Abstract
Background:
Subfertility among couples affected by HIV impacts on the wellbeing of couples who desire to have children and may prolong HIV exposure. Subfertility in the antiretroviral therapy era and its determinants have not yet been well characterized.
Objectives:
To investigate the burden and determinants of subfertility among HIV-affected couples seeking safer conception services in South Africa.
Study Design:
Non-pregnant women and male partners in HIV seroconcordant or HIV discordant relationships desiring a child were enrolled in the Sakh’umndeni safer conception cohort at Witkoppen Clinic in Johannesburg between July 2013—April 2017. Clients were followed prospectively through pregnancy (if they conceived) or until six months of attempted conception, after which they were referred for infertility services. Subfertility was defined as not having conceived within 6 months of attempted conception. Robust Poisson regression was used to assess the association between baseline characteristics and subfertility outcomes; inverse probability weighting (IPW) was used to account for missing data from women lost-to-safer conception care before 6-months of attempted conception.
Results:
Among 334 couples enrolled, 65% (IPW-weighted, 95% CI: 0.59–0.73) experienced subfertility, of which 33% were primary subfertility and 67% secondary subfertility. Compared to HIV-negative women, HIV-positive women not on antiretroviral therapy had a two-fold increased risk of subfertility (weighted and adjusted [w-aRR 2.00; 95% CI: 1.19–3.34). Infertility risk was attenuated in women on antiretroviral therapy but remained elevated even after ≥2 years on antiretroviral therapy (w-aRR 1.63; 95% CI: 0.98–2.69). Other factors associated with subfertility were female age (w-aRR 1.03, 95% CI 1.01–1.05 per year), male HIV-positive status (w-aRR 1.31; 95% CI: 1.02–1.68), male smoking (w-aRR 1.29; 95% CI: 1.05–1.60) and trying to conceive for ≥1 year (w-aRR 1.38; 95% CI: 1.13–1.68).
Conclusions:
Two in three HIV-affected couples experienced subfertility. HIV-positive women were at increased risk of infertility, even when on antiretroviral therapy. Both male and female HIV status were associated with subfertility. Sub-fertility is an underrecognized reproductive health problem in resource limited settings and may contribute to prolonged HIV exposure and transmission within couples. Low cost approaches for screening and treating subfertility is this population are needed.
Keywords: fertility, HIV, safer conception, subfertility, sub-Saharan Africa
INTRODUCTION
With increased access to highly active antiretroviral therapy (HAART), HIV-positive individuals across sub-Saharan Africa are living longer, healthier lives.1–3 This improved outlook is altering the context of childbearing among HIV affected couples, and many people affected by HIV now desire to have children.4 While public health efforts to eliminate mother-to-child transmission have made great progress, attention only recently focused on prevention of horizontal transmission among serodiscordant couples trying to conceive.5–8
Reduced fertility may alter horizontal transmission risks and is influenced by behavioral, environmental and biological factors. One of the strongest determinants is age, with an inverse relationship with fecundity in women, and to a lesser extent for men.2 Behavioral factors such as use of cigarettes, alcohol, caffeine, diet, and stress levels also influence fertility, along with occupational and environmental exposures.2
The degree to which biological and behavioral factors influence fertility in the context of HIV is not fully understood.3,9–11 Data from the pre-HAART and early HAART-era suggested reduced fertility in the context of HIV12–16 due to immune suppression, co-infection with sexually transmitted infections (STIs), reduced sperm motility and volume in men, and anovulation/menstrual cycle irregularities in women.2,12,17–22 The relationship between subfertility and HIV may be bi-directional in sub-Saharan Africa, as subfertility may precede HIV infection when personal desires alongside cultural and social pressures to bear children contribute to repeated condomless sex and multiple partnerships, thus increasing STI and HIV acquisition risks among women trying to conceive.14,19 Among HIV serodiscordant couples, which account for 30% of stable heterosexual couples in South Africa,23 20–50% indeed report pregnancy desires as a reason for engaging in condomless sex.24 In the HAART era, an undetectable viral load prevents HIV transmission, which is particularly important in the periconception period when horizontal and vertical transmission risks are elevated.5,25,26 Viral suppression can however not be assumed among individuals on HAART.
Understanding the prevalence and determinants of subfertility among HIV-affected couples is thus essential for decreasing HIV transmission resulting from prolonged periods of HIV exposure and to maximize the effectiveness of safer conception services.1,27 The objective of this analysis is to determine the burden and predictors of subfertility among HIV-affected couples seeking safer conception care.
MATERIALS AND METHODS
Study population and design
Study participants were clients enrolled into the Sakh’umndeni Safer Conception Cohort between July 2013 and April 2017. Sakh’umndeni was a nurse-run primary care safer conception service located at Witkoppen Clinic in northern Johannesburg, South Africa.5 Participants were recruited through posters, flyers, media outreach, and clinician referrals at Witkoppen Clinic, surrounding primary health clinics and within community gathering places (e.g. malls and taxi ranks). Non-pregnant women of reproductive age (18–49 years) and men were eligible to participate if they were in a relationship where one or both partners were HIV-positive, planned to become pregnant in the next six months, and had not previously received an untreated infertility diagnosis. Participants were provided a comprehensive package of pre-conception care and safer conception strategies (e.g. STI screening and treatment, HAART initiation/management, viral load monitoring, pre-exposure prophylaxis (PrEP), male medical circumcision, condomless sex timed to the periovulatory period and home-based self-insemination for partnerships in which the male partner was HIV-negative) and were followed up monthly. Once the couple was virally suppressed (<50 copies/mL) or stable on PrEP, and clinically ready for conception (e.g. STIs screened and treated), providers gave clients a “greenlight” to attempt conception. Following enrollment, clients were prospectively followed until delivery (if they conceived), until completion of 6-months of attempted conception at which time they were referred to outside fertility services, or until they voluntarily exited the service.
Clinic procedures and data collection
At enrollment into the safer conception clinic, participants underwent a medical examination, pap smear, syphilis testing, syndromic screening for STIs, a medical history assessment-including a full reproductive history, HIV counseling and testing (if HIV-negative or unknown status), HAART initiation (if HIV-positive and not on treatment) and viral load monitoring (if HIV-positive). PrEP was offered to HIV-negative partners after discussing risks and benefits. In all cases, patients with syndromic screening indicative of an STI or diagnostic diagnoses (syphilis, abnormal histology on cervical pap smears) were treated and advice was provided to delay conception until completion of STI management.
STI and reproductive histories, current symptoms, time spent previously trying to conceive and prior infertility procedures, consultations and diagnoses were considered by the attending clinician at enrollment. Participants with known untreated infertility were not eligible for the service and those with a strong underlying suspicion of infertility were referred immediately to gynecological and/or infertility services. However, the closest public fertility service was 50 kilometers away and even at reduced prices, cost prohibitive to most couples in this cohort. As such, we were conservative in our baseline referrals understanding that infertility was not established and in the absence of safer conception service most patients would fall out of preconception care.
Information was collected for all female (n=334) and male participants (n=192). Women unaccompanied by their male partners (n=142) provided information about her male partner’s age, behaviors (e.g. smoking, drinking), health, and reproductive history.
Measures
The primary outcome for this analysis was subfertility, defined as failure to conceive within six months from first reported condomless vaginal intercourse during safer conception follow-up. Subfertility is generally defined as “any form of reduced fertility with prolonged time of unwanted non-conception.”28 We chose six months because most pregnancies (85%) occur within the first six months of condomless sex during the fertile phase,28 the safer conception service aims to reduce prolonged HIV exposure to partners, and because women who fail to conceive within a six-month trial period were routinely referred to fertility services. The subfertility outcome includes both primary subfertility (among women who have never become pregnant) and secondary subfertility (among women who have been pregnant before). Exposures of interest included sociodemographic and partnership characteristics, behavioral factors and clinical and reproductive characteristics that were supported by the literature or hypothesized to be associated with fertility outcomes. All variables used were collected at baseline, with the exception of average number of sex acts and whether the male partner ever attended, which were summarized across study visits. Sociodemographic characteristics included female age and employment status. Partnership-level characteristics included HIV status within the partnership, age difference between partners, and relationship duration with the current partner. Clinical characteristics examined included: female and male partner HIV status (confirmed through clinical records/testing for enrolled participants and self-report for male partners not in attendance), HAART use, female weight, self-reported and baseline measured STI history, history of tuberculosis and male partner erectile dysfunction. Behavioral characteristics included: male partner cigarette smoking (current vs. non-smoker), alcohol and drug use (yes/no), average condomless sex acts per month, and whether the couple ever attended safer conception services together. Female reproductive history variables included: number of previous live births, previous pregnancies with current partner (yes/no), age at menarche, history of abortion or miscarriage, irregular and missed menstrual periods, average reported number of flow days per menstrual cycle, and history of inability to conceive over at least twelve months of trying.13,29,30
Statistical Analysis
Characteristics of study participants were described, and the prevalence of subfertility assessed. Univariate and multivariable robust Poisson regression models were used to estimate associations with subfertility. Inclusion of variables in the multivariable model was determined by the univariate model results (p-values<0.10), as well as predictors hypothesized a priori. Variance inflation factors (VIFs) were used to assess multicollinearity between variables of interest, and collinear variables (VIF>10) were excluded from the model.
Many women discontinued services prior to six months of attempted conception because they were no longer trying to conceive, due to partnership dissolution or in cases of loss-to-follow-up. Inverse probability weighting (IPW) was used to account for underrepresentation of those missing the full six months of attempted conception time.31 For IPW, multivariable robust Poisson regression was used to identify variables associated with having completed six months of follow-up.32 A multivariable logistic regression model was then used to estimate the IPWs. Each participant was assigned a weight equal to the inverse probability of having six months of attempted conception based on observed associated covariates for this outcome. Weights from the multivariable model were estimated, creating a new pseudo-population in which clients missing six-months of attempted conception are “replaced” by up-weighting those with complete attempted conception time, who have the same exposure covariates.31
A final IPW-weighted multivariable predictive model of subfertility was estimated using robust Poisson regression.
We conducted several sensitive analyses to assess the overall robustness of our findings. Sensitivity analyses were completed to account for potential selection bias, given that many women attending the service reported unsuccessfully trying to conceive for 12 months or more at enrollment. To explore whether this may have impacted our inferences, we reran our primary analyses in the restricted subset of women who did not report trying to conceive for 12 or more months at enrollment. Additionally, given that CD4 cell counts were only available among HIV-positive women, to assess the potential unaccounted for impact of CD4 cell count, following our primary analysis approach, we conducted a sensitivity analysis among HIV-positive women which included baseline CD4 count as a categorical variable in the model (≤200 cells/mm3, 201–349 cells/mm3, 350–499 cell/mm3, 500+ cell/mm3). Finally, due to collinearity, HAART use and duration could not be assessed simultaneously with partner serodynamics in any of the models. We thus conducted a sensitivity analysis to assess the joint effect of partner serodynamics (seroconcordant vs. serodiscordant) on sub-fertility.
IPW-weighted, univariate risk differences (RD) were estimated to assess the absolute risk of subfertility across predictors because RDs consider the population prevalence of exposures and provide a potentially clinically meaningful guide to subfertility screening. Predictors included in this analysis were those that were significant by univariate relative risk comparisons.
Data were entered into a REDCap database (Vanderbilt, TN, USA) and all analyses were conducted using Stata 14.1 (College Station, TX).
Ethics
This study was approved by the Human Research Ethics Committee (Medical) at the University of Witwatersrand, Johannesburg and the University of North Carolina. Participants completed written, informed consent.
RESULTS
Client characteristics
Overall, 334 women were enrolled into care and followed for pregnancy in the Sakh’umndeni Safer Conception Cohort from July 2013 to December 2017. Characteristics of individuals utilizing services are presented in Table 1. The median age of women and male partners at enrollment was 34 years (interquartile range [IQR]: 30–38) and 38 years (IQR: 34–42), respectively. The majority (74%, n=246) of couples reported having been together for more than two years, with a median relationship duration of 5 years (IQR: 2–10). Most women (n=291/334, 87%) and 58% of male partners (n=192/334) were HIV-positive; the status of 7% (n=23/334) of men was unknown. In terms of couples’ serostatus, 45% of couples were seroconcordant HIV-positive, 43% were serodiscordant or sero-unknown with an HIV-positive female partner and 13% were serodiscordant with an HIV-positive male partner. Of the HIV-positive women, 84% were on HAART at cohort enrollment, 61% had undetectable viral loads (<50 copies/mL) and 46% had a CD4 count of 500 or above. Reported STI history, baseline and syndromic diagnosis through follow up for women was 3% (n=9/334), 3% (n=11/334) and 12% (n=21/182) among women retained through six months of follow-up. Most women had previously been pregnant (77%, n=256/334) and 19% (n=63/334) reported a missed or irregular period within the past three months. Nine percent (n=30/334) of male partners reported erectile dysfunction. During follow-up, the median number of reported condomless sex acts per month was 2.3 (IQR: 1.0–4.3), which did not differ by couple partner serodynamics (p=0.99).
Table 1:
Characteristics of Sakh’umndeni Safer conception service female clients at Witkoppen Health and Welfare Centre, Johannesburg, South Africa (n=334)
| Early Exit from Study (N=152) | Completed Six months of follow-up (N=182) | Total (N=334) | P-value | |
|---|---|---|---|---|
| Individual Level Characteristics | ||||
| Age, median (IQR) | 34.0 (30.0–38.5) | 33.0 (30.0–37.0) | 34.0 (30.0–38.0) | 0.341 |
| Partner age, median (IQR) | 38.0 (33.0–43.0) | 37.9 (34.0–41.0) | 38.0 (34.0–42.0) | 0.331 |
| Employed, n (%) | 91 (59.9%) | 125 (68.7%) | 216 (64.7%) | 0.093 |
| Median monthly income, USD (IQR) | 344.0 (172.0–516.0) | 344.0 (215.0–516.0) | 344.0 (215.0–516.0) | 0.732 |
| Completed Education, n (%) | 0.216 | |||
| Grade school or less | 94 (61.8%) | 127 (69.8%) | 221 (66.2%) | |
| Completed secondary | 39 (25.7%) | 41 (22.5%) | 80 (24.0%) | |
| Tertiary | 19 (12.5%) | 14 (7.7%) | 33 (9.9%) | |
| Residence, n (%) | 0.877 | |||
| Northern Johannesburg | 132 (86.8%) | 157 (86.3%) | 289 (86.5%) | |
| Outside of northern Johannesburg | 20 (13.2%) | 25 (13.7%) | 45 (13.5%) | |
| Partnership Level Characteristics | ||||
| HIVstatus within the partnership, n (%) | 0.024 | |||
| F (−) | M (+) | 20 (13.2%) | 23 (12.6%) | 43 (12.9%) | |
| F (+) | M (+) | 56 (36.8%) | 93 (51.1%) | 149 (44.6%) | |
| F (+) | M (−) (unknown) | 76 (50.0%) | 66 (36.3%) | 142 (42.5%) | |
| Male partner HIV status, n(%) | 0.039 | |||
| HIV- | 63 (41.4%) | 56 (30.8%) | 119 (35.6%) | |
| HIV+ | 76 (50.0%) | 116 (63.7%) | 192 (57.5%) | |
| Unknown | 13 (8.6%) | 10 (5.5%) | 23 (6.9%) | |
| Age difference between partners | 0.357 | |||
| < 5 years | 87 (57.2%) | 95 (52.2%) | 182 (54.5%) | |
| > 5 years | 65 (42.8%) | 87 (47.8%) | 152 (45.5%) | |
| Relationship Duration with current partner, median (IQR) | 6.0 (2.0–10.0) | 4.0 (3.00–10.0) | 5.0 (2.0–10.0) | 0.507 |
| Clinical & Behavioral Characteristics | ||||
| a On Antiretroviral Therapy, n (%) | 0.656 | |||
| No | 20 (15.2%) | 27 (17.1%) | 47 (16.2%) | |
| Yes | 112 (84.8%) | 131 (82.9%) | 243 (83.8%) | |
| Female HIV status and HAART use | 0.905 | |||
| HIV- | 20 (13.2%) | 23 (12.6%) | 43 (12.9%) | |
| HIV+, no HAART | 20 (13.2%) | 28 (15.4%) | 48 (14.4%) | |
| HIV+, HAART 0–2 years | 53 (34.9%) | 58 (31.9%) | 111 (33.2%) | |
| HIV+, HAART 2+ years | 59 (38.8%) | 73 (40.1%) | 132 (39.5%) | |
| a, b Female Baseline Viral Load | 0.528 | |||
| undetectable, n (%) | 81 (63.3%) | 93 (59.6%) | 174 (61.3%) | |
| a, c Female CD4, n (%) | 0.405 | |||
| <200 | 13 (10.2%) | 11 (7.0%) | 24 (8.4%) | |
| 201–349 | 31 (24.2%) | 30 (19.1%) | 61 (21.4%) | |
| 350–499 | 31 (24.2%) | 37 (23.6%) | 68 (23.9%) | |
| 500 or more | 53 (41.4%) | 79 (50.3%) | 132 (46.3%) | |
| Weight in kilograms, median (IQR) | 70.9 (59.5–79.8) | 66.6 (58.6–79.3) | 69.0 (59.0–79.5) | 0.766 |
| Current or History of STI, n (%) | 9 (5.9%) | 9 (4.9%) | 18 (5.4%) | 0.694 |
| Smoker | 11 (7.2%) | 8 (4.4%) | 19 (5.7%) | 0.264 |
| Partner Smoker | 49 (32.2%) | 43 (23.6%) | 92 (27.5%) | 0.080 |
| Any alcohol consumption by partner | 66 (43.4%) | 55 (30.2%) | 121 (36.2%) | 0.010 |
| d Alcoholic drinks per week, median (IQR) | 0 (0–2) | 0 (0–1) | 0 (0–1) | <0.01 |
| History of TB, n (%) | 19 (12.5%) | 28 (15.4%) | 47 (14.1%) | 0.450 |
| Reported erectile dysfunction | 8 (5.3%) | 22 (12.1%) | 30 (9.0%) | 0.030 |
| Condomless sex acts per month, median (IQR) | 1.6 (0.0–3.4) | 2.9 (1.5–4.3) | 2.3 (1.0–4.3) | 0.182 |
| Accompanied by Partner in clinic | 73 (48.0%) | 119 (65.4%) | 192 (57.5%) | <0.01 |
| Reproductive History | ||||
| History of previous pregnancy | 111 (73.0%) | 145 (79.7%) | 256 (76.6%) | 0.153 |
| Previously been pregnant with current partner, n (%) | 46 (30.3%) | 70 (38.5%) | 116 (34.7%) | 0.117 |
| Number of live births | 0.016 | |||
| Zero | 62 (40.8%) | 50 (27.5%) | 112 (33.5%) | |
| One | 59 (38.8%) | 75 (41.2%) | 134 (40.1%) | |
| Two or more | 31 (20.4%) | 57 (31.3%) | 88 (26.3%) | |
| e Reported history of abortion | 6 (5.4%) | 16 (11.0%) | 22 (8.6%) | 0.111 |
| e Reported history of miscarriage | 37 (33.3%) | 44 (30.3%) | 81 (31.6%) | 0.610 |
| Age at Menarche, median (range) | 15 (14–16) | 15 (14–16) | 15 (14–16) | 0.408 |
| Flow days in Menstrual Cycle, median (range) | 4 (3–5) | 4 (3–5) | 4 (3–5) | 0.972 |
| Missed or irregular period in the past three months | 27 (17.8%) | 36 (19.8%) | 63 (18.9%) | 0.639 |
| Tried to conceive for at least one year and failed | 64 (42.1%) | 65 (35.7%) | 129 (38.6%) | 0.232 |
IQR: interquartile range, USD: United States Dollar, HAART: highly active antiretroviral therapy, STI: sexually transmitted infection, TB: Turberculosis, Undetectable viral load (<50 copies/mL)
Among HIV+ female clients (n=291)
Baseline viral loads available for 284 HIV+ women
CD4 counts available for 285 HIV+ women
Among partners with any reported alcohol (n=121)
Among clients who have history of previous pregnancy (n=256)
Retention throughout conception trial period
Overall, 152 women (46%) did not complete the six-month conception trial period and thus could not be classified with respect to subfertility. Reasons included loss to follow-up (n=70/152, 46%), referral for fertility work-up prior to six months based on clinician judgment (n=25/152, 16%), termination of relationship with partner (n=13/152, 9%) and combined other infrequently reported reasons (n=26/152, 17%). Study retention was associated with partner accompaniment to the clinic, erectile dysfunction, male HIV status, history of two or more live births, history of abortion, male partner cigarette and alcohol use. These factors and a priori determined variables of importance (female partner HIV status and duration of HAART use) were included in the multivariable IPW model (Supplemental Table 1), generating weights ranging from 1.09–6.20, with a median value of 1.83.
Subfertility
Two-thirds of women were classified as subfertile (crude proportion 64%, IPW-weighted estimate 65% (95% CI: 0.59–0.73) with primary and secondary subfertility contributing to 33% (n=39/117) and 67% (n=78/117) of cases respectively.
In univariate analysis, female age, HIV-positivity of the female partner (regardless of HAART use), having a male partner who is a smoker, and a history of unsuccessfully trying to conceive for at least one year prior to participation in safer conception services were all associated with greater subfertility, while having two or more children was associated with a decreased risk for subfertility (Table 2). In multivariable and IPW-multivariable adjusted models, female age, HIV-positivity of the female partner, male partner smoking, history of unsuccessfully trying to conceive for at least one year and parity remained associated with subfertility, as was male partner HIV-positivity (Table 2). The relationship between a woman’s HIV status and subfertility was present among both women who were HAART-experienced and HAART-naive at enrollment. Compared to HIV-negative women, the risk of subfertility was increased two-fold among HIV-positive women on HAART for less than two years at enrollment and by 63% among women on HAART for two or more years. Subfertility was 31% higher among women with HIV-positive male partners (a-wRR: 1.31, 95% CI: 1.02–1.68). For every one-year increase in women’s age there was a three percent increased risk of subfertility (a-wRR: 1.03, 95% CI: 1.01–1.05, p-value <0.01). Other factors associated with increased subfertility were male partner smoking (w-aRR 1.29; 95% CI:1.05–1.60) and a history of trying to conceive for ≥1 year prior to enrollment (w-aRR 1.38; 95% CI: 1.13–1.68). The overall findings -including relationships between HIV status and HAART use- were robust in a sensitivity analysis restricted to women who did not report unsuccessfully trying to conceive for 12-months or more at enrollment (Supplemental Table 2). Similarly, in a sensitivity analysis restricted to HIV-positive women, HAART use and duration remained unprotective after adjusting for CD4 count (table not shown).
Table 2:
Correlates of subfertility among women attending the safer conception cohort (n=182)
| Univariate | Multi variable | Weight Adjusted MuInvariable | ||||
|---|---|---|---|---|---|---|
| RR (95% Cl) | p-Value | RR (95%CI) | p-Value | RR (95%CI) | p-Value | |
| Individual & Partnership Characteristics | ||||||
| Female Age | 1.03 (1.01–1.05) | <0.01 | 1.03 (1.01–1.05) | <0.01 | 1.03 (1.01–1.05) | <0.01 |
| Employment status | ||||||
| Unemployed | REF | |||||
| Employed | 0.95 (0.75–1.19) | 0.646 | ||||
| Age difference between partners | ||||||
| <5 years | REF | REF | REF | |||
| > 5 years | 0.81 (0.65–1.02) | 0.072 | 0.86 (0.70–1.05) | 0.149 | 0.87 (0.71–1.07) | 0.177 |
| Length of Relationship with Current partner | ||||||
| 0–2 years | REF | |||||
| 2 or more years | 1.06 (0.82–1.39) | 0.652 | ||||
| Clinical & Behavioral Characteristics | ||||||
| Female HIV status and HAART use | ||||||
| HIV- | REF | REF | REF | |||
| HIV+, no HAART | 2.01 (1.16–3.47) | 0.012 | 2.00 (1.21–3.28) | <0.01 | 2.00 (1.19–3.34) | <0.01 |
| HIV+, HAART 0–2 years | 1.63 (0.94–2.82) | 0.080 | 1.76 (1.08–2.87) | 0.024 | 1.89 (1.14–3.10) | 0.013 |
| HIV+, HAART 2+ years | 1.72 (1.00–2.93) | 0.048 | 1.62 (0.99–2.65) | 0.056 | 1.63 (0.98–2.69) | 0.058 |
| Male partner HIV status | ||||||
| HIV- | REF | REF | REF | |||
| HIV+ | 1.11 (0.86–1.44) | 0.417 | 1.33 (1.04–1.71) | 0.025 | 1.31 (1.02–1.68) | 0.035 |
| Unknown | 1.36 (0.93–1.99) | 0.115 | 1.32 (0.87–2.00) | 0.189 | 1.34 (0.91–1.98) | 0.134 |
| Weight in kg | 1.00 (0.99–1.01) | 0.903 | ||||
| Current or History of STI | ||||||
| No | REF | |||||
| Yes | 1.04 (0.65–1.67) | 0.875 | ||||
| Partner Smoker | ||||||
| No | REF | REF | REF | |||
| Yes | 1.27 (1.03–1.57) | 0.028 | 1.31 (1.05–1.63) | 0.015 | 1.29 (1.05–1.60) | 0.017 |
| Any alcohol consumption by partner | ||||||
| No | REF | |||||
| Yes | 1.03 (0.81–1.30) | 0.827 | ||||
| History of TB | ||||||
| No | REF | |||||
| Yes | 1.07 (0.80–1.41) | 0.656 | ||||
| Partner reported erectile dysfunction | ||||||
| No | REF | |||||
| Yes | 0.68 (0.42–1.09) | 0.109 | ||||
| Condomless sex acts per month | 1.01 (0.97–1.06) | 0.542 | ||||
| Accompanied by partner in clinic | ||||||
| Unaccompanied | REF | |||||
| Accompanied | 0.95 (0.76–1.18) | 0.621 | ||||
| Reproductive History | ||||||
| Number of live births | ||||||
| Zero | REF | REF | REF | |||
| One | 0.82 (0.65–1.03) | 0.085 | 0.96 (0.77–1.21) | 0.736 | 0.98 (0.77–1.24) | 0.861 |
| Two or more | 0.67 (0.51–0.90) | <0.01 | 0.80 (0.60–1.07) | 0.133 | 0.77 (0.57–1.03) | 0.079 |
| Previously been pregnant with current partner | ||||||
| No | REF | REF | REF | |||
| Yes | 0.80 (0.63–1.02) | 0.072 | 0.92 (0.72–1.16) | 0.472 | 0.91 (0.72–1.15) | 0.431 |
| a Reported history of abortion | ||||||
| No | REF | |||||
| Yes | 1.14 (0.79–1.63) | 0.484 | ||||
| a History of miscarriage | ||||||
| No | REF | |||||
| Yes | 1.11 (0.85–1.45) | 0.447 | ||||
| Age at Menarche | 0.97 (0.92–1.03) | 0.356 | ||||
| Flow days in Menstrual Cycle | 1.02 (0.97–1.07) | 0.448 | ||||
| Missed or irregular period in the past three months | ||||||
| No | REF | REF | REF | |||
| Yes | 1.10 (0.86–1.42) | 0.447 | 1.20 (0.92–1.56) | 0.182 | 1.17 (0.90–1.51) | 0.240 |
| Tried to conceive for at least one year and failed | ||||||
| No | REF | REF | REF | |||
| Yes | 1.49 (1.22–1.82) | <0.001 | 1.42 (1.17–1.73) | <0.01 | 1.38 (1.13–1.68) | <0.01 |
HAART: highly active antiretroviral therapy, STI: sexually transmitted infection, TB: Turberculosis
Models estimated using robust Poisson regression. Weight adjusted multivariable models apply inverse probability weighting.
Among clients who have history of previous pregnancy (n=145)
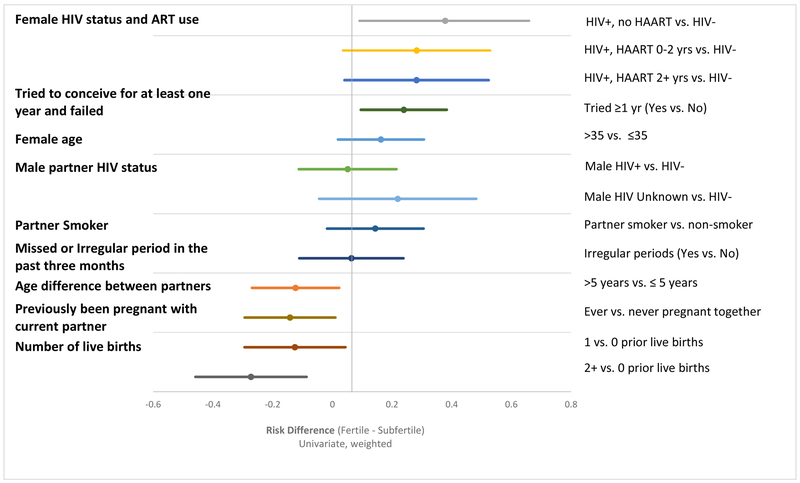
In a further sensitivity analysis assessing the joint effect of couples’ HIV status on sub-fertility (rather than the independent effects of the woman’s HIV status and the man’s HIV status), being in a seroconcordant positive relationship increased the risk of subfertility by 78% as compared to serodiscordant couples with HIV-negative female partners (w-aRR: 1.78, 95% CI: 1.10–2.90) (Supplemental Table 3). Subfertility was elevated, but not statistically significantly higher in partnerships in which only the female partner was living with HIV, as compared to serodiscordant relationships with an HIV-negative woman (w-aRR: 1.42, 95% CI: 0.85–2.38). Risk differences were calculated to assess the factors most strongly associated with absolute risk for subfertility (Figure 1). The predictors attributable to the largest fraction of subfertility were female HIV status and HAART duration, followed by failure to conceive after at least one year of trying at some point prior to safer conception care, female partner age, and male partner HIV status.
Figure 1:
Weighted univariate risk difference estimates for predictors of subfertility in the safer conception cohort
COMMENT
We found a very high burden of subfertility among HIV-affected couples trying to conceive, with two out of every three women accessing safer conception services failing to conceive within 6 months. HIV infection, in both women and men had a negative effect on fertility, even among women on HAART. HIV-positive seroconcordant couples had a 78% increased risk of subfertility compared to serodiscordant couples where only the male partner was HIV-positive. These data suggest that even in the HAART-era, HIV can have an important impact on fertility and potentially prolong attempted conception which could in turn increase HIV transmission risks between partners.
In terms of predictors of subfertility, older female age was significantly associated with subfertility, which is well established in the literature.33 HIV infection among women was associated with subfertility even when accounting for HAART use and duration. Compared to HIV-negative women, HIV-positive women not on HAART at enrollment or starting HAART in the past two years had a 2-fold increased risk of subfertility. Women on HAART for more than two years still had a 63% increased risk of subfertility compared to HIV-negative women. These findings support previous research which suggest that HAART use does not fully restore fertility among HIV-positive women to the level of HIV-negative women.34,35 Explanations for subfertility despite HAART use may include a sustained relative immune-compromised state or HAART drug-related toxicities. Data on these issues are limited and inconclusive.13
Prior work has shown that in 20–26% of couples, a male factor is the cause of subfertility.36 Few studies, however, have investigated the association of HIV infection on male fertility, and of these the evidence is mixed. Some studies report unimpaired semen parameters among men with asymptomatic HIV infection, while others report reduced sperm motility and semen volume.2,18 Although our study did not include a semen analysis, our findings add epidemiologic evidence of the impact of HIV on male subfertility, independent of their female partner’s HIV status. Women with partners who smoke were also at higher risk for subfertility, which is in line with prior evidence.37,38 Overall, our findings support the need to screen for male biological and behavioral characteristics when assessing potential risk for subfertility among HIV-affected couples instead of just assessing female factors.
We also found that over one in three couples had already failed to conceive for over one year, and these couples had a 34% increased risk of subfertility after six months of attempted conception. Timely referrals for couples with suspected fertility challenges to gynecologists or fertility specialists while simultaneously ensuring optimal clinical management of HIV within safer conception care is thus essential to reduce prolonged HIV transmission risks. However, specialized fertility services are frequently not accessible or affordable in the public sector in many resource-constrained settings, and anecdotal data from our service suggest that very few women referred out successfully received infertility-related workups and care. These findings suggest the importance of providing low-cost fertility services in resource-constrained settings to support reproductive health and reduce transmission among HIV-affected couples who may have prolonged HIV transmission risks when desired conception outcomes are not achieved. Strengths and limitations of this study should be considered while interpreting results. Although patients receiving safer conception services are advised to stop condomless sex until risk of transmission is minimized, clients often started attempting conception prior to being given the ‘greenlight’ from their healthcare providers. To account for this, our study began the six-month period of attempted conception at the start of reported condomless sex. This approach more accurately captures the time at risk for pregnancy but does not capture heterogeneity in the frequency of condomless sex, which may increase after the ‘greenlight’ was given and may underestimate time at risk for subfertility among women not reporting condomless sex prior to the “greenlight” period for social desirability purposes. Additionally, to account for insufficient follow-up time for individuals missing the full six months of attempted conception, IPWs were applied to address biases resulting from potential differences between clients with and without six months of attempted conception. As women were referred to outside care after six months of attempted conception, we limited our assessment to subfertility and were unable to prospectively assess infertility which is defined as attempted conception for 12 months. Our approach importantly accounts for characteristics of both the female and male partners, however for the subset of women who reported information on their male partner because he was not in attendance, misclassification of male characteristics is possible. Our study relied on many self-reported measures which may introduce bias, for example alcohol and smoking reports may underestimate usage. Additionally, biological measures including hormone levels, semen analysis and diagnostic screening for gonorrhea and chlamydia were not available. These findings highlight the importance of research to examine these underlying markers which may be impacting fertility. Furthermore, safer conception services may attract a population at higher risk of subfertility than the general population of HIV-affected couples, further emphasizing the need for providers to take detailed medical histories at HIV care entry to screen for potential infertility, and adequately counsel HIV couples about the risks and benefits of trying to conceive. We assessed the robustness of our findings among women who did not report previously trying to conceive for 12-months or more at enrollment and found our inferences to be unchanged. Even so, our results may not be generalizable to other settings if women choosing to use this service differ from those who may take up services in different contexts.
Our findings raise awareness of the burden of subfertility among HIV-affected couples and advocate for the development of subfertility screening. HIV serodynamics – including both the woman and man’s HIV status - and women’s treatment status are important determinants of absolute risk of subfertility in this population, along with prior failed conception attempts, female age and male partner smoking status. Screening for pre-existing fertility concerns, smoking cessation counseling, and low-cost ovulation predictor kits should be incorporated into HIV care and safer conception interventions to reduce the risk of HIV transmission within couples and to ensure opportunities for creating a family for all couples, independent of their HIV status.
Supplementary Material
Condensation.
HIV infection in women-including those on antiretroviral therapy-but also in men, is associated with a high burden of subfertility among HIV-affected couples trying to conceive.
AJOG at a Glance:
A. Safer conception care for couples with at least one HIV-positive partner promotes optimal pregnancy health and prevents onward HIV transmission to HIV-negative partners while trying to conceive and to potential infants, but may be negatively impacted by underlying subfertility.
B. Among couples engaged in safer conception services in South Africa, two-thirds did not conceive by 6-months of trying; subfertility was twice as high among HIV-positive women not on anti-retroviral therapy and was also independently associated with male partner HIV status.
C. HIV had a negative impact on fertility in the pre-antiretroviral therapy era, however more recent data have been limited; these findings suggest that even with growing antiretroviral therapy coverage, HIV-affected couples commonly experience subfertility, which may play a role in sustained HIV transmission risks among HIV serodiscordant couples trying to conceive.
Acknowledgments
We are grateful to the patients, study team and healthcare providers at Witkoppen Health and Welfare Centre for their participation and dedication towards this research. Support for this research was provided by the United States Agency for International Development (USAID) and the President’s Emergency Plan for AIDS Relief (PEPFAR) [award number AID: 674-A-12–00033], the UJMT Fogarty Grant/Fogarty International Center of the National Institutes of Health [award number R25TW009340] and the Johns Hopkins University Center for AIDS Research [award number P30AI094189]. The content is solely the responsibility of the authors and does not necessarily represent the official views of USAID/PEPFAR, the National Institutes of Health or the United States Government.
Role of the funding source: This work was supported through the President’s Emergency Plan for AIDS Relief (PEPFAR) through United States Agency for International Development (USAID) under the terms of AID-674-A-12–00033, the UJMT Consortium/Fogarty International Center of the National Institutes of Health (R25TW009340), and the Johns Hopkins University Center for AIDS Research through the National Institutes of Health (P30AI094189). The sponsor did not have any role in the design of the study, data collection, data analysis or interpretation of the data, write-up of results or the decision to submit the article for publication. The contents are the sole responsibility of Witkoppen Health and Welfare Centre and the authors and do not necessarily reflect the views of USAID, NIH or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The authors report no conflict of interest.
REFERENCES
- 1.Bekker L, Black V, Myer L, et al. Guideline on safer conception in fertile HIV-infected individuals and couples: guideline. Southern African Journal of HIV Medicine 2011; 12(2): 31–44. [Google Scholar]
- 2.van Leeuwen E, Prins JM, Jurriaans S, et al. Reproduction and fertility in human immunodeficiency virus type-1 infection. Human Reproduction Update 2006; 13(2): 197–206. [DOI] [PubMed] [Google Scholar]
- 3.McLean E, Price A, Chihana M, et al. Changes in Fertility at the Population Level in the Era of ART in Rural Malawi. Journal of acquired immune deficiency syndromes (1999) 2017; 75(4): 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wekesa E, Coast E. Fertility desires among men and women living with HIV/AIDS in Nairobi slums: a mixed methods study. PloS one 2014; 9(8): e106292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz SR, Bassett J, Sanne I, Phofa R, Yende N, Van Rie A. Implementation of a safer conception service for HIV-affected couples in South Africa. AIDS 2014; 28 Suppl 3: S277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz SR, West N, Phofa R, et al. Acceptability and preferences for safer conception HIV prevention strategies: a qualitative study. Int J STD AIDS 2016; 27(11): 984–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz SR, Bassett J, Holmes CB, et al. Client uptake of safer conception strategies: implementation outcomes from the Sakh’umndeni Safer Conception Clinic in South Africa. Journal of the International AIDS Society 2017; 20(S1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthews LT, Crankshaw T, Giddy J, et al. Reproductive counseling by clinic healthcare workers in Durban, South Africa: perspectives from HIV-infected men and women reporting serodiscordant partners. Infectious diseases in obstetrics and gynecology 2012; 2012: 146348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nattabi B, Li J, Thompson SC, Orach CG, Earnest J. A Systematic Review of Factors Influencing Fertility Desires and Intentions Among People Living with HIV/AIDS: Implications for Policy and Service Delivery. AIDS and Behavior 2009; 13(5): 949–68. [DOI] [PubMed] [Google Scholar]
- 10.Mumah JN, Ziraba AK, Sidze EM. Effect of HIV status on fertility intention and contraceptive use among women in nine sub-Saharan African countries: evidence from Demographic and Health Surveys. Global health action 2014; 7(1): 25579–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakayiwa S, Abang B, Packel L, et al. Desire for children and pregnancy risk behavior among HIV-infected men and women in Uganda. AIDS and behavior 2006; 10(4 Suppl): S95. [DOI] [PubMed] [Google Scholar]
- 12.Gray RH, Wawer MJ, Serwadda D, et al. Population-based study of fertility in women with HIV-1 infection in Uganda. The lancet 1998; 351(9096): 98–103. [DOI] [PubMed] [Google Scholar]
- 13.Kushnir VAMD Lewis WMD. Human immunodeficiency virus/acquired immunodeficiency syndrome and infertility: emerging problems in the era of highly active antiretrovirals. Fertility and Sterility 2011; 96(3): 546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Favot I, Ngalula J, Mgalla Z, Klokke AH, Gumodoka B, Boerma JT. HIV infection and sexual behaviour among women with infertility in Tanzania: a hospital-based study. International journal of epidemiology 1997; 26(2): 414–9. [DOI] [PubMed] [Google Scholar]
- 15.Dhont N, Muvunyi C, Luchters S, et al. HIV infection and sexual behaviour in primary and secondary infertile relationships: a case–control study in Kigali, Rwanda. Sexually transmitted infections 2011; 87(1): 28–34. [DOI] [PubMed] [Google Scholar]
- 16.Yeatman S, Eaton JW, Beckles Z, Benton L, Gregson S, Zaba B. Impact of ART on the fertility of HIV-positive women in sub-Saharan Africa. Trop Med Int Health 2016; 21(9): 1071–85. [DOI] [PubMed] [Google Scholar]
- 17.Pilatz A, Discher T, Lochnit G, et al. Semen quality in HIV patients under stable antiretroviral therapy is impaired compared to WHO 2010 reference values and on sperm proteome level. AIDS 2014; 28(6): 875–80. [DOI] [PubMed] [Google Scholar]
- 18.Kehl S, Weigel M, Müller D, Gentili M, Hornemann A, Sütterlin M. HIV-infection and modern antiretroviral therapy impair sperm quality. Archives of Gynecology and Obstetrics 2011; 284(1): 229–33. [DOI] [PubMed] [Google Scholar]
- 19.Ross A, Morgan D, Lubega R, Carpenter LM, Mayanja B, Whitworth JAG. Reduced fertility associated with HIV: the contribution of pre-existing subfertility. AIDS 1999; 13(15): 2133. [DOI] [PubMed] [Google Scholar]
- 20.Crittenden JA, Handelsman DJ, Stewart GJ. Semen analysis in human immunodeficiency virus infection**Supported in part by an AIDS research grant of the New South Wales Department of Health, Sydney, New South Wales, Australia.††Presented at the 23rd Annual Conference of the Australian Society for Reproductive Biology, Sydney, Australia, September 30 to October 2, 1991. Fertility and Sterility 1992; 57(6): 1294–9. [PubMed] [Google Scholar]
- 21.van Leeuwen E, Cornelissen M, de Vries JW, et al. Semen parameters of a semen donor before and after infection with human immunodeficiency virus type 1: Case report. Human Reproduction 2004; 19(12): 2845–8. [DOI] [PubMed] [Google Scholar]
- 22.Lo JC, Schambelan M. Reproductive Function in Human Immunodeficiency Virus Infection. The Journal of Clinical Endocrinology & Metabolism 2001; 86(6): 2338–43. [DOI] [PubMed] [Google Scholar]
- 23.Matthews LT, Moore L, Crankshaw TL, et al. South Africans with recent pregnancy rarely know partner’s HIV serostatus: implications for serodiscordant couples interventions. BMC Public Health 2014; 14(1): 843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hancuch K, Baeten J, Ngure K, et al. Safer conception among HIV-1 serodiscordant couples in East Africa: understanding knowledge, attitudes, and experiences. AIDS care 2018: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith SM, Baskin GB, Marx PA. Estrogen protects against vaginal transmission of simian immunodeficiency virus. The Journal of infectious diseases 2000; 182(3): 708–15. [DOI] [PubMed] [Google Scholar]
- 26.Consensus Statement. Risk of Sexual Transmission of HIV from a Person Living with HIV who has an Undetectable Viral Load: Messaging Primer & Consensus Statement. . 2016. https://www.preventionaccess.org/consensus (accessed August 15 2018).
- 27.Davies NE, Matthews LT, Crankshaw TL, Cooper D, Schwartz SR. Supporting HIV prevention and reproductive goals in an HIV-endemic setting: taking safer conception services from policy to practice in South Africa. J Int AIDS Soc 2017; 20(Suppl 1): 21271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gnoth C, Godehardt E, Frank-Herrmann P, Friol K, Tigges J, Freundl G. Definition and prevalence of subfertility and infertility. Human reproduction (Oxford, England) 2005; 20(5): 1144–7. [DOI] [PubMed] [Google Scholar]
- 29.Gizzo S, Andrisani A, Noventa M, et al. Menstrual cycle length: A surrogate measure of reproductive health capable of improving the accuracy of biochemical/sonographical ovarian reserve test in estimating the reproductive chances of women referred to ART; 2015. [DOI] [PMC free article] [PubMed]
- 30.Meczekalski B, Katulski K, Czyzyk A, Podfigurna-Stopa A, Maciejewska-Jeske M. Functional hypothalamic amenorrhea and its influence on women’s health. Journal of Endocrinological Investigation 2014; 37(11): 1049–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lippman SA, Shade SB, Hubbard AE. Inverse Probability Weighting in STI/HIV Prevention Research: Methods for Evaluating Social and Community Interventions. Sexually Transmitted Diseases 2010; 37(8): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol 2003; 3: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Louis GMB, Platt RW. Reproductive and perinatal epidemiology: Oxford University Press; 2011. [Google Scholar]
- 34.Maier M, Andia I, Emenyonu N, et al. Antiretroviral Therapy is Associated with Increased Fertility Desire, but not Pregnancy or Live Birth, among HIV+ Women in an Early HIV Treatment Program in Rural Uganda. AIDS & Behavior 2009; 13: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marston M, Zaba B, Eaton JW. The relationship between HIV and fertility in the era of antiretroviral therapy in sub-Saharan Africa: evidence from 49 Demographic and Health Surveys. Tropical Medicine & International Health 2017; 22(12): 1542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evers JLH. Female subfertility. The Lancet 2002; 360(9327): 151–9. [DOI] [PubMed] [Google Scholar]
- 37.Ramlau-Hansen CH, Thulstrup AM, Aggerholm AS, Jensen MS, Toft G, Bonde JP. Is smoking a risk factor for decreased semen quality? A cross-sectional analysis. Human Reproduction (Oxford, England) 2007; 22(1): 188–96. [DOI] [PubMed] [Google Scholar]
- 38.Sansone A, Di Dato C, Angelis Cd, et al. Smoke, alcohol and drug addiction and male fertility . Reproductive Biology and Endocrinology: RB&E 2018; 16(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.