Abstract
Objective(s):
To identify predictors of home oxygen use in preterm infants with bronchopulmonary dysplasia in a statewide cohort, identify hospital variation in home oxygen use, and determine the relationship between home oxygen use and NICU discharge timing.
Study design:
This was a secondary analysis of California Perinatal Quality Care Collaborative data. Infants were born <32 weeks’ gestation, diagnosed with bronchopulmonary dysplasia based on respiratory support at 36 weeks postmenstrual age, and discharged home. Risk factors for home oxygen use were identified using a logistic mixed model with center as random effect. Estimates were used to calculate each center’s observed to expected ratio of home oxygen use, and a Spearman coefficient between center median postmenstrual age at discharge and observed and expected proportions of home oxygen use.
Results:
3672/7846 (47%) infants with bronchopulmonary dysplasia were discharged with home oxygen. Higher odds of home oxygen use were seen with antenatal steroids, maternal hypertension, earlier gestational age, male sex, ductus arteriosus ligation, more ventilator days, nitric oxide, discharge from regional hospitals, and postmenstrual age at discharge (ROC area under the curve 0.85). Of 92 hospitals, home oxygen use ranged from 7–95%; 42% of observed home oxygen use was significantly higher or lower than expected given patient characteristics. The 67 community hospitals with higher observed rates of home oxygen had earlier median postmenstrual age at discharge (correlation −0.27, p=0.024).
Conclusion:
Clinical and hospital factors predict home oxygen use. Home oxygen use varies across California, with community centers using more home oxygen having a shorter length of stay.
Keywords: bronchopulmonary dysplasia, preterm, neonatal intensive care
Bronchopulmonary dysplasia (BPD) is a common complication of prematurity, occurring in up to 50% of very-low-birth-weight infants.1–3 Infants with more severe lung disease are at higher risk of adverse outcomes.4 For this reason, BPD is the major cause of prolonged length of stay for premature infants in the neonatal intensive care unit (NICU).5 Because the prevalence of BPD is known to vary widely across institutions, it is likely that the care of infants with BPD varies widely as well.6–8
Home oxygen is common therapy for infants with BPD, used in 1–37% of infants in the NICHD Neonatal Research Network.9, 10 A study from the Pediatrix Medical Group reported wide variation in use of home oxygen therapy at NICU discharge, ranging from 7–95% of infants with an oxygen requirement at 36 weeks post-menstrual age (PMA), which was significantly more variation than had been reported previously.11 Since that analysis, the 2010 publication of the SUPPORT trial suggested lower mortality at higher oxygen saturation targets, and home oxygen use may have become more common and less variable after that point.12 A population-based cohort encompassing both regional referral hospitals and community NICUs of all levels would offer more generalizable insights into how home oxygen therapy is used nationally. Home oxygen is a major component of post-NICU respiratory morbidity; understanding variation in its use will be important for patient counseling, identifying biomarkers predicting disease, and designing interventions.13 Specifically, the contribution of home oxygen use to NICU length of stay in a population-based cohort of preterm infants with BPD has not been assessed.
The objectives of this study were to use a population-based cohort to identify significant predictors of home oxygen use in preterm infants with BPD; determine the extent of hospital variation in home oxygen use in a statewide cohort; and determine the relationship between hospital home oxygen use and timing of NICU discharge.
Methods
This was a secondary analysis using the California Perinatal Quality Care Collaborative (CPQCC) database, which includes NICUs of all types in California. The CPQCC collects clinical data on infants ≤1500 grams at birth or <32 weeks’ gestational age in member hospitals with definitions aligned to those of the Vermont Oxford Network.14 We included infants in the database between January 2008 and December 2016 who were born between 22–31 weeks completed gestational age, diagnosed with BPD based on any respiratory support requirement at 36 weeks PMA, and discharged home alive from a CPQCC hospital. Infants with major congenital anomalies and those who were discharged from a non-CPQCC, currently closed, or unknown hospital were excluded.
The primary outcome was home oxygen at NICU discharge, which was a binary variable. Maternal characteristics included age, race/ethnicity, prenatal care, receipt of antenatal steroids, cesarean delivery, spontaneous labor, multiple births, diabetes mellitus, and hypertension. These variables are obtained from the medical record of the neonate by trained data abstractors. Neonatal characteristics included birth weight, gestational age by best obstetric estimate, small for gestational age, growth curves, number of transfers between hospitals, whether a hospital transfer happened within 7 days of birth, sex, Apgar score, surfactant use, post-delivery room respiratory support, days on mechanical ventilator, patent ductus arteriosus ligation, nitric oxide use, nosocomial infection, human milk for enteral feeding at discharge to home, and birth year. Hospital characteristics included the birth and discharge hospital NICU level of care as determined by California Children’s Services (CCS), average NICU volume, and proportion of infants in discharging hospital with BPD.
Statistical analyses
First, we performed bivariate analyses to determine how home oxygen use varied with maternal, neonatal, and hospital characteristics. Next, given the hierarchical data structure with infants nested within hospitals, we assessed which risk factors were associated with home oxygen use by using a logistic mixed model with discharging center as a random intercept. Risk factors with bivariate significance of p≤0.1 were entered in groups: first maternal risk factors, then infant illness, then hospital factors. Because of the common outcome of home oxygen use, other modeling strategies were considered including log binomial and Poisson mixed models. However, the Poisson model resulted in underdispersion and the log binomial did not converge. Instead, odds ratios from the logistic mixed model were converted to relative risk using the method described by Zhang and Yu.15 A P value < .05 was considered statistically significant. A C statistic was used to check the fit of the fixed effects portion of the model, and a receiver operating characteristic area under the curve was used to evaluate the fit of the overall model.
From the regression model, we calculated each hospital’s expected proportion of home oxygen use based on the fixed effects portion of the regression model, and compared it with that hospital’s observed proportion of home oxygen use in the raw data (O/E ratio). This was done for hospitals with at least 15 infants with BPD discharged during the study period, to ensure that comparisons were based on stable estimates. We then divided hospitals into community and regional NICUs because of anticipated differences in unmeasured patient characteristics that may not have been captured in the regression model. For each hospital type we calculated a Spearman rank correlation between each center’s median PMA at discharge and their observed and expected proportion of home oxygen use. We also divided centers in thirds into low, medium and high home oxygen use groups, and calculated the median discharge PMA in each group using a Kruskal-Wallis test for significance.
SAS version 9.4 (SAS Institute, Cary, NC) was used for all analyses. This study was approved by the Stanford University Institutional Review Board.
Results
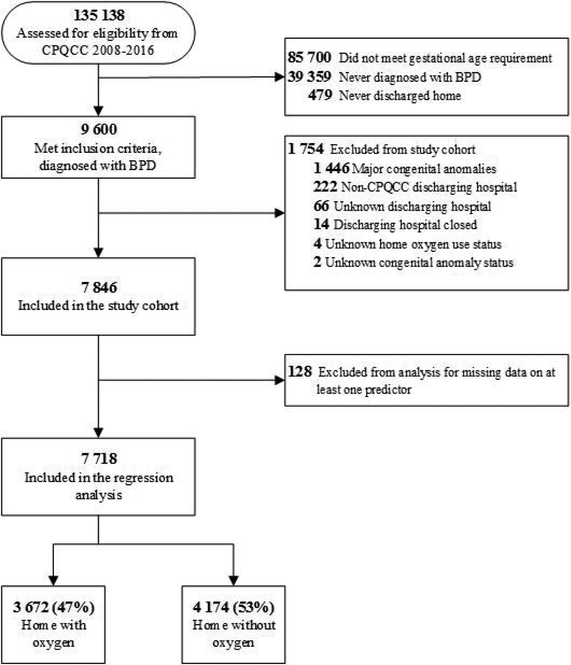
Out of 135,138 infants assessed for eligibility from the CPQCC data from 2008–16, 9600 infants met inclusion criteria of gestational age, diagnosis of BPD, and discharge home. After exclusions, the final study cohort included 7846 infants with BPD, of which 3672 (47%) were discharged with home oxygen and 4174 (53%) were discharged home in room air (Figure 1).
Figure I.
Study cohort flow diagram. Abbreviations: CPQCC, California Perinatal Quality Care Collaborative; BPD, bronchopulmonary dysplasia
Table I shows a bivariate analysis of characteristics of infants with BPD discharged with vs without home oxygen. For maternal characteristics, home oxygen use was more common in infants born to black mothers and those born vaginally, following spontaneous labor or maternal bleeding, placental abruption or placenta previa. For neonatal characteristics, a higher proportion of home oxygen use was associated with lower birth weight, earlier gestational age, more transfers prior to discharge, transfer within 7 days of birth, nosocomial infection, more days requiring mechanical ventilation, surfactant use, patent ductus arteriosus (PDA) ligation, nitric oxide use, and no breast milk at discharge. For hospital characteristics, higher proportions of home oxygen use were seen for infants born in non-CCS or regional hospitals. Home oxygen use varied by discharging center volume and proportion of infants with BPD in a non-linear fashion, with the lowest and highest centiles having the highest proportion of home oxygen use. Home oxygen use for infants with BPD decreased over time from 2008–2011, and then increased from 2011–2016.
Table I.
Maternal, infant and hospital factors associated with home oxygen use in California 2008 – 2016.*
| Factor | Level | n | Oxygen at Discharge (%) | P-value |
|---|---|---|---|---|
| MATERNAL FACTORS | ||||
| Maternal race and ethnicity | Black | 996 | 51% | 0.0273 |
| Hispanic | 3707 | 47% | ||
| White | 2000 | 44% | ||
| Asian, Native Hawaiian, Pacific Islander | 910 | 47% | ||
| Native American or Other | 200 | 46% | ||
| Cesarean delivery | Yes | 5829 | 46% | 0.0259 |
| No | 2017 | 49% | ||
| Spontaneous labor | Yes | 5359 | 48% | 0.0001 |
| No | 2471 | 44% | ||
| Bleeding / Abruption/ Previa | Yes | 1808 | 51% | <0.0001 |
| No | 6031 | 46% | ||
| INFANT FACTORS | ||||
| Birth weight | 500g or less | 207 | 63% | <0.0001 |
| 501 – 750g | 2521 | 57% | ||
| 751 – l000g | 2688 | 44% | ||
| 1001 – 1250g | 1415 | 37% | ||
| 1251 – 1500g | 682 | 35% | ||
| 1501g + | 333 | 38% | ||
| Gestational age | 24 or less | 1640 | 61% | <0.0001 |
| 25 | 1319 | 53% | ||
| 26 | 1312 | 48% | ||
| 27 | 1117 | 40% | ||
| 28 | 949 | 39% | ||
| 29 | 667 | 36% | ||
| 30 | 513 | 35% | ||
| 31 | 329 | 30% | ||
| Number of transfers before discharging to home | 0 | 5270 | 36% | <0.0001 |
| 1 | 1589 | 65% | ||
| 2 | 847 | 72% | ||
| 3 + | 140 | 84% | ||
| Transfer happened within 7 days of birth | Yes | 1327 | 63% | <0.0001 |
| No | 6459 | 43% | ||
| Nosocomial infection | Yes | 1675 | 56% | <0.0001 |
| No | 6166 | 44% | ||
| NICU length of stay | 79 Days Or Less | 2005 | 38% | <0.0001 |
| 80 – 98 | 2003 | 40% | ||
| 99 – 119 | 1877 | 49% | ||
| 120 + | 1961 | 61% | ||
| Days on ventilator | 4 Or Less | 2752 | 33% | <0.0001 |
| 5 – 16 | 1696 | 45% | ||
| 17 – 35 | 1703 | 50% | ||
| 36 + | 1695 | 67% | ||
| Breast milk provided at discharge | Yes | 4458 | 45% | 0.0029 |
| No | 3387 | 49% | ||
| Surfactant use | Yes | 6819 | 49% | <0.0001 |
| No | 1022 | 34% | ||
| PDA ligation | Yes | 1853 | 62% | <0.0001 |
| No | 5986 | 42% | ||
| NEC | Yes | 549 | 58% | <0.0001 |
| No | 7296 | 46% | ||
| Nitric oxide use | Yes | 550 | 59% | <0.0001 |
| No | 7295 | 46% | ||
| Delivery room oxygen support | None | 134 | 33% | <0.0001 |
| Oxygen/CPAP | 1033 | 35% | ||
| Bag/Mask/NIPPV | 1386 | 42% | ||
| Endotracheal Intubation | 4541 | 50% | ||
| Epinephrine or Cardiac Medications | 752 | 56% | ||
| Post delivery room respiratory support | Oxygen/High Flow Nasal Cannula | 108 | 23% | <0.0001 |
| CPAP | 374 | 31% | ||
| NIPPV | 235 | 26% | ||
| Intubated Mechanical Ventilation | 3179 | 39% | ||
| Oscillator | 3950 | 56% | ||
| PMA in weeks at discharge | 38 Or Less | 1939 | 45% | <0.0001 |
| 39 – 40 | 2176 | 38% | ||
| 41 – 42 | 1497 | 42% | ||
| 43 + | 1766 | 56% | ||
| HOSPITAL FACTORS | ||||
| Birth NICU CCS level | Non-CCS or Intermediate | 1214 | 55% | <0.0001 |
| Community | 4664 | 46% | ||
| Regional | 1968 | 44% | ||
| Discharging NICU CCS level | Non-CCS or Intermediate | 243 | 49% | <0.0001 |
| Community | 4464 | 42% | ||
| Regional | 3139 | 54% | ||
| Discharging NICU average volume (hospital quintiles) | 47 Or Less | 240 | 55% | <0.0001 |
| 48 – 75 | 536 | 34% | ||
| 76 – 107 | 1042 | 41% | ||
| 108 – 189 | 2040 | 48% | ||
| 190 + | 3988 | 49% | ||
| % BPD patients discharged across years (hospital quartiles) | 11.9% or Less | 2127 | 48% | <0.0001 |
| 11.9 – 14.5% | 1849 | 36% | ||
| 14.5 – 16.8% | 1962 | 50% | ||
| 16.8 + | 1908 | 53% | ||
| Birth Year | 2008 | 455 | 50% | <0.0001 |
| 2009 | 426 | 48% | ||
| 2010 | 444 | 47% | ||
| 2011 | 339 | 42% | ||
| 2012 | 362 | 42% | ||
| 2013 | 380 | 44% | ||
| 2014 | 438 | 47% | ||
| 2015 | 422 | 53% | ||
| 2016 | 406 | 49% | ||
Table 1 presents factors which were significantly associated with home oxygen use in bivariate analysis. Other factors considered that were not significant at p<0.05 level included prenatal care, antenatal steroids, gestational diabetes, maternal hypertension, multiple pregnancy, maternal age, small for gestational age, sex.
Abbreviations: NICU, neonatal intensive care unit; PDA, patent ductus arteriosus; CPAP, continuous positive airway pressure; NIPPV, nasal intermittent positive pressure ventilation; CCS, California children’s services; BPD, bronchopulmonary dysplasia; PMA, post-menstrual age.
Table 2 shows results from multivariable analyses. Statistically significant higher likelihood of home oxygen use was seen with antenatal steroids, maternal hypertension, earlier gestational age, male sex, PDA ligation, nitric oxide use, and more ventilator days. Infants transferred from their birth hospital to another facility within 7 days of birth, or discharged from a regional hospital were more likely to be discharged with home oxygen, adjusted for both clinical characteristics and center. Infants with the earliest and latest PMA at discharge were more likely to be discharged with home oxygen, adjusted for other characteristics. Birth year was a significant contributor to the overall model, although most individual year estimates did not significantly differ from reference. The fixed effects version of the final model had a Hosmer Lemeshow goodness of fit test p value of 0.5, the C statistic for the was >0.70, and the multilevel model had a receiver operating characteristic area under the curve of 0.85, indicating good model fit.
Table II.
Multivariate analysis of characteristics associated with home oxygen use.
| Factor | Level | Adjusted relative risk (95% confidence interval) | p value |
|---|---|---|---|
| Antenatal steroids | No | Reference | 0.0294 |
| Yes | 1.11 (1.01, 1.21) | ||
| Maternal hypertension | No | Reference | 0.0007 |
| Yes | 1.13 (1.05, 1.2) | ||
| Gestational age in weeks | 31 | Reference | <0.0001 |
| 30 | 1.13 (1.09, 1.21) | ||
| 29 | 1.07 (1.02, 1.17) | ||
| 28 | 1.18 (1.10,1.32) | ||
| 27 | 1.15 (1.06, 1.28) | ||
| 26 | 1.39 (1.25,1.60) | ||
| 25 | 1.43 (1.27, 1.66) | ||
| 24 or less | 1.49 (1.23, 1.89) | ||
| Sex | Female | Reference | 0.004 |
| Male | 1.09 (1.03,1.16) | ||
| PDA ligation | No | Reference | <0.0001 |
| Yes | 1.23 (1.14, 1.32) | ||
| Total days on ventilator (quartiles) | 4 or less | Reference | <0.0001 |
| 5 – 16 | 1.38 (1.26,1.5) | ||
| 17 – 35 | 1.48 (1.35, 1.62) | ||
| 36 + | 2.02 (1.87,2.15) | ||
| Nitric oxide use | No | Reference | <0.0001 |
| Yes | 1.3 (1.17, 1.42) | ||
| PMA at discharge in weeks | 38 or less | Reference | <0.0001 |
| 39 – 40 | 0.88 (0.88, 0.89) | ||
| 41 – 42 | 0.88 (0.88, 0.89) | ||
| 43 + | 1.05 (0.97, 1.15) | ||
| Discharging NICU CCS level | Community | Reference | <0.0001 |
| Non CCS or | |||
| Intermediate | 1.46 (1.17, 1.65) | ||
| Regional | 1.92 (2.44, 1.52) | ||
| Birth Year | 2008 | Reference | 0.0072 |
| 2009 | 0.95 (0.83, 1.08) | ||
| 2010 | 1 (0.87, 1.12) | ||
| 2011 | 0.86 (0.73, 0.99) | ||
| 2012 | 0.88 (0.75, 1.01) | ||
| 2013 | 0.9 (0.76, 1.03) | ||
| 2014 | 1.05 (0.91, 1.18) | ||
| 2015 | 1.11 (0.97, 1.25) | ||
| 2016 | 0.97 (0.82, 1.12) | ||
| No | Reference | <0.0001 | |
Adjusted relative risk was calculated from odds ratios from mixed logistic regression based on proportion, using the method of Zhang J, Yu KF. What’s the relative risk? A method of correcting odds ratio in cohort studies of common outcomes. JAMA 1998. Abbreviations: PDA, patent ductus arteriosus; PMA, post-menstrual age; BPD, bronchopulmonary dysplasia.
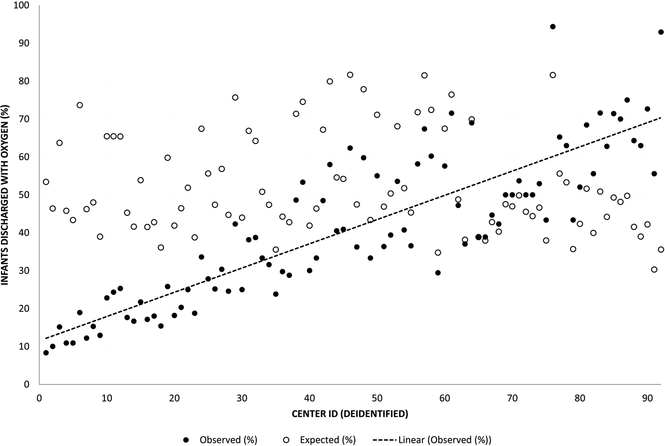
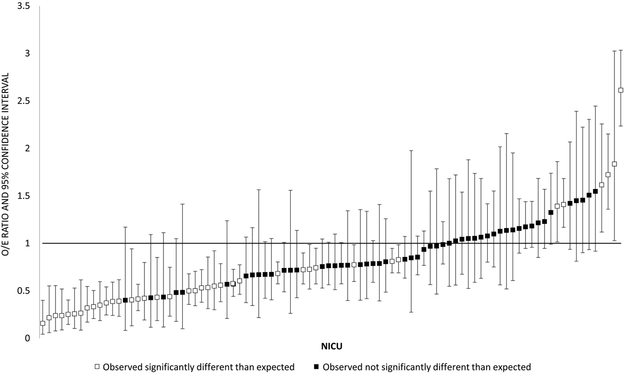
Figure 2 depicts variation in the observed and expected use of home oxygen among the 92 CPQCC centers discharging at least 15 patients with BPD over the study period. Observed home oxygen use ranged from 9–95%, with expected rates from the fixed effects portion of the model falling largely between 40–70% (Figure 2, A). 42% of hospitals’ observed proportions of home oxygen use fell significantly outside of the expected range based on the 95% confidence intervals around the observed to expected ratio of home oxygen use (Figure 2, B). Six of 92 hospitals had observed proportions of home oxygen use that were significantly more than expected; 33 hospitals had observed proportions of home oxygen use that were significantly less than expected. Our model resulted in an intra-class coefficient of 20%, meaning that 20% of the variation in probability of home oxygen use at discharge was accounted for at the hospital level. For the 23 regional hospitals, neither observed nor expected rates of home oxygen use were correlated with median PMA at NICU discharge. For the 67 community CCS hospitals with at least 15 discharged patients, centers using more home oxygen had an earlier median PMA at NICU discharge (Spearman correlation coefficient −0.27, p=0.024) (Table 3; available at www.jpeds.com). Community hospitals using the highest proportion of home oxygen use had a median discharge PMA of 39 weeks (IQR 37–41), 2 weeks shorter than hospitals using medium (41 weeks, IQR 39–43) or low (41 weeks, IQR 39–44) proportions of home oxygen use (Kruskal-Wallis test p<0.001).
Figure II. Observed and expected home oxygen use for NICUs discharging ≥15 infants with BPD.
In figure 2a, the x axis represents individual deidentified NICUs, arranged in order from lowest to highest observed home oxygen use. The y axis represents the proportion of discharge with home oxygen, either observed (black circles) or expected based on model estimates (white circles). The line represents a best fit through the observed rates of home oxygen use.
Figure 2b depicts the observed/expected (O/E) ratio for home oxygen use for 92 NICUs discharging ≥15 infants with BPD. The x axis depicts individual deidentified NICUs, arranged from lowest to highest O/E ratio. The y axis represents the O/E ratio. Squares are point estimates, with vertical bars representing 95% confidence intervals. Black squares are NICUs whose observed home oxygen use was not significantly different from expected based on the model, defined as an O/E ratio confidence interval that crosses 1. White squares are NICUs whose observed home oxygen use was significantly different than expected based on the model, defined as an O/E ratio confidence interval that does not cross 1.
Table 3.
Correlation between observed and expected home oxygen use and median PMA at discharge.
| Community NICUs (n=67) Correlation coefficient | p | Regional NICUs (n=23) Correlation coefficient | p | |
|---|---|---|---|---|
| Observed home oxygen use | −0.27 | 0.0024 | 0.16 | 0.471 |
| Expected home oxygen use | 0.38 | 0.002 | 0.36 | 0.093 |
Table 3 shows Spearman’s rank correlation coefficients for the relationship between median PMA at discharge and observed or expected proportion of home oxygen use for infants with BPD, for the 67 community CCS and 23 regional NICUs in the CPQCC with at least 15 discharged infants with BPD during the study period. The 2 non-CCS or intermediate level centers are not represented here due to small n.
Discussion
The purpose of this study was to identify predictors of home oxygen use, determine the extent of institutional variation in a statewide cohort, and identify potential relationships between home oxygen use and discharge timing for infants with BPD. We identified several clinical and hospital-level risk factors associated with higher use of home oxygen for infants with BPD. We found that hospital variation accounted for 20% of all variation in home oxygen use which was not explained by clinical factors or measured center characteristics. We also found that in community NICUs, centers with higher rates of home oxygen use had an earlier PMA at discharge.
Home oxygen use is a relatively common therapy for preterm infants in California, used in 47% of preterm infants with BPD in the state. This is similar to proportions of home oxygen use for infants with BPD in other reports.11, 13, 16 Previous studies from the Pediatrix Clinical Data Warehouse identified similar major risk factors including gestational age and days of mechanical ventilation.11, 17 One difference between this and prior studies is the greater number of clinical predictors associated with home oxygen use among infants with BPD. Laughon et al have previously shown the ability to predict BPD over time in the NICU.18 For infants with BPD, the ability to identify clinical risk factors associated with home oxygen use enables us to offer earlier anticipatory teaching and coordinated care services to parents likely to need to become familiar with this therapy. Events such as PDA ligation might serve as an anticipatory moment for clinicians to consider discussing the possibility of home oxygen with families. We also found that infants with the earliest and latest PMA at discharge had the highest odds of home oxygen use, adjusted for center. In addition to discussing complex discharge needs for infants with more severe lung disease, a relatively healthy infant with persistent oxygen needs who is acquiring early oral feeding skills may also prompt consideration of discharge with home oxygen.
In this population-based cohort, we found that hospital variation accounted for 20% of total variation in home oxygen use after adjusting for clinical characteristics, and nearly half of all centers had significantly different use of home oxygen than expected given their case mix. We observed an increase in home oxygen use from 2011–2016, which might correspond to the publication timing of the oxygen saturation targeting trials. We did not notice a large effect of birth year in multivariable modeling, although any effect of birth year was likely mediated by center-specific practice changes and we adjusted for center. Regardless, the variation in home oxygen use across NICUs even after adjusting for birth year and illness measures suggests that oxygen targets may not be standard across NICUs, home oxygen decision-making is based on more than oxygen saturation targets, or likely both. Lapcharoensap et al have previously shown that level of NICU care was associated with differences in an outcome of BPD.7 This study extends those findings by indicating that NICU characteristics are associated with differences in discharge planning as well.
We confirmed a previous finding that community hospitals with higher proportions of home oxygen use had an earlier median PMA at NICU discharge for infants with BPD. The Neonatal Research Network recently conducted an analysis of a propensity-matched cohort of infants under 27 weeks’ gestation discharged with and without home oxygen, in which infants discharged with home oxygen had the same neurodevelopmental outcomes, slightly better growth and slightly more readmissions.19 This provides important information on expected outcomes for infants where there is a clinical question of treatment with or without home oxygen. To extend these findings on a population level, we also need to consider the implications for infants outside of a matched cohort. For infants with more severe lung disease, we would expect a longer length of stay and more likely home oxygen use. What about infants with less severe lung disease? A previous study of infants of all gestational ages showed many infants ≥27 weeks discharged with home oxygen.17 In our model, infants with both the earliest and latest PMA at NICU discharge had highest rates of home oxygen use. The association between home oxygen use and earlier PMA at discharge in community NICUs suggests that for many infants, home oxygen is not just a marker of illness, but a discharge tool. Given the high cost of NICU care and potential for reimbursement changes, the association between home oxygen use and discharge timing has important implications. Lorch et al have shown that outpatient medical facilities are associated with differences in hospital readmission for preterm infants; centers with more associated comprehensive outpatient care and community health supports may be more likely to use therapies such as home oxygen to facilitate NICU discharge.20
There are several strengths to this study. First, the large sample size of this study allowed for evaluation of associations between multiple covariates and the outcome of home oxygen use. The CPQCC prospectively captures clinical data on at least 90% of very-low-birth-weight infants receiving all levels of neonatal intensive care in California, increasing external validity of the study. Similar rates of BPD and other common preterm morbidities between the CPQCC and other literature have been reported previously.
A number of limitations deserve comment. The CPQCC does not require the physiologic definition of BPD, which has been shown to decrease variation in diagnosis of BPD between practices.21 Similarly, the recently proposed revised definition of BPD was unavailable due to lack of data on fraction of inhaled oxygen and liter flow or non-invasive ventilation.22 We attempted to capture other predictors of BPD severity including gestational age, ventilator days, maximum post-delivery room respiratory support, male sex and PDA ligation, but a clearer definition of lung disease severity was not available in the data. Another limitation is the lack of information on institutional criteria for discharge, specific non-respiratory conditions such as poor oral feeding or apnea of prematurity prolonging discharge, or other reasons for variation in discharge practices which might influence comparisons between community and regional centers. We adjusted for birth year but were unable to account for possible changes in treatment patterns over time. Similarly, although we attempted to capture maternal characteristics associated with home oxygen use, we were limited by specific maternal health conditions available in the data. Unmeasured socioeconomic factors such as education, income, smoking, and access to transportation could be very important in understanding physician and family preferences for home oxygen use. Altitude was unavailable in these data. Although we had less than 2% missing data across all study covariates, missing data has the potential to introduce selection bias.
In conclusion, in a contemporary population-based cohort of preterm infants with BPD, several clinical factors were found to predict home oxygen use. Hospital practices vary widely across California, with community centers using more home oxygen having a shorter length of stay. Future research will be important to understand child health implications for home oxygen use in multiple types of patient populations, and factors affecting family preferences, in designing evidence-based strategies around the time of NICU discharge.
Acknowledgments
Supported by the National Institutes of Health (K23HL136525 to J.L.] and R01 HD087425 to H.L.]) and a Medical College of Wisconsin Presidential Faculty Scholar Award (to J.L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare no conflicts of interest.
Abbreviations:
- BPD
bronchopulmonary dysplasia
- NICU
neonatal intensive care unit
- PMA
postmenstrual age
- CPQCC
California Perinatal Quality Care Collaborative
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Jobe AH, Bancalari E Bronchopulmonary dysplasia. American journal of respiratory and critical care medicine. 2001;163:1723–9. [DOI] [PubMed] [Google Scholar]
- [2].] Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–60. [DOI] [PubMed] [Google Scholar]
- [4].Allen J, Zwerdling R, Ehrenkranz R, Gaultier C, Geggel R, Greenough A, et al. Statement on the care of the child with chronic lung disease of infancy and childhood. American journal of respiratory and critical care medicine. 2003;168:356–96. [DOI] [PubMed] [Google Scholar]
- [5].Hintz SR, Bann CM, Ambalavanan N, Cotten CM, Das A, Higgins RD, et al. Predicting time to hospital discharge for extremely preterm infants. Pediatrics. 2010;125:e146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ambalavanan N, Walsh M, Bobashev G, Das A, Levine B, Carlo WA, et al. Intercenter differences in bronchopulmonary dysplasia or death among very low birth weight infants. Pediatrics. 2011;127:e106–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lapcharoensap W, Gage SC, Kan P, et al. Hospital variation and risk factors for bronchopulmonary dysplasia in a population-based cohort. JAMA pediatrics. 2015;169:e143676. [DOI] [PubMed] [Google Scholar]
- [8].Vohr BR, Wright LL, Dusick AM, Perritt R, Poole WK, Tyson JE, et al. Center differences and outcomes of extremely low birth weight infants. Pediatrics. 2004;113:781–9. [DOI] [PubMed] [Google Scholar]
- [9].Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196:147 e1–8. [DOI] [PubMed] [Google Scholar]
- [10].Lemons JA, Bauer CR, Oh W, Korones SB, Papile L-A, Stoll BJ, et al. Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. Pediatrics. 2001;107:e1-e. [DOI] [PubMed] [Google Scholar]
- [11].Lagatta J, Clark R, Spitzer A. Clinical predictors and institutional variation in home oxygen use in preterm infants. The Journal of pediatrics. 2012;160:232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Target Ranges of Oxygen Saturation in Extremely Preterm Infants. New England Journal of Medicine. 2010;362:1959–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Keller RL, Feng R, DeMauro SB, Ferkol T, Hardie W, Rogers EE, et al. Bronchopulmonary Dysplasia and Perinatal Characteristics Predict 1-Year Respiratory Outcomes in Newborns Born at Extremely Low Gestational Age: A Prospective Cohort Study. The Journal of Pediatrics. 2017;187:89–97.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gould JB. The Role of Regional Collaboratives: The California Perinatal Quality Care Collaborative Model. Clinics in perinatology. 2010;37:71–86. [DOI] [PubMed] [Google Scholar]
- [15].Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. Jama. 1998;280:1690–1. [DOI] [PubMed] [Google Scholar]
- [16].Lodha A, Sauve R, Bhandari V, Tang S, Christianson H, Bhandari A, et al. Need for supplemental oxygen at discharge in infants with bronchopulmonary dysplasia is not associated with worse neurodevelopmental outcomes at 3 years corrected age. PloS one. 2014;9:e90843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lagatta JM, Clark RH, Brousseau DC, Hoffmann RG, Spitzer AR. Varying patterns of home oxygen use in infants at 23–43 weeks’ gestation discharged from United States neonatal intensive care units. The Journal of pediatrics. 2013;163:976–82 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, Kennedy KA, et al. Prediction of Bronchopulmonary Dysplasia by Postnatal Age in Extremely Premature Infants. American journal of respiratory and critical care medicine. 2011;183:1715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].DiMauro S Discharge on Oxygen and 2-Year Outcomes of Preterm Infants with Bronchopulmonary Dysplasia. Pediatric Academic Societies Platform, 20172018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lorch SA, Baiocchi M, Silber JH, Even-Shoshan O, Escobar GJ, Small DS. The Role of Outpatient Facilities in Explaining Variations in Risk-Adjusted Readmission Rates between Hospitals. Health Services Research. 2010;45:24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004;114:1305–11. [DOI] [PubMed] [Google Scholar]
- [22].Higgins RD, Jobe AH, Koso-Thomas M, Bancalari E, Viscardi RM, Hartert TV, et al. Bronchopulmonary Dysplasia: Executive Summary of a Workshop. The Journal of Pediatrics. 2018;197:300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]