Abstract
Background:
The trans-maternal exposure to tobacco, microbes, nutrients and other environmental factors shapes the fetal immune system through epigenetic processes. The gastric microbe Helicobacter pylori represents an ancestral constituent of the human microbiota that causes gastric disorders on the one hand, and is inversely associated with allergies and chronic inflammatory conditions on the other.
Objective:
Here, we investigate the consequences of trans-maternal exposure to H. pylori, in utero and/or during lactation, for susceptibility to viral and bacterial infection, predisposition to allergic airway inflammation, and the development of immune cell populations in the lung and lymphoid organs.
Methods:
We use experimental models of house dust mite- or ovalbumin-induced airway inflammation and influenza A virus or Citrobacter infection along with metagenomics analyses, multi-color flow cytometry and bilsufite pyrosequencing to study the effects of H. pylori on allergy severity and immunological and microbiome correlates thereof.
Results:
Perinatal exposure to H. pylori extract, or its immunomodulator VacA, confers robust protective effects against allergic airway inflammation not only in the first, but also the second offspring generation, but does not increase susceptibility to viral or bacterial infection. Immune correlates of allergy protection include skewing of regulatory over effector T-cells, expansion of Treg subsets expressing CXCR3 or RORγt, and demethylation of the FOXP3 locus. The composition and diversity of the gastrointestinal microbiota is measurably affected by perinatal H. pylori exposure.
Conclusion:
We conclude that exposure to H. pylori has consequences not only for the carrier, but also for subsequent generations that may be exploited for interventional purposes.
Keywords: Allergic airway inflammation, microbial interventions during pregnancy, immune regulation, immune tolerance, metagenomics, epigenetic regulation of allergy and asthma
Graphical Abstract
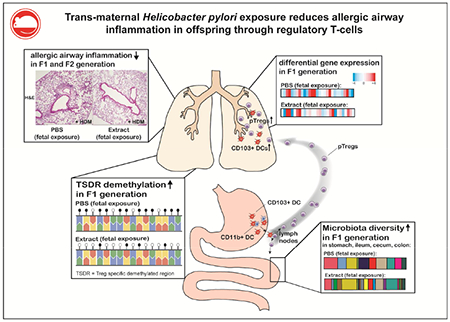
Capsule summary
Perinatal exposure to Helicobacter pylori or its immunomodulatory molecule VacA confers protection against allergic airway inflammation in mouse models. This protection is mediated by regulatory T-cells but is not indicative of generalized immunosuppression.
Introduction
The prevalence of allergic asthma has increased dramatically in recent decades, with ~235 million people affected worldwide.1 Environmental and lifestyle factors that include diet, exposure to antibiotics, sanitary conditions, country of birth, exposure to pets and livestock, the delivery mode and breastfeeding, sometimes collectively referred to as the “exposome”, have all been causally implicated in this trend.2, 3 Many of the environmental factors affecting asthma and allergy risk act early in life, i.e. leave their strongest marks on young adults, children, newborns and even the unborn fetus.2, 3 Others act not only on the exposed individual, but may manifest in subsequent generations. Examples of environmental factors that preferentially or exclusively act early in life include exposure to rural farming environments and livestock, which decreases the atopic sensitization and allergy risk of (directly exposed) children and the offspring of exposed pregnant mothers through mechanisms that appear to involve regulatory T-cells.4, 5 Other factors contributing to allergy risk early in life are the mode of birth (i.e. vaginal or Cesarean section delivery) as well as breast-feeding, which affect the establishment of a diverse human gut microbiota; recent studies suggest that microbial colonization of the GI tract and other mucosal surfaces may be initiated already in utero, continues at birth with the acquisition of microbes during vaginal delivery, and is completed during early postnatal life.6–9
It is now well-established that allergic infants and children exhibit a reduced diversity of their gastrointestinal microbiota that is characterized by a predominance of Firmicutes, and members of the Bacteroidaceae family.2 In both developed and developing countries, the decreasing prevalence of immunomodulatory microbes such as intestinal helminths10,11 or Helicobacter pylori12–15is significantly associated with an increased risk for allergic diseases, especially in pediatric populations. We have reported that neonatal infection of mice with H. pylori protects effectively against the airway hyper-responsiveness, pulmonary inflammation and goblet cell metaplasia that are hallmarks of experimental models of asthma induced by ovalbumin or house dust mite allergen sensitization and challenge.16–19 The experimental infection of adult animals had no or much weaker effects on allergy parameters, a finding that is in agreement with epidemiological data suggesting that older adults benefit less from harboring H. pylori than children, adolescents and young adults.12, 13, 20, 21 The underlying protective mechanism involves tolerogenic subsets of dendritic cells that -in the context of an H. pylori infection- drive the differentiation and suppressive activity of regulatory T-cells.16, 17, 19, 22 Interestingly, the immunomodulatory activity of H. pylori depends on two determinants, the vacuolating cytotoxin VacA and γ-glutamyl-transpeptidase GGT, that are on the one hand required for the protective effects of the live infection, and on the other hand recapitulate many of the benefits associated with live infection when administered in purified form.18, 22, 23
Here, we hypothesized that the perinatal, trans-maternal exposure (i.e. exclusively via the mother) to immunomodulatory molecules of H. pylori that occurs in utero or during lactation may recapitulate the benefits of H. pylori infection early in life. To test this idea, we perinatally exposed animals to H. pylori extract or purified VacA and evaluated their response to allergens in two models of allergen-induced airway inflammation. We further examined the immunological, microbial metagenomic and epigenetic correlates of perinatal exposure to H. pylori to identify possible determinants and biomarkers of trans-maternal immunomodulation. Finally, we used viral and bacterial infection models to address whether perinatal exposure to bacterial immunomodulators results in generalized immunosuppression.
Methods
Study approval
All animal experimentation was reviewed and approved by the Zurich Cantonal Veterinary Office (licenses 170/2014 and 140/2017 to A.M. and 210/2014 to C.M.).
Animal experimentation
C57BL/6 mice were purchased from Janvier and Foxp3eGFP-DTR (B6.129(Cg)-Foxp3tm3(DTR/GFP)Ayr/J, 016958) as well as OT-II (B6.Cg-Tg(TcraTcrb)425Cbn/J) mice were purchased from the Jackson Laboratory and included in experiments at 5-8 weeks of age. For the induction of acute house dust mite (HDM)-induced airway inflammation, mice were briefly anesthetized and subsequently subjected to six intranasal injections of house dust mite extract (Greer laboratories, XPB70D3A25 Dermatophagoides pteronyssinus) on day 0 (1 μg) and on days 8-12 (10-15 μg depending on extract lot). Mice were sacrificed on day 15. Transgenic Foxp3-eGFP-DTR mice were intraperitoneally treated 4 times with 1 μg diphteria toxin (DT, Sigma D0564-1MG) during days 5-12 of the HDM protocol. The ovalbumin (OVA)-induced model of airway inflammation involved sensitization with 20 μg OVA (Sigma A5503) adjuvanted with Alum Imject (Thermo Scientific 77161) by i.p. injection on days 0 and 14, followed by challenge on days 28, 29 and 30 with 1% aerosolized ovalbumin in PBS using an ultrasonic nebulizer. At the study endpoint, blood was collected and serum prepared. Lungs were lavaged via the trachea with 1 mL of PBS. Bronchoalveolar lavage fluid (BALF) cells were counted using trypan blue dye exclusion. Differential cell counts of macrophages, lymphocytes, neutrophils, and eosinophils were performed on cytocentrifuged preparations stained with the Microscopy Hemacolor-Set (Merck). One lung lobe was collected, homogenized and the total protein isolated, and the concentration determined using the BCA Protein Assay Kit (Thermo Scientific 23227). For histopathology, lungs were fixed by inflation and immersion in 10% (vol/vol) formalin and embedded in paraffin. Tissue sections were stained with H&E and periodic acid-Schiff and examined in blinded fashion on a BX40 Olympus microscope. Peribronchial inflammation was scored on a scale from 0 to 4. PAS-positive goblet cells were quantified per 1 mm of basement membrane as described previously.16 Scoring and goblet cell quantification was performed on 3-4 sections per mouse cut at different depths of 20 μm intervals, and ideally on more than one lung lobe (although this was not always possible).
For the production of H. pylori extract, bacterial cultures of the H. pylori strain PMSS1 were pelleted, washed with PBS, and subjected to three freeze-thaw cycles and homogenization using a pressure cell homogenizer (Stansted SPCH-18). The same procedure was used to produce E. coli (BL21, DE3) extract. The homogenate was centrifuged at 3000 × g, the resulting supernatant was filter-sterilized, and the protein concentration was determined as above. To prevent microbial contamination during cultivation of H. pylori, we generally used media supplemented with vancomycin and regularly checked for microbial contamination by streaking on selection plates containing antibiotics to which H. pylori is naturally resistant. Oligomeric s1m1 type VacA was purified from culture supernatants of H. pylori strain 60190 expressing Strep-tagged VacA via a streptactin resin as described previously.24 The dosage of extract and VacA was adjusted to the age of the mice and the application mode: extract p.o. 50 (neonates) −200 μg (adult mice), VacA i.p. 5-20 μg, VacA p.o. 5-20 μg. Neonates were treated with VacA or extract 1-2 times per week. Dams were treated 2-3 times per week during the entire pregnancy and lactation phase. For microbiota transplantation, the cecal content of trans-maternally treated or untreated adult mice was isolated, suspended in PBS, the weight per ml PBS adjusted, centrifuged at 300 x g and filtered through a cell strainer before gavaging it to 7 day-old pups.
IL-13 and HDM-specific IgE ELISA
The concentration of IL-13 in lung homogenate was determined by mouse IL-13 ELISA kit (eBioscience 88-7137-88). The quantification of serum HDM-specific IgE was performed by coating high affinity 96-well plates with 100 μl 25 μg/ml HDM extract in carbonate-bicarbonate coating buffer overnight at 4°C. After washing and blocking, diluted samples were incubated for 2 hours, before washing again and addition of an HRP-coupled IgE-specific antibody (GeneTex GTX77227) for detection. HRP substrate was added and the absorbance measured on a plate reader. Arbitrary units were calculated using a standard curve determined by serial dilution of a serum mix processed on the same plate.
Cytometric bead assay (CBA)
13 different cytokines were analyzed using the mouse Th cytokine panel legend plex assay (Biolegend, 740005).
TSDR methylation analysis
Total mesenteric lymph node cells of male C57BL/6 mice were isolated by means of collagenase type IV (Sigma C5138) digestion and filtering through a cell strainer. Male mice had to be used because in females, one X chromosome is inactivated by DNA methylation, which biases the results obtained for the second, active copy. After fixation, permeabilization and washing, cells were stained with anti-mouse CD4-FITC (Biolegend 100510) and Foxp3-APC antibodies (eBioscience 17-5773-82). CD4+Foxp3+ and CD4+Foxp3− cells were sorted on a FACS Aria. Genomic DNA was isolated from sorted cell subsets using the NucleoSpin® Tissue kit (Macherey-Nagel). An additional step was added to the manufacturer’s protocol to remove formaldehyde-induced crosslinking. Briefly, Chelex-100 beads (Biorad) were added after the lysis step and incubated at 95°C for 15 min in a shaker. Chelex-100 beads were spun down and the supernatant was transferred to a fresh tube. After addition of an adjusted amount of 100 % ethanol, additional purification steps were performed according to the manufacturer’s protocol. Genomic DNA was converted with bisulfite using the EZ DNA Methylation Kit (Zymo Research) according to the manufacturer’s instructions. The Treg-specific demethylated region (TSDR) was amplified by PCR and analyzed by pyrosequencing on a PSQ96MA instrument (Qiagen) as described;25 primers for sequencing were (in 5′ to 3′ direction), S1: CCATACAAAACCCAAATTC, S2: ACCCAAATAAAATAATATAAATACT, S3: ATCTACCCCACAAATTT, S4: AACCAAATTTTTCTACCATT), which cover CpG motifs 3-12 of the TSDR core region.
Flow cytometric analysis and cell sorting for qRT-PCR
Total mesenteric lymph node cells of C57BL/6 or Foxp3-eGFP-DTR mice were isolated by filtering through a cell strainer. Cells from perfused lungs were isolated by mincing the tissue followed by digestion with collagenase type IA (Sigma, C9891-500MG) and mechanical disruption using a syringe and pushing through a cell strainer. To assess DC allergen uptake and processing capacity, mice were intranasally challenged with 50 μl DQ-OVA (800 μg/ml, Thermo Fisher Scientific D12053) and AF647-OVA (800 μg/ml, Thermo Fisher Scientific 034784) ~ 15 hours before euthanization. After washing, the cells were stained in various combinations with mouse-specific antibodies targeting CD4 PerCP/Cy5.5 or BV 785 (Biolegend 116012 or 100552), CD45 BV650 (Biolegend 103151), the fixable viability dye eFluor780 (eBioscience 65-0865-14), TCR β chain PE/Cy7 (Biolegend 109222), siglecF PE or BB515 or BV421 (BD 552126, 56514, 562681), CD16/CD32 Fc Block (Biolegend, 101302), CD11c BV605 (Biolegend 117333), CD11b PerCP/Cy5.5 (Biolegend 101228), CD103 PE (Biolegend 121406), I-A/I-E AF700 (Biolegend 107622), F4/80 APC (Biolegend 123116), CD8α BV510 or PE/Cy7 (Biolegend 100752 or 100722), Neuropilin-1 BV421 (Biolegend 145209), CXCR-3 BV510 (Biolegend 126527), and CD3 Biotin (Biolegend 100244) in combination with Streptavidin BV711 (Biolegend 405241). After fixation, permeabilization and washing, cells were stained with antibodies for Foxp3 BV421 (Biolegend 126419) or Foxp3 FITC (eBioscience 11-577382), RORyt PE-eFluor 610 (eBioscience 61-6981), IRF4 PerCP-eFluor 710 (eBioscience 46-9858-80). In some experiments cells were re-stimulated in vitro in IMDM medium supplemented with Golgi-stop (BD 51-2092KZ), BFA (eBioscience 00-4506-51), PMA (Sigma P-8139) and ionomycin (Sigma I0634-1MG) before fixation and permeabilization for intracellular cytokine staining with anti-mouse IL-17 APC (Biolegend 506916) and IFNγ AF488 (Biolegend 505815). Samples were analyzed on a LSR II Fortessa instrument followed by detailed analysis using FlowJo software. For FACS-sorting of regulatory T-cells, mesenteric lymph node cells or lung cells from Foxp3eGFP-DTR mice were stained with the fixable viability dye eFluor780 (eBioscience 65-0865-14), and antibodies targeting CD4 BV711 (Biolegend 100550) and SiglecF BV421 (BD 562681) and sorted for live CD4+GFP+ cells on a FACS Aria. RNA of sorted cells was isolated using the RNeasy Mini Kit (Qiagen 74106), converted into cDNA, and subjected to TaqMan Real-Time PCR assay using the primers Mm03024075 (Hprt), Mm00475162 (Foxp3), Mm01178820 (Tgfb1) and Mm01288386 (IL10; all from Thermo Fisher Scientific). Samples were run on a Light Cycler 480 and normalized to the house-keeping gene Hprt.
RNA-sequencing
Lung regulatory T-cells were sorted as described above in section “Flow cytometric analysis and cell sorting for qRT-PCR”. RNA was isolated using the RNeasy micro kit (Qiagen) and RNA quality was assessed by Bioanalyzer 2100. The TruSeq RNA Sample Prep Kit v4 (Illumina) was used for library preparation, and sequencing was performed on the Illumina HiSeq 2500 instrument. RNA-seq reads were quality-checked with fastqc, which computes various quality metrics for the raw reads. RNA-seq reads were mapped to the GRCm38 mouse reference genome using STAR. Reads were counted according to Ensembl gene annotation using the featureCounts function in the Rsubread Bioconductor package. The EdgeR package was used to conduct statistical analysis of differential expression. The Treg RNA sequencing data reported in this paper are available in the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/gds) under the accession number GSE116116.
Influenza A Infection
Seven-week old mice were anesthetized by intraperitoneal (i.p.) injection of 0.05 mg/kg Fentanyl (Sintetica), 5 mg/kg Midazolam (Dormicum, Roche), 0.5 mg/kg Medetomidin (Dorbene, Gräub) and infected intranasally with 200pfu of Influenza A H1N1 strain A/Puerto Rico/8/1934 (PR8, Charles River) diluted in 30μl PBS (Gibco). Anesthesia was antagonised by i.p. injection of 2.5 mg/kg Atipamezolin (Alzane, Graub), 1.2 mg/kg Naloxon (Swissmedic), 0.5 mg/kg Flumazenil (Anexate, Roche). Mice were sacrificed by CO2 asphyxiation on day 9 post-infection. Blood was obtained by heart puncture. Lungs were perfused with cold PBS and extracted. Extracted lungs were cut into small pieces. After digestion with 2mg/ml Collagenase A (Sigma C9891) and 40μg/ml DNAse A (Roche 7002221) for 45min at 37°C, a single cell suspension was obtained by passing the tissue through a cell strainer of 40μm. Leukocytes were isolated by Percoll gradient centrifugation (GE Healthcare). Isolated leukocytes were restimulated in vitro in RPMI-1640 medium (Gibco) with 2μg/ml rat anti-mouse CD28 (BD 553294) and 10μg/ml PR8-specific epitopes NP1366–374 and HA211-225 or irrelevant control peptide OVA257-264 (peptides&elephants). Brefeldin A (BD) was added after one hour of restimulation at 5μg/ml. After an additional incubation time of 4h, cells were stained with FITC-labelled rat anti-mouse CD8 (eBioscience, clone 53-6.7), fixed and permeabilized following manufacturer’s instructions (BD Cytofix/Cytoperm kit, 554714) and intracellularly stained with PE-labelled rat anti-mouse IFNgamma (BD 554412). Cells were subjected to flow cytometric analysis using a FACS Canto II (BD). For the determination of PR8-specific IgG titres, adsorbent 96-well ELISA plates (NUNC) were coated with PR8 virus at 5×105 pfu/ml overnight at 4°C. Virus was inactivated by UV irradiation of 2× 240 mJ. Blood was collected in Microtainer tubes (BD 365967) and spun at 3500 rpm for 10 min to obtain serum. Serial dilutions of serum in PBS were distributed in the ELISA plates. Bound IgG was detected with HRP-coupled goat anti-mouse IgG (Jackson Laboratories) and visualized by incubation with substrate (TMB, Sigma). The reaction was stopped with H2SO4 1N solution and plates were acquired on a Tecan infinite M200 Pro reader at 450 nm and 620 nm. Values at 620 nm were subtracted from readings at 450 nm.
C. rodentium infection
The nalidixic acid (NAL) resistant Citrobacter rodentium strain ICC169 was grown over night at 37°C in Luria broth (LB) supplemented with NAL (50 μg/mL, Sigma). Mice were infected orally with 5×108 bacteria for 12 days. To assess C. rodentium colonization, cecal and colonic tissues were homogenized in PBS, diluted and plated on LB plates supplemented with NAL. Colonies were counted after 18h of culture at 37°C. Colonic and cecal bacterial loads were normalized to tissue weight. Colonic lamina propria preparations were stained and flow cytometrically analyzed as described under “Flow cytometric analysis”.
In vitro co-culture of naïve T-cells and CD11c+ APCs
Total spleen cells from OT-II mice were filtered through a 40μm cell strainer. After red blood cell lysis with ACK buffer, the remaining cells were stained with antibodies targeting CD4 BV711 (Biolegend 100549), CD25 FITC (Biolegend 102005), CD44 AF647 (Biolegend 103017), CD62L PE (Biolegend 104407), TCRb PE-Cy7 (Biolegend 109222), CD11c V450 (BD 560369), MHCII AF700 (Biolegend 107622) and the viability dye eFluor780 (eBioscience 65-0865-14). Naïve T-cells and CD11c+ MHCII+ APCs were FACS-sorted on a FACS aria and subsequently co-cultured at a ratio of 5:1 in the presence of 1 μg/ml OVA peptide 323-339 (EMC BAP-250) and 5ng/ml rmTGF-β1 (R&D Systems, 7666-MB) for 72 hours. Staining of regulatory T-cells and FACS analysis was conducted as described in “Flow cytometric analysis”.
Library preparation for microbiome analyses
Gastric tissue and ileal, cecal, and colonic tissue and contents were collected after necropsy and immediately frozen in liquid nitrogen and stored at −80°C until DNA extraction. DNA was extracted using the DNeasy PowerSoil HTP 96 Kit (Qiagen), and the V4 region of the bacterial 16S rRNA gene amplified in triplicate using barcoded fusion primers (F515/R806).26 Amplicon replicates were pooled, and the DNA was quantified using Quant-iT PicoGreen (Invitrogen). A maximum of 94 samples were then pooled at 20 nM concentration, purified using a Qiaquick PCR purification kit (Qiagen) and quantified using a Qubit 2.0 Fluorometer (Life Technologies). Finally, these samples were pooled at equal molar concentrations and sequenced on the Illumina MiSeq platform.
Microbiome Analysis
Utilizing QIIME 1.9.1, forward and reverse paired-end reads were trimmed and joined before being demultiplexed, filtered and analyzed. Open-reference OTU-picking was performed using the Greengenes Database Consortium (May 2017). Unweighted UniFrac distances were calculated and 2D-principal coordinate (PCoA) plots were generated in QIIME. Statistical significance was determined using the Adonis or Anosim tests. To determine OTUs that were significantly altered by treatment, a linear discriminate analysis (LDA) effect size (LEfSe) was performed. Taxa were classified as significantly different between groups when the log10 LDA score was >2.0, and the p-value <0.05 by the ANOVA test. Taxa were summarized at the genus level, and unclassified taxa were removed.
Statistical analysis
GraphPad Prism 6 was used for all statistical analyses. Unless specified otherwise, symbols in graphs represent individual mice and horizontal lines indicate medians. The Mann-Whitney test or ANOVA with Dunn’s correction for multiple comparisons were used to assess for significant differences. Stars are used to indicate the level of significance according to the p-value: * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001.
Results
Perinatal exposure to Helicobacter pylori extract reduces symptoms of house dust mite-induced allergic airway inflammation
To address whether administration of H. pylori extract to pregnant or lactating mice reduces allergy symptoms of their offspring upon allergen exposure later in life, we intragastrically treated females with twice-weekly doses of H. pylori extract generated by non-denaturing pressure homogenization. H. pylori extract was orally administered either during the three weeks of pregnancy, or during lactation, or both, with the first two treatments requiring litter swapping at birth (see schematic in suppl. Figure 1A). All offspring received an intranasal dose of house dust mite (HDM) allergen for the purpose of allergic sensitization once they had reached six weeks of age, followed by intranasal HDM challenge on days 8-12 post sensitization (suppl. Figure 1B). Both pre- and post-natal exposure of the offspring (i.e. in utero or during lactation), as well as the combined treatment, efficiently reduced all assessed parameters of airway inflammation, with strongly diminished overall leukocyte infiltration of the bronchoalveolar lavage fluid (BALF), strongly reduced eosinophilia, lower inflammation scores and fewer PAS-positive goblet cells counted in the airway epithelium (Figure 1A–F). HDM-specific serum IgE titers and the pulmonary expression of the Th2 cytokines IL-5 and IL-13 were reduced as well (suppl. Figure 1C–E); additional cytokines (IFN-γ, TNF-α, IL-2, -4, -6, -10, 17A, -21 and -22) were quantified by cytometric bead array of lung extracts, but did not change upon HDM sensitization and challenge (data not shown). The parallel administration of E. coli extract had no measurable effect on the various parameters of allergen-induced airway inflammation, suggesting that the observed protection is specific to H. pylori (Figure 1A–F, suppl. Figure 1C–E). Interestingly, we found the protective effects of extract exposure in utero or during lactation to be quite comparable in magnitude to the direct intraperitoneal or intragastric treatment of newborn pups with extract (suppl. Figure 1F–J) and to not be biased by litter sizes or gender (data not shown).
Figure 1. Perinatal trans-maternal exposure to H. pylori but not E. coli extract protects against house dust mite-induced allergic airway inflammation.
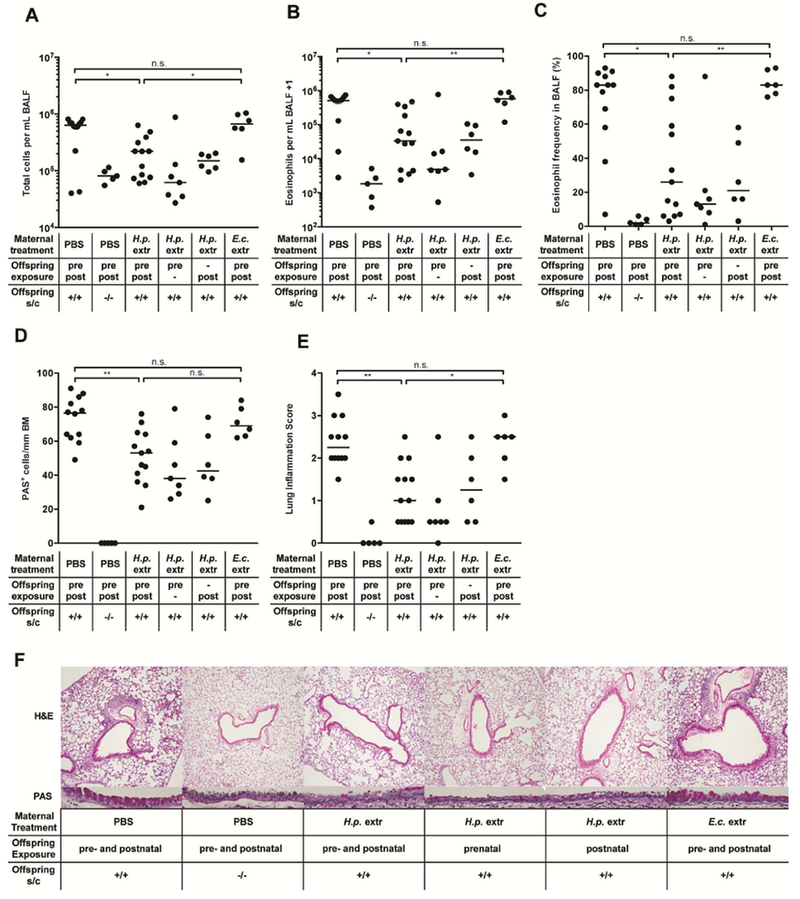
Mice were either pre- (pre) and/or postnatally (post/prepost) trans-maternally exposed to H. pylori- (H.p. extr), E. coli-extract (E.c. extr) or PBS treatment through 2-3 weekly oral gavages of the dams during pregnancy and/or lactation. Litter swaps were conducted at birth wherever necessary to avoid unwanted exposures. At six weeks of age, the offspring was sensitized and challenged (s/c) intranasally with house dust mite (HDM) allergen. Negative controls were sensitized and challenged with PBS only. (A) Total leukocytes in 1 ml of bronchoalveolar lavage fluid (BALF). (B) Total eosinophils in 1 ml of BALF. (C) Eosinophil frequencies in BALF. (D-F) Pulmonary inflammation and goblet cell metaplasia, as assessed on stained lung sections. BM, basement membrane. Representative sections are shown in F. In A-E, each symbol represents one mouse. The results were pooled from two independent experiments. Horizontal lines indicate medians; ANOVA with Dunn’s multiple comparison correction was used for calculation of p-values. * p<0.05, ** p<0.01.
To address whether perinatal extract treatment induces general immunosuppression and thereby increases the susceptibility of the offspring to pulmonary challenge with influenza A virus (IAV),27 we infected perinatally (pre- and postnatally) treated offspring with 200 PFU (0.4 HAU) of IAV. The infection triggered clearly detectable weight loss as well as high titers of PR8-specific IgG, with high titers correlating well with more pronounced weight loss (suppl. Figure 1K–M). The re-stimulation of lung leukocyte preparations with the influenza antigens NP1 and HA revealed strong influenza-specific CD8+ T cell responses (suppl. Figure 1N). Perinatal H. pylori extract treatment did not measurably affect the magnitude of the IAV-specific readouts (suppl. Figure 1K–N), suggesting that the reduced severity of allergic airway inflammation is not likely to be due to general immunosuppression. This conclusion was further supported by an acute bacterial infection model using Citrobacter rodentium; trans-maternally extract-treated animals were as effective at generating C. rodentium-specific Th1 and Th17 responses and at controlling the infection as PBS-treated animals (suppl. Figure 1O-Q).
Postnatal exposure to the H. pylori immunomodulator VacA reduces house dust mite-induced allergic airway inflammation
We have previously attributed the allergy-protective effects of H. pylori to its secreted immunomodulator VacA, which is both necessary and sufficient for conferring protection against ovalbumin and house dust mite-induced allergic airway inflammation on the one hand, and food allergy driven by ovalbumin or peanut allergen exposure on the other.18, 22, 28 As intraperitoneal injection was not feasible in pregnant dams, we administered VacA purified from culture supernatants of H. pylori either intraperitoneally during lactation or orally during pregnancy and lactation. Both treatments, administered twice weekly, reduced the bronchoalveolar infiltration and eosinophilia, as well as lung inflammation and goblet cell metaplasia associated with HDM-induced allergic airway inflammation (Figure 2A–E; suppl. Figure 2A–E). However, we found trans-maternally administered VacA to be less beneficial in terms of reducing allergy severity than the direct intraperitoneal treatment of newborn offspring with VacA (suppl. Figure 1F–J). The combined results suggest that the H. pylori immunomodulator VacA suppresses hallmarks of allergic airway inflammation not only when present in the context of a live infection, but also when administered in purified form to pregnant or lactating dams, or to pups during the first weeks of life.
Figure 2. Postnatal trans-maternal exposure to H. pylori VacA protects against house dust mite-induced allergic airway inflammation.
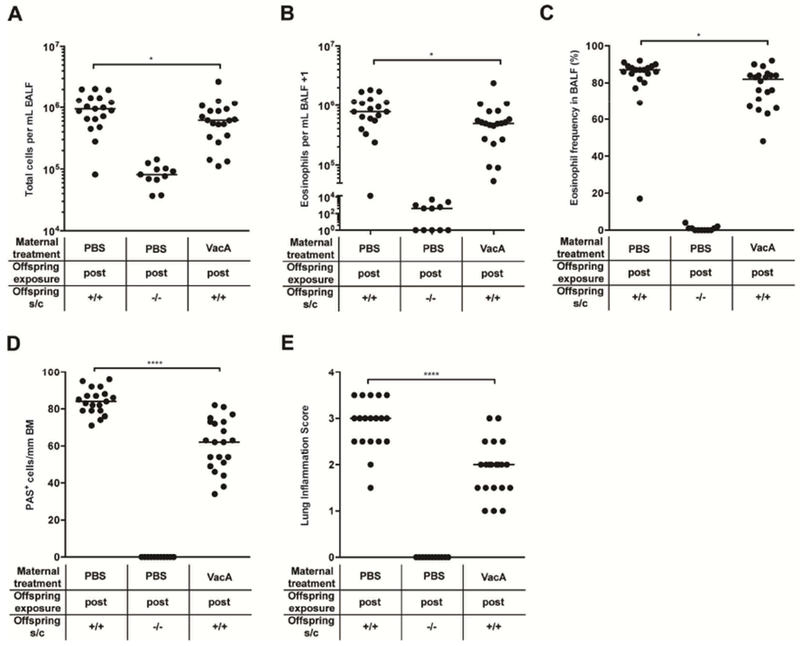
Mice were trans-maternally exposed postnatally (“post”) to H. pylori VacA through twice-weekly intraperitoneal treatments of the dams with 20 μg purified VacA during lactation. Offspring of PBS-treated dams were used as controls. At six weeks of age, the offspring was sensitized and challenged intranasally with HDM allergen. Negative controls were sensitized and challenged with PBS only. (A) Total leukocytes in 1 ml of BALF. (B) Total eosinophils in 1 ml of BALF. (C) Eosinophil frequencies in BALF. (D,E) Pulmonary inflammation and goblet cell metaplasia, as assessed on stained lung sections. BM, basement membrane. In A-E, each symbol represents one mouse. The results were pooled from three independent experiments. Horizontal lines indicate medians; an unpaired Mann-Whitney U test was used for statistical analyses. * p<0.05, ** p<0.01, *** p<0.001.
To address whether exposure of the offspring to (however minuscule) amounts of extract in utero or during lactation were required for the protective effects, or tolerizing treatment of the mother from her own neonatal period onwards was sufficient to confer protection, we initiated the treatment of prospective mothers at 6 days of age, but discontinued it before mating. The unexposed offspring of such tolerized mothers did not show evidence of protection against HDM-induced airway inflammation (suppl. Figure 2F–J), indicating that exposure to extract via the placenta or the milk is a prerequisite for protection. To rule out that trans-maternal exposure to H. pylori extract protects against house dust mite, but not other allergens, we examined possible protective effects in the ovalbumin model of allergic airway inflammation. Interestingly, the protective effects of trans-maternal exposure to H. pylori were just as strong in this model as in the house dust mite model (suppl. Figure 2K,L), indicating that this form of allergy protection is independent of the allergen used.
Perinatal exposure to H. pylori extract skews lung T-cell responses towards regulatory T-cells
We next performed a detailed analysis of the steady state (unchallenged) lung T-cell and myeloid compartments by multicolor flow cytometry to identify immune correlates of the differential propensity of extract-treated and control mice to respond to HDM allergen. Mice that had been exposed perinatally (i.e. in utero and until weaning) to H. pylori extract exhibited generally lower pulmonary CD4+ T-cell frequencies and lower Th1 and Th17 frequencies -as assessed by intracellular cytokine staining for the signature cytokines IFN-γ and IL-17 of ex vivo re-stimulated leukocyte preparations- than did the PBS-exposed offspring (Figure 3A,B). Although the overall Foxp3+ Treg frequencies did not differ among the two treatment groups, extract-exposed mice exhibited significantly higher frequencies of pulmonary neuropilin-negative Tregs (which are enriched for peripherally induced pTregs; see gating strategy in suppl. Figure 3A) and of two Treg subsets that are associated with particularly high suppressive activity, i.e. CXCR3+ and RORγt+ Tregs (Figure 3C–F).29–32 In contrast, the very abundant IRF4+ Treg subset was not significantly different, and if anything, underrepresented, in the lungs of extract-exposed mice (Figure 3G). The shifts in T-cell populations appeared to be specific to the lung and were not observed in the mesenteric lymph nodes (MLNs; suppl. Figure 3B–H).
Figure 3. Perinatal trans-maternal exposure to H. pylori extract skews lung T-cell responses towards specific regulatory T-cell subsets.
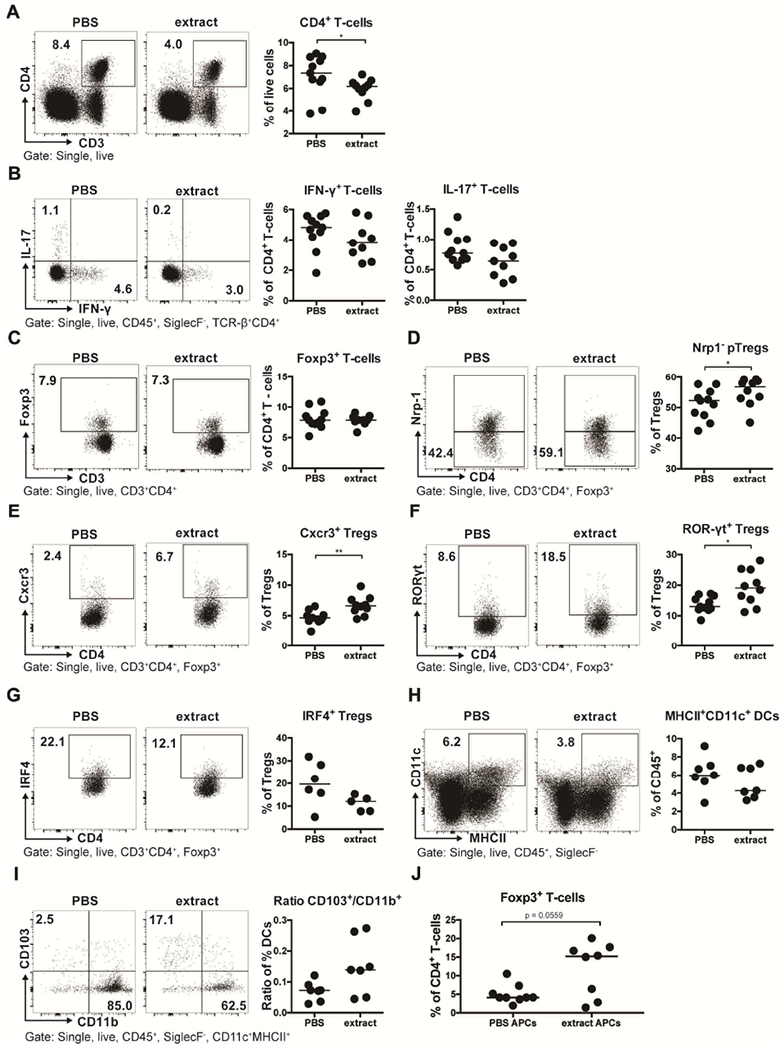
(A-I) Mice were trans-maternally pre- and postnatally exposed to H. pylori extract or PBS during pregnancy and lactation. At six weeks of age, lung leukocytes were analyzed by multi-color flow cytometry. (A) CD4+ T-cell frequencies among all leukocytes; representative FACS plots are shown on the left. (B) Th1 and Th17 frequencies among all CD4+ T cells, of the mice shown in A. (C) Foxp3+ Treg frequencies of the same mice. (D) Peripherally induced Treg (pTreg; Nrp-1−) frequencies among all Foxp3+ Tregs. (E-G) Frequencies of Cxcr3+, RORγT+ and IRF4+ Tregs among all CD4+FoxP3+ Tregs. (H) MHCII+CD11c+ dendritic cell (DC) frequencies among all pulmonary leukocytes. (I) Ratios of CD103+CD11b− over CD103−CD11b+ DCs, of all mice shown in H; representative FACS plots are shown on the left. (J) Frequencies of Foxp3+ T-cells among all CD4+ OT-II T-cells, after three days of co-culture with FACS-sorted splenic MHCII+CD11c+ DCs. Data are pooled from two studies (A-F) or show the results of a representative experiment of at least two (G-J). Horizontal lines indicate medians; an unpaired Mann-Whitney U test was used throughout. * p<0.05, ** p<0.01.
We next asked whether DC subsets were quantitatively or functionally different in the lungs of extract- and PBS-exposed mice. DCs are critically required for the development of peripheral tolerance to antigens and allergens, and we have reported previously that the CD103+ subset of DCs is over-represented in the lungs of mice that are protected against allergic airway inflammation due to live infection with H. pylori.22 Although the overall frequencies of MHCII+ CD11c+ pulmonary DCs were somewhat lower in extract-treated relative to control animals (Figure 3H), the ratio of CD103+ to CD11b+ DCs, which represent the two major resident DC populations, was higher (Figure 3I). CD103+CD11b+ DCs represent a minor (if any) population in the lung and do not change measurably upon extract exposure (suppl. Figure 3I). As observed for T-cells, DC populations in the MLNs did not differ depending on the perinatal exposure (suppl. Figure 3J–M). To assess the functionality of DCs in the lung, we administered recombinant ovalbumin (OVA) protein that is coupled either to a constitutive fluorophore (AF647) or to a fluorophore that requires OVA processing and presentation in the context of MHCII molecules (DQ-OVA). Perinatal extract exposure did not measurably impair the ability of MHCII+CD11c+ pulmonary DCs to sample or to process OVA (suppl. Figure 3N,O); however, the OVA-positive or DQ-OVA-positive DC populations recapitulated the general trend toward over-representation of CD103+ DCs (suppl. Figure 3Q–S). Overall, our immunological profiling efforts reveal the skewing of T-cell responses towards specific regulatory T-cell subsets in extract-treated animals, which is consistent with the observed shifts favoring tolerogenic over immunogenic DC populations. To examine possible direct systemic effects of trans-maternal extract exposure on DCs, we sorted splenic CD11c+ MHCII+ DCs from six week-old, trans-maternally extract-exposed or control offspring and co-cultured them with naïve splenic OT-II T-cells in the presence of OVA peptide and recombinant TGF-β. DCs from extract-exposed offspring showed a higher propensity to induce Foxp3 expression in naïve T-cells than their counterparts from control offspring (Figure 3J), indicating their more pronounced tolerogenic activity in this setting.
Perinatal exposure to H. pylori extract or VacA affects the diversity and composition of gastrointestinal bacterial community structures
To address whether the perinatal exposure to H. pylori extract or VacA not only has consequences for allergy severity and immunological correlates of protection, but may also affect the gastrointestinal microbiota, we performed 16S rRNA sequencing of 194 samples from the stomach, ileum, cecum and colon of 50 mice that were subjected to perinatal extract, VacA, or PBS treatment (i.e. their mothers were orally gavaged with extract, PBS or VacA throughout pregnancy and lactation). We obtained an average of 6407 reads per sample. The sequencing depth was comparable across organs; to include 190 (i.e. 98% of) samples, we rarified to 2,000 reads, but results were similar with greater read depth and fewer samples (data not shown). In total, we identified 512 observed operational taxonomic units (OTUs). Examination of beta-diversity using unweighted UniFrac analysis revealed a clear segregation of samples driven by anatomical site as well as by treatment modality (suppl. Figure 4A,B). For each organ studied, samples clustered significantly by the treatment group; this was most pronounced in the stomach and ileum (Figure 4A,C). Linear discriminant analysis (LDA) effect size (LEfSE) confirmed significant differences in relative abundances related to treatment. The largest differences were observed in the stomach and ileum; both VacA and extract treatment favored depletion of Firmicutes and Bacteroidetes in the stomach, while several taxa were strongly overrepresented at the class and order level (Figure 4B,D). In the stomach, VacA treatment resulted in depletion of Allobaculum and enrichment of Ruminococcaceae Clostridium (Figure 4B). VacA treatment was associated with depletion of the taxa Clostridiaceae Candidatus Arthromitus (Segmented Filamentous Bacteria) and treatment with either extract or VacA resulted in a depletion of Akkermansia and Desulfovibrio species in the ileum (Figure 4D). The results from our metagenomics analysis thus suggest that perinatal exposure to H. pylori immunomodulators has clearly discernible effects on bacterial communities in the gastrointestinal tract much later in life, which may either be a cause or consequence of the skewing of steady state immune parameters towards regulatory branches of the immune system. To assess possible causality, we transplanted the cecal content of perinatally extract-exposed adult animals into neonates and subjected these animals to HDM sensitization and challenge as adults. Cecal transplantation was not sufficient to confer protection against allergic airway inflammation (suppl. Figure 4C–G), suggesting that the changes in the GI tract microbiota are probably a consequence rather than the cause of the immunomodulation that manifests in reduced allergy symptoms.
Figure 4. Perinatal trans-maternal exposure to H. pylori extract induces shifts in the gastric and ileal microbiota.

(A-D) Mice were trans-maternally pre- and postnatally exposed to H. pylori extract, VacA or PBS through twice-weekly oral treatments of the dams during pregnancy and lactation. Gastric and ileal tissue was collected at necropsy and subjected to DNA extraction. The V4 region of the bacterial 16S rRNA gene was amplified and sequenced on the Illumina MiSeq platform. (A,C) Unweighted UniFrac distances in a principal coordinate analysis (PCA) plot, where samples were rarefied at 2000 reads for the stomach (A) and ileal (C) tissue. The Adonis test was used to compare community structures between all treatment groups at both sites. (B,D) OTUs that were significantly depleted or enriched as a consequence of the indicated treatments, for stomach (B) and ileum (D); LDA scores were generated using LefSE.
The systemic depletion of regulatory T-cells reduces the protective effects of H. pylori extract on house mite-induced allergic airway inflammation
Having observed that, in the steady state, perinatally extract-treated mice exhibit significantly higher frequencies of pulmonary Treg subsets with known or presumed suppressive activity than mock-treated controls, we next set out to deplete Tregs systemically during the challenge phase of our protocol of allergic airway inflammation. To this end, HDM-sensitized mice expressing GFP and the diphtheria toxin (DT) receptor under the control of the Foxp3 locus (Foxp3DTR)33 received a total of four doses of DT a few days prior to, and during, allergen challenge. The efficiency of Treg depletion, as judged by flow cytometric analysis of the residual GFP+CD4+ T-cell populations, was >80% in the lung and MLNs even when assessed three days after application of the last DT dose (suppl. Figure 5A,B). The beneficial effects of perinatal exposure to H. pylori extract on allergen-induced bronchoalveolar infiltration and eosinophilia, and on pulmonary inflammation and goblet cell metaplasia, were largely abolished by Treg depletion (Figure 5A–E), as were the effects on HDM-specific serum IgE titers and the pulmonary expression of the Th2 cytokine IL-13 (suppl. Figure 5C,D). In line with a previous report,34 the depletion of Tregs aggravated pulmonary inflammation and goblet cell metaplasia, but not airway eosinophilia also in mock-treated Foxp3DTR mice of the positive control group (Figure 5A–E). Furthermore, the same readouts showed a (albeit not significant) trend towards lower allergy even in trans-maternally H. pylori-exposed mice that were depleted of their Tregs, indicating that Treg-independent regulatory pathways may contribute to the reduction in allergy symptoms that is a hallmark of trans-maternally exposed animals. The combined results implicate Tregs in the suppression of excessive allergen-specific immune responses, in both trans-maternally exposed and mock-treated offspring.
Figure 5. The depletion of regulatory T-cells during HDM challenge abrogates allergy protection induced by perinatal trans-maternal exposure to H. pylori extract.
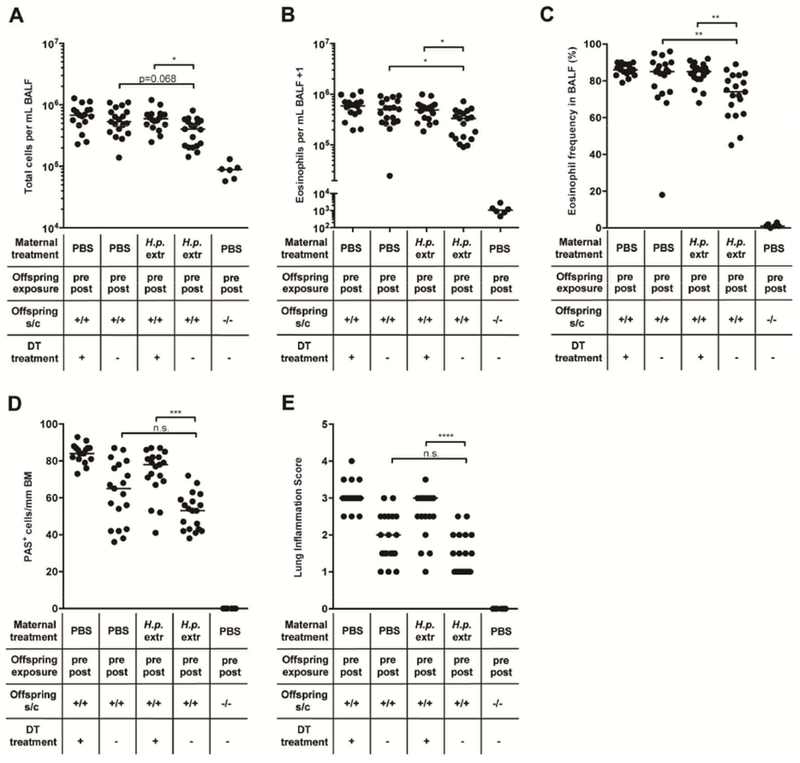
(A-E) Foxp3DTR mice were trans-maternally pre- and postnatally exposed to H. pylori extract (H.p. extr) or PBS. At six weeks of age, the offspring was sensitized and challenged intranasally with house dust mite (HDM) allergen. Where indicated, mice received a total of four doses (spread across eight days) of 1 μg DT just before and during HDM challenge. (A) Total leukocytes in 1 ml of BAL fluid (BALF). (B) Total eosinophils in 1 ml of BALF. (C) Eosinophil frequencies in BALF. (D,E) Pulmonary inflammation and goblet cell metaplasia, as assessed on stained lung sections. BM, basement membrane. In A-E, each symbol represents one mouse. The results were pooled from three independent experiments. Horizontal lines indicate medians; ANOVA with Dunn’s multiple comparisons correction was used for calculation of p-values. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001.
Perinatal extract and VacA treatment results in an enhanced demethylation of the TSDR in Foxp3+ Tregs and a specific transcriptional profile
Tregs contribute to allergy protection in mice that are perinatally tolerized with H. pylori extract or with VacA (Figure 5), and the frequencies of specific Treg subsets are elevated in the lungs as a consequence of the treatment (Figure 3). We therefore set out to assess whether the same interventions affect the epigenetic processes driving the differentiation and stability of Tregs. To this end, we quantified the methylation status of CpG motifs (Figure 6A) localized within an intronic enhancer region of the Foxp3 locus termed TSDR because of its selective demethylation in lineage-committed (stable) Tregs.35, 36 Tregs from pooled MLNs of five to ten male adult mice per group were FACS-sorted based on their CD4 and Foxp3 expression, and subjected to genomic DNA extraction, bisulfite conversion and TSDR-specific pyrosequencing. CD4+Foxp3− T-cells were sorted and analyzed in parallel. We used MLN Tregs since the cell numbers that were obtained from the lungs were far too low for methylation analyses. As expected, Foxp3− T-cells exhibited a demethylated TSDR, with ~95% methylated CpG motifs (Figure 6B); their methylation did not change upon intervention (data not shown). In contrast, Foxp3+ T-cells exhibited strong differences in their methylation status: whereas Foxp3+ T-cells that had been harvested from naive mice showed methylation levels varying between 25-40% depending on the experiment, this level was reduced to 5-15% due to perinatal intervention with extract or with VacA (Figure 6B). The ten analyzed CpG motifs of the TSDR showed very consistent methylation patterns within one sample, indicating an all-or-nothing mechanism of demethylation that encompasses the entire locus (note that not all regions could be analyzed in all samples, Figure 6B). Furthermore, a qRT-PCR-based analysis of the Treg-specific transcripts FoxP3, IL-10 and TGF-β conducted on CD4+Foxp3+ Tregs, sorted in parallel from lungs and MLNs, showed modestly higher TGF-β, but not Foxp3 and IL-10 expression due to the two interventions (suppl. Figure 6A,B). To obtain a more comprehensive picture of differential gene expression in pulmonary Tregs from extract vs. PBS-treated mice, we performed RNA sequencing on two extract- and three PBS-treated pools of 20.000 CD4+Foxp3+ Tregs each (combined from 3-4 mice per pool) (suppl. Figure 6C–E; GEO accession no. GSE116116). We found the samples to segregate based on treatment if the 2.000 most differentially expressed genes were used for unbiased clustering (suppl. Figure 6C); 248 genes were significantly differentially expressed in the two treatment groups with a fold change cutoff of log ratio >0.5 and p-value <0.01, with the majority of transcripts showing higher expression in extract-treated relative to control Tregs (suppl. Figure 6D). Among the differentially expressed genes were genes of the TGF-β signaling pathway (Tgfbi, Smad3), the IL-10 receptor a chain (Il10ra), several genes that had previously been linked to Tregs found in visceral adipose tissue (Ccrl, Cd36), and several genes known to be involved in Treg functionality and suppressive activity (Themis2, Runx3; suppl. Figure 6E). 37–39 We conclude from these data that epigenetic marks leading to stable expression of Foxp3 and to the definitive lineage commitment of Tregs, along with differential gene expression due to extract exposure, reflect and likely mediate the suppressive effects of H. pylori-specific perinatal interventions on allergic airway inflammation.
Figure 6. Perinatal trans-maternal exposure to H. pylori extract or VacA enriches for regulatory T-cells with a demethylated FOXP3 TSDR.
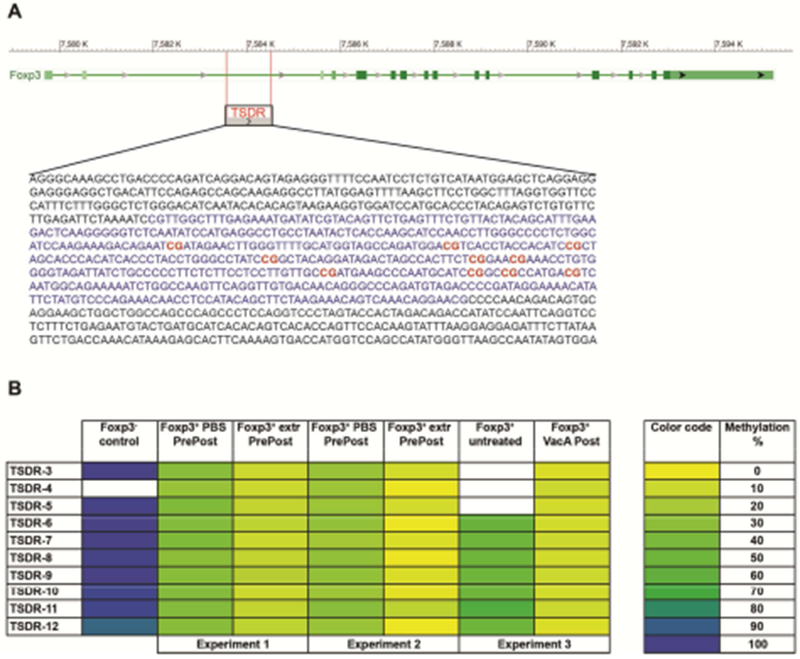
Mice were trans-maternally pre- and postnatally exposed to H. pylori extract or PBS, or postnatally only to VacA, through twice-weekly oral treatments of the dams during pregnancy and lactation (oral treatment with extract and PBS; intraperitoneal treatment with VacA). Foxp3+ Tregs and Foxp3− CD4+ T-cells were FACS-sorted from the mesenteric lymph nodes and subjected to DNA extraction. DNA was bisulfite converted and subjected to TSDR-specific pyrosequencing. (A) Schematic representation of the FOXP3 locus with the TSDR upstream of the TSS (retrieved using BLAST). The CG-rich region is marked in blue and CG motifs covered by pyrosequencing are labeled in red. (B) Color-coded (right panel) methylation pattern of ten CG dinucleotides within the TSDR region. White cells indicate sequences that failed to yield interpretable results due to technical problems. Data are from three independent experiments (labelled experiment 1 to 3, with experiments 1 and 2 testing the effects of extract and experiment 3 testing the effects of VacA); cells from five to ten male mice were pooled per treatment group prior to DNA extraction.
The decreased susceptibility to allergic airway inflammation by exposure to H. pylori extract is inter-generationally transmitted to the F2 generation
The enhanced demethylation of the TSDR in Foxp3+ Tregs from extract- and VacA-treated mice encouraged us to set up additional crosses, this time between perinatally extract-exposed (i.e. through oral gavage of their mothers) and naive offspring. Strikingly, we found that the severity of HDM-induced allergic airway inflammation, goblet cell metaplasia and eosinophilia was reduced (albeit not significantly in all readouts) even in the F2 generation born to perinatally extract-exposed offspring, which are grandchildren of extract-treated dams (Figure 7A–E). It did not matter in this context whether only the father, or only the mother, or both parents of HDM-sensitized and challenged offspring had been exposed in utero and during lactation to the H. pylori extract administered to their mother (Figure 7A–E). Also, both genders exhibited similar levels of protection, arguing that non-X-chromosome-encoded regions other than the TSDR might be involved in such inter-generational protective effects. Interestingly, immunophenotyping analyses of the pulmonary Treg compartment of F2 mice revealed similar differences as observed in the (protected) F1 generation (Figure 7F–I). Although overall Foxp3+ Treg frequencies did not differ significantly among the two treatment groups, extract-exposed mice exhibited higher frequencies of pulmonary neuropilin-negative pTregs and of CXCR3+ and RORγt+ Treg subsets (Figure 7F–I). In contrast, IRF4+ Tregs were underrepresented in the lungs of extract-exposed mice (data not shown).
Figure 7. H. pylori extract induces inter-generational protection against allergic airway inflammation and skews lung T-cell responses of the F2 generation.
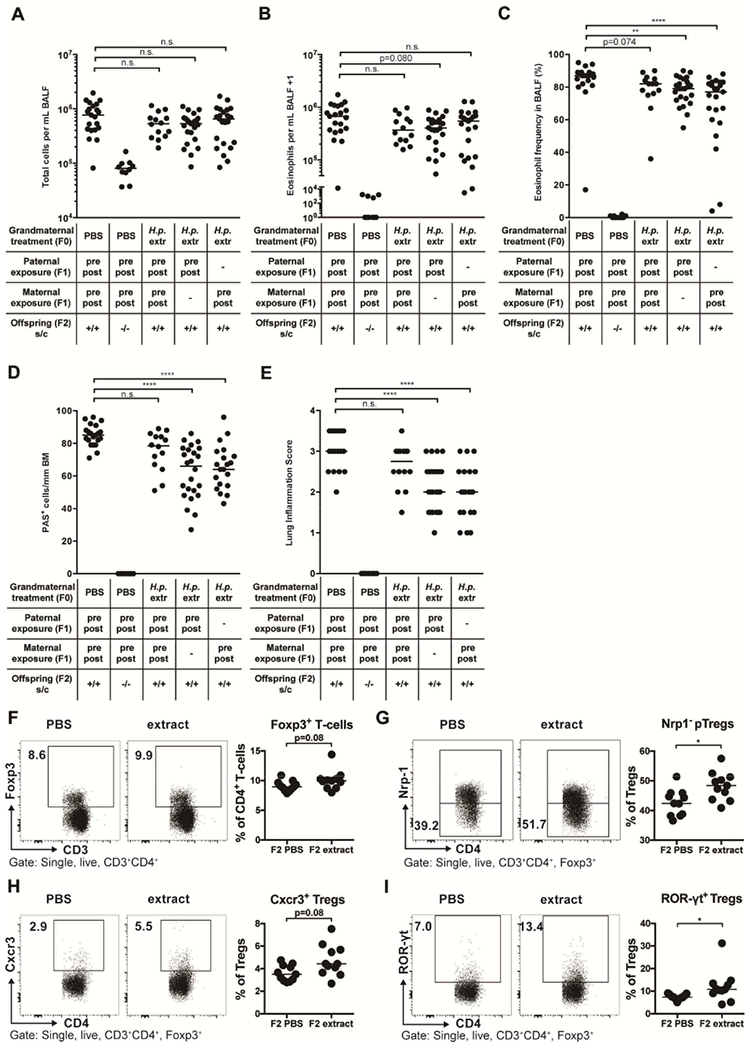
(A-I) F0 dams were subjected to twice-weekly oral gavage with H. pylori extract (H.p. extr) or PBS during pregnancy and lactation. Perinatally exposed F1 animals obtained in this manner were bred with each other or with naïve mates. At six weeks of age, F2 progeny were subjected to flow-cytometric analysis of the pulmonary T-cell compartment (F-I) or sensitized and challenged intranasally with house dust mite (HDM) allergen (A-E). (A) Total leukocytes in 1 ml of BAL fluid (BALF). (B) Total eosinophils in 1 ml of BALF. (C) Eosinophil frequencies in BALF. (D,E) Pulmonary inflammation and goblet cell metaplasia. (F) Foxp3+ Treg frequencies among all T cells. (G) Peripherally induced Treg (pTreg; Nrp-1−) frequencies among all Foxp3+ Tregs. (H,I) Frequencies of Cxcr3+ and RORγt+ Tregs among all CD4+FoxP3+ Tregs. In A-I each symbol represents one mouse. Results were pooled from three (A-E) or two (F-I) independent experiments. In A-E, ANOVA with Dunn’s multiple comparison correction was used for calculation of p-values. In F-I, an unpaired Mann-Whitney U test was used for calculation of p-values. * p<0.05, ** p<0.01, **** p<0.0001.
These experiments do not rule out that the F2 offspring benefited in terms of their reduced allergy severity because the gametes that form in the developing fetus in utero had been exposed to the tolerance-promoting components of H. pylori extract. Indeed, further interbreeding of F2 offspring with one another yielded a generation of mice (F3) that showed no evidence of protection whatsoever (suppl. Figure 7A–E), indicating that the protective effects of this type of environmental exposure is limited to directly exposed generations.
Discussion
In this study, we present the first experimental evidence for an intergenerational effect of the perinatal exposure to H. pylori-derived immunomodulatory molecules during pregnancy and lactation on several parameters of atopic sensitization and allergen-induced airway inflammation. We further report several immunological, meta-genomic and epigenetic correlates of perinatal H. pylori exposure that may at least partly account for the observed protective effects. The acquisition of H. pylori in early life has been implicated in epidemiological as well as experimental studies in having a clearly detectable influence on allergy risk and severity, especially in children and young adults and their murine counterparts in experimental models of allergic airway inflammation.12–19 Here, we have extended these findings and focused on possible exposures occurring even earlier in life, i.e. in utero and during lactation. To exclude the risk of vertical transmission of the infection from mother to offspring, a route that has been identified as the main route of H. pylori transmission in humans,40 we instead opted to treat pregnant or lactating dams with H. pylori extract or purified VacA, the main immunomodulatory molecule secreted by H. pylori that is known to skew T-cell responses towards Tregs and promote persistent colonization.18 Interestingly, we found that the direct trans-maternal exposure to H. pylori components -either in utero or during lactation- was required as discontinuation of the treatment of the (prospective) mother before mating failed to confer protection. This finding argues that immunomodulatory molecules produced by H. pylori, rather than tolerance-promoting factors produced by the mother, confer protection of the offspring.
The concept that direct perinatal exposure to specific intact microbes may be beneficial with respect to allergy risk and severity has been tested in experimental models with bacteria isolated from the farming environment, such as Acinetobacter Iwoffii, Lactobacillus lactis, Bacillus licheniformis, and Staphylococcus sciuri.3,41–43 Trans-maternal allergy-protective effects as shown here for H. pylori (with exposure occurring exclusively via the mother) were reported in experimental models with Acinetobacter lwoffii, and in those models were dependent on intact TLR signaling and IL-6, TNF-α, and IL-12 production in the mother and epigenetically regulated IFN-γ production in the offspring.44, 45 Observational studies in human subjects lend further support to the idea that prenatal microbial exposures (e.g. to farming environments) have extensive effects on innate and adaptive immune function that are regulated at least in part through epigenetic mechanisms.46, 47 Regulatory T-cells have been implicated in some of the benefits associated with prenatal exposure (via the mother) to farming environments; their numbers and functionality in cord blood were found to be significantly higher in offspring of farm-exposed relative to control mothers, which in turn was associated with lower Th2 cytokine secretion and lymphoproliferation upon innate stimulation.46 Conversely, reduced Foxp3 expression in fetus-derived placental tissues appears to predict allergic disease in infancy.47
Three pieces of evidence implicate Foxp3+ Tregs in the beneficial effects of perinatal trans-maternal exposure to H. pylori and its immunomodulator VacA. On the one hand, we observe in the steady state (i.e. prior to allergen sensitization and challenge) that the frequencies of specific Treg subsets -all associated with particularly strong suppressive activities-29–32 in the lung are higher than without such perinatal exposure. Secondly, we detected enhanced demethylation of the TSDR within the Foxp3 locus, which serves as an indicator of stable, irreversible differentiation towards the Treg lineage,35, 36 only in Tregs from H. pylori-exposed, not from naïve mice. Finally, the specific and selective depletion of Foxp3+ Tregs by DT administration during the challenge phase of the allergy protocol abrogates the benefits of trans-maternal exposure, indicating that the suppression of allergen-specific Th2 responses (and their signature cytokines, as determined in this study for IL-5 and IL-13) is driven by Tregs. The higher infiltration of H. pylori-exposed lungs with certain subsets of Tregs in the steady state correlates well with their lower T-effector cell frequencies; the preferential priming (or local expansion) of Tregs over effector T-cells may be attributable to the skewed ratio of DCs with tolerogenic vs. inflammatory activities. The CD103+ DCs that are over-represented in the lungs of H. pylori-exposed mice relative to CD11b+ DCs depend on the transcription factor basic leucine zipper ATF-like 3 (BATF3), and are known for their potency in driving CD8+ T cell immunity48–50 and for their role in priming Treg differentiation through the production of retinoic acid.51, 52 Although their exclusive, non-redundant role in promoting Treg-driven tolerance was recently questioned in an oral tolerization model,53 we have found their over-representation in the lung to be indicative of protection against airway inflammation in experimental models.22 Furthermore, the deficiency of CD103+ DCs in BATF3−/− mice abrogates the protective effects of live infection,22 and also of direct VacA treatment (unpublished data). More work will be required to elucidate which of the various differentially represented Treg subsets depend on CD103+ DCs for their priming or local expansion, and whether BATF3 proficiency is required for protection of trans-maternally exposed offspring to allergic airway inflammation.
We found three Treg subsets to be over-represented in the lungs of H. pylori-exposed animals despite similar overall Foxp3+ Treg frequencies. These were Nrp1− Tregs, i.e. cells that presumably arose in the periphery in a thymus-independent manner, as well as RORγt+ and CXCR3+ subsets of both peripherally induced Tregs and, to a lesser extent, thymus-dependent natural Tregs. Notably, in contrast to the GI tract,32 we found that RORγt and CXCR3 were not generally co-expressed in the pulmonary Treg compartment. Expression of the chemokine receptor CXCR3 is known to be driven by the transcription factor T-bet and can be used as a marker of cells that have at some stage of their ontogeny (transiently or stably) expressed T-bet.32 T-bet-dependent, CXCR3+ Tregs have recently been shown to develop in parallel to T-bet-positive Th1 cells during infection with Listeria monocytogenes,32 which similar to H. pylori is a strong Th1 inducer. Furthermore, the loss of T-bet+ Treg cells (by selective depletion of Foxp3 expression in T-bet+ Tregs) is sufficient to induce systemic autoimmunity, notably with strong T-effector cell infiltration into the lung.32 Our immunophenotyping data, albeit largely descriptive, are consistent with the novel concept that Tregs co-expressing Foxp3 and T-bet/CXCR3 have an essential immunosuppressive function and further suggest that not only autoimmunity, but also Th2-driven allergy, is controlled by these cells. In contrast, we found IRF4+ Tregs to be under-rather than over-represented in the lungs of H. pylori-exposed animals, largely as a consequence of the elevated frequencies of the other two (RORγt+ and CXCR3+) populations. As IRF4 expression in Tregs had previously been shown to endow these cells with the ability to selectively suppress Th2 responses,54 we had expected IRF4+ Tregs to increase in the setting of perinatal H. pylori exposure; however, this was not the case.
Interestingly, the observed shifts in immune cell populations that are a hallmark of mice trans-maternally exposed to H. pylori did not affect antiviral responses, which were normal in a model of pulmonary influenza infection as well as in a model of intestinal Citrobacter rodentium infection. We further speculated that the differences in CD4+ T-cell populations, in particular those affecting T-effector to Treg ratios, would have an influence on microbial communities in the upper airways as well as the GI tract. Indeed, we found both trans-maternal VacA and H. pylori extract exposure to measurably affect microbial communities in all examined sites of the GI tract. An unbiased analysis of community composition revealed largely distinct, non-overlapping changes due to treatment with extract and VacA, respectively; however, among the significant changes in relative abundance of individual taxonomic units that were detected, quite a few were found to occur with both VacA and extract treatment (e.g. depletion of Firmicutes and Bacteroidetes in the stomach and depletion of Akkermansia and Desulfovibrio species in the ileum). Whereas the described cecal transplantation experiments appear to rule out a causal role for the altered microbiota composition in preventing allergic airway inflammation, we found interesting that some of the shifts occurred in taxa that have previously been associated with healthy vs. diseased states, such as the segmented filamentous bacteria.
The most striking observation made in the course of our studies related to the intergenerational transmission of allergy protection. Such effects had previously been reported mostly for exposure to tobacco smoke, where not only maternal but also grand-maternal smoking is associated with increased risk for childhood asthma in humans.55 The results of the observational studies in humans have been corroborated in a rat model of nicotine-induced asthma, in which a trans-generational transmission to the F2 and F3 generations could be demonstrated following perinatal nicotine exposure of F0 dams.56
Collectively, our findings indicate that trans-maternal pre- and postnatal exposure to H. pylori and its main immunomodulator VacA has suppressive effects on the severity of allergic airway inflammation later in life, which likely is attributable to the suppressive activity of the pulmonary Tregs that are induced under such conditions. Our study suffers from three major limitations; on the one hand, we provide only descriptive, but not functional evidence of the suppressive role of pulmonary RORγt+ and CXCR3+ Tregs in airway inflammation. Secondly, we have not conducted methacholine assays due to the technical challenges associated with this procedure, and therefore cannot judge the effects on lung function of perinatal exposure to H. pylori. Finally, the negative data obtained upon cecal transplantation do not definitively rule out that shifts in the GI microbiota contribute to the effects of perinatal tolerization with H. pylori. Additional experiments to this end could involve co-housing or antibiotic pre-treatment prior to cecal transplantation in an effort to improve the colonization of transplanted communities.
Among the useful indicators of the reduced allergy risk associated with perinatal H. pylori exposure are shifts in the microbiota composition of various sites of the GI tract, as well as the epigenetic signature of the Foxp3 locus, which indicates qualitative or at least quantitative differences in the stability and functionality of Tregs due to this treatment. Importantly, perinatal exposure to H. pylori does not result in generalized immunosuppression and an elevated susceptibility to viral or bacterial infection; rather, acute infection with the lung pathogen influenza A virus or with the gastrointestinal pathogen C. rodentium readily breaks perinatally induced immune tolerance. We propose that the common human amphibiont H. pylori functions as an integral part of the early life “exposome” that skews the developing immune system towards immune tolerance.
Supplementary Material
Key messages.
Trans-maternal exposure to H. pylori reduces allergic airway inflammation in F1 and F2 offspring
Protection against allergy requires regulatory T-cells and is associated with robust shifts in the gastrointestinal microbiota, but not general immunosuppression
The analysis of lung T-cell and dendritic cell subsets reveals immunological correlates of allergy protection
Acknowledgments
Funding. This work was supported by the Swiss National Science Foundation Temporary Backup Schemes Consolidator Grant BSCGIO_157841/1 and the clinical research priority program on Human Hemato-Lymphatic Diseases, University of Zurich (both to A.M.). Additional funds were supplied by the National Institutes of Health (grants AI039657 and CA116087) and Dept. of Veterans Affairs BX000627 (all to T.L.C.), as well as the Swiss National Science Foundation (project grant 310030_162560 to C.M.). P.P. is supported by HFSPO (LT000438/2014) and Marie Curie Fellowships (PIEF-GA-2013-623055). We further acknowledge NIH support (U01AI22285, to MJB), training grant support (TL1TR001447, to TB) and the NYUMC Genome Technology Center for sequencing assistance (partially supported by P30CA016087), and support through the C&D fund.
Abbreviations
- BALF
bronchoalveolar lavage fluid
- HDM
house dust mite
- DT
diphtheria toxin
- IAV
influenza A virus
- MLNs
mesenteric lymph nodes
- OTU
operational taxonomic unit
- RORγt
RAR-related orphan receptor gamma t
- TSDR
Treg-specific demethylated region
- VacA
vacuolating cytotoxin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None of the authors declare a conflict of interest.
References
- 1.WHO asthma fact sheet http://www.who.int/mediacentre/factsheets/fs307/en/. 2017.].
- 2.Kyburz A, Muller A. The Gastrointestinal Tract Microbiota and Allergic Diseases. Dig Dis 2016; 34:230–43. [DOI] [PubMed] [Google Scholar]
- 3.Renz H, Holt PG, Inouye M, Logan AC, Prescott SL, Sly PD. An exposome perspective: Early-life events and immune development in a changing world. J Allergy Clin Immunol 2017; 140:24–40. [DOI] [PubMed] [Google Scholar]
- 4.Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med 2002; 347:869–77. [DOI] [PubMed] [Google Scholar]
- 5.Ege MJ, Bieli C, Frei R, van Strien RT, Riedler J, Ublagger E, et al. Prenatal farm exposure is related to the expression of receptors of the innate immunity and to atopic sensitization in school-age children. J Allergy Clin Immunol 2006; 117:817–23. [DOI] [PubMed] [Google Scholar]
- 6.Rautava S, Luoto R, Salminen S, Isolauri E. Microbial contact during pregnancy, intestinal colonization and human disease. Nat Rev Gastroenterol Hepatol 2012; 9:565–76. [DOI] [PubMed] [Google Scholar]
- 7.West CE. Gut microbiota and allergic disease: new findings. Curr Opin Clin Nutr Metab Care 2014; 17:261–6. [DOI] [PubMed] [Google Scholar]
- 8.West CE, Jenmalm MC, Prescott SL. The gut microbiota and its role in the development of allergic disease: a wider perspective. Clin Exp Allergy 2015; 45:43–53. [DOI] [PubMed] [Google Scholar]
- 9.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A 2010; 107:11971–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alcantara-Neves NM, de SGBG, Veiga RV, Figueiredo CA, Fiaccone RL, da Conceicao JS, et al. Effects of helminth co-infections on atopy, asthma and cytokine production in children living in a poor urban area in Latin America. BMC Res Notes 2014; 7:817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponte EV, Rasella D, Souza-Machado C, Stelmach R, Barreto ML, Cruz AA. Reduced asthma morbidity in endemic areas for helminth infections: a longitudinal ecological study in Brazil. J Asthma 2014; 51:1022–7. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med 2007; 167:821–7. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis 2008; 198:553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amberbir A, Medhin G, Abegaz WE, Hanlon C, Robinson K, Fogarty A, et al. Exposure to Helicobacter pylori infection in early childhood and the risk of allergic disease and atopic sensitization: a longitudinal birth cohort study. Clin Exp Allergy 2014; 44:563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amberbir A, Medhin G, Erku W, Alem A, Simms R, Robinson K, et al. Effects of Helicobacter pylori, geohelminth infection and selected commensal bacteria on the risk of allergic disease and sensitization in 3-year-old Ethiopian children. Clin Exp Allergy 2011. [DOI] [PubMed] [Google Scholar]
- 16.Arnold IC, Dehzad N, Reuter S, Martin H, Becher B, Taube C, et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest 2011; 121:3088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koch KN, Hartung ML, Urban S, Kyburz A, Bahlmann AS, Lind J, et al. Helicobacter urease-induced activation of the TLR2/NLRP3/IL-18 axis protects against asthma. J Clin Invest 2015; 125:3297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oertli M, Noben M, Engler DB, Semper RP, Reuter S, Maxeiner J, et al. Helicobacter pylori gamma-glutamyl transpeptidase and vacuolating cytotoxin promote gastric persistence and immune tolerance. Proc Natl Acad Sci U S A 2013; 110:3047–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oertli M, Sundquist M, Hitzler I, Engler DB, Arnold IC, Reuter S, et al. DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori-specific immune tolerance, and asthma protection. J Clin Invest 2012; 122:1082–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q, Yu C, Sun Y. The association between asthma and Helicobacter pylori: a meta-analysis. Helicobacter 2013; 18:41–53. [DOI] [PubMed] [Google Scholar]
- 21.Zhou X, Wu J, Zhang G. Association between Helicobacter pylori and asthma: a meta-analysis. Eur J Gastroenterol Hepatol 2013; 25:460–8. [DOI] [PubMed] [Google Scholar]
- 22.Engler DB, Reuter S, van Wijck Y, Urban S, Kyburz A, Maxeiner J, et al. Effective treatment of allergic airway inflammation with Helicobacter pylori immunomodulators requires BATF3-dependent dendritic cells and IL-10. Proc Natl Acad Sci U S A 2014; 111:11810–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kabisch R, Semper RP, Wustner S, Gerhard M, Mejias-Luque R. Helicobacter pylori gamma-Glutamyltranspeptidase Induces Tolerogenic Human Dendritic Cells by Activation of Glutamate Receptors. J Immunol 2016; 196:4246–52. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Rivera C, Campbell AM, Rutherford SA, Pyburn TM, Foegeding NJ, Barke TL, et al. A Nonoligomerizing Mutant Form of Helicobacter pylori VacA Allows Structural Analysis of the p33 Domain. Infect Immun 2016; 84:2662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang BH, Hagemann S, Mamareli P, Lauer U, Hoffmann U, Beckstette M, et al. Foxp3(+) T cells expressing ROR gamma t represent a stable regulatory T-cell effector lineage with enhanced suppressive capacity during intestinal inflammation. Mucosal Immunology 2016; 9:444–57. [DOI] [PubMed] [Google Scholar]
- 26.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 2012; 6:1621–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kenney AD, Dowdle JA, Bozzacco L, McMichael TM, St Gelais C, Panfil AR, et al. Human Genetic Determinants of Viral Diseases. Annu Rev Genet 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kyburz A, Urban S, Altobelli A, Floess S, Huehn J, Cover TL, et al. Helicobacter pylori and its secreted immunomodulator VacA protect against anaphylaxis in experimental models of food allergy. Clin Exp Allergy 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol 2009; 10:595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohnmacht C, Park JH, Cording S, Wing JB, Atarashi K, Obata Y, et al. MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science 2015; 349:989–93. [DOI] [PubMed] [Google Scholar]
- 31.Sefik E, Geva-Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM, et al. MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science 2015; 349:993–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levine AG, Medoza A, Hemmers S, Moltedo B, Niec RE, Schizas M, et al. Stability and function of regulatory T cells expressing the transcription factor T-bet. Nature 2017; 546:421–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol 2007; 8:191–7. [DOI] [PubMed] [Google Scholar]
- 34.Baru AM, Hartl A, Lahl K, Krishnaswamy JK, Fehrenbach H, Yildirim AO, et al. Selective depletion of Foxp3+ Treg during sensitization phase aggravates experimental allergic airway inflammation. Eur J Immunol 2010; 40:2259–66. [DOI] [PubMed] [Google Scholar]
- 35.Toker A, Engelbert D, Garg G, Polansky JK, Floess S, Miyao T, et al. Active demethylation of the Foxp3 locus leads to the generation of stable regulatory T cells within the thymus. J Immunol 2013; 190:3180–8. [DOI] [PubMed] [Google Scholar]
- 36.Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, et al. DNA methylation controls Foxp3 gene expression. Eur J Immunol 2008; 38:1654–63. [DOI] [PubMed] [Google Scholar]
- 37.Pedros C, Gaud G, Bernard I, Kassem S, Chabod M, Lagrange D, et al. An Epistatic Interaction between Themis1 and Vav1 Modulates Regulatory T Cell Function and Inflammatory Bowel Disease Development. J Immunol 2015; 195:1608–16. [DOI] [PubMed] [Google Scholar]
- 38.Sugai M, Aoki K, Osato M, Nambu Y, Ito K, Taketo MM, et al. Runx3 is required for full activation of regulatory T cells to prevent colitis-associated tumor formation. J Immunol 2011; 186:6515–20. [DOI] [PubMed] [Google Scholar]
- 39.Burzyn D, Benoist C, Mathis D. Regulatory T cells in nonlymphoid tissues. Nat Immunol 2013; 14:1007–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weyermann M, Rothenbacher D, Brenner H. Acquisition of Helicobacter pylori infection in early childhood: independent contributions of infected mothers, fathers, and siblings. Am J Gastroenterol 2009; 104:182–9. [DOI] [PubMed] [Google Scholar]
- 41.Debarry J, Garn H, Hanuszkiewicz A, Dickgreber N, Blumer N, von Mutius E, et al. Acinetobacter lwoffii and Lactococcus lactis strains isolated from farm cowsheds possess strong allergy-protective properties. J Allergy Clin Immunol 2007; 119:1514–21. [DOI] [PubMed] [Google Scholar]
- 42.Vogel K, Blumer N, Korthals M, Mittelstadt J, Garn H, Ege M, et al. Animal shed Bacillus licheniformis spores possess allergy-protective as well as inflammatory properties. J Allergy Clin Immunol 2008; 122:307–12, 12, e1–8. [DOI] [PubMed] [Google Scholar]
- 43.Hagner S, Harb H, Zhao M, Stein K, Holst O, Ege MJ, et al. Farm-derived Gram-positive bacterium Staphylococcus sciuri W620 prevents asthma phenotype in HDM- and OVA-exposed mice. Allergy 2013; 68:322–9. [DOI] [PubMed] [Google Scholar]
- 44.Brand S, Teich R, Dicke T, Harb H, Yildirim AO, Tost J, et al. Epigenetic regulation in murine offspring as a novel mechanism for transmaternal asthma protection induced by microbes. J Allergy Clin Immunol 2011; 128:618–25 e1–7. [DOI] [PubMed] [Google Scholar]
- 45.Conrad ML, Ferstl R, Teich R, Brand S, Blumer N, Yildirim AO, et al. Maternal TLR signaling is required for prenatal asthma protection by the nonpathogenic microbe Acinetobacter lwoffii F78. J Exp Med 2009; 206:2869–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaub B, Liu J, Hoppler S, Schleich I, Huehn J, Olek S, et al. Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J Allergy Clin Immunol 2009; 123:774–82 e5. [DOI] [PubMed] [Google Scholar]
- 47.Prescott SL, Tulic M, Kumah AO, Richman T, Crook M, Martino D, et al. Reduced placental FOXP3 associated with subsequent infant allergic disease. J Allergy Clin Immunol 2011; 128:886–7 e5. [DOI] [PubMed] [Google Scholar]
- 48.Edelson BT, Kc W, Juang R, Kohyama M, Benoit LA, Klekotka PA, et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med 2010; 207:823–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, et al. Origin of the lamina propria dendritic cell network. Immunity 2009; 31:513–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity 2009; 31:502–12. [DOI] [PubMed] [Google Scholar]
- 51.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 2007; 204:1757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joeris T, Muller-Luda K, Agace WW, Mowat AM. Diversity and functions of intestinal mononuclear phagocytes. Mucosal Immunol 2017. [DOI] [PubMed] [Google Scholar]
- 53.Veenbergen S, van Berkel LA, du Pre MF, He J, Karrich JJ, Costes LM, et al. Colonic tolerance develops in the iliac lymph nodes and can be established independent of CD103(+) dendritic cells. Mucosal Immunol 2016; 9:894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature 2009; 458:351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li YF, Langholz B, Salam MT, Gilliland FD. Maternal and grandmaternal smoking patterns are associated with early childhood asthma. Chest 2005; 127:1232–41. [DOI] [PubMed] [Google Scholar]
- 56.Rehan VK, Liu J, Sakurai R, Torday JS. Perinatal nicotine-induced transgenerational asthma. Am J Physiol Lung Cell Mol Physiol 2013; 305:L501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


