Abstract
Restless legs syndrome (RLS) is a common neurological disorder in the US. This disorder is characterized by an irresistible urge to move the legs, although the symptoms vary in a wide range. The pathobiology of RLS has been linked to iron (Fe) deficiency and dopaminergic (DAergic) dysfunction. Several genetic factors have been reported to increase the risk of RLS. Caenorhabditis elegans (C. elegans) is a well-established animal model with a fully sequenced genome, which is highly conserved with mammals. Given the detailed knowledge of its genomic architecture, ease of genetic manipulation and conserved biosynthetic and metabolic pathways, as well as its small size, ease of maintenance, speedy generation time and large brood size, C. elegans provides numerous advantages in studying RLS-associated gene-environment interactions. Here we will review current knowledge about RLS symptoms, pathology and treatments, and discuss the application of C. elegans in RLS study, including the worm homologous genes and methods that could be performed to advance the pathophysiology RLS.
Keywords: restless legs syndrome, RLS, C. elegans, iron deficiency, dopaminergic dysfunction
1. Introduction
Restless legs syndrome (RLS) is a neurological disorder affecting ~10% of people in the US, and ~2.5% of the population exhibit symptoms serious enough to seek medical attention (Allen, Bharmal, & Calloway, 2011). RLS is often neglected or misdiagnosed especially when the symptoms are intermittent or mild. The primary symptom of RLS is an irresistible urge to move one’s legs to relieve discomfort, with 4 common accompanying clinical features: (1) sensations starting at rest (e.g. creeping, crawling, burning, tingling, painful or indescribable); (2) symptoms worsen later during the day or at night; (3) partial or temporary relief with motor activity; and (4) leg twitching at night. ~80% of RLS patients have involuntary twitching leg movements either when asleep or awake that recurs on a semi-regular basis (Periodic Limb Movements, PLMs) (Allen et al., 2003). These symptoms often result in sleep deprivation, which can seriously impact life quality to a degree similar to osteoarthritis, hypertension or diabetes (Allen et al., 2005). Patients may ceaselessly walk at night with job tardiness, lost work productivity, unemployment, anxiety, depression and attention-deficit/hyperactivity disorder (ADHD) symptoms (Allen et al., 2011; Wagner, Walters, & Fisher, 2004). Finally, RLS may portend more serious consequences, including hypertension, heart disease and stroke (Y. Li et al., 2012; Pennestri, Montplaisir, Colombo, Lavigne, & Lanfranchi, 2007; Siddiqui et al., 2007; Walters & Rye, 2009).
RLS is associated with structural and biochemical changes in the brain. Iron (Fe) deficiency and altered dopaminergic (DAergic) system have been closely related to RLS. All studies to date support a role for diminished brain Fe in RLS, even when blood Fe stores appear to be normal (Connor et al., 2008). Fe is a cofactor of tyrosine hydroxylase (TH), the rate-limiting step in dopamine (DA) synthesis, and required for DA production. Fe deficiency causes DA receptor dysfunction (Beard, Erikson, & Jones, 2003). Autopsy studies have shown up-regulation of TH and decreased DA D2 receptors in the basal ganglia of RLS patients(Connor et al., 2009). Magnetic resonance imaging (MRI) studies have shown mild presynaptic and postsynaptic deficits in nigrostriatal DAergic pathways in RLS patients (Tan, 2006; Wetter, Eisensehr, & Trenkwalder, 2004). The DAergic agonists, pramipexole and ropinirole, have been approved to treat moderate to severe RLS. RLS exhibits both familial and non-familiar (idiopathic) forms, with ~60% of cases having a family history of the disease (Lazzarini et al., 1999; Montplaisir et al., 1997; Trenkwalder, Seidel, Gasser, & Oertel, 1996; Walters et al., 1996; Winkelmann, Stautner, Samtleben, & Trenkwalder, 2002). Independent genome-wide association studies (GWAS) reported single-nucleotide polymorphisms (SNPs) in 4 genes that impart increased risk of having RLS. These genes (and SNPs) are MEIS1 (rs2300478), BTBD9 (rs9357271), MAP2K5 (rs1026732), and PTPRD(Kemlink et al., 2009; Schormair et al., 2008; Stefansson et al., 2007; Winkelmann et al., 2007; Yang, Li, Chen, et al., 2011; Yang, Li, Yang, et al., 2011).
Caenorhabditis elegans (C. elegans) offers a rubust model for dissecting neuropathological mechanisms of RLS, given the simplicity of this model organism. First of all, C. elegans has ~20,000 genes, with up to 80% of corresponding human homologs, including many genetic risk factors of human diseases (Titus & Michael, 2006). Second, the small size, short generation time, large brood size and ease of maintenance, allow for a nearly limitless worm supply for cellular, molecular and genetic analysis. The adult nematode is only ~1 mm long and it takes 3 days from a fresh embryo to a reproductive adult, which can lay ~300 eggs when self-inseminated. The worm feed on bacterium-Escherichia coli (E. coli), and a large population (millions) of worms can be harvested on a 100 mm petri-dish within couple days. Third, the transparency of worms, when combined with fluorescent proteins, allows in vivo study of various cellular and intracellular activities, such as gene transcription, protein function, endocytosis, fat metabolism, axon guidance and neurodegeneration, etc. Fourth, many tools are available for various studies. The genome, cell lineage and 3D anatomy of C. elegans have been mapped out. A large pool of mutants, covering majority of the genome, can be directly ordered from C. elegans genetic center (CGC) or the national bioresource project (NBRP). Genome editing by CRISPR-Cas9 technology and conventional transgenic techniques can be readily performed (Mello, Kramer, Stinchcomb, & Ambros, 1991; Schwartz & Jorgensen, 2016). Over 90% of genes can be easily knocked down by bacteria feeding using the RNA interference (RNAi) library (Kamath et al., 2003). Fifth, the worm is sensitive to biometals and a wide-range of neuroactive drugs and xenobiotics, which faciliates the discovery of pharmaceutic compounds (Avery & Horvitz, 1990; Jayanthi et al., 1998; Lewis, 1980; Morgan, Sedensky, Meneely, & Cascorbi, 1988). Last but not the least, C. elegans has a simple and well-characterized nervous system. An adult hermaphrodite has a total of 302 neurons and ~ 8000 synapses, yet their neurotransmitters, Fe channels and synaptic components are highly conversed with human (Hobert, 2013). Regarding the DAergic system, C. elegans hermaphrodites possess 8 identifiable DAergic neurons (Sulston, Dew, & Brenner, 1975). All genes responsible for DA biosynthesis, packaging, reuptake and degradation have recognizable orthologues in the worm genome, including tyrosine hydroxylase (TH/cat-2), GTP cyclohydrolase I (GTPCH/cat-4), aromatic amino acid decarboxylase (AADC/bas-1), monoamine oxidase (MAO/amx-1,2,3), catechol-O-methyl transferase (COMT/comt-1,2,3,4,5), vesicular monoamine transporter (VMAT/cat-1) and DA transporter (DAT/dat-1) (Hobert, 2013; Jayanthi et al., 1998). These 8 neurons are more sensitive to environmental toxins than other neurons in worms (Benedetto, Au, Avila, Milatovic, & Aschner, 2010). Given these features, C. elegans has been widely used to decipher genetic and environmental aspects of neurological disorders, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), to name a few. In this review, we will address the symptoms, causes and treatment of RLS, and explore the potential techniques and methods to study the pathology of RLS in C. elegans.
2. RLS symptoms, potential causes and treatments
2.1. Symptoms
The main characteristics of RLS are symptoms of neurosensation. These include robust feelings of restiveness and distressful prickling-like sensations in the legs (Garcia-Borreguero & Cano-Pumarega, 2017). These are accompanied by an irresistible longing to move the body, particularly the legs, to relieve the unbearable sensations. Patients often describe these feelings of restiveness and distressful sensations as tingling, crawling tingling, creeping, pulling or painful within one or both lower limbs (Trenkwalder, Paulus, & Walters, 2005). RLS also affects other parts of the body as well. It is known that the longer the condition persists, the more likely the arms or the other regions of the body become symptomatic (Guo et al., 2017). These symptoms are said to typically become evident when patients are at rest, with a higher possibility of symptoms occurring during a relaxed or cosy state (Garcia-Borreguero & Cano-Pumarega, 2017; Guo et al., 2017; Trenkwalder et al., 2005). RLS symptoms commonly show a circadian rhythm, with symptoms being worse in the evenings, followed by short lessening after waking up in the morning (Garcia-Borreguero & Cano-Pumarega, 2017). Consequently, patient’s sleep is affected. The lack of proper sleep (insomnia) that accompanies RLS is the main reason behind a patient looking for medical consultation. Struggle to initiate sleep is the most common insomnia-related issues associated with RLS (Guo et al., 2017).
RLS symptoms differ substantially in regularity from barely once a month or year to daily. Also, symptoms show varying degree of severity from slightly disturbing to incapacitating, and symptoms may also attenuate for varying periods (Allen et al., 2014). Patient’s health-related quality of life (HRQoL) is affected depending on the severity of symptoms. In patients with mild RLS, the symptoms happen relatively less frequent, show reduced severity and only slightly influence sleep. Patients with severe RLS symptoms show obvious deficits in general health, vitality, physicality, emotional and social stability amongst other features (Trenkwalder et al., 2005). Mostly, these patients complain of having trouble in normal life schedules almost on daily basis, including poor performance on their jobs and social functions (Guo et al., 2017). Diagnosis of RLS is based on symptoms reported by patients. Current diagnostic criteria for RLS are highlighted in Table 1.
Table 1:
Diagnostic criteria for RLS
|
Essential diagnostic criteria (all must be met) ➢ An urge to move the legs usually but not always accompanied by or felt to be caused by uncomfortable and unpleasant sensations in the legs. ➢ The urge to move the legs and any accompanying unpleasant sensations begin or worsen during periods of rest or inactivity such as lying down or sitting. ➢ The urge to move the legs and any accompanying unpleasant sensations are partially or ➢ totally relieved by movement, such as walking or stretching, at least as long as the activity continues. ➢ The urge to move the legs and any accompanying unpleasant sensations during rest or inactivity only occur or are worse in the evening or night than during the day. ➢ The occurrence of the above features are not solely accounted for as symptoms primary to another medical or a behavioural condition (e.g., myalgia, venous stasis, leg oedema, arthritis, leg cramps, positional discomfort, habitual foot tapping). |
|
Specifiers for Clinical Course of RLS • Chronic-persistent RLS: symptoms left untreated would occur on average at least two times a week for the past year. • Intermittent RLS: symptoms left untreated treated would occur on average less than two time in a week for the past year, with at least 5 lifetime events. |
|
Specifier for Clinical Significance of RLS • RLS symptoms cause significant distress or impairment in social, occupational, educational or other important areas of functioning by the impact on sleep, energy/vitality, daily activities, behaviour, cognition or mood. |
2012 Revised International Restless Legs Syndrome Study Group (IRLSSG) Diagnostic Criteria for RLS. http://irlssg.org/diagnostic-criteria/
2.2. Causes
The pathology of RLS remains unclear. However, current research has identified several genetic risk factors, as well as anomalies in iron metabolism and DAergic neurotransmission. Additionally, other systems, including opiates, adenosine, orexin and glutamate may be involved in RLS pathogenesis.
RLS has been found to associate with both genetic and environmental risk factors. Several studies have reported robust association between onset of RLS and predisposing genetics. Genome-wide association studies (GWAS) have demonstrated several single nucleotide polymorphisms associated with RLS, including BTBD9 (on chromosome6p21.2), MEIS1 (on chromosome 2p14), MAP2K5/SCOR1 (on chromosome 15q23), PTPRD (on chromosome 9p24.1-p23), and chromosome 16q12.1 (Garcia-Borreguero & Cano-Pumarega, 2017; Guo et al., 2017). Deficiency in Fe has been repeatedly demonstrated to involved in RLS pathogenesis. Particularly Fe stores in the central nervous system (CNS) have been depleted in RLS patients, even when systemic Fe levels are normal. However, low serum Fe and/or ferritin levels have also been linked with several cases of RLS (Ward et la., 2014). The severity of RLS has been correlated with a reduction in brain Fe levels. MRI studies have shown that Fe levels decrease in the substantia nigra and putamen of RLS patients, particularly in severe cases (Moon, Chang, & Lee, 2014). These brain regions are the major sites of CNS dopaminergic neurotransmission, hence there is likely an association between low Fe in these regions and dopaminergic dysfunction in RLS. Fe is a cofactor for tyrosine hydroxylase (the rate-limiting enzyme in dopamine production), hence Fe deficiency may affect dopaminergic systems in the brain. However, this potential link largely remains unclear (Bogan & Cheray, 2013; Dauvilliers & Winkelmann, 2013)
The case for DAergic dysfunction in pathogenesis of RLS is mostly due to effects seen upon administration of drugs that modulates DAergic neurotransmission. DAergic medications have been reported to relieve RLS symptoms whereas antagonists of dopamine worsen the symptoms. Interestingly, the need for DAergic medications to be able to cross the blood brain barrier as a determinant for a positive effect on RLS demonstrates that DA actions in the central nervous system but not the peripheral nervous system is involved in RLS (Bogan & Cheray, 2013; Galbiati et al., 2015; Garcia-Borreguero et al., 2013; Garcia-Borreguero & Williams, 2014). The number of DA transporters may be decreased in RLS, as shown by a PET study (Earley et al., 2011). Additionally, lesion of the area where DAergic pathway projects to spinal cord (area A11) led to overactivity in a rodent model, although this could not be corroborated in autopsy study of RLS patients (Garcia-Borreguero & Cano-Pumarega, 2017; Guo et al., 2017).
2.2. Treatments
Treatment for RLS can be grouped into non-pharmacological and pharmacological approaches. Use of pharmacological substance should be started when patient’s quality of life, social functioning, daytime activities and sleep becomes impaired as a result of RLS symptoms. Non-pharmacological options should be used before drug options are considered. Non-pharmacological options may be adequate in cases of mild or rare occurrence of RLS symptoms, though there is dearth of evidence regarding their efficacy and practicability in management of RLS (Symvoulakis, Anyfantakis, & Lionis, 2010; Trenkwalder et al., 2005)
Non-pharmacological options are commonly targeted towards improving patient’s sleep quality primarily via activities that attain healthier sleeping habits (Guo et al., 2017; Symvoulakis et al., 2010). These usually require modifying patients’ overall lifestyle. Patients are encouraged to develop structured sleep routines, with consistent times for beginning sleep and waking up. Activities that could hinder particularly preceding sleeping times should be avoided, and sleeping environments should be made relaxed and quiet to encourage ease of sleep (Symvoulakis et al., 2010). Other lifestyle modifications may include reducing or avoiding intake of alcohol, nicotine or caffeine as these are known to intensify RLS symptoms. Additionally, medications that worsen RLS symptoms, such as antidepressants, antihistamines, DA antagonists, should be discontinued. Furthermore moderate levels of exercise daily, massages and use of hot baths may also result in respite from RLS symptoms (NIH, 2017; Symvoulakis et al., 2010).
Oral Fe supplementations and in some cases, intravenous Fe administration can be used, particularly in patients with low serum ferritin (NIH, 2017; Trenkwalder, Winkelmann, Inoue, & Paulus, 2015). However, DAergic agents are considered the first line of pharmacological treatments for RLS (Garcia-Borreguero & Cano-Pumarega, 2017). These drugs which have dopamine increasing effects have been demonstrated to attenuate RLS symptoms, particularly when taken at bedtime. Pramipexole, ropinirole and rotigotine are FDA approved medications for the treatment of moderate to severe RLS. A combination of levodopa and carbidopa have been shown to be effective following intermittent use (Garcia-Borreguero & Cano-Pumarega, 2017). These agents, while well tolerated, have their side effects. Common side effects include gastrointestinal complaints, nausea, daytime sleepiness and other short-time side effects. Additionally, long-term use of these agents may result in exacerbation of RLS symptoms(Garcia-Borreguero & Cano-Pumarega, 2017; NIH, 2017). Furthermore, some patients on these DAergic agonists may develop compulsive behaviours such as pathological gambling, eating, shopping or hypersexuality (Garcia-Borreguero & Cano-Pumarega, 2017; NIH, 2017; Trenkwalder et al., 2015). Besides, antiseizure agents are becoming a better choice of treatment for RLS. Gabapentin enacarbil has been approved by FDA for treatment of moderate to severe RLS. This drug is as effective as the DAergic agonists and with no reports of exacerbation of symptoms (Garcia-Borreguero & Cano-Pumarega, 2017; NIH, 2017).
Another class of medications used for RLS treatment includes opioids and benzodiazepines. Opioids such as oxycodone, are used in individuals with more severe RLS symptoms which do not get improved following other medications. There are several side effects associated with these agents as well as the risk of addiction. Benzodiazepines can be useful to obtain a more relaxing sleep; however, they may cause or worsen sleep apnea. These agents are mostly used as last line of therapy, particularly during severe augmentation (Garcia-Borreguero & Cano-Pumarega, 2017; NIH, 2017; Trenkwalder et al., 2005, 2015).
3. RLS genetics
RLS exhibits both familial and non-familiar (idiopathic) forms, with ~60% of cases having a family history of the disease (Lazzarini et al., 1999; Montplaisir et al., 1997; Trenkwalder et al., 1996; Walters et al., 1996; Winkelmann et al., 2002). In a familial study in a French Canadian population, siblings of an RLS patient had 3.6 times the risk of themselves having RLS, and an offspring, 1.8 times (Xiong et al., 2010). For early-onset RLS patients, Allen et al also reported a greater risk for RLS among the patients’ first-degree relatives compared to the first-degree relatives of late-onset RLS patients (23.6% v. 10.1%). The same study found that first-degree and second-degree relatives of RLS patients generally had higher risk of developing RLS than did the relatives of non-RLS suffering individuals (Allen, La Buda, Becker, & Earley, 2002). Twin studies also has revealed the genetic nature of RLS. Ondo et al showed a concordance rate of 83.3% between monozygotic twins for RLS, despite varying symptom descriptions and ages of onset. They also showed evidence for genetic anticipation -- patients’ having earlier onset of symptoms if they have family history of RLS (Ondo, Vuong, & Wang, 2000). Higher concordance rate was observed in monozygotic twins than dizygotic twins (Xiong et al., 2007). Female sex has been linked to increased risk of RLS as well. Even among both monozygotic and dizygotic twins, female predominance of RLS patients has been shown (Xiong et al., 2007). In fact, in a Turkish family, all affected individuals were women (Akpinar, Turker, Aygun, & Aytac, 2017).
Genome-wide association studies (GWAS) have reported single-nucleotide polymorphisms (SNPs) in several genes that impart increased risk of having RLS (Table 2). In German and French-Canadian RLS cases, three loci were identified: MEIS1, a homeobox gene, BTBD9 containing a BTB domain, and MAP2K5, a mitogen-activated protein kinase (Winkelmann et al., 2007). TOX3, which contains a high mobility group (HMG) box, and PTPRD, encoding for protein tyrosine phosphatase receptor type D, have also been shown to be associated with RLS (Moore et al., 2014). MEIS1, BTBD9, MAP2K5, and TOX3 polymorphisms and their association with RLS have been shown in other ethnic populations around the world as well (G. Li et al., 2017; Vilarino-Guell, Farrer, & Lin, 2008); (Haba-Rubio et al., 2016); (Yang, Li, Chen, et al., 2011). Stefansson et al showed that BTBD9 is associated with Fe depletion, which is consistent with currently accepted pathogenesis of RLS (Stefansson et al., 2007). MEIS1 has been shown to be associated with defective Fe homeostasis as well (Vilarino-Guell et al., 2009).
Table 2.
Genetic risk factors of RLS.
| RLS genetic risk factors | Proposed functions in the nervous system | Predicted C. elegans orthologs* |
|---|---|---|
| MEIS1 | Neurogenesis | UNC-62 |
| BTBD9 | Long-term memory potentiation, Fe homeostasis | HPO-9 |
| PTPRD | Axonal guidance/myelination, neural development | PTP-3 |
| MAP2K5 | Neurogenesis | MEK-2 |
| TOX3 | Neural protection in CNS injury, neurogenesis | HMG-6 |
, the orthologs are predicted based on their amino acid sequence similarity, but have not been individually verified.
Genes associated with common risk factors and treatment of RLS have been studied in association with RLS. Garcia-Martin et al showed that allelic variants of heme oxygenases-1 and 2, enzymes which degrade the heme protein and commonly associated with Parkinson’s disease, were lower in patients with RLS (Garcia-Martin et al., 2015). Given improvement on RLS symptoms with GABAergic medications, Jimenez-Jimenez et al showed that the frequencies of GABA receptor gene rho 3 allelic variant rs832032T was significantly higher in RLS patients than in controls (Jimenez-Jimenez et al., 2018).
4. C. elegans homologs of RLS and their function
C. elegans is a free-living nematode that was established as a model organism for the study of human disease pathophysiology by Sydney Brenner in middle of 20th century (Corsi, Wightman, & Chalfie, 2015). Since then, C. elegans has been studied extensively to shed light on processes such as establishing genetic bases of programmed cell death and stress-induced sleep (Ellis & Horvitz, 1986; Nelson et al., 2014). C. elegans has many advantages as a genetic tool, including inexpensive cost of maintenance, rapid lifecycle, and fully sequenced genome (Corsi et al., 2015).
Previous studies have revealed the function of several RLS genes in C. elegans and other animal models. Myeloid ectopic viral integration site 1, or MEIS1, is a homeoprotein belonging to a subfamily of three amino acid loop extension (TALE) homeoproteins, which are important in embryonic survival and development, including hematopoiesis and angiogenesis (Barber et al., 2013), (Azcoitia, Aracil, Martinez, & Torres, 2005). As a transcription factor, MEIS1 has also been implicated in the pathogenesis of malignancies (J. Zhu et al., 2017). In the nervous system, MEIS1 was shown to be critical in neurogenesis, and its inactivation leads to sudden cardiac deaths in mice secondary to altered sympathetic innervation of the heart (Barber & Rastegar, 2010; Bouilloux et al., 2016). The importance of MEIS1 in C. elegans has also been observed. Arata et al. confirmed that a Meis-related gene, psa-3, is essential in determining the fate of hypodermal cells, and Van Auken et al showed mutation in unc-62, an ortholog of MEIS1, causes early embryonic arrest or death (Arata et al., 2006; Van Auken et al., 2002). In C. elegans, UNC-62::GFP is expressed in a variety of tissues in the hermaphrodite, including the hypodermis, the intestine and the nervous system (Fig. 1) (Van Nostrand, Sánchez-Blanco, Wu, Nguyen, & Kim, 2013). Interestingly, UNC-62 is expressed in DAergic neurons (Fig 1), which facilitates study of its function in DAergic system. Using RNA interference, Catoire et al. showed that decreased expression of UNC-62 was associated with increased the expression of ferritin in C. elegans, supporting the derangement of Fe homeostasis in the pathophysiology of RLS (Catoire et al., 2011).
Figure 1.
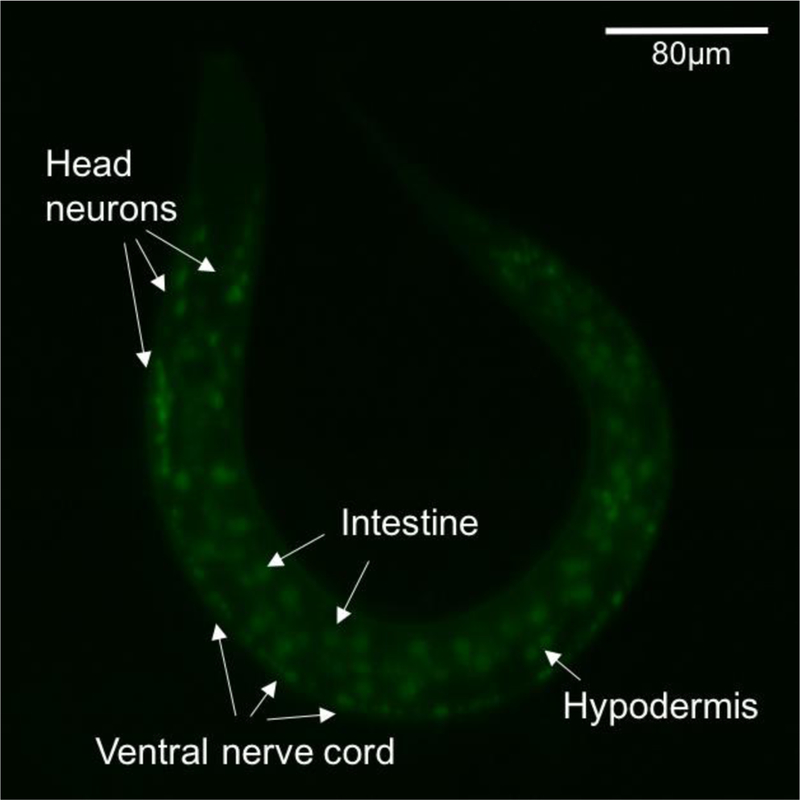
The expression pattern of UNC-62 in C. elegans. Strain SD1897 carries a translational GFP fusion UNC-62::GFP. At Larva 1 stage, GFP is seen in the intestine, hypodermis, head neurons and the ventral nerve cord. Image was taken using Zeiss LSM5 Live Duo Scan.
BTB domain containing 9, or BTBD9, gene encodes for a protein with two domains: at the N-terminus is a bric-a-brac, tramtrack (BTB), broad complex/pox virus and zinc finger domain, which has been associated with regulation of transcription, and at the C-terminus is a BTB and Kelch, associated with ubiquination (DeAndrade, Zhang, et al., 2012). The overall function of BTBD9, however, is currently not established (Y. Li, Doroodchi, Yang, & DeAndrade, 2011). In mice, BTBD9 deficiency has been shown to impair pre-synaptic activity and enhance long-term memory potentiation (DeAndrade, Zhang, et al., 2012). Btbd9 knock-out mice also has been established as a murine model for RLS, given their motor restlessness, resting-phase sensory alterations, altered Fe homeostasis, and symptomatic resolution upon administration of ropinirole, a dopaminergic agonist commonly used for the treatment of RLS (DeAndrade, Johnson, et al., 2012). HPO-9, the C. elegans ortholog of BTBD9, has been shown to be involved in embryonic and larval development and response to bacterial infection (Kao et al., 2011; McKay et al., 2003; Simmer et al., 2003). However, its role in the nervous system remains to be investigated. C. elegans model of RLS based on hpo-9 knockout showed alterations in egg-laying behavior and locomotion, but the nematode model has not been fully characterized (Y. Li et al., 2011). In addition, KEL-8, a BTB-Kelch containing protein, has been shown to regulate synaptic transmission via its association with GLR-1, a glutamate receptor. Specifically, mutants for kel-8 have increased GLR-1 levels in neurons and a higher frequency of spontaneous reversals of body bends (Schaefer & Rongo, 2006).
Protein tyrosine phosphatase, receptor type D (PTPRD), a member of the leukocyte common antigen-related subfamily of receptor protein tyrosine phosphatases, has been implicated in multiple disease processes, including non-alcoholic fatty liver disease, laryngeal squamous cell carcinoma, diabetes mellitus, and hypertension (Y. Li et al., 2015); (Nakajima et al., 2018); (Szaumkessel et al., 2017); (T. Chen et al., 2016); (Gong et al., 2015). In the CNS, PTPRD has been shown to play a role in axonal guidance and neuronal development. In mice, Shishikura et al. showed through immunohistochemistry that PTPRD is localized to olfactory bulb, cerebral cortex, hippocampus, cerebellum, and several brainstem nuclei. At the cellular level, PTPRD was shown to be expressed not only in the axons, but also in dendrites (Shishikura et al., 2016). Zhu et al demonstrated time and spatial variations in the expression of PTPRD in the embryonic and post-natal CNS of mice, and that PTPRD mutants had a defective axonal myelination process (Q. Zhu et al., 2015). In C. elegans, PTP-3 is the only known ortholog of PTPRD, and it also has been implicated in similar processes in the nervous system (Ackley et al., 2005). Nakamura et al showed that PTP-3 in C. elegans plays a role in cortical dendritic growth and axonal guidance (Nakamura et al., 2017). Ackley et al demonstrated that two isomers of PTP-3, PTP-3A and PTP-3B, may play a different role. PTP-3A was shown to localize specifically at neuronal synapses, while, PTP-3B, extrasynaptically. Furthermore, PTP-3B rescued axon guidance defect caused by ptp-3 mutation, whereas PTP-3A could not. Still, mutation in PTP-3A caused altered synaptic morphology (Ackley et al., 2005).
Mitogen-activated protein kinase kinase 5, or MAP2K5, belongs to the MAP kinase kinase family, and thus it plays a critical role in cellular signal transduction pathways (Zhou, Bao, & Dixon, 1995). In humans, MAP2K5 activity is associated with many disease states, including colorectal cancer, obesity, RLS, and prostate cancer (Diao et al., 2016; G. Li et al., 2017; Mehta et al., 2003; Rask-Andersen et al., 2012). In mice, Map2k5 has been implicated in cellular differentiation, apoptosis, sarcomere hypertrophy, among other processes (Kaneshiro, Otsuki, Yoshida, Yoshikawa, & Higuchi, 2015; Nicol et al., 2001; Sohn, Lewis, & Winoto, 2008). Knock-out of Map2k5 has been shown to enhance adult neurogenesis in the olfactory bulb on mice as well (Wang et al., 2015). The closest C. elegans ortholog of MAP2K5 is MEK-2, a MAP kinase kinase that has been shown to be associated in viability of larvae, development of the vulva, egg-laying, and immune system (Hsu, Zobel, Lambie, Schedl, & Kornfeld, 2002; Nicholas & Hodgkin, 2004; Wu, Han, & Guan, 1995). MEK-2’s role in the nervous system of the nematode has not been well-studied, however.
Along with its association with RLS, TOX high mobility group box family member 3, or TOX3, has been associated with breast cancer, Parkinson’s disease, and polycystic ovarian syndrome in humans (L. Li et al., 2018; Mohtashami et al., 2018; Ning, Jiayi, Jian, & Wanli, 2017). In the CNS of mice, Tox3 has been shown to be expressed mainly in the developing embryonic brain in the neural stem cells (Sahu et al., 2016). There is evidence that shows increased expression of Tox3 leads to neural protection and facilitates recovery in spinal cord injury in mice via protection of oligodendrocytes (Bastien et al., 2015). The closest predicted ortholog of TOX3 in C. elegans is HMG-6, which has been shown to be expressed in neurons and sperm (Blazie et al., 2017; Lockhead et al., 2016; Spencer et al., 2011). However, there has not been any evidence establishing its function in the nematode so far.
5. C. elegans methods for RLS study
Given the above features, C. elegans provides a robust model in delineating the pathogenesis, and progression of RLS, as well as testing therapeutic measures particularly aspects requiring genetic modifications. Below we present a few areas where this model organism is suitable to research into RLS.
5.1. Circadian rhythms
It is well established that RLS symptoms demonstrate a circadian pattern, with several symptoms worsening at bedtime. Understanding how circadian rhythms influence RLS symptoms is thus an essential to unravelling the disorder. Organisms demonstrate circadian rhythmicity in physiology and behaviour that is timed to an approximately 24-hour period, and this rhythm appears to be strongly conserved. Circadian rhythm in organisms appears to be driven by clock-controlled feedback loop of transcriptional and translational processes and/or through post-transcription or post-translation alterations. In this feedback loop, transcription factors drive expression of clock genes such as tim (timeless) and per (period) that then induces negative regulation of their own transcription, thus generating an oscillatory rhythms of gene expression within the approximately 24-hour period. These biological rhythms are synchronized by two major external cues (zeitgebers); light and temperature (Goya et al., 2010).
Several studies have examined circadian patterns in C. elegans. Though a true circadian rhythm is yet to be fully elucidated in this model, circadian rhythms have been demonstrated in several behavioural and physiological functions. These include defecation, swimming behaviours and general locomotor activity, as well as response to osmotic stress. Additionally, circadian patterns have been demonstrated in several other metabolic activities such as pharyngeal contractions, food and oxygen consumption (Migliori et al., 2011). Gene expression profiling studies afford a powerful tool to characterize clock genes under circadian transcriptional regulation as such profiling is not influenced by known assumptions of specific physiological and behavioural outcomes. A study using genome-wide expression profiling identified light and temperature controlled transcriptional rhythms in C. elegans, and demonstrated that subsets of such transcripts are controlled in a circadian pattern. The study also showed that expression of many genes in this organism is globally driven by these two zeitgebers, thus suggesting that C. elegans show systemic responses to light and temperature (Sengupta et al., 2010). A recent study applied an in vivo bioluminescence assay to demonstrate major features of the C. elegans circadian system. The study demonstrated that C. elegans expresses approximately 24-hour rhythms that are entrained by temperature and light/dark cycles in both single-nematode and population-based assays. The study also demonstrated that light and temperature detection involve the use of photoreceptors GUR-3 and LITE, as well as TAX-2 cyclic nucleotide-gated channel subunit. Importantly, this bioluminescence assay is extremely sensitive, non-invasive and has immediate resolution (Goya et al., 2016). Therefore, C. elegans can be optimized to elucidate the molecular mechanisms behind the circadian patterns of RLS symptoms using those characterized behavioural and physiological activities controlled by circadian rhythms in C. elegans. Genetic modifications of RLS genes in C. elegans can be elucidated using simple behavioural assessment of several activities that show circadian rhythmic patterns (Migliori et al., 2011) in C. elegans. Such behavioural assessment can include:
Pharyngeal pumping actions:
These actions are controlled by several mechanisms in C. elegans. The pharynx contracts rhythmically to aid feeding in worms. Rate of this activity commonly referred to as pharyngeal pumping can be measured manually counting pumps per minute under a dissecting microscope. A study reported that pharyngeal pumps in C. elegans show daily variations that is influenced by light/dark cycles (Migliori et al., 2011).
General locomotor activities:
Rate of movement (locomotion) in C. elegans can be assessed by quantifying (counting) body bends over a specified period of time. A body bend is described as change in the direction of the pharynx’s posterior bulb. Simply, it is counted every time the part of the worm just behind the pharynx reaches a maximum bend in the opposite direction from the bend last counted. Such assays can either be carried out on nematode growth media (NGM) or in water. Body bends can be assessed manually under dissecting microscope or with use of several available programs such as Worm Tracker, OptoTracker, Nemo, etc., on videotaped sessions (Chen et al., 2013; Hart, 2006).
5.2. DAergic function
DAergic dysfunction is implicated in the pathogenesis of RLS, however, specific molecular mechanisms are still unclear (Garcia-Borreguero & Cano-Pumarega, 2017; Garcia-Borreguero & Williams, 2014). C. elegans has a very simple nervous system. The DAergic system in C. elegans is particularly well studied. The C. elegans model can be harnessed to elucidate the role of DAergic neurotransmission in development of RLS symptoms. Structural and functional integrity of the DAergic system in C. elegans can be assessed using several tools described below.
5.2.1. Assessing DA neuronal morphology
Worms are transparent, thus allowing for easy in vivo imaging and visualisation of fluorescently tagged neurons. Interestingly, the C. elegans hermaphrodites have only 8 dopaminergic neurons, all of which can be visualized in vivo. Soma and processes of neurons can be visualized via expression of fluorescent proteins. Morphological changes including blebs, puncta, neuronal shrinkage, breaks or complete loss of neuronal processes, as well as vacuolation have been characterized as a phenotypic readout of neurodegenerative processes (Chen et al., 2013), which have been confirmed by transmission electronic microscopy (TEM) previously (Nass, Hall, Miller, & Blakely, 2002). Several worm strains are available for visualization of DA-producing neurons in C. elegans. These include but not limited to BY200 (dat-1:GFP (vtIs1) V.) and OH7193 (otIs181 [dat-1::mCherry + ttx-3::mCherry]) III) which expresses green fluorescent protein (GFP) and mCherry respectively in their dopaminergic neurons (Caito & Aschner, 2016; Ijomone et al., 2016). Homologues of RLS genes can be modified in these worms to understand the effect of RLS genes in dopaminergic morphology.
5.2.2. Assessing DAergic functions
Several behavioural paradigms are available to test DAergic functions using the C. elegans model. These can be used to unravel dopamine functions in C. elegans mutation of RLS homologue genes. These include;
Basal slowing response:
This is assay assessed bacterial mechanosensation as measure of DAergic functions (Sawin et al., 2000). DAergic neurotransmission causes worms to slow locomotor rate in the presence of food. C. elegans feed on bacterial. This assay is performed by counting number of body bends (to quantify locomotor rate) in a 20 s interval in the presence or absence of food. Worms with normal or intact DA systems down locomotion when they encounter food as opposed to worms with defective DA systems which do not slow down. Worms with deficient tyrosine hydroxylase homologue, cat-2, are used as positive control as they show defective bacterial mechanosensation (Caito & Aschner, 2016; Ijomone et al., 2016).
Dauer spontaneous movement:
In the absence of food and enabling environment, C. elegans morph into a developmental stage called dauer, after the larva-1 stage. Dauer worms are in a period of hibernation marked by almost complete inactivity. During this assay, dauers are considered to be moving if they make more than one body bend backwards or forwards within one minute. Increased dauer movement is reported to be a response to decreased DA levels (Chen et al., 2015).
Area restricted searching (ARS):
Wildtype C. elegans will expand search for food to neighbouring areas when food source in immediate vicinity is depleted. This behaviour is scored by reduction in regularity of high-angled turns when food in immediate vicinity is depleted and worms begin to explore farther areas. This behaviour which is referred to as area restricted searching (ARS) is DAergic dependent (Chen et al., 2013).
Habituation task/tap withdrawer response:
C. elegans growing on a plate respond to tapping of the plate by increasing forward or backwords movement. Continuous tapping result in habituation and decrease in number of reversals. All worms respond to this stimulus by reducing rate of reversals, however, the lag time in response is DA-dependent, as worms with defective DA systems habituate faster (Chen et al., 2013).
5.3. Measurement of neurotransmitters
Levels of neurotransmitters such as DA can be determined in worm extract using high-performance liquid chromatography (HPLC). This requires use of many worms (about several hundred thousands of Larva 1 stage worms), and they must be properly washed off bacteria (Chen et al., 2013).
5.4. Metal toxicity
Fe deficiency is implicated in RLS pathogenesis (Bogan & Cheray, 2013). Studies in several models including mouse, fly and worm, have demonstrated a functional interaction between homologues of RLS gene BTBD9 and Fe biology and DAergic activity (Catoire et al., 2011; Deandrade et al., 2012; Freeman et al., 2012). An association between RLS and PD has been proposed. MRI studies demonstrated slight presynaptic and postsynaptic deficits in nigrostriatal dopaminergic pathways in RLS patients without PD. Increased prevalence of PD is noted in RLS, and vice versa (Gao et al., 2010; Peeraully & Tan, 2012).
Metals, particularly manganese (Mn) have been indicated as risk factors for PD (Chen et al., 2015). Mn and Fe have similar characteristics, and share several homeostatic and transport pathways. Furthermore, Fe depletion, a key feature of RLS is associated with higher deposition of Mn in the brain. There is an established C. elegans model of Mn neurotoxicity (Chen et al., 2015; Ijomone et al., 2016; Peres et al., 2018; Ruszkiewicz et al., 2018). Given the association between Mn and Fe, this model can be harnessed to evaluate whether neuronal and/or systemic alteration in Mn may contribute to the pathophysiology of RLS. Potential modulation of neurotoxic impact of metals due to modifications of RLS genes in C. elegans can be assessed using several techniques including:
Survival assay:
This assay is used to determine lethal dose of a substance on C. elegans and how much toxicity is imparted by that substance. Specifically, survival assay could determine if modifications of RLS genes improves or worsens toxicity following exposures to heavy metals such as Mn. In performing this assay, a specific number of worms treated with Mn for example, are transferred onto NGM with food. The number of worms alive after 24 or 48 hours are counted and scored as percentage of initially transferred worms. Dose response curves are generated to determine if any genetic modifications influences survival of worms (Caito & Aschner, 2016; Ijomone et al., 2016)
Lifespan assay:
This assay particularly takes advantage of the short life span of the C. elegans (about 2–3 weeks). During this assay, a specified number of worms are grown to L4 stage (seen as day 0) and transferred to NGM plates. Number of surviving worms are scored every 24 or 48 hours till all worms are dead. To prevent confusion with progeny, the initial worms are transferred every two days or NGM plates are constituted with fluorodeoxyuridine (FUDR) to prevent eggs from hatching (Chen et al., 2015; Park et al., 2017). The impact of modification of RLS genes in C. elegans on lifespan can be evaluated in the absence or presence of cofounders such as Mn toxicity.
Inductively coupled plasma mass spectrometry (ICP-MS) analysis:
ICP-MS can be used to quantify metal content in C. elegans. This may require several thousands of worms (Peres et al., 2018). This method could be used to elucidate the influence of RLS gene modifications in C. elegans on levels of metals implicated in DAergic perturbations such as Fe and Mn.
6. Conclusion
RLS is common neurological disorder in the US. Although it is not a life-threatening disease, RLS can seriously impair life quality. The consequences include sleep deprivation, low work productivity, anxiety, depression and attention-deficit/hyperactivity disorder (ADHD) symptoms (Allen et al., 2011; Wagner et al., 2004). Finally, RLS may portend more serious consequences, including hypertension, heart disease and stroke (Y. Li et al., 2012; Pennestri et al., 2007; Siddiqui et al., 2007; Walters & Rye, 2009). It is undoubtedly that both environmental and genetic factors are involved, given the varied severity of symptoms. Although current research has revealed that brain Fe deficiency and DAergic dysfunction play important roles, it is far from drawing a conclusive understanding of the pathology of RLS. Biometals such as Fe, copper, zinc, Mn, usually share the transporters and complete for their influx and efflux of cells. It is very likely that Fe deficiency in the brain disrupts other metal homeostasis. Among these metals, Mn is the most similar one of Fe, given their biochemical property, metabolism and physiological functions in human. For example, Fe and Mn use the divalent metals transporter 1 (DMT1) and transferrin/transferrin receptor as the primary route of influx, and use ferroportin as the major efflux route (Chen, Bornhorst, & Aschner, 2018; Chen, Bornhorst, Diana Neely, & Avila, 2018; Chen, Parmalee, & Aschner, 2014). Low levels of Fe in the brain may activate expression of influx transporters in order to compensate for Fe deficiency, however, this also brings the risk of transporting more Mn in the brain. The accumulation of Mn has been reported to impair DAergic system and may cause neurological disorders such as Parkinson’s disease and dystonia (Chen et al., 2015; Chen, Chakraborty, Peres, Bowman, & Aschner, 2014; Chen, Culbreth, & Aschner, 2016; Chen, Miah, & Aschner, 2016). Therefore, it is necessary to investigate the levels of Mn and other metals in the brain of RLS patients to clarify whether Fe deficiency or high levels of other metals might play a role in the etiology of RLS.
The identified genetic risk factors are of great research value to elucidate the molecular mechanism of RLS, especially when combined with a suitable model organism. C. elegans provides numerous advantages in studying RLS-associated gene-environment interactions. These include its small size, short lifespan, a quick lifecycle and a simple nervous system. Additionally, the worm’s small and well-characterized genome is ideal for genetic manipulation. It contains all homologs of currently known RLS genetic risk factors (Table 2), as well as all genes necessary for DA biosynthesis, packaging and reuptake (Brenner, 1974; Sulston et al., 1975; White, Southgate, Thomson, & Brenner, 1976). More specifically, the circadian pattern of RLS symptoms can be studied in C. elegans via pharyngeal pumping actions and general locomotor activities, which re controlled by the worm circadian system. DAergic function can be accessed by studying individual neuronal morphology, behaviors regulated by DAergic neurons and measurement of neurotransmitters. Last, but not the least, the nematode is sensitive to metals and a wide-range of neuroactive drugs and xenobiotics, thus it can be applied to investigate the effect of Fe and other biometals, as well as to discover novel pharmaceutical compounds beneficial for RLS patients. Despite these advantages, very limited research on RLS has been reported in C. elegans. Therefore, we anticipate the involvement of C. elegans in RLS study will shed new light on the etiology and molecular mechanism of RLS, as well as identify novel drugs for treatment of RLS patients in the next decade.
Acknowledgements
The manuscript was supported by NIH grant NIEHS R01 10563, NIEHS R01 07331 and NIEHS R01 020852.
ABBREVIATIONS
- AD
Alzheimer’s disease
- ADHD
attention-deficit/hyperactivity disorder
- C. elegans
Caenorhabditis elegans
- CNS
central nervous system
- DA
dopamine
- DAergic
dopaminergic
- Fe
iron
- GFP
green fluorescent protein
- GWAS
genome-wide association studies
- HD
Huntington’s disease
- HPLC
high-performance liquid chromatography
- ICP-MS
Inductively coupled plasma mass spectrometry
- Mn
manganese
- MRI
magnetic resonance imaging
- PD
Parkinson’s disease
- RLS
restless legs syndrome
- SNPs
single-nucleotide polymorphisms
- TEM
transmission electronic microscopy
- TH
tyrosine hydroxylase
Footnotes
Conflict of interest
The authors declare no known or perceived conflict of interest.
References
- Ackley BD, Harrington RJ, Hudson ML, Williams L, Kenyon CJ, Chisholm AD, & Jin Y (2005). The two isoforms of the Caenorhabditis elegans leukocyte-common antigen related receptor tyrosine phosphatase PTP-3 function independently in axon guidance and synapse formation. J Neurosci, 25(33), 7517–7528. doi: 10.1523/jneurosci.2010-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akpinar CK, Turker H, Aygun D, & Aytac E (2017). Familial Restless Legs Syndrome: A Family with all Female Patients. Arch Iran Med, 20(2), 105–107. doi:0172002/aim.009 [PubMed] [Google Scholar]
- Allen RP, Bharmal M, & Calloway M (2011). Prevalence and disease burden of primary restless legs syndrome: results of a general population survey in the United States. Movement Disorders, 26(1), 114–120. doi: 10.1002/mds.23430 [DOI] [PubMed] [Google Scholar]
- Allen RP, La Buda MC, Becker P, & Earley CJ (2002). Family history study of the restless legs syndrome. Sleep Med, 3 Suppl, S3–7. [DOI] [PubMed] [Google Scholar]
- Allen RP, Picchietti DL, Garcia-Borreguero D, Ondo WG, Walters AS, Winkelman JW, … Lee HB (2014). Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: Updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria - history, rationale, description, and significance. Sleep Medicine, 15(8), 860–873. 10.1016/j.sleep.2014.03.025 [DOI] [PubMed] [Google Scholar]
- Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, & Montplaisi J (2003). Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med, 4(2), 101–119. doi:S1389945703000108 [pii] [DOI] [PubMed] [Google Scholar]
- Allen RP, Walters AS, Montplaisir J, Hening W, Myers A, Bell TJ, & Ferini-Strambi L (2005). Restless legs syndrome prevalence and impact: REST general population study. Archives of Internal Medicine, 165(11), 1286–1292. doi:165/11/1286 [pii] 10.1001/archinte.165.11.1286 [DOI] [PubMed] [Google Scholar]
- Arata Y, Kouike H, Zhang Y, Herman MA, Okano H, & Sawa H (2006). Wnt signaling and a Hox protein cooperatively regulate psa-3/Meis to determine daughter cell fate after asymmetric cell division in C. elegans. Dev Cell, 11(1), 105–115. doi: 10.1016/j.devcel.2006.04.020 [DOI] [PubMed] [Google Scholar]
- Avery L, & Horvitz HR (1990). Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. J Exp Zool, 253(3), 263–270. doi: 10.1002/jez.1402530305 [DOI] [PubMed] [Google Scholar]
- Azcoitia V, Aracil M, Martinez AC, & Torres M (2005). The homeodomain protein Meis1 is essential for definitive hematopoiesis and vascular patterning in the mouse embryo. Dev Biol, 280(2), 307–320. doi: 10.1016/j.ydbio.2005.01.004 [DOI] [PubMed] [Google Scholar]
- Barber BA, & Rastegar M (2010). Epigenetic control of Hox genes during neurogenesis, development, and disease. Ann Anat, 192(5), 261–274. doi: 10.1016/j.aanat.2010.07.009 [DOI] [PubMed] [Google Scholar]
- Barber BA, Liyanage VR, Zachariah RM, Olson CO, Bailey MA, & Rastegar M (2013). Dynamic expression of MEIS1 homeoprotein in E14.5 forebrain and differentiated forebrain-derived neural stem cells. Ann Anat, 195(5), 431–440. doi: 10.1016/j.aanat.2013.04.005 [DOI] [PubMed] [Google Scholar]
- Bastien D, Bellver Landete V, Lessard M, Vallieres N, Champagne M, Takashima A, … Lacroix S (2015). IL-1alpha Gene Deletion Protects Oligodendrocytes after Spinal Cord Injury through Upregulation of the Survival Factor Tox3. J Neurosci, 35(30), 10715–10730. doi: 10.1523/jneurosci.0498-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard J, Erikson KM, & Jones BC (2003). Neonatal iron deficiency results in irreversible changes in dopamine function in rats. Journal of Nutrition, 133(4), 1174–1179. [DOI] [PubMed] [Google Scholar]
- Benedetto A, Au C, Avila DS, Milatovic D, & Aschner M (2010). Extracellular Dopamine Potentiates Mn-Induced Oxidative Stress, Lifespan Reduction, and Dopaminergic Neurodegeneration in a BLI-3–Dependent Manner in Caenorhabditis elegans. PLoS Genet, 6(8), e1001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazie SM, Geissel HC, Wilky H, Joshi R, Newbern J, & Mangone M (2017). Alternative Polyadenylation Directs Tissue-Specific miRNA Targeting in Caenorhabditis elegans Somatic Tissues. Genetics, 206(2), 757–774. doi: 10.1534/genetics.116.196774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogan RK, & Cheray JA (2013). Restless Legs Syndrome : A Review of Diagnosis and Management in Primary Care, 125(3), 99–111. 10.3810/pgm.2013.05.2636 [DOI] [PubMed] [Google Scholar]
- Bouilloux F, Thireau J, Venteo S, Farah C, Karam S, Dauvilliers Y, … Marmigere F (2016). Loss of the transcription factor Meis1 prevents sympathetic neurons target-field innervation and increases susceptibility to sudden cardiac death. Elife, 5. doi: 10.7554/eLife.11627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S (1974). The genetics of Caenorhabditis elegans. Genetics, 77(1), 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caito SW, & Aschner M (2016). NAD+supplementation attenuates methylmercury dopaminergic and mitochondrial toxicity in Caenorhabditis elegans. Toxicological Sciences, 151(1), 139–149. 10.1093/toxsci/kfw030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catoire H, Dion PA, Xiong L, Amari M, Gaudet R, Girard SL, … Rouleau GA (2011). Restless legs syndrome-associated MEIS1 risk variant influences iron homeostasis. Ann Neurol, 70(1), 170–175. doi: 10.1002/ana.22435 [DOI] [PubMed] [Google Scholar]
- Catoire H, Dion PA, Xiong L, Amari M, Gaudet R, Girard SL, … Rouleau GA (2011). Restless legs syndrome-associated MEIS1 risk variant influences iron homeostasis. Annals of Neurology, 70(1), 170–175. 10.1002/ana.22435 [DOI] [PubMed] [Google Scholar]
- Chen P, Bornhorst J, & Aschner M (2018). Manganese metabolism in humans. Frontiers in bioscience (Landmark edition), 23, 1655–1679. [DOI] [PubMed] [Google Scholar]
- Chen P, Bornhorst J, Diana Neely M, & Avila DS (2018). Mechanisms and Disease Pathogenesis Underlying Metal-Induced Oxidative Stress. Oxidative medicine and cellular longevity, 2018, 3. doi: 10.1155/2018/7612172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Chakraborty S, Mukhopadhyay S, Lee E, Paoliello MMB, Bowman AB, & Aschner M (2015). Manganese homeostasis in the nervous system. Journal of Neurochemistry, 134(4), 601 610. doi: 10.1111/jnc.13170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Chakraborty S, Peres TV, Bowman AB, & Aschner M (2014). Manganese-induced Neurotoxicity: From C. elegans to Humans. Toxicology Research doi: 10.1039/C4TX00127C [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Culbreth M, & Aschner M (2016). Exposure, epidemiology, and mechanism of the environmental toxicant manganese. Environmental Science and Pollution Research, 23(14), 13802 13810. doi: 10.1007/s11356-016-6687-0 [DOI] [PubMed] [Google Scholar]
- Chen P, DeWitt MR, Bornhorst J, Soares FA, Mukhopadhyay S, Bowman AB, & Aschner M (2015). Age- And manganese-dependent modulation of dopaminergic phenotypes in a C. elegans DJ-1 genetic model of Parkinson’s disease. Metallomics, 7(2), 289–298. 10.1039/c4mt00292j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Martinez-finley EJ, Bornhorst J, Chakraborty S, & Aschner M (2013). Metal-induced neurodegeneration in C . elegans, 5(May), 1–11. 10.3389/fnagi.2013.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Miah MR, & Aschner M (2016). Metals and neurodegeneration. F1000Research, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Parmalee N, & Aschner M (2014). Genetic Factors and Manganese-Induced Neurotoxicity. Frontiers in Genetics, 5. doi: 10.3389/fgene.2014.00265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Xu J, Liu G, Liu H, Chen M, Qin Y, … Wang X (2016). Genetic variants in PTPRD and risk of gestational diabetes mellitus. Oncotarget, 7(46), 76101–76107. doi: 10.18632/oncotarget.12599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JR, Wang XS, Allen RP, Beard JL, Wiesinger JA, Felt BT, & Earley CJ (2009). Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain, 132(Pt 9), 2403–2412. doi: 10.1093/brain/awp125awp125 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JR, Wang XS, Neely EB, Ponnuru P, Morita H, & Beard J (2008). Comparative study of the influence of Thy1 deficiency and dietary iron deficiency on dopaminergic profiles in the mouse striatum. Journal of Neuroscience Research, 86(14), 3194–3202. doi: 10.1002/jnr.21758 [DOI] [PubMed] [Google Scholar]
- Corsi AK, Wightman B, & Chalfie M (2015). A Transparent Window into Biology: A Primer on Caenorhabditis elegans. Genetics, 200(2), 387–407. doi: 10.1534/genetics.115.176099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvilliers Y, & Winkelmann J (2013). Restless legs syndrome: Update on pathogenesis. Current Opinion in Pulmonary Medicine, 19(6), 594–600. 10.1097/MCP.0b013e328365ab07 [DOI] [PubMed] [Google Scholar]
- DeAndrade MP, Johnson RL Jr., Unger EL, Zhang L, van Groen T, Gamble KL, & Li Y (2012). Motor restlessness, sleep disturbances, thermal sensory alterations and elevated serum iron levels in Btbd9 mutant mice. Hum Mol Genet, 21(18), 3984–3992. doi: 10.1093/hmg/dds221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deandrade MP, Johnson RL, Unger EL, Zhang L, Van groen T, Gamble KL, & Li Y (2012). Motor restlessness, sleep disturbances, thermal sensory alterations and elevated serum iron levels in BTBD9 mutant mice. Human Molecular Genetics, 21(18), 3984–3992. 10.1093/hmg/dds221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAndrade MP, Zhang L, Doroodchi A, Yokoi F, Cheetham CC, Chen HX, … Li Y (2012). Enhanced hippocampal long-term potentiation and fear memory in Btbd9 mutant mice. PLoS One, 7(4), e35518. doi: 10.1371/journal.pone.0035518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao D, Wang L, Wan J, Chen Z, Peng J, Liu H, … Zou L (2016). MEK5 overexpression is associated with the occurrence and development of colorectal cancer. BMC Cancer, 16, 302. doi: 10.1186/s12885-016-2327-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley CJ, Kuwabara H, Wong DF, Gamaldo C, Salas R, Brasic J, … Allen RP (2011). The dopamine transporter is decreased in the striatum of subjects with restless legs syndrome. Sleep, 34(3), 341–347. 10.1093/sleep/34.3.341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis HM, & Horvitz HR (1986). Genetic control of programmed cell death in the nematode C. elegans. Cell, 44(6), 817–829. [DOI] [PubMed] [Google Scholar]
- Freeman A, Pranski E, Miller RD, Radmard S, Bernhard D, Jinnah HA, … Sanyal S (2012). Sleep fragmentation and motor restlessness in a drosophila model of restless legs syndrome. Current Biology, 22(12), 1142–1148. 10.1016/j.cub.2012.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati A, Marelli S, Giora E, Zucconi M, Oldani A, & Ferini-Strambi L (2015). Neurocognitive function in patients with idiopathic Restless Legs Syndrome before and after treatment with dopamine-agonist. International Journal of Psychophysiology, 95(3), 304–309. 10.1016/j.ijpsycho.2014.12.005 [DOI] [PubMed] [Google Scholar]
- Gao X, Schwarzschild MA, O’Reilly EJ, Wang H, & Ascherio A (2010). Restless legs syndrome and erectile dysfunction. Sleep, 33(1), 75–79. 10.1093/sleep/33.1.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Borreguero D, & Cano-Pumarega I (2017). New concepts in the management of restless legs syndrome. BMJ, 356 10.1136/bmj.j104 [DOI] [PubMed] [Google Scholar]
- Garcia-Borreguero D, & Williams A-M (2014). An update on restless legs syndrome (Willis-Ekbom disease). Current Opinion in Neurology, 27(4), 493–501. 10.1097/WCO.0000000000000117 [DOI] [PubMed] [Google Scholar]
- Garcia-Borreguero D, Kohnen R, Silber MH, Winkelman JW, Earley CJ, Högl B, … Allen RP (2013). The long-term treatment of restless legs syndrome/Willis-Ekbom disease: Evidence-based guidelines and clinical consensus best practice guidance: A report from the International Restless Legs Syndrome Study Group. Sleep Medicine, 14(7), 675–684. 10.1016/j.sleep.2013.05.016 [DOI] [PubMed] [Google Scholar]
- Garcia-Martin E, Jimenez-Jimenez FJ, Alonso-Navarro H, Martinez C, Zurdo M, Turpin-Fenoll L, … Agundez JA (2015). Heme Oxygenase-1 and 2 Common Genetic Variants and Risk for Restless Legs Syndrome. Medicine (Baltimore), 94(34), e1448. doi: 10.1097/md.0000000000001448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, McDonough CW, Beitelshees AL, El Rouby N, Hiltunen TP, O’Connell JR, … Johnson JA (2015). PTPRD gene associated with blood pressure response to atenolol and resistant hypertension. J Hypertens, 33(11), 2278–2285. doi: 10.1097/hjh.0000000000000714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goya ME, Romanowski A, Caldart CS, Bénard CY, & Golombek DA (2016). Circadian rhythms identified in Caenorhabditis elegans by in vivo long-term monitoring of a bioluminescent reporter. Proceedings of the National Academy of Sciences, 113(48), E7837–E7845. 10.1073/pnas.1605769113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Huang J, Jiang H, Han C, Li J, & Xu X (2017). Restless Legs Syndrome : From Pathophysiology to Clinical Diagnosis and Management, 9(June), 1–14. 10.3389/fnagi.2017.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haba-Rubio J, Marti-Soler H, Marques-Vidal P, Tobback N, Andries D, Preisig M, … Heinzer R (2016). Prevalence and determinants of periodic limb movements in the general population. Ann Neurol, 79(3), 464–474. doi: 10.1002/ana.24593 [DOI] [PubMed] [Google Scholar]
- Hart A (2006). Behavior. WormBook, 1–67. 10.1895/wormbook.1.87.1 [DOI]
- Hobert O (2013). The neuronal genome of Caenorhabditis elegans. WormBook, 1–106. doi: 10.1895/wormbook.1.161.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu V, Zobel CL, Lambie EJ, Schedl T, & Kornfeld K (2002). Caenorhabditis elegans lin-45 raf is essential for larval viability, fertility and the induction of vulval cell fates. Genetics, 160(2), 481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijomone OM, Miah MR, Peres TV, Nwoha PU, & Aschner M (2016). Null allele mutants of trt-1, the catalytic subunit of telomerase in Caenorhabditis elegans, are less sensitive to Mn-induced toxicity and DAergic degeneration. NeuroToxicology, 57 10.1016/j.neuro.2016.08.016 [DOI] [PubMed] [Google Scholar]
- Jayanthi LD, Apparsundaram S, Malone MD, Ward E, Miller DM, Eppler M, & Blakely RD (1998). The Caenorhabditis elegans gene T23G5.5 encodes an antidepressant- and cocaine-sensitive dopamine transporter. Mol Pharmacol, 54(4), 601–609. [PubMed] [Google Scholar]
- Jimenez-Jimenez FJ, Esguevillas G, Alonso-Navarro H, Zurdo M, Turpin-Fenoll L, Millan-Pascual J, … Garcia-Martin E (2018). Gamma-aminobutyric acid (GABA) receptors genes polymorphisms and risk for restless legs syndrome. Pharmacogenomics J doi: 10.1038/s41397-018-0023-7 [DOI] [PubMed] [Google Scholar]
- Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, … Ahringer J (2003). Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature, 421(6920), 231–237. doi:http://www.nature.com/nature/journal/v421/n6920/suppinfo/nature01278_S1.html [DOI] [PubMed] [Google Scholar]
- Kaneshiro S, Otsuki D, Yoshida K, Yoshikawa H, & Higuchi C (2015). MEK5 suppresses osteoblastic differentiation. Biochem Biophys Res Commun, 463(3), 241–247. doi: 10.1016/j.bbrc.2015.05.035 [DOI] [PubMed] [Google Scholar]
- Kao CY, Los FC, Huffman DL, Wachi S, Kloft N, Husmann M, … Aroian RV (2011). Global functional analyses of cellular responses to pore-forming toxins. PLoS Pathog, 7(3), e1001314. doi: 10.1371/journal.ppat.1001314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemlink D, Polo O, Frauscher B, Gschliesser V, Hogl B, Poewe W, … Winkelmann J (2009). Replication of restless legs syndrome loci in three European populations. Journal of Medical Genetics, 46(5), 315–318. doi:jmg.2008.062992 [pii] 10.1136/jmg.2008.062992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarini A, Walters AS, Hickey K, Coccagna G, Lugaresi E, Ehrenberg BL, … Johnson WG (1999). Studies of penetrance and anticipation in five autosomal-dominant restless legs syndrome pedigrees. Movement Disorders, 14(1), 111–116. [DOI] [PubMed] [Google Scholar]
- Lewis JA (1980). On the uses of small nematode worms. Neuroscience, 5(6), 961–966. doi:0306–4522(80)90179–7 [pii] [DOI] [PubMed] [Google Scholar]
- Li G, Tang H, Wang C, Qi X, Chen J, Chen S, & Ma J (2017). Association of BTBD9 and MAP2K5/SKOR1 With Restless Legs Syndrome in Chinese Population. Sleep, 40(4). doi: 10.1093/sleep/zsx028 [DOI] [PubMed] [Google Scholar]
- Li L, Guo G, Wang F, Lv P, Zhu M, Gu Y, … Pei X (2018). TOX high mobility group box family member 3 rs3803662 and breast cancer risk: A meta-analysis. J Cancer Res Ther, 14(Supplement), S208–s212. doi: 10.4103/0973-1482.167611 [DOI] [PubMed] [Google Scholar]
- Li Y, Doroodchi A, Yang Y, & DeAndrade MP (2011). Characterization of BTBD9 Homolog Knockout in C. elegans. Sleep Med, 12, S1–S130. [Google Scholar]
- Li Y, Walters AS, Chiuve SE, Rimm EB, Winkelman JW, & Gao X (2012). Prospective study of restless legs syndrome and coronary heart disease among women. Circulation, 126(14), 1689–1694. doi: 10.1161/CIRCULATIONAHA.112.112698CIRCULATIONAHA.112.112698 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang P, Choi TY, Park SK, Park H, Lee EJ, … Kim E (2015). Splicing-Dependent Trans-synaptic SALM3-LAR-RPTP Interactions Regulate Excitatory Synapse Development and Locomotion. Cell Rep, 12(10), 1618–1630. doi: 10.1016/j.celrep.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhead D, Schwarz EM, O’Hagan R, Bellotti S, Krieg M, Barr MM, … Goodman MB (2016). The tubulin repertoire of C. elegans sensory neurons and its context-dependent role in process outgrowth. Mol Biol Cell doi: 10.1091/mbc.E16-06-0473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay SJ, Johnsen R, Khattra J, Asano J, Baillie DL, Chan S, … Moerman DG (2003). Gene expression profiling of cells, tissues, and developmental stages of the nematode C. elegans. Cold Spring Harb Symp Quant Biol, 68, 159–169. [DOI] [PubMed] [Google Scholar]
- Mehta PB, Jenkins BL, McCarthy L, Thilak L, Robson CN, Neal DE, & Leung HY (2003). MEK5 overexpression is associated with metastatic prostate cancer, and stimulates proliferation, MMP-9 expression and invasion. Oncogene, 22(9), 1381–1389. doi: 10.1038/sj.onc.1206154 [DOI] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb DT, & Ambros VR (1991). Efficient gene transfer in C. elegans: Extrachromosomal maintenance and integration of transforming sequences. EMBO Journal, 10, 3959–3970. doi:citeulike-article-id:3275905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliori ML, Simonetta SH, Romanowski A, & Golombek DA (2011). Circadian rhythms in metabolic variables in Caenorhabditis elegans. Physiology and Behavior, 103(3–4), 315–320. 10.1016/j.physbeh.2011.01.026 [DOI] [PubMed] [Google Scholar]
- Mohtashami S, He Q, Ruskey JA, Zhou S, Dion PA, Allen RP, … Gan-Or Z (2018). TOX3 Variants Are Involved in Restless Legs Syndrome and Parkinson’s Disease with Opposite Effects. J Mol Neurosci, 64(3), 341–345. doi: 10.1007/s12031-018-1031-4 [DOI] [PubMed] [Google Scholar]
- Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, & Lesperance P (1997). Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Movement Disorders, 12(1), 61–65. doi: 10.1002/mds.870120111 [DOI] [PubMed] [Google Scholar]
- Moon H, Chang Y, & Lee Y (2014). T2 Relaxometry Using 3.0-Tesla Magnetic Resonance Imaging of the Brain in Early-and Late-Onset Restless Legs Syndrome. Journal of Clinical …, 10(3), 197–202. Retrieved from http://synapse.koreamed.org/DOIx.php?id=10.3988/jcn.2014.10.3.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H. t., Winkelmann J, Lin L, Finn L, Peppard P, & Mignot E (2014). Periodic leg movements during sleep are associated with polymorphisms in BTBD9, TOX3/BC034767, MEIS1, MAP2K5/SKOR1, and PTPRD. Sleep, 37(9), 1535–1542. doi: 10.5665/sleep.4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PG, Sedensky MM, Meneely PM, & Cascorbi HF (1988). The effect of two genes on anesthetic response in the nematode Caenorhabditis elegans. Anesthesiology, 69(2), 246–251. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Tanaka H, Sawada K, Hayashi H, Hasebe T, Abe M, … Okumura T (2018). Polymorphism of receptor-type tyrosine-protein phosphatase delta gene in the development of non-alcoholic fatty liver disease. J Gastroenterol Hepatol, 33(1), 283–290. doi: 10.1111/jgh.13820 [DOI] [PubMed] [Google Scholar]
- Nakamura F, Okada T, Shishikura M, Uetani N, Taniguchi M, Yagi T, … Strittmatter SM (2017). Protein Tyrosine Phosphatase delta Mediates the Sema3A-Induced Cortical Basal Dendritic Arborization through the Activation of Fyn Tyrosine Kinase. J Neurosci, 37(30), 7125–7139. doi: 10.1523/jneurosci.2519-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass R, Hall DH, Miller DM 3rd, & Blakely RD (2002). Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc Natl Acad Sci U S A, 99(5), 3264–3269. doi: 10.1073/pnas.042497999042497999 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MD, Lee KH, Churgin MA, Hill AJ, Van Buskirk C, Fang-Yen C, & Raizen DM (2014). FMRFamide-like FLP-13 neuropeptides promote quiescence following heat stress in Caenorhabditis elegans. Curr Biol, 24(20), 2406–2410. doi: 10.1016/j.cub.2014.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas HR, & Hodgkin J (2004). The ERK MAP kinase cascade mediates tail swelling and a protective response to rectal infection in C. elegans. Curr Biol, 14(14), 1256–1261. doi: 10.1016/j.cub.2004.07.022 [DOI] [PubMed] [Google Scholar]
- Nicol RL, Frey N, Pearson G, Cobb M, Richardson J, & Olson EN (2001). Activated MEK5 induces serial assembly of sarcomeres and eccentric cardiac hypertrophy. Embo j, 20(11), 2757–2767. doi: 10.1093/emboj/20.11.2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH. (2017). Restless Legs Syndrome Fact Sheet; NIH Publication No. 17–4847 Retrieved from https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Restless-Legs-Syndrome-Fact-Sheet#3237_2
- Ning Z, Jiayi L, Jian R, & Wanli X (2017). Relationship between abnormal TOX3 gene methylation and polycystic ovarian syndrome. Eur Rev Med Pharmacol Sci, 21(9), 2034–2038. [PubMed] [Google Scholar]
- Ondo WG, Vuong KD, & Wang Q (2000). Restless legs syndrome in monozygotic twins: clinical correlates. Neurology, 55(9), 1404–1406. [DOI] [PubMed] [Google Scholar]
- Park H-EH, Jung Y, & Lee S-JV (2017). Survival assays using Caenorhabditis elegans. Molecules and Cells, 40(2), 90–99. 10.14348/molcells.2017.0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeraully T, & Tan E-K (2012). Linking restless legs syndrome with Parkinson’s disease: clinical, imaging and genetic evidence. Transl Neurodegener, 1(1), 6 10.1186/2047-9158-1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennestri MH, Montplaisir J, Colombo R, Lavigne G, & Lanfranchi PA (2007). Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology, 68(15), 1213–1218. doi:68/15/1213 [pii] 10.1212/01.wnl.0000259036.89411.52 [DOI] [PubMed] [Google Scholar]
- Peres TV, Arantes LP, Miah MR, Bornhorst J, Schwerdtle T, Bowman AB, … Aschner M (2018). Role of Caenorhabditis elegans AKT-½ and SGK-1 in Manganese Toxicity. Neurotoxicity Research, 1–13. 10.1007/s12640-018-9915-1 [DOI] [PMC free article] [PubMed]
- Rask-Andersen M, Jacobsson JA, Moschonis G, Ek AE, Chrousos GP, Marcus C, … Schioth HB (2012). The MAP2K5-linked SNP rs2241423 is associated with BMI and obesity in two cohorts of Swedish and Greek children. BMC Med Genet, 13, 36. doi: 10.1186/1471-2350-13-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruszkiewicz JA, Pinkas A, Miah MR, Weitz RL, Lawes MJA, Akinyemi AJ, … Aschner M (2018). C. elegans as a model in developmental neurotoxicology. Toxicology and Applied Pharmacology 10.1016/j.taap.2018.03.016 [DOI] [PMC free article] [PubMed]
- Sahu SK, Fritz A, Tiwari N, Kovacs Z, Pouya A, Wullner V, … Methner A (2016). TOX3 regulates neural progenitor identity. Biochim Biophys Acta, 1859(7), 833–840. doi: 10.1016/j.bbagrm.2016.04.005 [DOI] [PubMed] [Google Scholar]
- Schaefer H, & Rongo C (2006). KEL-8 Is a Substrate Receptor for CUL3-dependent Ubiquitin Ligase That Regulates Synaptic Glutamate Receptor Turnover. Molecular Biology of the Cell, 17(3), 1250–1260. doi: 10.1091/mbc.e05-08-0794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schormair B, Kemlink D, Roeske D, Eckstein G, Xiong L, Lichtner P, … Winkelmann J (2008). PTPRD (protein tyrosine phosphatase receptor type delta) is associated with restless legs syndrome. Nature Genetics, 40(8), 946–948. doi:ng.190 [pii] 10.1038/ng.190 [DOI] [PubMed] [Google Scholar]
- Schwartz ML, & Jorgensen EM (2016). SapTrap, a Toolkit for High-Throughput CRISPR/Cas9 Gene Modification in Caenorhabditis elegans. Genetics, 202(4), 1277–1288. doi: 10.1534/genetics.115.184275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta P, van der Linden AM, Beverly M, Kadener S, Rodriguez J, Wasserman S, & Rosbash M (2010). Genome-wide analysis of light- and temperature-entrained circadian transcripts in Caenorhabditis elegans. PLoS Biology, 8(10). 10.1371/journal.pbio.1000503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishikura M, Nakamura F, Yamashita N, Uetani N, Iwakura Y, & Goshima Y (2016). Expression of receptor protein tyrosine phosphatase delta, PTPdelta, in mouse central nervous system. Brain Res, 1642, 244–254. doi: 10.1016/j.brainres.2016.03.030 [DOI] [PubMed] [Google Scholar]
- Siddiqui F, Strus J, Ming X, Lee IA, Chokroverty S, & Walters AS (2007). Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clinical Neurophysiology, 118(9), 1923–1930. doi:S1388–2457(07)00199-X [pii] 10.1016/j.clinph.2007.05.006 [DOI] [PubMed] [Google Scholar]
- Simmer F, Moorman C, van der Linden AM, Kuijk E, van den Berghe PV, Kamath RS, … Plasterk RH (2003). Genome-wide RNAi of C. elegans using the hypersensitive rrf-3 strain reveals novel gene functions. PLoS Biol, 1(1), E12. doi: 10.1371/journal.pbio.0000012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn SJ, Lewis GM, & Winoto A (2008). Non-redundant function of the MEK5-ERK5 pathway in thymocyte apoptosis. Embo j, 27(13), 1896–1906. doi: 10.1038/emboj.2008.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer WC, Zeller G, Watson JD, Henz SR, Watkins KL, McWhirter RD, … Miller DM 3rd. (2011). A spatial and temporal map of C. elegans gene expression. Genome Res, 21(2), 325–341. doi: 10.1101/gr.114595.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Rye DB, Hicks A, Petursson H, Ingason A, Thorgeirsson TE, … Stefansson K (2007). A genetic risk factor for periodic limb movements in sleep. N Engl J Med, 357(7), 639–647. doi: 10.1056/NEJMoa072743 [DOI] [PubMed] [Google Scholar]
- Sulston J, Dew M, & Brenner S (1975). Dopaminergic neurons in the nematode Caenorhabditis elegans. J Comp Neurol, 163(2), 215–226. doi: 10.1002/cne.901630207 [DOI] [PubMed] [Google Scholar]
- Symvoulakis E, Anyfantakis D, & Lionis C (2010). Restless legs syndrome : literature review. São Paulo Medical Journal, 128(3), 167–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaumkessel M, Wojciechowska S, Janiszewska J, Zemke N, Byzia E, Kiwerska K, … Giefing M (2017). Recurrent epigenetic silencing of the PTPRD tumor suppressor in laryngeal squamous cell carcinoma. Tumour Biol, 39(3), 1010428317691427. doi: 10.1177/1010428317691427 [DOI] [PubMed] [Google Scholar]
- Tan EK (2006). Restless legs syndrome and Parkinson’s disease: is there an etiologic link? Journal of Neurology, 253 Suppl 7, VII33–37. doi: 10.1007/s00415-006-7008-1 [DOI] [PubMed] [Google Scholar]
- Titus K, & Michael OH (2006). Finding function in novel targets: C. elegans as a model organism. Nature Reviews Drug Discovery, 5(5), 387–399. doi: 10.1038/nrd2031 [DOI] [PubMed] [Google Scholar]
- Trenkwalder C, Paulus W, & Walters AS (2005). The restless legs syndrome. Lancet Neurology 10.1016/S1474-4422(05)70139-3 [DOI] [PubMed]
- Trenkwalder C, Seidel VC, Gasser T, & Oertel WH (1996). Clinical symptoms and possible anticipation in a large kindred of familial restless legs syndrome. Movement Disorders, 11(4), 389–394. doi: 10.1002/mds.870110407 [DOI] [PubMed] [Google Scholar]
- Trenkwalder C, Winkelmann J, Inoue Y, & Paulus W (2015). Restless legs syndrome—current therapies and management of augmentation. Nature Reviews Neurology, 11(8), 434–445. 10.1038/nrneurol.2015.122 [DOI] [PubMed] [Google Scholar]
- Van Auken K, Weaver D, Robertson B, Sundaram M, Saldi T, Edgar L, … Wood WB (2002). Roles of the Homothorax/Meis/Prep homolog UNC-62 and the Exd/Pbx homologs CEH-20 and CEH-40 in C. elegans embryogenesis. Development, 129(22), 5255–5268. [DOI] [PubMed] [Google Scholar]
- Vilarino-Guell C, Chai H, Keeling BH, Young JE, Rajput A, Lynch T, … Lin SC (2009). MEIS1 p.R272H in familial restless legs syndrome. Neurology, 73(3), 243–245. doi: 10.1212/WNL.0b013e3181ae7c79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilarino-Guell C, Farrer MJ, & Lin SC (2008). A genetic risk factor for periodic limb movements in sleep. N Engl J Med, 358(4), 425–427. doi: 10.1056/NEJMc072518 [DOI] [PubMed] [Google Scholar]
- Wagner ML, Walters AS, & Fisher BC (2004). Symptoms of attention-deficit/hyperactivity disorder in adults with restless legs syndrome. Sleep, 27(8), 1499–1504. [DOI] [PubMed] [Google Scholar]
- Walters AS, & Rye DB (2009). Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep, 32(5), 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters AS, Hickey K, Maltzman J, Verrico T, Joseph D, Hening W, … Chokroverty S (1996). A questionnaire study of 138 patients with restless legs syndrome: the ‘Night-Walkers’ survey. Neurology, 46(1), 92–95. [DOI] [PubMed] [Google Scholar]
- Wang W, Lu S, Li T, Pan Y-W, Zou J, Abel GM, … Xia Z (2015). Inducible Activation of ERK5 MAP Kinase Enhances Adult Neurogenesis in the Olfactory Bulb and Improves Olfactory Function. The Journal of Neuroscience, 35(20), 7833–7849. doi: 10.1523/JNEUROSCI.3745-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RJ, Zucca FA, Duyn JH, Crichton RR, & Zecca L (2014). The role of iron in brain ageing and neurodegenerative disorders. The Lancet Neurology Elsevier Ltd; 10.1016/S1474-4422(14)70117-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetter TC, Eisensehr I, & Trenkwalder C (2004). Functional neuroimaging studies in restless legs syndrome. Sleep Med, 5(4), 401–406. doi: 10.1016/j.sleep.2004.01.009S1389945704000218 [pii] [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, & Brenner S (1976). The structure of the ventral nerve cord of Caenorhabditis elegans. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 275(938), 327–348. [DOI] [PubMed] [Google Scholar]
- Winkelmann J, Schormair B, Lichtner P, Ripke S, Xiong L, Jalilzadeh S, … Meitinger T (2007). Genome-wide association study of restless legs syndrome identifies common variants in three genomic regions. Nature Genetics, 39(8), 1000–1006. doi:ng2099 [pii] 10.1038/ng2099 [DOI] [PubMed] [Google Scholar]
- Winkelmann J, Stautner A, Samtleben W, & Trenkwalder C (2002). Long-term course of restless legs syndrome in dialysis patients after kidney transplantation. Movement Disorders, 17(5), 1072–1076. doi: 10.1002/mds.10231 [DOI] [PubMed] [Google Scholar]
- Wu Y, Han M, & Guan KL (1995). MEK-2, a Caenorhabditis elegans MAP kinase kinase, functions in Ras-mediated vulval induction and other developmental events. Genes Dev, 9(6), 742–755. [DOI] [PubMed] [Google Scholar]
- Xiong L, Jang K, Montplaisir J, Levchenko A, Thibodeau P, Gaspar C, … Rouleau GA (2007). Canadian restless legs syndrome twin study. Neurology, 68(19), 1631–1633. doi: 10.1212/01.wnl.0000261016.90374.fd [DOI] [PubMed] [Google Scholar]
- Xiong L, Montplaisir J, Desautels A, Barhdadi A, Turecki G, Levchenko A, … Rouleau GA (2010). Family study of restless legs syndrome in Quebec, Canada: clinical characterization of 671 familial cases. Arch Neurol, 67(5), 617–622. doi: 10.1001/archneurol.2010.67 [DOI] [PubMed] [Google Scholar]
- Yang Q, Li L, Chen Q, Foldvary-Schaefer N, Ondo WG, & Wang QK (2011). Association studies of variants in MEIS1, BTBD9, and MAP2K5/SKOR1 with restless legs syndrome in a US population. Sleep Med, 12(8), 800–804. doi: 10.1016/j.sleep.2011.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Li L, Yang R, Shen GQ, Chen Q, Foldvary-Schaefer N, … Wang QK (2011). Family-based and population-based association studies validate PTPRD as a risk factor for restless legs syndrome. Movement Disorders, 26(3), 516–519. doi: 10.1002/mds.23459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Bao ZQ, & Dixon JE (1995). Components of a new human protein kinase signal transduction pathway. J Biol Chem, 270(21), 12665–12669. [DOI] [PubMed] [Google Scholar]
- Zhu J, Cui L, Xu A, Yin X, Li F, & Gao J (2017). MEIS1 inhibits clear cell renal cell carcinoma cells proliferation and in vitro invasion or migration. BMC Cancer, 17(1), 176. doi: 10.1186/s12885-017-3155-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Tan Z, Zhao S, Huang H, Zhao X, Hu X, … Qiu M (2015). Developmental expression and function analysis of protein tyrosine phosphatase receptor type D in oligodendrocyte myelination. Neuroscience, 308, 106–114. doi: 10.1016/j.neuroscience.2015.08.062 [DOI] [PMC free article] [PubMed] [Google Scholar]


