Abstract
Objective:
To describe the clinical features of children who presented to Children’s Hospital Colorado (CHCO) with High-Altitude Pulmonary Edema (HAPE).
Study design:
We performed a retrospective chart review in children discharged from CHCO (an elevation of 1668m) with a clinical diagnosis of HAPE and a chest radiograph consistent with non-cardiogenic pulmonary edema. Descriptive statistics were used to describe the demographics, presentations, and treatment strategies.
Results:
From 2004 to 2014, 50 children presented to CHCO who were found to have a clinical diagnosis of HAPE and a chest radiograph consistent with non-cardiogenic pulmonary edema. Most (72%) patients were male and most (60%) of the children in the study were diagnosed with classic HAPE, 38% with re-entry HAPE, and 2% with high altitude resident pulmonary edema. Elevation at symptom presentation ranged from 1840–3536 m. Patients were treated with a variety of medications, including diuretics, steroids, and antibiotics. Four patients were newly diagnosed with structural heart findings: 2 patients with patent foramen ovale and 2 with atrial septal defects. Eleven patients had findings consistent with pulmonary hypertension at the time of echocardiography.
Conclusions:
HAPE symptoms may develop below 2500m, so providers should not rule out HAPE based on elevation alone. Structural heart findings and pulmonary hypertension are associated with HAPE susceptibility and their presence may inform treatment. Inappropriate use of antibiotics and diuretics in children with HAPE suggest that further education of providers is warranted.
Keywords: pediatrics, pulmonary hypertension, pulmonary edema, altitude, HAPE
At an average elevation of 2072 m,(1) Colorado has the greatest mean elevation of any state in the United States and welcomed 82.4 million visitors in 2016.(2) Visitors to Colorado are at risk of acute mountain sickness, high altitude cerebral edema, and high altitude pulmonary edema (HAPE).(3,4) HAPE is an acute non-cardiogenic pulmonary edema caused by patchy hypoxic pulmonary vasoconstriction.(5,6) Classic HAPE presents in people who travel from lower elevation to high elevation (>2500 m), and is related to altitude and rate of ascent.(4,7,8) People who live at high altitude can also develop HAPE, either when they return from lower elevations (re-entry HAPE)(3,9–11) or with a concurrent respiratory infection and no elevation change (High Altitude Resident Pulmonary Edema or HARPE).(12,13) Re-entry HAPE appears to be more common in children.(11) At Children’s Hospital Colorado (CHCO), we are in a unique position to comment on pediatric HAPE because we are the academic children’s hospital at the highest elevation in the country and we receive referrals from mountain communities at higher elevations.
The literature describing HAPE in children is sparse and has primarily been found in case reports and expert opinion.(3,5,11,14–16) Our objective was to better understand pediatric HAPE and to guide future studies for management and prophylaxis of HAPE.
Methods
We performed a retrospective chart review under an institutional review board approved protocol. Informatics for Integrating Biology & the Bedside (i2b2) was used to query the electronic medical record for the diagnosis (billing, encounter, and problem list) of “unspecified effects of high altitude” (ICD-9 code 993.2) from 2004 to 2014; this code includes diseases such as acute mountain sickness, barotrauma of ascent/descent, chronic mountain sickness, diving barotrauma, high altitude pulmonary edema, high altitude cerebral edema, high altitude pulmonary hypertension, high altitude retinopathy, and suit squeeze. We included 50 patients in this review (Figure 1). To be included, patients needed to have a clinical history consistent with HAPE documented by the treating provider and a chest radiograph suggestive of non-cardiogenic pulmonary edema (Figure 2). A pediatric radiologist with expertise in chest radiology reviewed the chest radiographs of all patients with a clinical diagnosis of HAPE to determine if chest radiographs had a high probability (eg, Figure 2), equivocal, or low probability of non-cardiogenic pulmonary edema. The chest radiographs were also evaluated for lobar pneumonia. Data collection from chart review included demographic data, clinical presentation, past medical history, examination findings, evaluation, treatment, and follow-up. Patients presented either directly from homes/hotels or from mountain community facilities. Data were entered into a REDCap database.(17) Echocardiograms and cardiac catheterizations were clinically performed at the discretion of the treating physician. All statistical tests were considered significant at the alpha=0.05 level. Demographic characteristics and clinical interventions were summarized as frequencies (%) and medians with range. Individuals were sub-grouped based on type of HAPE (classic, re-entry, or HARPE). Group comparisons were performed between classic and re-entry HAPE patients using Fisher exact test and Wilcoxon Rank Sum. All analyses were conducted using R version 3.4.1 software (R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/).
Figure 1.
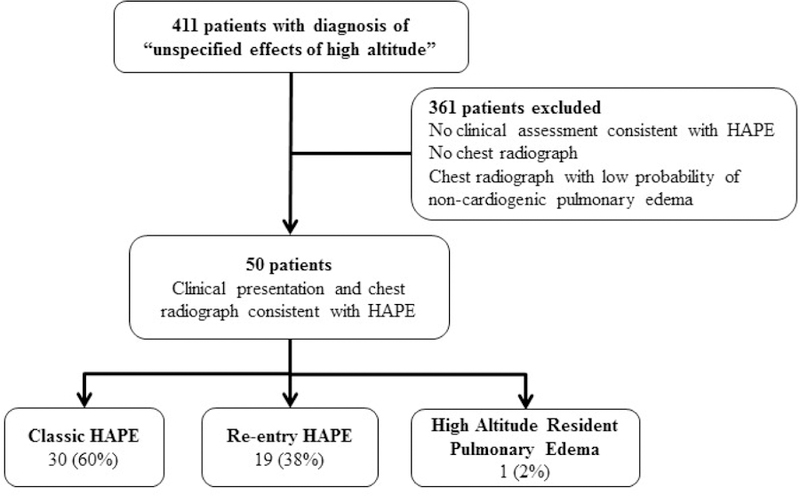
Consort diagram demonstrating the patients evaluated for the current study and the final study cohort.
Figure 2.
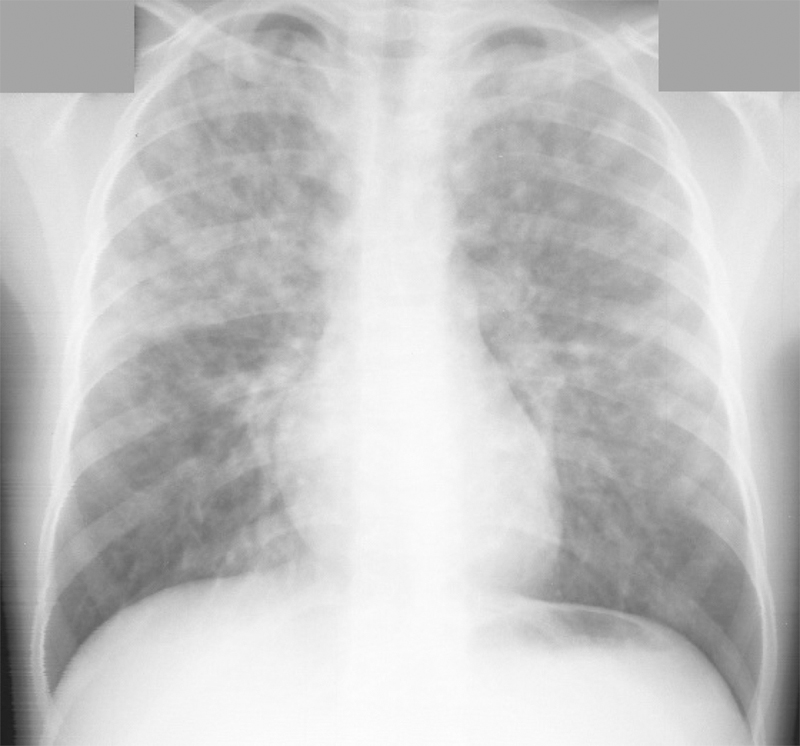
Chest radiograph demonstrating bilateral ill-defined nodular opacities consistent with high altitude pulmonary edema, in this case predominantly in the upper lobes.
Results
At CHOC 411 children were evaluated and were coded as 993.2 “other effects of high altitude.” However, 316 patients were excluded because they did not have a clinical presentation consistent with HAPE or a chest radiograph to suggest non-cardiogenic pulmonary edema (Figure 1). Many of the excluded patients were infants receiving oxygen. Fifty patients with clinical histories and chest radiographs suggestive of non-cardiogenic pulmonary edema (Figure 2) were included in the analysis. Twenty (40%) patients of the 50 patients had chest radiographs with findings suggestive of lobar pneumonia. Demographics (Table) are notable for a male predominance (n=36, 72%). This sex difference was most notable when comparing children <11 years and >11 years (p=0.004) This sex difference was also driven by patients with classic HAPE (p=0.007) rather than re-entry HAPE (P = .25). The most common symptoms in the entire cohort were shortness of breath (n=38, 76%), cough (n=36, 72%), and emesis (n=27, 54%) and are reported in the Table. Ten children (20%) presented with headache.
Table:
Demographics and presentation of 50 children who presented to Children’s Hospital Colorado with high altitude pulmonary edema.
| Median age in years [range] | 10.2 [0.6–19.2] |
| Sex N(%) | |
| Male : Female | 36 (72) : 14 (28) |
| Type of HAPE N(%) | |
| Classic | 30 (60) |
| Re-entry | 19 (38) |
| Resident | 1 (2) |
| Home elevation in meters [range] | 1655 [2.7–3139] |
| Elevation of symptom onset [range] | 2798 m [1840–3536] |
| Symptoms N(%) (49 patients reporting) | |
| Shortness of breath | 38 (78) |
| Coughing | 36 (73) |
| Emesis | 27 (55) |
| Fever | 13 (27) |
| Cyanotic | 13 (27) |
| Fast breathing | 12 (24) |
| Headache | 10 (20) |
Median age at presentation was 10.2 years (range 0.6 to 19 years). Half of the children in the cohort (n=26, 52%) were older than 10 years, 36% (n=18) were between 5–10 years, and 12% (n=6) were under 5 years. The youngest patient was a 7 month-old male originally from Texas who presented from a facility at 2500 m elevation with hypoxemia, respiratory infection, and chest radiograph with concerns for pulmonary edema.
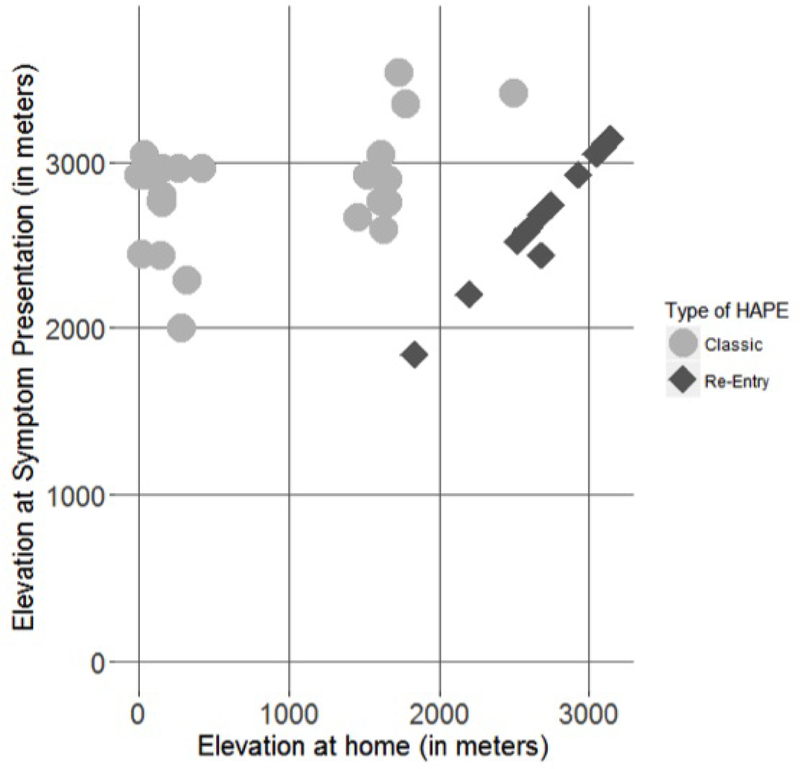
The median pulse oximetry in room air at first recorded presentation (either at CHOC at an outside hospital, or by emergency medical services) was 74% (range 20–94%). Symptoms of a concurrent respiratory infection were documented in 14 patients (28%). No patients were noted to have otitis media. Most patients had classic HAPE (n=30, 60%), followed by re-entry HAPE (n=19, 38%), and one patient had HARPE (n=1, 2%) (Figure 3). Children with re-entry HAPE spent a median length of 7 days (range 1–30 days) at lower altitudes. Most HAPE presentations occurred in the spring (46%), followed by winter (34%), summer (12%), and fall (8%). Thirteen of the children (26%) were skiing, 4 (8%) were camping, and 1 (2%) of the children was hiking. Other patients were visiting, snowshoeing, returning home, or mountain biking. Median elevation at symptom presentation was 2789 m (range 1840 to 3536 m) (Figure 3). The median home elevation was 1655 m (range 2.7 to 3139 m) (Figure 3). Eight children of the 50-patient cohort (16%) included in the analysis had a maximum elevation of <2500 m; 2 of these 8 reached a maximum elevation lower than 2000 m. Median duration of stay at Children’s Hospital Colorado for admitted patients was 2.2 days.
Figure 3.
Elevation of symptom presentation in meters by type of HAPE and home elevation in meters. Patients with classic HAPE typically lived at low elevations or moderate elevations (between 0–2000 m). Patients with re-entry HAPE lived at moderate to high (>2000 m) elevations. One patient had HARPE and lived at approximately 2926 m.
Seven children (14%) of the cohort had a history of structural heart findings prior to their HAPE presentation; three of these notably presented at altitudes lower than 2500 m. Of the seven with a history of structural heart findings, two had atrial septal defects (ASD), one had a patent foramen ovale (PFO) and an ASD, one had an absent pulmonary artery, one had a ventricular septal defect/coarctation of the aorta/bicuspid aortic valve, and two had pulmonary stenosis. There was no significant difference in presentation with classic versus re-entry HAPE between patients that had pre-existing heart conditions to those who did not prior to their HAPE diagnosis.
Children with classic and re-entry HAPE were additionally compared in terms of structural heart findings, season of presentation, and initial pulse oximetry, and there was no significant difference between groups.
Treatment
All patients presented to CHOC (elevation 1668 m) after descending from higher elevations. Level of respiratory support during HAPE episode and recovery varied for each patient. Six patients (12%) were not treated with oxygen upon evaluation at CHOC, as descent had been sufficient to treat their HAPE episode. Most patients (n=34 of 49 patients, 69%) were treated with oxygen, 6 children (12%) were initiated on non-invasive ventilation and four (8%) were intubated and invasively ventilated. Children who were treated with non-invasive or invasive ventilation (i.e. markers of severe disease,) versus no ventilation were compared, and there was no significant difference in type of HAPE, age, underlying heart disease, initial pulse oximetry, concurrent infection, and change in elevation from home.
Medication data during the acute HAPE episode were available from 41 patients and included furosemide (n=8, 20%), calcium channel blockers such as nifedipine or amlodipine (n=4, 10%), acetazolamide (n=3, 7%), systemic corticosteroids such as dexamethasone, prednisolone, or prednisone (n=9, 22%), and antibiotics (variety) (n=35, 85%).
Echocardiograms were conducted at CHOC in 24 patients (48%); 20patients had echocardiograms during the acute illness/recovery period (i.e. within 5 days of presentation). The remainder either had previous echocardiograms (two patients) or echocardiograms after recovery (2 patients). Eleven of the 24 who underwent echocardiograms displayed evidence of pulmonary hypertension defined as septal flattening and/or tricuspid regurgitation jet of 3 m/s or greater. One additional patient had evidence of pulmonary hypertension by cardiac catheterization (see below), but not by echocardiography. Several additional patients did not meet the above criteria for pulmonary hypertension, but had echo findings suggestive of pulmonary hypertension, including decreased right ventricular function, right ventricular dilation, and/or dilated pulmonary artery. Four patients (17%) of the 24 who underwent echocardiogram were diagnosed with new structural heart findings (new PFO in 2 and new ASD in 2).
Follow-up
Fifteen patients (30%) had a follow-up with cardiology clinic and 17 (34%) followed up in pulmonology clinic. Recommendations varied from patient to patient and included slow ascent, prophylaxis with medications, oxygen with sleep and/or at high altitudes, overnight pulse oximetry monitoring, and further evaluation including hypoxic echocardiography, cardiac catheterization, polysomnography, and cardiopulmonary exercise testing. Five patients were noted to have asthma. One patient was evaluated with polysomnogram and was determined to have borderline obstructive sleep apnea at 1668 m.
In cardiology or pulmonary follow-up, three patients had hypoxic echocardiograms (echocardiograms performed in hypoxic conditions, typically 16% oxygen or at 2766 m at rest) and all three had signs of pulmonary hypertension with the hypoxic challenge. Seven patients of the 50 (14%) underwent cardiac catheterizations (at 1668 m) either before the HAPE episode or following the HAPE episode. The mean pulmonary artery pressures median was 18.5 mmHg (range 15–22 mmHg, normal mean pulmonary pressures 11–12 mm Hg).(18) Not all patients were evaluated with hypoxia or oxygen, but those that were evaluated on oxygen had mean pulmonary artery pressures ranging from 13–15 mmHg, and those that were evaluated on 16% oxygen had mean pulmonary artery pressures ranging from 18–80 mmHg. Pulmonary vascular resistance index median was 3.06 Woods units (range 2.05 – 3.89 Woods units); five patients had Woods units of 3 or greater, indicative of pulmonary hypertension. One of these patients underwent cardiac catheterization prior to the HAPE episode and was noted to have pulmonary hypertension at that time (3.47 Woods Units)
At CHOC, children were prescribed a variety of prophylactic medications to prevent future HAPE episodes, including calcium channel blockers (nifedipine (n=10, 20%) or amlodipine (n=3, 6%)) and acetazolamide (n=9, 18%). The prescribing patterns were provider dependent and typically included starting 1–2 days prior to ascending in elevation and ending 1–2 days after reaching final elevation versus continuing the medication for the entire duration at high altitude. Eight patients suffered from known HAPE recurrence.
Discussion
At CHOC, approximately 5 children a year were evaluated with a clinical diagnosis of HAPE and chest radiograph suggestive of non-cardiogenic pulmonary edema. We found that HAPE may present at elevations lower than 2500 m, as reported in the literature.(19,20) Although HAPE is traditionally not an inflammatory process,(21) viral-induced inflammation leads to increased vascular permeability and may predispose children to HAPE.(20,22) In this retrospective cohort, males were more likely to present with HAPE, and this was especially true older patients with classic HAPE.
Our review is not a comprehensive review of the incidence of pediatric HAPE in Colorado as our study is limited by its retrospective design. The incidence is likely significantly higher than we report as many children had clinical histories consistent with HAPE and either had a chest radiograph at an outside facility that was not available for review or did not have a chest radiograph. Many more children may never have presented to CHOC if they were treated at local emergency departments and discharged on oxygen and/or advised to go to lower elevation (<2500 m). Other children may have never presented to care.
Ten (20%) of children in the cohort presented with headache and 27 (54%) presented with emesis suggestive of acute mountain sickness. There is crossover between these different manifestations of high altitude; in adults, 50% of patients with HAPE also have AMS, but this has not been well established in children.(3,4,23)
Antibiotics are not indicated for the treatment of HAPE,(3) however 35 patients (85%) were treated with antibiotics. HAPE may be misdiagnosed as pneumonia or pneumonia may be a comorbid disease process; in our cohort, 40% of patients had chest radiographs suggestive of lobar pneumonia. It is unclear whether these patients in fact had concurrent pneumonia or whether the findings of HAPE on radiography mimicked pneumonia. Eight patients were treated with furosemide which is not recommended for HAPE due to the risk of further volume depletion in patients with already low intravascular volume.(24) More education about HAPE recognition and treatment may be helpful for our emergency medicine, intensivist, hospitalist, cardiology, and pulmonary colleagues.
Children with pulmonary hypertension and structural heart findings may be predisposed to HAPE, and proper precautions should be given to these families. Evaluation for structural heart findings and pulmonary hypertension has been suggested following a HAPE episode, both for classic and re-entry HAPE.(13) Structural heart findings are a known contributor to HAPE, including unilateral absence of the pulmonary artery, ASD, coarctation of the aorta, and ventricular septal defect.(5,20,25–28) PFO is a known association with HAPE susceptibility (29) and is present in 10–35% of the population.(30) Eleven patients (22% of all patients; 46% of those who had an echocardiogram) in our study had structural heart findings (7 previously known, 4 newly diagnosed). Pulmonary hypertension is expected to be present during the acute episode of HAPE, but if it persists after recovery or with subsequent hypoxic challenge, then the child should be further evaluated for etiology of pulmonary hypertension and appropriately treated.(13,31) The prevalence of pulmonary hypertension in an otherwise healthy pediatric population is not well studied, but in extremely low birth weight infants (e.g. <1 kg), pulmonary hypertension affects 18% of children.(32) Nearly half of the patients did not undergo an echocardiogram, therefore pulmonary hypertension and structural heart findings may have been missed in these patients, or there may have been a bias to whom was evaluated with echocardiogram. Only 15 patients (30%) had follow-up with cardiology and 17 (34%) had follow-up with pulmonology, and contributors to pulmonary hypertension such as obstructive sleep apnea was assessed in only one patient.
We found that a significant majority of the patients were males. The sex difference was most notable in patients greater > 11 years of age, suggesting that sex hormones play a role in HAPE susceptibility, either that male sex hormones predispose patients to HAPE or that female sex hormones are protective. Prior pediatric case series have demonstrated a male predominance.(5) Young males have a higher respiratory morbidity than young females.(28) Although the literature supports a higher prevalence of HAPE in adult males,(7) this is typically thought to be because they are more likely to be at higher altitudes participating in recreational activities. In children, we think activity choice for this sex difference is unlikely as they are typically traveling with their families. We hope to further investigate this sex difference in future prospective studies.
We suggest that seasonal variation is reflective of vacation timing (ski vacations during winter break for lowlanders and sea-level travel during spring break for Coloradans) and respiratory illness season rather than differences in the seasons themselves, although atmospheric pressures—and therefore partial pressure of oxygen—are lowest in the winter, so there may be a geophysical explanation for some of the seasonal findings.
Our study was limited by its retrospective design, including lack of demographic information, missing data surrounding the presentation of HAPE, and limited long-term follow-up. A major limitation is that many children with HAPE would never have presented to CHOC and would therefore not be captured by the current study.
There is practice variation in terms of treatment and prophylaxis of HAPE and future studies should evaluate best practice patterns for type of both acute and prophylactic medication and timing as well as comparison with slower rate of ascent (i.e. spending a night in Denver) and use of nocturnal oxygen.
Use of antibiotics, steroids, and diuretics raise questions regarding limited knowledge about HAPE treatment guidelines, poor recognition of HAPE, and/or concern for co-existing medical conditions. This review suggests emergency medicine, intensivists, pulmonologists, cardiologists and hospitalists may benefit from additional education regarding HAPE diagnosis and treatment. This variability in terms of evaluation and both acute and prophylactic medications also creates opportunities for research into evidence based best practice.
Acknowledgements:
We are grateful for the patients who inspired this study and appreciative of the assistance of Deb Batson, a clinical research data warehouse architect in the Department of Research Informatics at the Children’s Hospital Colorado Research Institute, in generating patient data with i2b2.
Supported by the NIH/NCRR Colorado CTSI (UL1 TR001082); the Jayden de Luca Foundation; and the Frederick and Margaret Weyerhaeuser Foundation.
Abbreviations:
- HAPE
High altitude pulmonary edema
- PFO
patent foramen ovale
- ASD
atrial septal defect
- HARPE
high altitude resident pulmonary edema
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
Portions of this study were presented at the American Thoracic Society Conference, << >>, << >>.
References
- 1.Colorado Travel Facts [Internet] Available from: http://www.colorado.com/colorado-travel-facts
- 2.Colorado tourism sets all-time records for sixth consecutive year [Internet] 2017. Available from: https://www.colorado.com/news/colorado-tourism-sets-all-time-records-sixth-consecutive-year
- 3.Pollard AJ, Niermeyer S, Barry P, Bärtsch P, Berghold F, Bishop RA, et al. Children at high altitude: an international consensus statement by an ad hoc committee of the International Society for Mountain Medicine, March 12, 2001. High Alt Med Biol 2001;2(3):389–403. [DOI] [PubMed] [Google Scholar]
- 4.Bärtsch P, Swenson ER. Acute High-Altitude Illnesses. N Engl J Med 2013. June 13;368(24):2294–302. [DOI] [PubMed] [Google Scholar]
- 5.Das B, Wolfe R, Chan K-C, Larsen G, Reeves J, Dunbar I. High-Altitude Pulmonary Edema in Children With Underlying Cardiopulmonary Disorders and Pulmonary Hypertension Living at Altitude. Arch Pediatr Adolesc Med 2004. December;158:1170–6. [DOI] [PubMed] [Google Scholar]
- 6.Swenson ER, Bärtsch P. High‐Altitude Pulmonary Edema. Compr Physiol 2012;2:2753–73. [DOI] [PubMed] [Google Scholar]
- 7.Hultgren HN, Honigman B, Theis K, Nicholas D. High-altitude pulmonary edema at a ski resort. West J Med 1996;164(3):222. [PMC free article] [PubMed] [Google Scholar]
- 8.Luks AM, McIntosh SE, Grissom CK, Auerbach PS, Rodway GW, Schoene RB, et al. Wilderness Medical Society Consensus Guidelines for the Prevention and Treatment of Acute Altitude Illness. Wilderness Environ Med 2010. June 1;21(2):146–55. [DOI] [PubMed] [Google Scholar]
- 9.Scoggin C, Heyers T, Reeves JT, Grover RF. High-Altitude Pulmonary Edema in Children and Young Adults of Leadville, Colorado. N Engl J Med 1977;297:1269–72. [DOI] [PubMed] [Google Scholar]
- 10.Sophocles AM, Bachman J. High-altitude pulmonary edema among visitors to Summit County, Colorado. J Fam Pract 1983;17(6):1015–7. [PubMed] [Google Scholar]
- 11.Hultgren HN, Marticorena EA. High Altitude Pulmonary Edema. Chest 1978. October 1;74(4):372–6. [DOI] [PubMed] [Google Scholar]
- 12.Ebert-Santos C High-Altitude Pulmonary Edema in Mountain Community Residents. High Alt Med Biol [Internet] 2017; Available from: http://online.liebertpub.com/doi/full/10.1089/ham.2016.0100 [DOI] [PubMed]
- 13.Liptzin DR, Abman SH, Giesenhagen AM, Ivy DD. An Approach to Children with Pulmonary Edema at High Altitude. High Alt Med Biol 2018. February 22;19(1):91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marticorena E, Hultgren HN. Evaluation of therapeutic methods in high altitude pulmonary edema. Am J Cardiol 1979. February 1;43(2):307–12. [DOI] [PubMed] [Google Scholar]
- 15.Marticorena E, Tapia FA, Dyer J, Severino J, Banchero N, Gamboa R, et al. Pulmonary Edema by Ascending to High Altitudes. Dis Chest 1964. March 1;45(3):273–83. [DOI] [PubMed] [Google Scholar]
- 16.Sime F, Banchero N, Peñaloza D, Gamboa R, Cruz J, Marticorena E. Pulmonary hypertension in children born and living at high altitudes. Am J Cardiol 1963. February 1;11(2):143–9. [DOI] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009. April 1;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vargo TA. Cardiac Catheterization - Hemodynamic Measurements. In: Garson A, Bricker JT, McNamara DG, eds. The Science and Practice of Pediatric Cardiology Philadephia, PA: Lea & Febiger; 1990. p. 913–45. [Google Scholar]
- 19.Gabry A, Martin C. High-Altitude Pulmonary Edema. Chest 2003;124(4):1620–1. [DOI] [PubMed] [Google Scholar]
- 20.Durmowicz AG, Noordeweir E, Nicholas R, Reeves JT. Inflammatory processes may predispose children to high-altitude pulmonary edema. J Pediatr 130(5):838–40. [DOI] [PubMed] [Google Scholar]
- 21.Swenson ER, Maggiorini M, Mongovin S, Gibbs JSR, Greve I, Mairbäurl H, et al. Pathogenesis of High-Altitude Pulmonary Edema: Inflammation is not an etiologic factor. JAMA 2002;287(17):2228–35. [DOI] [PubMed] [Google Scholar]
- 22.Carpenter TC, Reeves JT, Durmowicz AG. Viral respiratory infection increases susceptibility of young rats to hypoxia-induced pulmonary edema. J Appl Physiol 1998;84(3):1048–54. [DOI] [PubMed] [Google Scholar]
- 23.Hultgren HN. High Altitude Medicine Stanford, CA: Hultgren Publications; 1997. [Google Scholar]
- 24.Hultgren HN. Furosemide for high altitude pulmonary edema. JAMA 1975. November 10;234(6):589–90. [DOI] [PubMed] [Google Scholar]
- 25.Hackett PH, Creagh CE, Grover RF, Honigman B, Houston CS, Reeves JT, et al. High-Altitude Pulmonary Edema in Persons without the Right Pulmonary Artery. N Engl J Med 1980. May 8;302(19):1070–3. [DOI] [PubMed] [Google Scholar]
- 26.Sebbane M, Wuyam B, Pin I, Pendlebury S, Plasse M, Durand C, et al. Unilateral agenesis of the pulmonary artery and high-altitude pulmonary edema (HAPE) at moderate altitude. Pediatr Pulmonol 1997;24:111–4. [DOI] [PubMed] [Google Scholar]
- 27.Rios B, Driscoll DJ, McNamara DG. High-Altitude Pulmonary Edema with Absent Right Pulmonary Artery. Pediatrics 1985. February 1;75(2):314. [PubMed] [Google Scholar]
- 28.Schoene R Fatal high altitude pulmonary edema associated with absence of the left pulmonary artery. High Alt Med Biol 1985;2:405–6. [DOI] [PubMed] [Google Scholar]
- 29.Allemann Y, Hutter D, Lipp E, Sartori C, Duplain H, Egli M, et al. Patent Foramen Ovale and High-Altitude Pulmonary Edema. JAMA 2006. December;296(24):2954–8. [DOI] [PubMed] [Google Scholar]
- 30.Fisher DC, Fisher EA, Budd JH, Rosen SE, Goldman ME. The Incidence of Patent Foramen Ovale in 1,000 Consecutive Patients. Chest 1995. June 1;107(6):1504–9. [DOI] [PubMed] [Google Scholar]
- 31.Ivy DD, Abman SH, Barst RJ, Berger RMF, Bonnet D, Fleming TR, et al. Pediatric Pulmonary Hypertension. Updat Pulm Hypertens 2013. December 24;62(25):D117–26. [DOI] [PubMed] [Google Scholar]
- 32.Bhat R, Salas AA, Foster C, Carlo WA, Ambalavanan N. Prospective Analysis of Pulmonary Hypertension in Extremely Low Birth Weight Infants. Pediatrics 2012. March 1;129(3):e682–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liptzin DR, Landau LI, Taussig LM. Sex and the lung: Observations, hypotheses, and future directions. Pediatr Pulmonol 50:1159–69. [DOI] [PubMed] [Google Scholar]