Abstract
Basal cell carcinoma (BCC) is the most common skin cancer, with an increasing incidence in Europe particularly in young individuals. Nodular basal cell carcinoma is the most common subtype and accounts for approximately 57.6-78.7% of all BCCs. We performed an observational, morphological study which involved 68 patients with the diagnosis of nodular BCC. The localization and diameter of the lesion, histological subtype of the lesion, dermoscopic patterns, Fitzpatrick skin type and sex of each patient were recorded. The most common dermoscopic pattern seen in nodular BCCs was irregular vascularity and, arborizing vessels (>0.2mm in diameter) being the most frequent irregular vascular pattern. The second most common dermoscopic feature in patients with nodular BCCs was translucency. The most common dermoscopic features of the 12 pigmented BCCs were: pigmented islands (blue-gray globules and blue-gray ovoid nests); the pigmented distribution pattern (with (maple leaf-like structures and spoke wheel-like areas); arborizing vessels and white streaks/white areas. The histopathological analysis of the 68 BCCs revealed that the nodular type was the most frequently identified for 71.7% of cases The differential diagnosis between basal cell carcinoma and other skin lesions and inflammatory skin diseases is very important, since serious morbidity may result from an undiagnosed tumor.
Keywords: Basal cell carcinoma, dermatoscopy, histology
Introduction
Basal cell carcinoma (BCC) is the most common skin cancer, with an increasing incidence in Europe particularly in young individuals.
As a result of the high prevalence, BCC represent an important health care problem [1].
Although a large number of basal cell carcinomas are diagnosed annually, metastases are extremely rare, accounting approximately 0.0028%.
In present, a universally accepted classification doesn’t exist for BCCs, and many different subtypes have been described.
Variants of BCC include nodular, superficial, infiltrative types, micronodular, adenoid, morpheaform, pigmented, or fibroepithelioma of Pinkus.
Nodular basal cell carcinoma is the most common subtype and accounts for approximately 57.6-78.7% of all BCCs [2].
The diagnosis of BCC can be suspected from clinical findings including pearly, rolled border, telangiectasia, and pink surface along with very slow growth mainly in sun-exposed areas especially in individuals with fair skin color.
Latest studies suggested that dermoscopy is useful for the management of the basal cell carcinoma, since it provides precious information about the histological subtype, the presence of the clinically undetectable pigmentation, the expansion of the tumor beyond clinically visible margins and the response of the tumor to non-ablative treatments.
The list of dermatoscopic criteria associated with basal cell carcinoma diagnosis has been several times updated and renewed.
Confirmation of diagnosis requires histopathologic examination of biopsy fragments.
Material and Methods
We performed an observational, morphological study which involved 68 patients with the diagnosis of nodular BCC hospitalized and treated in the Dermatology Department of Emergency Hospital from Craiova during March 2018-January 2019.
The localization and diameter of the lesion, histological subtype of the lesion, dermoscopic patterns, Fitzpatrick skin type and sex of each patient were recorded.
Tumors classification and staging was done according to the latest WHO classification.
Epidemiological data were collected from the Clinical Observation Form of these patients and for histopathological data were used the diagnostic registers from the Department of Pathology archive of the same hospital.
For all the patients included in the study group, the written informal consent was obtained and the working protocol meets the requirements of institutional ethics code.
Dermoscopic evaluation was performed by using a videodermoscope (FotoFinder, TeachScreen software GmbH, Bad Birnbach, Germany) and a manual dermatoscope (Heine Delta 20T, Heine Optotechnik, Herrsching, Germany).
The dermoscopy and dermoscopic features that were then agreed on, were performed by 3 independent observers using predefined criteria from the literature.
We selected nine dermoscopic features for analysis, including: irregular vascular pattern, white areas, translucency, milky-pink to red background, erosion/ulceration, blue-gray globules, blue-gray ovoid nests, maple leaf-like areas and spoke wheel-like areas.
For each lesion, a term that best described the global pattern was assigned.
These global vascular patterns were: clustered, scattered, homogenous, or avascular.
Background differences among white-red colors, observed within the lesions, were generally defined as white-red structureless areas.
After tumor excision, the tissues were fixed in formalin and embedded in paraffin.
After routine processing, the slides were stained with hematoxylin-eosin.
Results
Sixty-eight nodular BCCs out of 68 patients (29 males, 39 females) who attended our clinic over an 11-month period were evaluated.
The diagnosis was based on clinical, dermoscopical and histopathological findings.
Our population consisted of 29 men and 39 women ranging in age from 35-82 with a median age of 56.4.
It shows a clinical relevance as women tend to seek medical care earlier than males for cosmetically disfiguring lesions.
Thirty-eight cases were from rural area and thirty cases from urban area. In rural areas, the patients consider that the BCC lesions represent a minor cosmetic problem with an insignificant health impact and require medical advice only when the lesions become symptomatic or deforming.
Regarding the skin phototype 29% of the patients had phototype II and 71% had phototype III.
Considering the anatomical distribution of the 68 nodular BCCs; 92%, 4%, 3.5%, and 0.5% of the lesions were located in the area of head-face, neck, trunk, and limbs, respectively.
Classified frequencies of BCCs in the head-face area showed the highest and the lowest for nose and chin respectively People who developed BCC had occupations involving chronic sun exposure (farmers, construction workers, gardeners) or to artificial sources of ultraviolet (welders)-63% of cases.
There were no data about X-rays and chemicals exposure, burn scars or genodermatoses.
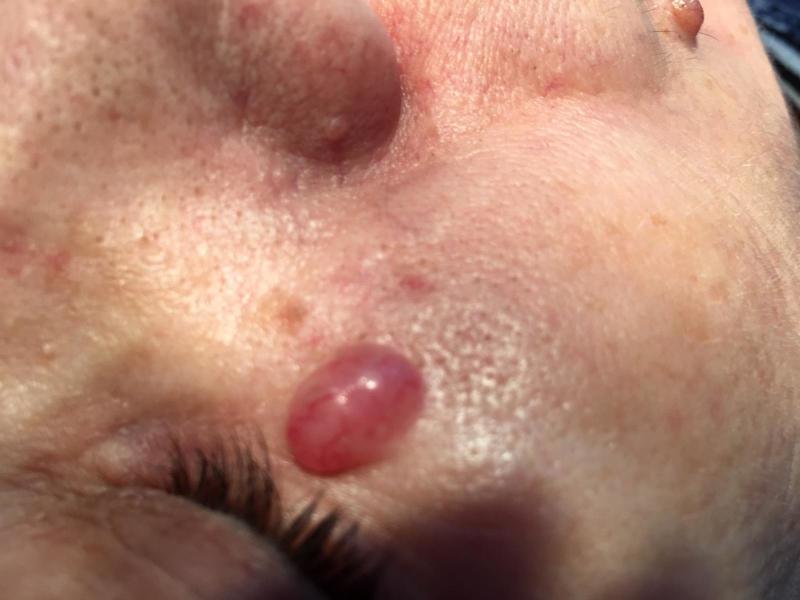
On clinical examination, nodular BCC appears as elevated, exophytic pearl-shaped nodules with overlying ulceration or telangiectatic vessels on the surface and periphery (Fig.1).
Figure 1.
Nodular basal cell carcinoma-clinical aspect
The size of lesions ranged from 0.5cm to 3.5cm in diameter. Majority of the lesions presented within 2 years are of size less than 2cm.
Majority of the lesions presented with more than 3 years of duration are of size more than 2cm.
All included tumors were primary BCCs.
The clinicopathologic aspects of the investigated basal cell carcinomas are detailed in Table 1.
Table 1.
Clinicopathologic aspects of the investigated basal cell carcinomas
|
Clinicopathological parameters |
Variable |
|
Age |
<50=10, >50=58 |
|
Gender |
males=39, females=29 |
|
Tumor location |
head and neck=65, trunk=2, members=1 |
|
Tumor size (cm) |
<2cm=40; 2-4cm=28 |
|
Histologic type |
nodular=48, pigmented=12, adenoid=5, keratotic=3 |
|
Skin Type of Fitzpatrick |
II=19; III=49 |
The histopathological analysis of the 68 BCCs revealed that the nodular type was the most frequently identified for 71.7% of cases.
Also, in the nodular type, the most common subtype was the conventional, observed for 52.6% of cases, followed by other forms such as adenoid, cystic, pigmented and keratotic.
Histopathological examination of nodular BCC showed nodular masses of basaloid cells extend from the epidermis into the dermis with surrounding connective tissue stroma.
In the tumour periphery was present a palisade arrangement of cells.
Sometimes as a result of tumor necrosis cystic spaces appeared.
The surrounding stroma can retract from the tumor mass forming the typical lacunae, a feature that aids in diagnosis (Fig.2).
Figure 2.
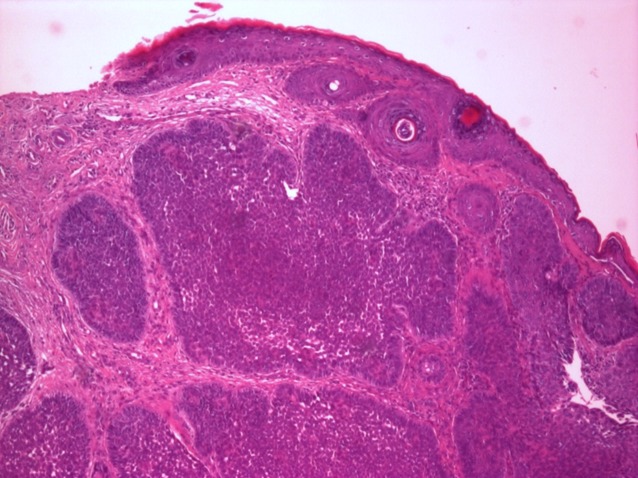
Nodular basal cell carcinoma, col HE, ob X 40
The surrounding stroma also can show a myxoid change, rarely can be fibrotic and may show calcification in discrete islands of tumor or in adjacent stroma.
Mitoses and individual cell necrosis are uncommon in basal cell carcinoma.
Transition to micronodular and other aggressive growth forms of BCC may be seen.
In pigmented BCCs the melanin is produced by melanocytes that colonize the tumour and is present in melanophages located in the surrounding stroma (Fig.3).
Figure 3.
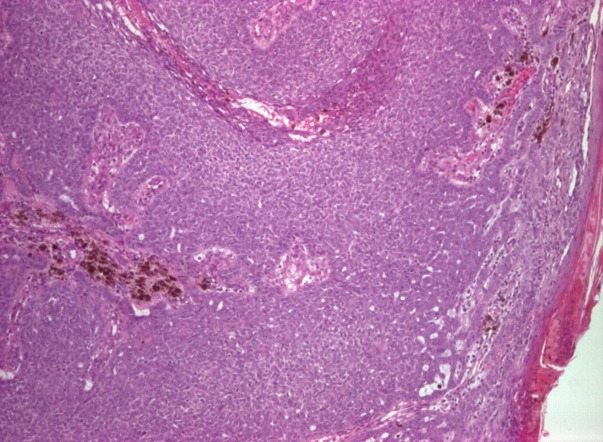
Nodular basal cell carcinoma with pigmented areas, col HE, ob X 40
Any histopathological type of BCC can be invasive, with the exception of superficial BCCs.
In our study, 5 cases were invasive, representing 7.35% of all tumors studied.
Of these, 4 were at the level of the cephalic extremity.
The degree of depth invasion meant extends into the dermis, hypodermis, striated muscle fibers or cartilage.
Histological examination revealed depth invasion in 2 cases of adenoid BCC (2.94%), 2 case of solid BCC (2.94 %) and 1 case of keratotic BCC (1.47%).
Regarding the local dermoscopic criteria and background colors, featureless areas (60%), atypical red vessels (61%), white-red structureless background (53%), arborizing vessels (51%), comma vessels (41%) and telangiectactic vessels (29%) were mostly presented in all BCCs (Table 2).
Table 2.
Dermoscopic features of BCCs
|
Dermoscopic Features |
Nodular |
Pigmented |
Mixed |
|
Irregular Vascular Pattern |
41 (61%) |
2 (2.94%) |
20 (29.4%) |
|
Arborizing Vessels |
34 (51%) |
2 (2.94%) |
16 (23.5%) |
|
Short fine telangiectasias |
28 (41%) |
- |
5 (7.3%) |
|
Arborizing microvessels |
20 (29%) |
- |
2 (2.9%) |
|
Featureless areas |
40 (60%) |
- |
15 (22%) |
|
Milky-pink to red background |
36 (53%) |
- |
10 (14.7%) |
|
Erosion |
5 (7.35%) |
- |
8 (11.7%) |
|
Blue-gray globules |
- |
12(17.64%) |
- |
|
Blue-gray ovoid nests |
12 (28.6%) |
- |
25 (36.7%) |
|
Maple-leaf like Structures |
1 (11.1%) |
3 (4.4%) |
8 (11.7%) |
|
Spoke wheel-like Structures |
- |
12 (17.64%) |
12 (17.6%) |
Non-pigmented BCCs were defined by the presence of brown⁄blue areas in <25% of the lesions.
The most common dermoscopic pattern seen in nodular BCCs was irregular vascularity and, arborizing vessels (>0.2mm in diameter) being the most frequent irregular vascular pattern. (Fig.4)
Figure 4.
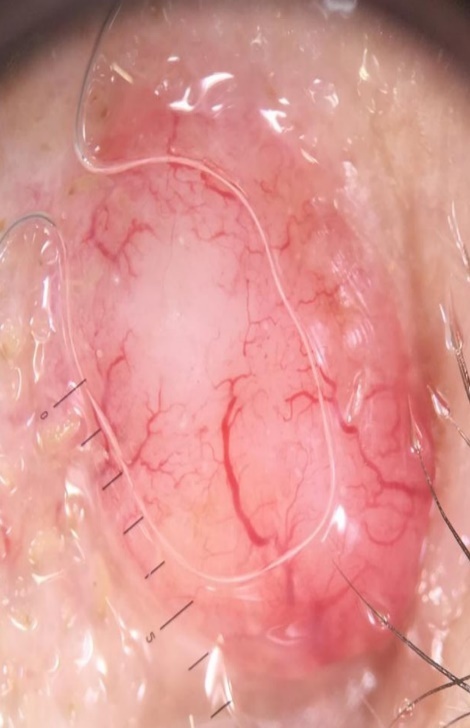
Nodular basal cell carcinoma-dermoscopy: featureless areas, atypical red vessels, white-red structureless background, arborizing vessels, comma vessels and telangiectactic vessels
The second most common dermoscopic feature in patients with nodular BCCs was translucency.
This study included 12 (6.6%) cases with pigmented BCCs.
Blue-gray globules and spoke wheel-like areas were observed in all of these lesions.
In 3 lesions we observed maple leaf-like structures and in 2 lesions arborizing vessels.
While these 12 patients are not a large enough sample to provide statistical significance, in our study, many of the patients with mixed BCCs (24%) also displayed a pigmented subtype. (Fig.5)
Figure 5.
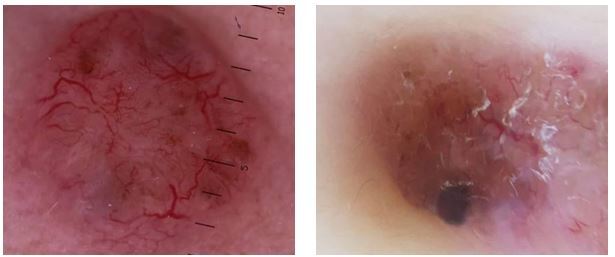
a,b. Pigmented basal cell carcinoma: pigmented islands, blue-gray globules and blue-gray ovoid nests; the pigmented distribution pattern with maple leaf-like structures and spoke wheel-like areas; arborizing vessels and white streaks/white areas
The most common dermoscopic features of these pigmented subtypes were: pigmented islands (blue-gray globules and blue-gray ovoid nests); the pigmented distribution pattern (with maple leaf-like structures and spoke wheel-like areas); arborizing vessels and white streaks/white areas (Fig.5 a,b).
Discussions
The incidence rate of non-melanoma skin cancers is increasing worldwide, with the highest rates reported in geographical areas of low latitude with significant Caucasian populations, such as Australia, followed by Europe and USA.
Prevalence was estimated to be 2.0% for Australia, 1.4% for Europe and 0.7% for USA [3].
Birch-Johansen et al. showed that the incidence of non-melanocytic cancers in Germany and Denmark is about 121/100000, while for Wales there was an incidence of 104 cases in males and 83 in women per 100 000 inhabitants [4].
Regarding localization, the data form the literature indicates the predominance of basal cell carcinomas at the cephalic extremity (70-80% of the cases).
Some studies indicate that approximately 80% of BCCs are located on the face, 30% on the nose, 22% in malar regions, 15% on the forehead, 5% in the periorbital area, 4% on the scalp and 4% at the neck level [5].
The results obtained in the study are in line with the data from the literature, most of the basal cell carcinomas being diagnosed in the head and neck, respectively in 96% of the cases; At the same time at the chest and limb level, 3.5% and 1.5% of the lesions were identified [6].
The differential diagnoses of nodular BCCs may include other tumors, such as dermal nevus, squamous cell carcinoma, epidermoid cyst, sebaceous hyperplasia, compound nevi and amelanotic melanoma.
BCC is a tumour of low degree of malignancy, but if it s left untreated, it can be locally aggressive, being an important factor of morbidity [7].
There are differences among authors when the terminology of histological types is concerned, but most of them described the following types: nodular, adenoid, superficial, keratotic, pigmented, morpheaform, infiltrative, cystic, metatypical, fibroepithelioma of Pinkus and basosquamous carcinoma.
There is also reported a classification of differentiated and non-differentiated BCCs [8].
In our study the nodular type was the most frequently identified, and it is in accordance with Meneses and associates who analyzed histopathological aspects in 269 patients with BCC, and found the nodular type in most cases. Also, Rigell et al. reported the nodular type of BCC in 60% of all histological subtypes [9].
Nodular BCC has always been the most common subtype (57.6-78.7%) which can have many components and differentiations such as cystic, pigmented, sebaceous, ecrine.
Superficial and infiltrative BCCs, comprising the aggressive subtypes, are less common [10].
Pigmentation is present in more than 50% of the basall cell carcinomas in skin of color, whereas less than 10% of BCCs in white population are pigmented.
Literature data show a lower recurrence rate for nodular and superficial subtypes compared to micronodular, infiltrative and morpheiform BCCs.
Dermatoscopy is a valuable tool in the evaluation of pigmented lesions that permits a magnified view of the epidermis and superficial dermis [11].
In their report Puspok-Schwarz et al. were described for the first time the dermoscopic features of pigmented BCCs.
In this study, they compared the dermoscopic features of 25 pigmented BCCs with 25 melanomas and arborizing telangiectasias were found in 52% of pigmented BCCs, being reported as the strongest model for diagnosis [12].
The diagnostic accuracy of dermatoscopy for pigmented basal cell carcinoma was done with the Menzies method achieving a sensitivity of 97% and a specificity of 92% for differentiating pigmented basal cell carcinoma from melanoma.
The specificity for differentiating BCC from nevi was 93% [13].
The Menzies method is based on the absence of a pigmented network and the presence of at least one of six positive features including: ulceration; multiple blue/gray globules; maple leaf-like areas; large blue/gray ovoid nests; spoke-wheel areas; and arborizing (treelike) telangiectasia.
In addition, the presence of a certain vascular pattern may be crucial for the dermoscopic diagnosis of basal cell carcinoma [14].
The classic BCC features described by Menzies did not include shiny white structures in the dermoscopic diagnosis of BCCs because they are better seen with polarized dermatoscopy than with traditional contact fluid dermatoscopy.
Navarette et al. found that the coexistence of shiny white blotches and strands is typical for BCCs [15].
Altamura et al. reported that arborizing telangiectasia, leaf-like areas and large blue/gray ovoid nests represent the most important BCC parameters. These three structures are defined as follows [16]:
1. Arborizing (treelike) telangiectasia is composed of a stem vessel and irregular treelike branching vessels completely in focus, running on the surface of the tumour. Arborizing vessels were reported as a dermoscopic prototypic feature of nodular, sclerodermiform, or cystic BCC, with high diagnostic accuracy. In a study on 504 cases of BCC, Micantonio et al., detected at least one vascular pattern in 91.5% of cases.
The authors compared vascular patterns of BCC and showed that arborizing vessels were more specific for nodular BCCs compared with superficial BCCs [17].
In BCC there were also detected hairpin vessels, minor vascular patterns included glomerular vessels, dotted, comma vessels and polymorphous pattern.
Zalaudek et al. showed that the arborizing vessels found in sclerodermiform BCC are more scattered and thinner with fewer branches compared with the arborizing vessels of nodular BCC [18].
2. Maple leaf-like areas are brown to gray/blue bulbous extensions connected at a base area, forming a leaf-like patern. They are not arising from a confluent pigmented tumor.
3. Large blue/gray ovoid nests are well-circumscribed, pigmented ovoid or elongated areas, larger than globules and not intimately connected to a pigmented tumour body. They also correspond to the basaloid tumoral nodules in dermis with pigment inside.
Enei et al described the dermoscopic association between yellowish structures and basal cell carcinoma. The authors reported a patient developing a BCC in a facial nevus sebaceous of Jadassohn. In addition, Menzies et al. found a 10% sensitivity for milia-like cysts in pigmented BCCs [19].
Lalloo and Sood demonstrated that 2-mm excision margins were adequate for complete tumour excision in more than 95% of cases [20].
The dermoscopic pattern that allows the definition of the peripheral borders of BCC is characterized by the interruption of the normal skin texture by tumour proliferation.
This aspect, appearing in many cases as greyish, glassy areas, represents a strongly suggestive pattern for BCCs.
Conclusion
Dermoscopy is a valuable tool for the diagnosis of basal cell carcinomas.
The presence of arborizing telagiectasia is the most common dermoscopic finding in BCCs, and these were most often seen in nodular BCCs.
The differential diagnosis between basal cell carcinoma and other skin lesions and inflammatory skin diseases is very important, since serious morbidity may result from an undiagnosed tumor.
References
- 1.Ciążyńska M, Narbutt J, Woźniacka A, Lesiak A. Trends in basal cell carcinoma incidence rates: a 16-year retrospective study of a population in central Poland. Postepy dermatologii i alergologii. 2018;35(1):47–52. doi: 10.5114/ada.2018.73164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dacosta Byfield, Chen D, Yim YM, Reyes C. Age distribution of patients with advanced non-melanoma skin cancer in the United States. Arch Dermatol Res. 2013;305:845–850. doi: 10.1007/s00403-013-1357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apalla Z, Lallas A, Sotiriou E, Lazaridou E, Ioannides D. Epidemiological trends in skin cancer. Dermatology practical and conceptual. 2017;7(2):1–6. doi: 10.5826/dpc.0702a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birch‐Johansen F, Jensen A, Mortensen L. Trends in the incidence of nonmelanoma skin cancer in Denmark 1978-2007: rapid incidence increase among young Danish women. Int J Cancer. 2010;127:2190–2198. doi: 10.1002/ijc.25411. [DOI] [PubMed] [Google Scholar]
- 5.Radespiel‐Tröger M, Meyer M, Pfahlberg A. Outdoor work and skin cancer incidence: a registry‐based study in Bavaria. Int Arch Occup Environ Health. 2009;82:357–363. doi: 10.1007/s00420-008-0342-0. [DOI] [PubMed] [Google Scholar]
- 6.Stoica LE, Georgescu CV, Pătraşcu V, Radu C, Tolea I, Mogoantă L. Basal cell carcinomas-clinical-evolutional and histopahotologic aspects. Current health sciences Journal. 2009;35(4):228–233. [PMC free article] [PubMed] [Google Scholar]
- 7.Lewin JM, Carucci JA. Advances in the management of basal cell carcinoma. F1000 prime reports. 2015;7:53–53. doi: 10.12703/P7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rigell DS , Cockerell CJ , Caruci J , et al. Actinic keratosis, basal cell carcinoma and squamous cell carcinoma . In: Bolognia JL , Jarizzo JL , Rapini RP , editors. Dermatology . 2 . St. Louis : Mosby Elsevier ; 2008 . pp. 1641 – 1660 . [Google Scholar]
- 9.Meneses N, Guides R, Moreira A, Mota G, Baptista A. Basal cell carcinoma: epidemiology from 269 cases. J Eur Acad Dermatol Venereol. 2010;24:1359–1360. doi: 10.1111/j.1468-3083.2010.03630.x. [DOI] [PubMed] [Google Scholar]
- 10.Arits AHMM, Schlangen MHJ, Nelemans PJ, Kelleners-Smeets NWJ. Trends in the incidence of basal cell carcinoma by histopathological subtype. Journal of the European Academy of Dermatology and Venereology. 2011;25(5):565–569. doi: 10.1111/j.1468-3083.2010.03839.x. [DOI] [PubMed] [Google Scholar]
- 11.Kittler H, Pehamberger H, Wolff K, Binder M. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3(3):159–165. doi: 10.1016/s1470-2045(02)00679-4. [DOI] [PubMed] [Google Scholar]
- 12.Puspok-Schwarz M, Steiner A, Binder M, Partsch B, et al. Statistical evaluation of epiluminescence microscopy criteria in the differential diagnosis of malignant melanoma and pigmented basal cell carcinoma. Melanoma Res. 1997;7:307–311. doi: 10.1097/00008390-199708000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Menzies SW. Dermoscopy of pigmented basal cell carcinoma. ClinDermatol. 2002;20:268–269. doi: 10.1016/s0738-081x(02)00229-8. [DOI] [PubMed] [Google Scholar]
- 14.Menzies SW, Westerhoff K, Rabinovitz H, Kopf AW, et al. Surface microscopy of pigmented basal cell carcinoma. Arch Dermatol. 2000;136:1012–1016. doi: 10.1001/archderm.136.8.1012. [DOI] [PubMed] [Google Scholar]
- 15.Navarrete-Dechent C, Bajaj S, Marchetti MA, Rabinovitz H, Dusza SW, Marghoob A. Association of Shiny White Blotches and Strands With Nonpigmented Basal Cell Carcinoma: Evaluation of an Additional Dermoscopic Diagnostic Criterion. JAMA dermatology. 2016;152(5):546–552. doi: 10.1001/jamadermatol.2015.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altamura D, Menzies S, Argenziano G, Zalaudek I, Soyer P, Sera F, Avramidis M, DeAmbrosis K, Fargnoli M, Peris K. Dermatoscopy of basal cell carcinoma: Morphologic variability of global and local features and accuracy of diagnosis. J Am Acad Dermatol. 2010;62(1):67–75. doi: 10.1016/j.jaad.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 17.Micantonio T, Gulia A, Altobelli E, Di Cesare, Fidanza R, Riitano A, Fargnoli MC, Peris K. Vascular patterns in basal cell carcinoma. J Eur Acad Dermatol Venereol. 2011;25(3):358–361. doi: 10.1111/j.1468-3083.2010.03734.x. [DOI] [PubMed] [Google Scholar]
- 18.Zalaudek I, Kreusch J, Giacomel J, Ferrara G, Catricala C, Argenziano G. How to diagnose nonpigmented skin tumors: a review of vascular structures seen with dermoscopy: part II. Nonmelanocytic skin tumors. J Am Acad Dermatol. 2010;63(3):377–386. doi: 10.1016/j.jaad.2009.11.697. [DOI] [PubMed] [Google Scholar]
- 19.Enei ML, Paschoal FM, Valdés G, Valdés R. Basal cell carcinoma appearing in a facial nevus sebaceous of Jadassohn: dermoscopic features. An Bras Dermatol. 2012;4:640–642. doi: 10.1590/s0365-05962012000400023. [DOI] [PubMed] [Google Scholar]
- 20.Lalloo MT, Sood S. Head and neck basal cell carcinoma: treatment using a 2-mm clinical excision margin. Clinical Otolaryngology and Allied Sciences. 2000;25(5):370–373. doi: 10.1046/j.1365-2273.2000.00376.x. [DOI] [PubMed] [Google Scholar]