Abstract
Ionotropic glutamate receptors in vertebrates are composed of three major subtypes – AMPA, kainate and NMDA receptors – and mediate the majority of fast excitatory neurotransmission at chemical synapses of the central nervous system. Among the three major families, native AMPA receptors function as complexes with a variety of auxiliary subunits, which in turn modulate receptor trafficking, gating, pharmacology and permeation. Despite the long history of structure-mechanism studies using soluble receptor domains or intact yet isolated receptors, structures of AMPA receptor-auxiliary subunit complexes have not been available until recent breakthroughs in single-particle cryo-electron microscopy. Single particle cryo-EM studies have, in turn, provided new insights into the structure and organization of AMPA receptor – auxiliary protein complexes and into the molecular mechanisms of AMPA receptor activation and desensitization.
Introduction
In the mammalian brain the majority of fast excitatory neurotransmission is carried out by α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA)-sensitive ionotropic glutamate receptors located within the post-synaptic density of glutamatergic synapses [1]. AMPA receptors open cation channels in response to binding of glutamate, thus depolarizing post-synaptic membranes. The AMPA receptor signaling complex is typically composed of tetrameric AMPA receptors and a broad range of auxiliary proteins, the latter of which modulate the trafficking, gating, pharmacology and permeation of receptors, leading to spatial and temporal fine-tuning of AMPA receptor function, which in turn is fundamental to synaptic plasticity, learning and memory [2,3].
At present, the group of AMPA receptor auxiliary subunits include transmembrane AMPA receptor regulatory proteins (TARPs) [4], the germ cell-specific gene 1-like protein (GSG1L) [5], cornichon homologs (CNIHs) [6], and the Shisa/cysteine-knot AMPA receptor modulating protein (CKAMP) family [7], with TARPs being the most widely expressed and extensively characterized auxiliary subunits [2,3]. Electrophysiological studies have shown that functional recapitulation of native-like AMPA receptor gating in a recombinant context requires co-expression of AMPA receptors with auxiliary proteins [8]. Therefore, elucidating molecular mechanisms of AMPA receptor function, under physiological conditions, requires structural ‘snapshots’ of AMPA receptor-auxiliary protein complexes in functionally defined conformations throughout the gating cycle. In this review, we discuss recent progress in elucidating the structures of AMPA receptor – auxiliary protein complexes using single particle cryo-electron microscopy (cryo-EM) and progress in elaborating the mechanisms of gating in AMPA receptors.
AMPA receptor-auxiliary protein assembly and stoichiometry
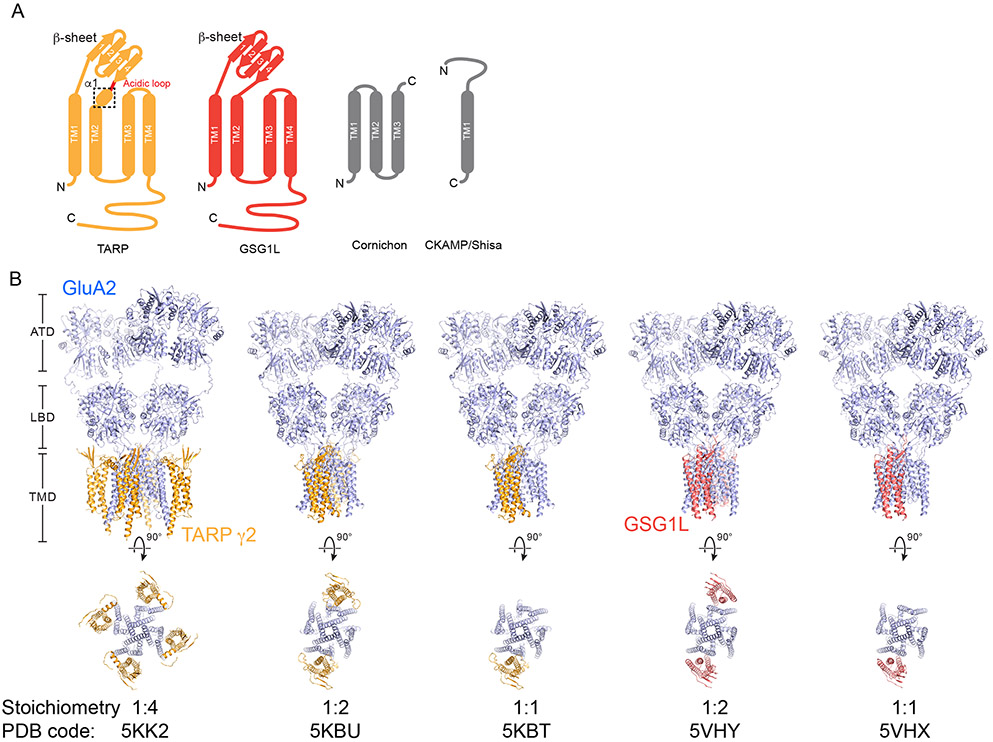
The prototypical AMPA receptor auxiliary protein TARP γ2, also known as stargazin, was discovered in the stargazer mutant mouse [9-11]. Disruption of TARP γ2 expression in cerebellar granule cells leads to insufficient surface delivery of functional AMPA receptors to synapses and a phenotype of absence epilepsy and ataxia [10]. Based on functional distinctions, canonical TARP homologs γ2, γ3, γ4 and γ8 are categorized as Type I, whereas Type II TARPs include γ5 and γ7 [3,12]. All members of the TARP family share structural similarity with the γ subunit of the L-type voltage-gated calcium channel [11], the tight junction protein claudins and GSG1L, and feature four transmembrane helices with a β-sheet decorated extracellular domain and both the amino- and the carboxyl- terminus located on the intracellular side of the membrane (Figure 1A). Interestingly, whereas TARPs potentiate the macroscopic steady-state current of AMPA receptors, GSG1L acts as an ‘inhibitory’ auxiliary protein. By contrast, the CNIHs and Shisa/CKAMPs auxiliary subunits are topologically distinct from TARPs, with three and one transmembrane helices, respectively (Figure 1 A) [2].
Figure 1.
Assembly and stoichiometry of AMPA receptor-auxiliary protein complexes. A, Topologies of auxiliary proteins. The α1 helix unique to TARPs is highlighted by a dashed box. B, Ribbon diagrams of structurally determined AMPA receptor-auxiliary protein complexes. Homomeric GluA2 receptors, TARP γ2 and GSG1L are colored in blue, orange and red, respectively.
The first AMPA receptor-auxiliary protein structures were elucidated using variants of the homomeric rat GluA2 receptor and TARP γ2 in the presence of the antagonist MPQX [13••,14••], where one study utilized co-expressed full-length receptor and TARP and the other used an engineered, covalently fused complex. These initial structures revealed a consistent complex architecture yet they harbored different receptor subunit to TARP subunit stoichiometries (Figure 1B). Solubilization and purification of the co-expressed complex using digitonin allowed for isolation of a fully occupied complex structure and provided structural views of how four TARPs surround the transmembrane periphery of the Y-shaped GluA2 receptor, with their extracellular domains juxtaposed under the receptor ligand binding domains (LBDs) [14••]. TARPs bind to the receptor predominantly through transmembrane interactions, thus following the ~4-fold symmetry of the TMD, yet are not structurally identical. The β-strands of opposing TARP pairs are poised to interact with the non equivalent A/C or B/D LBD pairs, giving rise to 2-fold related A’/C’ or B’/D’ TARP pairs. We speculate that these distinct TARP pairs likely elicit different functional action or effects on the receptor complex [14••]. The short α1 helix, unique to TARPs, positions an extracellular acidic loop for interaction with the conserved “lysine-glycine-lysine” motif in the receptor LBD, likely in an A/C or B/D position-dependent manner (Figure 1A and 1B) [13••,14••]. By contrast, when dodecyl-maltoside (DDM) is employed to isolate the receptor-TARP complex, despite the usage of covalently fused construct that presumably favors a fully-occupied complex, assemblies with either 1 or 2 TARPs per receptor were obtained [13••], underscoring the role of DDM in the partial disruption of the complex. In these complexes, the TARP subunits occupy sites such that their proximal receptor LBDs are from the B/D subunits, suggesting the receptor has higher apparent affinity for TARP γ2 subunits at these B’/D’ sites, in comparison to the unoccupied A’/C’ sites (Figure 1B). Thus, the initial structural studies of AMPA receptor – TARP complexes is consistent with biophysical and electrophysiological evidence indicating that a tetrameric AMPA receptor can bind 1 to 4 TARP γ2 subunits, perhaps dictated, at least in part, by TARP expression level [15•,16•].
Cryo-EM studies of the covalently fused GluA2-GSG1L complex resulted in two receptor-auxiliary protein stoichiometries – 1:2 and 1:1, despite the usage of digitonin, with GSG1L occupying either or both of the B’/D’ sites (Figure 1B) [17••]. However, it would be interesting to determine if “fully-occupied” complexes can be formed, as measured by independent biophysical approaches, as the initial structural studies cannot preclude the possibility that even digitonin is not sufficient in retaining GSG1L at the A’/C’ sites. GSG1L binds to the receptor complex at sites largely overlapping with the TARP γ2 binding site, underscoring the notion that interactions between AMPA receptors and their auxiliary proteins primarily involve hydrophobic interactions mediated by the transmembrane α-helices. In comparison to TARPs, the GSG1L subunits lack an α1 helix and an acidic loop but instead feature a longer loop between the β1 and β2 strands, structural differences that likely underpin the functional observation that the GSG1L subunits stabilize receptors in inactive, desensitized or desensitized-like states, rather than potentiating receptors and stabilizing active states, like the TARP subunits [13••, 14••, 17••]. Current studies have elucidated only a few AMPA receptor – auxiliary subunit complex structures and more effort is needed to achieve a complete structural understanding of AMPA receptors in complex with distinct auxiliary proteins, especially cornichons and Shisa/CKAMPs.
Activation mechanism of AMPA receptor-TARP complexes
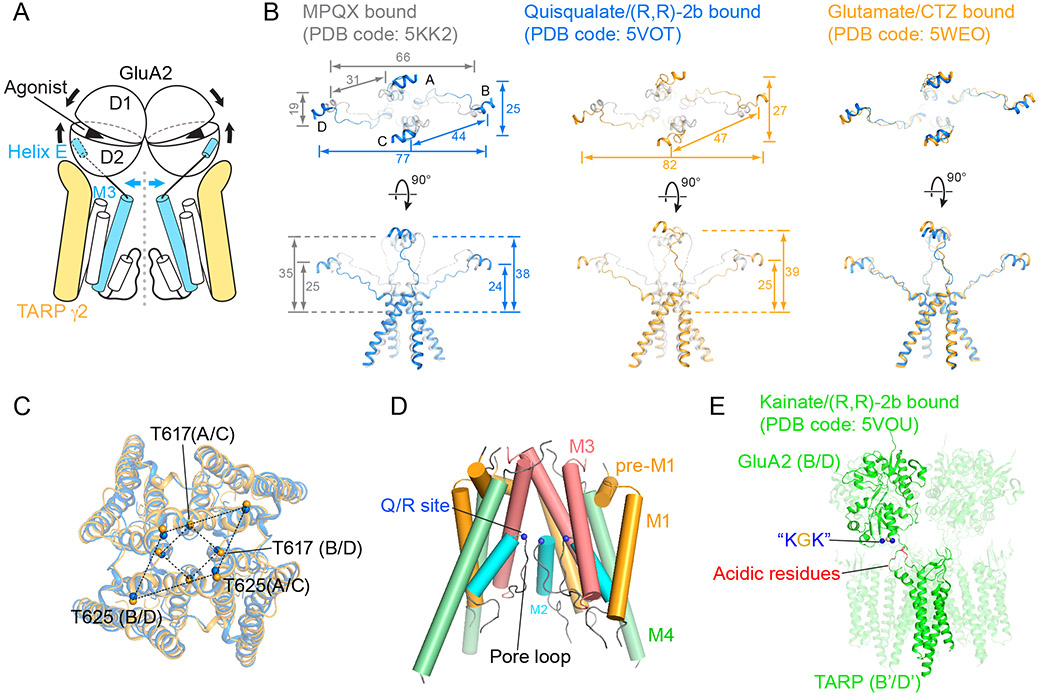
A holy grail in the structure-based mechanistic studies of AMPA receptors has been to elucidate the structure of an AMPA receptor arrested in a fully open, agonist-bound activated state. Initial models for receptor activation were inspired by comparison of crystal structures of the ‘clamshell’ apo- and holo-LBD dimers bound with agonists, which revealed that agonist-binding induces the closure of each LBD subunit and thus the separation of the lower D2 lobes [18-20]. In the context of intact receptors, it was hypothesized that LBD clamshell closure leads to an expansion of the “gating ring” consisting of four D2 lobes, each directly connected to the M3 gating helices by a linker extending from helix E, ‘pulling’ open the channel gate (Figure 2A) [21]. Indeed, gating ring expansion was observed in crystal structures of intact GluA2 receptors bound with agonists and a desensitization blocker (R,R)-2b [22]. However, contrary to the hypothesis, the channel gate clearly adopts a closed conformation under these conditions [22]. One plausible explanation for the closed ion channel gate despite bound agonists and positive allosteric modulators is that the tension on the helix E-M3 linker was ‘relaxed’ due to a progression of the gating ring towards the ion-channel domain. Perhaps these structures of the isolated receptor represent pre-activation states.
Figure 2.
Activation mechanism of the GluA2-TARP γ2 complex. A, Cartoon representation illustrating structural rearrangements upon receptor activation. Only two opposing subunits in the tetrameric assembly are shown. B, Comparison of helices E separation and elevation among MPQX-inhibited (grey), quisqualate-activated (cyan) and glutamate-activated (orange) complexes, superimposed using receptor TMD main-chain atoms as a reference. Whereas separations are measured as distances between COMs of helices E pairs, elevations are defined as vertical COM distances from helices E to a gating residue Thr617. Distances are in angstroms. C, Top-down view of super-positioned receptor TMD including the channel gate. Gating residues are labeled. D, Receptor pore structure defined by cryo-EM maps. E, Complementary electrostatic interactions formed between the LBD of receptor and extracellular domain of TARP at the B/D positions in the kainate-activated complex.
Recent breakthroughs in structural investigations of active states of AMPA receptors have provided the first insights into the open-gate conformations of the receptor. Two independent groups have reported single-particle cryo-EM structures of digitonin stabilized GluA2 receptors in complex with fully occupied TARP γ2 subunits and in the presence full-agonists and blockers of desensitization [23••,24••]. The two structures are largely consistent, unambiguously revealing dilated channel gates and confirming the aforementioned model of gating ring expansion upon full agonist-binding induced LBD clamshell closure (Figure 2A) [23••,24••]. TARPs prevent progression of LBDs toward the membrane, thus ensuring that the tension on the helix E-M3 linkers acts on the channel gate. The dimer-of-dimers configuration of the LBDs dictates that the B/D clamshell closure exerts a more profound pulling force on the gating helices, primarily directed parallel to the membrane plane, whereas the pulling force produced by A/C clamshell closure has a major perpendicular component, underpinning the notion that B/D positions play more important roles in receptor gating. Indeed, the channel gate is asymmetrically open, with the carboxyl terminal ends of the B/D gating helices unwinding by one more turn in comparison to the A/C helices, thus producing a 2-fold related M3 bundle-crossing in contrast to the ~4-fold related closed conformation [23••,24••].
To evaluate the extent of gating ring expansion, we compared the separation of opposing and adjacent helices E centers of mass (COM). In the complex composed of full-length GluA2 (flop isoform, arginine at Q/R site), full-length TARP γ2, (R,R)-2b and quisqualate (PDB code: 5VOT), helices E are separated by 25 Å, 77 Å and 44 Å along the A/C, B/D and A/D directions [23••], in comparison to 19 Å, 66 Å and 31 Å measured in the antagonist-bound state (PDB code: 5KK2) [14••], respectively (Figure 2B). We note that the conformations of helices E in the complex composed of a non-native GluA2 (flip isoform, glutamine at Q/R site) covalently fused with C-terminally truncated TARP γ2, cyclothiazide and glutamate (PDB code: 5WEO) deviates from the isolated LBD crystal structure (PDB code: 1MM6), whereas helices E from the quisqualate-activated complex (PDB code: 5VOT) do not. Nevertheless, there is greater separation of helices E in all three directions, as well as a more open channel gate, in the glutamate-activated complex [24••] (Figure 2B). Considering the LBD layers in both structures were at similar “elevations”, measured as the vertical distances between helices E and gating residues Thr617, a more expanded gating ring likely resulted in a more open channel gate, as illustrated by positions of residues Thr617 and Thr625 (Figure 2C). Nevertheless, glutamate and quisqualate, both of which are full agonists, yield structures with different extents of gate opening. We speculate that the variation in gate dilation might be the consequence of the discrepancy between the constructs used in these two independent structures. Further functional characterization of both complexes, to measure channel open probability, as well as higher resolution single-particle cryo-EM studies, to completely define 3D classes and to more accurately elucidate atomic-resolution structures, would be helpful to address these issues. Molecular dynamics simulations of the quisqualate-activated complex structure indeed confirmed that the dilated channel gate is wide enough to allow permeation of hydrated sodium ions [23••]. Indeed, the more open channel gate in the glutamate-activated complex should also be permeable to sodium ions. However, the gates in both structures are too narrow for unrestricted passage of fully hydrated potassium ions. Therefore, these structures likely represent partially open states.
Despite the progress in unveiling the open conformation of an AMPA receptor, several fundamental questions regarding the activation mechanism remain unanswered. A first question concerns a “fully open” conformation, a structure that would provide the greatest insights into the coupling between ligand-binding and ion channel gating. A second challenge is to elucidate the structural basis for well-documented subconductance levels [25,26]. Because of the lack of biophysical or biochemical assays to examine if purified receptor with or without auxiliary proteins recapitulates the observed subconductance behavior of the receptor in a membrane bilayer, one can only stabilize receptors or complexes in environments that are the closest mimics of the native biological membrane and hope to capture subconductance states by single-particle cryo-EM. Even if that is the case, resolving small yet important differences in the membrane/micelle embedded ion channel gate among fully open and subconductance states by classification may still be difficult. In fact, the reported partial open state reconstructions are possibly averages of subconductance states.
AMPA receptor pore structure
Despite previous structural determinations of intact AMPA receptors by x-ray crystallography and cryo-EM [13••,14••,21,22,27-29], structures of the ion channel pore, consisting of the short M2 helix and the pore loop lining the channel axis, remained largely elusive until the availability of the GluA2-TARP γ2 complex structure trapped in an active state. Continuous electron potential maps allowed improved structural modeling, resulting in reliable pore coordinates consistent with the notion that AMPA receptor pores resemble those of inverted potassium channels (Figure 2D) [23••,24••]. However, in sharp contrast with the ‘rigid’ potassium channel pore, structural comparison of the AMPA receptor pore among different conformational states of the gating cycle revealed substantial variation, consistent with the non-selective ion-permeability [24••]. The defined pore structure also revealed that the Q/R RNA editing site is positioned such that the side chains point into the vestibule ‘underneath’ the channel gate [23••,24••], thus providing a structural explanation for how the ‘edited form’ arginines fill the vestibule with positive charges, thus preventing divalent calcium cations from passing through the ion channel (Figure 2D).
TARP modulation of partial agonist efficacy
There are multiple structural mechanisms to describe partial agonist efficacy on AMPA receptors. Whereas crystallographic studies of isolated LBDs bound with partial or full agonists have illuminated a correlation between the efficacy and the extent of LBD clamshell closure [19], others have suggested that partial agonists can induce full LBD clamshell closure but at a lower occupancy or frequency [30]. A limitation of previous crystallographic studies has been the concern that interactions in the crystal lattice stabilize non-native conformations while the ‘free’ protein molecules outside of the crystal are more conformationally dynamic. In this regard, single-particle cryo-EM studies may better reveal the biologically relevant, agonist-bound conformations. The efficacy of kainate, a classic partial agonist of AMPA receptors, is elevated to 80% of a full-agonist efficacy in the presence of TARP γ2. Thus, a question in the field was whether kainate elicits more profound LBD clamshell closure in the receptor-TARP γ2 complex. Single-particle analysis of the full-length GluA2-TARP γ2 complex bound with (R,R)-2b and kainate did not result in any class featuring fully closed LBDs, but rather partially closed LBDs were observed, comparable to those found in crystal structures of kainate bound intact receptor and isolated LBDs [23••]. These observations support the notion that TARPs do not enhance kainate efficacy by altering the LBD clamshell closure, although it remains possible that the cryo-EM studies have not yet captured low occupancy, fully closed conformations.
Nevertheless, how does TARP γ2 enhance kainate efficacy without promoting more profound closure of LBD clamshells? Due to partially closed LBD clamshells, the gating ring found in the cryo-EM structure of the kainate/(R,R)-2b bound receptor-TARP γ2 complex is indeed not as expanded as that found in the full-agonist activated open state, as separations of the COMs of helices E are 19 Å, 75Å and 40 Å in A/C, B/D and A/D directions, respectively [23••]. However, these separations are 25 Å, 66 Å and 38 Å in the crystal structure of kainate-activated receptor alone (PDB code: 4U1W) [22], indicating that TARPs reshaped the gating ring by altering the LBD dimer-dimer interface, perhaps through complementary electrostatic interactions between TARP acidic loops and the “lysine-glycine-lysine” motif of receptor subunits at the B/D positions (Figure 2E). By achieving similar separations between helices E of the B and D subunits, yet compromising those between the A and C subunits, binding of kainate and (R,R)-2b opens the channel gate of the receptor-TARP complex to a similar extent as the full-agonist quisqualate [23••], consistent with the potentiated steady state currents elicited by kainate in the presence of TARP γ2. At present, the structural basis underlying the efficacy differences between kainate and quisqualate remain to be resolved, perhaps by future structural studies at a higher resolution and thus with a better ability to resolve structural classes with subtle differences.
Desensitization of AMPA receptor-auxiliary protein complexes
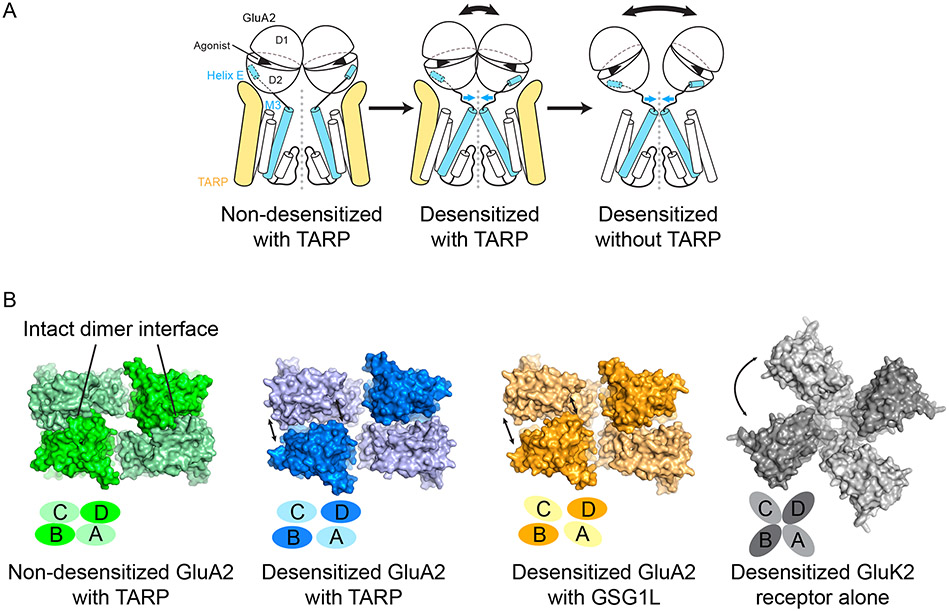
Rapid and profound desensitization following activation is the hallmark of AMPA receptor gating. TARP γ2 diminishes the extent of AMPA receptor desensitization by attenuating the entry of active GluA2 receptors into desensitized states and by accelerating the recovery from desensitization to re-form gating competent receptors [13••]. Studies of isolated LBD ‘clamshells’ suggest that desensitization is largely the consequence of rupture of the intradimer D1-D1 interface [31]. Stabilization of D1-D1 interactions by mutations or by small molecules blocks AMPA receptor desensitization, whereas trapping LBD dimers in conformations incompatible with intact D1-D1 interfaces eliminates agonist-evoked current [31]. Early crystallographic or cryo-EM investigations of isolated desensitized receptors have been limited to low resolution [22,29], presumably due to extensive conformational heterogeneity. Although the degree of clamshell closure and the conformation of the ion-channel gate were not resolved, single-particle classification analysis of desensitized receptor suggested that the LBDs undergo large-scale structural rearrangements (Figure 3A), and that the ATDs tend to lose their dimer-of-dimer configuration, with the two dimers apart from each other [29].
Figure 3.
Desensitization mechanism for AMPA receptor-auxiliary protein complexes. A, Cartoon illustration of structural rearrangements upon receptor desensitization. B, Structural comparison of the LBD dimerization interface among active and desensitized AMPA receptors as well as the closely related desensitized kainite receptor.
Recent cryo-EM studies of receptor-auxiliary protein complexes in putative desensitized states were reported, one using the native GluA2-TARP γ2 complex [23••] and the other using non-native, engineered GluA2-GSG1L complexes [17••]. In contrast with the antagonist-bound and active state structures, the desensitized state complexes in both studies were shown to exhibit conformational heterogeneity at the levels of single-particle projections, 2D class averages and 3D reconstructions, yet to a lesser extent in comparison to the isolated receptor [17••,23••]. For example, splayed open ATDs were only observed in a sub-population of the total GluA2-TARP γ2 complex particles, whereas most particles fell into 2D class averages showing features consistent with the dimer-of-dimer configuration. Subsequent analysis of both complexes focused on reconstructions using sub-populations of particles that yielded relatively well-defined structural features. These reconstructions unambiguously revealed fully closed LBD clamshells and shut channel gates, consistent with receptor desensitization [17••,23••]. Ruptured LBD dimer interfaces along with rotation of LBDs caused shrinking of the gating ring, thus releasing the tension exerted on M3 helices to allow closure of the channel gate (Figure 3A) [17••,23••]. Importantly, upon LBD dimer rupture, one LBD subunit rotated relative to the other differently between the TARP- and GSG1L-bound complexes (Figure 3B) [17••,23••]. The ‘disassociated’ LBD dimer in the GSG1L complex deviated more from its original 2-fold symmetry, creating a larger “gap” on the far side from the channel axis (Figure 3B) [17••]. It was hypothesized that TARPs spatially preclude the desensitized ensemble from undergoing large-scale LBD structural arrangements, thus facilitating reformation of the dimer-of-dimer configuration essential for gating competent receptors after agonist disassociation. GSG1Ls function to stabilize the desensitized ensemble by interactions with the extracellular domains, stabilizing the LBDs in conformations incompatible with ion channel gating.
Interestingly, among the desensitized ensemble of receptor-TARP and -GSG1L complexes, there is no clear evidence of any sub-population representing the ~4-fold related LBDs found with the desensitized state of the kainate receptor, a close relative of AMPA receptors (Figure 3B) [32•]. Indeed, trapping of receptor with and without TARPs by crosslinking in combination with electrophysiology has not only supported the hypothesis that TARPs restrict the scale of LBD rearrangement, but also raised the issue of the time duration of agonist exposure in structural studies [33•]. To date, all desensitized receptors or receptor complexes used for structural studies were prepared over durations of seconds or longer, well beyond the millisecond time scale associated with gating, in vivo, at chemical synapses.
Conclusion
This review summarizes the recent progress in elucidating structures of AMPA receptors in complex with auxiliary proteins, captured in specific functional states throughout the gating cycle, by single-particle cryo-EM. These structures shed light on how auxiliary subunits co-assemble with AMPA receptors and modulate receptor gating by sculpting the LBD layer. TARP γ2 subunits prevent the receptor LBDs from approaching the TMD layer, thus enhancing the coupling between agonist-binding and channel opening, giving rise to the first views of AMPA receptors in partially open conformations. These open states, together with antagonist-inhibited structures, provide an experimental basis to support the canonical AMPA receptor activation mechanism. Receptor desensitization results from rupture of the LBD intradimer interface, consistent with structural studies of the isolated LBDs, full-length receptors and receptor-auxiliary protein complexes. By contrast, the extent of receptor extracellular domain rearrangement upon LBD dimer disassociation varies dramatically between the isolated receptor and receptor – auxiliary protein complexes. Therefore, an important goal for the future involves capturing AMPA receptors, in their complexes with auxiliary subunits, in the most physiologically relevant conformational states.
Acknowledgement
We acknowledge Heidi Owen for help with proofreading and NIH for financial support. Eric Gouaux is an investigator with the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
The references of special (•) and outstanding (••) interest have been highlighted.
- 1.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R: Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev 2010, 62:405–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haering SC, Tapken D, Pahl S, Hollmann M: Auxiliary subunits: shepherding AMPA receptors to the plasma membrane. Membranes (Basel) 2014, 4:469–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson AC, Nicoll RA: The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron 2011, 70:178–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS: Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J Cell Biol 2003, 161:805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shanks NF, Savas JN, Maruo T, Cais O, Hirao A, Oe S, Ghosh A, Noda Y, Greger IH, Yates JR 3rd, et al. : Differences in AMPA and kainate receptor interactomes facilitate identification of AMPA receptor auxiliary subunit GSG1L. Cell Rep 2012, 1:590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwenk J, Harmel N, Zolles G, Bildl W, Kulik A, Heimrich B, Chisaka O, Jonas P, Schulte U, Fakler B, et al. : Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science 2009, 323:1313–1319. [DOI] [PubMed] [Google Scholar]
- 7.von Engelhardt J, Mack V, Sprengel R, Kavenstock N, Li KW, Stern-Bach Y, Smit AB, Seeburg PH, Monyer H: CKAMP44: a brain-specific protein attenuating short-term synaptic plasticity in the dentate gyrus. Science 2010, 327:1518–1522. [DOI] [PubMed] [Google Scholar]
- 8.Milstein AD, Nicoll RA: Regulation of AMPA receptor gating and pharmacology by TARP auxiliary subunits. Trends Pharmacol. Sci 2008, 29:333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA: Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature 2000, 408:936–943. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto K, Fukaya M, Qiao X, Sakimura K, Watanabe M, Kano M: Impairment of AMPA receptor function in cerebellar granule cells of ataxic mutant mouse stargazer. J Neurosci 1999, 19:6027–6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Letts VA, Felix R, Biddlecome GH, Arikkath J, Mahaffey CL, Valenzuela A, Bartlett FS 2nd, Mori Y, Campbell KP, Frankel WN: The mouse stargazer gene encodes a neuronal Ca2+-channel gamma subunit. Nat Genet 1998, 19:340–347. [DOI] [PubMed] [Google Scholar]
- 12.Kato AS, Gill MB, Yu H, Nisenbaum ES, Bredt DS: TARPs differentially decorate AMPA receptors to specify neuropharmacology. Trends Neurosci 2010, 33:241–248. [DOI] [PubMed] [Google Scholar]
- 13.Twomey EC, Yelshanskaya MV, Grassucci RA, Frank J, Sobolevsky AI: Elucidation of AMPA receptor-stargazin complexes by cryo-electron microscopy. Science 2016, 353:83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper reports the first cryo-EM structures of an AMPA receptor-TARP γ2 complex assembled at various stoichiometry at 0, 1 or 2 TARPs per receptor.
- 14.Zhao Y, Chen S, Yoshioka C, Baconguis I, Gouaux E: Architecture of fully occupied GluA2 AMPA receptor-TARP complex elucidated by cryo-EM. Nature 2016, 536:108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper reports the first cryo-EM structure of a fully-occupied AMPA receptor-TARP γ2 complex, showing 4 TARP molecules surround the ion-channel domain of receptor, potentially forming two types of interactions with receptor ligand-binding domains.
- 15.Hastie P, Ulbrich MH, Wang HL, Arant RJ, Lau AG, Zhang Z, Isacoff EY, Chen L: AMPA receptor/TARP stoichiometry visualized by single-molecule subunit counting. Proc Natl Acad Sci U S A 2013, 110:5163–5168. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper reports electrophysiological investigations of how varying AMPA receptor-TARP stoichiometry influences the function of receptor.
- 16.Shi Y, Lu W, Milstein AD, Nicoll RA: The stoichiometry of AMPA receptors and TARPs varies by neuronal cell type. Neuron 2009, 62:633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper reports evidence of varying stoichiometry of AMPA receptors and TARPs observed in single-molecule counting experiments.
- 17.Twomey EC, Yelshanskaya MV, Grassucci RA, Frank J, Sobolevsky AI: Structural Bases of Desensitization in AMPA Receptor-Auxiliary Subunit Complexes. Neuron 2017, 94:569–580 e565. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper reports the first cryo-EM structures of AMPA receptor-GSG1L complexes at stoichiometries of 1 or 2 GSG1Ls per receptor, underscoring the role of GSG1L in stabilizing the desensitized state.
- 18.Armstrong N, Gouaux E: Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron 2000, 28:165–181. [DOI] [PubMed] [Google Scholar]
- 19.Jin R, Banke TG, Mayer ML, Traynelis SF, Gouaux E: Structural basis for partial agonist action at ionotropic glutamate receptors. Nat Neurosci 2003, 6:803–810. [DOI] [PubMed] [Google Scholar]
- 20.Jin R, Horning M, Mayer ML, Gouaux E: Mechanism of activation and selectivity in a ligand-gated ion channel:Structural and functional studies of GluR2 and quisqualate. Biochemistry 2002, 41:15635–15643. [DOI] [PubMed] [Google Scholar]
- 21.Sobolevsky AI, Rosconi MP, Gouaux E: X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature 2009, 462:745–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dürr K, Chen L, Stein RA, De Zorzi R, Folea IM, Walz T, Mchaourab HS, Gouaux E: Structure and dynamics of AMPA receptor GluA2 in resting, pre-open and desensitized states. Cell 2014, 158:778–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen S, Zhao Y, Wang Y, Shekhar M, Tajkhorshid E, Gouaux E: Activation and Desensitization Mechanism of AMPA Receptor-TARP Complex by Cryo-EM. Cell 2017, 170:1234–1246 el214. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper reported the first cryo-EM structures of AMPA receptor-TARP γ2 complexes bound with full-agonist quisqualate or partial-agonist kainate in the presence of desensitization blocker (R,R)-2b, or with quisqualate alone. The quisqualate-activated complex structure, featuring a partially open gate and complete pore model, illustrated an activation mechanism; the kainate-activated complex structure explains how TARP enhances kainate efficacy; and the quisqualate-induced desensitized ensemble revealed how TARP restricts LBD rearragnements to facilitate recovery from desensitization.
- 24.Twomey EC, Yelshanskaya MV, Grassucci RA, Frank J, Sobolevsky AI: Channel opening and gating mechanism in AMPA-subtype glutamate receptors. Nature 2017, 549:60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper reported the first cryo-EM structure of an AMPA receptor-TARP γ2 complex in the presence of glutamate and cyclothiazide, providing the highest resolution electron potential map for a partially open state and revealing structrual details about receptor pore and an activation mechanism.
- 25.Smith TC, Wang LY, Howe JR: Heterogeneous conductance levels of native AMPA receptors. J Neurosci 2000, 20:2073–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shelley C, Farrant M, Cull-Candy SG: TARP-associated AMPA receptors display an increased maximum channel conductance and multiple kinetically distinct open states. J Physiol 2012, 590:5723–5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen L, Dürr K, Gouaux E: X-ray structures of AMPA receptor-cone snail toxin complexes illuminate activation mechanisms. Science 2014, 345:1021–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao P, Zhang HY, Soong TW: Alternative splicing of voltage-gated calcium channels: from molecular biology to disease. Pflugers Arch 2009, 458:481–487. [DOI] [PubMed] [Google Scholar]
- 29.Meyerson JR, Kumar J, Chittori S, Rao P, Pierson J, Bartesaghi A, Mayer ML, Subramaniam S: Structural mechanism of glutamate receptor activation and desensitization. Nature 2014, 514:328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed AH, Ptak CP, Fenwick MK, Hsieh C-L, Weiland GA, Oswald RE: Dynamics of cleft closure of the GluA2 ligand-binding domain in the presence of full and partial agonists revealed by hydrogen-deuterium exchange. J. Biol. Chem 2013, 288:27658–27666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armstrong N, Jasti J, Beich-Frandsen M, Gouaux E: Measurement of conformational changes accompanying desensitization in an ionotropic glutamate receptor. Cell 2006, 127:85–97. [DOI] [PubMed] [Google Scholar]
- 32.Meyerson JR, Chittori S, Merk A, Rao P, Han TH, Serpe M, Mayer ML, Subramaniam S: Structural basis of kainate subtype glutamate receptor desensitization. Nature 2016, 537:567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper reports a high-resolution cryo-EM structure of the kainate receptor in a desensitized state, providing direct evidence for approximately four-fold related ligand-binding domains, in turn raising the question whether the closely related AMPA recepotor undergoes similar conformational changes upon desensitization.
- 33.Baranovic J, Plested AJR: Auxiliary subunits keep AMPA receptors compact during activation and desensitization. Elife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper reports electrophysiological evidence showing that trapping of extracellular domains in 'open-up' configurations is favored with prolonged exposure to glutamate and in the absence of TARP γ2, suggesting the AMPA receptor extracellular domain likely remains compact upon desensitization under physiological conditions of brief glutamate pulses and in the presence of abundant TARPs.