuminated aperture, a food pellet is delivered to the magazine and the magazine light is illuminated. Pellet retrieval extinguishes the magazine light and starts the next trial. Incorrect responses and omissions (failure to respond) result in a time out (TO), during which the houselight is extinguished. The next trial begins at the end of the TO. Premature responses (occurring during the ITI) also result in a TO, but the same trial is reinitiated after a TO triggered by a premature response.
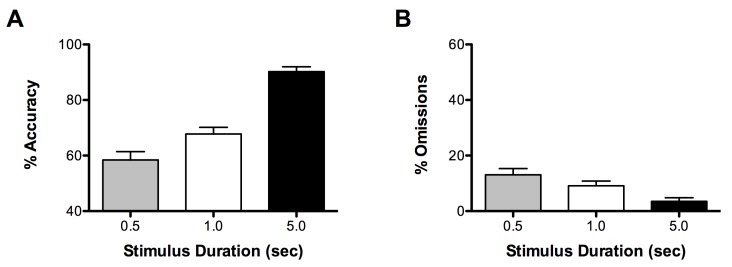 Figure 3.Effects of manipulating stimulus duration on attention. Decreasing the stimulus duration leads to decreases in attention as measure by a decrease in accuracy (A) and increase in omissions (B).
Figure 3.Effects of manipulating stimulus duration on attention. Decreasing the stimulus duration leads to decreases in attention as measure by a decrease in accuracy (A) and increase in omissions (B).
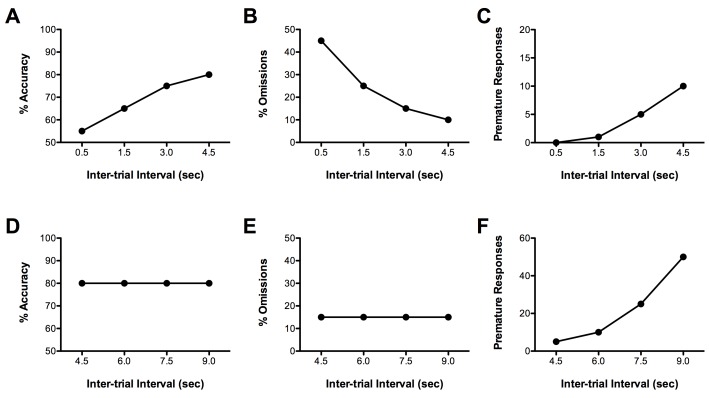 Figure 4.Hypothetical effects of manipulating the duration of the ITI on 5CSRTT performance. Decreasing the duration of the ITI and making it unpredictable decreases accuracy (A) and premature responses (C) and increases % omissions (B). Increasing the duration of the ITI and making it unpredictable does not reliably affect % accuracy (D) or % omissions (E) but increases premature responses (F).
Figure 4.Hypothetical effects of manipulating the duration of the ITI on 5CSRTT performance. Decreasing the duration of the ITI and making it unpredictable decreases accuracy (A) and premature responses (C) and increases % omissions (B). Increasing the duration of the ITI and making it unpredictable does not reliably affect % accuracy (D) or % omissions (E) but increases premature responses (F).
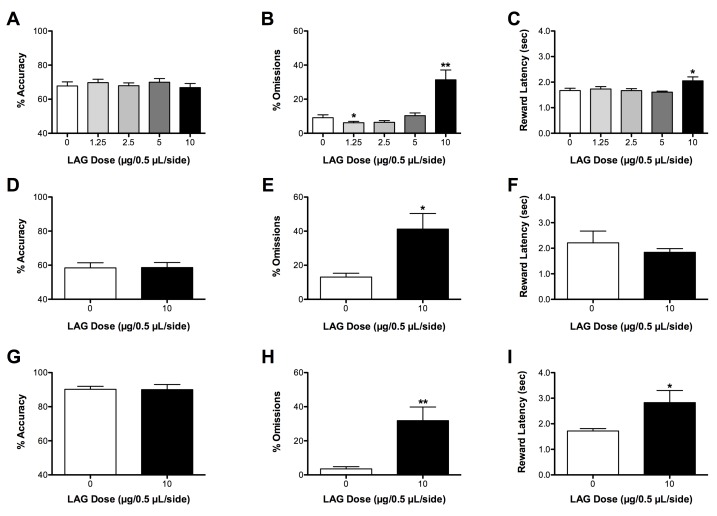 Figure 5.Effects of the GABA synthesis inhibitor on the Standard (A-C), Short (D-F), and Long (G-I) stimulus duration versions of the 5CSRTT. When the stimulus duration is shortened (D, E), % accuracy decreases and % omissions increase relative to the standard task (A, B). In contrast, when the stimulus duration is lengthened (G, H), % accuracy increased and % omissions decreased relative to the standard task. (This figure has been modified from Asinof & Paine12; reprinted with permission from Neuropharmacology).
Figure 5.Effects of the GABA synthesis inhibitor on the Standard (A-C), Short (D-F), and Long (G-I) stimulus duration versions of the 5CSRTT. When the stimulus duration is shortened (D, E), % accuracy decreases and % omissions increase relative to the standard task (A, B). In contrast, when the stimulus duration is lengthened (G, H), % accuracy increased and % omissions decreased relative to the standard task. (This figure has been modified from Asinof & Paine12; reprinted with permission from Neuropharmacology).
| Training Stage | Trials/Session | Stimulus Duration (SD) | ITI Duration | Limited Hold (LH) | Time Out (TO) | Criterion | |
| % Correct | % Omissions | ||||||
| TR-1 | 90 or 30 min | 30 | 2 | 30 | 2 | > 75 | < 10 |
| TR-2 | 90 or 30 min | 15 | 2 | 15 | 2 | > 75 | < 10 |
| TR-3 | 90 or 30 min | 15 | 3 | 15 | 3 | > 75 | < 10 |
| TR-4 | 90 or 30 min | 5 | 3 | 10 | 3 | > 75 | < 15 |
| TR-5 | 90 or 30 min | 2 | 3 | 5 | 3 | > 70* | < 15* |
| TR-6 | 90 or 30 min | 2 | 5 | 5 | 5 | > 65* | < 15* |
| TR-7 | 90 or 30 min | 1 | 5 | 5 | 5 | > 50 | < 20 |
Table 1.Training Schedule for Standard 5CSRTT. At each training stage, rats must perform at or above criterion for 2 consecutive days prior to being moved to the next training stage. % Correct is the number of correct trials divided by the total trials (i.e., correct, incorrect and omissions) x100 and % omissions is the number of omissions divided by the total trials x100. * indicates that not all rats will be able to attain this level of performance, however the better their performance at these training stages the more likely they will be to successfully complete the final stage of training. The duration of the stimulus, ITI, LH, and TO are all in seconds.
| Training Program | Cognitive Function | Stimulus Duration (SD) | ITI Duration | Limited Hold (LH) | Time Out (TO) |
| Short SD | Attention | 0.5 | 5 | 5 | 5 |
| Long SD | Attention | 5-Feb | 5 | 5 | 5 |
| Short ITI | Attention | 1 | 1.5 | 5 | 5 |
| Short Variable ITI | Attention | 1 | 0.5,1.5, 3.0, 4.5 | 5 | 5 |
| Long ITI | Impulsivity | 1 | 7 or 9 | 5 | 5 |
| Long Variable ITI | Impulsivity | 1 | 4.5, 6.0, 7.5, 9.0 | 5 | 5 |
Table 2. Common Test Day Challenges. The 5CSRTT parameters can be systematically manipulated in order to probe different cognitive functions. Decreasing the stimulus duration or decreasing the ITI can both be used to challenge attentional systems. If a shortened ITI is used, the ITI can either be of a fixed duration (e.g., 1.5 sec) or be variable (e.g., 0.5, 1.5, 3.0, and 4.5 sec). Lengthening the stimulus duration decreases attentional demands. Impulse control can be challenged by increasing the duration of the ITI. The ITI can either be of a fixed duration (e.g., 7 sec) or be variable (e.g., 4.5, 6.0, 7.5, 9.0 sec). If using a variable ITI each ITI duration should occur pseudo randomly and each rat should be exposed to equal number of trials at each ITI. The session length and/or number of trials should be increased in order to accommodate this. The duration of the stimulus, ITI, LH and TO all are in seconds.
Discussion
The 5CSRTT is a widely used task to assess attention and impulse control in rodents. Attention is most commonly measured by accuracy of responding1,7,10. Because accuracy of responding does not include omissions and because both correct and incorrect responses have the same response requirement (i.e., a nose poke in an aperture), accuracy is not influenced by locomotor ability, motivation or sedation. The % omissions can also be used as a measure of attention because well trained rodents will often withhold a response rather than ‘guess’ if they are uncertain of which hole was illuminated on a particular trial1,10. Problematically, sedation, decreased motivation, and locomotor impairments can also interfere with a rats’ ability to respond, making omissions difficult to interpret. To some extent, these factors can be ruled out by assessing other measures of performance or by implementing test day challenges. For example, motivational impairments may be indicated by a decrease in magazine entries and an increase in reward retrieval latencies and sedation and/or locomotor impairment can be indicated by a general decrease in responding (i.e., increased omissions, decreased premature responses and decreased magazine entries) and an overall increase in response latencies. That said, if it is not clear whether a change in omissions reflects an attention deficit or is the result of some other factor, it is advisable to implement a test day challenge.
Impulsivity is most commonly measured in the 5CSRTT using premature responses. Premature responses are those responses that occur during the ITI, prior to the stimulus presentation. It is thought that these responses reflect an inability to withhold the pre potent response to nose poke and thus reflect a form of impulsivity characterized by a lack of response inhibition1,9. Premature responses are thought to be analogous to impulsive responses made during other tasks of motor impulsivity such as go no go tasks1,8. Another measure of response inhibition in the 5CSRTT is perseverative responses; these are responses that occur in an aperture after a correct response has been made and the sugar pellet reward delivered. Perseverative responses are thought to reflect compulsive over responding rather than an impulsive responding per se1. Notably, these two measures of response inhibition are mediated, in part, by different neural substrates11; suggesting that they are in fact distinct measures of response inhibition.
Correct response latency (and/or incorrect response latency) is used to determine whether a manipulation causes a change in the rats’ processing speed or decision making time1,7. As with omissions, however, response latency can be affected by a number of other factors including sedation, motivation and locomotor ability. Thus, the overall pattern of results must be assessed to determine whether a particular manipulation affects processing or decision making speed.
There are several advantages to using the 5CSRTT in order to measure attention and impulse control in rodents; these likely explain why this task has been adopted by labs around the world. First, the 5CSRTT allows for concurrent investigation of multiple cognitive functions. Second, rats can be maintained at stable levels of performance for several weeks to months and can be easily retrained after a period of time off. Because baseline performance is stable it is possible to use a within-subjects design or to test the effects of chronic manipulations on task performance. Third, the versatility of the task is increased by the variety of test day challenges that can be implemented. Importantly, these manipulations result in predictable changes in performance, many of which have been replicated in a number of labs1,7,10,12. The test day challenges enable researchers to further characterize performance deficits (or improvements) observed on the standard task or can reveal changes in performance that aren’t observed when rats are tested on the standard task16. Fourth, because multiple behavioral measures are collected concurrently, researchers are able to assess the influence of other, potentially confounding, factors on task performance. Finally, because the operant chambers are controlled by an external computer there is enhanced control of stimulus presentation, accurate timing of events and unbiased collection of data7.
Despite its strengths, the 5CSRTT is not without its disadvantages. The primary shortcoming of the 5CSRTT is that it takes rats many weeks to complete training and attain stable levels of performance. Moreover, the prolonged training time can cause performance to become ‘automatic’ or responses habitual10; this can make performance on the standard task impervious to particular manipulations. Implementing test day challenges, which increase task demands or alter response contingencies, can minimize the influence of automatic responding on performance. The prolonged training does however result in performance that is relatively stable across time allowing for individual subjects to be tested repeatedly and serve as their own control. Thus, the time required to train an individual subject is mitigated by the amount of data that each subject can generate. Another disadvantage of the 5CSRTT is the requirement to use mild food restriction as a means of motivating task performance. As the session continues and subjects become sated the motivation to respond can be impacted; this can lead to changes in task performance25. Pre feeding subjects prior to testing is one means of determining whether satiety is mediating the effects of a particular manipulation7,25,47. Moreover, there can be concerns that chronic mild food restriction can cause undue stress to rodents; however there is growing evidence to the contrary48. Perhaps more problematically, mild food restriction can alter the very pathways that researchers are trying to investigate; this can influence the effects of drugs on task performance49.
It is important to note that different laboratories report differences in the baseline level of performance on the standard task. This may be due in part to differences in the operant chambers themselves, for example some labs use 9 hole boxes and occlude every other hole1,2,7, while other labs use 5-hole boxes12,23. In addition, the stimulus light in some chambers is an incandescent bulb2, while in other configurations it is a light emitting diode (LED)23. Differences in baseline levels of performance may also be due to variations in the training strategy implemented. Finally, differences in the strain of rat (or mouse) used may also account for differences in baseline levels of performance. Only a few laboratories have compared performance across rat strains with mixed results. For example, Sprague-Dawley rats have lower baseline levels of performance than do Lister hooded rats28. In contrast, Auclair and colleagues50 found that Sprague-Dawley rats acquired the task faster (i.e., in fewer training sessions) than did Long-Evans rats but both groups ultimately attained the same level of performance. Strain differences have also been observed when using mice; C57BL/6 mice are more accurate and make fewer premature responses than do DBA/2 mice when the stimulus duration is shortened51.
Although task acquisition and performance are similar for mouse and rats, slight deviations in the training strategy from that outlined here may be required. First, in order to accommodate their smaller size, the mouse 5CSRTT apparatus is smaller than the rat 5CSRTT5,6,36-39,51,52. Second, mice may require greater habituation to the operant chambers prior to initiating training5,6,52. Third, there is a greater risk of satiation and a loss of motivation to respond with mice5,6,52. In order to accommodate the risk of satiation smaller food pellets (e.g., 12 mg for mice versus 45 mg for rats) should be used52. Alternatively liquid reinforcers have also been described5,6,37. Finally, it may be necessary to run all sessions with the houselight off, this increases the discriminative properties of the stimulus lights6.
In summary, the 5CSRTT is a widely used operant conditioning based task used to study attention and impulse control in rodents. There are several advantages to using the task not the least of which is the ability to easily modify the task to more carefully investigate either attention or impulse control.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by a National Institutes of Health grant awarded to TAP (R15MH098246).
References
- Robbins T. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav. Brain. Res. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- Leonard JA. 5 choice serial reaction apparatus. Med. Res. Council. Appl. Psychol. Res. 1959. pp. 326–359.
- Muir JL. Attention and stimulus processing in the rat. Brain. Res. Cogn. Brain. Res. 1996;3:215–225. doi: 10.1016/0926-6410(96)00008-0. [DOI] [PubMed] [Google Scholar]
- Humby T, Laird FM, Davies W, Wilkinson LS. Visuospatial attentional functioning in mice: interactions between cholinergic manipulations and genotype. Eur. J. Neurosci. 1999;11:2813–2823. doi: 10.1046/j.1460-9568.1999.00701.x. [DOI] [PubMed] [Google Scholar]
- Humby T, Wilkinson L, Dawson G. Assaying aspects of attention and impulse control in mice using the 5-choice serial reaction time task. Curr. Protoc. Neurosci. 2005. Unit 8.5H. [DOI] [PubMed]
- Bari A, Dalley JW, Robbins TW. The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat. Protoc. 2008;3:759–767. doi: 10.1038/nprot.2008.41. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: Fronto-striatal systems and functional neurochemistry. Pharm. Biochem. Behav. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of Impulsivity. Psychopharmacology (Berl) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Amitai N, Markou A. Comparative effects of different test day challenges on performance in the 5-choice serial reaction time task. Behav. Neurosci. 2011;125:764–774. doi: 10.1037/a0024722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav. Brain. Res. 2003. pp. 105–119. [DOI] [PubMed]
- Asinof SK, Paine TA. Inhibition of GABA synthesis in the prefrontal cortex increases locomotor activity but does not affect attention in the 5-choice serial reaction time task. Neuropharmacology. 2013;65:39–47. doi: 10.1016/j.neuropharm.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Lääne K, Pena Y, Theobald DE, Everitt BJ, Robbins TW. Attentional and motivational deficits in rats withdrawn from intravenous self-administration of cocaine or heroin. Psychopharmacology (Berl) 2005;182:579–587. doi: 10.1007/s00213-005-0107-3. [DOI] [PubMed] [Google Scholar]
- Dalley JW, et al. Cognitive sequelae of intravenous amphetamine self-administration in rats: evidence for selective effects on attentional performance. Neuropsychopharmacology. 2005;30:525–537. doi: 10.1038/sj.npp.1300590. [DOI] [PubMed] [Google Scholar]
- Moreno M, et al. Divergent effects of D2/3 receptor activation in the nucleus accumbens core and shell on impulsivity and locomotor activity in high and low impulsive rats. Psychopharmacology (Berl) 2013. pp. 19–30. [DOI] [PMC free article] [PubMed]
- Lambe EK, Olausson P, Horst NK, Taylor JR, Aghajanian GK. Hypocretin and nicotine excite the same thalamocortical synapses in prefrontal cortex: correlation with improved attention in rat. J. Neurosci. 2005;25:5225–5229. doi: 10.1523/JNEUROSCI.0719-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarra R, et al. Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:34–41. doi: 10.1016/j.pnpbp.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Maddux JM, Holland PC. Effects of dorsal or ventral medial prefrontal cortical lesions on five-choice serial reaction time performance in rats. Behav. Brain. Res. 2011;221:63–74. doi: 10.1016/j.bbr.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis WL, Olmstead MC, Robbins TW. Selective deficits in attentional performance on the 5-choice serial reaction time task following pedunculopontine tegmental nucleus lesions. Behav. Brain. Res. 2001;123:117–131. doi: 10.1016/s0166-4328(01)00181-4. [DOI] [PubMed] [Google Scholar]
- Baunez C, Robbins TW. Bilateral lesions of the subthalamic nucleus induce multiple deficits in an attention task in rats. Eur. J. Neurosci. 1997;9:2086–2099. doi: 10.1111/j.1460-9568.1997.tb01376.x. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi on performance of a 5-choice serial reaction time task in rats: implications for theories of selective attention and arousal. Behav. Brain. Res. 1989;33:165–179. doi: 10.1016/s0166-4328(89)80048-8. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Everitt BJ, Robbins TW. Doubly dissociable effects of median- and dorsal-raphé lesions on the performance of the five-choice serial reaction time test of attention in rats. Behav. Brain. Res. 1997;89:135–149. doi: 10.1016/s0166-4328(97)00053-3. [DOI] [PubMed] [Google Scholar]
- Paine TA, Tomasiewicz HC, Zhang K, Carlezon WA., Jr Sensitivity of the five-choice serial reaction time task to the effects of various psychotropic drugs in Sprague-Dawley rats. Biol. Psychiatry. 2007;62:687–693. doi: 10.1016/j.biopsych.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Paine TA, Carlezon WA., Jr Effects of antipsychotic drugs on MK-801-induced attentional and motivational deficits in rats. Neuropharmacology. 2009;56:788–797. doi: 10.1016/j.neuropharm.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grottick AJ, Higgins GA. Assessing a vigilance decrement in aged rats: effects of pre-feeding, task manipulation, and psychostimulants. Psychopharmacology (Berl) 2002;164:33–41. doi: 10.1007/s00213-002-1174-3. [DOI] [PubMed] [Google Scholar]
- Hahn B, Shoaibm M, Stolerman IP. Nicotine-induced enhancement of attention in the five-choice serial reaction time task: the influence of task demands. Psychopharmacology (Berl) 2002;162:129–137. doi: 10.1007/s00213-002-1005-6. [DOI] [PubMed] [Google Scholar]
- Pattij T, Schetters D, Schoffelmeer AN, van Gaalen MM. On the improvement of inhibitory response control and visuospatial attention by indirect and direct adrenoceptor agonists. Psychopharmacology (Berl) 2012;219:327–340. doi: 10.1007/s00213-011-2405-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza NR, Bright JL. Nicotine-induced enhancements in the five-choice serial reaction time task in rats are strain-dependent. Psychopharmacology (Berl) 2001;154:8–12. doi: 10.1007/s002130000605. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Dalley JW, Robbins TW. Remediation of attentional dysfunction in rats with lesions of the medial prefrontal cortex by intra-accumbens administration of the dopamine D2/3 receptor antagonist sulpiride. Psychopharmacology (Berl) 2009;202:307–313. doi: 10.1007/s00213-008-1384-4. [DOI] [PubMed] [Google Scholar]
- Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J. Neurosci. 2000;20:1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine TA, Neve RL, Carlezon WA., Jr Attention deficits and hyperactivity following inhibition of cAMP-dependent protein kinase within the medial prefrontal cortex of rats. Neuropsychopharmacology. 2009;34:2143–2155. doi: 10.1038/npp.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson M, et al. Dissociable control of impulsivity in rats by dopamine d2/3 receptors in the core and shell subregions of the nucleus accumbens. Neuropsychopharmacology. 2010;35:560–569. doi: 10.1038/npp.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai N, Markou A. Chronic nicotine improves cognitive performance in a test of attention but does not attenuate cognitive disruption induced by repeated phencyclidine administration. Psychopharmacology (Berl) 2009;202:275–286. doi: 10.1007/s00213-008-1246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Eagle DM, Passetti F, Robbins TW. Deficits in impulse control associated with tonically-elevated serotonergic function in rat prefrontal cortex. Neuropsychopharmacology. 2002;26:716–728. doi: 10.1016/S0893-133X(01)00412-2. [DOI] [PubMed] [Google Scholar]
- Barbelivien A, Ruotsalainen S, Sirviö J. Metabolic alterations in the prefrontal and cingulate cortices are related to behavioral deficits in a rodent model of attention-deficit hyperactivity disorder. Cereb. Cortex. 2001;11:1056–1063. doi: 10.1093/cercor/11.11.1056. [DOI] [PubMed] [Google Scholar]
- Peña-Oliver Y, et al. Deletion of alpha-synuclein decreases impulsivity in mice. Genes. Brain. Behav. 2012;11:137–146. doi: 10.1111/j.1601-183X.2011.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueman RC, Dunnett SB, Jones L, Brooks SP. Five choice serial reaction time performance in the HdhQ92 mouse model of Huntington's disease. Brain. Res. Bull. 2012;88:163–170. doi: 10.1016/j.brainresbull.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Pattij T, Janssen MC, Loos M, Smit AB, Schoffelmeer AN, van Gaalen MM. Strain specificity and cholinergic modulation of visuospatial attention in three inbred mouse strains. Genes Brain Behav. 2007;6:579–587. doi: 10.1111/j.1601-183X.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- Romberg C, Mattson MP, Mughal MR, Bussey TJ, Saksida LM. Impaired attention in the 3xTgAD mouse model of Alzheimer's disease: rescue by donepezil (Aricept) J. Neurosci. 2011;31:3500–3507. doi: 10.1523/JNEUROSCI.5242-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Ricciardi J, Wetzler C, Hanania T. Sub-optimal performance in the 5-choice serial reaction time task in rats was sensitive to methylphenidate, atomoxetine and d-amphetamine, but unaffected by the COMT inhibitor tolcapone. Neurosci. Res. 2011;69:41–50. doi: 10.1016/j.neures.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Puumala T, Ruotsalainen S, Jäkälä P, Koivisto E, Riekkinen P, Jr, Sirviö J. Behavioral and pharmacological studies on the validation of a new animal model for attention deficit hyperactivity disorder. Neurobiol. Learn. Mem. 1996. pp. 198–211. [DOI] [PubMed]
- Amitai N, Markou A. Disruption of performance in the five-choice serial reaction time task induced by administration of N-methyl-D-aspartate receptor antagonists: Relevance to cognitive dysfunction in schizophrenia. Biol. Psychiatry. 2010;68:5–16. doi: 10.1016/j.biopsych.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, et al. Increased impulsivity during withdrawal from cocaine self-administration: role for DeltaFosB in the orbitofrontal cortex. Cereb. Cortex. 2009;19:435–444. doi: 10.1093/cercor/bhn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M, Bizarro L. Deficits in a sustained attention task following nicotine withdrawal in rats. Psychopharmacology (Berl) 2005;178:211–222. doi: 10.1007/s00213-004-2004-6. [DOI] [PubMed] [Google Scholar]
- Semenova S, Stolerman IP, Markou A. Chronic nicotine administration improves attention while nicotine withdrawal induces performance deficits in the 5-choice serial reaction time task in rats. Pharmacol. Biochem. Behav. 2007;87:360–368. doi: 10.1016/j.pbb.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academy Press. Washington, DC: 1996. National Academy Press. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- Nemeth CL, et al. Role of kappa-opioid receptors in the effects of salvinorin A and ketamine on attention in rats. Psychopharmacology (Berl) 2010;210:263–274. doi: 10.1007/s00213-010-1834-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland NE. Food or fluid restriction in common laboratory animals: balancing welfare considerations with scientific inquiry. Comp. Med. 2007;57:149–160. [PubMed] [Google Scholar]
- Carr KD. Chronic food restriction: enhancing effects on drug reward and striatal cell signaling. Physiol. Behav. 2007;91:459–472. doi: 10.1016/j.physbeh.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Auclair AL, Besnard J, Newman-Tancredi A, Depoortère R. The five choice serial reaction time task: comparison between Sprague-Dawley and Long-Evans rats on acquisition of task, and sensitivity to phencyclidine. Pharmacol. Biochem. Behav. 2009;92:363–369. doi: 10.1016/j.pbb.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Patel S, Stolerman IP, Asherson P, Sluyter F. Attentional performance of C57BL/6 and DBA/2 mice in the 5-choice serial reaction time task. Behav. Brain Res. 2006. pp. 197–203. [DOI] [PubMed]
- Higgins GA, Breysse N. Rodet model of attention: The 5-choice serial reaction time task. Current Protocols in Pharmacology. 2008. Unit 5.49. [DOI] [PubMed]


