Abstract
Introduction:
Lung cancer incidence is higher among AAs compared with European Americans in the United States. We, and others, previously demonstrated a relationship between immune and inflammation proteins with lung cancer in European Americans. Our aim was to investigate the etiological relationship between inflammation and lung cancer in African Americans.
Methods:
We adopted a two stage, independent study design (discovery n=316 cases and n=528 controls) (validation n=399 cases and 400 controls) and measured 30 inflammation proteins in blood using Mesoscale V-Plex multiplex assays.
Results:
We identified, and validated, ten proteins associated with lung cancer in AAs, some that were common between EA and AA (CRP (OR, 2.90; 95% CI, 1.99–4.22), IFN-γ (OR, 1.55; 95% CI, 1.10–2.19), IL-6 (OR, 6.28; 95% CI, 4.10–9.63), IL-8 (OR, 2.76; 95% CI, 1.92–3.98) and some that are only observed among AA (IL-10 (OR, 1.69; 95% CI, 1.20–2.38), IL-15 (OR, 2.83; 95% CI, 1.96–4.07), IP-10 (OR, 1.54; 95% CI, 1.09–2.18), MCP-4 (OR, 0.54; 95% CI, 0.38–0.76), MIP-1 α (OR, 1.57; 95% CI, 1.12–2.21), and TNF- β (OR, 0.52; 95% CI, 0.37–0.74). We did not find evidence that either menthol cigarette smoking or global genetic ancestry drove these population differences.
Conclusions:
Our results highlight a distinct inflammation profile associated with lung cancer in AAs compared with EAs. These data provide new insight into the etiology of lung cancer in AAs. Further work is needed to understand what drives this relationship with lung cancer and whether these proteins have utility in the setting of early diagnosis.
Introduction
Lung cancer is the leading cause of cancer-related death in the United States1–3. African Americans (AAs) have the highest lung cancer incidence and mortality rates compared with any other racial or ethnic group in the U.S.1. The reasons for these disparities are not fully understood4. Few studies have addressed differences in tumor biology between European Americans (EAs) and AAs. We recently identified transcriptomic differences between these two populations, some of which suggested differences in immune cell composition5, 6. Further, in an analysis of 10 serum cytokines, we observed differences in the relationship between immune proteins with lung cancer, including for IL-1b6. However, the study represented a small subset of inflammation proteins.
Several chronic inflammatory and infectious conditions are associated with an increased risk of lung cancer7–15. Indeed, detection of a systemic inflammation signal has been detected in lung cancer in populations of European descent, including C-reactive protein (CRP), interleukin-6 (IL-6), and interleukin-8 (IL-8)16–21. Inflammation protein levels fluctuate following exposure to tobacco smoke22–24. Smoking patterns are different between European Americans (EAs) and AAs: AAs smoke fewer cigarettes per day, start smoking later in life, are more likely to use menthol cigarettes and have a longer smoking duration4, 25–28. This suggests that the relationship between inflammation and lung cancer could be different between EAs and AAs. Recent evidence supports this possibility5, 6.
In this two-phase study design, we investigated the association of circulating levels of several key components of inflammation, including acute-phase proteins, pro- and anti-inflammatory cytokines, chemokines, growth factors, and angiogenesis factors, with lung cancer in AAs. In addition to providing etiologic insight into lung cancer health disparities, the discovery of circulating inflammation proteins associated with lung cancer in this case-control setting could identify circulating proteins that could be leveraged as biomarkers for lung cancer diagnosis and prognosis.
Materials and methods
Study design
For discovery, sera from 316 lung cancer cases and 509 population controls were selected from the National Cancer Institute-Maryland case control study. Detailed participant information is outlined in Table 1. All self-reported AAs enrolled since 1998 for whom a biospecimen was available were included in this study, therefore specific matching by age, gender or smoking was not possible. However, these variables were included in adjusted models. The validation phase included an independent study from the Wayne State University case control study. The sample size was chosen to allow sufficient power to replicate findings of the discovery dataset. The data and analyses in this study were prepared according to STARD guidelines.
Table 1:
Characteristics of participants in the discovery and validation case-control studies
| Characteristic | Discovery Study (NCI-MD) | Validation Study (WSU) | ||||
|---|---|---|---|---|---|---|
| P | P | |||||
| Age, years mean (range) | 64 (42–88) | 66 (43–86) | <0.001* | 63.6 (36–89) | 62.5 (46–87) | ns* |
| Gender, N (%) | ||||||
| Male | 177 (56%) | 346 (68%) | 177 (44%) | 177 (44%) | ||
| Female | 139 (44%) | 163 (32%) | 222 (56%) | 223 (56%) | ||
| Missing | 0 (0%) | 0 (0%) | <0.001† | 0 (0%) | 0 (0%) | ns† |
| Body Mass Index (kg/m2) N (%) | ||||||
| Underweight (<18.5) | 22 (7%) | 2 (<0.5%) | 38 (9.5%) | 3 (<1%) | ||
| Healthy (18.5–25) | 121 (38%) | 89 (18%) | 170 (42.5%) | 136 (34%) | ||
| Over-weight (25–30) | 105 (33%) | 180 (35%) | 110 (28%) | 131 (33%) | ||
| Obese (>30) | 68 (22%) | 238 (46.5%) | 81 (20.5%) | 130 (32.5%) | ||
| Missing | 0 (0%) | 0 (0%) | <0.001† | 0 (0%) | 0 (0%) | <0.001† |
| Smoking Status, N (%) | ||||||
| Never | 24 (8%) | 173 (34%) | 26 (6.5%) | 26 (6.5%) | ||
| Former | 114 (36%) | 244 (48%) | 159 (40%) | 123 (31%) | ||
| Current | 178 (56%) | 92 (18%) | 214 (53.5%) | 251 (62.5%) | ||
| Missing | 0 (0%) | 0 (0%) | <0.001† | 0 (0%) | 0 (0%) | 0.023† |
| Pack-years, mean ± SD (ever and never smokers only) | 39.19 ± 29.09 | 22.76 ± 18.36 | <0.001* | 36.64 ± 27.61 | 26.68 ± 20.33 | <0.001* |
| Menthol Cigarette Usage, N (%) | ||||||
| Menthol | 177 (60%) | 175 (52%) | 159 (42%) | 319 (85%) | ||
| Non- menthol | 96 (33%) | 125 (37%) | 29 (8%) | 54 (14%) | ||
| Both | 19 (7%) | 36 (11%) | - | - | ||
| Missing | 0 (0%) | 0 (0%) | 0.046† | 185 (50%) | 1 (<1%) | <0.001† |
| Filtered Cigarette Usage, Na (%) | ||||||
| Filtered | 248 (85%) | 285 (85%) | - | - | ||
| Non-filtered | 27 (9%) | 24 (7%) | - | - | ||
| Both | 17 (6%) | 27 (8%) | - | - | ||
| Missing | 0 (0%) | 0 (0%) | ns† | - | - | |
| History of emphysema, bronchitis, asthma, TB, N (%) | ||||||
| No | 184 (58%) | 398 (78%) | - | - | ||
| Yes | 132 (42%) | 111 (22%) | - | - | ||
| Missing | 0 (0%) | 0 (0%) | - | - | ||
| Stage, N (%) | ||||||
| I | 102 (32%) | - | 68 (17%) | - | ||
| II | 34 (11%) | - | 48 (12%) | - | ||
| III | 71 (23%) | - | 101 (25.5%) | - | ||
| IV | 62 (20%) | - | 168 (42%) | - | ||
| Missing | 47 (14%) | - | 14 (3.5%) | - | ||
| Histology, N (%) | ||||||
| Adenocarcinoma | 141 (45%) | - | 240 (60%) | - | ||
| Squamous | 90 (28%) | - | 108 (27%) | - | ||
| Other | 72 (23%) | - | 47 (12%) | |||
| Missing | 13 (4%) | 4 (<1%) | ||||
Statistically significant differences between cases and controls were determined using independent sample t-tests
Statistically significant differences between cases and controls were determined using chi-squared tests
NCI-Maryland Case Control Study
This is an ongoing case-control study within the Baltimore region of Maryland or Maryland Eastern shore and is designed address cancer health disparity research questions through its recruitment of both AAs and EAs. The study population accrual and eligibility criteria for the case-control study were previously described18, 29. Briefly, all cases had histologically confirmed non-small cell lung cancer. Controls were selected through the Maryland Department of Motor Vehicles records. Both case and control participants were approached by a trained interviewer to receive written informed consent for the collection of both biospecimens and demographic data. Following receipt of informed consent, participants completed an interviewer-administered questionnaire that covered lifestyle, medical, and demographic details. Eligible participants were enrolled within 24 months of diagnosis. Blood was also taken at the time of interview by a trained phlebotomist and collected in 2 red top tubes and centrifuged at 850g for 10 minutes to separate components. Serum was then removed and distributed into 2ml cryovials at 0.5ml per vial and stored at −80 degrees Celsius. Smoking was categorized as never, former and current. Never smokers were defined as individuals who had smoked fewer than 100 cigarettes in their lifetime. Former smokers were defined as individuals who had quit smoking for at least 1 year prior to interview. This study is registered as protocol number NCT00339859. A link to the full study protocol can be found here https://clinicaltrials.gov/ct2/show/NCT00339859. For this study, participants were recruited from 2000 through 2014.
Wayne State case control study
The first study (INHALE) enrolled cases and controls from 2012 to 201730. Lung cancer patients (cases) were enrolled from two institutions in the Detroit metropolitan area: the Karmanos Cancer Institute (KCI) and the Henry Ford Health System (HFHS). Informed consent was obtained from each participant. Eligible cases were 21–89 years of age at diagnosis and enrolled within 12 months of diagnosis. Controls were recruited to undergo CT scans through community-based methods and bronchoscopy clinics as part of an effort to understand the factors associated with lung disease. Controls were frequency matched on age (±5 years), sex, race and ever/never smoking status. This study was previously described in more detail31. Additional cases included those seen at the Karmanos Cancer Institute from 2010–2012, before the start of the INHALE study who consented to donate lung tumor tissue and a blood sample and to allow medical record abstraction. Where possible, controls were matched to the cases on sex, ever/never smoking status and age. Blood was collected in a 10ml K2 EDTA purple-top tube and centrifuged at 1500g × 10 minutes. The plasma was removed and placed in a 15ml tube which was centrifuged at 1500g × 10 minutes. Aliquots of 0.5ml were made and stored at −80C until needed. Smoking was defined as above and participant demographics are shown in Table 1.
Mortality and survival determination
For the NCI-MD study, date and cause of death were obtained from the National Death Index. The linkage process has been previously described29. Lung cancer-specific death was defined as an individual with lung cancer listed as the primary, secondary or tertiary cause of death or death due to another cancer within 2 years of the lung cancer diagnosis. TNM staging was re-classified using AJCC 7th edition. Survival time was calculated from date of surgery to date of either last known follow-up (last National Death Index update on 12/3½015) or date of death due to lung cancer. Cases from the Detroit studies were linked to Metropolitan Detroit Cancer Surveillance System (MDCSS), a participant in the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program. Through SEER, up to nine causes of death were collected and cases were followed up for vital status every 18 months using both passive and active methods. Survival time was calculated as date of lung cancer diagnosis to date of last follow-up or date of death.
Measurement of immune and inflammation proteins
Concentrations of 30 key immune and inflammation proteins were measured in the sera of patients enrolled in the NCI-MD study utilizing a highly sensitive and analytically validated electrochemiluminescence Mesoscale Discovery V-PLEX immunoassay (MSD® Rockville, MD). These 30 proteins were selected based on the multiplex matrices offered by the manufacturer based on key analytes that are important in inflammation response, the Th1/Th2 pathway, chemotaxis, the Th17 pathway, angiogenesis, and immune system regulation. Briefly, 25 μl of patient sera were assayed following the manufacturer’s protocol using the MSD® VPLEX Chemokine Panel, Pro-inflammatory Panel, Cytokine Panel Kits. Although IL-1ß is included in the V-Plex proinflammatory assay plate, we also measured it separately on an ultrasensitive kit (also MSD), as a run-in QC assay that we performed comparing ultrasensitive and V-Plex assays showed that the ultrasensitive assay for IL-1ß had a larger range of detection compared with the newer V-plex assay in our samples. CRP was also run on a single V-plex plate as standard. In stage two of the study, plasma samples from the WSU cohort were measured for significant proteins from the discovery study (CRP, Eotaxin-1, GM-CSF, IFN-γ, IL-5, IL-6, IL-7, IL-8, IL-10, IL-15, IP-10, MCP-4, MIP-1α, MIP-1β, TNF-α, TNF-β, and VEGFA) on a customized version of the MSD V-PLEX Plus Human Cytokine 30-Plex Kit (Mesoscale Discovery). Quality control measures are detailed in Table S1 and S2. Table S2 indicates the median levels among cases and controls.
Protein measurement quality control
Signal intensities were extrapolated to concentrations based on an 8-point standard curve run on each plate. To ensure quality data for further analyses and interpretation, detection level criteria were applied to all measurements (Table S1). Measurements that lay within the dynamic range were included within the analysis. Samples with values less than the lower limit of detection (LLOD), which is defined as two and a half standard deviations above the value of the background sample, were assigned half of the value of the plate-specific LLOD for that analyte. The number and percentage of samples deemed within the limits of detection are detailed in Table S1. Samples from all participants were randomly and blindly distributed across the plates. Control samples were also included on all plates to assess inter-plate variability. The median and quartile values of each protein among population controls were chosen as the cut-off values to classify high and low levels of the protein in accordance with previous studies16, 17. Proteins for which 50% of sample measurements lay outside of the range of quantification were dichotomized into ‘detect’ (concentration measurements within the range of quantification) and ‘non-detect’ (concentration measurements outside of the detection quantification) to classify high and low levels of the protein16. The detect/non-detect classification (i.e., when more than 50% of the samples fell outside the LLOD) criterion applied to 6 of the 30 proteins analyzed (Table S1).
Statistical Analysis
Cases vs. controls
Differences in protein concentrations between cases and controls were calculated using a Wilcoxon rank-sum test (Table S2). Univariable comparisons of characteristics between cases and controls were performed for continuous variables using Student’s t-test or the Kruskal-Wallis test for normally or non-normally distributed data, respectively. Comparisons for categorical variables were performed using the χ2 test (Table 1).
Lung cancer risk
Unconditional logistic regression models were constructed to estimate the relationship between lung cancer diagnosis and immune protein concentrations. Additional multivariable models were constructed with adjustment for age (continuous), gender (male/female), BMI (healthy, overweight, underweight), current smoking status (never, former, current), and pack-years of smoking (continuous). Adjustment of history of inflammatory conditions including tuberculosis, asthma and emphysema was also conducted for the NCI-MD study. Protein expression levels were divided into median or quartiles based on levels of the healthy control population. Analyses were not corrected for multiple comparisons. Analysis of an independent cohort was used to identify false-positive associations.
Lung cancer survival
The association between inflammation proteins and survival was assessed using the method of Kaplan and Meier and Log-rank tests of equality. The magnitude of association between inflammation proteins and lung cancer-specific survival were estimated using univariable and multivariable Cox proportional hazards regression modeling. Multivariable analyses were adjusted to control for potential confounding variables: age (continuous), gender (male/female), BMI (healthy, overweight, underweight), current smoking status (never, former, current), pack-years of smoking (continuous), stage (I, II, III, IV), and histology (adenocarcinoma, squamous, other). Time from lung cancer diagnosis was used to estimate survival timescales, failure was a lung cancer-specific death. Protein levels were first divided into median and quartiles based on levels within cases. Akaike information criteria (AIC) ranking was utilized to compare survival model performance32.
As correlations exist between inflammation proteins, for example CRP and IL-6, a logistic regression model was created with the 13 proteins from the discovery study (NCI-MD). Each variable was removed individually in a stepwise fashion and the contribution of each variable to the model was assessed using likelihood ratio tests comparing the full model to the model without the variable in question. Variables with Chi-squared P-values less than 0.10 were considered significant and retained in the model. This process of variable selection was continued until no more variables could be removed from the model. The intercept and beta values from these models were stored and used as prediction equations which was then applied to the data from the validation study (WSU study dataset) to produce a probability of being a case for every person. Receiver operator curves and area under the curve (AUC) values were generated. With sensitivity set at 95%, the predictive power was further assessed by calculating sensitivity, positive predictive value, and negative predictive value. All statistical analyses were performed using STATA® 14.0 (StataCorp, TX). Reported P-values were two-sided and the significance threshold level was specified as P=0.05.
Results
Association between circulating immune protein levels and lung cancer diagnosis in African Americans
Of the 30 proteins measured, statistically higher levels of CRP, IFN-γ, IL-1α, IL-1β, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-15, IL-17A, IP-10, MDC, GM-CSF, TARC, VEGFA, and MIP-1α, and lower levels of MCP-4 and TNF-β were detected in cases compared with controls (Table S2). Thirteen of these proteins were significantly associated with lung cancer diagnosis following adjustment for potential confounders (CRP, IFN-γ, IL-1β, IL-5, IL-6, IL-8, IL-10, IL-15, IP-10, MCP-4, MIP-1 α, TNF-α, and TNF- β) (Table S3). As many inflammation proteins are both quantitatively and functionally related, we generated a reduced model and eliminated potential redundancy across the 13 proteins using likelihood ratio tests (Supplementary Methods). This method produced a six-protein model (CRP, IL-6, IL-8, IL-15, MCP-4 and TNF-β), the AUC of which was 0.87 (0.85–0.90) (Table S4).
To test the robustness of our observations, we analyzed an independent, validation study (n=399 cases and n=400 controls). As this study had plasma biospecimens we initially conducted a runin experiment to evaluate potential discrepancies between protein expression levels from these two biospecimens (Fig S1). Here, we measured each of the significant proteins, in duplicate, from serum and plasma from the same patients (n=8). These pilot data demonstrated strong correlations between the two biospecimens (Fig S1).
Ten of the 13 proteins significantly associated with lung cancer in the discovery dataset were replicated within the independent WSU validation study: CRP (OR, 2.90; 95% CI, 1.99–4.22), IFN-γ (OR, 1.55; 95% CI, 1.10–2.19), IL-6 (OR, 6.28; 95% CI, 4.10–9.63), IL-8 (OR, 2.76; 95% CI, 1.92–3.98), IL-10 (OR, 1.69; 95% CI, 1.20–2.38), IL-15 (OR, 2.83; 95% CI, 1.96–4.07), IP-10 (OR, 1.54; 95% CI, 1.09–2.18), MCP-4 (OR, 0.54; 95% CI, 0.38–0.76), MIP-1 α (OR, 1.57; 95% CI, 1.12–2.21), and TNF- β (OR, 0.52; 95% CI, 0.37–0.74) (Table 2) (Fig 1) (Table S3). IL-1β concentrations were not analyzed in the validation study as this association has been previously validated in our laboratory6 and the detection of IL-1β on the MSD V-plex platform has a lower range of detection than previous assays. IL-5 and IL-7 did not validate. However, noteworthy differences in concentration levels were observed for these proteins between sera and plasma.
Table 2:
Relationship between circulating inflammation proteins and lung cancer diagnosis
| Characteristic | Discovery study (NCI-MD) | Validation study (WSU) | ||||
|---|---|---|---|---|---|---|
| P* | P* | |||||
| CRP | ||||||
| ≤Median | Reference | Reference | Reference | Reference | ||
| >Median | 2.90 | 1.99–4.22 | <0.0001 | 2.80 | 2.05–3.82 | <0.0001 |
| IFN-γ | ||||||
| ≤Median | Reference | Reference | Reference | Reference | ||
| >Median | 1.55 | 1.10–2.19 | 0.012 | 1.47 | 1.10–1.97 | 0.009 |
| IL-1β | ||||||
| ≤Median | Reference | Reference | ||||
| >Median | 1.92 | 1.36–2.73 | <0.0001 | |||
| IL-5 | ||||||
| Undetectable | Reference | Reference | Reference | Reference | ||
| Detectable | 0.67 | 0.46–0.98 | 0.041 | 1.14 | 0.85–1.54 | ns |
| IL-6 | ||||||
| ≤Median | Reference | Reference | Reference | Reference | ||
| >Median | 6.28 | 4.10–9.63 | <0.0001 | 4.72 | 3.35–6.24 | <0.0001 |
| IL-8 | ||||||
| ≤Median | Reference | Reference | Reference | Reference | ||
| >Median | 2.76 | 1.92–3.98 | <0.0001 | 2.23 | 1.65–3.02 | <0.0001 |
| IL-10 | ||||||
| ≤Median | Reference | Reference | Reference | Reference | ||
| >Median | 1.69 | 1.20–2.38 | 0.003 | 3.18 | 2.32–4.37 | <0.0001 |
| IL-15 | ||||||
| ≤Median | Reference | Reference | Reference | Reference | ||
| >Median | 2.83 | 1.96–4.07 | <0.0001 | 3.52 | 2.56–4.84 | <0.0001 |
| IP-10 | ||||||
| ≤Median | Reference | Reference | Reference | Reference | ||
| >Median | 1.54 | 1.09–2.18 | 0.014 | 1.79 | 1.33–2.41 | <0.0001 |
| MCP-4 | ||||||
| ≤Median | Reference | Reference | Reference | Reference | ||
| >Median | 0.54 | 0.38–0.76 | <0.0001 | 0.71 | 0.53–0.95 | 0.022 |
| MlP-1α | ||||||
| ≤Median | Reference | Reference | Reference | Reference | ||
| >Median | 1.57 | 1.12–2.21 | 0.01 | 1.78 | 1.32–2.39 | <0.0001 |
| TNF-α | ||||||
| ≤Median | Reference | Reference | Reference | Reference | ||
| >Median | 1.41 | 1.00–2.00 | 0.048 | 1.25 | 0.94–1.68 | ns |
| TNF-β | ||||||
| ≤Median | Reference | Reference | Reference | Reference | ||
| >Median | 0.52 | 0.37–0.74 | <0.0001 | 0.57 | 0.42–0.76 | <0.0001 |
(OR=Odds Ratio, CI=Confidence Interval); Bold text indicates statistically significance P <0.05.
Models adjusted for age, gender, smoking status, pack-years of smoking, and BMI
Fig 1.
Graphical representation of odds of lung cancer diagnosis for elevated protein expression levels (validated proteins only). Red lines indicate data from the discovery NCI-MD case control study. Black lines indicate data from the validation WSU dataset. * IL-1β was not analyzed in the validation study due to assay restrictions described in methods, however, IL-1β has been previously validated as a diagnostic protein in AA population on this MSD platform21.
We then tested the validity of the six-protein reduced model in the validation study using the beta values derived from the discovery study. Here, we again observed slightly lower, but robust, AUC and NPV values of 0.73 (0.70–0.76) and 82%, respectively (Table S4).
Findings were broadly consistent by histological subtype (Table S5). However, the relationships between CRP and MIP-1α were stronger for squamous cell carcinoma for both the discovery and validation studies.
Influence of host factors on inflammation protein expression in AAs
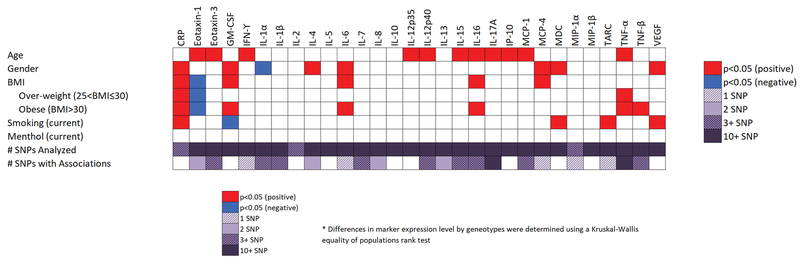
As the lung cancer-associated inflammation profile was different to that previously observed in EAs16, 18, 20, 33, we assessed genetic and non-genetic host factors that could drive the relationship. Specifically, as menthol cigarette use is higher among AAs34–36, we compared the effect of menthol cigarette use on circulating inflammation proteins. Using healthy population control current smokers to negate any effects of cancer on inflammation protein expression levels we found that use of menthol cigarettes did not modulate expression of inflammation proteins (Fig 2). No effects were observed among cases either (data not shown). Smoking status, age and gender were significantly associated with variability in several of the inflammation proteins, but not in a manner that differed from EAs37.
Fig 2.
Graphical representation of the influence of genetic and non-genetic host factors on circulating inflammation protein expression levels. * Differences in protein levels by genotype were determined using a Kruskal-Wallis equality of populations rank test.
We also assessed whether genetic host factors could influence circulating expression levels of inflammation proteins using a cis expression quantitative trait loci (eQTL) analysis (Table S6). As it was a cis eQTL analysis, we specifically focused on the inflammation proteins associated with lung cancer status in our study. However, no associations passed adjustment for multiple comparisons. The cis eQTL analysis suggested that genetic factors did not significantly impact the relationship between inflammation proteins with lung cancer in AA. To further confirm this observation, we adjusted our models for global genetic ancestry, but the risk estimates did not materially change (Table S4).
Circulating protein levels associated with lung cancer survival in AAs
After adjustment for potential confounding factors, IL-6 (HR, 1.43; 95% CI, 1.01–2.01), CRP (HR, 1.50; 95% CI, 1.06–2.13), VEGFA (HR, 1.60; 95% CI, 1.13–2.27) and MIP-1β (HR, 0.71; 95% CI, 0.51–0.99) (Table S7) were associated with survival. We replicated the relationship between IL-6 and CRP with survival in the validation study (IL-6 HR, 1.92; 95% CI, 1.46–2.53; CRP HR, 1.55; 95% CI, 1.19–2.02) (Table 3) (Table S7) (Fig 3). High levels of both IL-6 and CRP was associated with poor survival compared with patients with low levels of each protein (HR, 1.73; 95% CI, 1.14–2.64) (Table 3) (Table S7). We also validated this classifier in the WSU study (Table 3) (Fig 3). Adjustment of the survival analyses for history of co-morbidities including TB, asthma and emphysema, did not alter the estimates (data not shown).
Table 3:
Relationship between CRP and IL-6 with lung cancer survival
| Characteristic | Discovery study (NCI-MD) | Validation study (WSU) | ||||
|---|---|---|---|---|---|---|
| P* | P* | |||||
| IL-6 | ||||||
| ≤Median | Reference | Reference | Reference | Reference | ||
| >Median | 1.43 | 1.01–2.01 | 0.043 | 1.92 | 1.46–2.53 | <0.0001 |
| CRP | ||||||
| ≤Median | Reference | Reference | Reference | Reference | ||
| >Median | 1.50 | 1.06–2.13 | 0.023 | 1.55 | 1.19–2.02 | 0.009 |
| Classifier | ||||||
| Low for both | Reference | Reference | Reference | Reference | ||
| High for one | 1.34 | 0.86–2.09 | 0.201 | 1.50 | 1.04–2.16 | 0.030 |
| High for both | 1.73 | 1.14–2.64 | 0.010 | 2.03 | 1.50–2.77 | <0.0001 |
(HR=Ha8zard Ratio, CI=Confidence Interval); Bold text indicates statistically significance P<0.05
Models adjusted for age, gender, smoking status, pack-years, BMI, stage, and histology
Fig 3.
Kaplan Meier curves depicting lung cancer-specific survival among patients with a combined IL-6 and CRP classifier in the discovery and validation cohorts.
Discussion
Evidence from epidemiology, histopathology, inflammation profiling, functional studies and the efficacy of anti-inflammation drugs in cancer prevention imply a strong relationship between inflammation and lung cancer7–15. Earlier studies in European Americans detected a relationship between CRP, IL-6, IFN-g and IL-8 with lung cancer diagnosis16–18, 21, findings that we also observed in our study. Interestingly however, we noted that the effect size of the relationship is over 2-fold higher in AA in both the discovery and validation studies than previously observed in EA6, 16–18, 20, 21. Moreover, our analysis of a broader spectrum of immune and inflammation proteins revealed an inflammatory milieu associated with lung cancer in AA not previously described in EA. Specifically, our data suggest both a common (CRP, IL-6, IFN-γ and IL-8) and distinct (EA: SAA, sTNFRII, CXCL9) (AA: IL-1β, IL-10, IL-15, IP-10, MIP-1α, MCP-4 and TNF-β) relationship between inflammation and lung cancer in EA and AA.
It is not clear what drives this circulating immune profile in AAs. It could reflect the immune microenvironment within these tumors or the proteins secreted by tumor cells38–40. We noted that many of the inflammation proteins are macrophage-related, either attracting, activating or being secreted by these inflammatory cells (e.g. IFN-γ, IL-1β, IL-10, IL-15, and IP-10)41. Several studies have shown macrophage enrichment in AA tumors—including breast and prostate cancer42, 43—while our recent transcriptomic study of lung cancer in AA and EA suggested that tumors from AA were enriched for M2 macrophages5. However, definitive phenotyping of specific immune cells in tumors from AAs and EAs would be needed to conclude this.
Given that smoking can modulate the immune landscape and that smoking patterns are different between EA and AA we hypothesized that smoking—particularly menthol cigarette smoking given the distinct differences in usage between EAs and AAs—could perturb immune protein expression relative to non-menthol cigarette use. However, relative to non-menthol cigarette use, menthol cigarette smoking did not modulate inflammation protein expression among smokers, though it could impact inflammation proteins not studied here. Our analysis of genetic host factors, including cis eQTL and global genetic ancestry, suggested that genetic factors also did not drive this relationship. Interestingly, recent work by our group indicated racial differences in the effect of aspirin use on lung cancer44, though the analysis did not link this effect to different levels of circulating immune proteins. Our study examined the link between circulating levels of inflammation cytokines at one time-point. Thus, it is possible that individual immune cells could be primed to respond to environmental exposures and pathogens in a different manner and this could be addressed in future studies45.
Our data may have translational implications for both lung cancer diagnosis and treatment. First, as circulating levels of these inflammation proteins are elevated among cases, it is possible that the proteins could be useful for the early diagnosis of lung cancer in African Americans. In support of this, we conducted a sensitivity analysis restricting the data to early stage disease (i.e., stage I), and found that IL-6, IL-15 and MCP-4 were each indicators of early lung cancer (i.e., tumors < 3 cm in size) in both the discovery and validation studies (Table S8). As lung cancer screening with low dose CT screening becomes the one of the primary methods of lung cancer detection, assessing the utility of immune and inflammation biomarkers in this setting should also be considered. Biomarkers for risk stratification and discrimination between benign and malignant modules are needed for all populations, but especially African Americans given recent data suggesting they are less likely to eligible for screening with current guidelines46, 47. The identification of biomarkers that detect early-stage disease in AAs could help minimize health disparities and improve patient outcomes in this population. Thus, follow-up prospective studies should also examine changes in protein levels over time in relation to lung cancer risk.
Our data are also interesting from the perspective of cancer treatment. For example, as noted earlier, the effect size for the relationship between IL-6 with lung cancer was 2-fold higher in AAs than EAs. IL-6 is linked to the JAK/STAT pathway and important for leukocyte recruitment and apoptosis, proper T cell functioning, and inflammation activation of stromal tissues48, 49. Our observations raise the possibility that the relationship between IL-6 and lung cancer may reflect a difference in biology and cell signaling between EAs and AAs. Further, it suggests that patients with high levels of IL-6 could be good candidates for anti-IL-6 (such as siltuximab) or anti IL-6R (such as tocilizumab) drugs, several of which are in current clinical trials50. Second, a recent re-analysis of the CANTOS trial revealed that canakinumab, a monoclonal antibody targeting IL-1β, is associated with reduced incidence of fatal lung cancer51. Given that we observed a relationship between increasing IL-1β and lung cancer only among AAs, future studies examining the potential of IL-1 β in lung cancer treatment should consider including this population. Recombinant and receptor conjugated IL-15 is currently in clinical trials (NCT01727076; NCT03127098). As our study also identified a relationship between elevated IL-15 in AAs, further studies should examine whether AAs would be candidates for these therapies52.
Our study has several strengths and limitations. We profiled 30 inflammation proteins but note there may be others that play a role in lung cancer biology and disparities. To our knowledge, we have conducted the largest investigation on the relationship between inflammation and lung cancer in AAs and replicated our observations in a second, independent study population. While there were some demographic differences between these two studies, we adjusted for each of these factors in our analyses and this adjustment did not alter the main estimates. Further, while it wasn’t possible to match the cases and controls from the NCI-MD study based on smoking— due to availability of controls—the validation study was matched. Thus, the replication of our observation in an independent population, matched for smoking status, speaks to the robustness of our observations. To our knowledge we have conducted the first analysis of menthol cigarette exposure and circulating immune proteins. Our data replicated in both sera and plasma, which points towards the robustness of our observations. The relationship between IL-5, and TNF-α and lung cancer did not validate in the WSU study. One of the key differences between the test and validation datasets was the use of sera and plasma, respectively. A strength of our study is the run-in sera and plasma comparative analysis suggesting that decreased sensitivity in plasma could have affected the replication of certain observations, including IL-5. As observed in previous studies, we also broadly detected higher concentrations of these inflammation proteins in serum compared with plasma, partly due to some dilution by the anti-coagulant. However, rank order was generally preserved which speaks to the constituent differences between these two specimen types. Using ancestry informative analysis and self-reported race, we were able to assess both ancestral and societal determinants of disparities. For both studies, blood was drawn either at, before or after diagnosis, though most were following diagnosis. It is possible that inflammation protein levels could have been affected by surgery and any systemic therapies the patients underwent. However, the concordance in results between the discovery and validation studies suggests that any confounding, if it did occur, was minimal. Further, restricting our analysis to just those patients with a blood draw within 12 months of diagnosis did not meaningfully change the risk estimates or results (Table S9). It is possible that inflammation-related co-morbidities could have confounded the relationships we observed between immune proteins and lung cancer. However, adjustment for these variables did not alter the results from the main effects analysis (Table S10). Further, a recent study we conducted using samples from the National Lung Screening Trial in EAs21 also demonstrated a relationship between inflammation proteins and lung cancer diagnosis. In that study, we were able to adjust for up to 10 inflammatory conditions, none of which altered the relationship with lung cancer, suggesting that these relationships could be independent of that.
In summary, we report a distinct inflammation milieu associated with lung cancer in African Americans. Although this study relates to diagnosis in a case-control setting, it provides strong evidence of an inflammation signature that warrants future investigation to determine the utility of these proteins as biomarkers for risk stratification, nodule discrimination or prognosis. To assess utility for risk stratification, immune proteins would need to be assessed in a prospective study that includes minority populations and, ideally, studies with repeated blood draws would be ideal in order to assess the trajectory of levels over time. Importantly, such a study design would ensure that all samples tested were taken from a patient before their lung cancer diagnosis. A previous study on European Americans validated an association between CRP, SAA, sTNFRII, and CXCL9/MIG with lung cancer risk, but their inclusion in a risk model was of limited value17. While this could suggest immune and inflammation proteins have limited utility for risk stratification, the question remains unanswered in AAs. Additional work is also needed to understand why the etiological relationship between inflammation and lung cancer differs between European Americans and African Americans. Finally, given the differences we observed, our findings re-emphasize the importance of enrolling minority populations into clinical studies as differences in tumor biology could impact generalizability of results across populations.
Supplementary Material
Funding:
This work was supported by the Intramural Research Program of the National Cancer Institute and National Institute on Minority Health and Health Disparities, and by NIH grants R01CA141769 and P30CA022453, HHS contract HHSN261201300011I and the Herrick Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors do not have any conflicts of interest to disclose.
References
- 1.DeSantis CE, Siegel RL, Sauer AG, et al. Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. CA Cancer J Clin 2016;66:290–308. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018. [DOI] [PubMed] [Google Scholar]
- 3.Houston KA, Mitchell KA, King J, et al. Histologic Lung Cancer Incidence Rates and Trends vary by Race/Ethnicity and Residential County. J Thorac Oncol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan BM. Lung cancer health disparities. Carcinogenesis 2018;39:741–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell KA, Zingone A, Toulabi L, et al. Comparative Transcriptome Profiling Reveals Coding and Noncoding RNA Differences in NSCLC from African Americans and European Americans. Clin Cancer Res 2017;23:7412–7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pine SR, Mechanic LE, Enewold L, et al. Differential Serum Cytokine Levels and Risk of Lung Cancer Between African and European Americans. Cancer Epidemiol Biomarkers Prev 2016;25:488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engels EA. Inflammation in the development of lung cancer: epidemiological evidence. Expert Rev Anticancer Ther 2008;8:605–615. [DOI] [PubMed] [Google Scholar]
- 8.Le Jeune I, Gribbin J, West J, et al. The incidence of cancer in patients with idiopathic pulmonary fibrosis and sarcoidosis in the UK. Respir Med 2007;101:2534–2540. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz AG, Cote ML, Wenzlaff AS, et al. Chronic obstructive lung diseases and risk of non-small cell lung cancer in women. J Thorac Oncol 2009;4:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenner DR, Boffetta P, Duell EJ, et al. Previous lung diseases and lung cancer risk: a pooled analysis from the International Lung Cancer Consortium. Am J Epidemiol 2012;176:573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaturvedi AK, Caporaso NE, Katki HA, et al. C-reactive protein and risk of lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2010;28:2719–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang HY, Huang TB, Xu L, et al. Aspirin use and lung cancer risk: a possible relationship? Evidence from an updated meta-analysis. PLoS One 2015;10:e0122962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tammemagi CM, Neslund-Dudas C, Simoff M, et al. Impact of comorbidity on lung cancer survival. Int J Cancer 2003;103:792–802. [DOI] [PubMed] [Google Scholar]
- 16.Shiels MS, Pfeiffer RM, Hildesheim A, et al. Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst 2013;105:1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiels MS, Katki HA, Hildesheim A, et al. Circulating Inflammation Markers, Risk of Lung Cancer, and Utility for Risk Stratification. J Natl Cancer Inst 2015;107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pine SR, Mechanic LE, Enewold L, et al. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. J Natl Cancer Inst 2011;103:1112–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meaney CL, Zingone A, Brown D, et al. Identification of serum inflammatory markers as classifiers of lung cancer mortality for stage I adenocarcinoma. Oncotarget 2017;8:40946–40957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brenner DR, Fanidi A, Grankvist K, et al. Inflammatory Cytokines and Lung Cancer Risk in 3 Prospective Studies. Am J Epidemiol 2017;185:86–95. [DOI] [PubMed] [Google Scholar]
- 21.Brown D, Zingone A, Yu Y, et al. Relationship between circulating inflammation proteins and lung cancer diagnosis in the National Lung Screening Trial. Cancer Epidemiol Biomarkers Prev 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan Y, Jiang W, Liu L, et al. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 2015;160:62–73. [DOI] [PubMed] [Google Scholar]
- 23.Yanbaeva DG, Dentener MA, Creutzberg EC, et al. Systemic effects of smoking. Chest 2007;131:1557–1566. [DOI] [PubMed] [Google Scholar]
- 24.Kuschner WG, D’Alessandro A, Wong H, et al. Dose-dependent cigarette smoking-related inflammatory responses in healthy adults. Eur Respir J 1996;9:1989–1994. [DOI] [PubMed] [Google Scholar]
- 25.Ryan BM. Lung Cancer Health Disparities. Carcinogenesis 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med 2006;354:333–342. [DOI] [PubMed] [Google Scholar]
- 27.Thun MJ, Hannan LM, Adams-Campbell LL, et al. Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med 2008;5:e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blot WJ, Cohen SS, Aldrich M, et al. Lung cancer risk among smokers of menthol cigarettes. J Natl Cancer Inst 2011;103:810–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enewold L, Mechanic LE, Bowman ED, et al. Serum concentrations of cytokines and lung cancer survival in African Americans and Caucasians. Cancer Epidemiol Biomarkers Prev 2009;18:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz AG, Wenzlaff AS, Bock CH, et al. Admixture mapping of lung cancer in 1812 African-Americans. Carcinogenesis 2011;32:312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz AG, Lusk CM, Wenzlaff AS, et al. Risk of Lung Cancer Associated with COPD Phenotype Based on Quantitative Image Analysis. Cancer Epidemiol Biomarkers Prev 2016;25:1341–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindsey JK, Jones B. Choosing among generalized linear models applied to medical data. Stat Med 1998;17:59–68. [DOI] [PubMed] [Google Scholar]
- 33.Shiels MS, Katki HA, Freedman ND, et al. Cigarette smoking and variations in systemic immune and inflammation markers. J Natl Cancer Inst 2014;106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benowitz NL, Dains KM, Dempsey D, et al. Racial differences in the relationship between number of cigarettes smoked and nicotine and carcinogen exposure. Nicotine Tob Res 2011;13:772–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curtin GM, Sulsky SI, Van Landingham C, et al. Patterns of menthol cigarette use among current smokers, overall and within demographic strata, based on data from four U.S. government surveys. Regul Toxicol Pharmacol 2014;70:189–196. [DOI] [PubMed] [Google Scholar]
- 36.Finkenauer R, Pomerleau CS, Snedecor SM, et al. Race differences in factors relating to smoking initiation. Addict Behav 2009;34:1056–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ter Horst R, Jaeger M, Smeekens SP, et al. Host and Environmental Factors Influencing Individual Human Cytokine Responses. Cell 2016;167:1111–1124.e1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukuyama T, Ichiki Y, Yamada S, et al. Cytokine production of lung cancer cell lines: Correlation between their production and the inflammatory/immunological responses both in vivo and in vitro. Cancer Sci 2007;98:1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davalos AR, Coppe JP, Campisi J, et al. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev 2010;29:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seike M, Yanaihara N, Bowman ED, et al. Use of a cytokine gene expression signature in lung adenocarcinoma and the surrounding tissue as a prognostic classifier. J Natl Cancer Inst 2007;99:1257–1269. [DOI] [PubMed] [Google Scholar]
- 41.Mantovani A, Sica A, Sozzani S, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 2004;25:677–686. [DOI] [PubMed] [Google Scholar]
- 42.Koru-Sengul T, Santander AM, Miao F, et al. Breast cancers from black women exhibit higher numbers of immunosuppressive macrophages with proliferative activity and of crown-like structures associated with lower survival compared to non-black Latinas and Caucasians. Breast Cancer Res Treat 2016;158:113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carrio R, Koru-Sengul T, Miao F, et al. Macrophages as independent prognostic factors in small T1 breast cancers. Oncol Rep 2013;29:141–148. [DOI] [PubMed] [Google Scholar]
- 44.Erickson P, Gardner LD, Loffredo CA, et al. Racial and Ethnic Differences in the Relationship Between Aspirin Use and Non-Small Cell Lung Cancer Risk and Survival. Cancer Epidemiol Biomarkers Prev 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feairheller DL, Park JY, Sturgeon KM, et al. Racial differences in oxidative stress and inflammation: in vitro and in vivo. Clin Transl Sci 2011;4:32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryan BM. Differential eligibility of African American and European American lung cancer cases using LDCT screening guidelines. BMJ Open Respir Res 2016;3:e000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katki HA, Kovalchik SA, Berg CD, et al. Development and Validation of Risk Models to Select Ever-Smokers for CT Lung Cancer Screening. JAMA 2016;315:2300–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol 2015;16:448–457. [DOI] [PubMed] [Google Scholar]
- 49.Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest 2011;121:3375–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rossi JF, Lu ZY, Jourdan M, et al. Interleukin-6 as a therapeutic target. Clin Cancer Res 2015;21:1248–1257. [DOI] [PubMed] [Google Scholar]
- 51.Ridker PM, MacFadyen JG, Thuren T, et al. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017;390:1833–1842. [DOI] [PubMed] [Google Scholar]
- 52.Liu K, Catalfamo M, Li Y, et al. IL-15 mimics T cell receptor crosslinking in the induction of cellular proliferation, gene expression, and cytotoxicity in CD8+ memory T cells. Proc Natl Acad Sci U S A 2002;99:6192–6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.