Abstract
Background:
Sleep disturbances and low-quality diets are prevalent among children in low-income settings, yet the nature of their relationship remains unclear. In particular, whether aspects other than sleep duration- including timing and quality- are associated with dietary patterns has rarely been examined, especially among preschool-aged children.
Objective:
To evaluate whether nightly and total sleep duration, sleep timing, differences in timing and duration from weekdays to weekends, and sleep quality were related to dietary patterns.
Design:
A cross-sectional analysis of preschoolers. Parents completed questionnaires about children’s sleep habits as well as a semi-quantitative food frequency questionnaire (FFQ).
Participants/setting:
354 English-speaking children (49.9 % male) with no serious medical conditions aged 3-5 years enrolled in Head Start in Michigan (2009-2011) with complete information on sleep and diet.
Main outcome measures:
Dietary pattern scores derived from food frequency questionnaire
Statistical analyses performed:
Principal component analysis was used to identify dietary patterns. Separate linear regression models with dietary pattern scores as the dependent variable and continuous sleep measures as independent variables were used to evaluate associations between sleep and diet, adjusting for sex, age, parental education, and sleep hygiene.
Results:
Three dietary patterns were identified- Vegetables, Healthy Proteins & Sides; Breads & Spreads; and Processed & Fried. Longer average weekend sleep duration and a greater difference in weekend-to-weekday sleep duration was related to lower Vegetables, Healthy Proteins & Sides pattern scores. Later sleep midpoint during the weekday was related to lower Vegetables, Healthy Proteins & Sides pattern scores, while later sleep midpoint on the weekend was associated with higher Processed & Fried pattern scores. Similarly, a larger weekend-weekday midpoint difference was associated with higher Processed & Fried pattern scores.
Conclusions:
Later sleep timing and differences in sleep duration and timing from weekends to weekdays were related to less-optimal dietary pattern scores in young children.
Keywords: food frequency questionnaire, sleep timing, sleep hygiene
INTRODUCTION
Children from lower-socioeconomic environments have a higher likelihood of disordered sleep,1 poorer quality diets,2 and high rates of childhood obesity.3 Sleep disturbances and low diet quality are both predictive of obesity risk in children;4 thus, examining the associations between sleep and dietary patterns is an important step toward understanding the pathways to obesity risk, particularly in vulnerable populations.5,6 Further, sleep and dietary habits are established at a young age,7,8 and habits developed even as early as preschool age may have implications for later risk.9,10
In populations of older children, there is evidence of associations between sleep and diet. For example, cross-sectional studies of school-age children and adolescents in the US showed that short sleep duration was related to greater energy density, sugar-sweetened beverage, and added sugar intake.11,12 Similarly, studies among children in Portugal13 and Finland14 found that shorter sleep duration was related to energy-dense dietary patterns. Other aspects of sleep, including timing (i.e., late bedtimes and wake times; late midpoint of sleep), have also been related to lower quality diets.12,15 One comprehensive 12-country study among 9- to 11-year-olds16 found that shorter sleep duration, lower sleep efficiency, and later bedtimes were each associated with unhealthy dietary patterns high in added fats and sugars (e.g., fast food, hamburgers, soft drinks, sweets), while earlier bedtimes were related to healthy dietary patterns (vegetables, fruit, whole grains). A similarly conducted study of 9- to 11-year-olds from New Zealand showed that children in the late sleep/late wake category had a lower “Fruit and Vegetables” pattern score.17
In contrast to the growing number of studies on sleep and diet in school-aged children and adolescents, there are fewer studies in preschool-aged children. One potential reason for the lack of studies is that parents are assumed to have considerable control over their young children’s habits. Nonetheless, child eating behaviors (e.g. picky eating or temper tantrums) and temperament (e.g. negative emotionality)18 may influence parental feeding practices.19,20 Further, there is recent experimental evidence that short sleep alters dietary preference and intake even among young children.21 In this study, researchers found that US preschool children whose sleep was restricted by ~3 hours (bedtime delay and no nap) had a 20% increase in total calories the next day due to higher intake of sugar, carbohydrates and fat when compared to baseline. Whether these associations persist in free-living environments is less clear, although one observational study showed that longer sleep duration in 2 to 4-year-olds was associated with greater fat but lower carbohydrate intake, and that higher shifts in sleep duration from weekdays to weekends was related to higher calorie consumption.22,23 In contrast, a different study in low-income preschoolers did not report associations between nighttime sleep duration and diet after accounting for confounding factors.24
Thus, the overarching aim of our study was to evaluate how multiple indicators of sleep health related to diet in preschoolers from low-income households. We chose to focus on dietary patterns because foods naturally cluster in the diet (e.g. milk and cereal); thus, the analysis of dietary patterns may provide interpretations that are more intuitive and relevant for public health than examination of single foods.25 We hypothesized that multiple distinct dietary patterns would be identified within this dataset, and that longer sleep duration, earlier sleep timing, less difference in weekday to weekend sleep duration and timing, and higher sleep quality would each be associated with higher healthy dietary pattern scores and lower unhealthy dietary pattern scores.
METHODS
Study Population
Participants were children enrolled in a longitudinal study of stress and eating behavior; the present study is a secondary analysis of the recruitment visit, which occurred during the school year (October at the earliest) from 2009-2011. Families were recruited from Head Start, a federally-funded preschool program for low-income families. The study was approved by the University of Michigan Institutional Review Board; parents/legal guardians (typically mothers) provided written informed consent and families were compensated $90 for participating. Inclusion criteria were that the child was enrolled in Head Start (either in the first or second year), not in foster care, born at ≥ 35 weeks gestation without serious perinatal/neonatal complications, and had no serious medical problems affecting growth or appetite. Additional criteria were that the parent and child spoke English and that one parent did not have a 4-year college degree. All questionnaires were interviewer-administered.
Of the full sample of 380 children, 25 were excluded either due to missing or implausible dietary data.
Sleep Measures
A parent reported their child’s usual bedtime and wake time on weekdays and weekends, as well as the number of days per week their child napped and the typical nap duration. From these data, we calculated average weeknight sleep duration, average weekend-night sleep duration, difference between weekend and weeknight sleep duration, and total average 7-day sleep duration including naps. These were all reported in hours and minutes.
The midpoint of sleep, calculated as the median time of the sleep period (bedtime to wake time), was the measure of sleep timing. We also calculated the difference in midpoint from weekend to weekday.
Parents completed the 25-item Children’s Sleep-Wake Scale, which has been validated in preschoolers with sleep diaries and actigraphy, to assess sleep quality (Cronbach’s α based on the present sample=.89). The scale includes five components: Going to bed, falling asleep, maintaining sleep, reinitiating sleep, and returning to wakefulness; individual questions pertaining to these components were rated from 1=never to 6=always and a mean sleep quality score was generated.26 Higher scores indicated better sleep quality, and included items such as whether the child typically resists bedtime, has difficulty falling asleep, is restless during the night, or difficult to get out of bed in the morning.
Parents also completed the 22-item Children’s Sleep Hygiene Scale (Cronbach’s α based on present sample=.73), which has been assessed for internal consistency and content validity in preschoolers; items were rated from 1=never to 6=always and a mean sleep hygiene score was generated.27 Higher scores indicated more optimal sleep hygiene practices such as avoiding caffeine and maintaining a consistent sleep-wake schedule.
Child Dietary Pattern Scores
Parents completed the Harvard Service Food Frequency Questionnaire which estimates usual intake habits of preschool children via parental proxy and has been validated in low-income populations, with energy-adjusted correlation coefficients ranging from 0.26 for dietary fiber to 0.63 for magnesium.28 Parents were asked to recall how often in the past month the child typically consumed one serving of a standard portion size of 84 different food items. The dietary recall period included preschool meals that a parent was not present for, but per state regulations, Head Start classrooms were required to inform parents of the daily food offerings 29. Response options to the dietary frequency questions ranged from never to ≥6 times per day, which we converted into times/day (e.g. once per month=1/30.4= 0.03 times/day). United States Department of Agriculture Food Composition tables30 were also used to convert the portions/day into average daily total energy intake, and a trained nutritionist evaluated the plausibility of each participant’s total energy intake (<500 or >3500 calories deemed implausible) and response option variance (<1.27 overall variation deemed implausible).
Covariates
Parents provided relevant sociodemographic information, including child’s age and race/ethnicity, his/her highest level of education attained, and household income from all sources. Trained research assistants measured height (in meters) and weight (in kilograms) of children during home visits using calibrated equipment (Seca 213/217 portable stadiometer, Seca, Chino, California and Detecto Portable Scale Model #DR550C, Detecto, Webb City, Missouri, respectively). Child race/ethnicity was classified as non-Hispanic/Latino White, non-Hispanic/Latino Black, other non-Hispanic/Latino, and Hispanic/Latino of any race. Body mass index (BMI) z-scores for sex and age were calculated and weight status categories generated (overweight/obese as BMI ≥ 85th percentile for sex and age, vs. not overweight as BMI < 85th percentile for sex and age) based on the Center for Disease Control reference norms.31 Parental education was classified into three categories: did not complete a high school education or General Equivalency Diploma (GED), completed a high school diploma or GED, or completed at least some post-high school education. Income-to-needs ratio was calculated as the total reported household income divided by the federal poverty line for a family of the same size during the corresponding year. Household chaos was measured as the sum of 15 true/false items on the Confusion, Hubbub, and Order Scale32 (CHAOS; Cronbach’s α based on present sample=.79; theoretical range 0 to 15), with higher scores indicating more chaotic environments. Household routines were measured with the 14-item Child Routines Inventory33 which assesses how regularly the child engages in family routines that involve interaction with or supervision by a parent (e.g., eating together), on a 5-point scale (from 0 = “never” to 4 = “nearly always”), with higher scores indicating greater presence of routines (Cronbach's alpha based on present sample = .70).
Statistical Analyses
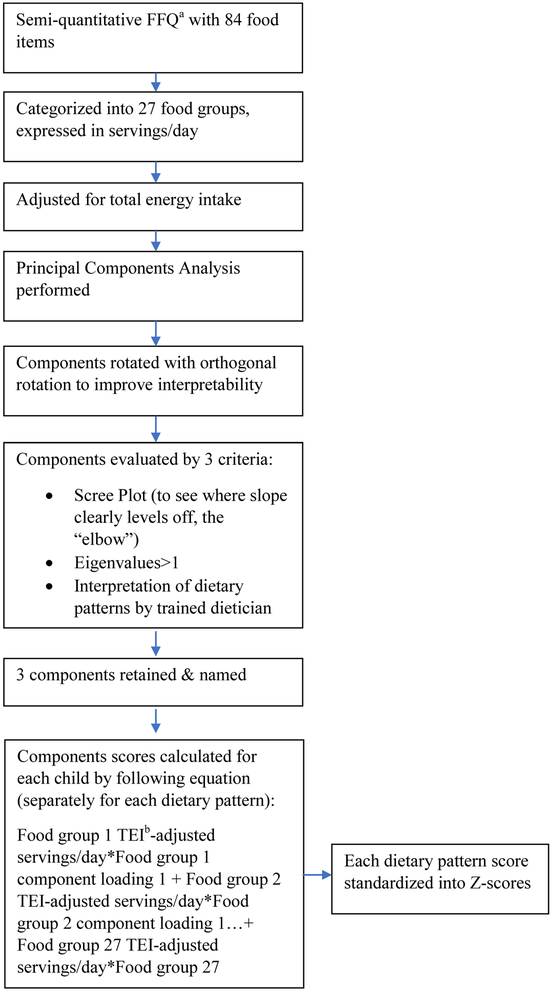
First, dietary patterns were identified based on how the intake of food groups correlate, using an often-used methodology.34 Figure 1 provides a flowchart of the process followed. Briefly, similar food items were first grouped into food groups based on nutritional similarity (Table 1), and total-energy adjusted food group intakes were computed using the residual method. 35 Next, a principal component analysis of the food groups was performed and the components were transformed with Varimax rotation to obtain uncorrelated components and improve interpretation. The number of components to retain was determined based on visual inspection of the Scree plot, eigenvalues>1, and interpretability of the components. The food groups with the highest component loadings enabled interpretation of the pattern; groups with component loadings ≥∣0.25∣ are shown in Table 2. To determine how closely each child’s diet aligned with the dietary pattern, scores were computed by multiplying the component loadings of each food group by the child’s frequency of intake in that food group and then summing (because there were food groups with negative component loadings, this meant that the overall raw scores could have been <0). Each child received a score for each of the dietary patterns, with higher scores representing a closer correspondence to the dietary pattern. Finally, each of the scores were converted to Z-scores with a mean of 0 and standard deviation of 1 to facilitate comparisons between the 3 dietary patterns.
Figure 1.
Flowchart depicting the creation of dietary pattern scores in a sample of 355 low-income Head Start preschoolers enrolled in a study on stress and eating behavior
a FFQ= Food Frequency Questionnaire
b TEI= total energy intake
Table 1 (Suggested Online-Only Table).
Foods in each food group, derived from a semi-quantitative food frequency questionnairea completed by parental proxy for 355 low-income preschoolers attending Head Start
| Food Groups | Foods |
|---|---|
| Potatoes | Potatoes; sweet potatoes or yams |
| French fries | French fries, fried potatoes, tater tots |
| Soup | Vegetable soup; other soup |
| Vegetables | Corn; peas; tomatoes, tomato sauce, salsa; peppers; carrots; broccoli; green beans; spinach; mixed vegetables; squash; zucchini; cabbage, coleslaw, cauliflower; lettuce salad |
| Rice | Rice |
| Legumes | Beans |
| Fruit | Banana; peaches; fruit cocktail, mixed fruit; orange or grapefruit; apple or pear; applesauce; grapes; strawberries; melon; pineapple; raisins or prunes |
| Juice | orange juice or grapefruit juice; other juice |
| Cheese | Cheese |
| Yogurt | Yogurt |
| Milk | Milk |
| Peanut butter and other nuts | Nuts; peanut butter |
| Hot Cereal | Hot cereal, grits |
| Cold Cereal | Cold cereal |
| Bread | Bread, toast, roll or pita; English muffin or bagel; biscuit; cornbread or tortilla |
| Salty snacks | Chips; popcorn or pretzels; crackers; |
| Mixed dish | Macaroni and cheese; pizza; spaghetti or other pasta; tacos, burritos |
| Processed meat | Hot dogs; sausage; cold cuts; fried chicken or turkey; bacon |
| Non-processed meat | Hamburger; other chicken or turkey; pork or ham; roast beef or steak; liver, organ meats |
| Fish | Canned tuna; fried fish, fish sticks; other fish |
| Spreads with fat | Mayonnaise; salad dressing; butter; margarine |
| Eggs | Eggs |
| Breakfast pastries | Donut; sweet roll or muffin; pancake, waffle or French toast |
| Sweetened dairy | Hot chocolate; ice cream; pudding |
| Sweets | Cookies or brownies; cake or cupcake; pie; chocolate or candy bar; other candy |
| Sugar-sweetened beverages | Fruit drinks; soda, soft drink, pop (not sugar free); other juice |
| Diet soda | Soda, soft drink, pop (sugar free) |
Food frequency questionnaire used was the Harvard Service Food Frequency Questionnaire
Table 2.
Component loadings for dietary patterns derived from semi-quantitative food frequency questionnaire data completed by parental proxy for 355 low-income preschoolers attending Head Start
| Component 1a | Component 2b | Component 3c | |
|---|---|---|---|
| Food Groups | Vegetables, Healthy Proteins & Sides |
Breads & Spreads |
Processed & Fried |
| Vegetables | 0.63 | ||
| Non-processed meat | 0.49 | ||
| Legumes | 0.48 | ||
| Fish | 0.48 | ||
| Sugar-sweetened beverages | −0.48 | ||
| Potato | 0.46 | ||
| Juice | −0.43 | ||
| Eggs | 0.43 | ||
| Rice | 0.39 | ||
| Cheese | −0.27 | ||
| Mixed dish | 0.25 | ||
| Bread | 0.68 | ||
| Peanut butter & other nuts | 0.51 | ||
| Fruit | −0.50 | ||
| Spreads with fat | 0.41 | ||
| Yogurt | −0.35 | ||
| Breakfast pastry | 0.27 | ||
| Milk | −0.58 | ||
| French fries | 0.51 | ||
| Processed meat | 0.45 | ||
| Salty snacks | 0.43 | ||
| Sweets | 0.33 | ||
| Cold cereal | −0.29 | ||
| % Variance Explained | 9% | 7% | 6% |
Component 1, called Vegetables, Healthy Proteins & Sides, is characterized by high energy-adjusted intake of vegetables, non-processed meat, legumes, fish, potatoes, eggs, and rice, and low frequency of sugar sweetened beverage and juice.
Component 2, called Breads & Spreads, is characterized by high energy-adjusted intake of bread, peanut butter and other nuts, spreads with fat, and breakfast pastry intake and low intake of fruit.
Component 3, called Processed & Fried, is characterized by high energy-adjusted intake French fries, processed meat, salty snacks, sweets, and diet soda, and low energy-adjusted intake of milk.
In bivariate analysis, the associations between dietary patterns and potential confounders were first examined by comparing the distributions or means ± standard deviation (SD) of the potential confounders in lower versus higher dietary pattern categories (split at the median of dietary pattern scores). To evaluate the primary study questions on sleep and diet, linear regression models were run with dietary pattern scores as the continuous dependent variable and sleep measures as a continuous independent variable (separate models for each sleep measure and for each dietary pattern). In multivariable models, child age, sex, race/ethnicity, parental education, and sleep hygiene were added to each of the models. These variables were selected as confounders based on the results of the bivariate confounder analysis and prior research.36,37
Three sets of sensitivity analyses were implemented. First, to evaluate the dose-response relationship between the sleep variables and dietary pattern scores, linear regression models were run with each sleep measure as a categorical variable (in quartiles). Because there was evidence of dose-response relationships, only the continuous β estimates are presented. Second, the following variables were added to the multivariable models separately to evaluate their potential confounding role: income-to-needs ratio, household CHAOS and household routines. Because the addition of these variables did not substantially alter the estimates, they were not included in the final multivariable models. Third, to evaluate whether associations with sleep duration and timing were independent, regression models were run with mutual adjustment for all sleep variables (separately for weekend versus weekday variables). Because estimates were not substantially different in mutually-adjusted models, analyses from separate models are presented. Analyses were conducted using Stata 14.0.38 The criterion of statistical significance was P<0.05.
RESULTS
The mean (SD) age of the children was 4.2 (0.5) years. The sample was nearly equally divided by sex (49.9% male). Other descriptive statistics are shown in Table 3.
Table 3.
Sociodemographic and sleep characteristics of a sample of 355 low-income Head Start preschoolers enrolled in a study on stress and eating behavior
| Variable | Mean (SD) or % |
|---|---|
| Child Age (years) | 4.23 (0.53) |
| Child Sex | |
| Male | 49.9 |
| Female | 50.1 |
| Child Race/Ethnicity | |
| White, non-Hispanic/Latino | 57.8 |
| Black, non-Hispanic/Latino or othera | 31.6 |
| Hispanic/Latino, any race | 10.7 |
| Child body mass index z-scoreb | 0.80 (1.04) |
| Child Weight Status | |
| Not overweight | 60.5 |
| At risk for overweight (>85th percentile) | 39.6 |
| Parental Education | |
| <High school | 15.5 |
| High school diploma or GEDc | 30.4 |
| Some education beyond high school | 54.1 |
| Household Income-to-Needs Ratio (1.0=poverty) | 0.87 (0.76) |
| Household CHAOSd (higher=more chaos; range= 0-15) | 4.09 (3.29) |
| Household Routine (higher=more routine; range=0-56) | 45.43 (6.23) |
| Weeknight Sleep Duration | 10 hr 22 min (47 min) |
| Weekend-night Sleep Duration | 10 hr 45 min (1 hr 8 min) |
| Total Sleep per day (hours per night + naps during the day) | 10 hr 39 min (2 hr 33 min) |
| Child’s Usual Bedtime (weekdays) | 8:35 pm (45 min) |
| Child’s Usual Waketime (weekdays) | 6:57 am (35 min) |
| Child’s Usual Midpoint (Median) of Sleep (weekday) | 1:45 am (33 min) |
| Child’s Usual Bedtime (weekend nights) | 9:40 pm (1 hr 7 min) |
| Child’s Usual Waketime (weekend days) | 8:25 am (1 hr 11 min) |
| Child’s Usual Midpoint (Median) of Sleep (weekend) | 3:01 am (60 min) |
| Sleep Hygiene (higher=better sleep hygiene; range= 0-10) | 4.84 (0.50) |
| Sleep Quality (higher=higher quality sleep; range= 0-10) | 4.13 (0.73) |
Includes Asian, Asian Pacific Islander, and Biracial, non-Hispanic
From Centers for Disease Control reference25
GED=General Equivalency Diploma
CHAOS= Confusion, Hubbub, and Order Scale
Using PCA, three dietary patterns that together explained 22% of the variance in the energy-adjusted food groups were identified: Vegetables, Healthy Proteins & Sides; Breads & Spreads; and Processed & Fried (Table 3). The Vegetables, Healthy Proteins & Sides pattern was characterized by high energy-adjusted intake of vegetables, non-processed meat, legumes, fish, potatoes, eggs, and rice, and low frequency of sugar sweetened beverage and juice. The Breads & Spreads pattern was marked by high energy-adjusted intake of bread, peanut butter and other nuts, spreads with fat, and breakfast pastry intake and low intake of fruit. The Processed & Fried pattern included high energy-adjusted intake French fries, processed meat, salty snacks, sweets, and diet soda, and low energy-adjusted intake of milk. Parental education was associated with the Breads & Spreads pattern, such that children with higher scores on the Breads & Spreads pattern were more likely to have parents who completed at least some post-high school education (Table 4) compared to children with lower scores on this pattern. In addition, Black, non-Hispanic (or other race/ethnicity) children were more likely to be in the upper half of the Processed & Fried pattern scores compared to White, non-Hispanic/Latino children.
Table 4 (Suggested Online-Only Table).
Cross-sectional associations between sociodemographic characteristics and dietary patterns among 355 low-income Head Start preschoolers enrolled in a study on stress and eating behavior
| Vegetables, Healthy Proteins & Sides Pattern |
Breads & Spreads Pattern |
Processed & Fried Pattern |
|||||
|---|---|---|---|---|---|---|---|
| Sociodemographic variables | n | Lower scores |
Higher scores |
Lower scores |
Higher scores |
Lower scores |
Higher scores |
| Child’s sex | |||||||
| Male | 177 | 50.0 | 49.7 | 51.7 | 48.0 | 47.8 | 52.0 |
| Female | 178 | 50.0 | 50.3 | 48.3 | 52.0 | 52.3 | 48.0 |
| P valuea | 0.96 | 0.49 | 0.43 | ||||
| Child’s race/ethnicity | |||||||
| White, non-Hispanic/Latino | 205 | 55.6 | 60.0 | 53.9 | 61.6 | 64.0 | 51.4 |
| Black, non-Hispanic/Latino or otherb | 112 | 36.0 | 27.1 | 31.5 | 31.6 | 25.3 | 37.9 |
| Hispanic/Latino, any race | 38 | 8.4 | 13.0 | 14.6 | 6.8 | 10.7 | 10.7 |
| P value | 0.12 | 0.05 | 0.03 | ||||
| Child’s overweight statusc | |||||||
| Not overweight | 214 | 58.4 | 62.5 | 64.6 | 56.3 | 60.5 | 60.5 |
| Overweight | 140 | 41.6 | 37.5 | 35.4 | 43.8 | 39.6 | 39.6 |
| P value | 0.43 | 0.11 | 0.99 | ||||
| Parental education | |||||||
| < high school | 55 | 16.9 | 14.1 | 14.0 | 17.0 | 12.9 | 18.1 |
| High school diploma or GEDd | 108 | 33.7 | 27.1 | 36.5 | 24.3 | 29.2 | 31.6 |
| Some education beyond high school | 192 | 49.4 | 58.8 | 49.4 | 58.8 | 15.5 | 50.3 |
| P, trend | 0.21 | 0.04 | 0.27 | ||||
| Child’s age, per year | 355 | 4.2 ± 0.5 | 4.3 ± 0.5 | 4.2 ± 0.5 | 4.2 ± 0.5 | 4.2 ± 0.6 | 4.3 ± 0.5 |
| P valuee | 0.10 | 0.79 | 0.40 | ||||
| Household income-to-needs ratio, per unit | 339 | 0.9 ± 0.9 | 0.9 ± 0.6 | 0.9 ± 0.9 | 0.9 ± 0.6 | 0.8 ± 0.6 | 0.9 ± 0.9 |
| P value | 0.85 | 0.81 | 0.47 | ||||
| Household CHAOS (higher=more chaos)e | 355 | 4.2 ± 3.2 | 4.0 ± 3.3 | 3.7 ± 3.1 | 4.4 ± 3.4 | 4.1 ± 3.3 | 4.1 ± 3.3 |
| P value | 0.67 | 0.05 | 0.83 | ||||
| Household routine (higher=more routine) | 343 | 45.2 ± 5.8 | 45.7 ± 6.6 | 45.6 ± 6.1 | 45.3 ± 6.3 | 46.1 ± 6.0 | 44.8 ± 6.4 |
| P value | 0.40 | 0.71 | 0.06 | ||||
P values for categorical variables are from Chi-square tests (for more than 3 categories, a significant P value indicates at least 2 of the categories are different from one another)
Includes Asian, Asian Pacific Islander, and Biracial, non-Hispanic
From Centers for Disease Control reference25
GED=General Equivalency Diploma
P values for continuous variables are from Wald tests
CHAOS= Confusion, Hubbub, and Order Scale
In regression analysis, one hour longer sleep duration during the weekend was related to a −0.13 lower Vegetables, Healthy Proteins & Sides SD after adjustment for potential confounders (95% Confidence Interval (CI) −0.22 to −0.04), and every one-hour greater difference in sleep duration from the weekend to weekdays was associated with −0.14 SD scores (95% CI −0.23 to −0.04, respectively; Table 5). Later sleep midpoint during the weekday was related to lower Vegetables, Healthy Proteins & Sides pattern scores, such that every one hour later in the sleep midpoint was related to a −0.20 SD lower scores (95% CI −0.40 to −0.01). Later sleep midpoints on the weekend was associated with higher Processed & Fried pattern scores; every one-hour later sleep midpoint was associated with a 0.18 SD higher Processed & Fried pattern score (95% CI 0.06 to 0.29). Similarly, each hour of difference in weekend-weekday midpoints was associated with a 0.21 SD higher Processed & Fried pattern score (95% CI 0.07 to 0.35). Sleep quality was not associated with dietary pattern scores.
Table 5.
Cross-sectional associations between sleep duration, timing, and quality and dietary patterns among 355 low-income Head Start preschoolers enrolled in a study on stress and eating behavior
| Vegetables, Healthy Proteins & Sides | Breads & Spreads | Processed & Fried | ||||
|---|---|---|---|---|---|---|
| Sleep measures | Unadjusted betaa (95% CI) |
Adjusted betab,c,d (95% CI) |
Unadjusted beta (95% CI) |
Adjusted betab,c,d (95% CI) |
Unadjusted beta (95% CI) |
Adjusted betab,c,d (95% CI) |
| Sleep duration | ||||||
| Weeknight Sleep (hr/night) | 0.05 (−0.09, 0.18) | 0.01 (−0.13, 0.14) | 0.00 (−0.13, 0.14) | −0.02 (−0.16, 0.11) | −0.05 (−0.19, 0.08) | −0.01 (−0.14, 0.13) |
| Weekend-night Sleep (hr/night) | −0.12 (−0.21, −0.02)* | −0.13 (−0.22, −0.04)** | −0.04 (−0.14, 0.05) | −0.04 (−0.13, 0.06) | 0.00 (−0.09, 0.10) | 0.02 (−0.08, 0.11) |
| Weekend-weekday duration difference | −0.14 (−0.23, −0.05)** | −0.14 (−0.23, −0.04)** | −0.05 (−0.14, 0.05) | −0.02 (−0.12, 0.07) | 0.03 (−0.06, 0.13) | 0.03 (−0.07, 0.12) |
| Total average sleep with naps (hr/day) | −0.04 (−0.18, 0.11) | −0.08 (−0.22, 0.07) | −0.04 (−0.19, 0.11) | −0.06 (−0.20, 0.09) | −0.03 (−0.17, 0.12) | 0.02 (−0.13, 0.16) |
| Sleep timing | ||||||
| Midpoint of sleep during weeke | −0.23 (−0.42, −0.04)* | −0.20 (−0.40, −0.01)* | 0.01 (−0.18, 0.21) | 0.05 (−0.14, 0.25) | 0.20 (0.01, 0.39)* | 0.13 (−0.06, 0.33) |
| Midpoint of sleep during weekende | −0.15 (−0.25, −0.04)** | −0.11 (−0.22, 0.01) | −0.08 (−0.19, 0.02) | −0.04 (−0.15, 0.08) | 0.21 (0.11, 0.32)*** | 0.18 (0.06, 0.29)** |
| Weekend-weekday midpoint difference | −0.13 (−0.26, 0.00) | −0.06 (−0.20, 0.08) | −0.15 (−0.28, −0.01)* | −0.10 (−0.24, 0.04) | 0.25 (0.12, 0.39)*** | 0.21 (0.07, 0.35)** |
| Sleep quality (higher is better) | 0.12 (−0.03, 0.26) | 0.08 (−0.10, 0.25) | −0.05 (−0.19, 0.09) | 0.00 (−0.17, 0.18) | −0.05 (−0.19, 0.10) | 0.02 (−0.15, 0.20) |
All estimates are from linear regression models with dietary pattern as the continuous outcome and sleep measure as a continuous predictor, separately for each sleep measure and dietary pattern combination
Adjusted for child age, sex, race, parental education level, and sleep hygiene
Sample sizes range from 345 to 352 in complete-case analysis
R2 for the adjusted models range from 0.02 to 0.06
Midpoint refers to the median time between bedtime and wake time
=P value<0.05
=P value<0.01
=P value<0.001
DISCUSSION
In this sample of low-income preschool children, three dietary patterns were identified - Vegetables, Healthy Proteins & Sides; Breads & Spreads; and Processed & Fried - that were related to several dimensions of sleep health reported by parents. In particular, after accounting for potential confounders, longer sleep duration on weekends, later sleep midpoints on both weekends and weekdays, and greater differences in sleep duration and timing from weekends to weekdays were related to less healthy diet quality- either lower Vegetables, Healthy Proteins & Sides dietary pattern scores or higher Processed & Fried pattern scores.
This study extends prior literature as it specifically focused on sleep and diet associations in preschool-aged children, and evaluated sleep duration, timing, and quality. One of the primary findings was that timing of sleep in young children may be associated with obesogenic eating behaviors. These associations are in line with recent findings among older children and young adults, showing that later sleep timing relates to lower intake of specific healthy foods and/or to higher intake of energy-dense foods.12,39–41 Similarly, a greater mismatch in sleep timing from weekend to weekday, also referred to as social jetlag, has also been related to obesity and unhealthy eating in older children42 and undergraduate students.43
There are several potential mechanisms to explain the findings with sleep timing, and it is important to consider the possibility of bi-directional associations.44 Regarding potential pathways linking sleep to diet, there is evidence that delayed sleep timing alters hormonal appetite regulators,45 which might affect food preferences.46 In addition, sleep timing may affect mood and stress levels,47 which have also been associated with eating behaviors in young children.48 Sleep timing can also affect the timing of food intake, and studies in adults show that the timing of food intake may have implications for energy metabolism and weight loss.49 In contrast, individual components in the dietary patterns could affect sleep. For example, the Vegetables, Healthy Proteins & Sides pattern contains sources of dietary fiber and polyunsaturated fat, which have been related to more favorable sleep measures.50,51 These nutrients may affect sleep by promoting melatonin production and regulation.52 In contrast, the Processed & Fried pattern contains foods high in saturated fat, which has been related to shorter sleep duration53 and less slow wave sleep50 in some studies.
Another finding was that longer sleep duration during the weekend only was associated with lower Vegetables, Healthy Proteins & Sides pattern scores. Although the finding was not in the hypothesized direction, there are some potential explanations. Children with longer sleep on the weekends may be compensating for shorter or lower quality sleep during the weekdays54, and these weekday sleep characteristics may be responsible for the association. Indeed, associations with sleep quality and duration during the weekdays were in this expected direction but were not statistically significant. Further, the finding that a greater difference in duration from weekend to weekday was associated with lower Vegetables, Healthy Proteins & Sides, and greater Processed & Fried pattern scores is in line with this explanation.
It remains a possibility that the sleep and diet associations we observed were due to confounding factors that are associated with dietary patterns as well as sleep health. For example, parents who promote healthy eating may be more likely to also maintain consistent bedtime routines. Residual confounding due to socioeconomic status (SES) is also a possibility. However, many of the associations between sleep and diet persisted in spite of adjusting for parental education as well as sleep hygiene. Additionally, in models adjusting for measures of routine and household chaos, the estimates were unaltered.
There are both strengths and limitations of this study. Strengths included querying multiple aspects of children’s sleep and examining several potential confounders (e.g., household chaos). In addition, the examination of dietary patterns is novel in this age group, and the percent variance explained by the components was in line other studies of adolescents and adults.55–58 The cross-sectional study design was a limitation as it means that temporality of the sleep and diet associations are unclear. There is a possibility of recall bias of self-reported measures. Parents may have reported their children's diet and/or sleep to appear more favorably (social desirability bias). Parent characteristics such as weight status or SES may also influence recall.59,60 Further, although menus of all meals and snacks provided at Head Start are made available, 29 parents may not be fully aware of food actually consumed at preschool. Although this is a notable limitation to our capacity to accurately measure child intake in the field, prior work has shown that parent FFQs even for children attending preschool show reasonable validity. 28,61–64 As well, although we cannot rule out systematic error, it may be that parents’ lack of knowledge on their child’s diet primarily introduces random measurement error, which would decrease precision of the study estimates but should not unduly bias the results.65 Finally, it is important to note that the generalizability of these findings may be limited, as the study sample consisted of low-income preschool-aged children attending Head Start in southeast Michigan.
CONCLUSION
In summary, in a sample of low-income preschoolers, delayed sleep timing, longer sleep duration on weekends, and greater differences in sleep timing and duration from weekends to weekdays were associated with less healthy dietary pattern scores. Longitudinal investigations are needed to further elucidate the relationship between sleep health and diet in young children.
RESEARCH SNAPSHOT.
Research Question:
Are measures of sleep- nightly and total sleep duration, sleep timing, and sleep quality- associated with dietary patterns in low-income preschool-aged children?
Key Findings:
Longer average weekend sleep duration and a greater difference in weekend-to-weekday sleep duration was related to lower Vegetables, Healthy Proteins & Sides pattern scores. Later sleep midpoint during the weekday was related to lower Vegetables, Healthy Proteins & Sides pattern scores, while later sleep midpoint on the weekend was associated with higher Processed & Fried pattern scores. Similarly, a larger weekend-weekday midpoint difference was associated with higher Processed & Fried pattern scores.
Acknowledgements
We would like to acknowledge Yu-Pu Chen and Julie Sturza for their assistance in the creation of the dietary patterns.
Funding/Financial Disclosures: NIH 1RC1DK086376 funded the study. Dr. Jansen was supported by a T32 grant from the National Institute of Diabetes and Digestive and Kidney Diseases (5T32DK071212-12).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None of the authors have any conflicts of interest to report.
Contributor Information
Erica C. Jansen, Department of Nutritional Sciences, University of Michigan School of Public Health, Ann Arbor, MI, janerica@umich.edu.
Karen E. Peterson, Department of Nutritional Sciences, Professor of Global Public Health, University of Michigan School of Public Health, University of Michigan, Ann Arbor, MI, karenep@umich.edu.
Julie C. Lumeng, Pediatrics and Communicable Diseases, University of Michigan Medical School, Professor, Department of Nutritional Sciences, University of Michigan School of Public Health, Research Professor, Center for Human Growth and Development, University of Michigan, Ann Arbor, MI, jlumeng@umich.edu.
Niko Kaciroti, Center for Human Growth and Development, University of Michigan, Ann Arbor, MI, nicola@umich.edu.
Monique K. LeBourgeois, University of Colorado Boulder, Boulder, CO, monique.lebourgeois@colorado.edu.
Kathleen Chen, University of Michigan, Ann Arbor, MI, kathchen@umich.edu.
Alison L. Miller, Health Behavior and Health Education, University of Michigan School of Public Health, Research Associate Professor, Center for Human Growth and Development, University of Michigan, Ann Arbor, MI, alimill@umich.edu.
References
- 1.Bagley EJ, Kelly RJ, Buckhalt JA, El-Sheikh M. What keeps low-SES children from sleeping well: the role of presleep worries and sleep environment. Sleep Med. 2015; 16(4):496–502. doi: 10.1016/j.sleep.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gasser CE, Kerr JA, Mensah FK, Wake M. Stability and change in dietary scores and patterns across six waves of the Longitudinal Study of Australian Children. Br J Nutr. 2017;117(08):1137–1150. doi: 10.1017/S0007114517000897. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Lim H. The global childhood obesity epidemic and the association between socio-economic status and childhood obesity. Int Rev Psychiatry. 2012;24(3):176–188. doi: 10.3109/09540261.2012.688195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quist JS, Sjödin A, Chaput J-P, Hjorth MF. Sleep and cardiometabolic risk in children and adolescents. Sleep Med Rev. 2016;29:76–100. doi: 10.1016/j.smrv.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Laposky AD, Van Cauter E, Diez-Roux AV. Reducing health disparities: The role of sleep deficiency and sleep disorders. Sleep Med. 2016. doi: 10.1016/j.sleep.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson C, Redline S, Emmons KM. Sleep as a Potential Fundamental Contributor to Disparities in Cardiovascular Health.; 2015. doi: 10.1146/annurev-publhealth-031914-122838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mindell JA, Meltzer LJ, Carskadon MA, Chervin RD. Developmental aspects of sleep hygiene: Findings from the 2004 National Sleep Foundation Sleep in America Poll. Sleep Med. 2009;10(7):771–779. doi: 10.1016/j.sleep.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Nicklas TA, Baranowski T, Baranowski JC, Cullen K, Rittenberry L, Olvera N. Family and Child-care Provider Influences on Preschool Children’s Fruit, Juice, and Vegetable Consumption. Nutr Rev. 2009;59(7):224–235. doi: 10.1111/j.1753-4887.2001.tb07014.x. [DOI] [PubMed] [Google Scholar]
- 9.Mamun AA, Lawlor DA, Cramb S, O’Callaghan M, Williams G, Najman J. Do Childhood Sleeping Problems Predict Obesity in Young Adulthood? Evidence from a Prospective Birth Cohort Study. Am J Epidemiol. 2007;166(12):1368–1373. doi: 10.1093/aje/kwm224. [DOI] [PubMed] [Google Scholar]
- 10.Kaikkonen JE, Mikkilä V, Magnussen CG, Juonala M, Viikari JSA, Raitakari OT. Does childhood nutrition influence adult cardiovascular disease risk?—Insights from the Young Finns Study. Ann Med. 2013;45(2):120–128. doi: 10.3109/07853890.2012.671537. [DOI] [PubMed] [Google Scholar]
- 11.Kjeldsen JS, Hjorth MF, Andersen R, et al. Short sleep duration and large variability in sleep duration are independently associated with dietary risk factors for obesity in Danish school children. Int J Obes (Lond). 2014;38(1):32–39. doi: 10.1038/ijo.2013.147. [DOI] [PubMed] [Google Scholar]
- 12.Thellman KE, Dmitrieva J, Miller A, Harsh JR, LeBourgeois MK. Sleep timing is associated with self-reported dietary patterns in 9- to 15-year-olds. Sleep Heal. 2017;3(4):269–275. doi: 10.1016/j.sleh.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pereira S, Katzmarzyk PT, Gomes TN, et al. Profiling physical activity, diet, screen and sleep habits in Portuguese children. Nutrients. 2015;7(6):4345–4362. doi: 10.3390/nu7064345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westerlund L, Ray C, Roos E. Associations between sleeping habits and food consumption patterns among 10–11-year-old children in Finland. Br J Nutr. 2009;102(10):1531. doi: 10.1017/S0007114509990730. [DOI] [PubMed] [Google Scholar]
- 15.Baron KG, Reid KJ, Kern AS, Zee PC. Role of Sleep Timing in Caloric Intake and BMI. Obesity. 2011;19(7):1374–1381. doi: 10.1038/oby.2011.100. [DOI] [PubMed] [Google Scholar]
- 16.Chaput J-P, Katzmarzyk PT, LeBlanc AG, et al. Associations between sleep patterns and lifestyle behaviors in children: an international comparison. Int J Obes Suppl. 2015;5:S59–S65. doi: 10.1038/ijosup.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrex HAL, Skeaff SA, Black KE, et al. Sleep timing is associated with diet and physical activity levels in 9–11-year-old children from Dunedin, New Zealand: the PEDALS study. J Sleep Res. November 2017. doi: 10.1111/jsr.12634. [DOI] [PubMed] [Google Scholar]
- 18.Tate AD, Trofholz A, Rudasill KM, Neumark-Sztainer D, Berge JM. Does child temperament modify the overweight risk associated with parent feeding behaviors and child eating behaviors?: An exploratory study. Appetite. 2016;101:178–183. doi: 10.1016/j.appet.2016.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kidwell KM, Kozikowski C, Roth T, Lundahl A, Nelson TD. Concurrent and Longitudinal Associations Among Temperament, Parental Feeding Styles, and Selective Eating in a Preschool Sample. J Pediatr Psychol. 2017. doi: 10.1093/jpepsy/jsx148. [DOI] [PubMed] [Google Scholar]
- 20.Bauer KW, Haines J, Miller AL, et al. Maternal restrictive feeding and eating in the absence of hunger among toddlers: A cohort study. Int J Behav Nutr Phys Act. 2017;14(1). doi: 10.1186/s12966-017-0630-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullins EN, Miller AL, Cherian SS, et al. Acute sleep restriction increases dietary intake in preschool-age children. J Sleep Res. 2017;26(1):48–54. doi: 10.1111/jsr.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrov ME, Vander Wyst KB, Whisner CM, et al. Relationship of Sleep Duration and Regularity with Dietary Intake Among Preschool-Aged Children with Obesity from Low-Income Families. J Dev Behav Pediatr. 2017;38(2):1. doi: 10.1097/DBP.0000000000000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez SM, Tschann JM, Butte NF, et al. Short Sleep Duration Is Associated With Eating More Carbohydrates and Less Dietary Fat in Mexican American Children. Sleep. 2017;40(2). doi: 10.1093/sleep/zsw057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hager ER, Calamaro CJ, Bentley LM, Hurley KM, Wang Y, Black MM. Nighttime Sleep Duration and Sleep Behaviors among Toddlers from Low-Income Families: Associations with Obesogenic Behaviors and Obesity and the Role of Parenting. Child Obes. 2016;12(5):392–400. doi: 10.1089/chi.2015.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schulze MB, Manson JE, Ludwig DS, et al. 2015 – 2020 Dietary Guidelines for Americans. Am J Clin Nutr. 2016. doi: 10.1097/NT.0b013e31826c50af. [DOI] [Google Scholar]
- 26.LeBourgeois MK, Harsh JR. Development and psychometric evaluation of the Children’s Sleep-Wake Scale. Sleep Heal. 2016;2(3):198–204. doi: 10.1016/j.sleh.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harsh JR, Easley A and LeBourgeois MK. A measure of sleep hygiene. Sleep. 2002;25:A316. [Google Scholar]
- 28.Blum RE, Wei EK, Rockett H, et al. Validation of a food frequency questionnaire in Native American and Caucasian children 1 to 5 years of age. Matern Child Heal J. 1999;3(3):167–172. [DOI] [PubMed] [Google Scholar]
- 29.Benjamin SE, Copeland KA, Cradock A, et al. Menus in Child Care: A Comparison of State Regulations with National Standards. J Am Diet Assoc. 2009;109(1):109–115. doi: 10.1016/j.jada.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 24; 2011. [Google Scholar]
- 31.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27. http://www.ncbi.nlm.nih.gov/pubmed/11183293. Accessed October 4, 2017. [PubMed] [Google Scholar]
- 32.Matheny AP, Wachs TD, Ludwig JL, Phillips K. Bringing order out of chaos: Psychometric characteristics of the confusion, hubbub, and order scale. J Appl Dev Psychol. 1995;16(3):429–444. doi: 10.1016/0193-3973(95)90028-4. [DOI] [Google Scholar]
- 33.Jordan S Further Validation of the Child Routines Inventory (CRI): relationship to parent practices, maternal distress, and child externalizing behavior. 2003. [Google Scholar]
- 34.Hu FB. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 Suppl):1220S–1228S; discussion 1229S-1231S. http://www.ncbi.nlm.nih.gov/pubmed/9094926. Accessed October 4, 2017. [DOI] [PubMed] [Google Scholar]
- 36.Patrick H, Nicklas TA. A Review of Family and Social Determinants of Children’s Eating Patterns and Diet Quality. J Am Coll Nutr. 2005;24(2):83–92. doi: 10.1080/07315724.2005.10719448. [DOI] [PubMed] [Google Scholar]
- 37.Bathory E, Tomopoulos S. Sleep Regulation, Physiology and Development, Sleep Duration and Patterns, and Sleep Hygiene in Infants, Toddlers, and Preschool-Age Children. Curr Probl Pediatr Adolesc Health Care. 2017;47(2):29–42. doi: 10.1016/j.cppeds.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 38.STATA (for Windows) [computer program]. Version 14.0. College Station, TX: StataCorp; 2016. [Google Scholar]
- 39.Golley RK, Maher CA, Matricciani L, Olds TS. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int J Obes. 2013;37(4):546–551. doi: 10.1038/ijo.2012.212. [DOI] [PubMed] [Google Scholar]
- 40.Sato-Mito N, Sasaki S, Murakami K, et al. The midpoint of sleep is associated with dietary intake and dietary behavior among young Japanese women. Sleep Med. 2011;12(3):289–294. doi: 10.1016/j.sleep.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 41.Fleig D, Randler C. Association between chronotype and diet in adolescents based on food logs. Eat Behav. 2009;10(2):115–118. doi: 10.1016/j.eatbeh.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Malone SK, Zemel B, Compher C, et al. Social jet lag, chronotype and body mass index in 14–17-year-old adolescents. Chronobiol Int. 2016;33(9):1255–1266. doi: 10.1080/07420528.2016.1196697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silva CM, Mota MC, Miranda MT, Paim SL, Waterhouse J, Crispim CA. Chronotype, social jetlag and sleep debt are associated with dietary intake among Brazilian undergraduate students. Chronobiol Int. 2016;33(6):740–748. doi: 10.3109/07420528.2016.1167712. [DOI] [PubMed] [Google Scholar]
- 44.Lundahl A, Nelson TD. Sleep and food intake: A multisystem review of mechanisms in children and adults. J Health Psychol. 2015;20(6):794–805. doi: 10.1177/1359105315573427. [DOI] [PubMed] [Google Scholar]
- 45.St-Onge M-P. Sleep-obesity relation: underlying mechanisms and consequences for treatment. Obes Rev. 2017;18:34–39. doi: 10.1111/obr.12499. [DOI] [PubMed] [Google Scholar]
- 46.Chaput J-P. Sleep patterns, diet quality and energy balance. Physiol Behav. 2014;134:86–91. doi: 10.1016/j.physbeh.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Kouros CD, El-Sheikh M. Daily mood and sleep: Reciprocal relations and links with adjustment problems. J Sleep Res. 2015;24(1):24–31. doi: 10.1111/jsr.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lumeng JC, Miller A, Peterson KE, et al. Diurnal cortisol pattern, eating behaviors and overweight in low-income preschool-aged children. Appetite. 2014;73:65–72. doi: 10.1016/j.appet.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garaulet M, Gómez-Abellán P, Alburquerque-Béjar JJ, Lee Y-C, Ordovás JM, Scheer FAJL. Timing of food intake predicts weight loss effectiveness. Int J Obes. 2013;37(4):604–611. doi: 10.1038/ijo.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.St-Onge MP, Roberts A, Shechter A, Choudhury AR. Fiber and saturated fat are associated with sleep arousals and slow wave sleep. J Clin Sleep Med. 2016;12(1):19–24. doi: 10.5664/jcsm.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Christian LM, Blair LM, Porter K, Lower M, Cole RM, Belury MA. Polyunsaturated fatty acid (PUFA) status in pregnant women: Associations with sleep quality, inflammation, and length of gestation. PLoS One. 2016;11(2). doi: 10.1371/journal.pone.0148752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peuhkuri K, Sihvola N, Korpela R. Dietary factors and fluctuating levels of melatonin. Food Nutr Res. 2012;56(0):1–9. doi: 10.3402/fnr.v56i0.17252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dashti HS, Follis JL, Smith CE, et al. Habitual sleep duration is associated with BMI and macronutrient intake and may be modified by CLOCK genetic variants. Am J Clin Nutr. 2015;101(1):135–143. doi: 10.3945/ajcn.114.095026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spruyt K, Molfese DL, Gozal D. Sleep Duration, Sleep Regularity, Body Weight, and Metabolic Homeostasis in School-aged Children. Pediatrics. 2011;127(2):e345–e352. doi: 10.1542/peds.2010-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Batis C, Mendez MA, Gordon-Larsen P, Sotres-Alvarez D, Adair L, Popkin B. Using both principal component analysis and reduced rank regression to study dietary patterns and diabetes in Chinese adults. Public Health Nutr. 2016. doi: 10.1017/S1368980014003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strate LL, Keeley BR, Cao Y, Wu K, Giovannucci EL, Chan AT. Western Dietary Pattern Increases, and Prudent Dietary Pattern Decreases, Risk of Incident Diverticulitis in a Prospective Cohort Study. Gastroenterology. 2017. doi: 10.1053/j.gastro.2016.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Emmett PM, Jones LR, Northstone K. Dietary patterns in the Avon Longitudinal Study of Parents and Children. Nutr Rev. 2015. doi: 10.1093/nutrit/nuv055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perng W, Fernandez C, Peterson KE, et al. Dietary Patterns Exhibit Sex-Specific Associations with Adiposity and Metabolic Risk in a Cross-Sectional Study in Urban Mexican Adolescents. J Nutr. 2017:jn256669. doi: 10.3945/jn.117.256669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sheikh MA, Abelsen B, Olsen JA. Differential recall bias, intermediate confounding, and mediation analysis in life course epidemiology: An analytic framework with empirical example. Front Psychol. 2016;7(NOV). doi: 10.3389/fpsyg.2016.01828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Livingstone MBE, Robson PJ, Wallace JMW. Issues in dietary intake assessment of children and adolescents. Br J Nutr. 2004;92(S2):S213. doi: 10.1079/BJN20041169. [DOI] [PubMed] [Google Scholar]
- 61.Parrish L a, Marshall J a, Krebs NF, Rewers M, Norris JM. Validation of a food frequency questionnaire in preschool children. Epidemiology. 2003;14(2):213–217. doi: 10.1097/01.EDE.0000041256.12192.23. [DOI] [PubMed] [Google Scholar]
- 62.Treiber FA, Leonard SB, Frank G, et al. Dietary assessment instruments for preschool children: reliability of parental responses to the 24-hour recall and a food frequency questionnaire. J Am Diet Assoc. 1990;90(6):814–820. doi:[1990, 90(6):814–820]. [PubMed] [Google Scholar]
- 63.Buch-Andersen T, Pérez-Cueto FJA, Toft U. Relative validity and reproducibility of a parent-administered semi-quantitative FFQ for assessing food intake in Danish children aged 3–9 years. Public Health Nutr. 2016;19(7):1184–1194. doi: 10.1017/S136898001500275X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wallace A, Kirkpatrick SI, Darlington G, Haines J. Accuracy of parental reporting of preschoolers’ dietary intake using an online self-administered 24-h recall. Nutrients. 2018. doi: 10.3390/nu10080987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hutcheon JA, Chiolero A, Hanley JA. Random measurement error and regression dilution bias. BMJ. 2010. doi: 10.1136/bmj.c2289. [DOI] [PubMed] [Google Scholar]