Abstract
Background and objectives: Rat experimentation is the first line of research by which a medical hypothesis is usually tested. Platelet Rich Fibrin (PRF) is a relatively new bio-material that has shown promise to enhance healing in the field of bone research and tissue engineering. In order to perform PRF based experiments on rats, a proper protocol of obtaining PRF from rats needs to be established. Materials and Methods: 35 Wistar rats were used to obtain PRF by using cardiac puncture blood draw and quick subsequent centrifugation. The PRF samples wereanalyzed and compared to standard literature PRF composition. Results: PRF samples analysis showed persistent results pertaining to known PRF composition. Conclusions: Our experiment has shown that our protocol of obtaining PRF is capable of providing high quality PRF from rats.
Keywords: Platelet-rich fibrin, tissue engineering, proof of concept study, experimental animal models, platelet count
Introduction
Platelet Rich Fibrin (PRF) is a new biomaterial that is obtained by a process of centrifugation from the patient’s own blood [1] that sets itself apart from the rest of platelet concentrates by the process of fibrin polymerization that encapsulates thrombocytes within it. The growth factors on the surface of the thrombocytes are slowly released and thus promote the healing processes that occur at the site of PRF application.
Research has been ongoing since its discovery with research areas including musculoskeletal injuries [2,3], oro-maxillo-facial surgery [4] or plastic surgery [5]. The common denominator in these field of research is that they make use of PRF’s capacity to be manipulated in its membrane form. This propriety gives the attending physician the possibility to use PRF in a multitude of clinical scenarios. The full range of therapeutic uses of PRFhave yet to be researched. In order to comply with scientific rigor any experimental use of PRF must first be performed in a controlled laboratory scenario, usually using rats as experimental subjects.
The rat is a good candidate for experimental bone research because it can fulfil the above-mentioned requirements besides having numerous other advantages such as: the relative ease with which it can be procured, manipulated, stored, fed and monitored. These advantages have propelled the rat at the forefront of bone healing research, with rat experimentation consisting of 38% of the total number of experiments [6] (as opposed to rabbits 19%, mice 13%, sheep 11%, dogs 9%, goats 4% and other 5%). This leads to a vast literature regarding numerous aspects of medical research with covered aspects including liver research [7], osseointegration of different materials [8,9] or stroke research [10,11,12,13].
The aim of this paper is to establish a protocol for obtaining PRF from rats that can be used by researchers in any field of laboratory or clinical scenarios.
Materials and Methods
For the purposes of this study we used 35 male Wistar rats. Approval of the Committee for Animal Welfare and Bioethics from the University of Pharmacy and Medicine from Craiova, Romania was obtained and the experiment was conducted at the Laboratory for Animal Research belonging to the University.
The weight of the rats was 320g in average (range: 220-420g). In order to comply with current good practices guidelines for the purpose at hand the rats were selected to be at least 6 months of age [14].
Due to the natural fast coagulation of rat blood all the materials had to be prepared for use and the centrifuge set up beforehand. The centrifuge (Process for PRF Duo Centrifuge©) was set according to the protocol for 1300rpm (400g) x 8min spin time [15].
The centrifuge was also primed with 3 sterile saline Vacutainers acting as counterweights for the PRF Vacutainer.
Given the 12-15ml total circulating blood in a rat [16] and the 10ml required for PRF preparation, the blood harvesting technique of choice was cardiac puncture [17]. This technique took advantage of the antigenicity compatibility [18] in between Wistar rats in order to produce sufficient PRF membrane from a single donor rat to suffice for 2-5 experiments with PRF, depending on the use.
Anesthesia was performed by subcutaneous injection of a mixture of Ketamine and Xylazine (dosage of 50mg/kg and 5mg/kg respectively). Ethical standards were complied, regarding the comfort of the test animals, by performing reflex tests such as the toe pinch test and corneal reflex [19] to ensure that full anesthesia is reached. Artificial tears were applied after the anesthesia set in order to prevent corneal dryness. This was reapplied when necessary.
We used a PRF Choukroun VacuTainer©, Vacutainer Eclipse Blood Collection Needles© and BD Vacutainer Single Use Needle holder© in order to obtain the necessary blood sample.
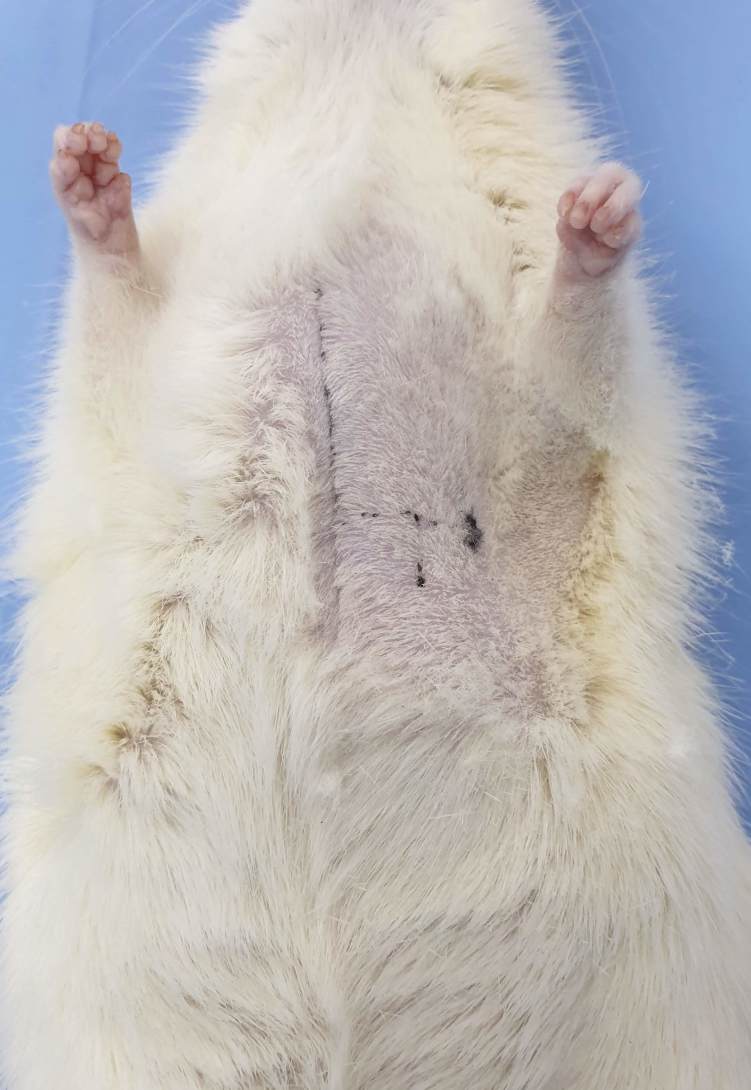
The anesthetized donor rat was placed in a dorsal recumbency position and we highlighted the apexian shock by using a marker. Starting from this point we drew a segment perpendicular to the median sternum line. We continued by finding the middle point of this segment and drew another perpendicular line starting from this middle point, in the caudal direction. We marked a point approximately 1cm distal on this line and we marked it as the place of needle insertion (Fig.1).
Figure 1.
Drawn lines according to the instructions
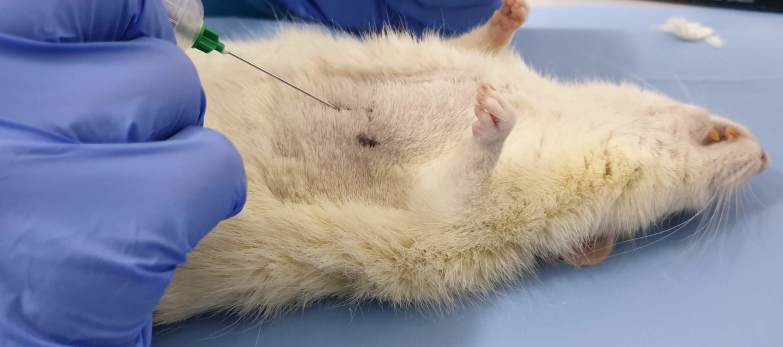
The needle was placed at 25-35° from the horizontal plane and the tip of the needle was cranially orientated (Fig.2).
Figure 2.
Needle positioning
If the insertion point was exactly over a rib, we adjusted the positioning in order to be in the intercostal space beneath the rib. We continued by carefully inserting the needle. After the needle was inside the heart we attached the vacutainer to the needle and waited for it to fill. This took a maximum of 3-4 seconds. The vacutainer was then transferred into the centrifuge and the PRF production protocol was initiated as quick as possible. In our experiments we have found that a time longer than 7-9 seconds from initial vacutainer connection to its placement in the centrifuge resulted in coagulated blood, that rendered the sample unsuitable for our purposes.
This was the reason for having the centrifuge prepared beforehand at the designated protocol of 1300rpm (400g)/8 minutes spin time.
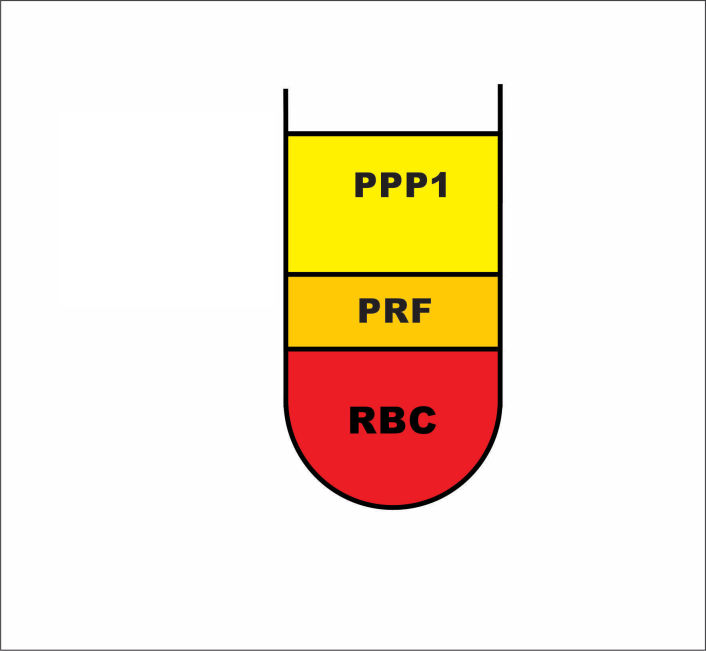
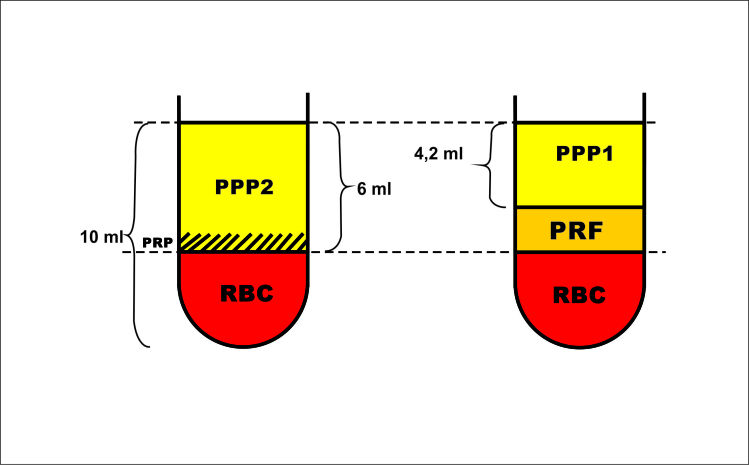
After the centrifugal process had finished, we removed the vacutainer and placed it in a tube stand where it sat for another 5 minutes, at room temperature, away from any direct light. After this, the vacutainer presented with 3 macroscopic layers:
the plasma layer (Platelet Pure Plasma-called PPP1 for future calculations)
the PRF clot
the red blood clot (RBC). (Fig.3)
Figure 3.
PRF centrifugation protocol, blood separation
We removed the cap of the vacutainer and, by using an anatomic surgical clasp we grabbed the PRF clot from the middle 1/3 of the liquid and carefully removed it from the tube. This mobilized the PRF clot together with the RBC clot. The two clots had to be separated at the junction line. If the procedure is difficult to perform with absolute precision (caused by an angled or somewhat irregular junction line), it is always advisable to perform the cut with a little tolerance by including a maximum of 1mm vertical height of RBC clot. This is performed because of the known spatial distribution of the platelets inside the clots (the spatial distribution of the platelets permeates the PRF-RBC clots junction by infiltrating in the upper most layer of the RBC) [20]. By performing the cut in this manner we were able to maximize the number of available platelets in the PRF clot.
After obtaining the PRF clot we placed it in a PRF box [21] in order to transform the clot into a membrane by pressing the exudate out. If kept wet, inside the sterile PRF box, with the adjacent exudate eliminated by the PRF clot, the resulted PRF membrane could be used for a period of up to 2.5-3 hours (Fig.4).
Figure 4.
PRF Box
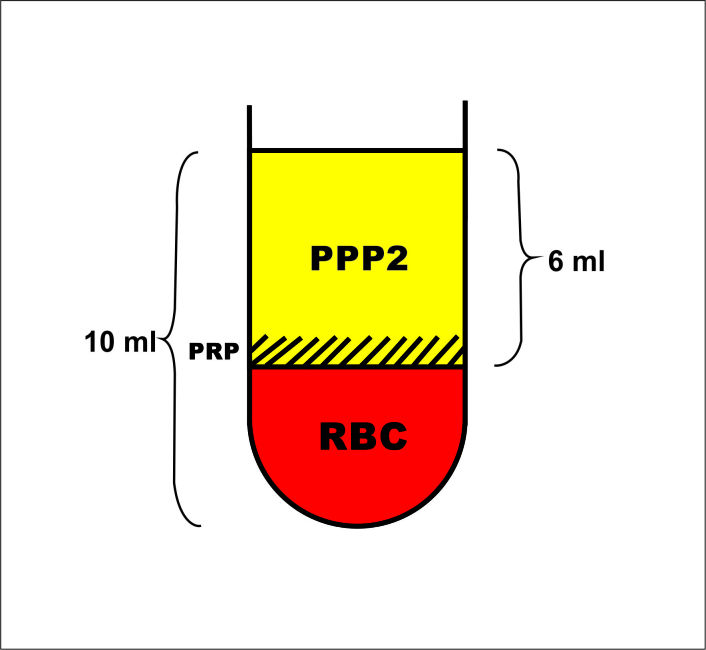
In order to measure the platelet concentration of the resulted PRF we used indirect deduction based on a separate production of Platelet Rich Plasma (PRP). We produced the PRP by centrifuging (12min at 450g) 10ml of blood collected in an identically shaped VacuTainer, primed with anticoagulant. After the centrifugal process the blood had separated into three macroscopic layers:
plasma layer (Platelet Pure Plasma-noted PPP2 for future calculations);
the thin buffy coat (platelet rich layer-PRP);
the RBC layer (Fig.5).
Figure 5.
PRP centrifugation protocol, blood separation
We collected the PRP+PPP2 and measured the platelet concentration of the resulting mixture and analyzed the platelet count of this mixture.
Results
We based our results on the following laboratory results measuring platelet concentrations:
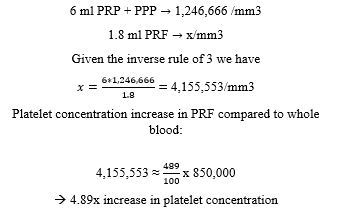
Mean platelet count of whole rat blood: 850,000/mm3, with a range of 702,000/mm3 to 998,000/mm3;
PPP1: trace platelets, for the purposes of calculation, 0/mm3, totaling a mean of 4.2ml;
PRP+PPP2: mean platelet count 1.246.666/mm3, totaling 6ml;
In order to find out the quantity of platelets that were lost in the red clot, if any, we perform the following calculations:
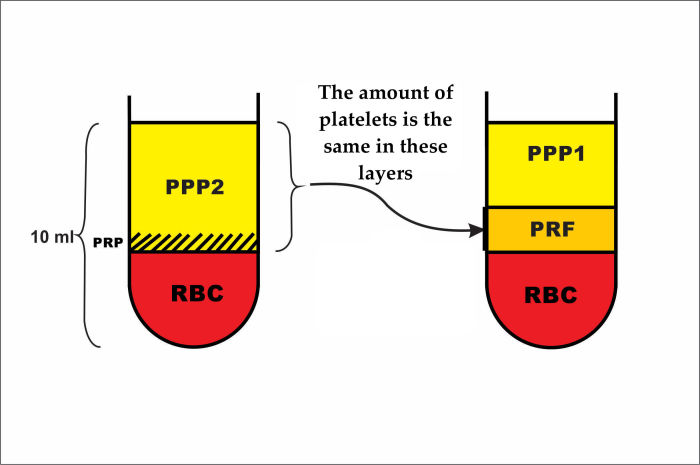
The red blood fractions (RBC) generated after PRF and PRP centrifugations were equal (Fig.6). We could thus extrapolate that:
Figure 6.
Schematic representation of PRP and PRF centrifugation of a 10 ml blood sample
PRF + PPP1 = PRP + PPP2 = 6 ml.
PPP1 = 4.2 ml -> PRF = 6-4.8 = 1.8 ml
PPP1 Platelet concentration was ~ 0 -> all of platelets that could be found in the PRP + PPP2 could now be found concentrated in the PRF. (Fig.7)
Figure 7.
Platelet distribution in the PRP and PRF

Thus, we could conclude that the mathematical calculations outlined resulted in a Platelet concentration of 4,155,553/mm3. When compared to the platelet concentration of whole blood we observed an increase in platelet concentration of 489% in the PRF clot. This proved that the protocol described above successfully producedPRF, with a relative platelet concentration of 4.89x compared to the baseline platelet concentration. The calculations delineated in Appendix A show that 12% of the available platelets in whole blood were lost in the RBC.
The advantages and disadvantages of our protocol can be seen in Table 1.
Table 1.
Advantages and disadvantages
|
Advantages |
Disadvantages |
|
Standardized method |
Terminal procedure for the donor rat |
|
Easily reproducible using step by step instructions |
Technique is sensible to user variation in method |
|
Proven platelet concentration increase of ~ 4.9 x |
|
|
Possibility to produce a large PRF membrane with a single centrifuge spin |
|
|
Identical PRF graft for 4-5 experimental procedures |
|
Discussion
PRF preparation protocols are based on the end point use. This can consist of the use of PRF as a naturally degradable membrane that acts as a barrier for guiding tissue regeneration, a source of growth factors delivered in a concentrated form or as scaffolding for tissue engineering. This results in the need for PRF to deliver both mechanical and biological proprieties. We performed this study in order to determine the best production method quality PRF.
The novel character of our protocol consists of the high quantity and quality of PRF that we were able to produce with a single donor rat. Upon review of the literature we have found other examples of researchers that have obtained PRF from rats for various research purposes, such as bone healing [22,23,24,25], nerve injury [26,27], intestinal anastomoses [28,29,30,31], Achilles tendon injuries [32] or perforated tympanic membrane [33]. The usual way of procuring blood from rats was by sub-orbital dripping or venous blood collection from the lateral tail vein [34]. These blood collecting techniques are able to produce a limited amount of blood and consequently a small PRF graft. The same can be said regarding another minimally invasive blood collecting technique from the rat, percutaneous drawing from the cranial vena cava [35]. This can generate additional issues such as difficulties to identify the PRF clot in the Vacutainer, difficulties in grabbing and subsequent manipulation of it. This in turn generated a very small PRF membrane that was very difficult to manipulate, rendering the process unfeasible, especially for large defects.
Our protocol also potentially eliminates the variability intrinsic to each donor animal by generating up to identical 4-5 PRF membranes that in turn can be used for 4-5 experiments. This can be achieved by cutting the PRF membranes into pieces that can fit the size for the experiment at hand. Thus, the PRF grafts used in these experiments are identical to each other, as they are cut from the same graft. By eliminating one of the variables of any experiment we can greatly improve the reliability of the outcome [36].
The use of a PRF box is a relatively new procedure that replaces the old spoon compression technique of obtaining PRF membranes manually and further enhancing the use of the obtained PRF membranes.
Our study relies on indirect mathematical induction and represents a personal variation of the standard subtraction technique of counting platelets and determining platelet concentration from PRF [37].
Further studies on different centrifugation protocols are also required to gather research regarding PRF variants, such as Intelligent PRF (I PRF), Advanced PRF (A-PRF), Leukocyte Rich PRF (L-PRF) [38].
Approximatively 12% of platelets were lost in the red blood fraction of the centrifugated vacutainer and thus unavailable for the platelet concentration process that occurred in the plasma layer, thus further studies need to be made in order to refine the PRF obtaining protocol, in a way that maximizes the available platelets.
Conclusions
Our study has successfully proven this method of obtaining PRF as a viable option with considerable advantages, especially for, but not limited to, research that involves the procurement of large batches of up to 4-5 identical PRF membranes.
Acknowledgment
Alexandru Florian Grecu, Eduard Mihai Ciuca and Adrian Camen contributed equally to this work.
References
- 1.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral RadiolEndod. 2006;101(3):e37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Zumstein MA, Berger S, Schober M, Boileau P, Nyffeler RW, Horn M, Dahinden CA. Leukocyte- and platelet-rich fibrin (L-PRF) for long-term delivery of growth factor in rotator cuff repair: review, preliminary results and future directions. Curr Pharm Biotechnol. 2012;13(7):1196–1206. doi: 10.2174/138920112800624337. [DOI] [PubMed] [Google Scholar]
- 3.Zumstein MA, Bielecki T, Dohan DM. The Future of Platelet Concentrates in Sports Medicine: Platelet-Rich Plasma, Platelet-Rich Fibrin, and the Impact of Scaffolds and Cells on the Long-term Delivery of Growth Factors. Oper Tech Sports Med. 2011;19:190–197. [Google Scholar]
- 4.Rastogi P, Saini H, Singhal R, Dixit J. Periodontal regeneration in deep intrabony periodontal defect using hydroxyapatite particles with platelet rich fibrin membrane-a case report. J Oral BiolCraniofac Res. 2011;1(1):41–43. doi: 10.1016/S2212-4268(11)60010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danielsen P, Jørgensen B, Karlsmark T, Jorgensen LN, Agren MS. Effect of topical autologous platelet-rich fibrin versus no intervention on epithelialization of donor sites and meshed split-thickness skin autografts: a randomized clinical trial. PlastReconstrSurg. 2008;122(5):1431–1440. doi: 10.1097/PRS.0b013e318188202c. [DOI] [PubMed] [Google Scholar]
- 6.O'Loughlin PF, Morr S, Bogunovic L, Kim AD, Park B, Lane JM. Selection and development of preclinical models in fracture-healing research. J Bone Joint Surg Am. 2008;90 Suppl 1:79–84. doi: 10.2106/JBJS.G.01585. [DOI] [PubMed] [Google Scholar]
- 7.Ionică FE, Mogoantă L, Nicola GC, ChiriŢă C, Negreş S, Bejenaru C, Turculeanu A, Badea O, Popescu NL, Bejenaru LE. Antifibrotic action of telmisartan in experimental carbon tetrachloride-induced liver fibrosis in Wistar rats. Rom J Morphol Embryol. 2016;57(4):1261–1272. [PubMed] [Google Scholar]
- 8.Manolea HO, CrăiŢoiu MM, Mogoantă L, Dascălu IT, Moraru AI, AgopForna D, MercuŢ R. An evaluation of a collagen-based material osseointegration. Rom J Morphol Embryol. 2017;58(1):161–165. [PubMed] [Google Scholar]
- 9.Obadan F, Craitoiu M, Manolea H, Mogoanta L, Osiac E, Rica R, Salan AI, Earar K. Osseointegration Evaluation of Two Socket Preservation Materials in Small Diameter Bone Cavities An in vivo lab rats study. REV.CHIM. Bucharest) 2018;69(10):2904–2909. [Google Scholar]
- 10.Popescu ES, Pirici I, Ciurea RN, Bălşeanu TA, Cătălin B, Mărgăritescu C, Mogoantă L, Hostiuc S, Pirici D. Three-dimensional organ scanning reveals brain edema reduction in a rat model of stroke treated with an aquaporin 4 inhibitor. Rom J Morphol Embryol. 2017;58(1):59–66. [PubMed] [Google Scholar]
- 11.Buchhold B, Mogoanta L, Suofu Y, Hamm A, Walker L, Kessler Ch, Popa-Wagner A. Environmental enrichment improves functional and neuropathological indices following stroke in young and aged rats. RestorNeurolNeurosci. 2007;25(5-6):467–484. [PubMed] [Google Scholar]
- 12.Kessler Ch, Junker H, Bălşeanu TA, Oprea B, Pirici D, Mogoantă L, Popa-Wagner A. Annexin A3 expression after stroke in the aged rat brain. Rom J Morphol Embryol. 2008;49(1):27–35. [PubMed] [Google Scholar]
- 13.Osiac E, Bălşeanu TA, Mogoantă L, Gheonea DI, Pirici I, Iancău M, Mitran SI, Albu CV, Cătălin B, Sfredel V. Optical coherence tomography investigation of ischemic stroke inside a rodent model. Rom J Morphol Embryol. 2014;55(3):767–772. [PubMed] [Google Scholar]
- 14.Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, Vidal JM, van de Vorstenbosch C. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J ApplToxicol. 2001;21(1):15–23. doi: 10.1002/jat.727. [DOI] [PubMed] [Google Scholar]
- 15.Yamashita Y, Chen K, Kuroda S, Kasugai S. Stability of Platelet-rich Fibrin in Vivo: Histological Study in Rats. J Tissue EngRegen Med. 2016;14(2):83–90. [Google Scholar]
- 16.Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med. 1985;26:72–76. [PubMed] [Google Scholar]
- 17.Parasuraman S, Raveendran R, Kesavan R. Blood sample collection in small laboratory animals. J PharmacolPharmacother. 2010;1(2):87–93. doi: 10.4103/0976-500X.72350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamada N, Brons G, Davies HS. Fully allogeneic liver grafting in rats induces a state of systemic nonreactivity to donor transplantation antigens. Transplantation. 1980;29(5):429–431. doi: 10.1097/00007890-198005000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Tsukamoto A, Serizawa K, Sato R, Yamazaki J, Inomata T. Vital signs monitoring during injectable and inhalant anesthesia in mice. ExpAnim. 2015;64(1):57–64. doi: 10.1538/expanim.14-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DohanEhrenfest DM, Del Corso, Diss A, Mouhyi J, Charrier JB. Three-dimensional architecture and cell composition of a Choukroun's platelet-rich fibrin clot and membrane. J Periodontol. 2010;81(4):546–555. doi: 10.1902/jop.2009.090531. [DOI] [PubMed] [Google Scholar]
- 21.DohanEhrenfest DM. How to optimize the preparation of leukocyte- and platelet-rich fibrin (L-PRF, Choukroun's technique) clots and membranes: introducing the PRF Box. Oral Surg Oral Med Oral Pathol Oral RadiolEndod. 2010;110:275–278. doi: 10.1016/j.tripleo.2010.05.048. [DOI] [PubMed] [Google Scholar]
- 22.Dülgeroglu TC, Metineren H. Evaluation of the Effect of Platelet-Rich Fibrin on Long Bone Healing: An Experimental Rat Model. Orthopedics. 2017;40(3):e479–e484. doi: 10.3928/01477447-20170308-02. [DOI] [PubMed] [Google Scholar]
- 23.Raafat SN, Amin RM, Elmazar MM, Khattab MM, El-Khatib AS. The sole and combined effect of simvastatin and platelet rich fibrin as a filling material in induced bone defect in tibia of albino rats. Bone. 2018;117:60–69. doi: 10.1016/j.bone.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Yu P, Yang X, Qi Z. Letter to the editor: The sole and combined effect of simvastatin and platelet rich fibrin as a filling material in induced bone defect in tibia of albino rats. Bone. 2019;120:533–533. doi: 10.1016/j.bone.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Raafat SN. Response to: Letter to the editor: The sole and combined effect of simvastatin and platelet rich fibrin as a filling material in induced bone defect in tibia of albino rats. Bone. 2019;120:534–534. doi: 10.1016/j.bone.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Torul D, Bereket MC, Onger ME, Altun G. Comparison of the Regenerative Effects of Platelet-Rich Fibrin and Plasma Rich in Growth Factors on Injured Peripheral Nerve: An Experimental Study. J Oral MaxillofacSurg. 2018;76(8):1823e1–1823e12. doi: 10.1016/j.joms.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Giannessi E, Coli A, Stornelli MR, Miragliotta V, Pirone A, Lenzi C, Burchielli S, Vozzi G, De Maria, Giorgetti M. An autologously generated platelet-rich plasma suturable membrane may enhance peripheral nerve regeneration after neurorraphy in an acute injury model of sciatic nerve neurotmesis. J ReconstrMicrosurg. 2014;30(9):617–626. doi: 10.1055/s-0034-1372483. [DOI] [PubMed] [Google Scholar]
- 28.zçay N, Özdemir H, Besim H. Role of platelet-rich fibrin on intestinal anastomosis wound healing in a rat. Biomed Mater. 2018;13(4):045006–045006. doi: 10.1088/1748-605X/aab8e1. [DOI] [PubMed] [Google Scholar]
- 29.Spartalis E, Prodromidou A, Spartalis M, Machairas N. Comment on 'Role of platelet-rich fibrin on intestinal anastomosis wound healing in a rat'. Biomed Mater. 2018;13(6):068001–068001. doi: 10.1088/1748-605X/aade75. [DOI] [PubMed] [Google Scholar]
- 30.zçay N. Reply to Comment on 'Role of platelet-rich fibrin on intestinal anastomosis wound healing in a rat'. Biomed Mater. 2018;13(6):068002–068002. doi: 10.1088/1748-605X/aade78. [DOI] [PubMed] [Google Scholar]
- 31.Daglioglu YK, Duzgun O, Sarici IS, Ulutas KT. Comparison of platelet rich plasma versus fibrin glue on colonic anastomoses in rats. Acta Cir Bras. 2018;33(4):333–340. doi: 10.1590/s0102-865020180040000005. [DOI] [PubMed] [Google Scholar]
- 32.Dietrich F, L Duré, P Klein, F Bampi, V Padoin, D Silva, Braga-Silva J. Platelet-Rich Fibrin Promotes an Accelerated Healing of Achilles Tendon When Compared to Platelet-Rich Plasma in Rat. World J PlastSurg. 2015;4(2):101–109. [PMC free article] [PubMed] [Google Scholar]
- 33.Ensari N, Gür ÖE, Öztürk MT, Süren D, Selçuk ÖT, Osma Ü. The effect of platelet-rich fibrin membrane on the repair of perforated tympanic membrane: an experimental study. ActaOtolaryngol. 2017;137(7):695–699. doi: 10.1080/00016489.2017.1282169. [DOI] [PubMed] [Google Scholar]
- 34.Lee G, Goosens KA. Sampling blood from the lateral tail vein of the rat. J Vis Exp. 2015;99:e52766–e52766. doi: 10.3791/52766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jekl V, Hauptman K, Jeklová E, Knotek Z. Blood sampling from the cranial vena cava in the Norway rat (Rattus norvegicus) Lab Anim. 2005;39:236–239. doi: 10.1258/0023677053739774. [DOI] [PubMed] [Google Scholar]
- 36.Chu H, Zeng L, Fetters MD, Li N, Tao L, Shi Y, Zhang H, Wang X, Li F, Zhao Y. How novice, skilled and advanced clinical researchers include variables in a case report form for clinical research: a qualitative study. BMJ Open. 2017;7(9):e016760–e016760. doi: 10.1136/bmjopen-2017-016760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe T, Isobe K, Suzuki T, Kawabata H, Nakamura M, Tsukioka T, Okudera T, Okudera H, Uematsu K, Okuda K, Nakata K, Kawase T. An Evaluation of the Accuracy of the Subtraction Method Used for Determining Platelet Counts in Advanced Platelet-Rich Fibrin and Concentrated Growth Factor Preparations. Dent J (Basel) 2017;5(1):E7–E7. doi: 10.3390/dj5010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DohanEhrenfest DM, Andia I, Zumstein MA, Zhang CQ, Pinto NR, Bielecki T. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014;4(1):3–9. [PMC free article] [PubMed] [Google Scholar]