Abstract
Age‐related changes in resting‐state (RS) neural rhythms in typically developing children (TDC) but not children with autism spectrum disorder (ASD) suggest that RS measures may be of clinical use in ASD only for certain ages. The study examined this issue via assessing RS peak alpha frequency (PAF), a measure previous studies, have indicated as abnormal in ASD. RS magnetoencephalographic (MEG) data were obtained from 141 TDC (6.13–17.70 years) and 204 ASD (6.07–17.93 years). A source model with 15 regional sources projected the raw MEG surface data into brain source space. PAF was identified in each participant from the source showing the largest amplitude alpha activity (7‐13 Hz). Given sex differences in PAF in TDC (females > males) and relatively few females in both groups, group comparisons were conducted examining only male TDC (N = 121) and ASD (N = 183). Regressions showed significant group slope differences, with an age‐related increase in PAF in TDC (R 2 = 0.32) but not ASD (R 2 = 0.01). Analyses examining male children below or above 10‐years‐old (median split) indicated group effects only in the younger TDC (8.90 Hz) and ASD (9.84 Hz; Cohen's d = 1.05). In the older ASD, a higher nonverbal IQ was associated with a higher PAF. In the younger TDC, a faster speed of processing was associated with a higher PAF. PAF as a marker for ASD depends on age, with a RS alpha marker of more interest in younger versus older children with ASD. Associations between PAF and cognitive ability were also found to be age and group specific.
Keywords: autism spectrum disorders, alpha, resting‐state, magnetoencephalography, maturation
1. INTRODUCTION
1.1. Background
Electroencephalographic (EEG) and magnetoencephalographic (MEG) studies show resting‐state (RS) oscillatory abnormalities in children with autism spectrum disorder (ASD; Cantor, Thatcher, Hrybyk, & Kaye, 1986; Coben, Clarke, Hudspeth, & Barry, 2008; Cornew, Roberts, Blaskey, & Edgar, 2012; Murias, Webb, Greenson, & Dawson, 2007; Orekhova et al., 2007; Rojas & Wilson, 2014). A potential problem, however, is that given maturational changes in RS brain activity in typically developing children (TDC; Clarke, Barry, McCarthy, & Selikowitz, 2001; Corbin & Bickford, 1955; Cragg et al., 2011; Eeg‐Olofsson, Petersen, & Sellden, 1971; Fisch, 1999; Gasser, Verleger, Bacher, & Sroka, 1988; Gibbs & Knott, 1949; Katada, Ozaki, Suzuki, & Suhara, 1981; Lopes da Silva, 2004; Matousek & Petersen, 1973; Matsuura et al., 1985), an abnormal RS profile identified in one ASD cohort may not generalize to children with ASD outside the studied age range (or even to all within the studied age range). This issue was examined via assessing RS parietal‐occipital alpha activity group differences in TDC and ASD across a wide age range.
In the eyes‐closed resting state, 8–13 Hz alpha oscillations are the dominant rhythms, most prominent in parietal‐occipital regions (Berger, 1929; Edgar et al., 2015; Haegens, Cousijn, Wallis, Harrison, & Nobre, 2014; Huang et al., 2014; Salmelin & Hari, 1994). A well‐established finding is that posterior RS alpha activity changes as a function of age. An age‐related increase in the frequency at which RS alpha oscillations show maximum power, often referred to as the peak alpha frequency (PAF), is notable in children. Among examined quantitative EEG and MEG parameters, the PAF is considered a robust signature of brain maturation (Szava et al., 1994; Valdes et al., 1992), with many studies showing an alpha peak at ~6 Hz in young children (5–7‐years‐old) and with an adult profile of a 10–12 Hz PAF not observed until at least 15‐years‐old (Miskovic et al., 2015; Somsen, van't Klooster, van der Molen, van Leeuwen, & Licht, 1997).
PAF is of clinical interest as it is one of the most heritable brain measures (Van Baal, De Geus, & Boomsma, 1996; van Beijsterveldt & van Baal, 2002). As an example, in a large sample of 16‐year‐old twins, Smit, Posthuma, Boomsma, and Geus (2005) obtained a heritability estimate of 0.81 for PAF, and with all nongenetic variance attributed to measurement unreliability rather than unique environmental factors. PAF is also of clinical interest given studies showing that PAF is associated with working memory and speed of information processing (Klimesch, 1997, 1999; Klimesch, Doppelmayr, Schimke, & Pachinger, 1996). Studies also indicate that the brain generates its own temporal structure via neural activity, with alpha rhythms providing the timing for communication within and between brain regions (e.g., (Klimesch, 2012)). Given that alpha rhythms and the circuits associated with alpha oscillations provide a scaffold for basic and more complex brain processes, an examination of alpha rhythms in neurodevelopmental disorders is of interest.
Two cross‐sectional studies have reported differences in the maturation of the PAF in TDC and children with ASD. Edgar et al. (2015) examined MEG source activity in a sample of 47 children with ASD and 41 TDC 6‐ to 14‐years‐old. Whereas TDCs showed the expected age‐related increase in parietal‐occipital PAF, PAF did not change as a function of age in the children with ASD. Using EEG and examining sensor activity in participants 2‐ to 12‐years‐old, Dickinson, DiStefano, Senturk, and Jeste (2018) also observed an age‐related increase in PAF in TDC (N = 38) but not children with ASD (N = 59).
Given age‐related PAF changes in TDC but not children with ASD, group‐differences might be expected to change as a function of age. Indeed, although Dickinson reported a higher PAF in TDC than ASD, examination of the Figure 4 regression lines in Dickinson et al. (2018) suggests that the group‐difference PAF effect is most pronounced in children with ASD older than 7 years, with either no group difference, or potentially a group difference in the opposite direction, in the younger children. (For a general discussion regarding the age dependence of brain electrophysiology measures see Szava et al. (1994) and John et al. (1980)).
Figure 4.
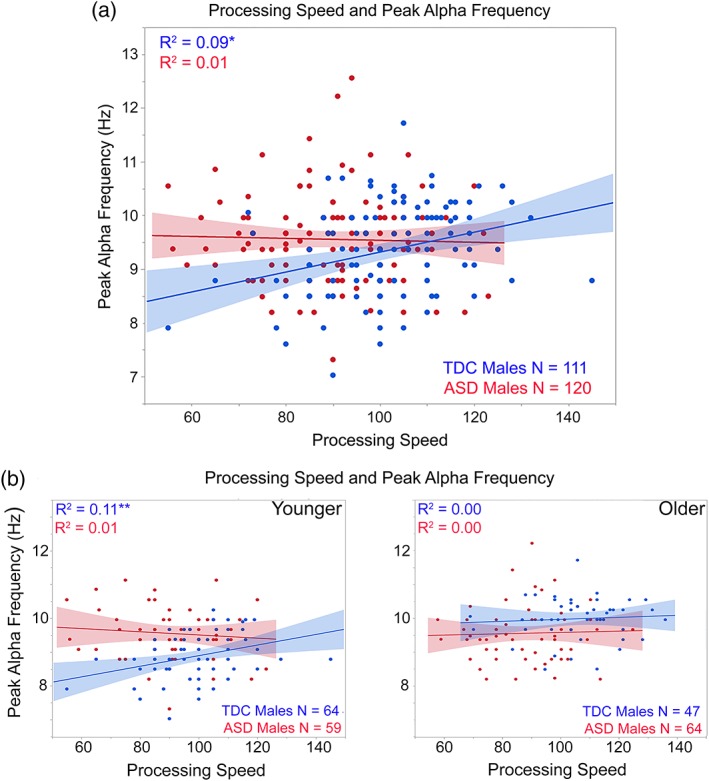
(a) Scatterplots showing associations between processing speed (x axis) and peak alpha frequency (y axis) for TDC (blue) and ASD (red). (b) Processing speed and peak alpha frequency associations shown for the younger (<10‐years‐old; left plot) and older children (>10‐years‐old; right plot). *p < 0.05, **p < 0.01 [Color figure can be viewed at http://wileyonlinelibrary.com]
The present study examined the above in a sample of TDC and children with ASD 2–3 times larger than any published study in order to (a) replicate previous PAF findings (samples in the present study did not overlap with the samples in Edgar et al. (2015)) and (b) determine if the PAF group‐difference pattern changes as a function of age. Hypothesizing an age‐related change in TDC but not ASD, group differences were expected to best characterize only a sub‐sample of the full population, thus indicating a “restricted” use of PAF as a clinical marker for ASD.1 Finally, given reported associations between cognitive ability (nonverbal IQ) and PAF in ASD but not TDC (Dickinson et al., 2018), exploratory analyses examined associations between cognitive ability and PAF.
2. METHODS AND MATERIALS
The study was approved by the local Institutional Review Board and all participants' families gave written informed consent. When competent to do so, children over the age of seven gave verbal assent to participate.
2.1. Subjects
TDC and ASD participants were selected according to the following criteria: (a) between the ages of 6 and 17 years, (b) no history of traumatic brain injury or other significant medical or neurological abnormality, (c) no active psychosis, (d) no magnetic resonance imaging (MRI) contraindications, (e) no sensory impairments (somatosensory, hearing, visual), and (f) native English speakers. ASD participants had a prior diagnosis, made by an expert clinician in CHOP's Regional Autism Center or by community providers according to DSM criteria. Given the extensive clinical evaluations upon which original ASD diagnosis was made, an abbreviated diagnostic battery confirmed the original diagnosis in the ASD group and ruled out ASD in the TDC group. ASD diagnostic classification was made using the Autism Diagnostic Observation Schedule (ADOS/ADOS‐2) and parent report on the Social Communication Questionnaire (SCQ; Lord et al., 2000; Lord, Rutter, & Le Couteur, 1994). In combination with the ADOS, exceeding empirically established cut‐offs by parent report on both the Social Responsiveness Scale and Autism Spectrum Rating Scale also led to ASD diagnostic confirmation if the SCQ did not corroborate diagnosis. The parent‐completed Autism Diagnostic Interview‐Revised (ADI‐R; Le Couteur, Lord, & Rutter, 2003) was administered for rare participants who entered the study without a formal ASD diagnosis made by an expert clinician (e.g., ASD educational classification only) and for any child with a prior ASD diagnosis for whom a diagnostic discordance existed (e.g., a child who exceeded ADOS diagnostic cut‐offs but was below SCQ and SRS‐2 cut‐offs). Dimensional symptom severity indices were obtained by parent report on the Social Responsiveness Scale (SRS/SRS‐2 (Constantino & Gruber, 2012)) and from the ADOS Calibrated Severity Score metric (Gotham, Pickles, & Lord, 2009).
Members of the TDC group were evaluated by licensed clinical psychologists who ruled out the presence of DSM‐IV‐TR/DSM‐5 Axis I disorders based on clinical judgment, review of the child's medical history form and parent screening interview. TDC‐specific inclusion criteria included scoring below the cut‐off for ASD on the ADOS‐2 as well as parent questionnaires, or, in some cases, just parent questionnaires when the ADOS‐2 was not administered. Additional TDC‐specific inclusion criteria included: no history of intellectual disability, speech/language disorder, learning disability, ADHD, or psychiatric disorders, and no first‐degree relatives with ASD.
To rule out global cognitive delay, all subjects recruited had to score at or above the second percentile (SS > 70) on at least one index of verbal or nonverbal intellectual functioning from the Wechsler Intelligence Scale for Children––fourth or fifth editions (WISC‐IV/WISC‐V; (Wechsler, 2003, 2014)), the Wechsler Abbreviated Scale of Intelligence‐2nd Edition (WASI; Wechsler, 2011), or the Differential Ability Scales––Second Edition (DAS‐II; Elliott, 2007).
For some of the studies, ASD participants withheld taking stimulant medication at least 24 hr prior to testing. Thirty‐eight (18.4%) of the children with ASD were prescribed stimulant medications, with 29% of these children withholding medication prior to testing. In addition to stimulant medication, 13.7% of children were taking some other class of psychotropic medications, including 2.9% taking nonstimulant medication for ADHD (e.g., alpha agonists), 6.4% taking SSRI's, and 4.4% taking atypical antipsychotics.
2.2. MEG and MRI data acquisition, co‐registration, and MEG forward modeling with BEM
Eyes‐closed RS MEG data were obtained from TDC and ASD children aged 6‐ to 17‐years‐old who have participated in studies conducted in our laboratory over the last 8 years. Participants were included if they had at least 2 min of RS eyes‐closed data collected using a 275‐channel MEG system (VSM MedTech Inc., Coquitlam, BC). Electro‐oculogram (EOG; vertical EOG on the upper and lower left sides) and electrocardiogram (ECG) were also obtained.
The participants' head position was monitored using head position indicator coils attached to the scalp. After a band‐pass filter (0.03–150 Hz), EOG and MEG signals were digitized at 600 Hz or 1,200 Hz (depending on study) with third‐order gradiometer environmental noise reduction. Participants were instructed to rest with their “eyes gently closed, like when you are sleeping” during a 2‐min or 5‐min RS exam, with length of recording depending on study. During the recording the EOG channel was monitored and if participants opened their eyes during the exam they were reminded to close their eyes. The majority of children were scanned in a supine position.
Eyes‐closed RS data were available from 155 TDC and 251 children with ASD. Of these, 15 TDC (7 females) and 50 ASD (7 females) participants did not keep their eyes closed during the task, fell asleep (and thus no clear alpha peak), or had too much movement or metal artifact. Of note, of the above with nonevaluable RS data, nearly half (45%) of participants prescribed stimulant medications did not complete the exam and/or their data was not evaluable due to the above noted reasons. Evaluable RS eyes‐closed data were thus available from 141 TDC (20 females; 19 left‐handed; 2 ambidextrous) and 204 children with ASD (21 females; 22 left‐handed, 3 ambidextrous).
2.3. Assessment of PAF
A two‐step process was employed for removal of muscle and movement artifact. First, participants' raw EOG data were visually examined and data contaminated by blinks, saccades, or other significant EOG activity were removed from the MEG data. Second, blind to diagnosis, participants' MEG data were visually inspected for muscle‐related activity (focusing especially on data from sensors close to the temporalis muscles), and data containing muscle activity were removed. To be included in the study, participants had to have at least 60 s of artifact‐free data, with a mean of 191 s (SD = 74) in the ASD group and a mean of 223 s (SD = 65) in the TDC group (data often “lost” in subjects with the shorter 2‐min RS exam). A t‐test showed that the groups differed with respect to amount of artifact‐free data (p < 0.01). To determine if group differences in the amount of artifact‐free data was a concern, the PAF values obtained using the total artifact‐free data were compared to PAF values obtained after first randomly removing one‐third of the epochs and followed by randomly removing two‐third of the epochs. These analyses were performed for a random selection of 26 TDC and 26 children with ASD. In this sample, the full data artifact‐free time ranged from 73 s to 298 s. For the analyses with two‐thirds of the epochs removed the artifact‐free time ranged from 22 s to 99 s. For the full sample, a Cronbach's Alpha value of 0.97 was observed. Exploring Cronbach's Alpha by group revealed a value of 0.98 for TDC and 0.95 for ASD. When PAF differences were observed within a subject, the PAF changed by only one frequency bin (frequency bin resolution = 0.29 Hz). The above analyses indicated that any potential PAF group difference finding was likely not due to the slight but significant group differences in the amount of evaluable data.
MEG data were further processed using BESA Research 6.1 (MEGIS Software GmbH, Grafelfing, Germany). To decompose the 275 channel data into a smaller number of measures for analysis, a source model with 15 regional sources (Figure 1) was applied to project each participant's raw MEG surface data into brain source space where the waveforms are the modeled source activities (given two orthogonal dipoles per regional source there are two time series at each location; Hoechstetter et al., 2004; Bulletin, 2003). These regional sources are not intended to correspond to precise neuroanatomical structures but rather to represent neural activity at coarsely defined regions and to provide measures of brain activity with better signal separation and with a greater signal‐to‐noise ratio than would be afforded at the sensor level (Scherg & Berg, 1996; Scherg & Ebersole, 1993). The locations of the regional sources in the model are such that there is an approximately equal distance between sources (3 cm), helping to separate signals originating from different brain regions.
Figure 1.
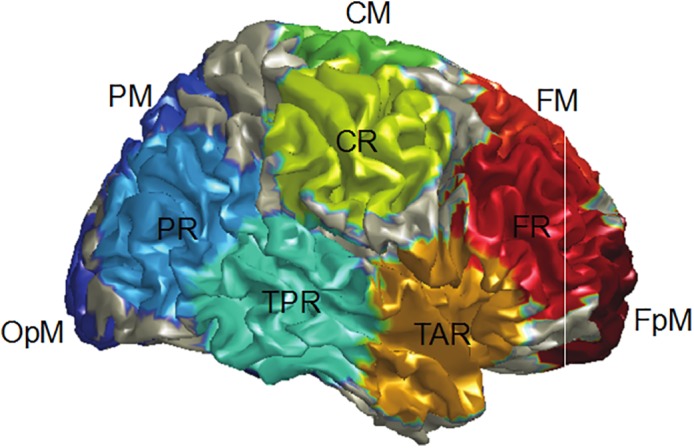
Regional source locations. Resting‐state alpha activity was measured for all 15 sources, with the PAF for each participant determined via identifying from the 15 alpha power spectra the frequency at the source with the most alpha activity within a 7–13 Hz range (typically the midline parietal or occipital source). The figure shows the locations for FpM fronto‐polar midline, FM frontal midline, FR frontal right, TAR temporal anterior right, CM central midline, CR central right, TPR temporal posterior right, PM parietal midline, PR parietal right, and OPM occipito‐polar midline. The left hemisphere contained analogous regional sources, though with region source labels ending with “L” instead of “R” [Color figure can be viewed at http://wileyonlinelibrary.com]
To transform MEG data from the time domain into the frequency domain, a Fast Fourier Transform was applied to artifact‐free 3.41 s epochs of continuous data for each of the two orthogonally oriented time series at each regional source. Each 3.41 s epoch overlapped 50% with the next epoch, and each epoch was multiplied by a cosine squared window. This combination of overlap and windowing ensured that each time point contributed equally to the mean power spectra. Where artifact‐free epochs were contiguous in time, then the windowing procedure was applied to “overlapping segments” of data. When the segment of data following an artifact‐free epoch was bad, there was no overlap between epochs and windowing was thus applied to the next available artifact‐free 3.41 s epoch.
The mean power spectra for the two orthogonally oriented time series at each regional source were summed to yield the power at a given frequency at that source. To assess study hypotheses, the PAF for each participant was determined via identifying the frequency at the source with the most alpha activity from the 15 power spectra within a 7–13 Hz range (typically the midline parietal or occipital source). PAF was determined blind to group status. Chi‐square analyses showed no group differences in the location of the PAF, with a PAF observed in the vast majority of the participants at midline Parietal (77%), midline Occipital (10%), and midline Central (9%)1. For one of the studies, in 34 participants (17 TDC and 17 ASD), the eye‐closed RS exam was administered twice at a single visit. For the 34 subjects with a repeated scan, an average PAF measure was obtained. Interclass correlation coefficient (ICC) analyses of these PAF measures showed very high reliability (ICC single measure = 0.88, ICC average measure = 0.94, ps < 0.001).
2.4. Cognitive assessment
As cognitive batteries were slightly different across studies, where possible, equivalent scores were determined from each battery in order to obtain the following for each participant: full scale IQ (FSIQ), verbal IQ (VIQ), nonverbal IQ (NVIQ), and processing speed (PS). Estimated Full Scale IQ was obtained from the WISC‐IV/WISC‐V General Ability Index (GAI) or from a highly reliable 4‐subtest WISC‐V short‐form (Sattler, Dunmont, & Coalson, 2016), as well as from the WASI‐II 4‐subtest FSIQ, and the General Conceptual Ability (GCA) composite from the DAS‐II. The Verbal Comprehension Index (VCI) from the WISC‐IV, WISC‐V, or WASI‐II was used across the majority of studies to represent VIQ; however in a subset of participants (N = 30) the Verbal Cluster score from the DAS‐II was accepted. NVIQ was represented by one of the following scores (selected according to the most reliable and equivalent measures available for each participant): the WISC‐IV or WASI‐II Perceptual Reasoning Index (PRI), the average of the WISC‐V Fluid Reasoning Index (FRI) and Visual Spatial Index (VSI), the WISC‐V FRI only, or the Special Nonverbal Composite score of the DAS‐II (SNC). Processing Speed scores were not collected for all studies; in cases where PS information was available, the Processing Speed Index (PSI) from the WISC‐IV/V was used or Symbol Search (SS) subtest scores served as a stand‐alone proxy for PSI. Speed of information processing scores from the DAS‐II were also accepted. Due to time constraints and also due to the fact that slightly different cognitive batteries were administered across studies, FSIQ scores were not obtained from 5 participants, VIQ from 3, NVIQ from 32, and PS from 89.
2.5. Group statistics
Between‐group t‐tests compared PAF values. To examine maturation changes, hierarchical regressions with age entered first, group second, and the interaction term last (formally assessing group slope differences), examined associations between PAF and age. Similar exploratory regressions examined associations between PAF and cognitive ability and between PAF and SRS and CSS scores.
3. RESULTS
Given many studies showing sex differences in RS alpha activity in children (Clarke et al., 2001; Gasser, Jennen‐Steinmetz, Sroka, Verleger, & Mocks, 1988; Matousek & Petersen, 1973; Matsuura et al., 1985; Petersen & Eeg‐Olofsson, 1971), preliminary analyses considered alpha and age differences with respect to sex. In TDC, a marginally significant finding indicated a higher PAF in females (9.95 Hz) than males (9.46 Hz), t(139) = 1.93; p = 0.06, and TDC females were significantly younger than TDC males, t(139) = 2.32; p < 0.05. In ASD, although no sex PAF difference was observed, t(202) = 1.39; p > 0.05, females with ASD were younger than males with ASD, t(227) = 2.38; p < 0.05. Due to marginally significant higher PAF in typically developing females than males, TDC and ASD age differences as a function of sex, and relatively few females in both groups (ASD (N = 21), TDC (N = 20)), all further analyses included only males. For comparison with previous and future studies, however, the online supplement presents the PAF findings for the entire male + female sample.
Considering only the male participants, as shown in Table 1, groups did not differ on age. As shown in Table 1, IQ scores were significantly higher in TDC than ASD. As expected, Social responsiveness scale (SRS) scores were higher in ASD than TDC.
Table 1.
Demographics for male sample
| TDC (N = 121) | ASD (N = 183) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 10.2 | 3.2 | 10.6 | 2.8 |
| Age range (years) | 6.1–17.7 | 6.1–17.9 | ||
| GAI/estimated full scale IQ** | 113.1 | 13.9 | 103.6 | 17.6 |
| Verbal IQ** | 111.0 | 12.9 | 100.3 | 17.7 |
| Nonverbal IQ* | 110.9 | 14.2 | 105.1 | 16.7 |
| Processing speed index** | 101.7 | 14.1 | 89.9 | 14.3 |
| SRS T‐score** | 43.4 | 4.6 | 73.87 | 12.4 |
Note. * p < 0.05. ** p < 0.01.
3.1. PAF group differences and associations with age
Simple effect analyses of a significant interaction term showed group slope differences, F(1,300) = 13.43, p < 0.001, with a positive relationship between age and PAF in TDC (r = 0.57, p < 0.001) and no age and PAF relationship in ASD (r = 0.10, p > 0.05; see Figure 2). Whereas in TDC the PAF increased at the rate of ~0.18 Hz per year (standard error 0.02 Hz) and thus predicted a PAF of ~8.5 Hz in 6‐year‐old male TDC and ~10 Hz in 12‐year‐old male TDC, PAF remained unchanged as a function of age in the male children with ASD (mean PAF value in ASD = ~9.95 Hz).
Figure 2.
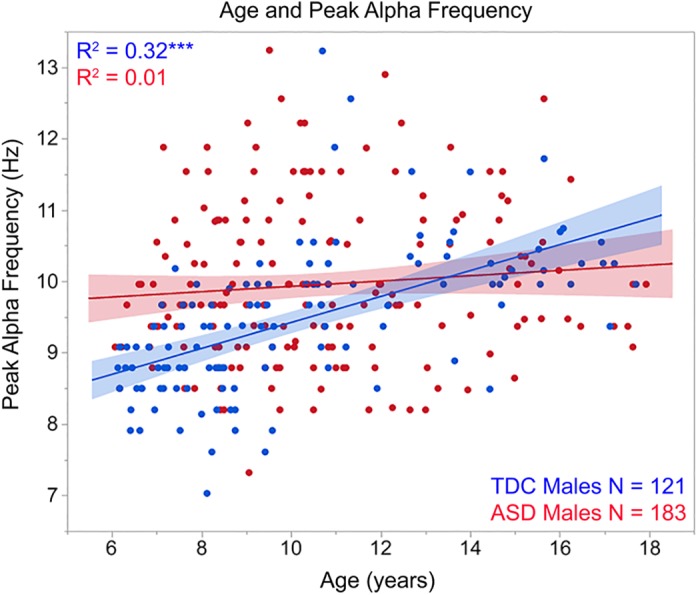
Scatterplots showing associations between age (x axis) and peak alpha frequency (y axis) for TDC (blue) and ASD (red). As detailed in Section 3, a significant interaction term indicated group slope differences. ***p < 0.001 [Color figure can be viewed at http://wileyonlinelibrary.com]
Although the regression analyses demonstrated group differences in the maturation of the PAF, a t‐test was performed to provide PAF group difference information for the full male sample; the t‐test showed a higher PAF in ASD (9.95 Hz) than TDC (9.46 Hz), t(302) = 3.93; p < 0.001. Examination of Figure 2 shows that given an age‐related change in PAF in TDC but not ASD, the ASD > TDC PAF finding was most representative of the male children between 6‐ and 10‐years‐old, with similar PAF values observed between the ages of 10‐ and 14‐years, and with some indication of lower PAF values in ASD than TDC in the children above 14 years. Analyses examining group differences in the male children below or above 10‐years of age (median age of entire sample = 10.07‐years‐old) indicated large group‐differences in the younger TDC (8.90 Hz) versus younger children with ASD (9.84 Hz; t[154] = 6.27, p < 0.001; Cohen's d = 1.05), and no group differences in the older TDC (10.19 Hz) and ASD (10.06), (t[146] = 0.72, p > 0.05).
Of note, the PAF group differences in younger participants may slightly overestimate the true group‐difference effect as in the younger group the children with ASD (8.32‐years‐old) were slightly but significantly older than the TDC (7.87‐years‐old), t(154) = 2.66, p < 0.01). However, given an estimated maturational change of 0.18 Hz per year, and thus an estimated difference in PAF of at most 0.09 Hz between children 0.5 years apart, this ~0.09 Hz difference likely does not account for the 0.94 Hz PAF group difference observed in the younger children.
3.2. Associations between clinical measures and PAF
Regressions were run with cognitive scores, SRS or ADOS CSS as the dependent variable and PAF entered in the first block, group in the second block, and the interaction term last. Considering FSIQ, NVIQ, and VIQ, simple effect analyses of very weakly trending interaction terms (ps = 0.09–0.12), suggested a positive relationship in ASD but not TDC between PAF and FSIQ (p = 0.08) and NVIQ (p = 0.08). To compare to the PAF and NVIQ associations reported in Dickinson et al. (2018), Figure 3a scatterplots show findings for NVIQ and PAF. Considering processing speed, and as shown in Figure 4a, simple effect analyses of a significant interaction term, F(1,227) = 6.60, p = 0.01, showed a positive relationship between age and processing speed in TDC (p < 0.001) but not ASD (p > 0.05).
Figure 3.
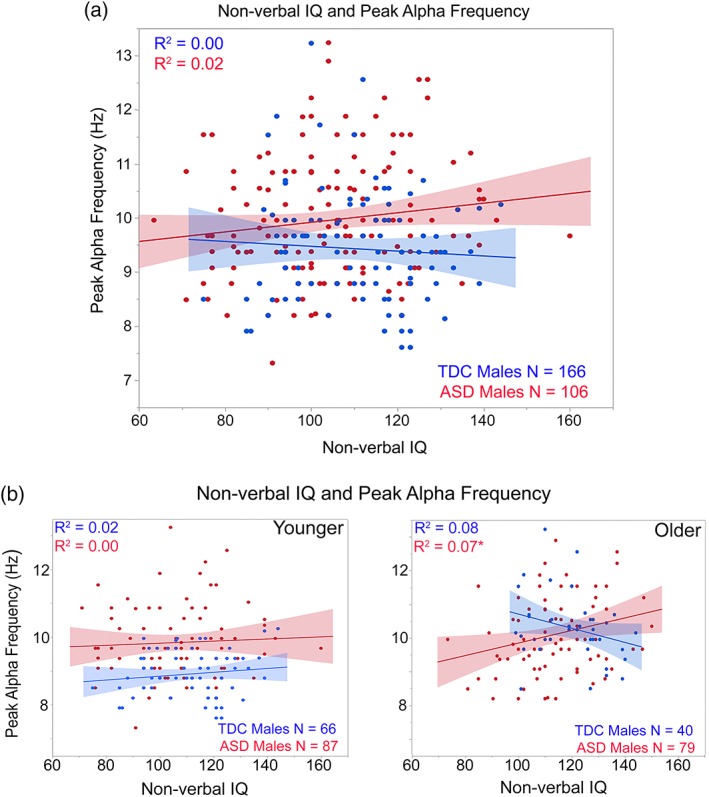
(a) Scatterplots showing associations between nonverbal IQ (x axis) and peak alpha frequency (y axis) for TDC (blue) and ASD (red). (b) Nonverbal IQ and peak alpha frequency associations shown for the younger (<10‐years‐old; left plot) and older children (>10‐years‐old; right plot). *p < 0.05 [Color figure can be viewed at http://wileyonlinelibrary.com]
Separately examining the younger and older groups, associations between FSIQ and NVIQ and PAF in the children with ASD were observed only in the older children (shown for NVIQ in Figure 3b subplots). In contrast, associations between processing speed and PAF were observed only in the younger TDC (shown in Figure 4b subplots). Finally, analyses separately examining each group showed no associations between SRS or ADOS CSS scores and PAF (ps > 0.05).
4. DISCUSSION
As hypothesized, the use of PAF as a marker for ASD depends on the age of the group investigated. Specifically, given no age‐related PAF change in male ASD, the younger male children with ASD (6‐ to 10‐years old) had a higher PAF than the younger male TDC (Cohen's d = 1.05). In contrast, the older male children with ASD (10–18 years) tended to have a lower PAF than the older male TDC (although this group difference was not significant in this sample). This pattern of findings is somewhat fortuitous, as a RS alpha marker is of more interest in younger versus older children with ASD.
PAF associations with cognitive ability were also found to be age‐specific. In particular, although a higher PAF in the young children with ASD appeared to reflect abnormal brain activity, in the older children with ASD a higher PAF was associated with a higher NVIQ. In contrast, in the TDC group, a higher PAF predicted faster processing speed, although this association was most prominent in the younger male TDC group. The text below considers present findings within the context of previous research.
PAF findings replicated the many previous studies observing age‐related PAF increases in typically developing children and adolescents (see Section 1). For example, examining EEG RS activity in a large sample of typically developing 7‐ to 11‐year‐old, Miskovic et al. (2015) observed an increase in PAF from 7 years (mean PAF = 8.89 Hz) to 11 years (mean PAF = 9.79 Hz), findings very consistent with the PAF values observed in the present study. The mean PAF for male children with ASD in this study was 9.95 Hz, with many 6‐ to 8‐years‐old male children with ASD thus showing an adult‐like PAF.
Although the present observation of an association between age and PAF in TDC but not ASD replicates Dickinson et al.'s (Dickinson et al., 2018) examination of PAF in 2–12 years old, several study differences are of note. First, whereas in the present study higher PAF values were observed in ASD than TDC, in Dickinson et al. lower PAF values were observed in ASD than TDC. In the present study, the PAF group‐difference was primarily driven by the younger participants, with the Figure 2 scatterplots indicating that a higher PAF in ASD than TDC was most prominent in the 6‐ to 10‐years‐old. As noted in Section 1, examination of Figure 4 in Dickinson et al. also suggests a reversal in the group‐difference PAF effect, although the interaction point in Dickinson occurs earlier, at around 4‐years‐old.
Thus, whereas the present study and Dickinson et al. both demonstrate that the pattern of group‐differences depends on the age of the sample, findings between the two studies are not completely equivalent. Study differences are likely due to the fact that the primary findings in the present study were restricted to males (as detailed in Section 3 there were too few females in the present study to perform female‐only analyses) whereas ~22% (ASD) and 33% (TDC) of the participants in the Dickinson study were female. Given the trend toward higher PAF in female versus male TDCs observed in the present study, the female controls in Dickinson et al. (2018) may contribute to the group‐difference pattern they reported. Another study difference is that given the age range examined in Dickinson et al. (~2–12 years) their RS data were obtained in an eyes‐open condition versus the eyes‐closed condition in the present study.
Present findings generally mirrored the Dickinson et al. (2018) finding of a higher NVIQ in the children with ASD with a higher PAF. Follow‐up analyses in the present study, however, showed this association only for the older children (see Figure 3b younger and older children scatterplots). This pattern compliments the PAF findings, indicating that a higher PAF in younger children with ASD reflects an abnormal neural circuit, but with a higher PAF in older children with ASD reflecting more normal RS alpha activity. Analogous to Dickinson et al., NVIQ was not associated with PAF in the TDC group.
The TDC group showed an association between PAF and processing speed (see Figure 4a). Given no associations with PAF and GAI, VIQ, or NVIQ in the TDC group, the association with processing speed in TDC appears to be specific to tests assessing the ability to complete tasks quickly versus tasks that rely on fluid or crystallized intelligence (Grandy et al., 2013; Klimesch et al., 1996). The loss of an association between PAF and processing speed in children with ASD (younger and older) perhaps accounts for the slower performance observed on processing speed tasks in some individuals with ASD (Mayes & Calhoun, 2003, 2008; Nyden, Billstedt, Hjelmquist, & Gillberg, 2001; Oliveras‐Rentas, Kenworthy, Roberson 3rd, Martin, & Wallace, 2012).
Studies exploring the relationship between PAF and aspects of cognition in adults have yielded mixed results. Some studies have found a positive relationship between PAF and processing speed (Grandy et al., 2013; Klimesch et al., 1996), memory (Grandy et al., 2013; Lebedev, 1994; Saletu & Grunberger, 1985), or general IQ (Mundy‐Castle, 1958) in adults of all ages. However, other studies have not observed a relationship between cognition and PAF in adults ((Posthuma, Neale, Boomsma, & de Geus, 2001); for review see (Vogel & Broverman, 1964)). Present results suggest that the lack of findings in some adult studies may be due to the fact that these associations are age specific. In particular, the PAF and processing speed association most prominent in the younger male TDC group (see Figure 4b) suggests a relationship between PAF and processing speed only as PAF matures in TDC (similar to how in young children age predicts basketball ability but in adolescents and adults age is a poor predictor of basketball ability).
Regarding the generation of alpha rhythms, thalamic, cortical, or thalamo‐cortical models have been proposed (for a detailed review see Valdes‐Hernandez et al. (2010)). There is mounting evidence that the PAF is determined, in part, by thalamo‐cortical pathways (Lopes da Silva, Vos, Mooibroek, & Van Rotterdam, 1980; Roberts & Robinson, 2008; Robinson et al., 2003; Steriade, Gloor, Llinas, Lopes de Silva, & Mesulam, 1990). For example, Valdes‐Hernandez et al. (2010) observed associations between posterior and superior corona radiata and the splenium of the corpus callosum white matter (fractional anisotropy) and PAF in a sample of 89 adults, suggesting an influence of myelination of posterior white‐matter tracks (or fiber density) on the parietal‐occipital PAF. These findings were replicated by Jann et al. (2012) who observed associations between PAF and white matter in the genu and splenium of the corpus callosum as well as the right superior longitudinal fascicle. That increased white‐matter would predict a higher PAF (and perhaps also a faster speed of processing) needs further examination in future studies.
Earlier myelination in ASD may account for the higher PAF observed in the younger children with ASD than TDC in the present study. Studies have shown, on average, larger brain volume in ASD versus TDC, at least in early childhood. Volumetric differences are observed in studies of head circumference (Dawson et al., 2007; Dementieva et al., 2005) and total brain volume (Aylward, Minshew, Field, Sparks, & Singh, 2002; Bloss & Courchesne, 2007; Courchesne et al., 2001; Courchesne, Carper, & Akshoomoff, 2003; Frith, 2003; Piven et al., 1995; Redcay & Courchesne, 2005; Sparks et al., 2002) and may be indicative of disturbances of synaptogenesis and neural pruning in ASD (Belmonte et al., 2004; Courchesne et al., 2001; Frith, 2003). Hazlett et al. (2017) found increased cortical surface expansion in 6–12‐month‐old infants later diagnosed with ASD compared to TD infants, with cortical surface expansions especially marked in the left and right middle occipital gyrus and the right cuneus, areas of the brain implicated as alpha generators (Edgar et al., 2015; Huang et al., 2014; Salmelin & Hari, 1994). Research has also indicated that this increased brain volume found in infants and young children with ASD is due to abnormally rapid brain maturation in ASD, including increased white matter in infants with ASD compared to typically developing infants (Courchesne et al., 2001, 2003; Hazlett et al., 2005; Hendry et al., 2006; Herbert, 2005; Herbert et al., 2004; Hughes, 2007; Ouyang et al., 2016). Of note, however, Courchesne (2004), Courchesne et al. (2001), and Redcay and Courchesne (2005) reported that the brain overgrowth during the first 2 years of life in infants with ASD is followed by abnormally slow or arrested growth. During this period of arrested growth, white matter volume no longer increases at the rate that would be expected in a typically developing child (Courchesne et al., 2001; Ouyang et al., 2016).
On the above account, increased PAF in the young children with ASD suggests alpha neural circuits that mature too early, perhaps as a result of abnormally rapid white‐matter myelination, and with the abnormally slow myelination in ASD after infancy accounting for no age‐related change in PAF (at least in some children with ASD). Given that alpha rhythms are thought to provide the basic structure for cognitive processes (Dickinson et al., 2018; Haegens et al., 2014; Klimesch, 1999; Mierau, Klimesch, & Lefebvre, 2017), premature development of these circuits may result in a brain that is not optimized for later acquisition of higher‐order cognitive skills including language and social skills. Future studies examining the alpha rhythms in young children first diagnosed with ASD would contribute to understanding the high PAF in children with ASD. Multimodal studies examining associations between PAF and white‐matter would help elucidate the contribution of white‐matter to alpha abnormalities in ASD.
Although there is growing evidence for a contribution of posterior white‐matter pathways to RS alpha, as reviewed in Edgar et al. (2015), other mechanisms may contribute to RS alpha activity. These include a role for cortical inhibitory interneurons in maintaining alpha oscillations (Lorincz, Kekesi, Juhasz, Crunelli, & Hughes, 2009), a role for thalamic “pacemarker” neurons (e.g., see (Anderson & Sears, 1964; Jahnsen & Llinas, 1984; Steriade & Deschenes, 1984)), and an influence of the mGluR1a subtype of the metabotropic glutamate receptor located postsyntactically to corticothalamic fibers (Hughes, Blethyn, Cope, & Crunelli, 2002). Studies investigating the above in individuals with ASD report abnormalities in all the above systems including an imbalance of excitatory (e.g., glutamatergic) and inhibitory (e.g., GABAergic) activity in inhibitory interneuron and pyramidal cell cortical networks (Casanova, Buxhoeveden, Switala, & Roy, 2002; Levitt, Eagleson, & Powell, 2004), and studies reporting abnormal thalamic structure and connectivity in ASD (Hardan et al., 2006, 2008; Nair, Treiber, Shukla, Shih, & Muller, 2013; Tsatsanis et al., 2003).
Several study limitations are of note. First, given the small number of TDC and ASD females, findings are specific to males. Although the total sample findings presented in the Online Supplement suggest in the full sample a pattern of findings similar to the male‐only sample, the findings are more evident in males, with larger N studies comparing RS alpha activity in female TDC and ASD needed. Another study limitation is that MEG data were co‐registered to a standard template MRI instead of each individual's MRI. Analysis (not shown) comparing PAF measures obtained using a template MRI versus individual MRI show that the PAF is similar across methods (the same or differing only by one frequency bin [0.29 Hz in the present study]). Douw, Nieboer, Stam, Tewarie, and Hillebrand (2018) also reported for RS MEG measures no bias or inconsistency between template and individual MRI. Another study limitation is that for many participants a relatively short eyes‐closed RS exam was used; given more data loss in the children with ASD due to motion and metal artifact, recordings longer than 2 min are needed to obtain evaluable RS data. Finally, as the study design was cross sectional, rate‐of‐change measures (slopes) were available only at the group level. As previously noted, future studies obtaining longitudinal data are of interest (especially from older toddlers to ~10‐year‐old children), with the hypothesis that PAF rate‐of‐change will better differentiate diagnostic groups as well as better predict current and future cognitive impairment in children with ASD.
To conclude, a primary finding was that the use of PAF as a marker for ASD depends on the age (and perhaps sex) of the group investigated. Associations between PAF and cognitive ability were also found to be age (and group) specific. This pattern of PAF group differences, most pronounced in the younger children, is somewhat fortuitous, as RS alpha biomarkers are of more interest in younger versus older children with ASD. In sum, findings demonstrated that normal maturational changes in brain function throughout childhood and adolescence need to be considered in child studies, with present results indicating that with large enough samples our data indicate the age range(s) when clinical imaging markers are of most use.
Supporting information
Appendix S1: Online Supplement (analyses for the full male + female sample)
Supplementary Figure S1: Online Supplement Figure 1. Scatterplots showing associations between age (x axis) and peak alpha frequency (y axis) for full sample TDC (blue) and ASD (red). ***p < 0.001
Online Supplement Figure 2. (a) Scatterplots showing associations between non‐verbal IQ (x axis) and peak alpha frequency (y axis) for full sample TDC (blue) and ASD (red). (b) Non‐verbal IQ and peak alpha frequency associations shown for the younger (< 10‐years‐old; left plot) and older children (>10‐years‐old; right plot). *p < 0.05
Online Supplement Figure 3. (a) Scatterplots showing associations between processing speed (x axis) and peak alpha frequency (y axis) for full sample TDC (blue) and ASD (red). (b) Processing speed and peak alpha frequency associations shown for the younger (< 10‐years‐old; left plot) and older children (>10‐years‐old; right plot). *p < 0.05, ** p < 0.01
ACKNOWLEDGMENTS
DISCLAIMER: Dr. Berman reports a consultancy with McGowan Associates. Dr. Roberts declares his position on the advisory boards of (a) CTF MEG, (b) Ricoh, (c) Spago Nano Medical, and (d) Prism Clinical Imaging. Dr. Roberts and Dr. Edgar also declare intellectual property relating to the potential use of electrophysiological markers for treatment planning in clinical ASD. This study was supported in part by NIH grant R01DC008871 (TR), NIH grant R01MH107506 (JCE), NICHD grant R01HD093776 (JCE), NIH grant R21MH098204 (JCE), NIH grant R21 NS090192 (JCE), and the Clinical Translational Core and the Neuroimaging Core of the Intellectual and Developmental Disabilities Research Center funded by NICHD grant 5U54HD086984 (to RTS and TR; principal investigator, M. Robinson). Dr. Roberts gratefully acknowledges the Oberkircher Family for the Oberkircher Family Chair in Pediatric Radiology at CHOP.
Edgar JC, Dipiero M, McBride E, et al. Abnormal maturation of the resting‐state peak alpha frequency in children with autism spectrum disorder. Hum Brain Mapp. 2019;40:3288–3298. 10.1002/hbm.24598
Funding information Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Numbers: R01HD093776, 5U54HD086984; National Institute of Mental Health, Grant/Award Numbers: R01DC008871, R01MH107506, R21MH098204, NS090192
Footnotes
Although in the present study the analysis strategy allowed determination of PAF in each individual, this strategy did not allow assessment of group differences in alpha power. This is because PAF for each participant was determined via identifying the regional source showing the strongest alpha activity. As such, in addition to brain source location differing across subjects, the selection in each participant of the source showing the largest alpha activity results in a confound with respect to examining group differences in alpha amplitude. In future studies, distributed source localization will be used to examine group differences in alpha power across the brain (as in Edgar et al. (2015)).
REFERENCES
- Anderson, P. , & Sears, T. A. (1964). The role of inhibition in the phasing of spontaneous thalamocortical discharge. The Journal of Physiology, 173, 459–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward, E. H. , Minshew, N. J. , Field, K. , Sparks, B. F. , & Singh, N. (2002). Effects of age on brain volume and head circumference in autism. Neurology, 59, 175–183. [DOI] [PubMed] [Google Scholar]
- Belmonte, M. K. , Cook, E. H., Jr. , Anderson, G. M. , Rubenstein, J. L. , Greenough, W. T. , Beckel‐Mitchener, A. , … Tierney, E. (2004). Autism as a disorder of neural information processing: Directions for research and targets for therapy. Molecular Psychiatry, 9, 646–663. [DOI] [PubMed] [Google Scholar]
- Berger, H. (1929). Über das elektrenkephalogramm des menschen. Archiv für Psychiatrie Und Nervenkrankheiten, 87, 527–570. [Google Scholar]
- Bloss, C. S. , & Courchesne, E. (2007). MRI neuroanatomy in young girls with autism: A preliminary study. Journal of the American Academy of Child and Adolescent Psychiatry, 46, 515–523. [DOI] [PubMed] [Google Scholar]
- Cantor, D. S. , Thatcher, R. W. , Hrybyk, M. , & Kaye, H. (1986). Computerized EEG analyses of autistic children. Journal of Autism and Developmental Disorders, 16, 169–187. [DOI] [PubMed] [Google Scholar]
- Casanova, M. F. , Buxhoeveden, D. P. , Switala, A. E. , & Roy, E. (2002). Minicolumnar pathology in autism. Neurology, 58, 428–432. [DOI] [PubMed] [Google Scholar]
- Clarke, A. R. , Barry, R. J. , McCarthy, R. , & Selikowitz, M. (2001). Age and sex effects in the EEG: Development of the normal child. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 112, 806–814. [DOI] [PubMed] [Google Scholar]
- Coben, R. , Clarke, A. R. , Hudspeth, W. , & Barry, R. J. (2008). EEG power and coherence in autistic spectrum disorder. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 119, 1002–1009. [DOI] [PubMed] [Google Scholar]
- Constantino, J. , & Gruber, C. P. (2012). Social responsiveness scale (2nd ed.). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Corbin, H. P. , & Bickford, R. G. (1955). Studies of the electroencephalogram of normal children: Comparison of viscal and automatic frequency analyses. Electroencephalography and Clinical Neurophysiology, 7, 15–28. [DOI] [PubMed] [Google Scholar]
- Cornew, L. , Roberts, T. P. , Blaskey, L. , & Edgar, J. C. (2012). Resting‐state oscillatory activity in autism spectrum disorders. Journal of Autism and Developmental Disorders, 42, 1884–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne, E. (2004). Brain development in autism: Early overgrowth followed by premature arrest of growth. Mental Retardation and Developmental Disabilities Research Reviews, 10, 106–111. [DOI] [PubMed] [Google Scholar]
- Courchesne, E. , Carper, R. , & Akshoomoff, N. (2003). Evidence of brain overgrowth in the first year of life in autism. JAMA, 290, 337–344. [DOI] [PubMed] [Google Scholar]
- Courchesne, E. , Karns, C. M. , Davis, H. R. , Ziccardi, R. , Carper, R. A. , Tigue, Z. D. , … Courchesne, R. Y. (2001). Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology, 57, 245–254. [DOI] [PubMed] [Google Scholar]
- Cragg, L. , Kovacevic, N. , McIntosh, A. R. , Poulsen, C. , Martinu, K. , Leonard, G. , & Paus, T. (2011). Maturation of EEG power spectra in early adolescence: A longitudinal study. Developmental Science, 14, 935–943. [DOI] [PubMed] [Google Scholar]
- Dawson, G. , Munson, J. , Webb, S. J. , Nalty, T. , Abbott, R. , & Toth, K. (2007). Rate of head growth decelerates and symptoms worsen in the second year of life in autism. Biological Psychiatry, 61, 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dementieva, Y. A. , Vance, D. D. , Donnelly, S. L. , Elston, L. A. , Wolpert, C. M. , Ravan, S. A. , … Cuccaro, M. L. (2005). Accelerated head growth in early development of individuals with autism. Pediatric Neurology, 32, 102–108. [DOI] [PubMed] [Google Scholar]
- Dickinson, A. , DiStefano, C. , Senturk, D. , & Jeste, S. S. (2018). Peak alpha frequency is a neural marker of cognitive function across the autism spectrum. The European Journal of Neuroscience, 47, 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douw, L. , Nieboer, D. , Stam, C. J. , Tewarie, P. , & Hillebrand, A. (2018). Consistency of magnetoencephalographic functional connectivity and network reconstruction using a template versus native MRI for co‐registration. Human Brain Mapping, 39, 104–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar, J. C. , Heiken, K. , Chen, Y. H. , Herrington, J. D. , Chow, V. , Liu, S. , … Roberts, T. P. (2015). Resting‐state alpha in autism spectrum disorder and alpha associations with thalamic volume. Journal of Autism and Developmental Disorders, 45, 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeg‐Olofsson, O. , Petersen, I. , & Sellden, U. (1971). The development of the electroencephalogram in normal children from the age of 1 through 15 years. Paroxysmal activity. Neuropadiatrie, 2, 375–404. [DOI] [PubMed] [Google Scholar]
- Elliott, C. D. (2007). Differential ability scales (2nd ed.). San Antonio, TX: Pearson. [Google Scholar]
- Fisch, B. (1999). Fisch and Spehlmann's EEG Primer In Basic principles of digital and analog EEG (3rd ed.). San Diego, California: Elsevier. [Google Scholar]
- Frith, C. (2003). What do imaging studies tell us about the neural basis of autism? Novartis Foundation Symposium, 251, 149–166 discussion 166–176, 281–197. [PubMed] [Google Scholar]
- Gasser, T. , Jennen‐Steinmetz, C. , Sroka, L. , Verleger, R. , & Mocks, J. (1988). Development of the EEG of school‐age children and adolescents. II. Topography. Electroencephalography and Clinical Neurophysiology, 69, 100–109. [DOI] [PubMed] [Google Scholar]
- Gasser, T. , Verleger, R. , Bacher, P. , & Sroka, L. (1988). Development of the EEG of school‐age children and adolescents. I. Analysis of band power. Electroencephalography and Clinical Neurophysiology, 69, 91–99. [DOI] [PubMed] [Google Scholar]
- Gibbs, F. A. , & Knott, J. R. (1949). Growth of the electrical activity of the cortex. Electroencephalography and Clinical Neurophysiology, 1, 223–229. [PubMed] [Google Scholar]
- Gotham, K. , Pickles, A. , & Lord, C. (2009). Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders, 39, 693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandy, T. H. , Werkle‐Bergner, M. , Chicherio, C. , Lovden, M. , Schmiedek, F. , & Lindenberger, U. (2013). Individual alpha peak frequency is related to latent factors of general cognitive abilities. NeuroImage, 79, 10–18. [DOI] [PubMed] [Google Scholar]
- Haegens, S. , Cousijn, H. , Wallis, G. , Harrison, P. J. , & Nobre, A. C. (2014). Inter‐ and intra‐individual variability in alpha peak frequency. NeuroImage, 92, 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardan, A. Y. , Girgis, R. R. , Adams, J. , Gilbert, A. R. , Keshavan, M. S. , & Minshew, N. J. (2006). Abnormal brain size effect on the thalamus in autism. Psychiatry Research, 147, 145–151. [DOI] [PubMed] [Google Scholar]
- Hardan, A. Y. , Girgis, R. R. , Adams, J. , Gilbert, A. R. , Melhem, N. M. , Keshavan, M. S. , & Minshew, N. J. (2008). Brief report: Abnormal association between the thalamus and brain size in Asperger's disorder. Journal of Autism and Developmental Disorders, 38, 390–394. [DOI] [PubMed] [Google Scholar]
- Hazlett, H. C. , Gu, H. , Munsell, B. C. , Kim, S. H. , Styner, M. , Wolff, J. J. , … Statistical, A. (2017). Early brain development in infants at high risk for autism spectrum disorder. Nature, 542, 348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett, H. C. , Poe, M. , Gerig, G. , Smith, R. G. , Provenzale, J. , Ross, A. , … Piven, J. (2005). Magnetic resonance imaging and head circumference study of brain size in autism: Birth through age 2 years. Archives of General Psychiatry, 62, 1366–1376. [DOI] [PubMed] [Google Scholar]
- Hendry, J. , DeVito, T. , Gelman, N. , Densmore, M. , Rajakumar, N. , Pavlosky, W. , … Nicolson, R. (2006). White matter abnormalities in autism detected through transverse relaxation time imaging. NeuroImage, 29, 1049–1057. [DOI] [PubMed] [Google Scholar]
- Herbert, M. R. (2005). Large brains in autism: The challenge of pervasive abnormality. Neuroscientist, 11, 417–440. [DOI] [PubMed] [Google Scholar]
- Herbert, M. R. , Ziegler, D. A. , Makris, N. , Filipek, P. A. , Kemper, T. L. , Normandin, J. J. , … Caviness, V. S., Jr. (2004). Localization of white matter volume increase in autism and developmental language disorder. Annals of Neurology, 55, 530–540. [DOI] [PubMed] [Google Scholar]
- Hoechstetter, K. , Bornfleth, H. , Weckesser, D. , Ille, N. , Berg, P. , & Scherg, M. (2004). BESA source coherence: A new method to study cortical oscillatory coupling. Brain Topography, 16, 233–238. [DOI] [PubMed] [Google Scholar]
- Huang, M. X. , Huang, C. W. , Robb, A. , Angeles, A. , Nichols, S. L. , Baker, D. G. , … Lee, R. R. (2014). MEG source imaging method using fast L1 minimum‐norm and its applications to signals with brain noise and human resting‐state source amplitude images. Neuroimage, 84, 585–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, J. R. (2007). Autism: The first firm finding = underconnectivity? Epilepsy & Behavior, 11, 20–24. [DOI] [PubMed] [Google Scholar]
- Hughes, S. W. , Blethyn, K. L. , Cope, D. W. , & Crunelli, V. (2002). Properties and origin of spikelets in thalamocortical neurones in vitro. Neuroscience, 110, 395–401. [DOI] [PubMed] [Google Scholar]
- Jahnsen, H. , & Llinas, R. (1984). Electrophysiological properties of Guinea‐pig thalamic neurones: An in vitro study. The Journal of Physiology, 349, 205–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann, K. , Federspiel, A. , Giezendanner, S. , Andreotti, J. , Kottlow, M. , Dierks, T. , & Koenig, T. (2012). Linking brain connectivity across different time scales with electroencephalogram, functional magnetic resonance imaging, and diffusion tensor imaging. Brain Connectivity, 2, 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John, E. R. , Ahn, H. , Prichep, L. , Trepetin, M. , Brown, D. , & Kaye, H. (1980). Developmental equations for the electroencephalogram. Science, 210, 1255–1258. [DOI] [PubMed] [Google Scholar]
- Katada, A. , Ozaki, H. , Suzuki, H. , & Suhara, K. (1981). Developmental characteristics of normal and mentally retarded children's EEGs. Electroencephalography and Clinical Neurophysiology, 52, 192–201. [DOI] [PubMed] [Google Scholar]
- Klimesch, W. (1997). EEG‐alpha rhythms and memory processes. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 26, 319–340. [DOI] [PubMed] [Google Scholar]
- Klimesch, W. (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res Brain Res Rev, 29, 169–195. [DOI] [PubMed] [Google Scholar]
- Klimesch, W. (2012). Alpha‐band oscillations, attention, and controlled access to stored information. Trends in Cognitive Sciences, 16, 606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch, W. , Doppelmayr, M. , Schimke, H. , & Pachinger, T. (1996). Alpha frequency, reaction time, and the speed of processing information. Journal of Clinical Neurophysiology: Official Publication of the American Electroencephalographic Society, 13, 511–518. [DOI] [PubMed] [Google Scholar]
- Le Couteur, A. , Lord, C. , & Rutter, M. (2003). The autism diagnostic interview – Revised (ADI‐R). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Lebedev, A. N. (1994). The neurophysiological parameters of human memory. Neuroscience and Behavioral Physiology, 24, 254–259. [DOI] [PubMed] [Google Scholar]
- Levitt, P. , Eagleson, K. L. , & Powell, E. M. (2004). Regulation of neocortical interneuron development and the implications for neurodevelopmental disorders. Trends in Neurosciences, 27, 400–406. [DOI] [PubMed] [Google Scholar]
- Lopes da Silva, F. H. , Vos, J. E. , Mooibroek, J. , & Van Rotterdam, A. (1980). Relative contributions of intracortical and thalamo‐cortical processes in the generation of alpha rhythms, revealed by partial coherence analysis. Electroencephalography and Clinical Neurophysiology, 50, 449–456. [DOI] [PubMed] [Google Scholar]
- Lord, C. , Risi, S. , Lambrecht, L. , Cook, E. H. , Leventhal, B. L. , DiLavore, P. C. , … Rutter, M. (2000). The autism diagnostic observation schedule ‐ generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30, 205–223. [PubMed] [Google Scholar]
- Lord, C. , Rutter, M. , & Le Couteur, A. (1994). Autism diagnostic interview ‐ revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24, 659–685. [DOI] [PubMed] [Google Scholar]
- Lorincz, M. L. , Kekesi, K. A. , Juhasz, G. , Crunelli, V. , & Hughes, S. W. (2009). Temporal framing of thalamic relay‐mode firing by phasic inhibition during the alpha rhythm. Neuron, 63, 683–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matousek, M. , & Petersen, I. (1973). Automatic evaluation of EEG background activity by means of age‐dependent EEG quotients. Electroencephalography and Clinical Neurophysiology, 35, 603–612. [DOI] [PubMed] [Google Scholar]
- Matsuura, M. , Yamamoto, K. , Fukuzawa, H. , Okubo, Y. , Uesugi, H. , Moriiwa, M. , … Shimazono, Y. (1985). Age development and sex differences of various EEG elements in healthy children and adults—quantification by a computerized wave form recognition method. Electroencephalography and Clinical Neurophysiology, 60, 394–406. [DOI] [PubMed] [Google Scholar]
- Mayes, S. D. , & Calhoun, S. L. (2003). Analysis of WISC‐III, Stanford‐Binet:IV, and academic achievement test scores in children with autism. Journal of Autism and Developmental Disorders, 33, 329–341. [DOI] [PubMed] [Google Scholar]
- Mayes, S. D. , & Calhoun, S. L. (2008). WISC‐IV and WIAT‐II profiles in children with high‐functioning autism. Journal of Autism and Developmental Disorders, 38, 428–439. [DOI] [PubMed] [Google Scholar]
- Mierau, A. , Klimesch, W. , & Lefebvre, J. (2017). State‐dependent alpha peak frequency shifts: Experimental evidence, potential mechanisms and functional implications. Neuroscience, 360, 146–154. [DOI] [PubMed] [Google Scholar]
- Miskovic, V. , Ma, X. , Chou, C. A. , Fan, M. , Owens, M. , Sayama, H. , & Gibb, B. E. (2015). Developmental changes in spontaneous electrocortical activity and network organization from early to late childhood. NeuroImage, 118, 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy‐Castle, A. C. (1958). Electrophysiological correlates of intelligence. Journal of Personality, 26, 184–199. [DOI] [PubMed] [Google Scholar]
- Murias, M. , Webb, S. J. , Greenson, J. , & Dawson, G. (2007). Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biological Psychiatry, 62, 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair, A. , Treiber, J. M. , Shukla, D. K. , Shih, P. , & Muller, R. A. (2013). Impaired thalamocortical connectivity in autism spectrum disorder: A study of functional and anatomical connectivity. Brain, 136, 1942–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidermeyer, E. , Lopes da Silva, F. H. (2004). Electroencephalography: Basic Principles, Clinical Applications, and Related Fields (5th ed.). Lippincott Williams and Wilkins, Philadelphia. [Google Scholar]
- Nyden, A. , Billstedt, E. , Hjelmquist, E. , & Gillberg, C. (2001). Neurocognitive stability in Asperger syndrome, ADHD, and reading and writing disorder: A pilot study. Developmental Medicine & Child Neurology, 43, 165–171. [PubMed] [Google Scholar]
- Oliveras‐Rentas, R. E. , Kenworthy, L. , Roberson, R. B., 3rd , Martin, A. , & Wallace, G. L. (2012). WISC‐IV profile in high‐functioning autism spectrum disorders: Impaired processing speed is associated with increased autism communication symptoms and decreased adaptive communication abilities. Journal of Autism and Developmental Disorders, 42, 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orekhova, E. V. , Stroganova, T. A. , Nygren, G. , Tsetlin, M. M. , Posikera, I. N. , Gillberg, C. , & Elam, M. (2007). Excess of high frequency electroencephalogram oscillations in boys with autism. Biological Psychiatry, 62, 1022–1029. [DOI] [PubMed] [Google Scholar]
- Ouyang, M. , Cheng, H. , Mishra, V. , Gong, G. , Mosconi, M. W. , Sweeney, J. , … Huang, H. (2016). Atypical age‐dependent effects of autism on white matter microstructure in children of 2‐7 years. Human Brain Mapping, 37, 819–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, I. , & Eeg‐Olofsson, O. (1971). The development of the electroencephalogram in normal children from the age of 1 through 15 years. Non‐paroxysmal activity. Neuropadiatrie, 2, 247–304. [DOI] [PubMed] [Google Scholar]
- Piven, J. , Arndt, S. , Bailey, J. , Havercamp, S. , Andreasen, N. C. , & Palmer, P. (1995). An MRI study of brain size in autism. The American Journal of Psychiatry, 152, 1145–1149. [DOI] [PubMed] [Google Scholar]
- Posthuma, D. , Neale, M. C. , Boomsma, D. I. , & de Geus, E. J. (2001). Are smarter brains running faster? Heritability of alpha peak frequency, IQ, and their interrelation. Behavior Genetics, 31, 567–579. [DOI] [PubMed] [Google Scholar]
- Redcay, E. , & Courchesne, E. (2005). When is the brain enlarged in autism? A meta‐analysis of all brain size reports. Biological Psychiatry, 58, 1–9. [DOI] [PubMed] [Google Scholar]
- Roberts, J. A. , & Robinson, P. A. (2008). Modeling distributed axonal delays in mean‐field brain dynamics. Physical Review. E, Statistical Physics, Plasmas, Fluids, and Related Interdisciplinary Topics, 78, 051901. [DOI] [PubMed] [Google Scholar]
- Robinson, P. A. , Rennie, C. J. , Rowe, D. L. , O'Connor, S. C. , Wright, J. J. , Gordon, E. , & Whitehouse, R. W. (2003). Neurophysical modeling of brain dynamics. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 28(Suppl 1), S74–S79. [DOI] [PubMed] [Google Scholar]
- Rojas, D. C. , & Wilson, L. B. (2014). Gamma‐band abnormalities as markers of autism spectrum disorders. Biomarkers in Medicine, 8, 353–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saletu, B. , & Grunberger, J. (1985). Memory dysfunction and vigilance: Neurophysiological and psychopharmacological aspects. Annals of the New York Academy of Sciences, 444, 406–427. [DOI] [PubMed] [Google Scholar]
- Salmelin, R. , & Hari, R. (1994). Characterization of spontaneous MEG rhythms in healthy adults. Electroencephalography and Clinical Neurophysiology, 91, 237–248. [DOI] [PubMed] [Google Scholar]
- Sattler, J. M. , Dunmont, R. , & Coalson, D. (2016). In Jerome M. (Ed.), Assessment of children: WISC‐V and WPPSI‐IV. La Mesa, CA: Sattler Publisher, Inc. [Google Scholar]
- Scherg, M. , & Berg, P. (1996). New concepts of brain source imaging and localization. Electroencephalography and Clinical Neurophysiology, 46, 127–137. [PubMed] [Google Scholar]
- Scherg, M. , & Ebersole, J. S. (1993). Models of brain sources. Brain Topography, 5, 419–423. [DOI] [PubMed] [Google Scholar]
- Smit, D. J. , Posthuma, D. , Boomsma, D. I. , & Geus, E. J. (2005). Heritability of background EEG across the power spectrum. Psychophysiology, 42, 691–697. [DOI] [PubMed] [Google Scholar]
- Somsen, R. J. , van't Klooster, B. J. , van der Molen, M. W. , van Leeuwen, H. M. , & Licht, R. (1997). Growth spurts in brain maturation during middle childhood as indexed by EEG power spectra. Biological Psychology, 44, 187–209. [DOI] [PubMed] [Google Scholar]
- Sparks, B. F. , Friedman, S. D. , Shaw, D. W. , Aylward, E. H. , Echelard, D. , Artru, A. A. , … Dager, S. R. (2002). Brain structural abnormalities in young children with autism spectrum disorder. Neurology, 59, 184–192. [DOI] [PubMed] [Google Scholar]
- Steriade, M. , & Deschenes, M. (1984). The thalamus as a neuronal oscillator. Brain Research, 320, 1–63. [DOI] [PubMed] [Google Scholar]
- Steriade, M. , Gloor, P. , Llinas, R. R. , Lopes de Silva, F. H. , & Mesulam, M. M. (1990). Report of IFCN committee on basic mechanisms. Basic mechanisms of cerebral rhythmic activities. Electroencephalography and Clinical Neurophysiology, 76, 481–508. [DOI] [PubMed] [Google Scholar]
- Szava, S. , Valdes, P. , Biscay, R. , Galan, L. , Bosch, J. , Clark, I. , & Jimenez, J. C. (1994). High resolution quantitative EEG analysis. Brain Topography, 6, 211–219. [DOI] [PubMed] [Google Scholar]
- (2003). Magnetoencephalography and magnetic source imaging: Presurgical localization of epileptic lesions and presurgical functional mapping. Bulletin, 20, 1–3. [PubMed] [Google Scholar]
- Tsatsanis, K. D. , Rourke, B. P. , Klin, A. , Volkmar, F. R. , Cicchetti, D. , & Schultz, R. T. (2003). Reduced thalamic volume in high‐functioning individuals with autism. Biological Psychiatry, 53, 121–129. [DOI] [PubMed] [Google Scholar]
- Valdes, P. , Valdes, M. , Carballo, J. A. , Alvarez, A. , Diaz, G. F. , Biscay, R. , … Quesada, M. E. (1992). QEEG in a public health system. Brain Topography, 4, 259–266. [DOI] [PubMed] [Google Scholar]
- Valdes‐Hernandez, P. A. , Ojeda‐Gonzalez, A. , Martinez‐Montes, E. , Lage‐Castellanos, A. , Virues‐Alba, T. , Valdes‐Urrutia, L. , & Valdes‐Sosa, P. A. (2010). White matter architecture rather than cortical surface area correlates with the EEG alpha rhythm. NeuroImage, 49, 2328–2339. [DOI] [PubMed] [Google Scholar]
- Van Baal, G. C. , De Geus, E. J. , & Boomsma, D. I. (1996). Genetic architecture of EEG power spectra in early life. Electroencephalography and Clinical Neurophysiology, 98, 502–514. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt, C. E. , & van Baal, G. C. (2002). Twin and family studies of the human electroencephalogram: A review and a meta‐analysis. Biological Psychology, 61, 111–138. [DOI] [PubMed] [Google Scholar]
- Vogel, W. , & Broverman, D. M. (1964). Relationship between Eeg and test intelligence: A critical review. Psychological Bulletin, 62, 132–144. [DOI] [PubMed] [Google Scholar]
- Wechsler, D. (2003). Wechsler intelligence scale for children. Administration and scoring manual (4th ed.). San Antonio: Harcourt Assessment, Inc. [Google Scholar]
- Wechsler, D. (2011). Wechsler abbreviated scale of intelligence (2nd ed.). San Antonio, Texas: Pearson. [Google Scholar]
- Wechsler, D. (2014). Wechsler intelligence scale for children—(WISC‐V): Technical and interpretive manual (5th ed.). Bloomington, MN: Pearson Clinical Assessment. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Online Supplement (analyses for the full male + female sample)
Supplementary Figure S1: Online Supplement Figure 1. Scatterplots showing associations between age (x axis) and peak alpha frequency (y axis) for full sample TDC (blue) and ASD (red). ***p < 0.001
Online Supplement Figure 2. (a) Scatterplots showing associations between non‐verbal IQ (x axis) and peak alpha frequency (y axis) for full sample TDC (blue) and ASD (red). (b) Non‐verbal IQ and peak alpha frequency associations shown for the younger (< 10‐years‐old; left plot) and older children (>10‐years‐old; right plot). *p < 0.05
Online Supplement Figure 3. (a) Scatterplots showing associations between processing speed (x axis) and peak alpha frequency (y axis) for full sample TDC (blue) and ASD (red). (b) Processing speed and peak alpha frequency associations shown for the younger (< 10‐years‐old; left plot) and older children (>10‐years‐old; right plot). *p < 0.05, ** p < 0.01


