Abstract
Background:
Risk-prediction models specifically for hospitalized heart failure with preserved ejection fraction (HFpEF) are lacking.
Methods and Results:
We analyzed data from the Atherosclerosis Risk in Communities (ARIC) Study Heart Failure Community Surveillance to create and validate a risk score predicting mortality in patients ≥55 years of age admitted with acute decompensated HFpEF (EF ≥50%). A modified version of the risk-prediction model for acute heart failure developed from patients in the Enhanced Feedback for Effective Cardiac Treatment (EFFECT) study was used as a composite predictor of 28-day and one-year mortality and evaluated together with other potential predictors in a stepwise logistic regression. The derivation sample consisted of 1,852 hospitalizations from 2005–2011 (mean age 77, 65% women, 74% white). Risk scores were created from the identified predictors and validated in hospitalizations from 2012–2013 (n=821). Mortality in the derivation and validation sample was 11% and 8% at 28 days and 34% and 31% at one year. The modified EFFECT score including age, systolic blood pressure, BUN, sodium, cerebrovascular disease, COPD and hemoglobin, was a powerful predictor of mortality. Another important predictor for both 28-day and one-year mortality was hypoxia. The risk scores were well calibrated and had good discrimination in the derivation sample (AUC: 0.76 for 28-day, and 0.72 for one-year mortality) and validation sample (AUC: 0.73 and 0.71, respectively).
Conclusions:
Mortality following acute decompensation in patients with HFpEF is high, with one-third of patients dying within a year. A prediction tool may allow for greater discrimination of the highest risk patients.
Clinical Trial Registration Information: URL: https://www.clinicaltrials.gov Unique identifier NCT00005131.
Subject terms: Heart failure, mortality/survival, risk factors, acute decompensated heart failure, preserved ejection fraction, risk prediction, mortality, risk score
Heart failure (HF) is a leading cause of hospital admissions and mortality among older adults in the US1. Half of patients presenting with acute decompensated heart failure (ADHF) are estimated to have preserved ejection fraction (HFpEF)2, 3. Several risk prediction models in HF exist4–8, some of which are derived from clinical trials and thus may be less applicable to the general HF population. Most are also restricted to patients with reduced ejection fraction (HFrEF) and some are developed for ambulatory patients limiting the use for hospitalized patients. From the Enhanced Feedback for Effective Cardiac (EFFECT) study risk scores were developed for 30-day and one-year mortality in ADHF regardless of EF9. Though the EFFECT cohort consisted of both HFrEF and HFpEF, the scores have not been validated in a strict HFpEF population. Comprehensive risk-prediction models specifically developed for hospitalized HFpEF patients are lacking.
No treatment has yet been convincingly shown to improve outcomes in HFpEF or in ADHF. A better insight into which factors relate to poor outcomes may help refine phenotypes for targeting with existing and potential novel treatment options. Moreover, early risk assessment at the time of hospital presentation may guide clinician, patient and family decision making and identify patients in need of more intensive monitoring and therapy or palliative interventions.
Therefore, we used the Heart Failure Community Surveillance in the Atherosclerosis Risk in Communities (ARIC) Study to identify predictors of mortality and to create a risk prediction model in patients with HFpEF hospitalized for ADHF.
METHODS
Study population
Beginning in 2005, the Heart Failure Community Surveillance component of the ARIC study enumerates and validates HF hospitalizations from 21 hospitals from 4 United States communities (Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland). Methods of event ascertainment and classification have been described previously.10 A stratified random sample of eligible hospitalizations for HF is selected based on a HF-related International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) codes in any position (Appendix table 1), age ≥55 years at the time of hospital discharge, and home address within the ARIC communities. The sampling fractions vary by ICD-9-CM discharge codes (428 and non-428), ARIC field center, sex, and race (by race in Forsyth County and Jackson only) to achieve similar standard errors for HF event rates across these strata. Medical records are abstracted by trained medical personnel. Abstracted hospitalizations are classified by physician review or computer algorithm as definite or possible ADHF, chronic stable HF, and HF unlikely or unclassifiable. ADHF is classified if there is evidence of worsening HF symptoms requiring augmentation of therapy, while chronic stable HF is selected if there is evidence of HF without change in symptoms.
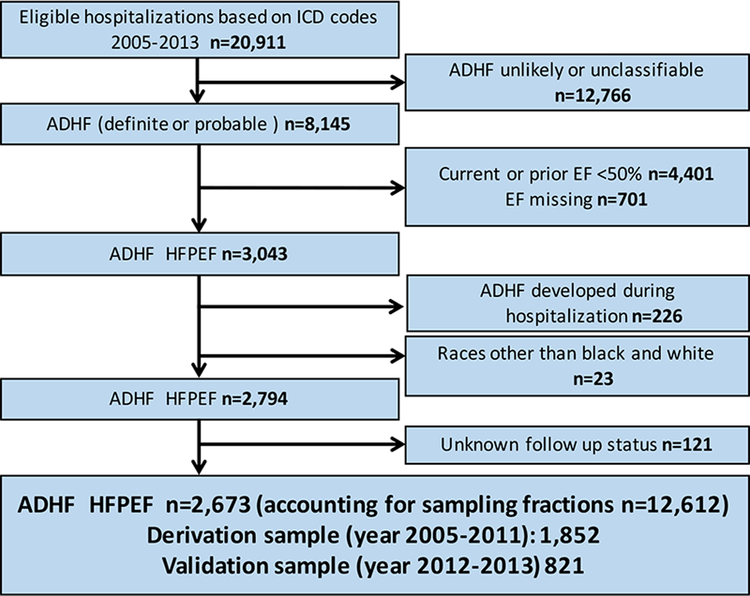
We excluded hospitalizations with EF <50%, EF ≥50 % with prior EF <50%, and EF missing. We further excluded hospitalizations of ADHF developed during hospitalization (rather than at time of admission), with unknown status at follow up, and race other than black or white. Hospitalizations from 2005–2011 were used as a derivation sample and from 2012–2013 as a validation sample (Flow chart; Figure 1). EF was based on inpatient diagnostic tests or, when absent, preadmission imaging studies (within 2 years before hospitalization). Direct linkage of individual patients to hospitalizations was not possible, thus the study is based on hospitalizations and not unique patients.
Figure 1. Flow chart: Selection of derivation and validation sample.
ICD, international classification of disease, ADHF, acute decompensated heart failure; ARIC, atherosclerosis risk in the commuity; EF, ejection fraction
Statistical methods
Baseline characteristics were compared between patients alive vs dead at 28-days and one-year post admission using t-test and Pearson chi-squared statistic with Rao-Scott correction for survey data11. Data were reported as mean ± standard error for continuous variables and percentages for categorical variables. Because of skewed distribution, BNP and NT-proBNP were log-transformed for modeling and geometric means are shown for descriptive purposes.
The index date was date of hospital admission. Outcomes were all-cause mortality within 28 days and within one year from index date. We used variables from the previously developed EFFECT score that were available in our dataset and created a modified EFFECT score using the scoring system from the EFFECT risk-prediction model (Appendix table 2)9. The variables in the modified EFFECT score included age, systolic blood pressure (SBP), blood urea nitrogen (BUN) sodium, cerebrovascular disease (defined as stroke/transitory ischemic attack [TIA] in ARIC), chronic obstructive pulmonary disease (COPD) and hemoglobin. Variables from the EFFECT score not available in our dataset were respiratory rate, dementia, hepatic cirrhosis and cancer. To improve model performance in HFpEF, we selected twenty-seven clinically relevant baseline variables as potential predictors that could be added to the modified EFFECT score. The modified EFFECT score-variable and the other candidate variables were used in a stepwise forward logistic regression with a p value of 0.2 as criteria for entering the model. Then the variables were eliminated in a stepwise fashion until discrimination was impacted (defined as a drop in area under the curve [AUC] by more than 0.015 from the full model). For variables with more than 5% missing, we performed simple imputation using the sample mean for missing values.
Continuous variables were fitted as continuous in the initial models unless there was clear evidence of non-linearity. The appropriateness of the linearity assumption was tested using spline analysis with the best fitting number of knots (3–5). Body mass index (BMI) showed a non-linear pattern and was categorized in 4 categories according to prior literature (underweight BMI <18.5, normal BMI 18.5–24.9, overweight BMI 25.0–29.9, obese BMI ≥30 kg/m2). When included in the modified EFFECT score, sodium and hemoglobin were categorized as in the original EFFECT score (Sodium < or ≥ 136mEq/L and Hemoglobin < or ≥ 10.0 g/dL respectively).
Because some hospitals use BNP and others NT-ProBNP, these variables were combined, first assessing a log transformation and standardization and then combining the variables into one. NT-ProBNP was log transformed for two age categories (≤75 and >75). The assessed odds ratios for BNP and NT-proBNP are per increase of one standard deviation of the log transformed values. For hospitalizations with both BNP and NT-ProBNP (n=4), BNP was used in the analysis. In ARIC, hypoxia is defined as oxygen saturation <90% or the term hypoxia stated in the medical record. Anemia is defined as previous hemoglobin levels of <12.0 g/dl for women and 13.0 g/dl for men or history of anemia stated in the record. For blood pressure and heart rate, the first documented levels in the record at the day of admission are recorded.
The reduced logistic regression models for 28-day and one-year mortality were used to create risk scores for predicting short and intermediate term mortality after admission. We converted the coefficients in the models into integer points in a risk score. Each integer is a rounding of the coefficient in the logistic regression models making the log odds ratio 0.1 equivalent to 1 point. For the risk scores, continuous variables were grouped into convenient intervals.
The risk score is directly related to the mortality at 28 days and one year in the two models respectively. Zero points represent the lowest risk and the score increases by an integer amount for each risk factor level above the lowest risk.
Discrimination of the risk scores was assessed by calculating AUC values and calibration by plotting predicted versus observed mortality by deciles of predicted probability and by the Hosmer-Lemeshow statistic. Temporal validation of the developed risk scores and the modified EFFECT score alone and was performed by assessing discrimination and calibration in the latest additions to the dataset (year 2012–2013). Because of a relatively small sample size, the calibration plots and Hosmer Lemeshow statistic are performing using quintiles in the validation sample.
All statistical analyses accounted for the stratified sampling design and weighted the observations by the inverse of the sampling fractions. Analyses were performed using Stata version 14.1 (Stata Corp., College Station, TX, USA). Institutional Review Board (IRB) approvals were obtained by each participating ARIC Study field center (the Universities of MS and MN, Wake Forest University, and Johns Hopkins University) and the coordinating center (University of NC). The research was conducted in accordance with the principles described in the Declaration of Helsinki.
RESULTS
Baseline characteristics and mortality
From 2005–2013 there were 20,911 eligible hospitalizations sampled in ARIC which represented a larger population of 87,342 hospitalizations. Of these, 8,145 sampled hospitalizations were defined as probable or definite ADHF. After additional exclusions as above, a study sample of 2,673 HFpEF hospitalizations representing a weighted sample of 12,612 remained (Flow chart, Figure 1). EF was based on inpatient diagnostic tests in 80% of the hospitalizations, in the remaining 20% EF was based on pre-admission evaluation performed no longer than two years before the current hospitalization. The derivation sample comprised 1,852 hospitalizations from 2005–2011 and the validation sample comprised 821 hospitalizations from 2012–2013.
Table 1 depicts baseline characteristics for the derivation sample by 28-day and one-year mortality. Mean age was 77 65% were women and 74% were white. Sixty-five percent had a prior diagnosis of HF and 29% had a prior hospitalization for HF. BMI was missing in 16% of the hospitalizations and the combined variable of BNP and NT-proBNP was missing in 12%. Simple imputation was performed on these variables; all other variables had <5% missing and complete case analysis was performed.
Table 1.
Baseline characteristics of Acute HFpEF Hospitalizations: ARIC Community Surveillance, 2005–2011 (Derivation Sample)
| Variable | 28 days | One year | ||||
|---|---|---|---|---|---|---|
| Dead n=180 |
Alive n=1,672 | p value | Dead n=620 |
Alive n=1,232 |
p value | |
| Demographics | ||||||
| Age | 82±0.7 | 76±0.3 | <0.001 | 80±0.4 | 75±0.3 | <0.001 |
| Sex (female) | 62% | 65% | 0.45 | 64% | 66% | 0.54 |
| Race (white) | 87% | 73% | <0.001 | 80% | 71% | <0.001 |
| BMI | 28±0.8 | 32±0.3 | <0.001 | 30±0.5 | 32±0.4 | <0.001 |
| BMI categories | <0.001 | <0.001 | ||||
| underweight | 8% | 2% | 6% | 1% | ||
| normal | 38% | 26% | 34% | 23% | ||
| overweight | 22% | 26% | 25% | 25% | ||
| obese | 33% | 47% | 35% | 50% | ||
| Health insurance | 99% | 97% | 0.14 | 99% | 97% | 0.030 |
| Heart failure related | ||||||
| Prior diagnosis of HF | 66% | 65% | 0.42 | 72% | 61% | <0.001 |
| Prior hospitalization for HF | 29% | 29% | 0.41 | 32% | 27% | 0.001 |
| Current LVEF | 58±0.7 | 58±0.2 | 0.93 | 58±0.4 | 58±0.3 | 0.73 |
| Vital signs at admission | ||||||
| Systolic blood pressure | 137±2.7 | 149±0.9 | <0.001 | 142±1.4 | 151±1.1 | <0.001 |
| Diastolic blood pressure | 70±1.6 | 77±0.5 | <0.001 | 73±0.9 | 78±0.6 | <0.001 |
| Heart rate | 89±1.9 | 86±0.6 | 0.15 | 88±1.1 | 85±0.7 | 0.014 |
| Increasing/new onset of symptoms at admission | ||||||
| Shortness of breath | 93% | 95% | 0.43 | 95% | 94% | 0.68 |
| Edema | 71% | 69% | 0.61 | 72% | 67% | 0.083 |
| PND | 8% | 15% | 0.009 | 9% | 17% | <0.001 |
| Orthopnea | 27% | 35% | 0.043 | 28% | 38% | <0.001 |
| Hypoxia | 70% | 47% | <0.001 | 62% | 43% | <0.001 |
| Signs and symptoms (any time during hospitalization) | ||||||
| JVD | 35% | 27% | 0.044 | 31% | 26% | 0.021 |
| Rhonchi | 38% | 28% | 0.014 | 35% | 26% | <0.001 |
| Rales (more than basilar) | 46% | 32% | <0.001 | 37% | 31% | 0.020 |
| Wheezing | 45% | 44% | 0.95 | 44% | 45% | 0.83 |
| Chest pain | 13% | 23% | 0.012 | 17% | 25% | 0.001 |
| Lab values, worst during hospitalization† | ||||||
| Hemoglobin (g/dL) | 9,8±0.1 | 10,6±0.1 | <0.001 | 10.0±0.1 | 10.8±0.1 | <0.001 |
| BNP * (pg/mL) | 857±83.6 | 540±17.8 | <0.001 | 719±37.6 | 501±19.3 | <0.001 |
| NT-proBNP * (pg/mL) | 5131±2040.0 | 3687±334.0 | 0.42 | 4379±769.0 | 3567±364.9 | 0.31 |
| Sodium (mEq/L) | 136±0.4 | 136±0.2 | 0.12 | 136±0.2 | 136±0.2 | 0.048 |
| Creatinine (mg/dL) | 2.2±0.2 | 2.1±0.1 | 0.30 | 2.3±0.1 | 2.0±0.1 | 0.003 |
| BUN (mg/dL) | 50±2.6 | 38±0.6 | <0.001 | 45±1.2 | 36±0.7 | <0.001 |
| Status at discharge | ||||||
| Deceased at discharge | 48% | 0% | <0.001 | 16% | 0% | <0.001 |
| Medical history | ||||||
| Anemia | 48% | 33% | <0.001 | 43% | 30% | <0.001 |
| Smoking (history of) | 6% | 12% | 0.035 | 8% | 13% | 0.005 |
| Asthma | 12% | 10% | 0.27 | 8% | 11% | 0.20 |
| COPD | 40% | 38% | 0.70 | 44% | 36% | 0.003 |
| Sleep apnea | 10% | 15% | 0.064 | 13% | 15% | 0.26 |
| Atrial fibrillation/flutter | 50% | 38% | 0.005 | 48% | 36% | <0.001 |
| Hypertension | 86% | 88% | 0.47 | 85% | 89% | 0.028 |
| Pulmonary hypertension | 26% | 20% | 0.088 | 26% | 18% | <0.001 |
| Peripheral vascular disease | 12% | 12% | 0.92 | 14% | 11% | 0.088 |
| Valvular heart disease | 36% | 26% | 0.013 | 34% | 24% | <0.001 |
| Diabetes | 41% | 49% | 0.07 | 45% | 50% | 0.085 |
| Stroke/TIA | 23% | 21% | 0.59 | 22% | 20% | 0.37 |
| Depression | 24% | 21% | 0.41 | 25% | 19% | 0.010 |
| Ischemic heart disease | 54% | 54% | 0.99 | 56% | 53% | 0.29 |
| Myocardial infarction | 17% | 19% | 0.64 | 20% | 18% | 0.37 |
| HF treatment at admission | ||||||
| RAS antagonist (ACEi and/or ARB) | 40% | 48% | 0.062 | 42% | 50% | 0.007 |
| Beta-blockers | 60% | 64% | 0.38 | 61% | 64% | 0.22 |
| Digoxin | 11% | 11% | 0.95 | 12% | 11% | 0.33 |
| Diuretics | 76% | 69% | 0.11 | 73% | 69% | 0.082 |
| MRA | 4% | 4% | 0.71 | 4% | 4% | 0.94 |
| Statins | 35% | 45% | 0.027 | 40% | 46% | 0.023 |
Values represent mean ±standard error or % unless stated. The numbers and percentages listed are weighted to account for sampling fractions (total 8,578 weighted and 1,852 non-weighted sampled events)
Variables typed in bold are used as potential covariates for the models
geometric means
worst value refers to the highest value with the exception of hemoglobin and sodium, where it refers to the lowest value
HFpEF, heart failure with preserved ejection fraction; ARIC, atherosclerosis in the community; BMI, body mass index; HF, heart failure; LVEF, left ventricular ejection fraction; PND, paroxysmal nocturnal dyspnea; JVD, jugular venous distension; BNP, brain natriuretic peptide; NT-proBNP, n-terminal prohormone brain natriuretic peptide; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; TIA, transitory ischemic attack; RAS, renin angiotensin system; ACEi, angiotensin converting enzyme; ARB, angiotensin receptor blocker; MRA, mineralocorticoid receptor antagonist;
Mortality in the derivation sample was 11% at 28 days and 34% at one year. In-hospital mortality was 6%. Those who died were older and more likely to be white. They were also more likely to be underweight and have a history of atrial fibrillation/flutter, anemia, pulmonary hypertension and valvular heart disease, and higher natriuretic peptide levels. Excluding in-hospital deaths, 28-day and one-year mortality was 6% and 30% respectively. Baseline characteristics for the validation sample is depicted in Appendix table 3.
Predictors of mortality
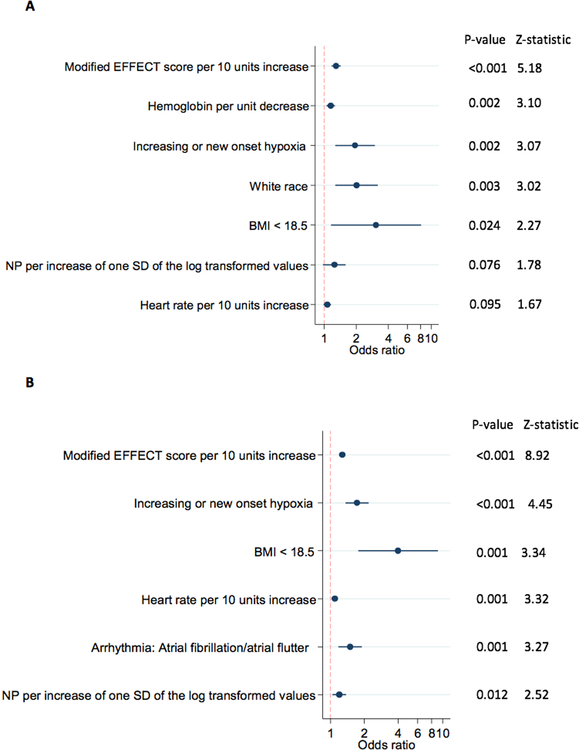
After imputation, <7% of the observations had any of the remaining variables missing and the stepwise logistic regression models were based on a total of 1,720 non-weighted (i.e. representing 7,957 weighted) observations. Following the stepwise regression, 4 and 8 variables were removed to result in the final models for 28-day and one-year mortality, respectively. The modified EFFECT score as a composite variable was the most powerful predictor of both 28-day and one-year mortality (Figure 2).
Figure 2. Predictors of (a) 28-day and (b) one-year mortality in order of significance.
Modified EFFECT score include age, systolic blood pressure, blood urea nitrogen (BUN) sodium, cerebrovascular disease and chronic obstructive pulmonary disease (COPD) and hemoglobin (the latter only for one-year mortality).
BMI, body mass index; SD, standard deviation
Hypoxia defined as oxygen saturation <90%
Higher heart rate, underweight (defined as BMI<18.5) and higher natriuretic peptide levels were predictors in both models whereas white race was identified as a predictor in the 28-day mortality model and history of atrial fibrillation/flutter was identified predictors in the one-year mortality model.
Discrimination of the models measured as AUC values was 0.76 for 28-day mortality and 0.72 for one-year mortality. To maintain appropriate discrimination of the models, two predictors with p values <0.10 were kept in the model for 28-day mortality (natriuretic peptides, p=0.076 and heart rate, p=0.095). All other predictors had p values <0.05.
Risk Score
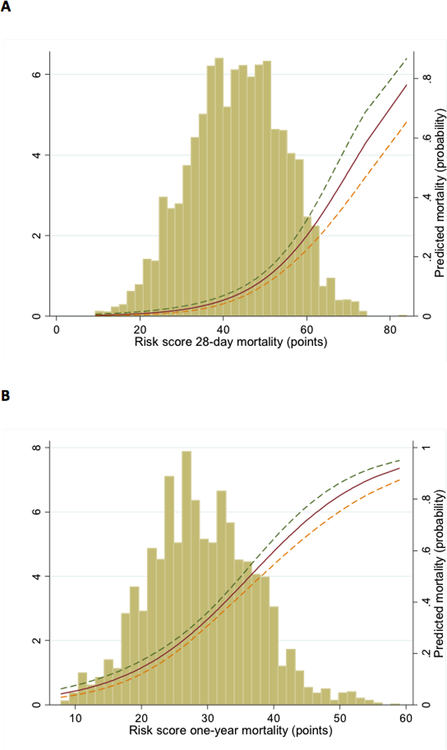
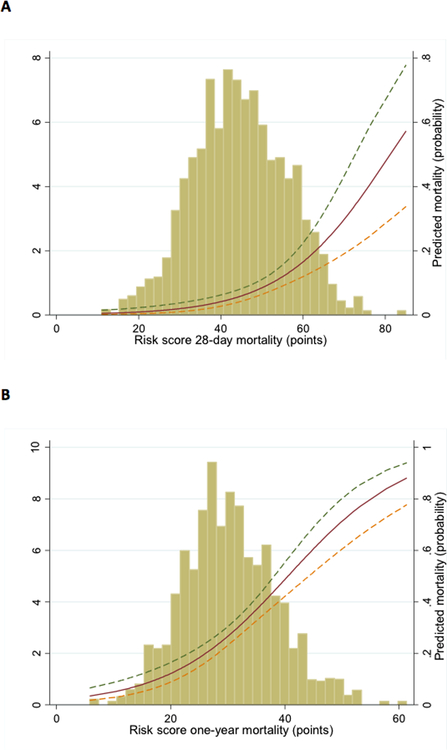
From the identified predictors, separate risk scores for 28-day and one-year mortality were created. The probability of dying within 28 days and one year was estimated by summing the points assigned for each value of the predictors. A web-based calculator is available at https://www2.cscc.unc.edu/aric/, providing a simple tool for prediction of mortality at 28 days and one year (The scoring system is shown in Appendix table 4). The distribution of the risk scores for 28-day and one-year mortality and their association with the risk of dying is shown in Figure 3. For 28-day mortality the median risk score was 44 with a range of 9–84. For one-year mortality, the median risk score 29, range 8–59.
Figure 3. Distribution of Risk score for (a) 28-day mortality and (b) one-year mortality and the association with predicted mortality.
The x-axis represents points of risk score, the left y-axis represents distribution of risk score (%) and the right y-axis represents predicted mortality (probability).
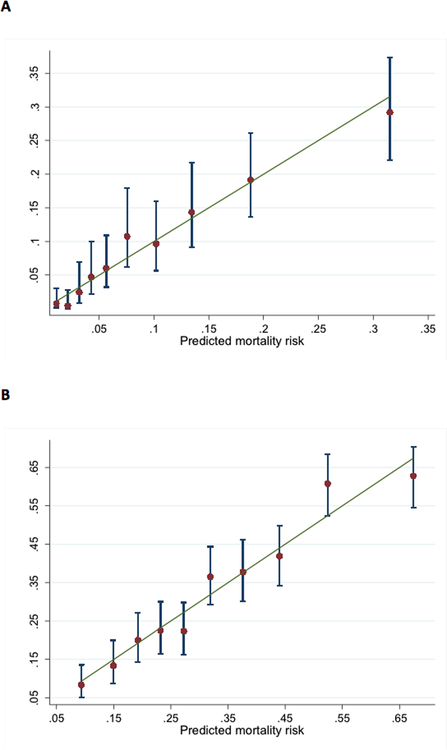
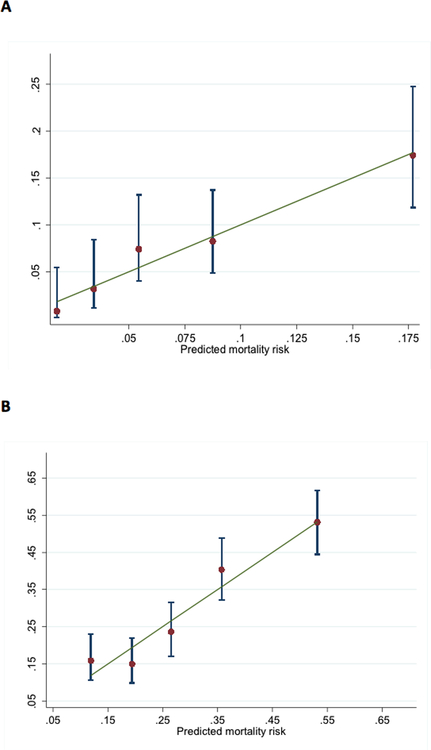
Overall, the mean observed 28-day and one-year mortality in the derivation sample was 11% and 34% respectively and the corresponding mean predicted numbers using the risk score were 10% and 33%. Discrimination of the risk scores was good (AUC 0.76 and 0.72 for 28-day and one-year mortality respectively). Calibration was also acceptable as shown with Hosmer-Lemeshow statistics (risk score p value 0.48 and 0.33 for 28-day and one-year mortality) and the plots of predicted vs. observed mortality by deciles of predicted mortality (Figure 4). The observed mortality increased by more than 30-fold across deciles of risk score.
Figure 4. Predicted vs. observed mortality by deciles of risk score for (a) 28-day mortality and (b) one-year mortality.
Error bars indicate 95% confidence intervals in each decile of risk score
Validation of the risk score
Mortality in the validation sample was 8% at 28 days and 31% at one year, the corresponding mean predicted numbers using the risk score were 7% and 29%. Distribution of the risk scores in the validation sample and the association with predicted mortality are shown in Figure 5. Risk score discrimination in the validation sample was only slightly weaker than in the derivation sample (AUC 0.73 and 0.71 for 28-day and one-year mortality respectively). Calibration was acceptable as shown with Hosmer Lemeshow statistics (risk score p value 0.51 and 0.29 for 28-day and one-year mortality) and the plots of predicted vs. observed mortality in Figure 6.
Figure 5. Validation sample: Distribution of risk score for (a) 28-day mortality and (b) one-year mortality and the association with predicted mortality.
The x-axis represents points of risk score, the left y-axis represents distribution of risk score (%) and the right y-axis represents predicted mortality (probability).
Figure 6. Validation sample: Predicted vs. observed mortality by quintiles of risk score for (a) 28-day mortality and (b)one-year mortality.
Error bars indicate 95% confidence intervals in each quintile of risk score
Discrimination of the modified EFFECT score alone in the validation sample was weaker than our developed models (AUC 0.70 and 0.68 for 28-day and one-year mortality respectively). Calibration of the modified EFFECT score alone was acceptable (Hosmer Lemeshow p value 0.70 and 0.99 for 28-day and one-year mortality respectively)
DISCUSSION
In this large generalizable community sample of patients ≥55 years of age hospitalized with ADHF and preserved EF from the Heart Failure Community Surveillance in the ARIC Study, mortality was 11% at 28 days and 34% at 1 year. Simple clinical variables at hospital admission were shown to be strongly associated with increased mortality. We generated risk scores that provide a simple and clinically useful tool to evaluate 28-day and one-year risk of death at the time of hospital presentation for HFpEF patients with ADHF.
The high mortality for patients hospitalized with ADHF and preserved EF of 6%, 11% and 34% in-hospital, 28-day and one-year, respectively, are consistent with data from the Olmsted county on hospitalized HFpEF patients and the EFFECT study from Ontario, Canada on ADHF, including both HFpEF and HFrEF patients2, 9. Studies with both ambulatory and hospitalized patients like the Meta-analysis Global Group in Chronic Heart Failure (MAGGIC) and the Swedish Heart Failure Registry report lower mortality in HFpEF12, 13. The much lower mortality rates in HFpEF trials such as CHARM-Preserved14 and I-PRESERVE15 are likely explained by a different patient selection including younger populations, lower comorbidity-burden and clinically stable patients. The Heart Failure Community Surveillance in ARIC distinctly differentiates between ADHF and chronic stable HF which most likely contributes to a higher specificity in identifying true ADHF hospitalizations than other classification criteria (Framingham) or simply ICD-codes. The ARIC classification of ADHF requires clear evidence of HF with active decompensation. Furthermore, 28-day and one-year mortality are from admission date and in-hospital deaths are included in these numbers. Exclusion of in-hospital deaths (n=100 non-weighted hospitalizations) gave slightly lower mortality rates at 28 days and one year.
Since the EFFECT score has been shown to be a good risk prediction tool in ADHF including the whole EF spectrum, we used the available variables from the EFFECT score as a basis for developing our new HFpEF risk score. Indeed, the composite modified EFFECT score variable including age, SBP, BUN, sodium, cerebrovascular disease and COPD was a powerful predictor of mortality also in HFpEF. By adding covariates to this composite variable we could improve model performance compared to the modified EFFECT score alone. The potential additional covariates included in our analyses were chosen based on prior knowledge and clinical relevance, and specifically, because of availability in the initial hours of hospitalization for early risk prediction, prioritization and triage. Since EF has been shown to be less prognostic when higher than 40–45%12, 16 and our inclusion criterion was EF ≥50%, EF was not included in the list of potential covariates.
Despite their dominant role in diagnosis and as treatment targets in ADHF, HF signs and symptoms have not frequently been evaluated as covariates for risk prediction in other studies. In ADHERE, dyspnea at rest was evaluated as a potential risk predictor, whereas Get with the Guidelines (GWTG) and OPTIMIZE-HF did not evaluate any signs or symptoms as potential predictors of mortality. We included shortness of breath, edema and hypoxia at admission and found hypoxia to be an important and to our knowledge novel predictor of both 28-day and one-year mortality.
Poor and worsening renal function, higher heart rate, and lower hemoglobin, BMI and SBP have all previously been shown to be strong predictors of mortality in HF9, 17–21. White race was a predictor of increased 28-day mortality, consistent with the findings from the risk prediction models from GWTG and OPTIMIZE-HF17, 19.
Comorbidities frequently associated with mortality in chronic HF such as diabetes and COPD were not confirmed to be significant predictors. These comorbidities may be relatively less important in an acute setting, where hemodynamic, metabolic and cardio-renal parameters are more reflective of the severity and progression of the heart failure syndrome and poor short and intermediate term outcomes. However, COPD was a significant predictor in the EFFECT score and thus included in our model as part of the modified EFFECT score. Interestingly, the most powerful predictors in our model represent different organ systems and aspects of the proposed pathophysiology if HFpEF.
Risk scores in HF are useful beyond improved discrimination and calibration of risk. They simplify and standardize the risk assessment in an otherwise heterogeneous and complex group of patients and encourage more rigorous and quantitative clinical assessment22. Most risk scores have been geared toward chronic HF and HFrEF for distinct purposes such as transplantation selection4, 23. In contrast, our risk score was developed for a strict HFpEF population where so far no convincing evidence-based treatment exists. The potential use is therefore in ADHF trial design (where HFpEF and HFrEF are often both included). Furthermore, HFpEF is a growing public health concern affecting elderly and comorbid patients24 where patient triage, decisions regarding in-patient and post-discharge health care resource utilization, and patient and family preferences and decision making are all informed by pragmatic and simple yet comprehensive tools for 28-day and one-year prognostication.
Our findings must be considered in the context of some limitations. First, due to limitations of the dataset, we were not able to link individual patients to hospitalizations. Thus, the study is based on hospitalizations and not unique patients. With a mean sampling fraction of 0.213 and a prior HF hospitalization in about 30%, around 6% of the events could possibly be rehospitalizations from patients already existing in the dataset. Since rehospitalized patients are known to have worse outcomes, our mortality rates may be slightly overestimated. Second, biochemical variables in ARIC are recorded as “worst” and “last” during hospitalization. We have used “worst” in the prediction models assuming that these variables are from admission/early part of hospitalization. We consider this an acceptable assumption considering that these patients have an acute condition at admission. Due to the complexity of our data and the variable selection process employed, we chose to perform simple imputation of missing values using the sample mean for BMI and natriuretic peptides, despite the fact that single imputation is generally considered inferior to multiple imputation. This may have resulted in biased estimates of the two parameters and their variances. The developed risk scores were validated using hospitalized acute HFpEF in the last two years of this study period, year 2012 through 2013, comprising 821 events including 64 deaths at 28 days and 252 at one year. This is a small validation sample with few outcome events, but sufficient to validate the risk scores with good discrimination and calibration. An important next step is to validate the risk score in a different cohort.
Since not all variables from the EFFECT model were available in our data we could not validate the complete EFFECT score and we can only compare our risk scores with the “modified EFFECT score”.
Among the strengths of our report is the use of a large biracial community sample from more than 20 hospitals in 4 diverse United States Communities, leading to generalizable and externally valid findings. Record abstraction is rigorously standardized with HF hospitalizations systematically classified and adjudicated by a panel of physician reviewers, lending reliability and internal validity to the findings.
In conclusion, this study provides further evidence that patients hospitalized with new onset or worsening symptoms of HFpEF face a high risk of death. The modified version of the EFFECT score, including age, systolic blood pressure, BUN, sodium, cerebrovascular disease, COPD and hemoglobin was a powerful predictor of mortality. The novel risk scores provide estimates of mortality that can guide clinician decision making regarding in hospital monitoring and treatment as well as early follow-up after discharge.
Supplementary Material
CLINICAL PERSPECTIVE.
What is new?
Our study provides further evidence that patients hospitalized with new onset or worsening symptoms of HFpEF face a high risk of death with about one-third of the patients being dead within a year.
Several risk scores in heart failure exist, but comprehensive risk-prediction models specifically developed for HFpEF patients are lacking.
We created a risk-prediction model for patients with HFpEF hospitalized for acute decompensated heart failure.
What are the clinical implications?
A better insight into which factors relate to poor outcomes may help refine phenotypes for targeting with existing and potential novel treatment options. Hence, a potential use of a risk-prediction model in this population may be in future trial design of HFpEF and ADHF.
Despite the lack of evidence-based treatment in HFpEF, risk-prediction models may be useful in daily practice, guiding clinical decision making regarding early patient triage, in-hospital monitoring and treatment, as well as early follow-up after discharge.
Estimates of mortality may provide patients and family realistic expectations regarding prognosis.
ACKNOWLEDGMENTS:
The authors thank the staff and participants of the ARIC study for their important contributions. The authors also thank Emanuel Ravemyr at the Royal Institute of Technology, Sweden, for programming the web-based calculator.
FUNDING SOURCES: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). T.T. was supported by the Swedish Heart-Lung foundation [grants 20080409 and 20100419 to L.H.L.’s institution] and the Stockholm County Council [grants 00556–2009 and 20110120 to L.H.L.´s institution].
Footnotes
DISCLOSURES: No conflicts of interest.
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB, American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics−−2015 update: A report from the american heart association. Circulation. 2015;131:e29–322 [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259 [DOI] [PubMed] [Google Scholar]
- 3.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43:317–327 [DOI] [PubMed] [Google Scholar]
- 4.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The seattle heart failure model: Prediction of survival in heart failure. Circulation. 2006;113:1424–1433 [DOI] [PubMed] [Google Scholar]
- 5.O’Connor CM, Whellan DJ, Wojdyla D, Leifer E, Clare RM, Ellis SJ, Fine LJ, Fleg JL, Zannad F, Keteyian SJ, Kitzman DW, Kraus WE, Rendall D, Pina IL, Cooper LS, Fiuzat M, Lee KL. Factors related to morbidity and mortality in patients with chronic heart failure with systolic dysfunction: The hf-action predictive risk score model. Circ Heart Fail. 2012;5:63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Connor CM, Hasselblad V, Mehta RH, Tasissa G, Califf RM, Fiuzat M, Rogers JG, Leier CV, Stevenson LW. Triage after hospitalization with advanced heart failure: The escape (evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness) risk model and discharge score. J Am Coll Cardiol. 2010;55:872–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pocock SJ, Wang D, Pfeffer MA, Yusuf S, McMurray JJ, Swedberg KB, Ostergren J, Michelson EL, Pieper KS, Granger CB. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27:65–75 [DOI] [PubMed] [Google Scholar]
- 8.Rohde LE, Goldraich L, Polanczyk CA, Borges AP, Biolo A, Rabelo E, Beck-Da-Silva L, Clausell N. A simple clinically based predictive rule for heart failure in-hospital mortality. J Card Fail. 2006;12:587–593 [DOI] [PubMed] [Google Scholar]
- 9.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: Derivation and validation of a clinical model. JAMA. 2003;290:2581–2587 [DOI] [PubMed] [Google Scholar]
- 10.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (aric) study: A comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.K Rao AJS JN. On chi-squared tests for multiway contingency tables with cell proportions estimated from survey data. . Annals of Statistics 1984;12:46–60 [Google Scholar]
- 12.Meta-analysis Global Group in Chronic Heart F. The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: An individual patient data meta-analysis. Eur Heart J. 2012;33:1750–1757 [DOI] [PubMed] [Google Scholar]
- 13.Lund LH, Benson L, Dahlstrom U, Edner M, Friberg L. Association between use of beta-blockers and outcomes in patients with heart failure and preserved ejection fraction. JAMA. 2014;312:2008–2018 [DOI] [PubMed] [Google Scholar]
- 14.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Investigators C, Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: The charm-preserved trial. Lancet. 2003;362:777–781 [DOI] [PubMed] [Google Scholar]
- 15.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A, Investigators IP. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467 [DOI] [PubMed] [Google Scholar]
- 16.Solomon SD, Anavekar N, Skali H, McMurray JJ, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S, Pfeffer MA, Candesartan in Heart Failure Reduction in Mortality I. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112:3738–3744 [DOI] [PubMed] [Google Scholar]
- 17.Peterson PN, Rumsfeld JS, Liang L, Albert NM, Hernandez AF, Peterson ED, Fonarow GC, Masoudi FA, American Heart Association Get With the Guidelines-Heart Failure P. A validated risk score for in-hospital mortality in patients with heart failure from the american heart association get with the guidelines program. Circ Cardiovasc Qual Outcomes. 2010;3:25–32 [DOI] [PubMed] [Google Scholar]
- 18.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC, Committee ASA, Investigators. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: A report from the acute decompensated heart failure national registry (adhere) database. J Am Coll Cardiol. 2006;47:76–84 [DOI] [PubMed] [Google Scholar]
- 19.Abraham WT, Fonarow GC, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O’Connor CM, Sun JL, Yancy CW, Young JB, Investigators O-H, Coordinators. Predictors of in-hospital mortality in patients hospitalized for heart failure: Insights from the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (optimize-hf). J Am Coll Cardiol. 2008;52:347–356 [DOI] [PubMed] [Google Scholar]
- 20.Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Kober L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN, Meta-Analysis Global Group in Chronic Heart F. Predicting survival in heart failure: A risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;34:1404–1413 [DOI] [PubMed] [Google Scholar]
- 21.Thorvaldsen T, Benson L, Stahlberg M, Dahlstrom U, Edner M, Lund LH. Triage of patients with moderate to severe heart failure: Who should be referred to a heart failure center? J Am Coll Cardiol. 2014;63:661–671 [DOI] [PubMed] [Google Scholar]
- 22.Lund LH, Stehlik J. Risk scores and biomarkers in heart failure: A journey to predictive accuracy and clinical utility. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation 2016;35:711–713 [DOI] [PubMed] [Google Scholar]
- 23.Parikh MN, Lund LH, Goda A, Mancini D. Usefulness of peak exercise oxygen consumption and the heart failure survival score to predict survival in patients >65 years of age with heart failure. Am J Cardiol. 2009;103:998–1002 [DOI] [PubMed] [Google Scholar]
- 24.Butler J, Fonarow GC, Zile MR, Lam CS, Roessig L, Schelbert EB, Shah SJ, Ahmed A, Bonow RO, Cleland JG, Cody RJ, Chioncel O, Collins SP, Dunnmon P, Filippatos G, Lefkowitz MP, Marti CN, McMurray JJ, Misselwitz F, Nodari S, O’Connor C, Pfeffer MA, Pieske B, Pitt B, Rosano G, Sabbah HN, Senni M, Solomon SD, Stockbridge N, Teerlink JR, Georgiopoulou VV, Gheorghiade M. Developing therapies for heart failure with preserved ejection fraction: Current state and future directions. JACC Heart Fail. 2014;2:97–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.