Abstract
Genetics strongly implicate the amyloid β-peptide (Aβ) in the pathogenesis of Alzheimer’s disease. Dominant missense mutation in the presenilins and the amyloid precursor protein (APP) cause early-onset familial Alzheimer’s disease (FAD). As presenilin is the catalytic component of the γ-secretase protease complex that produces Aβ from APP, mutation of the enzyme or substrate that produce Aβ leads to FAD. However, the mechanism by which presenilin mutations cause FAD has been controversial, with gain of function and loss of function offered as binary choices. This overview will instead present the case that presenilins are dysfunctional in FAD. γ-Secretase is a multi-functional enzyme that proteolyzes the APP transmembrane domain in a complex and processive manner. Reduction in a specific function—the carboxypeptidase trimming of initially formed long Aβ peptides containing most of the transmembrane domain to shorter secreted forms—is an emerging common feature of FAD-mutant γ-secretase complexes.
Keywords: amyloid, protease, genetics, biochemistry
Introduction
The deposition of extracellular amyloid plaques and neurofibrillary tangles in the brain are cardinal pathological features of Alzheimer’s disease (AD) (Goedert and Spillantini, 2006). The former are composed primarily of the amyloid β-peptide (Aβ), while the latter are comprised of filaments of the otherwise microtubule-associated protein tau. The general consensus is that Aβ and tau are both critical to the neurodegenerative process of AD, with Aβ being upstream of tau in the pathway. However, the deposits per se may not be pathogenic. Indeed, amyloid plaques numbers or location do not seem to correlate with disease onset or progression. (Lue et al., 1999; McLean et al., 1999; Naslund et al., 2000). In the past two decades, evidence has mounted suggesting that soluble oligomeric forms of Aβ cause synaptic dysfunction and are more detrimental than the plaques (Masters and Selkoe, 2012). Nevertheless, the specific pathogenic form of Aβ remains unknown, and the molecular means by which pathogenic Aβ triggers downstream synapto- and neurotoxic events are unclear (Benilova et al., 2012). Regardless of the exact forms responsible for eliciting synaptic and neuronal toxicity, genetic mutations associated with autosomal-dominant familial AD (FAD) clearly point to alterations in Aβ being sufficient for neurodegenerative disease (Rademakers et al., 2012; Tanzi and Bertram, 2005).
APP Processing by Secretases
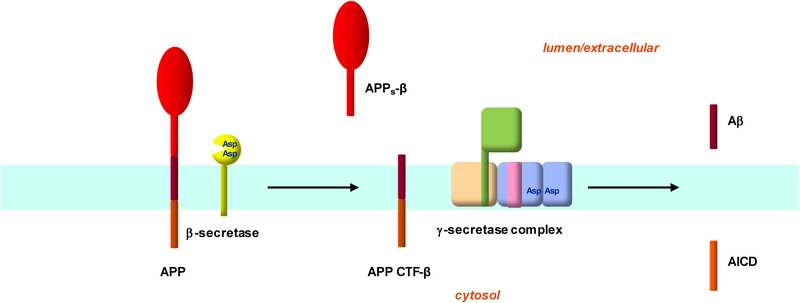
Aβ is a 38–43 amino acid secreted peptide derived from the amyloid β-protein precursor (APP), a 695–770 amino acid single-pass transmembrane protein (Masters and Selkoe, 2012) (Figure 1). The large extracellular/lumenal domain of APP is shed from the membrane by either α-secretase or β-secretase activity. α-Secretases are membrane-anchored metalloproteases that cut APP within the Aβ region, thus precluding Aβ production, leaving an APP C-terminal fragment (APP CTF-α) in the membrane. β-Secretase cuts further away from the transmembrane domain to generate the N-terminal region of Aβ as part of APP CTF-β. Both APP CTF-α and -β are subsequently cleaved by γ-secretase to produce p3 (from CTF-α) and Aβ (from CTF-β) and the APP intracellular domain (AICD). The biological roles of APP and its proteolytic fragments are largely unknown (Muller and Zheng, 2012).
Figure 1. Production of Aβ from APP.
APP is an integral membrane protein, with the Aβ portion derived in part from the single transmembrane domain. β-Secretase is a membrane-tethered aspartyl protease in the pepsin family that cleaves APP outside the membrane to release the large extracellular domain of APP (APPs-β). The membrane-bound remnant APP CTF-β) is then cleaved by γ-secretase to produce Aβ and the APP intracellular domain (AICD). γ-Secretase a membrane-embedded aspartyl protease composed of four different membrane proteins. Presenilin NTF/CTF heterodimer (blue) is the catalytic component of the γ-secretase complex that carries out proteolysis within the APP transmembrane domain.
The predominant form of Aβ is composed of 40 amino acids, while ~5–10% is 42 amino acids, with the difference at the C-terminus, which is formed from the transmembrane domain of APP by γ-secretase (De Strooper et al., 2012). Although Aβ42 is a minor variant, this peptide is much more prone to aggregation than is Aβ40 (Jarrett et al., 1993) and is the primary component of the amyloid plaques in the AD brain (Iwatsubo et al., 1994). Aβ42 has been extensively studied for many years to understand the aggregation process; the structure of the monomer, oligomers, protofibrils, fibrils and plaques (Roychaudhuri et al., 2009); the neurotoxic species and possible neurotoxic pathways (Mucke and Selkoe, 2012). The current dogma is that one or more soluble oligomeric forms of Aβ42 are the neurotoxic entity, although this leading hypothesis has been met with reasonable skepticism (Benilova et al., 2012).
Genetics of Familial AD
The strongest evidence for the pathogenicity of Aβ in AD comes from genetic studies of early-onset familial AD (FAD) (Tanzi and Bertram, 2005). Dominant missense mutations in the APP gene are associated with FAD. Although APP is a large membrane protein, all the FAD mutations in APP are found in and around the small Aβ region and alter the production or aggregation propensity of Aβ peptides. An FAD double mutation found immediately proximal to the N-terminus of Aβ is cleaved more effectively by β-secretase, leading to increased Aβ production (Cai et al., 1993; Citron et al., 1992). Recently, a protective mutation in this region was discovered, substantially reducing the risk of developing AD, even when carriers reach their 80s or 90s (Jonsson et al., 2012). This protective mutation decreases cleavage by β-secretase, leading to lower production of Aβ. Other mutations found within the Aβ sequence increase its aggregation propensity [e.g., (Massi et al., 2002)]. Still others, found near the γ-secretase cleavage site, lead to increased ratios of Aβ42 to Aβ40 [e.g., (Suzuki et al., 1994)], thereby increasing the aggregation propensity as well.
Dominant FAD missense mutations are also found in genes encoding presenilin-1 and −2 (PS1 and PS2), multi-pass membrane proteins that comprise the catalytic component of γ-secretase (Tanzi and Bertram, 2005). Over 200 such mutations have been identified, and some can cause AD even before age 30 (Moehlmann et al., 2002). Like those FAD mutations in APP near the γ-secretase cleavage site, FAD-mutant PS1 and PS2 lead to increased Aβ42/Aβ40 and overall increased aggregation propensity (Borchelt et al., 1996; Citron et al., 1997; Tomita et al., 1997; Xia et al., 1997). Other than the strong heredity factor and early age of onset, these dominant missense mutations in APP and the presenilins result in a similar course of disease onset, progression and brain pathology, (including neurofibrillary tangles containing tau filaments) that are observed in sporadic, late-onset AD.
While the FAD cases are very rare, because the presentation, course and pathology are similar to sporadic AD, the mechanisms by which the FAD mutations result in neurodegeneration and dementia should be informative about the pathogenic mechanism of AD in general. For this reason, Aβ and Aβ production, particularly via proteolytic cleavage by γ-secretase, has been such a major focus in the field. FAD mutations alter Aβ or Aβ production, with the large majority of these mutations found in the presenilins, which alter γ-secretase activity to increase Aβ propensity to aggregation. In the next section, we discuss the details of γ-secretase biochemistry and the effects of FAD mutations on its activity.
The γ-Secretase Complex
As mentioned above, presenilin is the catalytic component of γ-secretase. Specifically, two completely conserved transmembrane aspartic acids are required for proteolytic activity (Wolfe et al., 1999), placing γ-secretase in the aspartyl protease family, even though it otherwise bears no resemblance to the many well-known soluble aspartyl proteases (e.g., pepsin, renin, HIV protease). The presence of the two transmembrane aspartates within a multi-pass membrane protein is consistent with the ability of γ-secretase to cleave within the transmembrane domain of APP in generating Aβ. Three other membrane proteins are components of γ-secretase along with presenilin: nicastrin, Aph-1 and Pen-2 (Edbauer et al., 2003; Kimberly et al., 2003; Takasugi et al., 2003). After all four components assemble in the endoplasmic reticulum, presenilin undergoes autoproteolysis (Fukumori et al., 2010; Wolfe et al., 1999) into an N-terminal fragment (NTF) and C-terminal fragment (CTF) to generate the active γ-secretase complex (Figure 1). Each of these presenilin subunits contributes one of the catalytic aspartates to the active site (Esler et al., 2000; Li et al., 2000).
γ-Secretase has many more substrates besides APP (Haapasalo and Kovacs, 2011). The most important of these is signaling from the Notch family of receptors (Selkoe and Kopan, 2003). Notch receptors are single-pass membrane proteins like APP, and proteolytic processing of Notch, triggered by interaction with cognate ligands on neighboring cells, leads to release of its intracellular domain. This released domain then translocates to the nucleus and interacts with specific transcription factors that control the expression of genes involved in cell differentiation. These signaling pathways, particularly from Notch1 receptors, are essential to proper development in all multi-cellular animals.
Processive Proteolysis by γ-Secretase
Presenilin mutations that cause FAD were first identified over 20 years ago, but the pathogenic mechanisms remain controversial and unknown. One hypothesis is that these dominant mutations cause a gain of function, specifically an increase in the ratio of 42- or 43-residue amyloid β-peptides (Aβ42 or Aβ43) to the 40-residue form (Aβ40) (Selkoe and Hardy, 2016), while an alternative hypothesis argues that loss of proteolytic function of γ-secretase is responsible for pathogenesis (Kelleher and Shen, 2017). However as we will discuss, presenilin and the γ-secretase complex have multiple proteolytic functions and produce other Aβ peptides ranging from 38–49 amino acids. Gain of function and loss of function are genetic terms that do not address the biochemical complexity of proteolytic processing of APP and other substrates by γ-secretase.
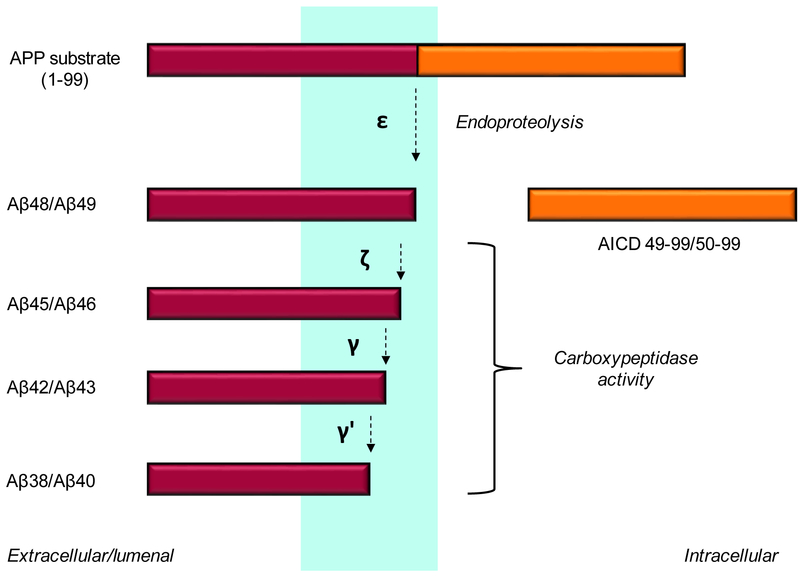
In recent years, the proteolysis of APP substrates by γ-secretase has been revealed to be much more complicated than initially believed. As noted earlier, secreted forms of Aβ range from 38–43 amino acids. However, identification of the N-terminus of the other product of γ-secretase cleavage of APP, AICD, revealed that proteolysis also occurs between residues 49 and 50 (Aβ numbering) (Weidemann et al., 2002), close to the cytosolic end of the transmembrane domain, and this cleavage event is also γ-secretase-dependent. Mass spectrometric analysis of AICD showed that a minor degree of cleavage also occurs between residues 48 and 49 (Sato et al., 2003) (Figure 2). Interestingly, FAD mutations were found to increase the proportion of AICD beginning at residue 49 over that beginning at residue 50, similar to the ability of these mutations to increase Aβ42 over Aβ40 (Sato et al., 2003). Thus, γ-secretase cleaves the APP transmembrane domain at least twice, and both of these cleavage events are altered by FAD mutations.
Figure 2. Processive proteolysis of the APP transmembrane domain by γ-secretase.
An endoproteolytic activity of the enzyme cleaves at the ε site within the transmembrane domain close to the membrane-cytosol interface to give long Aβ peptides Aβ48 and Aβ49 and the APP intracellular domain (AICD). The carboxypeptidase activity of γ-secretase then trims Aβ48 and Aβ49 in 3–4 amino acid increments.
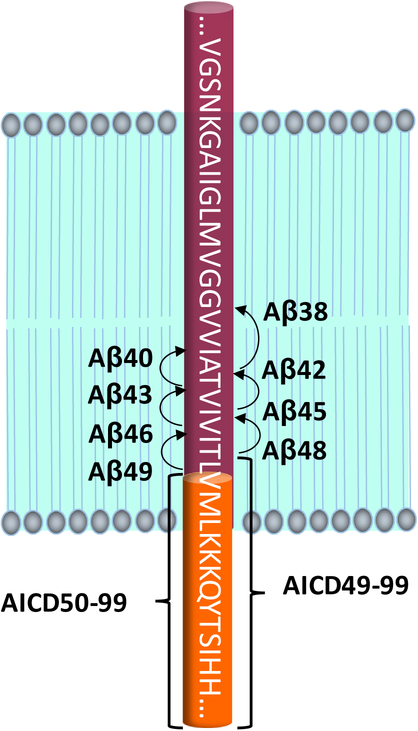
In the analysis of γ-secretase cleavage products, however, some residues were unaccounted for. Most Aβ peptides are Aβ40, while most AICD begins at residue 50. The discovery of longer forms of Aβ was an important step in solving this mystery. Modified gel electrophoresis methods allowed resolution of Aβ peptides to one amino acid resolutions, and such analysis revealed Aβ45, Aβ46, Aβ48 and Aβ49 (Kakuda et al., 2006; Qi-Takahara et al., 2005). Thus, Aβ peptides ranging all the way to where AICD begins suggested that initial proteolysis occurs near the C-terminal end of the transmembrane domain, producing AICD and either Aβ48 or Aβ49. These long Aβ peptides are then trimmed through a carboxypeptidase function of γ-secretase to produce the secreted forms of Aβ (Funamoto et al., 2004; Kakuda et al., 2006) (Figure 2) Further studies refined this idea by suggesting two pathways for Aβ production: Aβ49→Aβ46→Aβ43→Aβ40 and Aβ48→Aβ45→Aβ42→Aβ38 (Yagishita et al., 2008) (Figure 3). The expected tri- and tetrapeptides formed in this process have been detected by mass spectrometry in cell-free γ-secretase assays (Takami et al., 2009). Interestingly, the small levels of long Aβ peptides released by the protease complex before complete trimming are found in cell membrane fractions (Qi-Takahara et al., 2005), as might be expected given that these peptides contain most of the APP transmembrane domain.
Figure 3. Dual-pathway proteolysis of APP by γ-secretase.
Cleavage at the ε site occurs at one of 2 sites, resulting in Aβ49 and AICD50–99 or Aβ48 and AICD49–99. Subsequent carboxypeptidase trimming leads to two Aβ product lines: Aβ49→Aβ46→Aβ43→Aβ40 and Aβ48→Aβ45→Aβ42→Aβ38.
Reports of dissociation between Aβ42 and Aβ38 led to doubts about the precursor-product relationship between these two peptides (Czirr et al., 2008; Page et al., 2008), and other reports of dissociation between Aβ and AICD have questioned the carboxypeptidase activity of γ-secretase and the dual-pathway model [e.g., (He et al., 2010)]. However, as mentioned above, unambiguous evidence that γ-secretase converts Aβ42 to Aβ38 has come from in vitro assays (Okochi et al., 2013). Furthermore, a dissociation of Aβ and AICD is a stoichiometric impossibility, as cleavage of APP CTF-β must give one Aβ molecule for every AICD, and this has been formally proven to be the case through careful quantification (Kakuda et al., 2006). Moreover, we have found that synthetic peptides ranging from Aβ45-Aβ49 are converted to Aβ40 and Aβ42 by purified γ-secretase and in the same proportions as expected from the dual-pathway model (Fernandez et al., 2014). These results were seen whether using detergent solubilized γ-secretase preparations or purified protease complex reconstituted into lipid vesicles. Thus, the carboxypeptidase activity of γ-secretase and the dual-pathway model are unambiguously established as intrinsic properties of the enzyme independent of the membrane.
FAD γ-Secretases and Carboxypeptidase Activity
Recent findings on the more detailed effects of presenilin FAD mutations on Aβ production suggest how these mutations may cause disease. In a study from our lab (Quintero-Monzon et al., 2011), five different FAD PS1-mutant γ-secretase complexes were compared to wild type using in vitro assays and PAGE analysis of the range of Aβ peptides as well as AICD. Four of the five FAD mutations caused a reduction in AICD and total Aβ production compared to the wild-type enzyme, consistent with previous findings that the majority of PS1 mutations cause a “loss of function” in proteolysis. One of these mutations, however, produced AICD and Aβ to the same degree as the wild-type enzyme, demonstrating that such general reduction of proteolytic function is not essential for pathogenicity. However, all five mutations skewed Aβ production in favor of the longer forms (>Aβ42), suggesting that the mutations all caused a deficiency in the carboxypeptidase function of γ-secretase. Another report from the lab of Bart De Strooper (Chavez-Gutierrez et al., 2012) confirmed these findings, that presenilin FAD mutations do not necessarily inhibit general proteolysis by γ-secretase but do cause qualitative changes in the types of Aβ peptides produced, in favor of longer forms. We have further found that PS1 FAD-mutant γ-secretase complexes are dramatically deficient in their ability to trim Aβ48 and Aβ49 to Aβ40 and Aβ42 (Fernandez et al., 2014), providing important confirmation of reduced carboxypeptidase function as the common feature of disease-causing γ-secretase, independent of initial cleavage of APP CTF-β to form AICD and Aβ48/49.
Collectively, these findings show that the increase in Aβ42-to-Aβ40 observed by presenilin FAD mutations may be a consequence of reduced trimming function. However, the relationship between these two Aβ peptides is not the only change. In general, the proportion of longer forms of Aβ, including membrane-associated forms Aβ45-Aβ49, are substantially increased, raising the question of what pathogenic role, if any, these Aβ peptides may play. In any event, the apparent reduction in carboxypeptidase function caused by presenilin FAD mutations suggests that therapeutic agents targeting γ-secretase should correct this biochemical defect; that is, rather than searching for inhibitors of γ-secretase, stimulators of the carboxypeptidase function should be sought. A class of γ-secretase modulators do this, but only for the last of the trimming step (e.g., Aβ42→Aβ38), making their therapeutic potential entirely dependent on Aβ42 being the primary pathogenic species. A more general stimulator of γ-secretase carboxypeptidase activity might be more appropriate, as it would not depend on knowing exactly which form of Aβ is responsible for the cascade of events that result in neurotoxicity, neurodegeneration and dementia.
Pathogenic and Therapeutic Implications
Presenilin and the γ-secretase complex are central to the pathogenesis of AD, as dominant mutations in the presenilins alter γ-secretase activity and Aβ production and cause hereditary AD in midlife that is otherwise indistinguishable from sporadic, late-life AD. This membrane-embedded protease has been considered an AD therapeutic target for AD for over 15 years. Potent brain-penetrant inhibitors have been developed and brought into clinical trials. However, these compounds showed serious toxic effects due to inhibition of proteolysis and signaling from the Notch1 receptor (Coric et al., 2012; Doody et al., 2013). More worrisome, cognitive worsening was observed, which mouse models suggest may be due to increased levels of APP γ-secretase substrates (Mitani et al., 2012). Modulators of γ-secretase have been discovered that selectively lower Aβ42 and some have entered clinical trials (Bursavich et al., 2016). Although these compounds are apparently safe, their potential to prevent or treat AD is dependent on Aβ42 being the primary pathogenic entity. New findings on the effects of AD-causing presenilin mutations reveal a common defect in carboxypeptidase function, resulting in increased proportions of other Aβ peptides, even longer than Aβ42. The pathogenicity of these longer Aβ peptides is unknown.
While possible roles of these long Aβ peptides in AD should be investigated, parallel efforts to identify compounds that rescue the deficient carboxypeptidase function of FAD-mutant γ-secretase complexes may lead to the development of effective therapeutics for the prevention of FAD. Regardless of the pathogenic form of Aβ, rescuing deficient carboxypeptidase function so the mutant enzyme works more like the wild-type enzyme should be beneficial to those carrying these mutations. Presenilin mutation carriers are fated to develop this devastating form of the disease in mid-life. While only 1–2% of all AD cases are FAD, this still represents many tens of thousands of individuals who otherwise currently have no hope of escaping this fate. The discovery of the specific biochemical defect of FAD-mutant γ-secretase paves the way for drug discovery efforts specifically for FAD.
References
- Benilova I, Karran E, De Strooper B, 2012. The toxic Abeta oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat Neurosci 15, 349–357. [DOI] [PubMed] [Google Scholar]
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS, 1996. Familial Alzheimer’s disease-linked presenilin 1 variants elevate Abeta1–42/1–40 ratio in vitro and in vivo. Neuron 17, 1005–1013. [DOI] [PubMed] [Google Scholar]
- Bursavich MG, Harrison BA, Blain JF, 2016. Gamma Secretase Modulators: New Alzheimer’s Drugs on the Horizon? J Med Chem 59, 7389–7409. [DOI] [PubMed] [Google Scholar]
- Cai XD, Golde TE, Younkin SG, 1993. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science 259, 514–516. [DOI] [PubMed] [Google Scholar]
- Chavez-Gutierrez L, Bammens L, Benilova I, Vandersteen A, Benurwar M, Borgers M, Lismont S, Zhou L, Van Cleynenbreugel S, Esselmann H, Wiltfang J, Serneels L, Karran E, Gijsen H, Schymkowitz J, Rousseau F, Broersen K, De Strooper B, 2012. The mechanism of gamma-secretase dysfunction in familial Alzheimer disease. EMBO J 31, 2261–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ, 1992. Mutation of the beta-amyloid precursor protein in familial Alzheimer’s disease increases beta-protein production. Nature 360, 672–674. [DOI] [PubMed] [Google Scholar]
- Citron M, Westaway D, Xia W, Carlson G, Diehl T, Levesque G, Johnson-Wood K, Lee M, Seubert P, Davis A, Kholodenko D, Motter R, Sherrington R, Perry B, Yao H, Strome R, Lieberburg I, Rommens J, Kim S, Schenk D, Fraser P, St George Hyslop P, Selkoe DJ, 1997. Mutant presenilins of Alzheimer’s disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nat Med 3, 67–72. [DOI] [PubMed] [Google Scholar]
- Coric V, van Dyck CH, Salloway S, Andreasen N, Brody M, Richter RW, Soininen H, Thein S, Shiovitz T, Pilcher G, Colby S, Rollin L, Dockens R, Pachai C, Portelius E, Andreasson U, Blennow K, Soares H, Albright C, Feldman HH, Berman RM, 2012. Safety and tolerability of the gamma-secretase inhibitor avagacestat in a phase 2 study of mild to moderate Alzheimer disease. Arch Neurol 69, 1430–1440. [DOI] [PubMed] [Google Scholar]
- Czirr E, Cottrell BA, Leuchtenberger S, Kukar T, Ladd TB, Esselmann H, Paul S, Schubenel R, Torpey JW, Pietrzik CU, Golde TE, Wiltfang J, Baumann K, Koo EH, Weggen S, 2008. Independent generation of Abeta42 and Abeta38 peptide species by gamma-secretase. J Biol Chem 283, 17049–17054. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Iwatsubo T, Wolfe MS, 2012. Presenilins and γ-Secretase: Structure, Function, and Role in Alzheimer Disease. Cold Spring Harb Perspect Med 2, a006304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doody RS, Raman R, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, He F, Sun X, Thomas RG, Aisen PS, Siemers E, Sethuraman G, Mohs R, 2013. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N Engl J Med 369, 341–350. [DOI] [PubMed] [Google Scholar]
- Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C, 2003. Reconstitution of gamma-secretase activity. Nat Cell Biol 5, 486–488. [DOI] [PubMed] [Google Scholar]
- Esler WP, Kimberly WT, Ostaszewski BL, Diehl TS, Moore CL, Tsai J-Y, Rahmati T, Xia W, Selkoe DJ, Wolfe MS, 2000. Transition-state analogue inhibitors of γ-secretase bind directly to presenilin-1. Nature Cell Biology 2, 428–434. [DOI] [PubMed] [Google Scholar]
- Fernandez MA, Klutkowski JA, Freret T, Wolfe MS, 2014. Alzheimer presenilin-1 mutations dramatically reduce trimming of long amyloid beta-peptides (Abeta) by gamma-secretase to increase 42-to-40-residue Abeta. J Biol Chem 289, 31043–31052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumori A, Fluhrer R, Steiner H, Haass C, 2010. Three-amino acid spacing of presenilin endoproteolysis suggests a general stepwise cleavage of gamma-secretase-mediated intramembrane proteolysis. J Neurosci 30, 7853–7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funamoto S, Morishima-Kawashima M, Tanimura Y, Hirotani N, Saido TC, Ihara Y, 2004. Truncated carboxyl-terminal fragments of beta-amyloid precursor protein are processed to amyloid beta-proteins 40 and 42. Biochemistry 43, 13532–13540. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, 2006. A century of Alzheimer’s disease. Science 314, 777–781. [DOI] [PubMed] [Google Scholar]
- Haapasalo A, Kovacs DM, 2011. The many substrates of presenilin/gamma-secretase. J Alzheimers Dis 25, 3–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G, Luo W, Li P, Remmers C, Netzer WJ, Hendrick J, Bettayeb K, Flajolet M, Gorelick F, Wennogle LP, Greengard P, 2010. Gamma-secretase activating protein is a therapeutic target for Alzheimer’s disease. Nature 467, 95–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y, 1994. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43). Neuron 13, 45–53. [DOI] [PubMed] [Google Scholar]
- Jarrett JT, Berger EP, Lansbury PT Jr., 1993. The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer’s disease. Biochemistry 32, 4693–4697. [DOI] [PubMed] [Google Scholar]
- Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, Stefansson H, Sulem P, Gudbjartsson D, Maloney J, Hoyte K, Gustafson A, Liu Y, Lu Y, Bhangale T, Graham RR, Huttenlocher J, Bjornsdottir G, Andreassen OA, Jonsson EG, Palotie A, Behrens TW, Magnusson OT, Kong A, Thorsteinsdottir U, Watts RJ, Stefansson K, 2012. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 488, 96–99. [DOI] [PubMed] [Google Scholar]
- Kakuda N, Funamoto S, Yagishita S, Takami M, Osawa S, Dohmae N, Ihara Y, 2006. Equimolar production of amyloid beta-protein and amyloid precursor protein intracellular domain from beta-carboxyl-terminal fragment by gamma-secretase. J Biol Chem 281, 14776–14786. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ 3rd, Shen J, 2017. Presenilin-1 mutations and Alzheimer’s disease. Proc Natl Acad Sci U S A 114, 629–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberly WT, LaVoie MJ, Ostaszewski BL, Ye W, Wolfe MS, Selkoe DJ, 2003. γ-Secretase is a membrane protein complex comprised of presenilin, nicastrin, aph-1, and pen-2. Proc Natl Acad Sci U S A 100, 6382–6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YM, Xu M, Lai MT, Huang Q, Castro JL, DiMuzio-Mower J, Harrison T, Lellis C, Nadin A, Neduvelil JG, Register RB, Sardana MK, Shearman MS, Smith AL, Shi XP, Yin KC, Shafer JA, Gardell SJ, 2000. Photoactivated gamma-secretase inhibitors directed to the active site covalently label presenilin 1. Nature 405, 689–694. [DOI] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J, 1999. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol 155, 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massi F, Klimov D, Thirumalai D, Straub JE, 2002. Charge states rather than propensity for beta-structure determine enhanced fibrillogenesis in wild-type Alzheimer’s beta-amyloid peptide compared to E22Q Dutch mutant. Protein Sci 11, 1639–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters CL, Selkoe DJ, 2012. Biochemistry of Amyloid beta-Protein and Amyloid Deposits in Alzheimer Disease. Cold Spring Harb Perspect Med 2, a006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL, 1999. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol 46, 860–866. [DOI] [PubMed] [Google Scholar]
- Mitani Y, Yarimizu J, Saita K, Uchino H, Akashiba H, Shitaka Y, Ni K, Matsuoka N, 2012. Differential Effects between gamma-Secretase Inhibitors and Modulators on Cognitive Function in Amyloid Precursor Protein-Transgenic and Nontransgenic Mice. J Neurosci 32, 2037–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehlmann T, Winkler E, Xia X, Edbauer D, Murrell J, Capell A, Kaether C, Zheng H, Ghetti B, Haass C, Steiner H, 2002. Presenilin-1 mutations of leucine 166 equally affect the generation of the Notch and APP intracellular domains independent of their effect on Abeta 42 production. Proc Natl Acad Sci U S A 99, 8025–8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucke L, Selkoe DJ, 2012. Neurotoxicity of amyloid beta-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med 2, a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller UC, Zheng H, 2012. Physiological functions of APP family proteins. Cold Spring Harb Perspect Med 2, a006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, Buxbaum JD, 2000. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline [see comments]. Jama 283, 1571–1577. [DOI] [PubMed] [Google Scholar]
- Okochi M, Tagami S, Yanagida K, Takami M, Kodama TS, Mori K, Nakayama T, Ihara Y, Takeda M, 2013. gamma-secretase modulators and presenilin 1 mutants act differently on presenilin/gamma-secretase function to cleave Abeta42 and Abeta43. Cell Rep 3, 42–51. [DOI] [PubMed] [Google Scholar]
- Page RM, Baumann K, Tomioka M, Perez-Revuelta BI, Fukumori A, Jacobsen H, Flohr A, Luebbers T, Ozmen L, Steiner H, Haass C, 2008. Generation of Abeta38 and Abeta42 is independently and differentially affected by familial Alzheimer disease-associated presenilin mutations and gamma-secretase modulation. J Biol Chem 283, 677–683. [DOI] [PubMed] [Google Scholar]
- Qi-Takahara Y, Morishima-Kawashima M, Tanimura Y, Dolios G, Hirotani N, Horikoshi Y, Kametani F, Maeda M, Saido TC, Wang R, Ihara Y, 2005. Longer forms of amyloid beta protein: implications for the mechanism of intramembrane cleavage by gamma-secretase. J Neurosci 25, 436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero-Monzon O, Martin MM, Fernandez MA, Cappello CA, Krzysiak AJ, Osenkowski P, Wolfe MS, 2011. Dissociation between the processivity and total activity of gamma-secretase: implications for the mechanism of Alzheimer’s disease-causing presenilin mutations. Biochemistry 50, 9023–9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademakers R, Neumann M, Mackenzie IR, 2012. Advances in understanding the molecular basis of frontotemporal dementia. Nat Rev Neurol 8, 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychaudhuri R, Yang M, Hoshi MM, Teplow DB, 2009. Amyloid beta-protein assembly and Alzheimer disease. J Biol Chem 284, 4749–4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Dohmae N, Qi Y, Kakuda N, Misonou H, Mitsumori R, Maruyama H, Koo EH, Haass C, Takio K, Morishima-Kawashima M, Ishiura S, Ihara Y, 2003. Potential link between amyloid beta-protein 42 and C-terminal fragment gamma 49–99 of beta-amyloid precursor protein. J Biol Chem 278, 24294–24301. [DOI] [PubMed] [Google Scholar]
- Selkoe D, Kopan R, 2003. Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci 26, 565–597. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Hardy J, 2016. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8, 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Cheung TT, Cai XD, Odaka A, Otvos L Jr., Eckman C, Golde TE, Younkin SG, 1994. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science 264, 1336–1340. [DOI] [PubMed] [Google Scholar]
- Takami M, Nagashima Y, Sano Y, Ishihara S, Morishima-Kawashima M, Funamoto S, Ihara Y, 2009. gamma-Secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment. J Neurosci 29, 13042–13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, Takahashi Y, Thinakaran G, Iwatsubo T, 2003. The role of presenilin cofactors in the gamma-secretase complex. Nature 422, 438–441. [DOI] [PubMed] [Google Scholar]
- Tanzi RE, Bertram L, 2005. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell 120, 545–555. [DOI] [PubMed] [Google Scholar]
- Tomita T, Maruyama K, Saido TC, Kume H, Shinozaki K, Tokuhiro S, Capell A, Walter J, Grunberg J, Haass C, Iwatsubo T, Obata K, 1997. The presenilin 2 mutation (N141I) linked to familial Alzheimer disease (Volga German families) increases the secretion of amyloid beta protein ending at the 42nd (or 43rd) residue. Proc Natl Acad Sci U S A 94, 2025–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann A, Eggert S, Reinhard FB, Vogel M, Paliga K, Baier G, Masters CL, Beyreuther K, Evin G, 2002. A Novel var epsilon-Cleavage within the Transmembrane Domain of the Alzheimer Amyloid Precursor Protein Demonstrates Homology with Notch Processing. Biochemistry 41, 2825–2835. [DOI] [PubMed] [Google Scholar]
- Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ, 1999. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature 398, 513–517. [DOI] [PubMed] [Google Scholar]
- Xia W, Zhang J, Kholodenko D, Citron M, Podlisny MB, Teplow DB, Haass C, Seubert P, Koo EH, Selkoe DJ, 1997. Enhanced production and oligomerization of the 42-residue amyloid beta-protein by Chinese hamster ovary cells stably expressing mutant presenilins. J Biol Chem 272, 7977–7982. [DOI] [PubMed] [Google Scholar]
- Yagishita S, Morishima-Kawashima M, Ishiura S, Ihara Y, 2008. Abeta46 is processed to Abeta40 and Abeta43, but not to Abeta42, in the low density membrane domains. J Biol Chem 283, 733–738. [DOI] [PubMed] [Google Scholar]