Abstract
Histopathological changes associated with dilated cardiomyopathy (CMD) are frequently nonspecific and often only present in the terminal stage of the disease. The study followed the histopathological and morphometric quantification of fibrosis and nuclear pleomorphism in CMD. We analyzed left ventricle myocardial fragments harvested during autopsy, from 35 cases with clinical diagnosis of CMD and 5 cases of normal myocardium. Fibrosis was present in all CMD cases, with higher values compared with control cases. Nuclear pleomorphism was identified in 18 cases (45%), two of the analyzed parameters, respectively the ratio of nuclear diameters and roundness of nucleus, revealing significant differences in CMD compared to the control cases. Myocardial fibrosis present in all cases of CMD represents a major feature of the disease. The nuclear pleomorphism due to the nuclei change in diameters and size was more pronounced in the vicinity of fibrosis areas, possibly related to this alteration.
Keywords: Dilated cardiomyopathy, fibrosis, nuclear pleomorphism
Introduction
The histopathological characteristics of dilatative cardiomyopathy (CMD) are nonspecific, the histopathological diagnosis being an exclusion one [1].
In CMD were observed changes in both compartments of myocardocytes and extracellular matrix.
The histopathological characteristics identified in various studies are present only in the myocardium of patients in the terminal stage of the disease [1,2,3,4,5,6,7].
Histopathological aspects associated with CMD are often nonspecific and often not all present. Therefore, endomyocardial biopsy has limited utility in these situations, the different aspects being related only partial to the clinical evolution [8,9,10,11].
However, some studies have suggested that quantitative microscopy can be useful by correlating the volume of myofibrils or the myocyte area with the contractile function and prognosis [12].
In order to characterize fibrosis and nuclear pleomorphism, the present study followed the histopathological and morphometric quantification of the fibrosis area and the evaluation of some morphometric parameters of myocardial nuclei from CMD.
Material and methods
This study included 35 patients diagnosed with CMD in Cardiology Clinic of Emergency Clinical County Hospital Craiova and 5 control cases represented by normal myocardial fragments.
The studied material was represented by myocardial fragments harvested during left ventricle autopsy, which were fixed in 10% buffered formalin and processed by classical paraffin embedding technique, followed by Hematoxylin-Eosin and Masson's trichrome staining (histoprocesor and histostainer BioOptica).
For the histopathological assessment of the fibrosis area and nuclear pleomorphism, we used the following scale for affected tissue: grade I≤10%, grade II-10-50%, grade III>50%.
The acquisition of images was done using Nikon 90i microscope equipped with 16MP resolution CMOS DS-Ri2 sensor (Elta 90, Bucharest, Romania).
Subsequently we followed the morphometric quantification of the fibrosis area as well as the nuclear pleomorphism for which we tested several morphometric indicators in order to look for indices that can precisely characterize the nuclear changes in the hearts of these patients.
To highlight the differences in nuclear morphology, on high resolution images (60x) we first separated the contours of all clearly visible nuclei of the myocardocytes by encircling them with a manual separation tool in the Image ProPlus AMS package.
The regions of interest thus defined were then quantified as area, perimeter, ratio of maximum and minimum diameters, and the roundness factor (perimeter 2/4×π×area).
The data was summed on each image, averaged per specimen, then average values were generated for each case, and as separate groups for the CMD cases and controls.
The values of the two groups were compared with a Student's t test.
All data are represented on graphs as average±standard deviation of the mean (SD).
Results
The study of the 40 cases included in study revealed the presence of fibrosis in 35 of the analyzed cases.
Fibrosis was present with a diffuse or focal pattern, with interstitial or perivascular topography.
In the case of interstitial fibrosis, we observed quite variable collagen in the intermuscular spaces, from a finely perimyocytes distribution to stretched scars with infiltrative limits.
The collagen has individually surrounded myocardial fibers that have gained a "ragged" appearance due to corrugated cell membranes.
In case of perivascular fibrosis, the collagen has accumulated in the adventitia of coronary arteries and intracardiac coronary arterioles, which had normal morphology, but in fibrous areas occasionally showed thickening intima (Fig.1).
Figure 1.
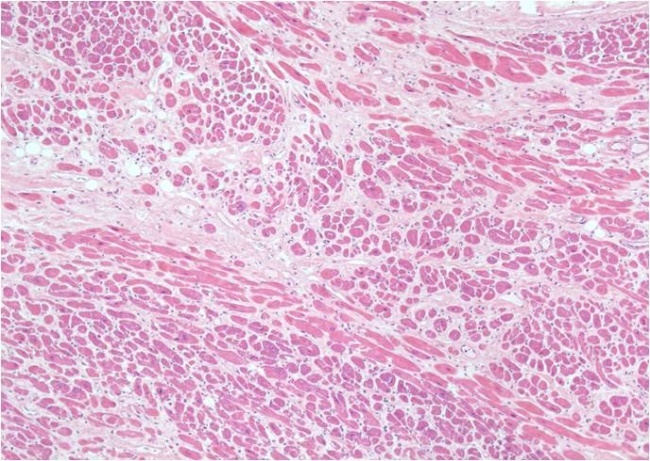
Interstitial fibrosis, HE staining, x40
Depending on the magnitude of the tissue alteration, we identified grade I fibrosis with focal areas in 8 cases (20%), grade II that had a zonal pattern in 21 cases (52.5%), or extended grade III fibrosis in 6 cases (15%) and absence in 5 cases (12.5%).
The presence of collagen fibrosis was then confirmed by Masson's trichrome staining, which allowed more accurate identification of the areas of interest and their morphometric quantification (Fig.2).
Figure 2.
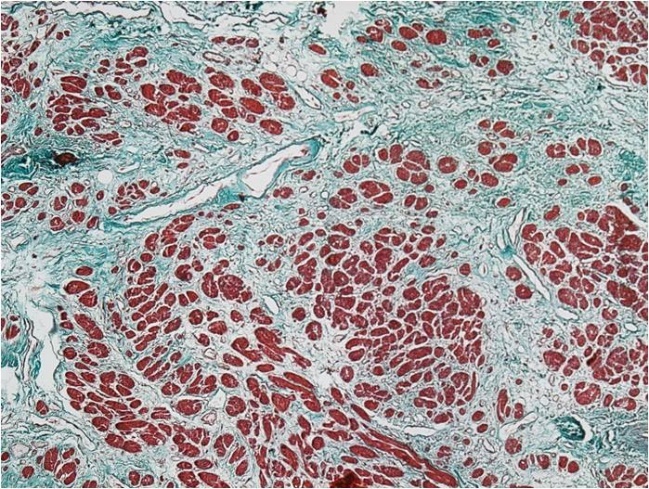
Perivascular and interstitial fibrosis, Masson's trichrome staining, x40
The area of the collagen trichrome component indicated higher values in CMD (318685.91±117482.55μ2) versus control (1616.0±2107.95 μ2) (Student's t test, p <0.05) (Table 1, Fig.3).
Table 1.
Morphometric features
|
Parameter |
Dilated cardiomyopathy group |
Control group |
p |
|
Fibrosis |
318685.91±117482.55µ2 |
1616.0±2107.95 µ2 |
0.05 |
|
Mean nuclear area |
542.28± 234.85 µ2 |
451.26± 13.67 µ2 |
0.31 |
|
Mean nuclear perimeter |
103.29±9.31µ |
93.42± 0.80 µ |
0.29 |
|
Mean nuclear diameter |
3.39±0.52 µ |
2.72±0.32 µ |
0.05 |
|
Mean nuclear roundness |
1.79±0.14 |
1.60±0.07 |
0.05 |
Figure 3.
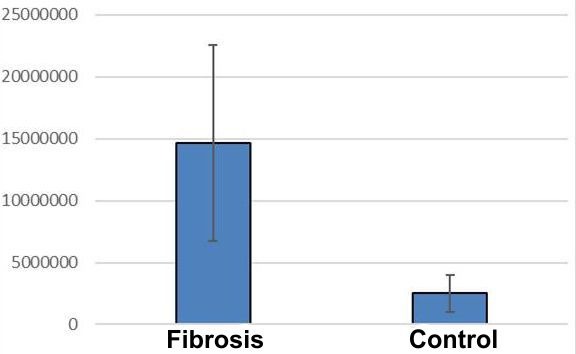
Distribution of fibrosis in dilated cardiomyopathy and control group, IOD values on trichrome staining
The analysis of nuclear pleomorphism revealed its presence in 18 cases (45%), out of which 11 cases of grade I (27.5%), 5 cases of grade II (12.5%), 2 cases of grade III (5%) and in 22 cases it was absent (55%).
The myocytes nuclei presented changes, both in shape and volume, but also regarding the tinctoriality.
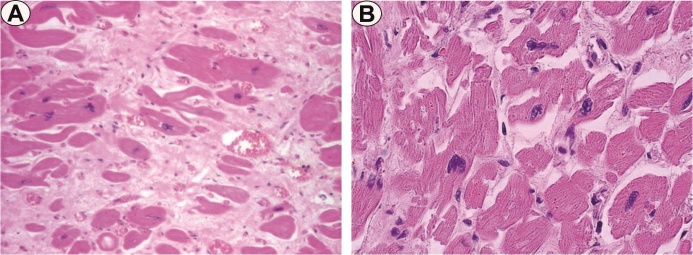
Their size and tinctoriality were variable, but in general the nuclei were large and hyperchromic, sometimes even with bizarre shapes, more pronounced in near the fibrosis areas (Fig. 4A-B).
Figure 4.
A. Pleomorphism and binucleate cells, HE staining, x200; B. Nuclear pleomorphism, HE staining, x200
We rarely noticed the existence of binucleate cells.
We also tested several morphometric indicators to look for indices that can characterize the nuclear alterations occurring in the CMD heart (Table 1).
The statistical analysis for the mean nuclear area revealed average values of 542.28±234.85µ2 in CMD, compared to 451.26±13.67µ2 in the normal control cases, with no significant differences between the CMD and controls nuclei (Student's t test, p=0.31).
For the nuclear perimeter we identified a mean values of 103.29±9.31μ in CMD, compared to 93.42±0.80μ in the normal control cords, also without statistical significant difference (Student's t test, p=0.29).
Investigation of nuclear diameters revealed average values of 3.39±0.52 in CMD cords, compared to 2.72±0.32μ control cases.
Unlike previous nuclear parameters, the ratio of nuclear diameters revealed significant differences between CMD nuclei in versus controls (Student's t test, p <0.05) (Fig.5).
Figure 5.
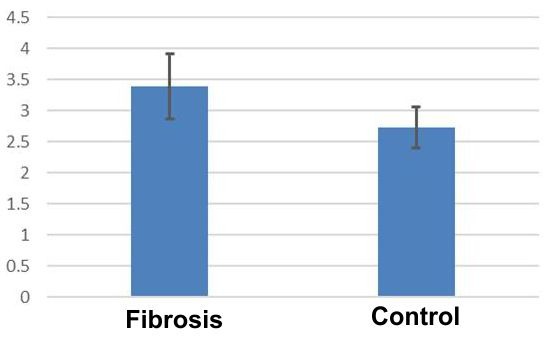
Distribution of diameters ratio in dilated cardiomyopathy related to the control group
Nuclear roundness analysis indicated mean values of 1.79±0.14 in CMD, compared to 1.60±0.07 in control cases.
Also, the mean roundness of the nuclei revealed significant differences between CMD cords versus the control cases (Student's t test, p<0.05) (Fig.6).
Figure 6.
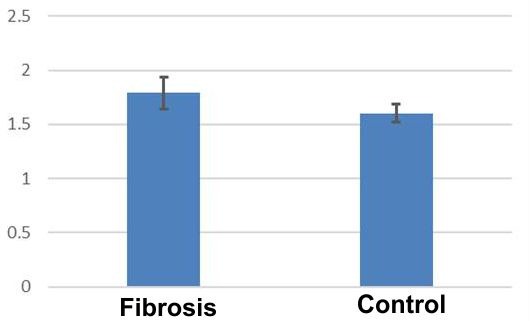
Distribution of average nuclear roundness in dilated cardiomyopathy in relation to the control group
Discussions
Morphometric studies that are concerned with the analysis of changes related to fibrosis and nucleus aspect in CMD are quite rare [13,14,15,16,17], probably due to controversy on the prognostic significance of results on endomyocardial biopsy fragments in these patients.
The most typical change in CMD is the development of interstitial and perivascular fibrosis of varying degrees [6,18].
Myocardial fibrosis is a major feature in CMD, and therefore proper involvement in the onset and progression of the disease is inevitable [19].
Wu KC et al. consider that focal fibrosis reflects the transition from the compensated form to the decompensated disease, associated with unfavorable prognosis [20].
In CMD, as in other cardiac diseases, fibrosis is an essential component of the myocardial remodeling process but also has some distinctive characteristics. Myocardial fibrosis in CMD is a secondary change due to degeneration and loss of myocytes, which occurs in the advanced stage of the disease in order to fill the remaining space due to the myocytes loss [3,21].
In this study, fibrosis was variable in all cases clinically diagnosed with CMD. The integrated optical density (IOD) of the collagen trichromatic component revealed higher values in dilated cardiomyopathy compared to control (Student's t test, p<0.05).
Johnson RA et al. reported fibrosis in CMD patients in only 28% of endometrial biopsy specimens and in 50% of autopsy sections [22].
In contrast, Sekiguchi M et al. found the presence of fibrosis in 100% of cases in both endomyocardial biopsy specimens and autopsy [23].
Following some studies, in the advanced stage of the disease, the collagen is increased by two to five times compared to controls [24,25].
In one study, myocardial scarring represented by segmental replacement by interstitial fibrosis occupied about 20% of each ventricle and indicated myocardial cell loss, but the number of myocytes was not reduced and the mean cell volume increased by two times in both ventricles [26].
Another study reported a percentage fibrosis area of 18.71% (7.76) in CMD patients compared to 13.39% (9.55) in normal [12].
Zorca M. et al. found out that the volume of interstitial tissue density was almost two times higher in patients with CMD who died 3 years after surgery compared to patients who survived 7 years after this moment (0.20±0.023 vs. 0.13±0.02, p<0,01) [27].
A comparative study of the collagen area indicated that it was higher in all CMR groups (7.36±1.09%) compared to control cases (1.12±0.18%, p <0.05) [17].
The nuclear pleomorphism is frequently described in CMD, characterized by dysmetric and dysmorphic nuclei [2,4,5,6,7,14].
Some authors have even suggested that nuclear abnormalities can constitute the main event in the pathogenesis of the disease [13].
However, the morphometric studies that deal with this aspect are rather rare [12,13,14,15].
Arbustini E. et al. analyzed the large nuclei with bizarre morphology, and found that interstitial fibrosis was similarly represented in cases with or without nuclear changes, but endocardial fibrosis had the highest values in cases of nuclear alteration [14].
In this study we found that the nuclei ratio of diameters and roundness indicated significant differences between CMD and control cases (Student's t test, p<0.05).
The various studies carried out have pursued different nuclear parameters. One such study identified two groups of patients-group A with oversized and bizarre nucleus (114 cases) and group B without these characteristics (52 cases) [14].
The authors reported that the width of the myocytes, the nuclear diameter and the nucleus/sarcoplasm ratio were significantly higher in group A patients, but without correlation with the clinical condition of the patients [14].
Also, the increase in nuclear area of myocytes was approximately 1.5 times in the dilated cardiomyopathy group compared to the control group [16].
In a study which analyzed 12 endomyometrial biopsy fragments from CMD patients, based on morphometric measurements, the authors reported statistically significant differences regarding the number of nuclei/mm2 of myocardial tissue, the diameter and the nuclear area in patients with dilatated cardiomyopathy compared to normal myocardium [12].
The authors reported the mean CMD myocytes nuclear area of88.27±33.12m2 versus 55.56±14.08m2 in controls, concluding that the mean nuclear area in the CMD group was increased by 59% compared to the control group (p <0.005).
In another study, the authors observed reduced nuclear density (18%, p<0.05), but the nuclear profile area was significantly increased (85%, p<0.001), the nucleus/cytoplasmic relation being thus modified [13].
Other authors reported similar values, the mean nuclear area being significantly higher in CMD (88.7±27.1µ2) compared to controls (45.0±9.2µ2) [15].
After analyzing the myocytes mean nuclear diameter in CMD, Di Somma S. et al. reported values of 10.45±1.98μ versus 8.35±1.06μl in controls, the aspect being statistically insignificant (p<0.01) [12].
In one study, the nuclear shape was significantly smaller (indicating a more irregular contour) in the heart of CMD patients (0.28±0.09) compared to controls (0.36±0.04) [15].
Conclusions
The myocardial fibrosis present in all cases of CMD is a major characteristic of CMD, its involvement in the onset and progression of the disease being inevitable.
The nuclear pleomorphism, variable as extension, was due to the alteration of nuclei diameter and shape, being more pronounced in the vicinity of fibrosis areas, possibly related to this change.
Reactive myocyte processes and architectural rearrangement of the myocardium appear to be determinant changes of ventricular remodeling in CMD.
References
- 1. Dilated Cardiomyopathy Pathology 2015. Available from: http://emedicine.medscape.com/ article/2017823-overview.
- 2.Rochael MC, Higuchi ML, Lopes EA. Bizarre nuclei of myocardial fibers in an infant with dilated cardiomyopathy. Light, electron-microscopic, and immunoperoxidase studies of a necropsy case. Bizarre nuclei of myocardial fibers in an infant with dilated cardiomyopathy. Light, electron-microscopic, and immunoperoxidase studies of a necropsy case. Am J Cardiovasc Pathol. 1990;3(4):321–324. [PubMed] [Google Scholar]
- 3.de Leeuw, Ruiter DJ, Balk AH, de Jonge, Melchers WJ, Galama GJ. Histopathologic findings in explantedheart tissue from patients with end-stage idiopathic dilated cardiomyopathy. Transpl Int. 2000;14(5):299–306. doi: 10.1007/s001470100339. [DOI] [PubMed] [Google Scholar]
- 4.Hughes SE. New insights into the pathology of inherited cardiomyopathy. Heart. 2005;91(2):257–264. doi: 10.1136/hrt.2004.040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basso C, Perazzolo MM, Thiene G. Myocardial clefts, crypts, or crevices: once again, you see only what you look for. Circ Cardiovasc Imaging. 2014;7(2):217–219. doi: 10.1161/CIRCIMAGING.114.001744. [DOI] [PubMed] [Google Scholar]
- 6. Hershberger RE , Morales A . In: Dilated Cardiomyopathy Overview . Pagon RA , Adam MP , Ardinger HH , et al., editors. Seattle : GeneReviews [Internet] ; 2017 . [Google Scholar]
- 7.Ryan TD, Gupta A, Gupta D, Goldenberg P, Taylor MD, Lorts A, Jefferies JL. Dilated cardiomyopathy in a 32-year-old woman with Russell-Silver syndrome. Cardiovasc Pathol. 2014;23(1):21–27. doi: 10.1016/j.carpath.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Perazzolo MM, De Lazzari, Rizzo S, Tona F, Tarantini G, Thiene G, Corrado D, Iliceto S, Basso C. The prognostic value of myocardial fibrosis in nonischemic dilated cardiomyopathy: a study by endomyocardial biopsy and cardiac magnetic resonance. Eur Heart J. 2013;34(1):P1867–P1867. [Google Scholar]
- 9.Broch K, Andreassen AK, Hopp E, Leren TP, Scott H, Müller F, Aakhus S, Gullestad L. Results of comprehensive diagnostic work-up in ‘idiopathic’ dilated cardiomyopathy. Open Heart. 2015;2(1):e000271–e000271. doi: 10.1136/openhrt-2015-000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu LA, Lapeyre AC, Cooper LT. Current role of endomyocardial biopsy in the management of dilated cardiomyopathy and myocarditis. Mayo Clin Proc. 2001;76(10):1030–1038. doi: 10.4065/76.10.1030. [DOI] [PubMed] [Google Scholar]
- 11.From AM, Maleszewski JJ, Rihal CS. Current status of endomyocardial biopsy. Mayo Clin Proc. 2011;86(11):1095–1102. doi: 10.4065/mcp.2011.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scholz D, Diener W, Schaper J. Altered nucleus/cytoplasm relationship and degenerative structural changes in human dilated cardiomyopathy. Cardioscience. 1994;5(2):127–138. [PubMed] [Google Scholar]
- 13.Di Somma, Marotta M, Salvatore G, Cudemo G, Cuda G, De Vivo, Di Benedetto, Ciaramella F, Caputo G, de Divitiis. Changes in myocardial cytoskeletal intermediate filaments and myocyte contractile dysfunction in dilated cardiomyopathy: an in vivo study in humans. Heart. 2000;84(6):659–667. doi: 10.1136/heart.84.6.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arbustini E, Gavazzi A, Pozzi R, Grasso M, Pucci A, Campana C, Graziano G, Martinetti M, Cuccia M, Salvaneschi L, et al. The morphologic spectrum of dilated cardiomyopathy and its relation to immune-response genes. Am J Cardiol. 1989;64(16):991–995. doi: 10.1016/0002-9149(89)90796-0. [DOI] [PubMed] [Google Scholar]
- 15.Rowan RA, Masek MA, Billingham ME. Ultrastructural morphometric analysis of endomyocardial biopsies. Idiopathic dilated cardiomyopathy, anthracycline cardiotoxicity, and normal myocardium. Am J Cardiovasc Pathol. 1988;2(2):137–144. [PubMed] [Google Scholar]
- 16.Yan SM, Finato N, Di Loreto, Beltrami CA. Nuclear size of myocardial cells in end-stage cardiomyopathies. Anal Quant Cytol Histol. 1999;21(2):174–180. [PubMed] [Google Scholar]
- 17.Sanderson JE, Olsen EG, Gatei D. Dilated cardiomyopathy and myocarditis in Kenya: an endomyocardial biopsy study. Int J Cardiol. 1993;41(2):157–163. doi: 10.1016/0167-5273(93)90156-b. [DOI] [PubMed] [Google Scholar]
- 18.Louzao-Martinez L, Vink A, Harakalova M, Asselbergs FW, Verhaar MC, Cheng C. Characteristic adaptations of the extracellular matrix in dilated cardiomyopathy. Int J Cardiol. 2016;220:634–646. doi: 10.1016/j.ijcard.2016.06.253. [DOI] [PubMed] [Google Scholar]
- 19.Wu KC, Weiss RG, Thiemann DR, Kitagawa K, Schmidt A, Dalal D, Lai S, Bluemke DA, Gerstenblith G, Marban E, Tomaselli GF, Lima JA. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51(25):2414–2421. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sheppard MN . Cardiac hypertrophy, heart failure and cardiomyopathy . In: XXXX XX , editor. ractical Cardiovascular Pathology . 2 . London : Hodder Arnold ; 2011 . pp. 133 – 192 . [Google Scholar]
- 21.Johnson RA, Palacios I. Dilated cardiomyopathies of the adult (second of two parts) N Engl J Med. 1982;307(18):1119–1126. doi: 10.1056/NEJM198210283071804. [DOI] [PubMed] [Google Scholar]
- 22.Sekiguchi M, Hiroe M, Morimoto S. On the standardization of histopathological diagnosis and semiquantitative assessment of the endo-myocardium obtained by endomyocardial biopsy. Bull Heart Inst Jpn. 1980;21:55–85. [Google Scholar]
- 23.Bishop JE, Greenbaum R, Gibson DG, Yacoub M, Laurent GJ. Enhanced deposition of predominantly type I collagen in myocardial disease. J Mol Cell Cardiol. 1990;22(10):1157–1165. doi: 10.1016/0022-2828(90)90079-h. [DOI] [PubMed] [Google Scholar]
- 24.Unverferth DV, Baker PB, Swift SE, Chaffee R, Fetters JK, Uretsky BF, Thompson ME, Leier CV. Extent of myocardial fibrosis and cellular hypertrophy in dilated cardiomyopathy. Am J Cardiol. 1986;57(10):816–820. doi: 10.1016/0002-9149(86)90620-x. [DOI] [PubMed] [Google Scholar]
- 25.Beltrami CA, Finato N, Rocco M, Feruglio GA, Puricelli C, Cigola E, Sonnenblick EH, Olivetti G, Anversa P. The cellular basis of dilated cardiomyopathy in humans. J Mol Cell Cardiol. 1995;27(1):291–305. doi: 10.1016/s0022-2828(08)80028-4. [DOI] [PubMed] [Google Scholar]
- 26.Zorc M, Vraspir-Porenta O, Zorc-Pleskovic R, Radovanović N, Petrovic D. Apoptosis of myocytes and proliferation markers as prognostic factors in end-stage dilated cardiomyopathy. Cardiovascular Pathol. 2003;12(1):36–39. doi: 10.1016/s1054-8807(02)00134-5. [DOI] [PubMed] [Google Scholar]
- 27.Soufen HN, Salemi VM, Aneas IM, Ramires FJ, Benício AM, Benvenuti LA, Krieger JE, Mady C. Collagen content, but not the ratios of collagen type III/I mRNAs, differs among hypertensive, alcoholic, and idiopathic dilated cardiomyopathy. Braz J Med Biol Res. 2008;41(12):1098–1104. doi: 10.1590/s0100-879x2008001200009. [DOI] [PubMed] [Google Scholar]