Abstract
BACKGROUND
Besides body mass index (BMI), other discriminators of cardiovascular risk are needed in obese patients, who may or may not undergo consideration for bariatric surgery. Coronary microvascular dysfunction (CMD), defined as impaired coronary flow reserve (CFR) in the absence of flow-limiting coronary artery disease, identifies patients at risk for adverse events independently of traditional risk factors.
OBJECTIVES
The study sought to investigate the relationship among obesity, CMD, and adverse outcomes.
METHODS
Consecutive patients undergoing evaluation for coronary artery disease with cardiac stress positron emission tomography demonstrating normal perfusion (N = 827) were followed for median 5.6 years for events, including death and hospitalization for myocardial infarction or heart failure.
RESULTS
An inverted independent J-shaped relationship was observed between BMI and CFR, such that in obese patients CFR decreased linearly with increasing BMI (adjusted p < 0.0001). In adjusted analyses, CFR but not BMI remained independently associated with events (for a 1-U decrease in CFR, adjusted hazard ratio: 1.95; 95% confidence interval: 1.41 to 2.69; p < 0.001; for a 10-U increase in BMI, adjusted hazard ratio: 1.20; 95% confidence interval: 0.95 to 1.50; p = 0.125) and improved model discrimination (C-index 0.71 to 0.74). In obese patients, individuals with impaired CFR demonstrated a higher adjusted rate of events (5.7% vs. 2.6%; p = 0.002), even in those not currently meeting indications for bariatric surgery (6.4% vs. 2.6%; p = 0.04).
CONCLUSIONS
In patients referred for testing, CMD was independently associated with elevated BMI and adverse outcomes, and was a better discriminator of risk than BMI and traditional risk factors. CFR may facilitate management of obese patients beyond currently used markers of risk.
Keywords: bariatric surgery, body mass index, coronary microvascular dysfunction, obesity, prognosis
More than half a billion adults worldwide are obese, many at increased cardiovascular risk over their lifespan (1). Obesity, defined as a body mass index (BMI) of 30 kg/m2 or higher, is closely associated with chronic metabolic disease, yet some obese patients do not have evident cardiometabolic effects and have been deemed “metabolically healthy” (2,3). BMI is a convenient measure that is widely used to diagnose obesity and assess patient candidacy for medical and surgical (4–6) interventions, but it may not be a reliable marker of cardiovascular prognosis in all patients with increased adiposity (7). The pathophysiological mechanisms underlying increased risk in obesity are complex, and may involve excess secretion of adipocyterelated factors leading to increased vascular oxidative stress, up-regulated neurohormonal activity, and low-grade systemic inflammation, which may lead to increased sympathetic nervous system activation, altered vascular tone, and coronary microvascular dysfunction (CMD) (1).
Emerging data from small studies have demonstrated the presence of CMD in some obese individuals (8–10). Coronary flow reserve (CFR), quantified as the ratio of hyperemic to rest myocardial blood flow, provides a combined physiological measure of large-and small-vessel ischemia, and in the absence of obstructive coronary artery disease (CAD), is a noninvasive marker of CMD. CFR measurements by stress testing with cardiac positron emission tomography (PET) distinguish patients at low or high risk for major adverse cardiovascular events including cardiac death (11,12), beyond comprehensive clinical assessment, left ventricular ejection fraction (LVEF), traditional measures of stress-induced ischemia, or plaque severity on coronary invasive angiography (13). Impaired CFR is also associated with systemic inflammation (14,15), low-level troponin elevation (16), and left ventricular diastolic dysfunction (17), and may precede high-risk CAD or heart failure, especially in diabetic (18) or obese patients.
We sought to investigate the relationship between BMI and CMD, and their contributions to adverse events in patients with and without obesity. We hypothesized that CMD, as assessed by impaired CFR, is associated with higher BMI and increased cardiovascular risk independently of BMI. We also explored the prognostic value of impaired CFR across guideline-directed BMI thresholds used in patient selection for bariatric surgery (19).
METHODS
STUDY POPULATION.
The study population included consecutive patients at Brigham and Women’s Hospital (Boston, Massachusetts) who underwent PET myocardial perfusion imaging for evaluation of suspected CAD based on clinical symptoms between 2007 and 2014. The most common indication for testing was the evaluation of chest pain, dyspnea, or their combination. Patient history, BMI, medication use, and select laboratory values were ascertained at the time of PET imaging. From 2,474 patients, a final cohort of 827 was established after excluding those with known CAD, including prior revascularization or myocardial infarction; prior history of heart failure or severe valvular disease; history of active malignancy, kidney disease with estimated glomerular filtration rate (eGFR) <45 ml/min/1.73 m2, or end-stage liver or lung disease; PET evidence of flow-limiting CAD (semi-quantitative perfusion summed stress score >2) or LVEF <40%; or no clinical follow-up (Online Figure 1). The eGFR was determined using the Chronic Kidney Disease Epidemiology Collaboration equation (20). The study was approved by the Partners HealthCare Institutional Review Board and conducted in accordance with institutional guidelines.
OBESE PATIENT SUBSET.
After assessing the relationships among BMI, CFR, and adverse outcomes in the overall population, we sought to explore how CFR may stratify risk in obese patients, particularly as related to bariatric surgery candidacy. Among 398 obese patients (BMI ≥30 kg/m2), patients were stratified into 2 categories: those recommended (n = 233) versus not recommended (n = 165) for bariatric surgery as defined by guidelines from the American Association of Clinical Endocrinologists, the Obesity Society, and the American Society for Metabolic and Bariatric Surgery (19). Those patients meeting a grade A recommendation for bariatric surgery (i.e., those with BMI ≥40 kg/m2 or those with BMI 35 to 39 kg/m2 and 1 or more severe obesity-related comorbidities) were considered eligible for bariatric surgery for this analysis (Online Figure 1).
CARDIAC STRESS PET AND MEASUREMENT OF CFR.
Patients were imaged with a standard hybrid whole-body PET-computed tomography scanner (Discovery RX or STE LightSpeed 64, GE Healthcare, Milwaukee, Wisconsin) with 13N-ammonia or 82rubidium as flow tracers at rest and pharmacologic stress, as previously described (21). Summed rest, stress, and difference scores, with higher scores reflecting larger areas of myocardial scar, scar plus ischemia, or ischemia, respectively, were computed; summed stress scores ≤2 were considered normal (22). Rest LVEFs were calculated from gated myocardial perfusion images with commercially available software (Corridor4DM, INVIA Medical Imaging Solutions, Ann Arbor, Michigan). Coronary hyperemia was achieved with vasodilation using standard protocols. Absolute global myocardial blood flow (in ml/min/g of tissue) was quantified at rest and at peak hyperemia using commercial software, as previously described (21). Per-patient global CFR was calculated as the ratio of stress to rest absolute myocardial blood flow for the entire left ventricle. Quantitative measures of CFR were obtained from routine post-processing of PET scans at no additional clinical cost, imaging time, or radiation exposure to patients.
OUTCOMES.
Patients were followed for the occurrence of a first major adverse event, defined as a composite of death or hospitalization for nonfatal myocardial infarction or heart failure. Ascertainment of clinical endpoints was determined by blinded expert committee adjudication of the longitudinal medical record, Partners HealthCare Research Patient Data Registry, the National Death Index, mail surveys, and telephone calls. For an event to be classified as admission for nonfatal myocardial infarction or heart failure, discharge with a primary hospitalization diagnosis of myocardial infarction or heart failure, respectively, was required. In addition, only events meeting the 2012 Third Universal Definition of Myocardial Infarction (23) or defined clinical criteria for the presence of symptoms, signs, and escalation of therapy for heart failure, were classified as such. All hospitalization events occurred >30 days following imaging.
STATISTICAL ANALYSIS.
Baseline characteristics were reported as rate with percentage (%) for categorical variables and median with interquartile ranges for continuous variables. We used chi-square and Wilcoxon rank sum tests to evaluate for differences in categorical and continuous baseline characteristics, respectively. BMI and CFR were treated as continuous variables. Unadjusted and multivariable-adjusted relationships between BMI and CFR were evaluated using restricted cubic spline linear regression models with 3 knots. The relationships between BMI or CFR and events were evaluated using unadjusted and multivariable-adjusted Poisson regression models, and plotted using adjusted Poisson regression models with restricted cubic splines with 3 knots. The variables and number of knots were selected based on optimal values of the Akaike information criterion after including clinically important covariates. Cox proportional hazards models were used to examine the association between covariates and events, sequentially adding demographic and clinical factors, followed by BMI and then CFR. The final model was adjusted for important demographic factors (age, sex, race) and clinical factors (history of hypertension, diabetes, peripheral vascular disease, chronic obstructive pulmonary disease, atrial fibrillation, tobacco use, beta-blocker use, eGFR, and LVEF), as well as BMI and CFR. We also assessed for an interaction between continuous measures of BMI and CFR in the adjusted model.
In the cohort of obese patients, we performed an exploratory analysis where we stratified patients by cutoffs of BMI and CFR and compared the rate of adjusted annualized events. From the primary analysis, a CFR cutpoint of 1.7 was identified as an optimal threshold above which there was a significantly increased hazard, with an annualized event risk of approximately 3%, the threshold of high risk in patients being evaluated for suspected ischemic heart disease (24). A CFR of 1.7 has also been described as representing an ischemic threshold in patients undergoing clinical evaluation for symptoms (25). Adjusted annualized event rates in those with CFR <1.7 versus ≥1.7 were compared among categories of obese patients: 1) BMI ≥40 kg/m2, bariatric surgery eligible; and 2) BMI 30 to 39 kg/m2, may be bariatric surgery eligible depending on obesity-related comorbidity, as previously described (19). Event rates were adjusted using the same model as in the primary analysis. A value of p < 0.05 was consistent with statistical significance, and all tests were 2 sided. Stata software version 14.2 (StataCorp, College Station, Texas) was used for all analyses.
RESULTS
BASELINE CHARACTERISTICS.
The distribution of baseline characteristics in the overall and obese cohorts is shown in Table 1. The median age of patients in the overall cohort was 62 (IQR: 54 to 72) years, 70% were women, 56% were white, and the median BMI was 30 (IQR: 25 to 36) kg/m2. The 3 most common obesity-related comorbidities among participants were hypertension (79%), dyslipidemia (58%), and diabetes mellitus (32%). The median LVEF was 63% (IQR: 57% to 70%). The median stress and rest global myocardial blood flow were 2.2 (IQR: 1.7 to 2.9) ml/min/g and 1.1 (0.8 to 1.4) ml/min/g, respectively, with a median CFR of 2.1 (1.6 to 2.5). Obese patients (48%) were more likely to be younger, female, and nonwhite, with higher rates of hypertension, diabetes, use of antihypertensive agents and insulin, higher eGFR and lower CFR (median 2.0 [IQR: 1.5 to 2.5]). CFR values presented did not significantly differ with correction for resting rate-pressure product.
TABLE 1.
Baseline Characteristics
| Obese |
||||
|---|---|---|---|---|
| Overall (N = 827) | No (n = 429) | Yes (n = 398) | p Value* | |
| Demographics | ||||
| Age, yrs | 62.2 (53.5–71.5) | 65.0 (55.3–75.2) | 59.6 (51.2–66.6) | <0.01 |
| Female | 576 (69.6) | 278 (64.8) | 298 (74.9) | <0.01 |
| White | 459 (55.5) | 266 (62.0) | 193 (48.5) | <0.01 |
| Body mass index, kg/m2 | 29.6 (25.4–36.0) | 25.6 (23.0–27.7) | 36.3 (32.7–42.7) | <0.01 |
| Medical history | ||||
| Hypertension | 654 (79.1) | 309 (72.0) | 345 (86.7) | <0.01 |
| Dyslipidemia | 477 (57.7) | 238 (55.5) | 239 (60.1) | 0.18 |
| Diabetes | 263 (31.8) | 84 (19.6) | 179 (45.0) | <0.01 |
| Peripheral vascular disease | 43 (3.8) | 21 (4.9) | 10 (2.5) | 0.07 |
| Stroke | 39 (4.7) | 26 (6.1) | 13 (3.3) | 0.06 |
| Chronic obstructive pulmonary disease | 81 (9.8) | 35 (8.2) | 46 (11.6) | 0.10 |
| Atrial fibrillation | 26 (3.1) | 11 (2.6) | 15 (3.8) | 0.32 |
| Tobacco use | 72 (8.7) | 36 (8.4) | 36 (9.0) | 0.74 |
| Medications | ||||
| Aspirin | 434 (52.5) | 236 (55.0) | 198 (49.7) | 0.13 |
| Beta-blocker | 349 (42.2) | 168 (39.2) | 181 (45.5) | 0.07 |
| RAS blocker | 247 (29.9) | 102 (23.8) | 145 (36.4) | <0.01 |
| Statin | 414 (50.1) | 207 (48.3) | 207 (52.0) | 0.28 |
| Insulin | 90 (10.9) | 22 (5.1) | 68 (17.1) | <0.01 |
| Nitrate | 49 (5.9) | 26 (6.1) | 23 (5.8) | 0.86 |
| Laboratory values | ||||
| Serum creatinine, mg/dl | 0.8 (0.7–0.9) | 0.8 (0.7–1.0) | 0.8 (0.7–0.9) | 0.41 |
| eGFR, ml/min/1.73 m2 | 84.4 (70.4–97.1) | 81.9 (70.0–94.2) | 87.5 (70.9–99.7) | <0.01 |
| Noninvasive imaging parameters | ||||
| Left ventricular ejection fraction, % | 63.0 (57.0–70.0) | 63.0 (57.0–70.0) | 62.0 (57.0–68.0) | 0.09 |
| Rest myocardial blood flow, ml/min/g | 1.1 (0.8–1.4) | 1.1 (0.9–1.5) | 1.0 (0.8–1.3) | <0.01 |
| Stress myocardial blood flow, ml/min/g | 2.2 (1.7–2.9) | 2.5 (1.9–3.1) | 2.0 (1.5–2.6) | <0.01 |
| Stress coronary vascular resistance, mm Hg/(ml/min/g)† | 39.7 (30.3–54.1) | 36.0 (27.3–46.0) | 45.4 (35.3–61.7) | <0.01 |
| Coronary flow reserve | 2.1 (1.6–2.5) | 2.1 (1.7–2.6) | 2.0 (1.5–2.5) | 0.02 |
Values are median (interquartile range) or n (%).
Comparison between obese groups, and based on the chi-square test for categorical variables and the Wilcoxon rank sum test for continuous variables.
Stress coronary vascular resistance is calculated by dividing stress mean arterial pressure by coronary flow reserve.
eGFR = estimated glomerular filtration rate; RAS = renin-angiotensin system.
RELATIONSHIP BETWEEN BMI AND CFR.
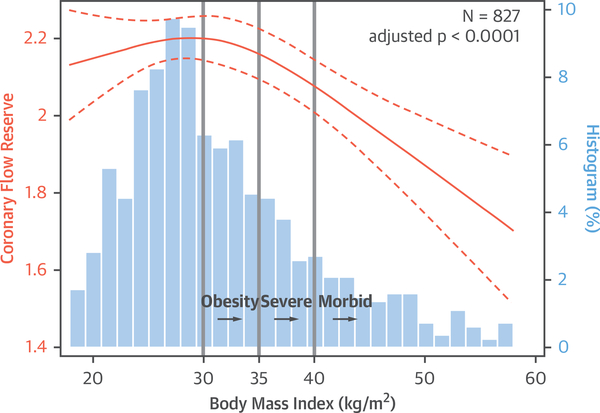
Figure 1 illustrates the independent relationship between BMI and CFR in the overall cohort across the distribution of BMI, adjusted for demographic and clinical risk factors. An inverted J curve was seen, with an inflexion point occurring at BMI ≥30 kg/m2, such that higher BMI was independently associated with lower CFR (adjusted p < 0.0001). In obese patients, CFR decreased linearly with increasing BMI.
FIGURE 1. Coronary Flow Reserve Is Inversely Associated With Body Mass Index in Obese Patients.
An inverted J–shaped relationship between coronary flow reserve and body mass index is illustrated using a restricted cubic spline linear regression model with 95% confidence intervals (orange); patient frequency histograms are shown for body mass index (blue). Model is adjusted for demographic and clinical risk factors (age, sex, race, hypertension, diabetes, peripheral vascular disease, chronic obstructive pulmonary disease, atrial fibrillation, tobacco use, estimated glomerular filtration rate, beta-blocker use, and left ventricular ejection fraction).
BMI, CFR, AND CLINICAL EVENTS.
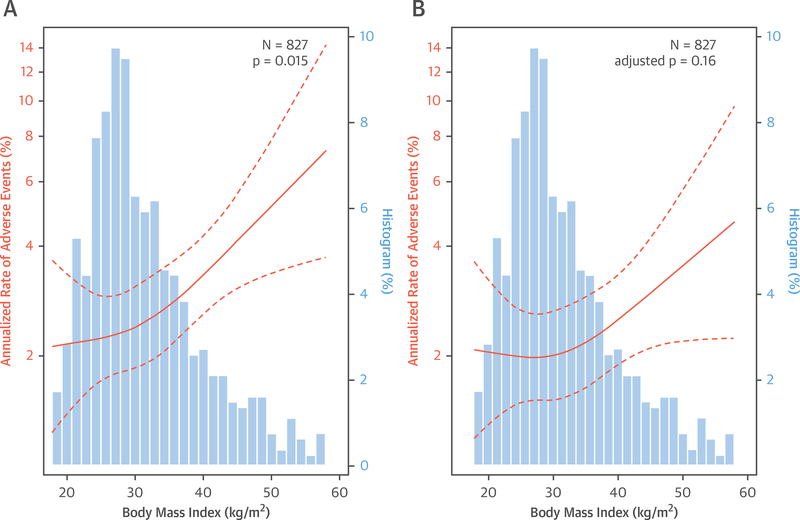
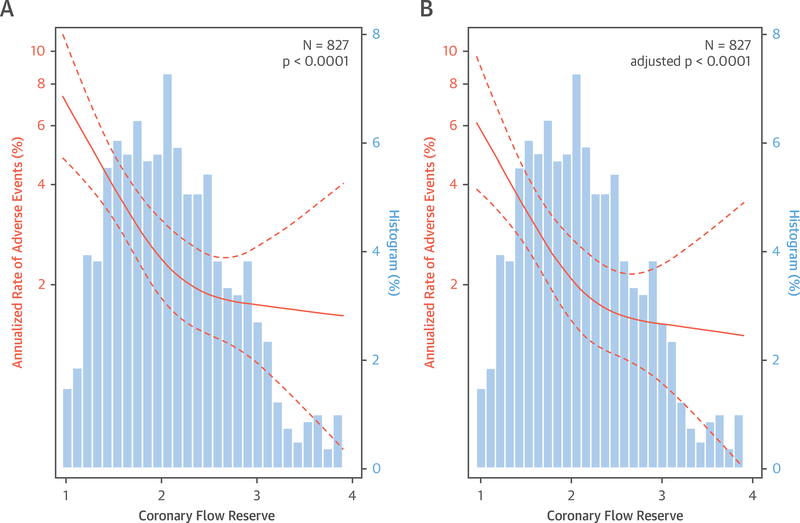
Over a median follow-up of 5.6 (IQR: 3.9 to 7.1) years, 135 patients met the primary composite endpoint of death or hospitalization for nonfatal myocardial infarction or heart failure, including 72 deaths (Online Table 1, Online Figure 2). In age-adjusted analysis, the annualized rate of composite events was significantly associated with both increasing BMI (p = 0.01) (Figure 2A) and decreasing CFR (p < 0.01) (Figure 3A).
FIGURE 2. Relationship Between Body Mass Index and Annualized Rate of Adverse Events.
Relationship (A) adjusted for age only and (B) adjusted for demographic and clinical risk factors, including coronary flow reserve (also age, sex, race, hypertension, diabetes, peripheral vascular disease, chronic obstructive pulmonary disease, atrial fibrillation, tobacco use, estimated glomerular filtration rate, beta-blocker use and left ventricular ejection fraction). Restricted cubic spline Poisson regression models with 95% confidence intervals are shown in orange; patient frequency histograms appear in blue.
FIGURE 3. Relationship Between Coronary Flow Reserve and Annualized Rate of Adverse Events.
Relationship (A) adjusted for age only and (B) adjusted for demographic and clinical risk factors, including body mass index (also age, sex, race, hypertension, diabetes, peripheral vascular disease, chronic obstructive pulmonary disease, atrial fibrillation, tobacco use, estimated glomerular filtration rate, beta-blocker use, and left ventricular ejection fraction). Restricted cubic spline Poisson regression models with 95% confidence intervals are shown in orange; patient frequency histograms appear in blue.
We sequentially added demographic and clinical factors, followed by BMI and then CFR to Cox proportional hazards models. For BMI, associations remained significant after the inclusion of clinically and statistically important covariates into a multivariable model including age, sex, race, and history of hypertension, diabetes, peripheral vascular disease, chronic obstructive pulmonary disease, atrial fibrillation, tobacco use, beta-blocker use, eGFR, and LVEF (adjusted hazard ratio [HR] for 10-U increase in BMI: 1.31; 95% confidence interval [CI]: 1.05 to 1.64; p = 0.018) (Table 2). Accordingly, after adjustment for baseline demographic and clinical variables, patients with increased BMI experienced increased annualized rates of events.
TABLE 2.
Multivariable-Adjusted Associations of BMI and CFR With Adverse Events
| Model Statistics |
|||||||
|---|---|---|---|---|---|---|---|
| Sequential Models for Composite Events | BMI* Hazard Ratio (95% CI) | p Value | CFR† Hazard Ratio (95% CI) | p Value | Likelihood Ratio Chi-Square | p Value‡ | Harrell’s C-Index |
| Multivariable model 1: demographic factors | – | – | – | – | 59.20 | <0.001 | 0.65 |
| Multivariable model 2: model 1 + clinical factors | – | – | – | – | 108.67 | <0.001 | 0.71 |
| Multivariable model 3: model 2 + BMI | 1.31 (1.05–1.64) | 0.018 | – | – | 114.02 | <0.001 | 0.71 |
| Multivariable model 4: model 3 + CFR | 1.20 (0.95–1.50) | 0.125 | 1.95 (1.41–2.69) | <0.001 | 132.17 | <0.001 | 0.74 |
Model 1: adjusted for demographic factors including age, sex, and race. Model 2: adjusted for demographic and clinical factors including age, sex, race, hypertension, diabetes, peripheral vascular disease, chronic obstructive pulmonary disease, tobacco use, atrial fibrillation, estimated glomerular filtration rate, b-blocker use, and left ventricular ejection fraction. Model 3: adjusted for demographic and clinical factors as in model 2, and body mass index (BMI). Model 4: adjusted for demographic, clinical factors and BMI as in model 3, and coronary flow reserve (CFR). No significant interaction was observed between CFR and BMI.
Per +10 U.
Per −1 U.
For likelihood ratio test between sequential models; the first value is for comparison of multivariable model 1 to the univariate analysis of BMI.
CI = confidence interval.
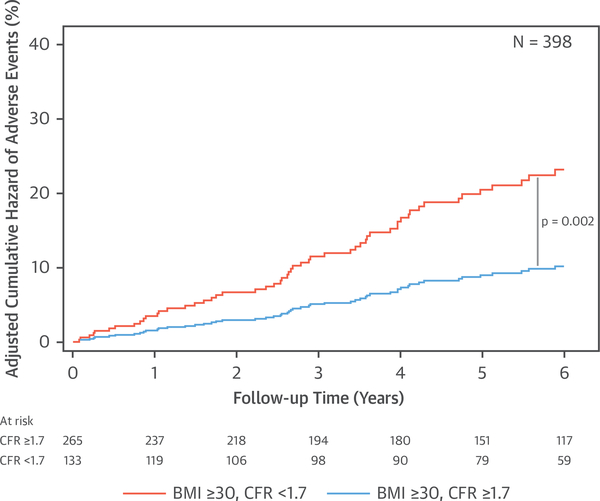
Subsequent addition of CFR into the Cox proportional hazards model with BMI led to further incremental improvements in model statistics, including model discrimination (C-index 0.71 to 0.74) (Table 2). After adjusting for CFR (adjusted HR for 1-U decrease in CFR: 1.95; 95% CI: 1.41 to 2.69; p < 0.001), the effect of BMI on outcomes decreased and was no longer statistically significant (adjusted HR for 10-U increase in BMI: 1.20; 95% CI: 0.95 to 1.50; p = 0.125). No significant interaction was observed between CFR and BMI on events. In the final model, CFR had a larger prognostic contribution than any traditional risk factors, including BMI, as compared using the partial chi-square statistic minus the predictor degrees of freedom (15.6 and 1.4 for CFR and BMI, respectively). The adjusted relationships between BMI and adverse events (p = 0.16) or CFR and adverse events (p < 0.0001) from the final model are illustrated in Figures 2B and 3B, respectively. A CFR value below 1.7 was associated with a steep increase in the annualized event risk to approximately 3%, the threshold of high risk in patients being evaluated for suspected ischemic heart disease (24). When obese patients were stratified by CFR, those with impaired CFR <1.7 demonstrated a significantly increased unadjusted and adjusted cumulative hazard of events (unadjusted HR: 2.22; 95% CI: 1.36 to 3.61; p = 0.001; adjusted HR: 2.28; 95% CI: 1.36 to 3.81; p = 0.002) (Figure 4).
FIGURE 4. Adjusted Cumulative Hazard of Adverse Events in Obese Patients According to Coronary Flow Reserve.
Obese patients with impaired coronary flow reserve experienced increased adjusted risk of events. Curves are adjusted for age, sex, race, hypertension, diabetes, peripheral vascular disease, chronic obstructive pulmonary disease, atrial fibrillation, tobacco use, estimated glomerular filtration rate, beta-blocker use, and left ventricular ejection fraction. BMI = body mass index; CFR = coronary flow reserve.
RISK STRATIFICATION OF OBESE PATIENTS BY CFR ACROSS CATEGORIES OF ELEVATED BMI.
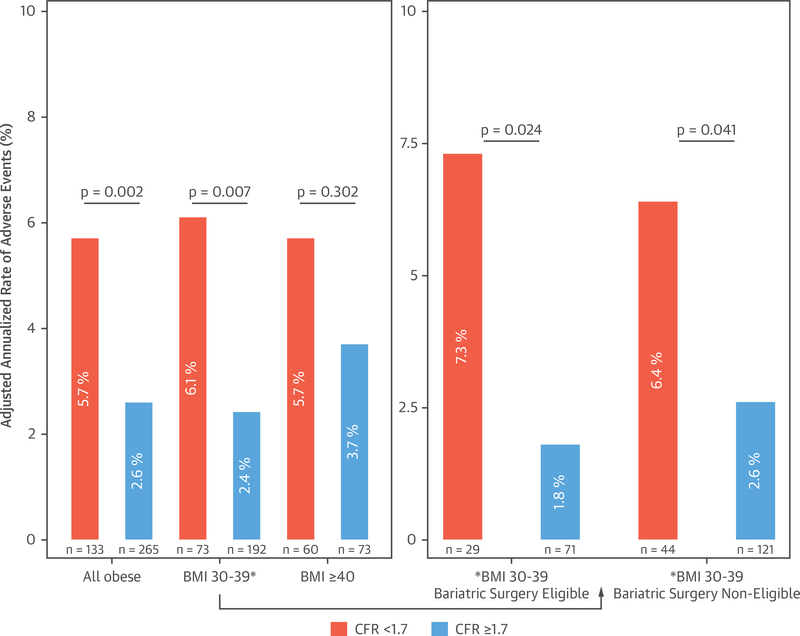
We next explored the prognostic value of impaired CFR in obese patients across categories of elevated BMI to better understand clinical risk and possible implications for management, including patient selection for bariatric surgery. Among 398 patients with BMI ≥30 kg/m2, 233 met current grade A recommendations (19) for bariatric surgery, 57% with BMI ≥40 kg/m2. In patients with BMI <40 kg/m2 meeting eligibility with a severe obesity-related comorbidity, 91% had hypertension or diabetes. Baseline characteristics of obese patients by bariatric surgery eligibility are presented in Online Table 2. Compared with other obese patients, those deemed eligible for bariatric surgery were younger with higher rates of diabetes and lower CFR. A total of 66 obese patients met the primary composite endpoint of death or hospitalization for nonfatal myocardial infarction or heart failure, including 27 deaths. In obese patients, those with impaired CFR demonstrated a significantly higher adjusted annualized rate of adverse events (5.7% vs. 2.6%; overall p = 0.002). In those without extreme obesity (BMI 30 to 39 kg/m2), this occurred irrespective of whether they met current indications for bariatric surgery (Figure 5).
FIGURE 5. Adjusted Annualized Rate of Adverse Events Among Categories of Obese Patients by Coronary Flow Reserve.
In obese patients, those with impaired coronary flow reserve demonstrated a higher adjusted annualized rate of adverse events (overall p = 0.002). In those without extreme obesity (BMI 30 to 39 kg/m2), this occurred irrespective of whether they met current indications for bariatric surgery. Plots are adjusted for age, sex, race, hypertension, diabetes, peripheral vascular disease, chronic obstructive pulmonary disease, atrial fibrillation, tobacco use, estimated glomerular filtration rate, beta-blocker use, and left ventricular ejection fraction. Abbreviations as in Figure 4.
DISCUSSION
We demonstrate an inverted J–shaped relationship between BMI and CFR in patients referred for cardiac stress testing, such that higher BMI in obese patients was associated with worsening coronary microvascular function, independently of clinical risk factors. BMI and CFR both appeared to be prognostically important in unadjusted and partially adjusted models, but only CFR improved model discrimination and remained independently associated with events in fully adjusted analyses. Indeed, in obese patients, only those with impaired CFR demonstrated a significantly increased risk of events; this was particularly evident in patients without extreme obesity (BMI 30 to 39 kg/m2), in whom impaired CFR was associated with a significant ≥2.5-fold increased adjusted rate of events.
Although BMI is a convenient measure used worldwide to diagnose obesity and assess patient candidacy for interventions, it is problematic as a risk marker (7). BMI may not distinguish between patients with more metabolically malignant versus benign body fat profiles and distribution (or even between excess body fat vs. lean mass), which may vary substantially by geographical region and sex (4). Alternative methods, such as hydrostatic weighing, dual-energy x-ray absorptiometry, and air displacement plethysmography, have been proposed to more accurately detect excess body fat, but these lack ability to detect physiologic alterations in vascular function to more directly assess pathological obese states (26).
Beyond an association with atherosclerosis, there is growing recognition of the impact of obesity, metabolic dysregulation, and low-grade inflammation (via adipocytokines including leptin, adiponectin, interleukin-6, and tumor necrosis factor alpha) on multiorgan microvascular function, including the heart (27–29). The current study advances prior observations in experimental animal models (30), ex vivo tissue (31), and cross-sectional patient studies (8–10) linking obesity and CMD. These prior studies were limited by small numbers and incompletely adjusted associations, and evaluated neither continuous relationships of BMI and CFR nor outcomes. Our data demonstrate that although obesity is an independent risk factor for CMD, it is not independently associated with adverse events after adjusting for the effect of CMD on outcomes. Indeed, the patients at greatest cardiovascular risk were those with CMD. Although there was significant phenotypic overlap among patients with CMD, obesity, and comorbid conditions such as hypertension and diabetes, some obese patients did not demonstrate comorbid conditions and may not have been considered for bariatric surgery. Yet, recent data suggest that even obese individuals without apparent risk factors may still be at higher risk of adverse events and are not truly “metabolically healthy” (32). When stratified by CFR, it became apparent that obese patients in this cohort represent a heterogeneous group with varying levels of risk (i.e., some patients have severe CMD and may potentially benefit from aggressive management strategies, including bariatric surgery) (4,5,33), whereas others may be closer to representing a “metabolically healthy” phenotype in which close patient monitoring and less invasive approaches may be reasonable (Central Illustration). Along with surgical weight loss (29), novel therapies targeting residual cholesterol or inflammatory risk (34), and neurohormonal activation or glucose handling in the kidneys, may improve CMD in this sector of patients and shift individuals from a high- to low-risk obesity phenotype. This approach may be especially impactful in women, who appear to be over-represented in terms of CMD (16,35), obesity, and heart failure events (17,36), and in whom new evidence-based approaches to reduce risk are needed (37,38). Early preliminary data support that bariatric surgery may improve CFR in selected patients (29), and it remains to be tested whether obese individuals with impaired CFR may derive an even greater benefit from undergoing bariatric surgery.
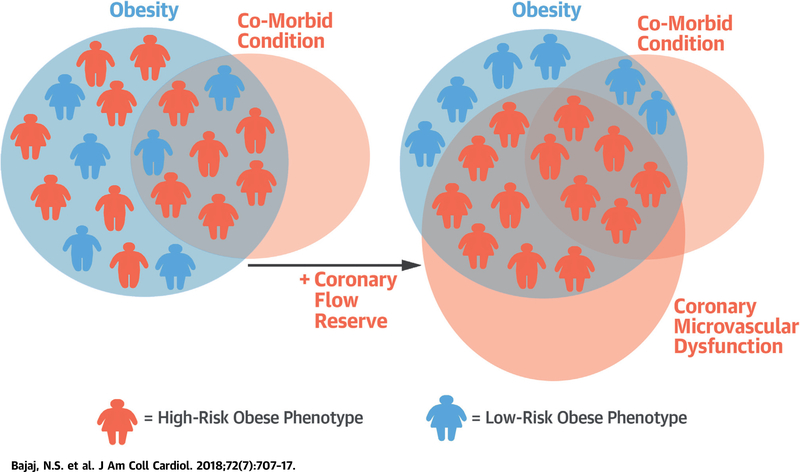
CENTRAL ILLUSTRATION. Obesity, Coronary Microvascular Dysfunction, and Cardiovascular Risk.
Schematic of heterogeneity of risk among obese patients, and a potential role for coronary flow reserve to better phenotype patients by identifying those with coronary microvascular dysfunction. Higher-risk obese patients may be more likely to benefit from interventions such as bariatric surgery than lower-risk obese patients.
STUDY LIMITATIONS.
Limitations of this study include its single-center observational design, in which subjects were patients clinically referred for PET myocardial perfusion imaging. Cardiac PET is widely available at our institution and is often used in patients who cannot exercise and require stress testing with imaging. To focus on the effects of CMD in structurally normal hearts, we excluded from analysis patients with known CAD, severe valvular disease, or heart failure. PET represents one of the most sensitive and diagnostically accurate tests available for noninvasive evaluation of ischemia, even in very obese patients (7,39,40). CMD was therefore defined as the presence of impaired CFR in the absence of flow-limiting CAD. Although it is conceivable that some patients in this cohort harbored severe, flow-limiting multivessel CAD without perfusion abnormalities, our clinical experience with PET suggests this to be unlikely (13). To minimize the confounding effect of weight loss and death, we also excluded patients with malignancy, end-stage liver or lung disease, and renal disease. For the exploratory analysis, we defined bariatric surgery-eligible patients as those meeting a grade A guideline recommendation for surgery (19), but recognize that individual patient characteristics and local clinician expertise impact on patient selection for surgery in important ways. This study did not address the impact of obesity-related noncardiac comorbidities, which may be substantial in these patients. In addition, the cohort size limited extensive subgroup analysis. As with other nonrandomized analyses, residual confounding may persist despite adjustment for baseline differences due to unmeasured (e.g., humoral and structural) factors, and causation cannot be discerned. Understanding important limitations, these results may have clinical implications for risk stratification and management of obese patients, a prevalent and growing sector of the population.
CONCLUSIONS
In patients referred for cardiac stress testing, CMD was independently associated with elevated BMI and adverse outcomes, and served as a better discriminator of risk than BMI. Prospective studies are needed to investigate a possible role for the use of CFR as a marker of vascular health in the risk management of obese patients beyond BMI and traditional risk factors.
Supplementary Material
PERSPECTIVES:
COMPETENCY IN MEDICAL KNOWLEDGE:
In obese patients, CMD worsened with increasing BMI independently of demographic and clinical risk factors. After adjustment for these risk factors, CMD but not BMI was independently associated with adverse events.
TRANSLATIONAL OUTLOOK:
Further studies are needed to determine whether CMD identifies obese individuals most likely to benefit from bariatric surgery and other weight loss interventions.
Acknowledgments
This work was supported by National Institutes of Health grant numbers T32 HL094301 to Drs. Bajaj, Gupta, Bravo, and Vita; T32 HL076136 to Dr. Osborne; R01HL132021 to Dr. Di Carli; and K23HL135438 to Dr. Taqueti. This independent research was supported in part by the Gilead Sciences Research Scholars Program in Cardiovascular Disease. Dr. Tavakkoli has served as a consultant for and received consulting fees from Medtronic. Dr. Dorbala has served as a consultant for GE Healthcare. Dr. Bhatt has served on the advisory board for Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, and Regado Biosciences; has served on the board of directors of the Boston VA Research Institute and Society of Cardiovascular Patient Care; has served as the chair of American Heart Association Quality Oversight Committee; has served on the data monitoring committees of the Cleveland Clinic, Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, and Population Health Research Institute; has received honoraria from the American College of Cardiology (senior associate editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor-in-Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor-in-Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (guest editor; associate editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (chief medical editor, Cardiology Today Intervention), Society of Cardiovascular Patient Care (secretary/treasurer), and WebMD (CME steering committees); has served as the deputy editor for Clinical Cardiology; has served as the chair of the NCDR-ACTION Registry Steering Committee and VA CART Research and Publications Committee; has received research funding from Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Ironwood, Ischemix, Lilly, Medtronic, Pfizer, Roche, Sanofi, and The Medicines Company; has received royalties from Elsevier (editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); has served as a site co-investigator for Biotronik, Boston Scientific, and St. Jude Medical (now Abbott); has served as a trustee of the American College of Cardiology; and has performed unfunded research for FlowCo, PLx Pharma, and Takeda. Dr. Di Carli has received research grant support from Spectrum Dynamics; and consulting fees from Sanofi and General Electric. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- BMI
body mass index
- CAD
coronary artery disease
- CFR
coronary flow reserve
- CI
confidence interval
- CMD
coronary microvascular dysfunction
- eGFR
estimated glomerular filtration rate
- HR
hazard ratio
- IQR
interquartile range
- LVEF
left ventricular ejection fraction
- PET
positron emission tomography
Footnotes
APPENDIX For supplemental figures and tables, please see the online version of this paper.
REFERENCES
- 1.Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med 2017;376:254–66. [DOI] [PubMed] [Google Scholar]
- 2.Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular disease. Circ Res 2016;118:1752–70. [DOI] [PubMed] [Google Scholar]
- 3.Stefan N, Haring HU, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol 2013;1:152–62. [DOI] [PubMed] [Google Scholar]
- 4.Kim J, Eisenberg D, Azagury D, Rogers A, Campos GM. American Society for Metabolic and Bariatric Surgery position statement on long-term survival benefit after metabolic and bariatric surgery. Surg Obes Relat Dis 2016;12:453–9. [DOI] [PubMed] [Google Scholar]
- 5.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012; 366:1567–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pareek M, Schauer PR, Kaplan LM, Leiter LA, Rubino F, Bhatt DL. Metabolic surgery: weight loss, diabetes, and beyond. J Am Coll Cardiol 2018;71:670–87. [DOI] [PubMed] [Google Scholar]
- 7.Taqueti VR. Myocardial perfusion imaging in extreme obesity: leveraging modern technologies to meet a modern challenge. J Nucl Cardiol 2017. June 19 [E-pub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 8.Motivala AA, Rose PA, Kim HM, et al. Cardiovascular risk, obesity, and myocardial blood flow in postmenopausal women. J Nucl Cardiol 2008;15:510–7. [DOI] [PubMed] [Google Scholar]
- 9.Schindler TH, Cardenas J, Prior JO, et al. Relationship between increasing body weight, insulin resistance, inflammation, adipocytokine leptin, and coronary circulatory function. J Am Coll Cardiol 2006;47:1188–95. [DOI] [PubMed] [Google Scholar]
- 10.Tona F, Serra R, Di Ascenzo L, et al. Systemic inflammation is related to coronary microvascular dysfunction in obese patients without obstructive coronary disease. Nutr Metab Cardiovasc Dis 2014; 24:447–53. [DOI] [PubMed] [Google Scholar]
- 11.Murthy VL, Naya M, Foster CR, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 2011;124:2215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziadi MC, Dekemp RA, Williams KA, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol 2011;58:740–8. [DOI] [PubMed] [Google Scholar]
- 13.Taqueti VR, Hachamovitch R, Murthy VL, et al. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation 2015; 131:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Recio-Mayoral A, Mason JC, Kaski JC, Rubens MB, Harari OA, Camici PG. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur Heart J 2009;30: 1837–43. [DOI] [PubMed] [Google Scholar]
- 15.Taqueti VR, Ridker PM. Inflammation, coronary flow reserve, and microvascular dysfunction: moving beyond cardiac syndrome X. J Am Coll Cardiol Img 2013;6:668–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taqueti VR, Everett BM, Murthy VL, et al. Interaction of impaired coronary flow reserve and cardiomyocyte injury on adverse cardiovascular outcomes in patients without overt coronary artery disease. Circulation 2015;131:528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taqueti VR, Solomon SD, Shah AM, et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J 2018;39:840–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murthy VL, Naya M, Foster CR, et al. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation 2012;126:1858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient–2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery.Obesity 2013;21 Suppl 1:S1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El Fakhri G, Kardan A, Sitek A, et al. Reproducibility and accuracy of quantitative myocardial blood flow assessment with (82)Rb PET: comparison with (13)N-ammonia PET. J Nucl Med 2009; 50:1062–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovasc Imaging 2002; 18:539–42. [PubMed] [Google Scholar]
- 23.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation 2012;126:2020–35. [DOI] [PubMed] [Google Scholar]
- 24.Gibbons RJ, Chatterjee K, Daley J, et al. ACC/ AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Chronic Stable Angina). J Am Coll Cardiol 1999;33:2092–197. [DOI] [PubMed] [Google Scholar]
- 25.Gould KL, Johnson NP, Bateman TM, et al. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol 2013;62:1639–53. [DOI] [PubMed] [Google Scholar]
- 26.Romero-Corral A, Somers VK, Sierra-Johnson J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes 2008;32:959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorop O, Olver TD, van de Wouw J, et al. The microcirculation: a key player in obesity-associated cardiovascular disease. Cardiovasc Res 2017;113:1035–45. [DOI] [PubMed] [Google Scholar]
- 28.Badimon L, Bugiardini R, Cenko E, et al. Position paper of the European Society of Cardiology-working group of coronary pathophysiology and microcirculation: obesity and heart disease. Eur Heart J 2017;38:1951–8. [DOI] [PubMed] [Google Scholar]
- 29.Quercioli A, Montecucco F, Pataky Z, et al. Improvement in coronary circulatory function in morbidly obese individuals after gastric bypass-induced weight loss: relation to alterations in endocannabinoids and adipocytokines. Eur Heart J 2013;34:2063–73. [DOI] [PubMed] [Google Scholar]
- 30.Trask AJ, Katz PS, Kelly AP, et al. Dynamic micro- and macrovascular remodeling in coronary circulation of obese Ossabaw pigs with metabolic syndrome. J Appl Physiol 2012;113:1128–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell DJ, Somaratne JB, Prior DL, et al. Obesity is associated with lower coronary microvascular density. PLoS One 2013;8:e81798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caleyachetty R, Thomas GN, Toulis KA, et al. Metabolically healthy obese and incident cardiovascular disease events among 3.5 million men and women. J Am Coll Cardiol 2017;70:1429–37. [DOI] [PubMed] [Google Scholar]
- 33.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med 2017; 376:641–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taqueti VR, Ridker PM. Lipid-lowering and anti-inflammatory benefits of statin therapy: more than meets the plaque. Circ Cardiovasc Imaging 2017;10:e006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murthy VL, Naya M, Taqueti VR, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 2014;129:2518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taqueti VR, Shaw LJ, Cook NR, et al. Excess cardiovascular risk in women relative to men referred for coronary angiography is associated with severely impaired coronary flow reserve, not obstructive disease. Circulation 2017;135:566–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taqueti VR, Dorbala S, Wolinsky D, et al. Myocardial perfusion imaging in women for the evaluation of stable ischemic heart disease-state-of-the-evidence and clinical recommendations. J Nucl Cardiol 2017;24:1402–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmaier AA, Taqueti VR. A lack of reserve: recognizing the large impact of small vessels in the heart. Circulation 2018;138:424–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chow BJ, Dorbala S, Di Carli MF, et al. Prognostic value of PET myocardial perfusion imaging in obese patients. J Am Coll Cardiol Img 2014;7:278–87. [DOI] [PubMed] [Google Scholar]
- 40.Harnett DT, Hazra S, Maze R, et al. Clinical performance of Rb-82 myocardial perfusion PET and Tc-99m-based SPECT in patients with extreme obesity. J Nucl Cardiol 2017. March 29 [E-pub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.