Abstract
Voltage-gated sodium channels are the molecular components of electrical signaling in the body, yet the molecular origins of Na+-selective transport remain obscured by diverse protein chemistries within this family of ion channels. In particular, bacterial and mammalian sodium channels are known to exhibit similar relative ion permeabilities for Na+ over K+ ions, despite their distinct signature EEEE and DEKA sequences. Atomic-level molecular dynamics simulations using high-resolution bacterial channel structures and mammalian channel models have begun to describe how these sequences lead to analogous high field strength ion binding sites that drive Na+ conduction. Similar complexes have also been identified in unrelated acid sensing ion channels involving glutamate and aspartate side chains that control their selectivity. These studies suggest the possibility of a common origin for Na+ selective binding and transport.
Keywords: Voltage-gated sodium channel, Acid sensing ion channel, Ion permeation, Ion selectivity, Molecular dynamics simulation
Introduction
Voltage gated sodium (Nav) channels are essential in the initial depolarization of excitable cells during the generation and propagation of action potentials. Their isoforms can be found in all excitable tissues in the body (Goldin, 1999), and are necessary for a wide range of physiological processes, including regulation of heartbeat, pain sensation, transmission of nervous signals and enablement of cognitive processes (Hille, 2001). This ubiquity makes Nav channels of particular interest in the development of pharmaceutical compounds, and they have been identified as targets for epilepsy, cardiac arrhythmias, chronic and neuropathic pain, migraine, spasticity, neurodegenerative disease, bipolar disorder, anxiety, obsessive-compulsive disorder, dystonia, schizophrenia and restless legs syndrome, among others (Kyle and Ilyin, 2007; Zuliani et al., 2009). Central to the role of these ion channels is their ability to selectively conduct Na+ ions across cell membranes in preference to other ionic species. Understanding how this selectivity arises from the structure and chemistry of the protein is of primary interest. With atomic resolution structures of different Na+-selective channels emerging, we are beginning to learn the fundamental rules governing Na+ selectivity in membrane transport.
The structure of a sodium channel and its ion permeation pore
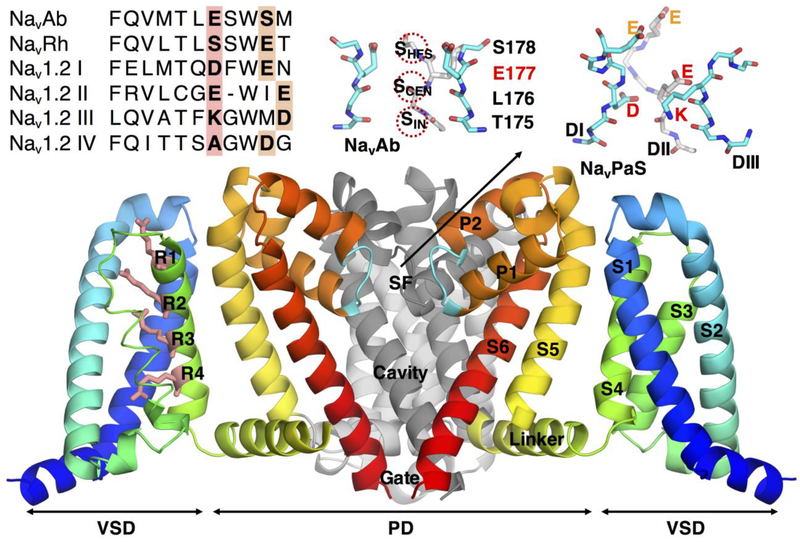
The mammalian Nav channel family comprises 9 isoforms (Nav1.1 to Nav1.9), with a high sequence similarity, suggesting shared features of structure and function. Unfortunately, high-resolution structural data for mammalian Nav channels is still lacking. Recently, publication of near-atomic resolution cryo-Em structures of the channels NavPaS (Shen et al., 2017), from cockroach, and EeNav1.4 (Yan et al., 2017), from electric eel, provided a new source of structural information for eukaryotic Nav channels, but with limited resolution in key regions, such as the selectivity filter (SF). These channels display an architecture typical of other voltage gated ion channels (VGIC) that possess atomic resolution structures, such as voltage-gated potassium (Kv) channels (Long et al.), and more recently, bacterial Nav channels (Bagneris et al., 2014; McCusker et al., 2012; Payandeh et al., 2012; Payandeh et al., 2011; Shaya et al., 2014; Zhang et al., 2012). The structures exhibit a pore forming subunit composed of 4 homologous domains (DI to DIV), each made up of 6 trans-membrane segments (S1 to S6; Fig.1). Segments S1 to S4 form 4 voltage-sensing domains (VSD), and segments S5 and S6 arrange themselves around a central opening to form the pore domain (PD). In the eukaryotic NavPaS, segments S5 and S6 are linked together by 2 short helices (P1 and P2), some large flexible extracellular loops and a short-coiled structure forming the channel’s selectivity filter (SF; Fig.1 upper insets). This narrow part of the permeation pathway is formed by a highly conserved sequence of amino acids, within which can be found a DEKA (Asp-Glu-Lys-Ala or equivalent residues, carried by domains DI to DIV, respectively) ring of residues, responsible for ion discrimination in eukaryotic channels (Chiamvimonvat et al., 1996a; Favre et al., 1996; Schlief et al., 1996; Tsushima et al., 1997a), with ion conduction and selectivity determined by the inclusion and positions of at least one carboxylate-containing side chain (the E or D) and the lysine (Favre et al., 1996). This DEKA selectivity signature is accompanied by an additional ring of negatively charged residues (EEDD) higher up in the pore, above the SF, known to play an important role in ion conduction (Khan et al., 2002; Noda et al., 1989; Schlief et al., 1996; Terlau et al., 1991), probably due to their ability to attract cations into the SF (Khan et al., 2002), although all four residues do not affect conduction equally (Chiamvimonvat et al., 1996a; Schlief et al., 1996), and there is contrasting evidence for (Chiamvimonvat et al., 1996a; Chiamvimonvat et al., 1996b; Tsushima et al., 1997a) and against (Penzotti et al., 1998; Schlief et al., 1996) a role for these residues in selectivity. Residues of the DEKA and EEDD rings appear staggered asymmetrically around the central axis (Fig.1 upper right inset), but the orientation of their side chains remains unclear in the recent eukaryotic NavPaS, limiting direct insight into channel permeation and selectivity mechanisms (Shen et al., 2017) without simulation studies.
Figure 1:
The structure of Nav channels. Main structural elements of the bacterial channel NavAb showing the arrangment of segments S1 to S6 forming the pore domain (PD) and the volatge-sensing domain (VSD). For clarity, the segments forming the PD of one subunit and the VSD of another have been omitted. In the VSD, the key arginines playing a role in gating are represented as pink sticks and labelled. More detailed representations of the SFs are added as inserts (colored sticks, C in cyan and gray for back subunit, N in blue and O in red, 3 subunits shown), displaying NavAb (top center), with its specific sequence of residues TLES and the 3 binding sites for Na+ SHFS, SCEN and SIN as red dashed circles, and NavPaS (top right), with the key residues of the DEKA locus indicated (D, E, and K, the subunit carrying A, DIV, is not shown), as well as the extracellular ring of charged residues (E and E, as DIII doesn’t carry a charged residue in NavPaS), the name of each domain DI, DII and DIII is indicated. To the left a sequence alignment of NavBaCs (NavAb and NavRh) and human Nav1.2 is shown. Bold and highlighted letters indicate key DEKA and EEEE rings, as well as the vestibular EEDD ring.
Our understanding of Nav channels has been greatly enhanced by the discovery of bacterial channels, NavBaC, in the early 2000s (Ito et al., 2004; Koishi et al., 2004; Ren et al., 2001), soon followed by the publication of high resolution X-ray structures of various bacterial channels: NavAb (Payandeh et al., 2011) (Payandeh et al., 2012), NavMs (Bagneris et al., 2014; McCusker et al., 2012; Sula et al., 2017), NavRh (Zhang et al., 2012) and NavAe (Shaya et al., 2014). While their physiological functions in bacteria remain somewhat obscure (Ito et al., 2004), these channels have a high level of sequence similarity, share related architectures, exhibit comparable single channel conductances (NaChBac ~12 pS (Ren et al., 2001), mammalian Nav ~4–24 pS (Hille, 2001)) and robust Na+ selectivity, albeit with more variability within the bacterial family (Payandeh et al., 2011; Shaya et al., 2014; Ulmschneider et al., 2013). Unlike their eukaryotic counterparts, bacterial channels are made up of 4 independent and identical subunits, arranged symmetrically around a central pore (see Fig.1), with a conformation similar to that of the NavPaS channel (Flood et al., 2018). The SF is the most striking departure from mammalian channels, with the inner DEKA locus replaced by a symmetrical ring of glutamates, EEEE (with the exception of NavRh), and the outer vestibular EEDD (EEEE in NavRh (Zhang et al., 2012); see Fig.1 upper insets) ring being absent. As for DEKA, this EEEE ring plays an essential role for conduction and selectivity in the bacterial case. For instance, in bacterial NavMs, EEEE mutation to DDDD reduced both selectivity and single channel conductance (Naylor et al., 2016); an observation consistent with the homologous non-selective NsvBa bacterial channel that presents a DDDD ring (DeCaen et al., 2014). The divergence in the core functional part of the permeation pathways of mammalian and bacterial Navs, despite their shared functional roles as Na+-selective channels, raises the intriguing question as to what causes Na+-selective permeation.
Conserved function within the sodium channel family
Functionally, eukaryotic and prokaryotic channels share the same gating process, where the charged residues (Arg) carried by segments S4 of the VSDs (pink residues R1 to R4 in Fig.1) move in response to a depolarizing membrane potential, pulling at the S4-S5 linker and opening the gate in the PD (Yarov-Yarovoy et al., 2012). Following gating (within milliseconds to seconds), the channel spontaneously enters a non-conductive, or inactive, state. This inactivation process, often targeted by blockers, can happen on two distinct timescales. Fast inactivation, exclusive to eukaryotic channels, occurs when the cytoplasmic loop linking domains DIII and DIV (Benz et al., 1997; Kellenberger et al., 1996; Rohl et al., 1999; Vassilev et al., 1988; West et al., 1992) docks into the gate formed by segments S6 (Hilber et al., 2002), blocking the flow of ions. Slow inactivation, common to both families of channels, happens on longer timescales (milliseconds to minutes (Ellerkmann et al., 2001; Ulbricht, 2005)) via a less localized mechanism, involving the SF (Balser et al., 1996; Kambouris et al., 1998; Ong et al., 2000; Zhang et al., 2003) and some residues of segments S6 (Carboni et al., 2005; Vedantham and Cannon, 2000; Wang and Wang, 1997; Zarrabi et al., 2010), leading to a generalized PD collapse. Interestingly, Nav families are found to share their sensitivity to channel inhibitors that target inactivation, suggesting a role for pore-based slow inactivation in drug activity for both bacterial and mammalian sodium channels (Boiteux and Allen, 2016; Boiteux et al., 2014b; Carboni et al., 2005; Chen et al., 2000; Fozzard et al., 2005; Lee et al., 2012; Sheets et al., 2011). It has also been shown that key drug-binding binding regions are remarkably conserved among mammalian and bacterial channels (Bagneris et al., 2014; Payandeh et al., 2011), making NavBaCs reliable models to study drug activities (Bagneris et al., 2014; Barber et al., 2014; Lee et al., 2012; Raju et al., 2013). This has allowed the use of computational simulations that rely on well-defined bacterial channel structures to shed light on drug binding sites and pathways (Bagneris et al., 2014; Boiteux et al., 2014b; Martin and Corry, 2014).
When combined with the comparable selective ion permeabilities of bacterial and mammalian Nav channels (Finol-Urdaneta et al., 2014; Ren et al., 2001; Yue et al., 2002), overall there exists widespread correspondence between bacterial and mammalian sodium channels that suggests a greater role for simulations of bacterial structures in understanding function, and in particular in uncovering the rules governing selective ion permeation.
Ion permeation and selectivity in bacterial Nav channels
Thermodynamic selectivity for ion binding is known to be controlled by the nature of coordinating ligands, where the weaker fields provided by backbone carbonyl groups (as found in potassium channels) favor the larger K+, and strong fields created by charged carboxylate groups (as found in sodium channels) favor the smaller Na+ (Eisenman, 1962; Roux et al., 2011). However, selective permeation is not governed solely by the thermodynamic stability of an ion in a single site, but by the free energy surface governing the multi-ion permeation mechanism. In the SFs of bacterial Nav channels these carboxylates are highly dynamic, involving concerted changes in the rotameric states of side chains during ion translocation, enabling rapid exchange between configurational states to create lower activation barriers (Boiteux et al., 2014a; Chakrabarti et al., 2013; Furini and Domene, 2018; Ke et al., 2014), corresponding to higher permeation rates. This collaborative movement of ions and protein groups can only be observed on the timescales of conduction itself, and was not seen in earlier simulation studies of bacterial Navs that described ion binding and movement in the SF (Carnevale et al., 2011; Corry and Thomas, 2012; Furini and Domene, 2012; Qiu et al., 2012; Stock et al., 2013; Ulmschneider et al., 2013; Zhang et al., 2013). These studies focused primarily on the role of ligand chemistry and geometry of the binding sites within a crystal structure-like conformation of the SF, appearing to favor partly-hydrated Na+ binding due to the strength of interaction and snug-fit in the site formed by high field strength (HFS) glutamate side chains (Corry and Thomas, 2012; Furini and Domene, 2012; Ulmschneider et al., 2013). Such simulation studies demonstrated the ability to bind 2 ions concurrently within this SHFS site, with increased affinity for Na+ leading to a reduced apparent permeation barrier for Na+ compared to K+ ions (Furini and Domene, 2012), and suggested a 3-ion conduction mechanism (Finol-Urdaneta et al., 2014). However, simulations on the microsecond scale have captured protein movements that break the symmetric arrangement of Glu side chains (Boiteux et al., 2014a; Chakrabarti et al., 2013; Furini and Domene, 2018; Ke et al., 2014) and suggest coupling between ionic movements and the conformation of the filter, with isomerization of glutamates catalyzing efficient Na+ permeation.
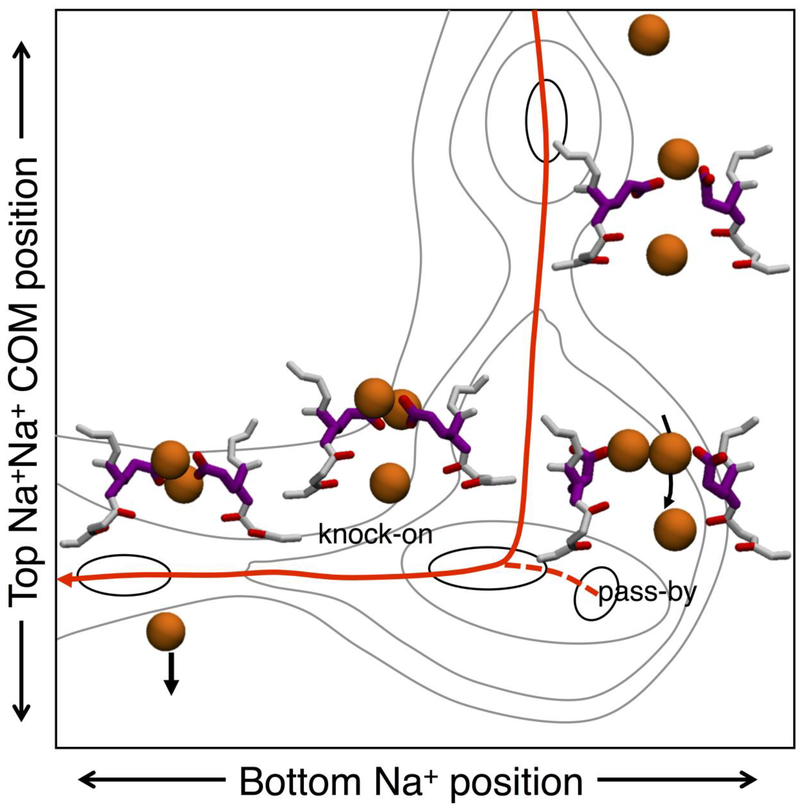
This flexibility of the SF leads to structural rearrangements that allow side-chains to form Na+ specific complexes during ion translocation, with the side chains continuously coordinating multiple ions to apparently ‘carry’ them across the narrowest part of the pore (Boiteux et al., 2014a; Chakrabarti et al., 2013). In bacterial channels, glutamates from the EEEE ring come together to create complexes with multiple ions that are less favorable for K+, but attractively bind Na+ ions in SHFS (Boiteux et al., 2014a). Simulations in the presence of Na+ ions show filter occupancies alternating between 2 and 3 ions in bacterial channels NavAb (Boiteux et al., 2014a; Chakrabarti et al., 2013) and NaChBac (Guardiani et al., 2017), leading to an efficient knock-on ion permeation process. In the presence of 2 ions in the SF, free movement is observed between 3 binding sites: SHFS, formed by the side chains of E177; a central cite, SCEN, involving by L176 backbone carbonyls; and an inner site, SIN, created by the backbone carbonyls and hydroxyl groups of T175 (Fig.1 upper center inset). However, no permeation through the SF is seen in this 2-ion state. The entrance of a third ion triggers the conduction process (Fig.2). This ion movement from extracellular medium to the channel’s central cavity beneath the SF can occur via 2 different mechanisms. The third ion can enter the SF from above, join an ion at the level of the glutamate ring, electrostatically repelling and knocking down the bottom ion into the cavity (knock-on). Alternatively, the entering ion can pass the ion bound to the glutamates (joining it in a 2-ion complex in SHFS) before pushing the bottom ion into the cavity (see Fig.2; ‘pass by’). In both cases, the ions encounter low free energy barriers (<1 kcal/mol), leading to an efficient Na+ conduction mechanism. This efficiency relies on the ability of carboxylate groups in SHFS to bind 2 ions simultaneously; a consequence of the width of the Nav pore and flexibility of the E177 side-chains.
Figure 2:
Conduction of sodium in NavAb. 2D free energy projection for Na+ ions when 3 ions occupy the filter, with Na+-Na+ corresponding to the center of mass position of the two upper ions. The lowest free energy pathway is via knock-on conduction, indicated with a solid red curve, with pass-by conduction indicated with a dashed curve. Illustrations are schematic and based on free energy results presented in (Boiteux et al., 2014a), with contouring at 0.5 kcal/mol.
In contrast, K+ ions are unable to form the stable multi-carboxylate/multi-ion complexes, reducing the occupancy of the NavAb SF to 2 ions. While the channel may still conduct K+ ions with this reduced occupancy, it has been suggested that it may do so at a reduced rate due to slight increase in permeation barrier (Boiteux et al., 2014a). Thus, the existence of multi-ion/multi-carboxylate complexes, as seen for Na+ in Fig.2, appears to be central to the ion selectivity in the bacterial Nav channel. Interestingly, only 2 of the 4 glutamates side chains in the bacterial EEEE ring are involved in this high field strength binding, which happens to be the number available in the mammalian signature DEKA sequence, suggesting the possibility of a common ion binding complex. However, as we shall see below, new simulation studies suggest that high field strength binding is not limited to just the carboxylates from the DEKA signature in mammalian channels.
Ion permeation and selectivity in mammalian Nav channels
Computational investigations into ion permeation in mammalian sodium channels have been impeded by a lack of high-quality structural data. The structures available of eukaryotic channels, the recently solved NavPaS and EeNav1.4, suffer from lower resolution, with key charged side chains in the SF unresolved in NavPaS, which is also lacking one of the important residues in the vestibular EEDD ring seen in all mammalian channels (DIII-Asp). It does, however, offer a useful reference for validating sodium channel models, such as those based on the well-defined bacterial structures. These channels offer a stable structural scaffold for supporting the core functional regions of the protein; notably the SF and vestibular sequences. This includes models where the mammalian ring DEKA has been substituted into the NavRh SF (Li et al., 2016; Xia et al., 2013), while other models have threaded the amino acids from the entire PD onto the backbone of NavAb (Mahdavi and Kuyucak, 2015). These studies have primarily focused on shorter simulations, but have suggested a multi-ion Na+ knock-on mechanism. They have also suggested a role for the DEKA lysine in physically occluding the pore, finding that only when the Lys is constrained to EIV is the SF permeable to ions (Li et al., 2016). They have also hypothesized that this constrained Lys can repel Na+ to promote stronger interactions with carboxylates, leading to selective binding (Li et al., 2016).
New studies of a model human Nav1.2 channel have demonstrated a more direct role for the lysine on longer timescales (Flood et al., 2018). In this model, the SF and vestibular sequence (including both DEKA and EEDD rings) were grafted onto the bacterial NavRh, chosen because it presents a vestibular EEEE ring, and has a shorter SF/P2 sequence, better matching that of Nav1.2 (Fig.1 inset), and a stable model for several μs of simulation (Flood et al., 2018). Here the SF and vestibule residues were seen to relax in an asymmetric fashion around the pore axis, similar to their distribution in the NavPaS cryo-EM structure (Shen et al., 2017), while large rearrangements of the side chains reveal flexibility reminiscent of bacterial channels (Boiteux et al., 2014a; Chakrabarti et al., 2013). Side chains from both inner and vestibular rings coordinate ions, leading to a wide-spanning binding site, unlike the more localized SHFS seen in NavAb.
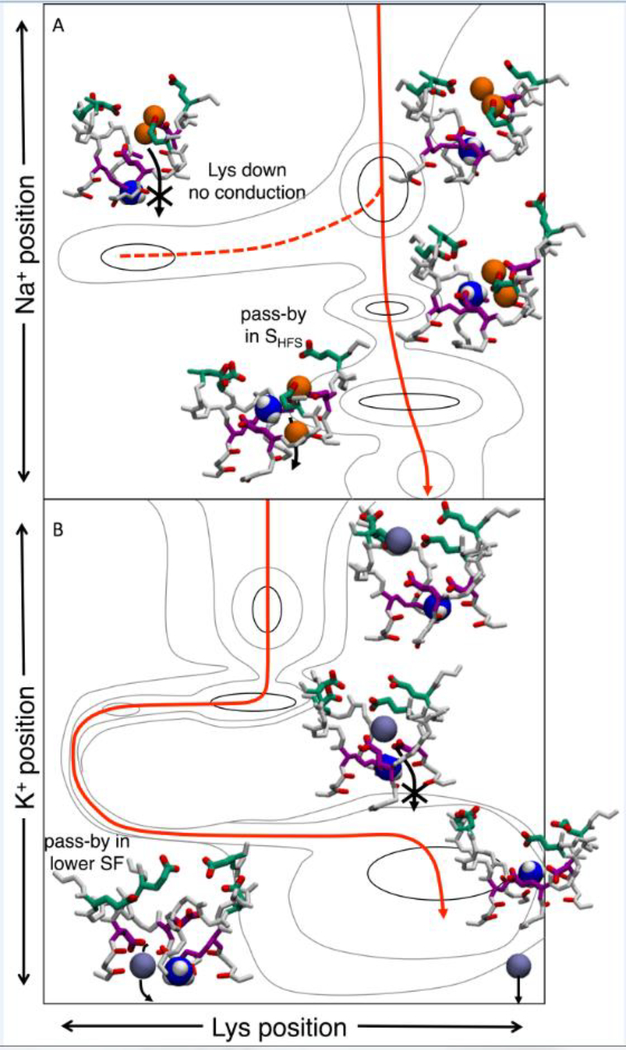
The presence of a positively charged lysine in the inner DEKA ring seems an odd foundation for the selective permeation of cations, yet its contribution to ion selectivity has long been established (Favre et al., 1996; Lipkind and Fozzard, 2008; Schlief et al., 1996; Sun et al., 1997). Microsecond-scale simulations (Flood et al., 2018) have revealed that Lys participates by contributing its ammonium group as an additional ion (n.b. Nav channels conduct ammonium nearly as well as Na+, with relative permeability PNH4+/PNa+=0.16 (Hille, 1971)). Formation of a Na+-Lys+/multi-carboxylate complex allows Na+ to permeate the SF via a pass-by mechanism, with low energy barrier (Fig.3a). In contrast, K+ is unable to form this multi-ion complex and must wait for the Lys to move downward, unblocking the pore (Fig.3b). Without the help of the attractive fields established by the carboxylates in SHFS, it experiences a higher energy barrier.
Figure 3:
Ion conduction in model human Nav1.2 for Na+ (A) and K+ (B). Insets show configurations during conduction. A) Two ion occupancy dominates for Na+. As the ions enter the SF they bind to carboxylates and create multi-ion/multi carboxylate complexes. Lysine creates a stable state with Na+ in the SHFS to allow pass-by conduction (solid red curve). When lysine is in the lower pore no conduction is observed (dashed curve). B) The K+ ion singly binds to the carboxylates and waits for lysine to undergo structural isomerization to pass lysine in the lower SF (solid red curve). Illustrations are schematic and based on free energy maps presented in (Flood et al., 2018), with contouring at 0.5 kcal/mol.
Experimentally, investigation of the concentration dependence on conduction in mammalian Nav channels has previously suggested the existence of a permeation mechanism involving multiple binding sites within the channel (Begenisich and Cahalan, 1980a, b; French et al., 1994; Ravindran et al., 1992). Some of these investigations have shown a saturating concentration dependence suggestive of a single-ion mechanism (Begenisich and Cahalan, 1980a, b; French et al., 1994), whereas when a wide range of concentrations are investigated, a complex relationship suggestive of a multi-ion mechanism has been observed (Ravindran et al., 1992). Furthermore, investigations of bionic mixtures have shown anomalous mole fraction effects in bacterial Navs (Finol-Urdaneta et al., 2014) whereas mammalian Navs appear to lack this effect (Ravindran et al., 1992). Anomalous mole fraction effects have previously been explained by a multi-ion permeation mechanism in a single file channel (Eisenman et al., 1986), but may also arise from preferential and localized ion binding (Gillespie and Boda, 2008; Gillespie et al., 2009; Nonner et al., 1998). Single file conduction, as seen in potassium channels, is unlikely in Nav channels considering their width (Hille, 1971) and flexibility (Tsushima et al., 1997b), consistent with the decoupling of ion and water fluxes seen in recent simulations (Ulmschneider et al., 2013). The lack of a single file multi-ion mechanism, and the possibility of distinct and largely independent pathways for conduction of different ion species, could conceivably conceal such ion cooperativity from anomalous mole fraction experiments.
Ion permeation and selectivity in acid sensing ion channels
Investigation of ion selectivity in an unrelated Na+-selective channel of the acid sensing family (ASIC1a; Fig.4) bore surprisingly similar observations to the Nav channels, despite vastly different architectures. The ASIC1a channel is a homo-trimeric protein, with a large extracellular domain and a shorter PD formed by 2 trans-membrane helices (Fig.4a), thought to enable selectivity at the level of a short inner coil (GAS belt) (Baconguis et al., 2014; Kellenberger and Schild, 2002), based on a size-selection principle involving partial dehydration of the ions, despite the presumption that the nature of the ligands (carbonyls) would generally be expected to favor K+ over Na+. While the most recent x-ray structure of ASIC seems to support this hypothesis with its narrow GAS belt helix swap (Baconguis et al., 2014), it has never been verified experimentally (Lynagh et al., 2017). Instead, combined computational and experimental studies have recently shown that selectivity is rather linked to 2 rings of carboxylates (from residues E18’ and D21’) at the intracellular end of the pore (Lynagh et al., 2017).
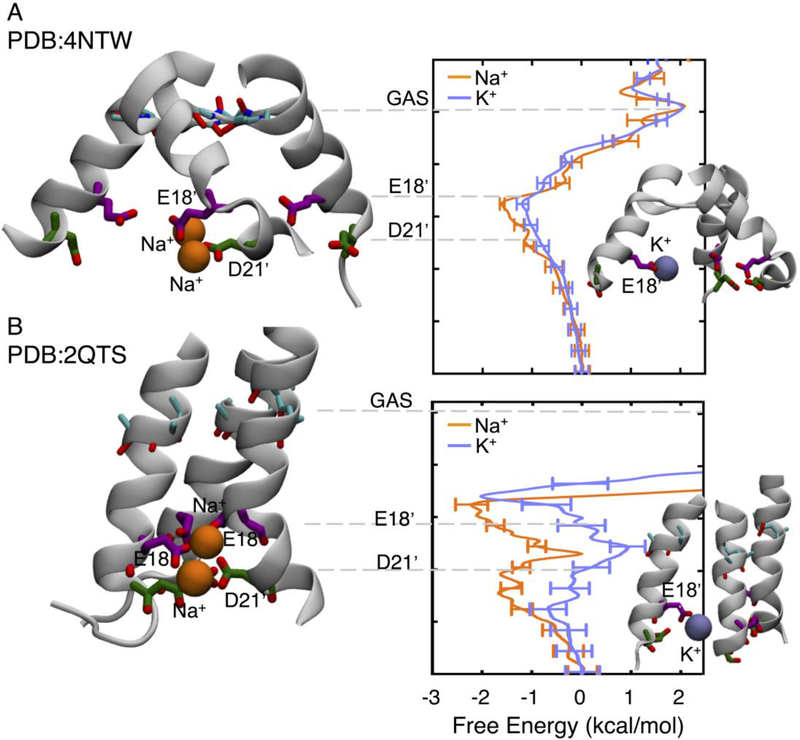
Figure 4:
Free energy profile for Na+ (orange) and K+ (purple) in ASIC PDB:4NTW (A) and PDB:2QTS (B), based on data presented in (Lynagh et al., 2017). Insets show Na+ and K+ in key sites and labels indicate the residues that the respective ions are binding to.
Simulations of ASIC1a in the presence of Na+ and K+ have shown that both species encounter similar free energy barriers at the level of the GAS belt. Surprisingly there is no thermodynamic difference between the ions in this belt, owing to partial compensation of dehydration losses through interactions with backbone carbonyls (Lynagh et al., 2017). However, Na+ displays a larger affinity for two rings of residues found at the intracellular entrance of the pore domain, E18’ and D21’, also shown to affect selectivity by mutagenesis (Lynagh et al., 2017). In this region there exists a preference for Na+ over K+ by of the order of 1 kcal/mol in the proposed open structure (Lynagh et al., 2017) (PDB:4NTW; Fig.4a), that is increased dramatically in a structure with narrower lower pore (PDB:2QTS; Fig.4b) that can form Nav-like multi-ion/multi-carboxylate complexes across subunits that promote Na+ conduction over K+.
Conclusion
Recent structural determination using x-ray crystallography and cryo-EM for prokaryotic (Bagneris et al., 2014; McCusker et al., 2012; Payandeh et al., 2012; Payandeh et al., 2011; Shaya et al., 2014; Zhang et al., 2012) and eukaryotic (Baconguis et al., 2014; Jasti et al., 2007; Shen et al., 2017) Na+ channels has enabled atomistic simulations that have shed light on channel function, and have helped us learn the fundamentals controlling selective Na+ transport in nature. Simulations of disparate proteins have been seen to exhibit common underlying foundations of selective binding of Na+ ions, centered on the preferential formation of multi-ion/multi-carboxylate complexes.
Notwithstanding apparent divergences in structures, bacterial channels, with their inner EEEE ring, and mammalian channels with their double DEKA and EEDD asymmetric rings, these channels appear to rely on a similar mechanism, involving binding to a flexible carboxylate-rich site to achieve discriminatory ion binding. While the lysine in the mammalian Nav SF appears to differentiate it from the bacterial case, new studies suggest formation of an ion-lysine complex enables rapid Na+ conduction. Overall, these Na+-selective multi-ion/multi-carboxylate complexes appear to be shared by a range of ion transport proteins, including in evolutionally unrelated acid-sensing ion channels, and perhaps broader afield.
Acknowledgments
This work was supported by National Institutes of Health (U01- HL126273-01/02), National Health and Medical Research Council (APP1104259 and APP1141974), Australian Research Council (DP170101732), DE Shaw Anton (PSCA17045P; NRBSC, NIH RC2GM093307), National Computational Initiative (dd7), and the Medical Advances Without Animals Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baconguis I, Bohlen CJ, Goehring A, Julius D, and Gouaux E (2014). X-ray structure of acid-sensing ion channel 1-snake toxin complex reveals open state of a Na+-selective channel. Cell 156, 717–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagneris C, DeCaen PG, Naylor CE, Pryde DC, Nobeli I, Clapham DE, and Wallace BA (2014). Prokaryotic NavMs channel as a structural and functional model for eukaryotic sodium channel antagonism. Proc Natl Acad Sci USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balser JR, Nuss HB, Chiamvimonvat N, Perez-Garcia MT, Marban E, and Tomaselli GF (1996). External pore residue mediates slow inactivation in μ1 rat skeletal muscle sodium channels. J Physiol 494 (Pt 2), 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AF, Carnevale V, Klein ML, Eckenhoff RG, and Covarrubias M (2014). Modulation of a voltage-gated Na+ channel by sevoflurane involves multiple sites and distinct mechanisms. Proc Natl Acad Sci USA 111, 6726–6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begenisich T, and Cahalan M (1980a). Sodium channel permeation in squid axons. I: Reversal potential experiments. The Journal of physiology 307, 217–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begenisich T, and Cahalan M (1980b). Sodium channel permeation in squid axons. II: Non-independence and current-voltage relations. The Journal of physiology 307, 243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz I, Beck W, Kraas W, Stoll D, Jung G, and Kohlhardt M (1997). Two types of modified cardiac Na+ channels after cytosolic interventions at the α-subunit capable of removing Na+ inactivation. Eur Biophys J 25, 189–200. [DOI] [PubMed] [Google Scholar]
- Boiteux C, and Allen TW (2016). Understanding Sodium Channel Function and Modulation Using Atomistic Simulations of Bacterial Channel Structures. Curr Top Membr 78, 145–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiteux C, Vorobyov I, and Allen TW (2014a). Ion conduction and conformational flexibility of a bacterial voltage-gated sodium channel. Proc Natl Acad Sci USA 111, 3454–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiteux C, Vorobyov I, French RJ, French C, Yarov-Yarovoy V, and Allen TW (2014b). Local anesthetic and antiepileptic drug access and binding to a bacterial voltage-gated sodium channel. Proc Natl Acad Sci U S A 111, 13057–13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni M, Zhang Z-S, Neplioueva V, Starmer CF, and Grant AO (2005). Slow sodium channel inactivation and use-dependent block modulated by the same domain IV S6 residue. J Membr Biol 207, 107–117. [DOI] [PubMed] [Google Scholar]
- Carnevale V, Treptow W, and Klein ML (2011). Sodium Ion Binding Sites and Hydration in the Lumen of a Bacterial Ion Channel from Molecular Dynamics Simulations. Journal of Physical Chemistry Letters 2, 2504–2508. [Google Scholar]
- Chakrabarti N, Ing C, Payandeh J, Zheng N, Catterall WA, and Pomes R (2013). Catalysis of Na+ permeation in the bacterial sodium channel NavAb. Proc Natl Acad Sci U S A 110, 11331–11336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Ong BH, Kambouris NG, Marban E, Tomaselli GF, and Balser JR (2000). Lidocaine induces a slow inactivated state in rat skeletal muscle sodium channels. J Physiol 524 Pt 1, 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiamvimonvat N, Perez-Garcia MT, Ranjan R, Marban E, and Tomaselli GF (1996a). Depth asymmetries of the pore-lining segments of the Na+ channel revealed by cysteine mutagenesis. Neuron 16, 1037–1047. [DOI] [PubMed] [Google Scholar]
- Chiamvimonvat N, Perez-Garcia MT, Tomaselli GF, and Marban E (1996b). Control of ion flux and selectivity by negatively charged residues in the outer mouth of rat sodium channels. J Physiol 491 (Pt 1), 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corry B, and Thomas M (2012). Mechanism of ion permeation and selectivity in a voltage gated sodium channel. J Am Chem Soc 134, 1840–1846. [DOI] [PubMed] [Google Scholar]
- DeCaen PG, Takahashi Y, Krulwich TA, Ito M, and Clapham DE (2014). Ionic selectivity and thermal adaptations within the voltage-gated sodium channel family of alkaliphilic Bacillus. Elife 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman G (1962). Cation selective glass electrodes and their mode of operation. Biophys J 2, 259–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman G, Latorre R, and Miller C (1986). Multi-ion conduction and selectivity in the high-conductance Ca2+-activated K+ channel from skeletal muscle. Biophys J 50, 1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerkmann RK, Riazanski V, Elger CE, Urban BW, and Beck H (2001). Slow recovery from inactivation regulates the availability of voltage-dependent Na+ channels in hippocampal granule cells, hilar neurons and basket cells. The Journal of Physiology 532, 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre I, Moczydlowski E, and Schild L (1996). On the structural basis for ionic selectivity among Na+, K+, and Ca2+ in the voltage-gated sodium channel. Biophysical Journal 71, 3110–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finol-Urdaneta RK, Wang Y, Al-Sabi A, Zhao C, Noskov SY, and French RJ (2014). Sodium channel selectivity and conduction: prokaryotes have devised their own molecular strategy. J Gen Physiol 143, 157–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood E, Boiteux C, and Allen TW (2018). Selective ion permeation involves complexation with carboxylates and lysine in a human sodium channel. Submitted to PLOS Computational Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozzard HA, Lee PJ, and Lipkind GM (2005). Mechanism of local anesthetic drug action on voltage-gated sodium channels. Curr Pharm Des 11, 2671–2686. [DOI] [PubMed] [Google Scholar]
- French R, Worley J, Wonderlin W, Kularatna AS, and Krueger B (1994). Ion permeation, divalent ion block, and chemical modification of single sodium channels. Description by single-and double-occupancy rate-theory models. The Journal of general physiology 103, 447–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furini S, and Domene C (2012). On conduction in a bacterial sodium channel. PLoS Comput Biol 8, e1002476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furini S, and Domene C (2018). Ion-triggered selectivity in bacterial sodium channels. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D, and Boda D (2008). The anomalous mole fraction effect in calcium channels: a measure of preferential selectivity. Biophysical Journal 95, 2658–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D, Giri J, and Fill M (2009). Reinterpreting the anomalous mole fraction effect: the ryanodine receptor case study. Biophysical journal 97, 2212–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin AL (1999). Diversity of mammalian voltage-gated sodium channels. Ann N Y Acad Sci 868, 38–50. [DOI] [PubMed] [Google Scholar]
- Guardiani C, Rodger PM, Fedorenko OA, Roberts SK, and Khovanov IA (2017). Sodium Binding Sites and Permeation Mechanism in the NaChBac Channel: A Molecular Dynamics Study. J Chem Theory Comput 13, 1389–1400. [DOI] [PubMed] [Google Scholar]
- Hilber K, Sandtner W, Kudlacek O, Schreiner B, Glaaser I, Schutz W, Fozzard HA, Dudley SC, and Todt H (2002). Interaction between fast and ultra-slow inactivation in the voltage-gated sodium channel. Does the inactivation gate stabilize the channel structure? J Biol Chem 277, 37105–37115. [DOI] [PubMed] [Google Scholar]
- Hille B (1971). The permeability of the sodium channel to organic cations in myelinated nerve. The Journal of general physiology 58, 599–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B (2001). Ionic channels of excitable membranes, 3rd edn (Sunderland, Sinauer; ). [Google Scholar]
- Ito M, Xu H, Guffanti AA, Wei Y, Zvi L, Clapham DE, and Krulwich TA (2004). The voltage-gated Na+ channel NavBP has a role in motility, chemotaxis, and pH homeostasis of an alkaliphilic Bacillus. Proc Natl Acad Sci U S A 101, 10566–10571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasti J, Furukawa H, Gonzales EB, and Gouaux E (2007). Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature 449, 316–323. [DOI] [PubMed] [Google Scholar]
- Kambouris NG, Hastings LA, Stepanovic S, Marban E, Tomaselli GF, and Balser JR (1998). Mechanistic link between lidocaine block and inactivation probed by outer pore mutations in the rat μ1 skeletal muscle sodium channel. J Physiol 512 (Pt 3), 693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke S, Timin EN, and Stary-Weinzinger A (2014). Different inward and outward conduction mechanisms in NaVMs suggested by molecular dynamics simulations. PLoS Comput Biol 10, e1003746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S, Scheuer T, and Catterall WA (1996). Movement of the Na+ Channel Inactivation Gate during Inactivation. Journal of Biological Chemistry 271, 30971–30979. [DOI] [PubMed] [Google Scholar]
- Kellenberger S, and Schild L (2002). Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82, 735–767. [DOI] [PubMed] [Google Scholar]
- Khan A, Romantseva L, Lam A, Lipkind G, and Fozzard HA (2002). Role of outer ring carboxylates of the rat skeletal muscle sodium channel pore in proton block. J Physiol 543, 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koishi R, Xu H, Ren D, Navarro B, Spiller BW, Shi Q, and Clapham DE (2004). A superfamily of voltage-gated sodium channels in bacteria. J Biol Chem 279, 9532–9538. [DOI] [PubMed] [Google Scholar]
- Kyle DJ, and Ilyin VI (2007). Sodium channel blockers. J Med Chem 50, 2583–2588. [DOI] [PubMed] [Google Scholar]
- Lee S, Goodchild SJ, and Ahern CA (2012). Local anesthetic inhibition of a bacterial sodium channel. J Gen Physiol 139, 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu H, Xia M, and Gong H (2016). Lysine and the Na+/K+ Selectivity in Mammalian Voltage-Gated Sodium Channels. PLoS One 11, e0162413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkind GM, and Fozzard HA (2008). Voltage-gated Na channel selectivity: the role of the conserved domain III lysine residue. J Gen Physiol 131, 523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SB, Tao X, Campbell EB, and MacKinnon R (2007). Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature 450, 376–382. [DOI] [PubMed] [Google Scholar]
- Lynagh T, Flood E, Boiteux C, Wulf M, Komnatnyy VV, Colding JM, Allen TW, and Pless SA (2017). A selectivity filter at the intracellular end of the acid-sensing ion channel pore. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdavi S, and Kuyucak S (2015). Mechanism of ion permeation in mammalian voltage-gated sodium channels. PloS one 10, e0133000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, and Corry B (2014). Locating the route of entry and binding sites of benzocaine and phenytoin in a bacterial voltage gated sodium channel. PLoS Comput Biol 10, e1003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker EC, Bagnéris C, Naylor CE, Cole AR, D’Avanzo N, Nichols CG, and Wallace BA (2012). Structure of a bacterial voltage-gated sodium channel pore reveals mechanisms of opening and closing. Nature Comm 3, 1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor CE, Bagneris C, DeCaen PG, Sula A, Scaglione A, Clapham DE, and Wallace BA (2016). Molecular basis of ion permeability in a voltage-gated sodium channel. EMBO J 35, 820–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M, Suzuki H, Numa S, and Stuhmer W (1989). A single point mutation confers tetrodotoxin and saxitoxin insensitivity on the sodium channel II. FEBS Lett 259, 213–216. [DOI] [PubMed] [Google Scholar]
- Nonner W, Chen DP, and Eisenberg B (1998). Anomalous mole fraction effect, electrostatics, and binding in ionic channels. Biophysical Journal 74, 2327–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong B-H, Tomaselli GF, and Balser JR (2000). A Structural Rearrangement in the Sodium Channel Pore Linked to Slow Inactivation and Use Dependence. The Journal of General Physiology 116, 653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payandeh J, Gamal El-Din TM, Scheuer T, Zheng N, and Catterall WA (2012). Crystal structure of a voltage-gated sodium channel in two potentially inactivated states. Nature 486, 135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payandeh J, Scheuer T, Zheng N, and Catterall WA (2011). The crystal structure of a voltage-gated sodium channel. Nature 475, 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzotti JL, Fozzard HA, Lipkind GM, and Dudley SC Jr. (1998). Differences in saxitoxin and tetrodotoxin binding revealed by mutagenesis of the Na+ channel outer vestibule. Biophys J 75, 2647–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H, Shen R, and Guo W (2012). Ion solvation and structural stability in a sodium channel investigated by molecular dynamics calculations. Biochim Biophys Acta 1818, 2529–2535. [DOI] [PubMed] [Google Scholar]
- Raju SG, Barber AF, LeBard DN, Klein ML, and Carnevale V (2013). Exploring volatile general anesthetic binding to a closed membrane-bound bacterial voltage-gated sodium channel via computation. PLoS Comput Biol 9, e1003090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran A, Kwiecinski H, Alvarez O, Eisenman G, and Moczydlowski E (1992). Modeling ion permeation through batrachotoxin-modified Na+ channels from rat skeletal muscle with a multi-ion pore. Biophysical journal 61, 494–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Navarro B, Xu H, Yue L, Shi Q, and Clapham DE (2001). A prokaryotic voltage-gated sodium channel. Science 294, 2372–2375. [DOI] [PubMed] [Google Scholar]
- Rohl CA, Boeckman FA, Baker C, Scheuer T, Catterall WA, and Klevit RE (1999). Solution structure of the sodium channel inactivation gate. Biochemistry 38, 855–861. [DOI] [PubMed] [Google Scholar]
- Roux B, Berneche S, Egwolf B, Lev B, Noskov SY, Rowley CN, and Yu H (2011). Ion selectivity in channels and transporters. J Gen Physiol 137, 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlief T, Schönherr R, Imoto K, and Heinemann SH (1996). Pore properties of rat brain II sodium channels mutated in the selectivity filter domain. European Biophysics Journal 25, 75–91. [DOI] [PubMed] [Google Scholar]
- Shaya D, Findeisen F, Abderemane-Ali F, Arrigoni C, Wong S, Nurva SR, Loussouarn G, and Minor DL Jr. (2014). Structure of a prokaryotic sodium channel pore reveals essential gating elements and an outer ion binding site common to eukaryotic channels. J Mol Biol 426, 467–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets PL, Jarecki BW, and Cummins TR (2011). Lidocaine reduces the transition to slow inactivation in Nav1.7 voltage-gated sodium channels. Br J Pharmacol 164, 719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Zhou Q, Pan X, Li Z, Wu J, and Yan N (2017). Structure of a eukaryotic voltage-gated sodium channel at near-atomic resolution. Science 355. [DOI] [PubMed] [Google Scholar]
- Stock L, Delemotte L, Carnevale V, Treptow W, and Klein ML (2013). Conduction in a biological sodium selective channel. J Phys Chem B 117, 3782–3789. [DOI] [PubMed] [Google Scholar]
- Sula A, Booker J, Ng LC, Naylor CE, DeCaen PG, and Wallace BA (2017). The complete structure of an activated open sodium channel. Nat Commun 8, 14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YM, Favre I, Schild L, and Moczydlowski E (1997). On the structural basis for size-selective permeation of organic cations through the voltage-gated sodium channel. Effect of alanine mutations at the DEKA locus on selectivity, inhibition by Ca2+ and H+, and molecular sieving. J Gen Physiol 110, 693–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terlau H, Heinemann SH, Stuhmer W, Pusch M, Conti F, Imoto K, and Numa S (1991). Mapping the site of block by tetrodotoxin and saxitoxin of sodium channel II. FEBS Lett 293, 93–96. [DOI] [PubMed] [Google Scholar]
- Tsushima RG, Li RA, and Backx PH (1997a). Altered ionic selectivity of the sodium channel revealed by cysteine mutations within the pore. Journal of General Physiology 109, 463–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsushima RG, Li RA, and Backx PH (1997b). P-loop flexibility in Na+ channel pores revealed by single-and double-cysteine replacements. The Journal of general physiology 110, 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbricht W (2005). Sodium channel inactivation: molecular determinants and modulation. Physiol Rev 85, 1271–1301. [DOI] [PubMed] [Google Scholar]
- Ulmschneider MB, Bagneris C, McCusker EC, DeCaen PG, Delling M, Clapham DE, Ulmschneider JP, and Wallace BA (2013). Molecular dynamics of ion transport through the open conformation of a bacterial voltage-gated sodium channel. P Natl Acad Sci USA 110, 6364–6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev PM, Scheuer T, and Catterall WA (1988). Identification of an intracellular peptide segment involved in sodium channel inactivation. Science 241, 1658–1661. [DOI] [PubMed] [Google Scholar]
- Vedantham V, and Cannon SC (2000). Rapid and slow voltage-dependent conformational changes in segment IVS6 of voltage-gated Na+ channels. Biophys J 78, 2943–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SY, and Wang GK (1997). A mutation in segment I-S6 alters slow inactivation of sodium channels. Biophys J 72, 1633–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JW, Patton DE, Scheuer T, Wang Y, Goldin AL, and Catterall WA (1992). A cluster of hydrophobic amino acid residues required for fast Na+ channel inactivation. Proc Natl Acad Sci U S A 89, 10910–10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M, Liu H, Li Y, Yan N, and Gong H (2013). The mechanism of Na+/K+ selectivity in mammalian voltage-gated sodium channels based on molecular dynamics simulation. Biophys J 104, 2401–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Zhou Q, Wang L, Wu J, Zhao Y, Huang G, Peng W, Shen H, Lei J, and Yan N (2017). Structure of the Nav1.4-beta1 Complex from Electric Eel. Cell 170, 470–482 e411 [DOI] [PubMed] [Google Scholar]
- Yarov-Yarovoy V, DeCaen PG, Westenbroek RE, Pan C-Y, Scheuer T, Baker D, and Catterall WA (2012). Structural basis for gating charge movement in the voltage sensor of a sodium channel. Proc Natl Acad Sci USA 109, E93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L, Navarro B, Ren D, Ramos A, and Clapham DE (2002). The cation selectivity filter of the bacterial sodium channel, NaChBac. J Gen Physiol 120, 845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrabi T, Cervenka R, Sandtner W, Lukacs P, Koenig X, Hilber K, Mille M, Lipkind GM, Fozzard HA, and Todt H (2010). A molecular switch between the outer and the inner vestibules of the voltage-gated Na+ channel. J Biol Chem 285, 39458–39470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ren W, DeCaen P, Yan C, Tao X, Tang L, Wang J, Hasegawa K, Kumasaka T, and He J (2012). Crystal structure of an orthologue of the NaChBac voltage-gated sodium channel. Nature 486, 130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Xia M, Li Y, Liu H, Jiang X, Ren W, Wu J, DeCaen P, Yu F, Huang S, et al. (2013). Analysis of the selectivity filter of the voltage-gated sodium channel NavRh. Cell Res 23, 409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Xu Y, Dong PH, Sharma D, and Chiamvimonvat N (2003). A negatively charged residue in the outer mouth of rat sodium channel determines the gating kinetics of the channel. Am J Physiol Cell Physiol 284, C1247–1254. [DOI] [PubMed] [Google Scholar]
- Zuliani V, Patel MK, Fantini M, and Rivara M (2009). Recent advances in the medicinal chemistry of sodium channel blockers and their therapeutic potential. Curr Top Med Chem 9, 396–415. [DOI] [PubMed] [Google Scholar]