Abstract
Objective:
To determine whether prenatal sex hormones from maternal saliva are associated with birth weight-for-gestational age.
Study Design:
We measured salivary progesterone, testosterone, estradiol, dehydroepiandrosterone (DHEA) and cortisone in 504 pregnant women in a Mexico City cohort in the. We performed linear and modified Poisson regression to examine associations of log-transformed hormones with birth weight-for-gestational age z-scores and the risk of small-for-gestational age (SGA) and large-for-gestational age (LGA) adjusting for maternal age, sex, BMI, parity, smoking, education and socioeconomic status.
Results:
15% of infants were SGA and 2% were LGA. Each interquartile range increment in testosterone/estradiol ratio was associated with a 0.12 decrement in birth weight-for-gestational age z-score (95% CI: −0.27, −0.02) and a 50% higher risk of SGA versus appropriate-for-gestational age (AGA) (95% CI: 1.13, 1.99).
Conclusion:
Higher salivary testosterone/estradiol ratios may affect fetal growth, and identifying the predictors of hormone levels may be important to optimizing fetal growth.
Keywords: sex hormones, birth weight-for-gestational age, SGA, LGA, pregnancy
INTRODUCTION
Infants born small-for-gestational age (SGA) or large-for-gestational age (LGA) have increased likelihood of chronic illness,1 lower academic or professional attainment,2, 3 and metabolic disorders.4 Mother are more likely to have an SGA or LGA infants if they are older, smoke, have lower educational status, preeclampsia, higher BMI or diabetes.5, 6, 7 Nevertheless, the underlying mechanisms of maladaptive fetal growth are incompletely understood.
Given the complex hormonal milieu of pregnancy, understanding the role of maternal hormones in the regulation of fetal growth is critical. Endocrine responses in the mother, placenta and fetus are important in regulating fetal development and growth.8, 9 Maternal levels of androgens and estrogens increase throughout pregnancy 10 and play key roles in maintenance of pregnancy and induction of labor. 11, 12, 13 Surprisingly, limited information is available regarding sex hormones and their relationship to birth weight-for-gestational age. The few animal and human studies which have investigated this relationship have focused primarily on testosterone or progesterone. Two studies found higher prenatal exposure to testosterone (one in sheep and the other in rats) to be associated with reduced body size, as measured by birth weight and height.14, 15 These results were not replicated in a study of monkeys.16 In humans, higher prenatal testosterone levels have been associated with lower birth weight and birth weight-for-gestational age z-scores.17, 18 Although dehydroepiandrosterone (DHEA) is a precursor of testosterone only one prior study has examined prenatal DHEA and birth size and found no association.17 Retrospectively, DHEA levels in early childhood have been found to be higher in children who were born SGA and lower in children who were born LGA, as compared to their appropriate-for-gestational age (AGA) counterparts.19 Progesterone is commonly administered in pregnancy to prevent preterm birth and prolong pregnancy, yet little is known about its independent effects on birth weight-for-gestational age. This administration could disrupt normal feedback loops and disrupt endocrine signaling for sex hormones. It has been suggested that higher maternal progesterone and estradiol are associated with higher birth weight.20, 21, 22 Most of the extant literature on prenatal hormones and birth size is limited by consideration of birth weight and length only without consideration of the length of gestation. Furthermore most studies adjust birth weight for gestational age with a linear term, when fetal growth is non-linear throughout pregnancy which may induce bias in the final analysis.23
Given this background, we sought to determine whether maternal salivary hormones in pregnancy can predict birth weight-for-gestational age or risk for SGA or LGA. We hypothesized that higher levels of androgens (testosterone or DHEA) would be associated with lower birth weight-for-gestational age. We also hypothesized that higher progesterone or estradiol would be associated with higher birth weight-for-gestational age. These potential relationships would then reflect higher or lower risk for delivering an SGA or LGA infant.
METHODS
Study population
Participants included in this analysis are mother and infant pairs enrolled in the Programming Research in Obesity, Growth Environment and Social Stress (PROGRESS) birth cohort study. In 2007–2011, we enrolled 948 pregnant women through the Mexican Social Security System (IMSS), who delivered a live infant. Women were eligible if they were less than 20 weeks’ gestation, at least 18 years of age, and planned to reside in Mexico City for the next 3 years. Women were excluded if they had a history of heart or kidney disease, consumed alcohol on a daily basis, used steroids or anti-epilepsy drugs or had a multiple gestation pregnancy. All women provided, written, informed consent. A description of the recruitment process can be found in detail elsewhere.24, 25 This analysis focuses on the subset of women who had at least one salivary hormone measured and a documented infant birth (n=504). This study was approved by the IRB Committees at the participating institutions: National Institute of Public Health (INSP), Icahn School of Medicine at Mount Sinai (ISMMS), Harvard T.H Chan School of Public Health (HSPH) and the Brigham and Women’s Hospital.
Salivary hormones
Between 16 to 32 weeks of gestation (mean (SD): 18.3 (0.9)), women collected salivary samples into Salicaps (IBL International, Hamburg, Germany) using a passive drool technique described elsewhere in detail.24 Samples were frozen at −70°C and then shipped on dry ice for analysis. Hormones were measured using liquid chromatography mass spectrometry (LC-MS/MS). We measured progesterone, testosterone, estradiol, DHEA, and cortisone in each sample.
We considered a participant’s daily hormone concentration to be the geometric mean of 3 hormone measures collected longitudinally over 2 days. As part of our salivary cortisol protocol we collected saliva at 5 time points (upon awakening, 45 minutes after waking, 4 hours after awakening, 8 hours after awakening and bedtime). These time points were chosen to capture cortisol variation across the day. For this study, we focused on 3 time points (upon awakening, 45 minutes after awakening, and bedtime) to minimize cost by not using all 5 samples from the two consecutive days.24 To reduce the influence of outliers, we capped all values to between ±3SD from the population mean on the log scale using ±3SD as the minimum and maximum values.26 Seven to 13 outliers were reassigned for each hormone (0.5% to 0.9% of samples). In addition, for each participant, we excluded any hormone value that differed from the other two measurements of the same hormone by more than ±2SD on the log scale. We excluded 18 to 47 measurements for each hormone (1.4% to 3.1% of samples). Additionally, due to observed positive correlations of all hormones measured we normalized each participant’s hormone level by dividing it by the geometric mean of the other hormones measured. In this way we preserved the relationship of each hormone to the other hormones in the participant’s sample, thereby addressing issues such as hydration status. If a participant was missing one or more of the 5 salivary hormones, the population mean of that hormone was imputed for the purpose of normalization (34 to 47 measurements for each hormone). The final sample size for each hormone ranged from 482 to 504. We also calculated ratios of progesterone/estradiol and testosterone/estradiol ratios as these hormones are co-regulated and rise in pregnancy until just before parturition when estradiol increases.11, 12 Due to skewness we conducted analyses of log-transformed geometric means of hormones and hormone ratios.
Outcome and covariate ascertainment
We calculated birth-weight-for-gestational age z-scores based using the Fenton reference growth curve.23 We calculated gestational age based on the last menstrual period (LMP) and date of birth. We used a standard physical assessment (Capurro method)27 of gestational age when gestational age differed by more than 3 weeks from that assigned by the LMP.27, 28 Of the 508 participants, 21 gestational ages were reassigned based on the Capurro assessment.
We collected information on sociodemographic characteristics and maternal risk factors for poor fetal growth including age, education, socioeconomic status (SES), smoking, secondhand smoke exposure, and parity through standardized questionnaires given during the 2nd trimester. Maternal height and weight were measured by study staff during the 2nd trimester and used to calculate BMI (kg/m2), then categorized as follows to take into consideration weight gain during pregnancy; normal (<27 kg/m2), overweight (27–32 kg/m2) and obese (>32 kg/m2).29 The socioeconomic index was based on 13 questions related to the characteristics of the household which classifies families into six socioeconomic levels.30 For the purpose of this analysis these six levels were reduced to three categories to represent a low, medium and high socioeconomic status relative to the distribution of the study population.
Statistical Analysis
Descriptive statistics of the participants and salivary hormones are reported in table 1 and 2. We performed non-parametric test (Mann-Whitney U test) to compare differences in geometric means across birth weight-for-gestational age categories and regression models were run on log transformed salivary hormones and hormone ratios due to skewness. We used linear regression to evaluate the relationship between each interquartile range (IQR) increment of log-transformed hormone or log-transformed hormone ratio with birth weight-for-gestational age z-scores. We used modified Poisson regression to calculate risk ratios of SGA or LGA versus AGA per IQR increment of log-transformed hormone or log-transformed hormone ratio. We also tested for differences by analytical batch and gestational age at time of collection as potential confounding variables. To test for sex differences, we performed analyses with an interaction term and also stratified models by sex.
Table 1.
Maternal and infant characteristics by infant birth weight-for-gestational age category, PROGRESS birth cohort, Mexico City.
| Covariates | Overall (n=504) | SGA (n=75) | AGA (n=419) | LGA (n=10) |
|---|---|---|---|---|
| Maternal age (years) | n (%) | Row % | ||
| < 25 | 172 (34.1) | 15.1 | 83.1 | 1.7 |
| 25 – 34 | 269 (53.4) | 15.2 | 83.3 | 1.5 |
| ≥ 35 | 63 (12.5) | 12.7 | 82.5 | 4.8 |
| Maternal 2nd trimester | ||||
| BMI (kg/m2) | ||||
| Normal (< 27) | 283 (56.2) | 15.9 | 83.0 | 1.1 |
| Overweight (27 – 32) | 161 (31.9) | 14.3 | 81.4 | 4.4 |
| Obese (> 32) | 60 (11.9) | 11.7 | 88.3 | 0.0 |
| Parity | ||||
| Nulliparous | 240 (47.6) | 14.2 | 84.2 | 1.7 |
| Multiparous | 264 (52.4) | 15.5 | 82.2 | 2.3 |
| Secondhand smoke exposure | ||||
| No | 365 (72.4) | 15.1 | 82.5 | 2.5 |
| Yes | 139 (27.6) | 14.4 | 84.9 | 0.7 |
| Education | ||||
| < High school | 193 (38.3) | 18.1 | 79.8 | 2.1 |
| High school | 187 (37.1) | 15.0 | 83.4 | 1.6 |
| > High school | 124 (24.6) | 9.7 | 87.9 | 2.4 |
| SES index | ||||
| Low | 257 (51.0) | 16.3 | 81.7 | 2.0 |
| Medium | 186 (36.9) | 14.5 | 84.4 | 1.1 |
| High | 61 (12.1) | 9.8 | 85.3 | 4.9 |
| Infant sex | ||||
| Male | 248 (49.2) | 14.9 | 83.5 | 1.6 |
| Female | 256 (50.8) | 14.8 | 82.8 | 2.3 |
Abbreviations: SGA - small-for-gestational age, AGA - appropriate-for-gestational age and LGA - large-for-gestational age.
Table 2.
Maternal salivary hormone concentrations by infant birth weight-for-gestational age category
| Salivary hormones (ρg/mL) | Overall† | SGA | AGA | LGA |
|---|---|---|---|---|
| GM (SD) | GM (SD) | GM (SD) | GM (SD) | |
| Progesterone | 399.6 (447.1) | 435.5 (405.5) | 393.8 (457.2) | 370.1 (310.6) |
| Testosterone | 24.1 (31.6) | 30.4 (45.0) | 23.1 (28.7) | 20.5 (21.6) |
| Estradiol | 48.8 (54.8) | 52.7 (76.8) | 47.8 (49.6) | 59.8 (66.4) |
| DHEA | 169.1 (337.0) | 165.1 (255.1) | 171.1 (353.1) | 112.0 (122.3) |
| Cortisone | 6232.6 (3635.5) | 6262.1 (3430.1) | 6197.4 (3680.4) | 7487.2 (3315.4) |
| Ratios | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Progesterone /Estradiol | 11.8 (14.5) | 14.4 (15.0)* | 11.4 (14.6) | 7.9 (5.3) |
| Testosterone/Estradiol | 0.6 (0.5) | 0.7 (0.4)** | 0.6 (0.5) | 0.4 (0.4)* |
P-value<0.10
P-value<0.05, Mann-Whitney U test (rank score), SGA vs. AGA and LGA vs. AGA.
Number of participants ranged from 481 to 504 for each hormone and ratio.
RESULTS
Maternal and infant characteristics as well as proportions of SGA, AGA, and LGA are presented in Table 1. Just over half of the women were between 25–34 years (53.4%), had a normal BMI (<27kg/m2) (56.2%), were multiparous (52.4%), and had a lower SES index (51.0%). Approximately one third were exposed to secondhand smoke during pregnancy (27.6%) and had lower than high school education (38.3%). Mean (SD) birth weight-for-gestational age z-score was −0.4 (0.9), and more infants were SGA (15.0%) than LGA (2.0%). Compared to women who delivered an AGA or LGA infant, women who delivered an SGA infant tended to have less than high school education (p-value = 0.06) but were otherwise similar.
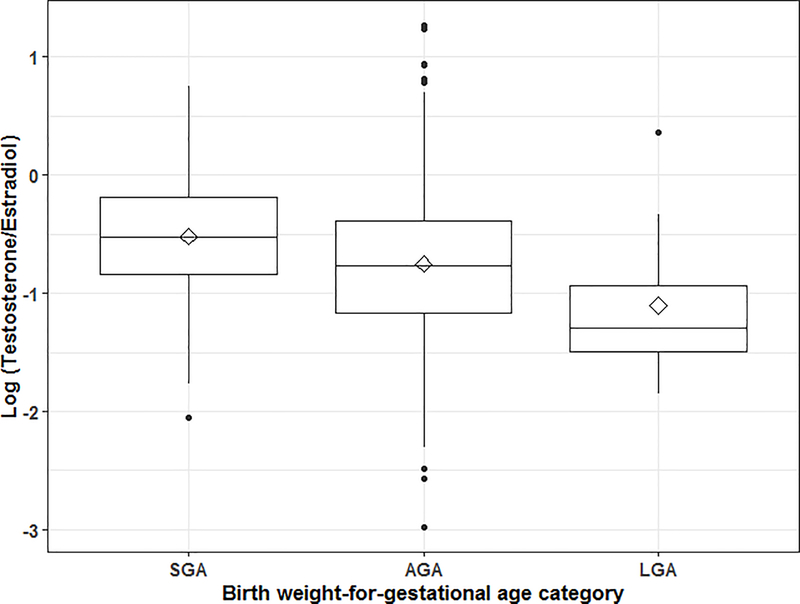
Across all salivary hormone measurements there were 13 outliers which were reassigned with the value of ±3SD from the population mean (0.5% to 0.9% of samples). We also excluded 18 to 47 measurements for each hormone which differed from the other two measurements of the same hormone (1.4% to 3.1% of samples). Salivary hormone levels did not vary by participant characteristics except for progesterone, estradiol and progesterone/estradiol ratio which were higher in women with male infants (Table 2). Lower levels of testosterone/estradiol ratios were observed among women not exposed to secondhand smoke. Women who were nulliparous and younger than 25 years of age had higher levels of DHEA. Women younger than 25 years also had higher cortisone levels (Table S2). Higher testosterone/estradiol ratios were observed among women who delivered an SGA infant compared to women who delivered an AGA or LGA infant (p-value < 0.002). We found slightly higher levels of progesterone, and progesterone/estradiol ratios among women with an SGA infant, but these differences were not statistically significant (Table 2).
Linear regression models revealed that higher testosterone/estradiol ratios were associated with lower birth-weight-for-gestational age z-scores (β: −0.11; 95%CI: −0.21, −0.02 per IQR of the hormone ratio) (Table 3 and Figure 1). This association remained significant after adjusting for maternal age, BMI, parity, secondhand smoke exposure, education, SES, and infant sex (β: −0.12; 95%CI: −0.22, −0.02). We did not find significant associations between birth-weight-for-gestational age z-scores and the other hormones or hormone ratios. No differences were found in our results when adjusting the models for analytical batch or gestational age at the time of saliva collection. Therefore, we did not include these variables in the final models. No differences were found between male and female infants when adding an interaction term with each hormone or in sex-stratified models.
Table 3.
Maternal salivary hormones in association with infant birth-weight-for-gestational age z-scores
| Unadjusted models | Adjusted models‡ | |||
|---|---|---|---|---|
| Log transformed normalized salivary hormones (median, IQR) | β† | 95% CI | β† | 95% CI |
| 1. Progesterone (2.42, 0.94) | 0.02 | −0.07, 0.11 | 0.02 | −0.07, 0.11 |
| 2. Testosterone (−1.12, 0.97) | −0.06 | −0.17, 0.04 | −0.07 | −0.17, 0.03 |
| 3. Estradiol (−0.13, 0.80) | 0.08 | −0.02, 0.18 | 0.08 | −0.02, 0.18 |
| 4. DHEA (1.04, 1.26) | −0.02 | −0.10, 0.05 | −0.02 | −0.09, 0.06 |
| 5. Cortisone (−2.44, 0.77) | −0.003 | −0.10, 0.09 | −0.003 | −0.10, 0.09 |
| Log ratios | ||||
| 1. Progesterone/Estradiol (2.05, 1.20) | −0.03 | −0.13, 0.06 | −0.03 | −0.12, 0.07 |
| 2. Testosterone/Estradiol (−0.73, 0.79) | −0.11 | −0.21, −0.02 | −0.12 | −0.22, −0.02 |
Beta coefficient per IQR increment of log transformed normalized hormones or ratios.
Adjusted by maternal age, BMI, parity, secondhand smoke exposure, education, SES, and child’s sex.
Figure 1.
Testosterone/Estradiol ratio by birth weight-for-gestational age categories
Modified Poisson regression models revealed that women with higher testosterone/estradiol ratios had a 50% increased risk of delivering an SGA infant per IQR of the hormone ratio (adjusted RR: 1.50; 95%CI: 1.13, 2.00). Women with higher testosterone/estradiol ratios also had a 38% decreased risk of delivering an LGA infant, even though there were only 10 LGA infants in our study and this difference was not statistically significant (adjusted RR: 0.62; 95% CI: 0.28, 1.38). No other hormones or ratios were associated with the risk of delivering an SGA or LGA infant (Table 4).
Table 4.
Maternal salivary hormones and risk of SGA and LGA†
| SGA vs. AGA‡ | LGA vs. AGA‡ | |||
|---|---|---|---|---|
| Log transformed normalized salivary hormones | RR | 95% CI | RR | 95% CI |
| Progesterone | 1.15 | 0.88–1.50 | 1.11 | 0.50–2.44 |
| Testosterone | 1.25 | 0.92–1.70 | 0.70 | 0.28–1.74 |
| Estradiol | 0.81 | 0.59–1.09 | 1.16 | 0.50–2.70 |
| DHEA | 0.97 | 0.77–1.20 | 1.01 | 0.54–1.87 |
| Cortisone | 0.97 | 0.74–1.28 | 1.28 | 0.60–2.74 |
| Log ratios | ||||
| Progesterone/Estradiol | 1.26 | 0.94–1.69 | 1.02 | 0.44–2.34 |
| Testosterone/Estradiol | 1.50 | 1.13–2.00 | 0.62 | 0.28–1.38 |
RR per IQR of log transformed hormones or hormone ratios.
Adjusted for maternal age, BMI, parity, secondhand smoke exposure, education, SES, and infant sex.
Participants included in this analysis (n=504) did not differ from rest of the participants in the cohort (n=444) with the exception of their being less likely to have been exposed to secondhand smoke (27.6% vs. 36.2%) and less likely to deliver preterm (9.9% vs. 15.3%) (Table S1). Very few women in our sample reported smoking during pregnancy (n=2) and secondhand smoke was more common (27.6%). Therefore, we performed a sensitivity analysis excluding women who smoked or were exposed to secondhand smoke during pregnancy, and results remained similar (adjusted RR for SGA = 1.53; 95% CI: 1.10, 2.14 per IQR log testosterone/estradiol ratio when we excluded smoke-exposed women versus 1.50; 95% CI: 1.13, 2.00 in the overall study sample). Because risk factors for SGA infants may vary according to gestational age5 and because there were fewer preterm births in our sample (9.9%) compared to the overall cohort (12.5%), we also performed a sensitivity analysis excluding infants born preterm, and results remained similar (adjusted RR for SGA = 1.48; 95% CI: 1.09, 2.01 per IQR log testosterone/estradiol ratio). In our dataset, only 5 infants were SGA and also premature, and testosterone/estradiol ratios did not differ between term and preterm infants.
DISCUSSION
We found that higher maternal salivary testosterone/estradiol ratios measured midpregnancy were associated with lower birth weight-for-gestational age z-scores and an increased risk of delivering an SGA infant. For each IQR increment of testosterone/estradiol ratios, birth weight-for-gestational age z-score was 0.12 lower. To put this finding into context, for a male infant born at 40 weeks of gestation with an average birth weight of 3368 grams (z-score of 0; 50th percentile), a change of 0.12 in birth weight z-score represents a decrease in birth weight of 50–60 grams and would place this male infant at the 46th percentile for birth weight. This is similar to the decrement of 36 grams in birth weight observed in a prior study of infants with prenatal secondhand smoke exposure and is approximately one third of the 168 gram decrement observed among infants born to women who smoke during pregnancy.31
Our main findings are consistent with the existing literature. One study of sheep demonstrated fetal growth retardation following prenatal testosterone treatments.14 Two human studies found higher maternal testosterone levels to be associated with lower birth weight. Carlsen et al., estimated that an increase from the 25th to the 75th percentile in testosterone levels during the 3rd trimester of pregnancy was associated with a decrease of 115 grams in birth weight and Voegtline et al., reported a 0.04 decrease in birth weight-for-gestational age z-scores per each pg/ml increment.17, 18 The finding of an association between higher testosterone and lower birthweight is consistent with the fact that women with clinical hyperandrogenism, for example, from polycystic ovarian syndrome (PCOS),32 have increased risk of delivering SGA infants. Similarly, women with preeclampsia also have increased risk of delivering SGA infants,33, 34 possibly due to impaired placental aromatase, which converts androgen into estrogens, and therefore results in an increase of circulating maternal testosterone levels.12, 35, 36 Our results also suggest that it may be the balance between testosterone and estradiol that impact fetal growth. Testosterone and estradiol seem to have the opposite effect on birth size as increased estradiol during pregnancy has been associated with larger birth size.21, 22 Hence, the combination of relatively higher testosterone and lower estradiol levels may be a better predictor of risk of an SGA infant than the individual components.
Alterations both in the fetal and maternal production of hormones have been shown to contribute to maladaptive fetal growth.12 It has been demonstrated that rodents administered testosterone during pregnancy have reduced fetal and placental weights suggesting that maternal testosterone has an effect on both placenta and fetus.37, 38 Testosterone is a lipophilic agent and can pass the placenta. 8 The existent literature is not clear whether maternal testosterone levels exert both a direct effect on fetal growth by increasing the testosterone levels in the fetus or indirectly through changes in placental and/or maternal metabolism. 8, 38 Our findings may be particularly important given the rise in environmental exposure to endocrine disrupting chemicals (EDCs) that impact hormone levels.39 For example, exposure to metals, such as arsenic and cadmium, has also been associated with placental expression of genes related to hormone secretion and SGA status.40, 41 Also, EDCs can inhibit human placental aromatase activity which produces estradiol from testosterone and stimulates placental growth.42 It may be that exposure to EDCs or metals during pregnancy contributes to maladaptive fetal growth by disrupting the maternal and placental hormone levels.43
We did not identify associations for progesterone, DHEA, or cortisone with birth size. Previous studies have demonstrated positive associations of maternal progesterone at 27 weeks’ gestation with birth weight and length.21, 22 Likewise, lower progesterone during the 1st trimester was associated with greater risk for low birth weight in a study of 131 mother and infant pairs.44 However, these studies only evaluated birth weight and not birth weight-for-gestational age. We examined birth weight-for-gestational age which is a more comprehensive way to evaluate size at birth because it accounts for infant’s sex, length of gestation and the non-linearity of fetal growth.23 Two previous studies found that SGA infants had higher DHEA levels at two years of life and in adulthood when compared to children born appropriate-for-gestational age (AGA).19, 45 It could be that disruption of certain maternal hormones during pregnancy have long-term effects through intrauterine fetal programming (perhaps via epigenetic mechanisms) that could result in later life health effects.8 We did not observe sex-specific differences in the association between hormones or hormone ratios and birth weight-for-gestational age. This is consistent with a study in sheep14 and one human study which did not report any differences between male and female offspring in the association between maternal testosterone levels during pregnancy and lower birth weight.17 However, the literature has mixed findings with two other studies that have reported differences by infant sex in the relationship between hormones and birth weight.18, 20
Our sample has a limited number of LGA infants, so while we observed higher testosterone/estradiol ratio to be associated with lower risk of having an LGA infant, effect estimates were imprecise. Generalizability to LGA babies is limited. While we collected multiple samples throughout the day, we only collected samples at one time-point during pregnancy. There is evidence that some hormones increase in the second half of gestation.11 In our study the mean (sd) gestational age at saliva collection was 18.4 ± 1.7 weeks and most participants (98%) provided saliva samples before or at 19 week of gestation. Nonetheless, six participants who provided saliva samples in their 3rd trimester of pregnancy did not have outlying testosterone levels (GM (SD): 14.1 (11.3)), estradiol levels (GM (SD): 66.3 (74.3)), or birth weight-for-gestational age (mean (SD): −0.66 (0.54)). Therefore, the differences in timing of measuring testosterone and estradiol in our sample were unlikely to affect our main findings. We are unable to determine if hormone levels at other time points in pregnancy or changes in hormone levels throughout pregnancy affects the risk of SGA. More frequent prenatal salivary collections in future studies may help to identify critical windows during which the fetus may be most susceptible to hormonal changes. While we do not have data on maternal hormone treatment during pregnancy, this was a population-based cohort of women seeking routine prenatal care and in overall healthy pregnancies at the time of recruitment. The possibility still exists that a woman developed a high-risk pregnancy after recruitment and received hormone treatment. On the other hand, the most common hormone that is administered in pregnancy is progesterone which was not associated with birth weight-for-gestational age in our study.46
There are limited data on normative values of salivary hormones during pregnancy. Our study sample had lower levels of testosterone and progesterone but similar levels of DHEA compared to a prior study of 28 healthy pregnant women in which salivary hormones were measured 3 weeks before delivery.10 Levels of progesterone in our study were similar and estradiol was higher compared to another study which measured levels in pregnant women at 24 weeks’ gestation.47 Also, our participants’ samples had lower testosterone levels than those measured at 36 weeks’ gestation in a previous study analyzing associations with birth size, specifically 24.1 versus 97.1 (ρg/mL).18 These differences may be explained by the fact that sex steroids tend to increase during pregnancy with the highest levels just before delivery, and we measured salivary hormones earlier in pregnancy.10, 47 The discrepancy may also be explained by differences in sex steroids by ethnicity. For example, US Hispanic and African-American women have higher androgen levels as compared to White women.48 Hormone levels can also vary according to maternal characteristics such as stress, diet, or BMI.49 It would be important for future studies to address the gap of normative salivary androgens and estrogens concentrations throughout pregnancy in a population-wide study while also identifying predictors of those concentrations such as maternal and fetal characteristics.
Strengths of our study include that it was conducted in a large prospective cohort with information on multiple potential covariates. Three salivary samples were measured from each participant, and we applied a careful pre-processing of the data to consider variations within and between participants’ hormone values. Most previous studies on hormones and birth weight-for-gestation have been smaller in sample size varying from 50 to 300 participants, and have focused on birth weight without considering gestational age at birth.
In conclusion, we found that higher maternal testosterone/estradiol ratios during pregnancy were associated with lower birth weight-for-gestational age and increased risk of SGA. Understanding the determinants of hormone levels during pregnancy such as maternal and fetal characteristics, and external factors such as environmental exposures is warranted. If our results are replicated, determining the utility of measuring testosterone/estradiol ratios to predict SGA may be clinically important.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grants R01 ES014930, R01 ES026033, R01 ES021357, R24 ES028522, R01 ES013744, R01 ES023450, P30 ES023515, K99ES027508, K23ES024803, K23ES022242. Co-Investigators and staff at the INSP were also supported and received partial funding from the National Institute of Public Health/Ministry of Health of Mexico. We acknowledge Dr. Clemens Kirschbaum for performing the steroid hormone panel on the saliva samples. We acknowledge the American British Cowdray Medical Center for providing research facilities which made it possible to conduct the study.
Abbreviations:
- SGA
small-for-gestational age
- AGA
appropriate-for-gestational age
- LGA
large-for-gestational age
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
‘Supplementary information is available at JPER’s website’
REFERENCES
- 1.Pallotto EK, Kilbride HW. Perinatal outcome and later implications of intrauterine growth restriction. Clinical obstetrics and gynecology 2006, 49(2): 257–269. [DOI] [PubMed] [Google Scholar]
- 2.Larroque B, Bertrais S, Czernichow P, Léger J. School difficulties in 20-year-olds who were born small for gestational age at term in a regional cohort study. Pediatrics 2001, 108(1): 111–115. [DOI] [PubMed] [Google Scholar]
- 3.Strauss RS. Adult functional outcome of those born small for gestational age: twenty-six-year follow-up of the 1970 British Birth Cohort. Jama 2000, 283(5): 625–632. [DOI] [PubMed] [Google Scholar]
- 4.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005, 115(3): e290–296. [DOI] [PubMed] [Google Scholar]
- 5.Clausson B, Cnattingius S, Axelsson O. Preterm and term births of small for gestational age infants: a population-based study of risk factors among nulliparous women. British journal of obstetrics and gynaecology 1998, 105(9): 1011–1017. [DOI] [PubMed] [Google Scholar]
- 6.Jolly MC, Sebire NJ, Harris JP, Regan L, Robinson S. Risk factors for macrosomia and its clinical consequences: a study of 350,311 pregnancies. European Journal of Obstetrics and Gynecology and Reproductive Biology 2003, 111(1): 9–14. [DOI] [PubMed] [Google Scholar]
- 7.Surkan PJ, Hsieh C-C, Johansson ALV, Dickman PW, Cnattingius S. Reasons for Increasing Trends in Large for Gestational Age Births. Obstetrics & Gynecology 2004, 104(4): 720–726. [DOI] [PubMed] [Google Scholar]
- 8.Fowden A, Forhead A. Endocrine mechanisms of intrauterine programming. Reproduction 2004, 127(5): 515–526. [DOI] [PubMed] [Google Scholar]
- 9.Fowden AL, Forhead AJ, Sferruzzi-Perri AN, Burton GJ, Vaughan OR. Review: Endocrine regulation of placental phenotype. Placenta 2015, 36 Suppl 1: S50–59. [DOI] [PubMed] [Google Scholar]
- 10.Hampson E, Phillips SD, Soares CN, Steiner M. Steroid concentrations in antepartum and postpartum saliva: normative values in women and correlations with serum. Biology of sex differences 2013, 4(1): 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darne J, McGarrigle HH, Lachelin GC. Saliva oestriol, oestradiol, oestrone and progesterone levels in pregnancy: spontaneous labour at term is preceded by a rise in the saliva oestriol:progesterone ratio. British journal of obstetrics and gynaecology 1987, 94(3): 227–235. [DOI] [PubMed] [Google Scholar]
- 12.Hakim C, Padmanabhan V, Vyas AK. Gestational Hyperandrogenism in Developmental Programming. Endocrinology 2017, 158(2): 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makieva S, Saunders PT, Norman JE. Androgens in pregnancy: roles in parturition. Human reproduction update 2014, 20(4): 542–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, et al. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology 2004, 145(2): 790–798. [DOI] [PubMed] [Google Scholar]
- 15.Wolf CJ, Hotchkiss A, Ostby JS, LeBlanc GA, Gray JLE. Effects of Prenatal Testosterone Propionate on the Sexual Development of Male and Female Rats: A Dose-Response Study. Toxicological Sciences 2002, 65(1): 71–86. [DOI] [PubMed] [Google Scholar]
- 16.Herman RA, Jones B, Mann DR, Wallen K. Timing of prenatal androgen exposure: anatomical and endocrine effects on juvenile male and female rhesus monkeys. Hormones and behavior 2000, 38(1): 52–66. [DOI] [PubMed] [Google Scholar]
- 17.Carlsen SM, Jacobsen G, Romundstad P. Maternal testosterone levels during pregnancy are associated with offspring size at birth. European journal of endocrinology 2006, 155(2): 365–370. [DOI] [PubMed] [Google Scholar]
- 18.Voegtline KM, Costigan KA, Kivlighan KT, Henderson JL, DiPietro JA. Sex-specific associations of maternal prenatal testosterone levels with birth weight and weight gain in infancy. Journal of developmental origins of health and disease 2013, 4(4): 280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nordman H, Voutilainen R, Antikainen L, Jaaskelainen J. Prepubertal children born large for gestational age have lower serum DHEAS concentrations than those with a lower birth weight. Pediatric research 2017, 82(2): 285–289. [DOI] [PubMed] [Google Scholar]
- 20.Hartwig IR, Pincus MK, Diemert A, Hecher K, Arck PC. Sex-specific effect of first-trimester maternal progesterone on birthweight. Human reproduction (Oxford, England) 2013, 28(1): 77–86. [DOI] [PubMed] [Google Scholar]
- 21.Mucci LA, Lagiou P, Tamimi RM, Hsieh C-C, Adami H-O, Trichopoulos D. Pregnancy estriol, estradiol, progesterone and prolactin in relation to birth weight and other birth size variables (United States). Cancer Causes & Control 2003, 14(4): 311–318. [DOI] [PubMed] [Google Scholar]
- 22.Lagiou P, Samoli E, Hsieh CC, Lagiou A, Xu B, Yu GP, et al. Maternal and cord blood hormones in relation to birth size. European journal of epidemiology 2014, 29(5): 343–351. [DOI] [PubMed] [Google Scholar]
- 23.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC pediatrics 2013, 13: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braun JM, Wright RJ, Just AC, Power MC, Tamayo YOM, Schnaas L, et al. Relationships between lead biomarkers and diurnal salivary cortisol indices in pregnant women from Mexico City: a cross-sectional study. Environmental health : a global access science source 2014, 13(1): 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burris HH, Braun JM, Byun HM, Tarantini L, Mercado A, Wright RJ, et al. Association between birth weight and DNA methylation of IGF2, glucocorticoid receptor and repetitive elements LINE-1 and Alu. Epigenomics 2013, 5(3): 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilcox RR. Estimating measure of location and scale In: Wilcox RR (ed). Introduction to robust estimation and hypothesis testing Elsevier Inc: Waltham, 2012, pp 43–101. [Google Scholar]
- 27.Capurro H, Konichezky S, Fonseca D, Caldeyro-Barcia R. A simplified method for diagnosis of gestational age in the newborn infant. The Journal of pediatrics 1978, 93(1): 120–122. [DOI] [PubMed] [Google Scholar]
- 28.Sanders AP, Burris HH, Just AC, Motta V, Svensson K, Mercado-Garcia A, et al. microRNA expression in the cervix during pregnancy is associated with length of gestation. Epigenetics 2015, 10(3): 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Institute of Medicine National Research Council Committee to Reexamine IOMPWG. The National Academies Collection: Reports funded by National Institutes of Health In: Rasmussen KM, Yaktine AL (eds). Weight Gain During Pregnancy: Reexamining the Guidelines. National Academies Press (US) National Academy of Sciences.: Washington (DC), 2009. [PubMed] [Google Scholar]
- 30.Carrasco A The Amai system of classifying households by socio-economic level: the experience of Mexico and its comparison with Brazil and Argentina. ESOMAR 2002. [Google Scholar]
- 31.Ward C, Lewis S, Coleman T. Prevalence of maternal smoking and environmental tobacco smoke exposure during pregnancy and impact on birth weight: retrospective study using Millennium Cohort. BMC Public Health 2007, 7(1): 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sir-Petermann T, Hitchsfeld C, Maliqueo M, Codner E, Echiburu B, Gazitua R, et al. Birth weight in offspring of mothers with polycystic ovarian syndrome. Human reproduction (Oxford, England) 2005, 20(8): 2122–2126. [DOI] [PubMed] [Google Scholar]
- 33.Salamalekis E, Bakas P, Vitoratos N, Eleptheriadis M, Creatsas G. Androgen levels in the third trimester of pregnancy in patients with preeclampsia. European Journal of Obstetrics & Gynecology and Reproductive Biology 2006, 126(1): 16–19. [DOI] [PubMed] [Google Scholar]
- 34.McCowan L, Horgan RP. Risk factors for small for gestational age infants. Best practice & research Clinical obstetrics & gynaecology 2009, 23(6): 779–793. [DOI] [PubMed] [Google Scholar]
- 35.Perez-Sepulveda A, Monteiro LJ, Dobierzewska A, Espana-Perrot PP, Venegas-Araneda P, Guzman-Rojas AM, et al. Placental Aromatase Is Deficient in Placental Ischemia and Preeclampsia. PloS one 2015, 10(10): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Perez-Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Human reproduction (Oxford, England) 2002, 17(10): 2573–2579. [DOI] [PubMed] [Google Scholar]
- 37.Sathishkumar K, Elkins R, Chinnathambi V, Gao H, Hankins GD, Yallampalli C. Prenatal testosterone-induced fetal growth restriction is associated with down-regulation of rat placental amino acid transport. Reproductive biology and endocrinology : RB&E 2011, 9: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun M, Maliqueo M, Benrick A, Johansson J, Shao R, Hou L, et al. Maternal androgen excess reduces placental and fetal weights, increases placental steroidogenesis, and leads to long-term health effects in their female offspring. American journal of physiology Endocrinology and metabolism 2012, 303(11): E1373–1385. [DOI] [PubMed] [Google Scholar]
- 39.Craig ZR, Wang W, Flaws JA. Endocrine-disrupting chemicals in ovarian function: effects on steroidogenesis, metabolism and nuclear receptor signaling. Reproduction 2011, 142(5): 633–646. [DOI] [PubMed] [Google Scholar]
- 40.Deyssenroth MA, Gennings C, Liu SH, Peng S, Hao K, Lambertini L, et al. Intrauterine multi-metal exposure is associated with reduced fetal growth through modulation of the placental gene network. Environment international 2018, 120: 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Everson TM, Kappil M, Hao K, Jackson BP, Punshon T, Karagas MR, et al. Maternal exposure to selenium and cadmium, fetal growth, and placental expression of steroidogenic and apoptotic genes. Environmental research 2017, 158: 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen L, Chen X, Chen X, Hu Z, Li X, Su Y, et al. Ziram inhibits aromatase activity in human placenta and JEG-3 cell line. Steroids 2017, 128: 114–119. [DOI] [PubMed] [Google Scholar]
- 43.Veiga-Lopez A, Kannan K, Liao C, Ye W, Domino SE, Padmanabhan V. Gender-Specific Effects on Gestational Length and Birth Weight by Early Pregnancy BPA Exposure. The Journal of clinical endocrinology and metabolism 2015, 100(11): E1394–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He S, Allen JC Jr., Malhotra R, Ostbye T, Tan TC. Association of maternal serum progesterone in early pregnancy with low birth weight and other adverse pregnancy outcomes. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 2016, 29(12): 1999–2004. [DOI] [PubMed] [Google Scholar]
- 45.Meuwese CL, Euser AM, Ballieux BE, van Vliet HA, Finken MJ, Walther FJ, et al. Growth-restricted preterm newborns are predisposed to functional adrenal hyperandrogenism in adult life. European journal of endocrinology 2010, 163(4): 681–689. [DOI] [PubMed] [Google Scholar]
- 46.Rode L, Langhoff-Roos J, Andersson C, Dinesen J, Hammerum MS, Mohapeloa H, et al. Systematic review of progesterone for the prevention of preterm birth in singleton pregnancies. Acta Obstetricia et Gynecologica Scandinavica 2009, 88(11): 1180–1189. [DOI] [PubMed] [Google Scholar]
- 47.Vanston CWN, Zava DT. Sex steroid monitoring during pregnancy using a saliva test.. Seattle: Nurse Practitioners in Women’s Health 11th Annual Premiere Women’s Health Conference; 2008. [Google Scholar]
- 48.Potischman N, Troisi R, Thadhani R, Hoover RN, Dodd K, Davis WW, et al. Pregnancy hormone concentrations across ethnic groups: implications for later cancer risk. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2005, 14(6): 1514–1520. [DOI] [PubMed] [Google Scholar]
- 49.Sferruzzi-Perri AN, Sandovici I, Constancia M, Fowden AL. Placental phenotype and the insulin-like growth factors: resource allocation to fetal growth. The Journal of physiology 2017, 595(15): 5057–5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.