Abstract
Background
Higher resting heart rate (HR) and increases in HR over time in patients with heart failure (HF) are associated with adverse outcomes. Whether these relationships between HR and prognosis are also observed in patients with HF and preserved ejection (HFpEF) requires further assessment.
Objective
To examine the relationship between baseline HR, changes in HR from a preceding visit, and time-updated HR with subsequent outcomes in patients with HFpEF in the TOPCAT trial.
Methods and Results
In 1767 patients enrolled in the TOPCAT trial from the Americas, we examined the association between baseline resting HR, as well as change in HR from the preceding visit and clinical outcomes using Cox proportional hazards models, as well the association between HR at each visit and outcome. Both baseline HR (adjusted hazard ratio (AHR) 1.08 (HR 1.04–1.12) and change in HR from the preceding visit (AHR 1.09, 95% confidence intervals 1.05–1.14, p < 0.001 per 5 bpm higher HR), after adjusting for covariates, were associated with a higher risk of primary endpoint of cardiovascular death, hospitalization for HF or aborted cardiac arrest. Time-updated resting HR at each visit was also associated with risk (AHR 1.11, 95% CI 1.07–1.15; p<0.001, per 5 bpm higher HR). Furthermore, we observed a rise in resting HR of approximately 10 bpm beginning approximately 10 days prior to the primary endpoint.
Conclusions
Baseline resting HR and change in HR over time predict outcome in patients with HFpEF, as does time-updated HR during follow-up. These data suggest that frequent outpatient monitoring of HR, possibly with remote technologies, may identify patients with HFpEF who may be at increased risk of rehospitalization or death.
Keywords: Heart rate, heart failure, outcome research
Introduction:
Elevated resting heart rate (HR) is a known risk factor for adverse outcome in patients with cardiovascular (CV) disease (1–5). The association between temporal changes in HR and mortality has been assessed in subjects without known CV disease (6,7) as well as in subjects with hypertension (8) and heart failure (HF) (9). These studies showed that an increase in HR over time was associated with a higher risk of adverse events.
Whether baseline HR and changes in HR over time could be a useful prognostic marker in patients with a contemporary definition of HFpEF requires further assessment. The availability of new remote monitoring strategies and devices that can measure and track HR provide a practical platform for assessing HR in real time in patients with HFpEF. Thus the objective of this analysis was to determine whether temporal changes in HR from the preceding visit are of prognostic importance independent of baseline resting HR in patients with HFpEF within the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial(10).
Methods:
The TOPCAT Trial
The details of the design, and overall findings have been previously reported(10). In brief, the TOPCAT trial enrolled a total of 3445 patients with a contemporary definition of HFpEF (symptomatic heart failure and a left ventricular EF ≥45%) with either an admission with decompensated HF in the last 12 months or an elevated naturetic peptide (brain natriuretic peptide [BNP] level ≥100 pg/ml or N-terminal pro-BNP [NTproBNP] level ≥360 pg/ml) were assigned to either spironolactone (15 to 45 mg daily) or placebo. The primary outcome was a composite of death from CV causes, aborted cardiac arrest, or hospitalization for the management of HF.
The main exclusion criteria were severe systemic illness with a life expectancy of less than 3 years, severe renal dysfunction (an estimated glomerular filtration rate [GFR] of <30 ml/min/1.73 M2 of body-surface area or a serum creatinine level that was ≥2.5 mg/DL [221 mol/l]), and specific coexisting conditions, medications, or acute events (11).
The scheduled follow-up consisted of up to 16 trial visits, including an initial baseline visit followed by visit 2 and 3 occurring at 4 and 8 weeks, visit 4 at four months and subsequent visits occurring every 4 months thereafter, up to visit 16. The median time interval between visits was 135 days, (IQR 61–182 days). The follow-up time from the initial visit was a median of 3.3 years. Resting HR was recorded at each visit as part of the physical examination. At each visit, HR was recorded at rest by palpation, auscultation of the heart, or ECG.
Heart rate at any clinic visit and calculation of temporal changes in heart rate
To assess temporal changes in HR, we created a time-updated covariate representing the most recent available HR value for each patient at each visit over the course of the trial. We named this time-updated variable as HR at any visit. A patient’s baseline HR was carried forward until the day of the first follow-up visit, at which time the new “current” value was used and subsequently carried forward until the next visit. As there were up to 16 trial visits in the program, the resting HR was updated up to 15 times after baseline for each patient. We calculated temporal changes in resting HR from the preceding visit (ΔHR) by subtracting the time-updated visit HR value from the value from the preceding visit. These changes in HR reflect changes occurring in between visits.
Statistical analysis
As previously reported significant regional differences between the Americas and Russia and Georgia, and with very few events in Russia and Georgia(12), we performed the data analysis on subjects recruited from the Americas (N=1767) to have a more homogenous group of subjects with contemporary definition of HFpEF. Sensitivity analyses were also performed using subjects from Russia and Georgia (N=1678) as well as the full TOPCAT cohort (N=3445), with data presented within the supplements section.
We related resting baseline HR, HR at any visit (i.e. time-updated HR) and ΔHR to several clinical outcomes. These included the primary endpoint of the TOPCAT trial, the composite of CV death, aborted cardiac arrest and admission for worsening HF and also the outcome of all-cause mortality, CV death, hospitalization for HF, non-CV death, fatal and non-fatal myocardial infarction and stroke. The basis for using the non-CV death as an outcome was to assess whether ΔHR was predictive of general ill health or whether it was specific to CV outcomes alone. Incidence rates were calculated, per 100 patient-years.
The association between outcomes and resting baseline HR, HR at any visit and ΔHR as continuous covariates were assessed using multivariable Cox proportional hazards models. For baseline HR, HR at anytime and ΔHR the estimated hazard ratio for each of these covariates correspond to a 5 bpm difference in HR. we also modelled as five categories of HR change (decrease >10 beats/minute (bpm), decrease of 5–10 bpm, < 5 bpm change, increase of 5–10 bpm, increase >10 bpm), based on the rationale that changes in HR greater than 5 bpm would be considered as clinically meaningful. In models using categorical covariates, the no change in HR category (<5 bpm change) was used as the reference for changes in HR from the preceding visit.
The multivariable analysis adjusted for factors that had a significant difference (P value of <0.05) across the tertiles of baseline heart rate. Thus we adjusted for age, gender, previous hospitalization for heart failure in last 12 months prior to randomization, history of previous myocardial infarction, previous percutaneous coronary intervention and use at baseline of beta-blocker and rate-limiting calcium antagonist, and time-updated diastolic blood pressure and weight. We also created second multivariate model that adjusted for additional covariates from our initial model. These included the following baseline NYHA functional class III/IV versus I/II, race, smoking status, chest X-ray (signs of congestion), presence of atrial fibrillation at baseline, QRS duration, LV hypertrophy at baseline, paroxysmal nocturnal dyspnea, treatment with spironolactone, diabetes mellitus, peripheral vascular disease at baseline, baseline creatinine and time-updated use and dose change of beta-blocker and rate-limiting calcium antagonist to account for starting or stopping and dose changes. For the second model we also adjusted for time–updated systolic and diastolic blood pressure and time-updated NYHA functional class at each visit. For the second model, outcomes without myocardial infarction (MI) Interval non-fatal myocardial infarction (MI) was also included in the model. The results of the second model are reported in the supplements section of the manuscript. We also controlled for baseline HR when modelling for HR at any time for the initial model and also the second model; however, we replaced baseline HR with HR from the preceding visit when modelling ΔHR.
An adjusted model using a restricted cubic spline with 5-knots was constructed to flexibly display the relationship between the hazards of developing an outcome and continuous covariate of HR at any time, using a reference value of 60 bpm. For ΔHR, 0 was used as the reference. Interaction testing was used to determine whether the relationship between ΔHR and outcomes varied in different subgroups (patients recruited from the Americas versus Russia/Georgia, with or without atrial fibrillation, diabetes mellitus at baseline, ejection fraction above or equal to 55%, with or without beta-blocker use at any time).
We also performed an analysis to relate the value of resting HR up to 30 days before the occurrence of the primary end-point of the study, thus this analysis excluded patients who did not have the primary end-point. Relative to the time of randomization, all patients follow a similar study visit schedule. However, because clinical events can occur at any time between scheduled study visits, the amount of time lapsed between a clinical event and that patient’s prior study visit is continuous. Furthermore, for each patient who experienced a clinical event, multiple prior heart rate values are available, each collected at a different number of days prior to the event. We estimated the average values of HR as a function of the number of days prior to a primary endpoint. This was done using a mixed effects linear regression model, accounting for multiple observations per patient using patient ID’s as random intercept terms. The exposure variable was the date of the confirmed primary endpoint subtracted from the date of the HR measurement (i.e. days prior to the event). The outcome variable was the reported HR. This methodology has been used previously to estimate changes in other biomarkers prior to an event (13). No measurements obtained after the event were used for this analysis. We conducted sensitivity analyses using only data collected within 30 days of the primary event. To allow for potentially non-linear changes over time, the exposure variable (days prior to event) was modelled using restricted cubic spline terms.
Differences between baseline characteristics of patients were compared using trend tests. Continuous variables were described using median and interquartile ranges (IQR) and categorical variables were expressed as counts and percentages. Assessment of the proportional hazards assumption was performed using Schoenfeld residuals (14). All p values were 2-sided, and a p<0.05 was used to determine statistical significance. Analysis was performed using STATA (version 13.1, StataCorp LP, College Station, Texas).
Results
Baseline characteristics of the patients
The key baseline characteristics of the study group comprised of subjects from the Americas are summarized in Table 1. The median age of the cohort was 72 (64–79) years, and half of the population were female. At baseline 72% had entered the study based on a HF admission in the past 12-months. Ninety percent of the patients had a history of hypertension, and one fifth had a previous myocardial infarction. The mean LV ejection fraction was 58±8%. Atrial fibrillation was present in just over forty percent at baseline, and a third of the patients were anticoagulated.
Table 1.
Characteristics of patients within the TOPCAT Americas presented as total cohort and by tertiles of baseline heart rate
| Total (N=1767) | Lower Tertile | Middle Tertile | Highest Tertile | P Value | |
|---|---|---|---|---|---|
| Median Baseline HR | 68 (60–75) | 59(54–60) | 68 (65–70) | 78 (74–84) | |
| Mean Baseline HR | 68±11 | 57±5 | 67±2 | 80±8 | |
| Age (years) | 72 (64–79) | 73 [65–80) | 73 (65–80) | 71 (63–79) | 0.006 |
| Females, n (%) | 882 (50) | 264 (47) | 248 (49) | 310 (54) | 0.03 |
| NYHA class, n (%) | |||||
| III/ IV | 620 (35) | 183 (32) | 177 (35) | 259 (38) | 0.14 |
| Paroxysmal Nocturnal Dyspnea, n (%) | 255 (14.4) | 76 (14) | 75 (15) | 104 (15) | 0.69 |
| Chest X-ray with Congestion, n (%) | 582 (33) | 188 (33) | 174 (34) | 219 (32) | 0.72 |
| Mean LVEF ±sd | 58±8 | 59±8 | 58±8 | 58±8 | 0.13 |
| HR at any time (bpm) | 68 (61–76) | N/A | N/A | N/A | |
| ΔHR (bpm) | 0 (−6 – 6) | N/A | N/A | N/A | |
| Blood pressure (mmHg) | |||||
| Systolic | 129 (118–138) | 130 (118–140) | 130 (118–139) | 128 (117–138) | 0.28 |
| Diastolic | 70 (62–80) | 69 (60–78) | 70 (63–80) | 74 (64–81) | <0.001 |
| Body Mass Index (Kg/M2) | 34±8 | 33±7 | 34±8 | 35±9 | 0.001 |
| Medical history, n (%) | |||||
| Hospitalization for CHF | 976 (72) | 287 (51) | 278 (54) | 409 (60) | 0.008 |
| Myocardial infarction | 893 (20) | 143 (25) | 105 (21) | 111 (16) | 0.001 |
| Stroke | 158 (9) | 49 (9) | 49 (10) | 60 (9) | 0.83 |
| Diabtetes mellitus | 788 (45) | 235 (42) | 232 (46) | 320 (47) | 0.19 |
| Hypertension | 1588 (90) | 517 (92) | 451 (88) | 618 (90) | 0.24 |
| Atrial fibrillation | 743 (42) | 233 (41) | 211 (41) | 297 (43) | 0.47 |
| Pacemaker | 242 (14) | 64 (11) | 79 (16) | 99 (14) | 0.11 |
| Implantable defibrillator | 42 (2) | 8 (1) | 18 (4) | 16 (2) | 0.08 |
| PCI | 344 (20) | 135 (24) | 103 (20) | 106 (15) | <0.001 |
| CABG | 336(19) | 120 (23) | 96(19) | 141 (16) | 0.007 |
| Current smoker | 117 (7) | 31 (6) | 31 (6) | 55 (8) | 0.17 |
| Previous Cancer | 69 (4) | 23 (4) | 14 (3) | 30 (4) | 0.32 |
| Left ventricular Hypertrophy | 167 (9) | 54 (10) | 50 (10) | 63 (9) | 0.93 |
| peripheral artery disease | 207 (12) | 76 (14) | 64 (13) | 67 (10) | 0.11 |
| Medical treatment at randomization, n (%) | |||||
| ACE inhibitor | 891 (51) | 290 (51) | 265 (52) | 334 (49) | 0.46 |
| Angiotensin receptor blocker | 698 (20.3) | 188 (33) | 157 (31) | 206 (30) | 0.42 |
| β-blocker | 1387 (79) | 461 (82) | 412 (81) | 513 (75) | 0.004 |
| Spironolactone | 886 (51) | 291 (52) | 257 (50) | 336 (49) | 0.66 |
| Calcium antagonist | 682 (39) | 240 (43) | 207 (41) | 233 (34) | 0.004 |
| Oral anticoagulants | 592 (34) | 190 (34) | 162 (32) | 240 (35) | 0.5 |
| Diuretic | 1573 (89) | 493 (87) | 450 (88) | 627 (91) | 0.06 |
The baseline characteristics of the patients by tertiles of baseline HR is also described in Table 1. Patients within the highest tertile of baseline HR, as compared to lower tertiles of HR, had higher diastolic pressure, higher body mass index, and more frequently had a history of hospitalization for HF, had a lower prevalence of ischaemic heart disease, and were more frequently on diuretics. There was a higher prevalence of atrial fibrillation in patients within the highest tertile of HR.
Resting heart rate measured at any time and temporal changes in heart rate
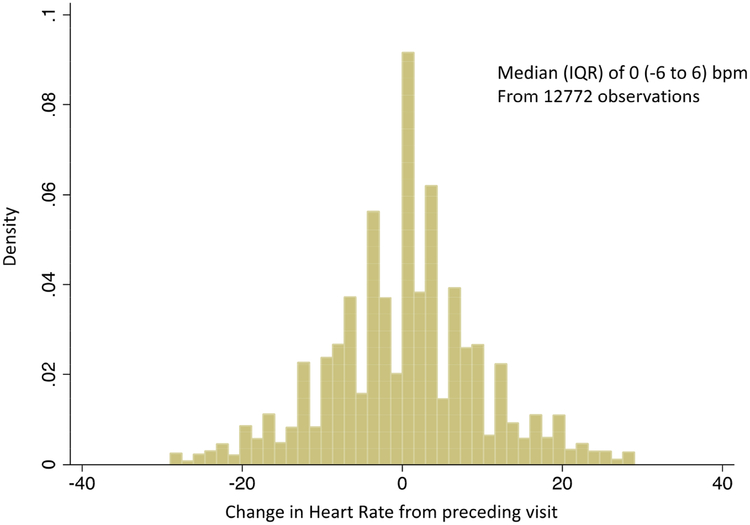
The median values of resting HR measured at any visit for the total Americas cohort were almost identical to the resting baseline HR of 68 bpm (Interquartile range 61–76 bpm), Table 1. The distribution of ΔHR occurring over a median of 135 (IQR 61–182) days is summarized in Figure 1. The majority of patients within the cohort did not change their HR from the preceding visit (median of zero (−6 to 6) bpm).
Figure 1: Histogram of the distribution of changes in HR from preceding visit (ΔHR) in subjects from the Americas.
The majority of patients have no change in HR from the preceding visit.
Temporal changes in heart rate from the preceding visit (ΔHR) and outcomes
Over a median follow up of 3.3 years, 522 patients experienced the primary endpoint of the study, a composite of CV death, aborted cardiac arrest or hospitalization for HF. The number events for other outcomes are summarized (Table2). As a continuous covariate, change in HR from the preceding visit were associated with all outcomes measures, except for fatal and non-fatal MI (Table 2). For example for each 5 bpm increase in HR from the preceding visit was associated with 9% higher risk of the primary outcome and a 17% higher risk of all-cause mortality. Furthermore, each 5 bpm increase in HR from the preceding visit was also associated with a 14%, 11% and 20% higher risk of CV death, hospitalization for HF and non-CV death respectively (Table 2).
Table 2:
Number of events, event rate and adjusted hazard ratio with 95% confidence interval for per 5 bpm increase in baseline HR, HR at any visit, changes in HR from preceding visit as continuous covariates and outcomes
| Events(n) | Event rate*(95% CI) | Adjusted hazard ratio (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline HR | P value | HR at any visit | P value | AHR | P value | |||
| All-Cause Mortality | 385 | 7.1 (6.8–7.8) | 1.12 (1.07–1.18) | <0.001 | 1.17 (1.12–1.22) | <0.001 | 1.17 (1.12–1.22) | <0.001 |
| CV Death or Hospitalization for HF or aborted cardiac arrest | 522 | 11.5 (10.5–12.5) | 1.08 ((1.04–1.12) | 0.001 | 1.11 (1.07–1.14) | <0.001 | 1.09(1.05–1.14) | <0.001 |
| CV Death | 223 | 4.3 (3.7–4.9) | 1.12 (1.06–1.20) | <0.001 | 1.14 (0.98–1.20) | <0.001 | 1.14 (0.97–1.21) | <0.001 |
| Hospitalization for HF | 400 | 8.8 (7.9–9.7) | 1.06 (1.01–1.11) | 0.01 | 1.13 (1.09–1.18) | <0.001 | 1.11 (1.06–1.16) | <0.001 |
| Non-CV death | 162 | 3.0 (2.6–3.5) | 1.123(1.04–1.21) | 0.002 | 1.20 (1.14–1.27) | <0.001 | 1.20 (1.14–1.28) | <0.001 |
| Fatal and Non fatal MI | 94 | 1.8 (1.5–2.3) | 0.97 (0.87–1.07) | 0.515 | 1.01 (0.91–1.12) | 0.86 | 1.02 (0.91–1.14) | 0.34 |
| Fatal and Non Fatal Stroke | 77 | 1.5 1.2–1.9) | 1.01 (0.90–1.12) | 0.905 | 1.11 (1.00–1.22) | 0.031 | 1.11 (0.99–1.24) | 0.052 |
Abbreviations: Confidence interval (CI); heart rate (HR); heart failure (HF); cardiovascular (CV), Myocardial infarction (MI), change in HR from the preceding visit(ΔHR)
Event rate per 100-patient years.
Adjusted for Age, sex, previous hospitalization for heart failure, previous MI, beta-blocker and rate limiting calcium channel blocker dose
Time updated disatolic blood pressure, weight,
For change in HR from the previous visit, the previous visit HR was included in the model and for HR at anytime baseline HR was included in the model.
For all reported hazard ratios above, no significant violations of the proportional hazards assumption were detected (p>0.05 for all)
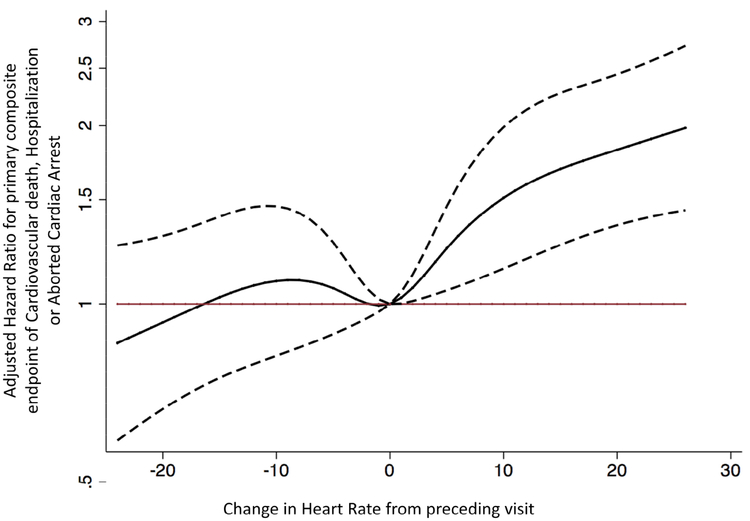
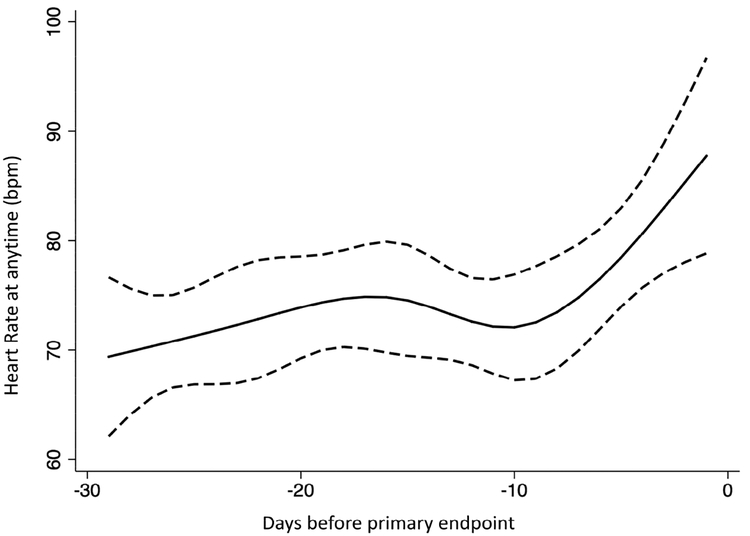
The restricted cubic spline model showed that the relationship between ΔHR and the primary endpoint was non-linear (Figure 2a). Any rise in HR was associated with elevated risk; however, a decline in HR was not significantly associated with lower risk of the primary endpoint. In patients experiencing the primary endpoint, a rise in heart rate of >10-bpm was visualized 5 to 10 days prior to the primary endpoint (Figure 2b).
Figure 2a: Association of Changes in HR from preceding visit (ΔHR) and the composite of CV death, HF hospitalization or aborted cardiac arrest in an adjusted cubic spline model in subjects from the Americas.
The adjusted cubic spline model, demonstrates the flexible relationship between changes in HR from preceding visit (ΔHR) and the hazard of developing the primary end-point in the TOPCAT trial, the composite of CV death, hospitalization for HF or aborted cardiac arrest. when no change in HR is taken as the reference (i.e. 0 bpm). This curve (solid black line) displays a near linear relationship between ΔHR and risk of the composite of CV death or hospitalization for HF, such that any rise in HR (>1 bpm) from the preceding visit appears to increase the risk significantly. A decrease in HR from the preceding visit was not associated with a significant reduction in risk. The dashed black curves represent the upper and lower 95% confidence limits, respectively. The horizontal red line represents the hazard ratio of 1. The domain was defined by excluding the smallest 1% and the largest 1% of values of ΔHR values.
Figure 2b: Restricted cubic spline model of the averaged heart rate at any time against time before the occurrence of the primary end-point (Time zero) in subjects from the Americas.
This figure demonstrates the relationship between resting HR at any visit in days before the primary event defined as the occurrence of either CV death, hospitalization for HF or aborted cardiac arrest. Within 10 days before an event, the HR appears to increase by more 5–10 bpm. The dashed black curves represent the upper and lower 95% confidence limits respectively.
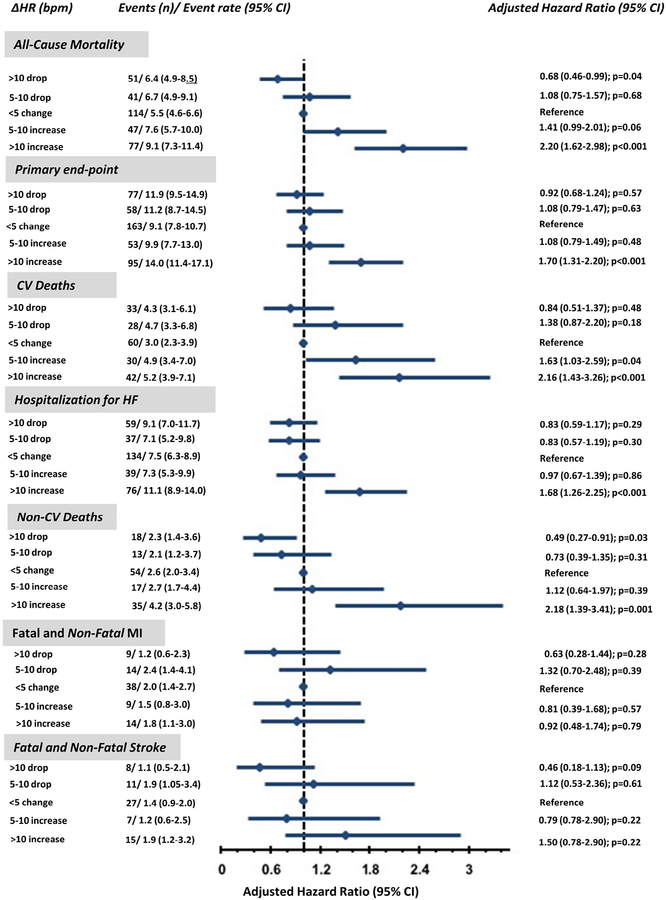
When we analysed the data categorically, ΔHR was also associated with outcomes (Figure 3). A >10 bpm rise in HR from the preceding visit compared to the no change in HR group was associated with a 70% higher risk of the primary endpoint and a 120%, 116% and 118% higher risk of all-cause mortality, CV death or non-CV death, respectively (Figure 3). A >10 bpm rise in HR from the preceding visit compared to the no change in HR group was associated with 60% higher risk of hospitalization for HF. A drop in heart rate >10 bpm, was significantly associated with a 32% and 51% reduced risk of all-cause mortality and non-CV death. There was no association with drop in HR and reduction in risk of CV death.
Figure 3: Association between changes in heart rate from the preceding visit and outcomes in subjects from the Americas.
Adjusted hazard ratio and 95% confidence intervals (95% CI) for six outcomes across the 5 categories of changes in HR from preceding visit (ΔHR).
The use of a beta-blocker at baseline in the study did not affect the relationship between ΔHR and outcomes (P for interaction of 0.90 for the primary endpoint) nor did the presence of atrial fibrillation at the time of randomization (P for interaction 0.41) for the primary endpoint. Ejection fraction above or equal/below 55% did not modify the relationship between change in HR from the preceding visit and the primary endpoint (P for interaction of 0.9). No interactions were detected between the other specified subgroups and ΔHR such as those with or without Diabetes mellitus.
Sensitivity analysis assessing the relationship between ΔHR and primary endpoint within the total cohort of the TOPCAT study detected no significant interaction between geographic region and the relationship between ΔHR and primary endpoint (p for interaction of 0.86), see table 1 of the supplements section. Furthermore sensitivity analysis using the second model, which adjusted for time updated changes in doses of beta-blocker and rate-limiting calcium antagonist and many more covariates had very minor differences in the quality of the results, see online table 2.
Heart rate at any time and outcome
As a continuous covariate, both baseline HR and time-updated HR, which represents HR at any visit were associated with most adverse outcomes (Table 2). However, baseline HR was not associated with fatal and non-fatal MI nor with fatal and non-fatal stroke and HR at anytime was not associated with fatal and non-fatal MI. For every 5 bpm higher HR at any time, the primary end-point and all-cause mortality were 11% and 17% higher, respectively.
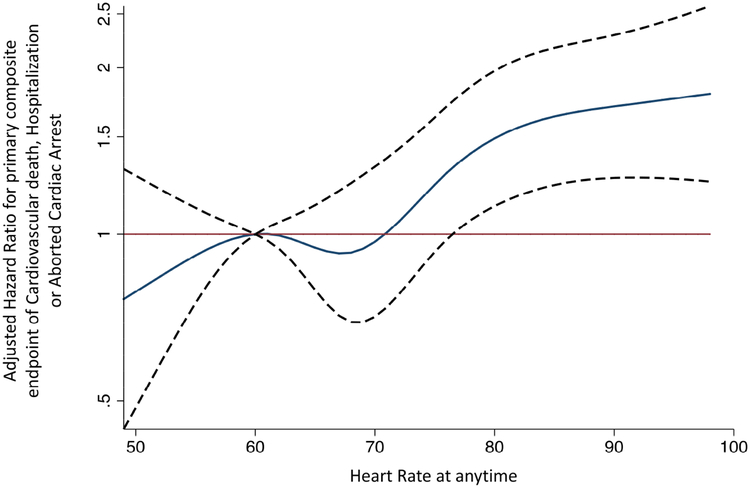
A resting HR of 61–76 bpm at any time during follow-up was not associated with a higher risk of the primary endpoint compared with a HR of 60 bpm (Figure 2c). However, a resting HR above 76 bpm at any time during follow-up was associated with a higher risk of the primary endpoint (Figure 2c). In patients with AF at baseline, a higher HR at any time was associated with the primary end-point, such that for every 5-bpm higher HR, the adjusted hazard ratio for the primary endpoint was 1.09 (1.03–1.16); p<0.01. The presence of atrial fibrillation at baseline did not modify the relationship between HR at anytime and the primary outcome (P for interaction of 0.50). The presence of diabetes mellitus nor preserved versus reduced EF or presence of beta-blocker have an interaction between HR at anytime and the primary endpoint.
Figure 2c: Association of heart rate at any visit (time-updated heart rate) and composite of CV death, HF hospitalization or aborted cardiac arrest in an adjusted cubic spline model in subjects from the Americas.
The adjusted cubic spline model, demonstrates the flexible relationship between resting HR at any visit and the hazard of developing the primary endpoint of the TOPCAT study, a composite of CV death, HF hospitalization or aborted cardiac arrest, when a resting HR of 60 bpm is the reference. This curve (solid black) shows that a resting HR of 61 to 76 bpm was not associated with an elevated risk of CV death, HF hospitalization or aborted cardiac arrest relative to a HR of 60 bpm. However, a resting HR above 76 bpm is associated with a steep rise in risk. The dashed black curves represent the upper and lower 95% confidence limits, respectively. The horizontal red line represents the hazard ratio of 1.
Sensitivity analysis using the total cohort of the TOPCAT study demonstrated that geographic region was associated with a differential relationship between baseline HR and the primary endpoint (P for interaction of <0.01) and also on the relationship between HR at anytime and the primary endpoint (P for interaction of <0.01), online table 1. The magnitude of the hazard ratio associated with baseline HR was larger in subjects from Russia/Georgia compared to subjects from the Americas.
Discussion
In a large cohort of patients with HFpEF, we found that baseline resting HR and changes in resting HR from the preceding clinic visit occurring over a median of 135 (IQR 61–182) days were independent predictors of the composite endpoint of CV death, aborted cardiac arrest or hospitalization for HF, and other endpoints such as all-cause mortality and non-CV death. While increases in HR were associated with higher risk, reductions in HR were not significantly associated with lower risk. Higher HR at any time was also predictive of adverse outcomes. These findings suggest that resting HR may be a useful and easily measured prognostic biomarker in the management of patients with HFpEF.
Our study further supports that changes in HR over time from the preceding clinic visit are of prognostic importance in patients with HFpEF, irrespective of cardiac rhythm. Patients with HFpEF and atrial fibrillation were at a similar risk compared to those in sinus rhythm; the presence of atrial fibrillation did not modify the relationship between baseline HR, HR at any time or change in HR from the preceding visit and adverse outcome. Increases in HR in patients with HF may reflect higher sympathetic tone due to decompensated HF or further progression of HF (15). Another possible cause of an increase in HR is the onset of atrial arrhythmias such as atrial fibrillation. In contrast, a decline in HR may reflect improving cardiac function and lower sympathetic tone. However, in this cohort a decrease in HR was only associated with a lower risk of non-CV death, but not of any of the CV adverse outcomes.
We controlled for both the use and dose changes of beta-blockers and rate-lowering calcium antagonists in a time-updated analysis as shown in online table 2. Thus, the risk associated with changes in HR were independent of both the use of and dose changes of these drugs. Furthermore, interaction testing showed that the use of beta-blocker at any time in the study did not modify the relationship between ΔHR and outcome.
As previously demonstrated in the analysis of HR and its change over time in the CHARM program (9), an increase in HR from the preceding visit was also associated with increased risk of non-CV death, which further supports that such a HR increase is likely to be a non-specific signal of deteriorating health or episodes of acute infection or other systemic stress.
Our study also showed that a temporal drop in HR over a median of 135 days was only associated with reduced risk of non-CV death, but was not associated with lower risk of CV adverse events. The explanation for these findings is unclear and the results differ from those found in the CHARM population, where a decline in HR heart was associated with reduced risk of the composite of CV-death or hospitalization for HF as well as for non-CV death. Perhaps the decrease in HR over time only has prognostic significance in patients with HF and reduced ejection fraction, but this will require further examination. Another explanation that could be considered is the presence of chronotropic incompetence in patients with HFpEF, which is poorly tolerated. In this study, beta-blocker therapy was associated with poorer outcome (unadjusted hazard ratio of 1.11 (1.04–1.18), p=0.001).
Our analysis provides additional evidence that the value of HR recorded at any time during the study was also of prognostic importance, in keeping with findings in the CHARM program(9). In the TOPCAT trial, patients with HR at any time below 76 bpm had the lowest risk of adverse outcome when 60 bpm is taken as the reference point. This finding is identical to that found from the analysis of the CHARM program. Thus, a resting HR >76 bpm appears to be associated with a higher risk of adverse events. In our analysis, HR appeared to be stable over time except that 5 to 10 days before an adverse event occurs, the resting HR appears to rise as demonstrated in Figure 2b. This method of analysis provides further support that a rise in HR is a marker of adverse events and could provide a warning sign to both patient and provider of such impending events. Our observation suggests that monitoring of HR over time, as a biomarker of severity of HF, in the clinic setting or perhaps remotely, may be useful in identifying HFpEF patients at greatest risk of readmission and death. Further research is required to assess the utility of such an approach.
In our sensitivity analysis, presented in the supplements section, we found that geographic region did not produce a differential relationship between the primary endpoint and change in HR, while region did modify the relationship between baseline HR and the primary endpoint and also between HR at anytime and the primary endpoint, online Table 1. The magnitude of the hazard ratio for both baseline HR and HR at anytime for the primary endpoint were larger in subjects from Russia/Georgia compared to subjects from the Americas, suggesting that baseline HR and HR at anytime is more important predictors of the primary endpoint in subjects from Russian/Georgia compared to the Americas. A plausible explanation for this relationship could be partly be explained by greater heterogeneity of the TOPCAT population from Russia/Georgia, with patients with true HFpEF having a higher baseline HR and going on to develop the primary event, see online figure 1.
Some limitations of the current analysis should be recognized. First, we were dependent on investigator-reported HR at each visit, which may have been measured in different ways, at different times of the day, and under different circumstances. Another limitation is that time-updated analysis limits the detailed characterization of the patients that have an increase or decline in HR from baseline or the preceding visit. However, an advantage of time-updated analysis is that it uses HR data from all visits, allowing the calculation of changes in HR over short periods of time, and the total time spent in each category with the associated number of events.
Strengths of this study included the large sample of patients with HFpEF including a large number who experienced adverse events. In addition, our analysis controlled for rate lowering medications such as beta-blockers and calcium antagonists using a time-updated analysis, taking into account both the use and changes in dose of these drugs. Thus, the importance of HR change appears to be independent of the use of beta-blockers and calcium antagonists.
Conclusions
In patients with HFpEF, inclusive those with atrial fibrillation, both baseline resting HR and changes in resting HR over time from the preceding clinic visit were independently associated with clinical outcomes, such that a higher baseline HR and an increase in HR were associated with elevated risk of CV events. However, a decline in HR over time was not associated with a lower risk of CV events. Our findings support the importance of measuring resting HR in every day clinical practice, and potentially with remote monitoring, as a way to identify HFpEF patients at greatest risk for readmission and death.
Supplementary Material
Perspectives.
Clinical Perspective
Resting HR and a change in HR over time were associated with adverse outcomes in patients with contemporary definition of HFpEF. Furthermore, we observed a rise in resting HR of approximately 10 bpm beginning approximately 10 days prior to the endpoint of HF hospitalisation, aborted sudden cardiac death and CV death. These data suggest that frequent outpatient monitoring of HR, possibly with remote technologies, may identify patients with HFpEF who may be at increased risk of rehospitalization or death.
Translational Outlook
Prospective studies are required to assess whether remote tracking of resting HR in patients with HFpEF can help identify those at increased risk of hospitalisation or death.
Acknowledgments
None
Funding Source: TOPCAT was funded by the National Institutes of Health, National Heart, Lung, and Blood Institute (NHLBI), Bethesda. Contract Number HHSN268200425207C
Abbreviation List:
- AHR
Adjusted Hazard ratio
- BNP
Brain natriuretic peptide
- ΔHR
Change in Heart from the preceding visit
- CV
Cardiovascular disease
- IQR
Inter-quartile range
- HF
Heart failure
- HFpEF
Heart Failure with preserved Ejection Fraction
- HR
Heart Rate
- NYHA
New York Heart Association
- NTproBNP
N-terminal pro-BNP
- TOPCAT
Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer: The contents of this manuscript are solely the responsibility of the authors and do not necessarily reflect the official views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the US government.
Disclosures
AV, BC, IA, JCF, NS, JR, JF, SS, BP and SDS have no conflicts of interest to disclose. MAP has received consulting fees from Aastrom, Abbott Vascular, Amgen, Bristol-Myers Squibb, Cerenis, Concert, Fibrogen, Genzyme, GlaxoSmithKline, Hamilton Health Sciences, Medtronic, Merck, Novo Nordisk, Roche, Salix, Sanderling, Serono, Servier, Teva, University of Oxford and received grant support from Amgen, Celladon, Novartis and Sanofi Aventis.
Reference
- 1.Castagno D, Skali H, Takeuchi M et al. Association of heart rate and outcomes in a broad spectrum of patients with chronic heart failure: results from the CHARM (Candesartan in Heart Failure: Assessment of Reduction in Mortality and morbidity) program. Journal of the American College of Cardiology 2012;59:1785–95. [DOI] [PubMed] [Google Scholar]
- 2.Bohm M, Swedberg K, Komajda M et al. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet 2010;376:886–94. [DOI] [PubMed] [Google Scholar]
- 3.Fox K, Borer JS, Camm AJ et al. Resting heart rate in cardiovascular disease. Journal of the American College of Cardiology 2007;50:823–30. [DOI] [PubMed] [Google Scholar]
- 4.Poole-Wilson PA, Uretsky BF, Thygesen K et al. Mode of death in heart failure: findings from the ATLAS trial. Heart 2003;89:42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palatini P, Julius S. Elevated heart rate: a major risk factor for cardiovascular disease. Clinical and experimental hypertension 2004;26:637–44. [DOI] [PubMed] [Google Scholar]
- 6.Nauman J, Janszky I, Vatten LJ, Wisloff U. Temporal changes in resting heart rate and deaths from ischemic heart disease. JAMA : the journal of the American Medical Association 2011;306:2579–87. [DOI] [PubMed] [Google Scholar]
- 7.Jouven X, Empana JP, Escolano S et al. Relation of heart rate at rest and long-term (>20 years) death rate in initially healthy middle-aged men. The American journal of cardiology 2009;103:279–83. [DOI] [PubMed] [Google Scholar]
- 8.Paul L, Hastie CE, Li WS et al. Resting heart rate pattern during follow-up and mortality in hypertensive patients. Hypertension 2010;55:567–74. [DOI] [PubMed] [Google Scholar]
- 9.Vazir A, Claggett B, Jhund P et al. Prognostic importance of temporal changes in resting heart rate in heart failure patients: an analysis of the CHARM program. European heart journal 2015;36:669–75. [DOI] [PubMed] [Google Scholar]
- 10.Pitt B, Pfeffer MA, Assmann SF et al. Spironolactone for heart failure with preserved ejection fraction. The New England journal of medicine 2014;370:1383–92. [DOI] [PubMed] [Google Scholar]
- 11.Shah SJ, Heitner JF, Sweitzer NK et al. Baseline characteristics of patients in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circulation Heart failure 2013;6:184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfeffer MA, Claggett B, Assmann SF et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015;131:34–42. [DOI] [PubMed] [Google Scholar]
- 13.Lewis EF, Claggett B, Parfrey PS et al. Race and ethnicity influences on cardiovascular and renal events in patients with diabetes mellitus. American heart journal 2015;170:322–9. [DOI] [PubMed] [Google Scholar]
- 14.Schoenfeld D Partial residuals for the proportional hazards regression model. Biometrika 1982;69:239–241. [Google Scholar]
- 15.Kaye DM, Lambert GW, Lefkovits J, Morris M, Jennings G, Esler MD. Neurochemical evidence of cardiac sympathetic activation and increased central nervous system norepinephrine turnover in severe congestive heart failure. Journal of the American College of Cardiology 1994;23:570–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.