Abstract
Objective:
To determine the influence of delivery hospital on the rate of vaginal birth after cesarean (VBAC).
Study Design:
This retrospective cohort study used claims data from Blue Cross and Blue Shield of Michigan. Women with a prior cesarean and a singleton livebirth between 2012 and 2016 were included. We calculated the hospital-specific risk-standardized VBAC rates and median odds ratio as a measure of variation.
Result:
Hospital-level adjusted rates varied nearly 10-fold (3.7%-35.5%). Compared to the lowest volume hospitals (1st quartile), the likelihood of VBAC increased for those in the 2nd (adjusted OR 2.75 [95% CI 1.23-6.17]), 3rd (adjusted OR 3.73 [95% CI 1.59-8.75]), and 4th quartiles (adjusted OR 2.9 [95% CI 1.11-7.72]). The median OR suggested significant variation by hospital after adjustment.
Conclusion:
The delivery hospital itself explains a large amount of the variation in rates of VBAC after adjustment for patient and hospital characteristics.
Introduction
Nearly one-third of births in the United States in 2015 were cesarean deliveries [1]. Historically, offering trial of labor after cesarean (TOLAC) has decreased the population-level cesarean rate [2]. After peaking in the mid-1990s, the rate of vaginal birth after cesarean (VBAC) in the United States is now around 9-12%, and many facilities have stopped offering TOLAC [2, 3].
While patient characteristics influence the likelihood of a successful VBAC, the reduction in VBAC rates over time is primarily driven by changes in the number of women attempting TOLAC [4, 5]. Factors associated with lower rates of TOLAC and VBAC include limited understanding of risks and benefits of TOLAC, perceived provider preference, physician cognitive traits, and provider call schedules [6-8]. The type of obstetric care provider may also play a role [9].
The reduction of repeat cesarean is an explicit Healthy People 2020 goal, but there is no sustained systematic effort to achieve this metric [2, 10]. There is limited recent data on variation in VBAC across institutions, although VBAC rates ranged from 0-45% across California hospitals with a median VBAC rate of 5% [11, 12]. Similar hospital-level variation has been demonstrated in other obstetric outcomes including “low-risk” cesarean delivery (2.4-36.5%) and episiotomy [13, 14]. Hospital factors previously associated with VBAC include obstetric volume, level of neonatal care, rural location, and teaching status [12, 15].
Our objective was to assess VBAC rates and the relationship between delivery hospital and VBAC among a large, contemporary population. We hypothesized that after adjustment for patient and hospital-level characteristics, there is a significant and clinically meaningful variation in VBAC between hospitals.
Materials and Methods
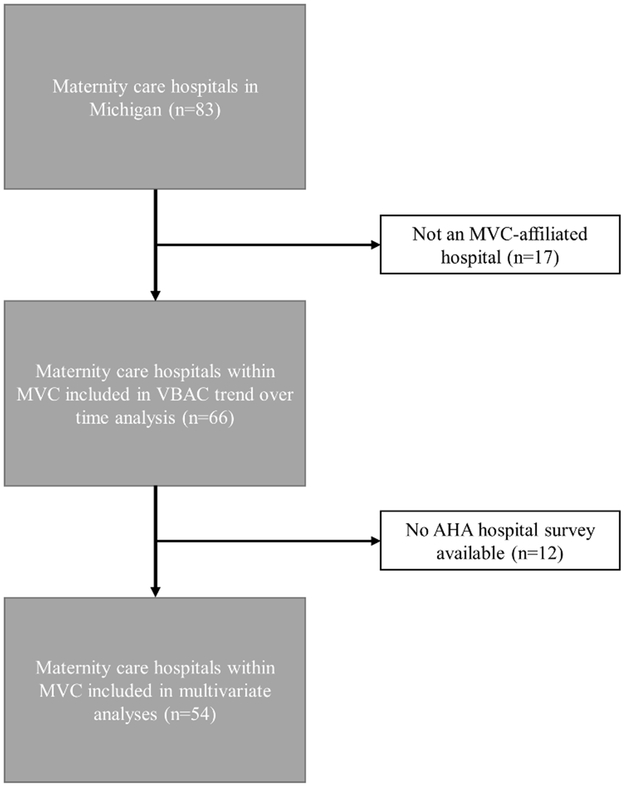
We performed a retrospective study using Blue Cross Blue Shield of Michigan (BCBSM) Preferred Provider Organization claims data from 2012-2016 for women receiving maternity services at one of the 66 Michigan Value Collaborative (MVC)-participating hospitals that provide those services. MVC is a voluntary statewide consortium funded by BCBSM and comprised of acute care hospitals with the goal of improving patient outcomes through data analytics and collaborative learning. The participating hospitals account for the majority of maternity care hospitals in the state of Michigan (66/83, 79.5%, Figure 1).
Figure 1. Selection of hospitals included in analyses of vaginal birth after cesarean across the state of Michigan (2012-2016).
AHA, American Hospital Association; MVC, Michigan Value Collaborative; VBAC, vaginal birth after cesarean
Claims were obtained for women with BCBSM Preferred Provider Organization coverage and contained the complete 30- and 90-day episode payments for obstetrical care, as well as diagnostic and procedural codes. The claims-based algorithm for identification of episodes of care has previously been validated [16]. BCBSM provided the claims data but did not contribute to the design or analysis of the study. Based on the study period, both ICD-9 and ICD-10 diagnostic codes were used. This study used de-identified patient information for the purpose of quality assurance and improvement, which meets criteria for not regulated status by the University of Michigan Institutional Review Board–Medical (HUM00133809).
We identified women who had a history of cesarean delivery (ICD-9 654.20, 654.21, 654.23; ICD-10 O34219, O34311, O34212, O34219, O3421) and a delivery episode of a singleton livebirth during the study period. Only women older than 18 years of age were included based on our data use agreement. We excluded women based on ICD-9 and ICD-10 codes if their index delivery included codes for malpresentation (breech, transverse, shoulder), placenta previa, or vasa previa because these women were not medically eligible for TOLAC. Amongst this cohort, VBAC was identified using the following CPT codes: 59400, 59409, 59410, 59610, 59612, and 59614. The VBAC rate was calculated as the number of women with VBAC divided by the number of women with a history of prior cesarean delivery. ICD codes do not allow ascertainment of parity or prior number or type of cesarean deliveries.
We obtained patient characteristics including age, obesity, diabetes, and hypertension [4, 17]. Race and ethnicity were not available. Maternal ICD codes for fetal conditions that are known prior to delivery and may affect VBAC were obtained including excessive growth/large for gestational age and fetal growth restriction. Urban/rural status of the facility was based on the American Hospital Association (AHA)/Centers for Medicare and Medicaid Services designation. Annual delivery volume by hospital was obtained from the Michigan Department of Health & Human Services. The quartile rank, with 1st quartile being lowest volume, was based on average annual total births from 2012-2016 across all MVC hospitals. Obstetric and neonatal care levels were obtained from the 2015 voluntary annual AHA survey, which was representative of the care levels over the 5-year study period. Obstetric care level is designated in three tiers. Level 1 “provides services for uncomplicated maternity and newborn cases”; level 2 “provides service for all uncomplicated and most complicated cases”; and level 3 “provides services for all serious illnesses and abnormalities.” Neonatal levels of care were treated as separate variables because a single facility could provide care in both an intermediate care unit and an intensive care unit. Teaching hospital status was based on the presence of a residency program in obstetrics and gynecology, which was determined by cross-matching the lists of Michigan hospitals in MVC and those known to have a residency program by the American College of Obstetricians and Gynecologists in 2016. Hospital websites were also reviewed to verify existence of a residency program.
To examine the trend in observed VBAC rates over time in the entire cohort, we used ordinary least squares regression to test for a significant trend between 2012 and 2016 using episode year as the independent variable.
Of the 66 MVC-participating hospitals that provided maternity services during the study period, 54 also had available AHA data and were therefore included in the subsequent analyses. We performed bivariate analyses of patient and hospital factors considered to be associated with VBAC using Chi-square or Fisher’s exact tests for categorical variables, and analysis of variance for continuous variables. Normality of variables was assessed by reviewing skew, kurtosis, and quantile-quantile plots to examine the need for non-parametric testing.
Clinically and statistically relevant (p<0.05) patient and hospital characteristics were considered in a stepwise logistic regression model. A hierarchical multivariable logistic regression model with hospital random effects was fit to account for clustering of deliveries within hospitals and avoid overestimation of the significance of statistical associations. Using the final model, we calculated the hospital-specific risk-standardized VBAC rates using methodology from the Centers for Medicare and Medicaid Services [18]. Risk and reliability-adjustment provides better estimates for low-volume hospitals, as outcome rates are “shrunk” towards the average to minimize extreme values due to small sample size. We calculated Clopper-Pearson 95% CIs on the risk and reliability-adjusted hospital-specific VBAC rate. To illustrate variation in rates and identify outliers with respect to the MVC reference rate, we graphed hospitals on a caterpillar plot. If a 95% CI did not cross the statewide reference VBAC rate, that hospital was considered a statistical outlier.
The intraclass correlation coefficient was measured to determine the percentage of variation in VBAC attributable to the delivery hospital. Given the limitations of this measure in interpreting the proportion of variance explained for dichotomous outcomes (such as VBAC versus repeat cesarean), the median odds ratio (OR) was also calculated. The median OR is a more interpretable measure of the level of variation due to delivery hospital [19]. The median OR is always greater than or equal to one; however, the further it is from one indicates stronger hospital-level differences. The median OR was chosen as a measure of hospital variation as the value is easily interpretable on an odds ratio scale, which can be compared to patient- and hospital-level factors’ adjusted odds ratios [20]. This analytic technique has been used previously to study hospital- and regional-level variation in clinical outcomes [21, 22]. Since deliveries are clustered in hospitals, we constructed an 80% interval OR for significant hospital-level variables to determine if a given factor had a significant impact on the variation in VBAC rate [19]. If the 80% interval OR crosses one, then that factor has minimal influence on the variation in VBAC rate. This is a complementary measure to the adjusted OR [20]. Model fit was assessed with the C-statistic. All statistical analyses were performed using Statistical Analysis System (SAS Version 9.4, Cary, NC). All statistical tests were two-sided and performed with α=0.05. Code availability: risk and reliability adjustment, median OR, and 80% interval OR available by request to corresponding author.
Results
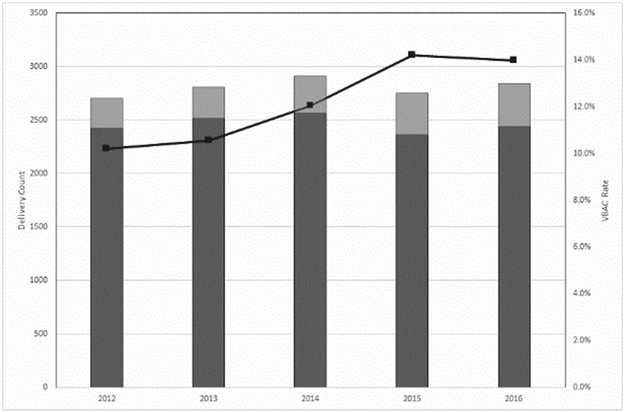
During the study period, there were 14,012 deliveries among women with a history of cesarean that occurred at 66 maternity care hospitals. Of those, 1709 (12.2%) were VBAC. Amongst the 66 maternity care hospitals, eight (12.1%) had no VBACs in the five-year period. The observed VBAC rate among hospitals with at least one VBAC was 0.6-24.8%. Overall, the incidence of VBAC increased over time by 1.1% per year, from 10.2% in 2012 to 14.0% for 2016 (p=0.01, R2 0.91, Figure 2).
Figure 2. Rate of vaginal birth after cesarean (2012-2016).
Observed delivery counts are shown in bars on the primary axis with repeat cesarean in dark gray and VBAC in light gray. The observed VBAC rate is shown in the line on the secondary axis (black line). There was a significant increase in VBAC rate over time by linear regression (p=0.01).
VBAC, vaginal birth after cesarean
Fifty-four hospitals had AHA survey results regarding level of obstetric and neonatal care and were included in the subsequent analyses. There were 12,526 deliveries among women with a history of cesarean at those hospitals (89.4% of the total population). Women who had a VBAC were younger (p=0.002) and less likely to be obese (p<0.001) than women who had a repeat cesarean (Table 1). Medical comorbidities including diabetes (p<0.001), chronic hypertension (p<0.001), pre-eclampsia (p=0.001), and gestational hypertension (p<0.001) were less frequent among women who had a VBAC. VBAC was less likely in pregnancies complicated by large for gestational age (0.9% vs. 3.0%, p<0.001); there was no relationship between growth restriction and mode of delivery in our population (p=0.40).
Table 1.
Demographic, obstetric, and hospital characteristics by mode of delivery
| Characteristics | Repeat Cesarean (n=10 979) |
VBAC (n=1547) |
P-value |
|---|---|---|---|
| Maternal age, years | 32.5 ± 4.7 | 32.1 ± 4.5 | 0.002 |
| Obesity | 913 (8.3) | 51 (3.3) | <0.001 |
| Diabetes1 | 1890 (17.2) | 140 (9.1) | <0.001 |
| Chronic hypertension | 438 (4.0) | 24 (1.6) | <0.001 |
| Pre-eclampsia | 300 (2.7) | 21 (1.4) | 0.001 |
| Gestational hypertension | 391 (3.6) | 27 (1.8) | <0.001 |
| Large for gestational age | 332 (3.0) | 14 (0.9) | <0.001 |
| Fetal growth restriction | 50 (0.5) | 4 (0.3) | 0.40 |
| Annual delivery volume (quartile) | <0.001 | ||
| 1st (281-534 births) | 574 (5.2) | 18 (1.2) | |
| 2nd (549-995 births) | 1247 (11.4) | 102 (6.6) | |
| 3rd (996-1975 births) | 2875 (26.2) | 401 (25.9) | |
| 4th (1989-7657 births) | 6283 (57.2) | 1026 (66.3) | |
| Hospital location | <0.001 | ||
| Urban | 9860 (89.8) | 1468 (94.9) | |
| Rural | 1119 (10.2) | 79 (5.1) | |
| Teaching hospital | 5421 (49.4) | 927 (59.9) | <0.001 |
| Level of obstetric care | <0.001 | ||
| Level 1 | 1893 (17.2) | 156 (10.1) | |
| Level 2 | 3199 (29.1) | 375 (24.2) | |
| Level 3 | 5887 (53.6) | 1016 (65.7) | |
| Neonatal intensive care | 7058 (64.3) | 1174 (75.9) | <0.001 |
| Neonatal intermediate care | 2380 (21.7) | 388 (25.1) | 0.003 |
Data presented as mean ± standard deviation, or n (%). P-values are calculated using analysis of variance, χ2 test, or Fisher’s exact test.
VBAC, vaginal birth after cesarean
Gestational and pre-existing diabetes
At urban hospitals, the VBAC rate was 13.0% over the study period versus 6.6% in rural hospitals (p<0.001). The VBAC rate increased across quartiles of delivery volume (3.0% vs. 7.6% vs. 12.2% vs. 14.0%, p<0.001). VBAC was also more common at teaching hospitals (14.6%) than at non-teaching hospitals (10.0%, p<0.001). VBAC was more frequent in hospitals with neonatal intermediate (14.0 vs. 11.9%, p=0.003) and intensive (14.3 vs. 8.7%, p<0.001) care units, respectively. The VBAC rate increased with higher level obstetric care (7.6% vs. 10.5% vs. 14.7%, p<0.001).
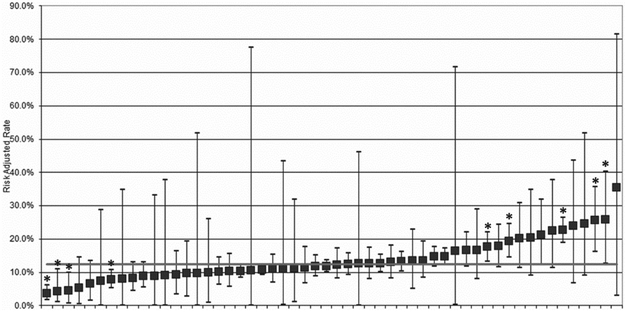
Observed VBAC rates across the 54 hospitals ranged from 0% to 24.8%, with 7 hospitals having 0 VBAC in the study period. Risk and reliability-adjusted VBAC rates ranged from 3.7% (95% exact CI 1.6-6.2%) to 35.5% (95% exact CI 2.9%-81.4%, Figure 3). The mean adjusted VBAC rate across the cohort was 12.4%.
Figure 3. Risk and reliability-adjusted rates of vaginal birth after cesarean.
The 54 hospitals responding to the American Hospital Association survey are ranked from left to right by lowest to highest adjusted VBAC rate. The error bars demonstrate the 95% exact CI. The solid gray line is the mean VBAC rate. Nine statistical outliers are identified (*); four are below the mean and five are above the mean.
VBAC, vaginal birth after cesarean
The obstetric and hospital factors included in the multivariable hierarchical model are shown in Table 2. Teaching status and urban-rural designation were not retained in the model. Increasing age, obesity, diabetes, hypertensive disorders, and large for gestational age were all associated with decreased odds of VBAC. Both adjusted OR and 80% interval OR are shown for hospital factors included in the model. Only annual delivery volume remained significantly associated with VBAC in the multivariate analysis, with increasing adjusted odds ratios for VBAC for higher volume quartiles. Compared to the 1st quartile, the 3rd quartile for delivery volume had an adjusted OR of 3.73 (95% CI 1.59-8.75) and the 80% interval OR did not cross one—suggesting that volume significantly drives variation in VBAC. The adjusted OR for the 2nd and 4th quartile of delivery volume demonstrated a significant association between volume and VBAC, but the 80% interval OR was not significant for each quartile. Level of obstetric care and neonatal levels of care were not significantly associated with VBAC or variation in VBAC rates.
Table 2.
Adjusted odds ratio and 80% interval odds ratio of patient and hospital-level factors on vaginal birth after cesarean
| Factors | Odds Ratio (95% CI) | P-value | 80% Interval OR1 |
|---|---|---|---|
| Maternal age, years | 0.98 (0.97-0.99) | <0.001 | |
| Obesity | 0.58 (0.41-0.84) | 0.003 | |
| Diabetes2 | 0.65 (0.52-0.81) | <0.001 | |
| Chronic hypertension | 0.42 (0.28-0.64) | <0.001 | |
| Pre-eclampsia | 0.50 (0.32-0.78) | 0.002 | |
| Gestational hypertension | 0.52 (0.35-0.78) | 0.002 | |
| Large for gestational age | 0.32 (0.18-0.55) | <0.001 | |
| Annual delivery volume (quartile) | |||
| 1st (281-534 births) | reference | reference | reference |
| 2nd (549-995 births) | 2.75 (1.23-6.17) | 0.02 | 0.92-8.17 |
| 3rd (996-1975 births) | 3.73 (1.59-8.75) | 0.003 | 1.25-11.07 |
| 4th (1989-7657 births) | 2.92 (1.11-7.72) | 0.03 | 0.98-8.69 |
| Level of obstetric care3 | |||
| Level 1 | reference | reference | reference |
| Level 2 | 1.02 (0.55-1.92) | 0.08 | 0.34-3.04 |
| Level 3 | 1.07 (0.51-2.23) | 0.18 | 0.36-3.17 |
| Neonatal intensive care | 1.95 (0.89-4.27) | 0.09 | 0.66-5.80 |
| Neonatal intermediate care | 1.60 (0.92-2.77) | 0.09 | 0.54-4.75 |
C-statistic 0.67 for the hierarchical multivariable model
CI, confidence interval; OR, odds ratio
80% interval OR only computed for hospital-level factors to assess effect on variation in vaginal birth after cesarean
Gestational and pre-existing diabetes
Level 1 “provides services for uncomplicated maternity and newborn cases”; Level 2 “provides service for all uncomplicated and most complicated cases”; Level 3 “provides services for all serious illnesses and abnormalities
To assess the effect of the delivery hospital on likelihood of VBAC after adjusting for patient and hospital-level factors, we calculated the intraclass correlation coefficient and the median OR. The intraclass correlation coefficient of the null model including only the random effects of the delivery hospitals was 16.2%, which was reduced to 9.9% in the fully-adjusted model. The median OR was 1.77. In other words, if two patients with identical obstetric characteristics presented for TOLAC to two random hospitals, we would expect the woman at the hospital with higher VBAC propensity to have 77% greater odds of VBAC than the woman at the other hospital.
Discussion
In this large cohort of commercially insured women, we determined that the delivery hospital itself explains a significant degree of the variation in VBAC rates between hospitals, after adjusting for measurable patient and hospital characteristics. We found a ten-fold difference in risk-adjusted rates of VBAC across a region, yet measured hospital factors besides delivery volume did not contribute significantly to that variation.
The degree of variation that is attributable to the delivery hospital in our study is impressive. The odds of VBAC is 77% higher at a randomly-selected hospital compared to a lower-performing hospital. Using a similar statistical approach, other studies have found the odds of sepsis mortality increased approximately 20% by hospital, and the odds of mortality after out-of-hospital cardiac arrest increased 40% depending on county [20, 21].
There is an opportunity to increase access to VBAC given that variation in VBAC rates is likely driven by differences in institutional and organizational characteristics between hospitals. Hospital outliers with very high rates of VBAC may have practices that could be studied and adopted by institutions with lower VBAC rates. There are several specific areas that may increase TOLAC and VBAC. Standardization of counseling about the risks and benefits of TOLAC could minimize the influence of perceived provider preference and increase the likelihood of TOLAC [6]. Hospitals could alter staffing models to include night float or midwifery services given these models have been associated with increased VBAC rates [8, 9, 12]. The success of regional referral centers for neonatal care may also provide a model for increasing access to TOLAC [2, 22]. There is also an imperative to monitor maternal and fetal outcomes—particularly at outlier institutions—so that the goal is not only to increase VBAC, but to improve maternal and neonatal outcomes without increasing harms.
This study has several strengths. We had a large sample size with data from diverse facilities across a region. We included women with medical conditions associated with decreased success of VBAC (e.g., preeclampsia), as these conditions are not contraindication to TOLAC, and these women were excluded from a prior analysis of hospital variation in VBAC [12]. There is extremely limited data on VBAC rates or variation across facilities in the past 10 years, so providing data on changing VBAC rates through 2016 is a useful contribution [12, 23]. Our statistical approach is also a strength. The median OR is readily interpretable on a familiar odds ratio scale and takes into account both known and random effects of the delivery hospitals. It is thus easier to compare the hospital effects to clinical factors that are known to alter risk of VBAC as opposed to presenting the attributable percent of variation. This method has been used in health care quality literature to assess regional or facility-level variation in important clinical outcomes but is novel in obstetric literature [20, 21].
The limitations of this study are primarily related to the nature of claims data. We were unable to ascertain intended mode of delivery and can comment only on actual route of delivery. Parity and the number of prior cesarean deliveries is not identifiable by ICD codes, and these factors may alter who is a candidate for TOLAC at different hospitals. More detailed patient information such as race, ethnicity, and labor characteristics were not available to more fully adjust for odds of VBAC. There is also under-coding for some comorbidities, particularly obesity. The availability of anesthesia is not readily discernible from our data sources; however, the level of obstetric care may be a proxy. Per the American College of Obstetricians and Gynecologists practice bulletin, TOLAC can be offered in facilities that are at least level 1 (basic obstetric care) [2]. Lastly, the women included in this study all had commercial insurance, so our results may not be generalizable to all populations.
In this study, we found that delivery hospital significantly influences the VBAC rate. Fundamental questions, including 1) should it matter where a woman delivers her baby?; and 2) is the delivery hospital the woman’s choice? need to be addressed in the context of a public health goal of decreasing the cesarean rate. There is an urgent need to identify and disseminate institutional practices associated with increased access to TOLAC and VBAC in order to reverse the trend of increasing repeat cesarean deliveries and concomitant maternal morbidity. Future research on implementation of those models and assessment of maternal and neonatal outcomes is warranted.
Acknowledgements:
Ms. Sarah Block assisted in preparation of this manuscript. Mr. Ryan Jakubowski assisted with graphic design for the figures.
Funding: Dr. Moniz receives support from the Agency for Healthcare Research and Quality (AHRQ) under award number K08 HS025465.
Footnotes
Conflict of interest: Dr. Langen, Dr. Moniz, Dr. Morgan, Mr. Kamdar, and Mr. Syrjamaki receive salary support from Blue Cross Blue Shield of Michigan (BCBSM). Mr. Kamdar is a consultant for Lucent Surgical. The other authors report no potential conflict of interest.
References:
- 1.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Mathews TJ. Births: Final Data for 2015. Natl Vital Stat Rep. 2017;66:1. [PubMed] [Google Scholar]
- 2.American College of Obstetricians and Gynecologists. Practice Bulletin No. 184. Vaginal birth after cesarean delivery. Obstet Gynecol. 2017;130:e217–33. [DOI] [PubMed] [Google Scholar]
- 3.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: Final Data for 2016. Natl Vital Stat Rep. 2018;67:1–54. [PubMed] [Google Scholar]
- 4.Grobman WA, Lai Y, Landon MB, Spong CY, Leveno KJ, Rouse DJ, et al. Development of a nomogram for prediction of vaginal birth after cesarean delivery. Obstet Gynecol. 2007;109:806–12. [DOI] [PubMed] [Google Scholar]
- 5.Grobman WA, Lai Y, Landon MB, Spong CY, Rouse DJ, Varner MW, et al. The change in the rate of vaginal birth after caesarean section. Paediatr Perinat Epidemiol. 2011;25:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein SN, Matalon-Grazi S, Rosenn BM. Trial of labor versus repeat cesarean: are patients making an informed decision? Am J Obstet Gynecol. 2012;207:204.e1–6. [DOI] [PubMed] [Google Scholar]
- 7.Yee LM, Liu LY, Grobman WA. Relationship between obstetricians’ cognitive and affective traits and delivery outcomes among women with a prior cesarean. Am J Obstet Gynecol. 2015;213:413.e1–7. [DOI] [PubMed] [Google Scholar]
- 8.Yee LM, Liu LY, Grobman WA. Obstetrician call schedule and obstetric outcomes among women eligible for a trial of labor after cesarean. Am J Obstet Gynecol. 2017;216:75e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metz TD, Stoddard GJ, Henry E, Jackson M, Holmgren C, Esplin S. How do good candidates for trial of labor after cesarean (TOLAC) who undergo elective repeat cesarean differ from those who choose TOLAC? Am J Obstet Gynecol. 2013;208:458.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Office of Disease Prevention and Health Promotion, Healthy People 2020. Maternal, Infant, and Child Health. Available from: https://www.healthypeople.gov/2020/topics-objectives/topic/maternal-infant-and-child-health/objectives. Accessed Feb 8, 2018.
- 11.Guise JM, Eden K, Emeis C, Denman M, Marshall N, Fu R, et al. Vaginal birth after cesarean: new insights Evidence Report/Technology Assessment No. 191. (Prepared by the Oregon Health & Science University Evidence-based Practice Center under Contract No. 290-2007-10057-I; ). AHRQ Publication No. 2010;10–E003. [Google Scholar]
- 12.Rosenstein MG, Kuppermann M, Gregorich SE, Cottrell EK, Caughey AB, Cheng YW. Association between vaginal birth after cesarean delivery and primary cesarean delivery rates. Obstet Gynecol. 2013;122:1010–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozhimannil KB, Law MR, Virnig BA. Cesarean delivery rates vary tenfold among US hospitals; reducing variation may address quality and cost issues. Health Aff (Millwood). 2013;32:527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman AM, Ananth CV, Prendergast E, D’Alton ME, Wright JD. Variation in and factors associated with use of episiotomy. JAMA. 2015;313:197–9. [DOI] [PubMed] [Google Scholar]
- 15.DeFranco EA, Rampersad R, Atkins KL, Odibo AO, Stevens EJ, Peipert JF, et al. Do vaginal birth after cesarean outcomes differ based on hospital setting? Am J Obstet Gynecol. 2007;197:440.e1-6. [DOI] [PubMed] [Google Scholar]
- 16.Ellimoottil C, Syrjamaki JD, Voit B, Guduquntla V, Miller DC, Dupree JM. Validation of a claims-based algorithm to characterize episodes of care. Am J Manag Care. 2017;23:e382–6. [PubMed] [Google Scholar]
- 17.Landon MB, Leindecker S, Spong CY, Hauth JC, Bloom S, Varner MW, et al. The MFMU Cesarean Registry: factors affecting the success of trial of labor after previous cesarean delivery. Am J Obstet Gynecol. 2005;193:1016–23. [DOI] [PubMed] [Google Scholar]
- 18.Agency for Healthcare Research and Quality. Re-Engineered Discharge (RED) Toolkit: How CMS Measures the “30-Day All Cause Rehospitalization Rate” on the Hospital Compare Web Site. Available from: https://www.ahrq.gov/professionals/systems/hospital/red/toolkit/redtool-30day.html. Accessed Feb 8, 2018.
- 19.Larsen K, Petersen JH, Budtz-Jørgensen E, Endahl L. Interpreting parameters in the logistic regression model with random effects. Biometrics. 2000;56:909–14. [DOI] [PubMed] [Google Scholar]
- 20.Merlo J, Chaix B, Ohlsson H, Beckman A, Johnell K, Hjerpe P, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60:290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girotra S, van Diepen S, Nallamothu BK, Carrel M, Vellano K, Anderson ML, et al. Regional Variation in Out-of-Hospital Cardiac Arrest Survival in the United States. Circulation. 2016;133:2159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prescott HC, Kepreos KM, Wiitala WL, Iwashyna TJ. Temporal Changes in the Influence of Hospitals and Regional Healthcare Networks on Severe Sepsis Mortality. Crit Care Med. 2015;43:1368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American College of Obstetricians and Gynecologists. Obstetric Care Consensus No. 2. Levels of maternal care. Obstet Gynecol. 2015;125:502–15. [DOI] [PubMed] [Google Scholar]