Abstract
A key question in addiction research concerns how, in some individuals, initial recreational or casual patterns of drug use may change brain and psychological function in ways that promote a transition to the problematic patterns of use that define substance use disorders (addiction). In preclinical studies this is modeled using self-administration procedures. However, most cocaine self-administration procedures produce continuously high brain concentrations of drug, whereas in people bouts of use are thought to be more intermittent. Here we ask whether such temporal pharmacokinetic factors matter, by comparing and contrasting the neuropsychological consequences of intermittent vs. long access cocaine self-administration experience. It turns out the temporal pattern of cocaine use has profound effects on a number of outcomes. First, despite much less total drug consumption, intermittent access to cocaine is more effective in producing addiction-like behavior. Second, intermittent and long access cocaine self-administration change the brain in very different ways to influence motivated behavior. We argue that intermittent access self-administration procedures might be better suited than traditional self-administration procedures for isolating drug-induced changes in neuropsychological function that contribute to the transition to cocaine addiction.
Keywords: addiction, preclinical, self-administration, cocaine, dopamine, intermittent-access
“Study of the neural implementation of behavior is best investigated after [the] behavioral work” (p. 480, Krakauer et al. 2017).
Why Do We Need Preclinical Models of Addiction?
One of the most fundamental questions in addiction research concerns how, in susceptible individuals, initial recreational patterns of drug use may change brain and psychological function in ways that promote a transition to the problematic patterns of use that define addiction. This is particularly difficult to address in humans, for a number of reasons. First, following initial drug use, it is hard to predict who will go on to develop addictive patterns of use. Second, once addiction has developed, and after drugs have been used for decades, it may be too late to determine how drug use changed neuropsychological function in the first place, leading to years of problematic use. Third, people with addiction have often experienced decades of poor nutrition/health, poly-drug use and stressful conditions, all of which can also change the brain. Finally, the environmental context and cues associated with drug use powerfully modulate their effects (Stewart and Eikelboom 1987; Weiss et al. 2000; Panlilio et al. 2005; Caprioli et al. 2009; Badiani 2013; Childs and de Wit 2013; Leyton and Vezina 2014), and in humans it is difficult to study the neurobiological effects of drugs in the environmental context in which they are usually used. For these reasons, preclinical models are especially important for isolating drug-induced changes in neuropsychological function that contribute to the transition to addiction.
Preclinical models of addiction typically use drug self-administration procedures, and laboratory animals readily learn to self-administer most drugs humans use (e.g., Weeks 1962; Schuster and Thompson 1969). Drug self-administration studies have provided valuable information about the neural systems that mediate the reinforcing and incentive motivational effects of drugs. However, mere self-administration of a drug is not tantamount to addiction – most people take potentially addictive drugs at some time in their lives, but most do not become addicted (Anthony et al. 1994). There has been considerable effort, therefore, to develop self-administration procedures that produce addiction-like behavior, often assessed by modeling DSM diagnostic criteria (Deroche-Gamonet and Piazza 2014; Lynch 2018; Everitt et al. 2018; see Table 1).
Table 1.
DSM-V criteria for a diagnosis of substance use disorder (addiction) and corresponding behaviors in laboratory animals that self-administer drug.
| 1) Recurrent failure to fulfill role obligations | ||
| 2) Recurrent substance use in hazardous situations | ||
| 3) Continued substance use despite persistent or recurrent social or interpersonal problems | ||
| 4) Tolerance | ||
| 5) Withdrawal | ||
| 6) The substance is often used in larger amounts or over a longer period than intended | ||
| 7) Persistent desire or unsuccessful effort to cut down on substance | ||
| 8) Craving | ||
| 9) Considerable time spent in obtaining the substance or using, or recovering from its effects | ||
| 10) Important social, work, or recreational activities given up because of use | ||
| 11) Continued use despite recognition of problems resulting from use | ||
| Diagnosis | ||
| 0–1 criteria | Unaffected | |
| 2–3 | Mild Substance Use Disorder | |
| 4–5 | Moderate Substance Use Disorder | |
| 6 or more | Severe Substance Use Disorder | |
| (Note that according to the DSM no single criterion is necessary for a diagnosis of a substance use disorder) | ||
| Using DSM Criteria to Assess the Development of Addiction-Like Behavior in Laboratory Animals | ||
|
Addiction-Like Behavior in Laboratory Animals |
Example Measures |
Relevant DSM Criteria |
| • High motivation to procure drugs |
Breakpoint (progressive ratio schedule) Behavioral economic metrics, Pmax and α |
7,8,9 |
| • High propensity to reinstate drug-seeking |
Renewed drug-seeking produced by the drug, drug cues, or stress after a period of abstinence |
7,8,9 |
| • Continued drug-seeking/taking despite adverse consequences | Continued drug-taking despite, for example, receiving an electrical shock; Max Charge | 2, 11 |
| • Continued drug-seeking when drug is unavailable | Resistance to extinction; Continued responding during signaled ‘Drug Unavailable’ periods | 7,8,9 |
| • Increased consumption when drug is available | Escalation of intake | 6 |
| • Choice between a drug vs. alternative reinforcers | Choice tasks | 9, 10 |
| • Amount of drug required to produce its desired effect | Preferred consumption at minimal price Behavioral economic metric, Qo | 4,6 |
| • Discomfort associated with the discontinuation of use | Physiological signs of withdrawal Elevated ICSS thresholds | 4,5 |
Arguably, the most widely accepted rodent model of addiction in use today involves so-called ‘Long Access’ (LgA) self-administration procedures (Ahmed and Koob 1998; Edwards and Koob 2013), by which rats are allowed to self-administer drugs for ~6 hours/day, generally under a fixed ratio schedule of reinforcement. Although other long-access/unlimited access procedures have been used in rats [e.g., continuous cocaine access for 9–72 hours (Weiss et al. 1992; Tornatzky and Miczek 2000)] and non-human primates [e.g., continuous access for 23 hours (Johanson et al. 1976)], we focus here specifically on studies using the LgA procedure developed by Ahmed and Koob in rats (1998). Rats with LgA experience develop a number of the symptoms of addiction described in Table 1, relative to animals tested under ‘Short Access’ (ShA; 1–2 hours/day) conditions. The thesis of this Perspective, however, is that typical animal self-administration procedures, and LgA in particular, are actually not very good for studying drug-induced changes in brain and behavior responsible for the transition to addiction. We suggest this is because they might not reflect the temporal patterns of use (pharmacokinetics) within a bout of cocaine consumption in humans during this transition – and it turns out this matters. In the following we briefly discuss studies involving LgA (and ShA) procedures. We then present an alternative, intermittent access (IntA) procedure, which is more effective than LgA in producing addiction-like behavior, and which challenges a number of assumptions underlying LgA models. (A caveat: we focus on studies using cocaine, and it remains to be seen how well the conclusions will apply to other drugs of abuse).
Long Access Self-Administration (LgA)
Although there are exceptions (e.g., Shaham et al. 1994; Gratton and Wise 1994; Heyne and Wolffgramm 1998), until the late 1990’s many experiments on neural systems that mediate the reinforcing and motivational effects of drugs used self-administration sessions that lasted 1–2 hours - what would now be referred to as ShA. However, as was well known at that time, under ShA conditions, rats generally show very stable levels of drug intake over many weeks. In 1998, Ahmed and Koob published a pioneering study in which they compared rats allowed to self-administer cocaine under ShA conditions with those allowed to self-administer for 6 hours/day (LgA). The ShA group showed stable levels of intake over 22 days of testing, as expected, but the LgA group escalated their intake, and after a period of abstinence they escalated intake even further. Ahmed and Koob (1998) concluded that LgA, “may provide an animal model for studying the development of excessive drug intake and the basis of addiction” (p. 298). Ahmed and Koob (1998) focused on one addiction-like behavior – escalation of intake, but there are reports that, relative to ShA, LgA produces other addiction-like behaviors described in Figure 1 (Edwards and Koob 2013, for review). However, evidence that LgA experience increases motivation for cocaine is somewhat mixed, which is the reason for the question mark in Fig. 1. For example, some researchers report that LgA increases motivation for cocaine, relative to ShA (e.g., Paterson and Markou 2003; Zimmer et al. 2012), whereas others report that LgA only produces a very transient increase in motivation (Bentzley et al. 2014; James et al. 2018a), no change (Kawa and Robinson 2018), or actually decreases motivation (Oleson and Roberts 2009 for discussion).
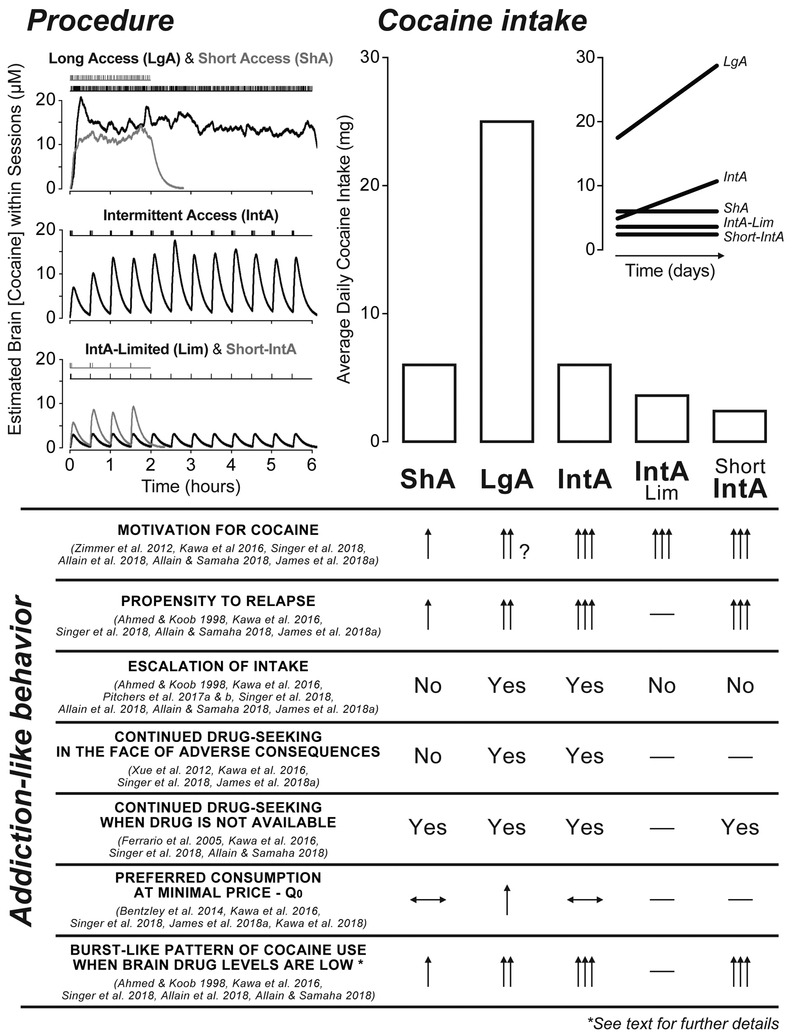
Figure 1.
A summary of the effects of different cocaine self-administration experiences on cocaine intake, and on the development of addiction-like behaviors (see Table 1). Representative patterns of cocaine intake and estimated brain concentrations of cocaine produced by different self-administration procedures are on the top left. In the first 1–2 hours, LgA animals achieve higher brain cocaine concentrations than ShA only after escalation of intake. Under LgA and ShA conditions, there are some fluctuations in brain cocaine concentrations, reflecting the inter-injection interval, which is influenced by unit dose. This is consistent with findings that there are cyclical fluctuations in dopamine-related electrochemical signals in the nucleus accumbens when rats are self-administering cocaine under continuous-access conditions (Gratton and Wise 1994; also see Keramati et al. 2017). However, the fluctuations in brain cocaine concentrations under LgA and ShA are very small relative to the large spikes obtained with IntA. Note also that LgA and ShA conditions would produce high brain concentrations of drug throughout each self-administration session, but concentrations would then drop until the next session. This would model the intermittency of drug use between bouts of consumption reported in human cocaine users (see text). However, human cocaine use is intermittent both between and within bouts, and this is modeled by intermittent access self-administration. The top right panel shows the relative total cocaine intake/session produced by these different procedures (and the inset shows the pattern of change in consumption over time). Below that, addiction-like behaviors typically assessed in laboratory animals are listed and the relative ability of the different self-administration procedures to produce each behavior is indicated. When arrows are used, a single arrow indicates the ‘baseline’ effect, and then the number of arrows indicates the relative size of a given effect. When quantitative comparisons were not possible a “Yes” or “No” indicates whether a procedure produces a given effect. Horizontal lines with a double arrowhead indicate no effect, and the downward and upward facing arrowheads a decrease and increase, respectively. Dashes indicate no data are available.
The figure highlights two main points discussed in the text. (1) LgA results in greater drug consumption than all the other procedures. (2) IntA experience is more effective in increasing motivation for drug, based on a number of metrics, than ShA or LgA experience, despite much less consumption than LgA.
As the LgA procedure became accepted as a model of addiction, researchers began to focus on the neurobiological consequences of LgA experience. Early after the discontinuation of LgA, or other high dose procedures (e.g., Calipari et al. 2014a), rats show a marked decrease (tolerance) in a number of measures of dopamine function. This includes decreases in cocaine-induced inhibition of dopamine uptake, electrically-evoked dopamine release, cocaine-induced dopamine overflow measured with microdialysis and some (but not all) measures of cocaine-induced psychomotor activation (Ferris et al. 2011; Calipari et al. 2013, 2014a). In addition, LgA experience can also decrease phasic dopamine release in the striatum during cocaine self-administration (Willuhn et al. 2014). Tolerance to cocaine-induced inhibition of dopamine uptake subsided within 14 and 60 days, but could be reinstated by a single re-exposure to the drug (Siciliano et al. 2016). Although there are exceptions to reports that LgA produces marked tolerance in dopamine neurotransmission (e.g., Ahmed et al. 2003; Kawa et al. 2018), reports of tolerance have been interpreted as support for the view that addictive behavior is a consequence of a drug-induced hypodopaminergic state, and that continued drug-seeking is motivated by a desire to overcome this dopamine deficiency (Dackis and Gold 1985; U.S. Department of Health and Human Services 2016; Volkow et al. 2016; Koob and Volkow 2016).
Relative to ShA, LgA cocaine self-administration is also especially effective in producing ‘incubation of cocaine craving’, which refers to a progressive increase in cue-induced reinstatement of drug-seeking behavior during protracted withdrawal (Grimm et al. 2003; Lu et al. 2004). This incubation effect is seen both when abstinence is forced or voluntary (Venniro et al. 2016, 2018). A series of studies by Marina Wolf and her colleagues suggests that this time-dependent increase in the motivational properties of a cocaine cue following LgA experience is due to a time-dependent increase in the number of calcium-permeable AMPA receptors in the nucleus accumbens core (Conrad et al. 2008; Wolf 2016). Wolf (2016) states, “incubation of cocaine craving is accompanied by increased recruitment of NAc core neurons that fire in a manner that is time-locked to cue-induced cocaine-seeking” … and concludes, “a probable cellular underpinning of this is the strengthening of excitatory synapses onto MSNs in the NAc core” (p. 3). Glutamate plasticity may also be related to the enhanced expression of psychomotor sensitization when rats are tested long after the discontinuation of LgA experience (Ferrario et al. 2005). In summary, although more research is required, the neurobiological (and behavioral) consequences of LgA experience may vary considerably as a function of time following the discontinuation of self-administration, from those reflective of tolerance to those suggestive of hyperexcitability (sensitization).
Shortcomings of LgA in Modeling the Transition to Addiction
A fundamental assumption underlying the use of LgA procedures is that the amount of drug exposure is the critical factor responsible for the transition to addiction. This was put clearly by Ahmed (2012), who stated, “addiction-causing neuropathological processes could be set in motion only when rats can expose themselves sufficiently to cocaine to cross the ‘threshold of addiction’—the minimum level of drug exposure required for inducing addiction. Conversely, below this critical level of cocaine exposure, there would be no drug-induced neuropathological changes, and drug use would remain under control, at least in the majority of drug-exposed individuals” (p. 110). Similarly, Edwards and Koob (2013) state, “excessive drug exposure likely remains an indispensable element driving the development of addiction” (p. 360). More recently, Allen et al. (2018) say, “this animal model [LgA] provides support for the loss of control over drug intake and is particularly useful … to study the transition to compulsive-like drug seeking behavior” (p. 2). However, recent studies challenge these assumptions.
Beyond the amount of drug exposure, two other pharmacokinetic factors might be more important in predicting the transition to addiction – how fast drug reaches the brain, and how often drug reaches the brain - that is, the temporal pattern of drug use (Allain et al. 2015). Here we focus only on the latter. The temporal pattern of drug use is especially important in the present context, because the pharmacokinetics associated with LgA, and the majority of self-administration studies using fixed ratio schedules, might not reflect temporal patterns of drug use in humans. Most self-administration studies use schedules of reinforcement in which animals maintain continuously elevated brain levels of drug for the duration of daily sessions, be they 1–2 hour (ShA) or 6+ hour sessions (LgA; see Fig. 1). A shortcoming of this approach is that in humans, intermittent patterns of cocaine use are thought to be more typical, certainly between bouts of use, which is captured by LgA, but also within bouts of use, which is not captured by LgA. Periods of cocaine use are frequently interspersed with periods of non-use (e.g., Cohen and Sas 1994; Simon et al. 2002), and even during a bout of use, cocaine users tend to take drug intermittently, with brain levels rising and then falling between ‘hits’ (Beveridge et al. 2012). Much more quantitative information is needed regarding the microstructure of drug use patterns, especially during the transition to addiction, but as put by Ward et al. (1997), “Cocaine users … can clearly describe the advantages of waiting a long time between doses…” (p. 380). This would suggest that cocaine users do not usually maintain uniformly high brain levels of drug for hours on end. Furthermore, intermittent patterns of use are probably especially pronounced during the transition to addiction, prior to regular use. Because LgA and IntA sessions are typically given once a day for ~6 h, both are characterized by intermittency of drug intake between self-administration sessions, as drug is not available in the ~18-h lapse between sessions. However, IntA also achieves intermittent drug intake within each session. The important question, therefore, is whether such temporal factors matter in promoting the transition to addiction. Recent preclinical studies suggest that they matter a lot.
Intermittent Access Self-Administration (IntA)
To better model an intermittent pattern of cocaine use within a bout of intoxication, Benjamin Zimmer and colleagues, working in Dave Roberts’ laboratory, developed what they called an Intermittent Access (IntA) self-administration procedure. This followed the group’s initial work using a discrete trials procedure, where rats could self-administer cocaine during 2 to 5 discrete 10-min trials per hour, 24 hours/day for several weeks (Roberts et al. 2002). In contrast, IntA consists of cycles of drug available (5 min) and no drug available (25 min) periods, but for ~6 hours/day (Zimmer et al. 2011, 2012). This imposes an intermittent pattern of drug use during each self-administration session that results in repeated ‘spikes’ in brain cocaine concentrations (see Fig. 1). It turns out these differences in temporal pattern of use have profound consequences on the ability of LgA vs. IntA to produce addiction-like behavior, and how they change the brain.
Motivation.
Zimmer and colleagues (2012) initially asked how IntA experience influenced subsequent incentive motivation for cocaine, relative to ShA and LgA experience, using a behavioral economic indicator of cocaine demand. LgA resulted in much greater total cocaine intake than ShA, but total drug intake was comparable during IntA and ShA (Fig. 1). LgA did produce greater motivation for cocaine than ShA (although see Oleson and Roberts 2009), but importantly, despite much less cocaine consumption, IntA experience produced even greater motivation for cocaine than LgA experience (also see Kawa et al. 2018). IntA experience also produced more motivation for cocaine than ShA. This showed that the temporal pattern of cocaine intake does matter, independent of total intake. More recently, Allain et al. (2018) used an IntA procedure that limited total cocaine intake even more, such that the IntA-Limited rats took 8 times less cocaine than LgA rats (~60 mg/kg relative to ~470 mg/kg cumulative drug intake). Nevertheless, subsequent motivation for cocaine (assessed using a progressive ratio schedule) was still greater after IntA-Limited, than LgA experience (Allain et al. 2018; Fig. 1). In addition, the increase in motivation for cocaine seen after IntA experience persists for a long period of time (months) after withdrawal, but is short-lived (days) following LgA experience (Bentzley et al. 2014; James et al. 2018a).
Escalation.
Another assumption of the LgA model is that high levels of cocaine consumption are necessary to produce escalation of intake (Ahmed and Koob 1998; Ahmed 2012). However, this is not the case - IntA experience can also produce escalation of intake (Kawa et al. 2016, 2018, Pitchers et al. 2017a, c; Singer et al. 2018; Allain et al. 2018; Allain and Samaha 2018) (Fig. 1). Interestingly, during IntA nearly all cocaine consumption takes place in the first 60–90 sec of each 5 min drug available period (Kawa et al. 2016), and escalation of intake is confined to this period (Kawa et al. 2016; Allain et al. 2018; Allain and Samaha 2018). Furthermore, because brain cocaine concentrations drop several times within each session (Fig. 1), IntA experience also promotes the development of multiple burst-like episodes of drug intake, whereby rats take several injections within a few seconds each time drug becomes available. In addition, the IntA-Limited procedure described above does not permit escalation, but still increases subsequent motivation for cocaine to a greater extent than LgA, and to a similar degree as produced by standard IntA experience with escalation (Allain et al. 2018). Similarly, decreasing the length of IntA self-administration sessions to 2 hours (Short-IntA) precludes escalation of intake, but still produces burst-like patterns of drug-taking, psychomotor sensitization, and levels of motivation for cocaine and cocaine-induced reinstatement that are similar to those seen with 6-hour IntA sessions (Allain and Samaha 2018). In conclusion, “Taking large and escalating quantities of cocaine does not appear necessary to increase incentive motivation for the drug. Taking cocaine in an intermittent pattern—even in small amounts—is more effective in producing this addiction-relevant change” … “Escalation might be a consequence, rather than a cause in the transition to addiction” (Allain et al. 2018; also see Beckmann et al. 2012).
Incentive-Sensitization.
Recent studies have addressed how other measures of addiction-like behavior change over time with increasing IntA experience (Fig. 1). Not only does IntA produce (i) escalation of intake; but also (ii) a progressive increase in the amount of effort rats are willing to expend to maintain preferred brain levels of cocaine; (iii) a decrease in the elasticity of the cocaine demand curve; (iv) continued drug-taking in the face of an adverse consequence; (v) continued responding when drug is not available; and (vi) especially robust cue-induced reinstatement of drug-seeking (Kawa et al. 2016, 2018; Singer et al. 2018; James et al. 2018a). There is, however, considerable individual variation in the development of addiction-like behavior with IntA experience. For example, both male and female rats develop addiction-like behaviors with IntA experience, but this occurs more rapidly and more robustly in females (Kawa and Robinson 2018), consistent with the “telescoping effect” described in the clinical literature (Griffin et al. 1989; Kosten et al. 1993; McCance-Katz et al. 1999).
In addition, studies using only male rats have shown that the development of addiction-like behaviors following IntA are most pronounced in rats that meet multiple criteria for addiction (Kawa et al. 2016; Singer et al. 2018). Using DSM-like criteria to assess individual variation in the development of addiction-like behavior, as described in Table 1, has been championed most prominently by Deroche-Gamonet, Piazza and colleagues (e.g., Piazza and Deroche-Gamonet 2013; Deroche-Gamonet and Piazza 2014 for review). In these studies rats self-administer cocaine for long periods of time (e.g., 50–70 days). They are then assessed for the development of addiction-like behavior, as indicated by three DSM criteria that capture ‘loss of control’ over drug-taking behavior: “(i) an inability to refrain from drug seeking, (ii) high motivation for the drug, and (iii) maintained drug use despite negative consequences” (p. 440, Deroche-Gamonet and Piazza 2014), using measures described in Table 1. A rat in the top 35th percentile for a criterion is said to meet that criterion. Each criterion is given equal weight. Thus, rats are assigned to groups meeting 0, 1, 2 or 3 criteria for addiction, which the authors suggest reflects a range from “non-addicted” (0 criteria) to “fully addicted” (3 criteria). This is similar to the approach laid out in the DSM-V; symptoms are given equal weight, and severity of the substance use disorder is estimated by the number of symptoms present. Importantly, only a relatively small number of animals meet 3 criteria (approx. 20%), with 40% meeting 0 criteria and 40% 1–2 criteria.
There is similar variation in the development of addiction-like behavior following IntA experience in that rats meeting 2–3 criteria for addiction were more susceptible to incentive-sensitization than 0–1 criteria rats (Kawa et al. 2016; Singer et al. 2018). Relative to rats meeting 0–1 criteria, 2–3 criteria rats showed a greater increase in motivation for cocaine with IntA experience, as well as greater drug (Kawa et al. 2016) and cue-induced reinstatement, and resistance to extinction (Singer et al. 2018). More work is required, in animals of both sexes, to explore the extent of individual variation in the ability of IntA experience to produce incentive-sensitization and addiction-like behavior, and what factors contribute to such variation. Interestingly, in their studies using this “multi-symptomatic model”, Deroche-Gamonet, Piazza and colleagues also use a cocaine self-administration procedure that has features similar to IntA. They typically allow rats three 40 min drug available periods, interspersed with two 15 min drug not available periods. This would produce fluctuations in brain cocaine concentration. Also similar to IntA, the cocaine self-administration procedure used in the “multi-symptomatic model” produces a burst-like pattern of drug intake, most prominently in 3 criteria rats, and this pattern of intake is thought to precede and contribute to the development of addiction-like behavior (Belin et al. 2009; Martín-García et al. 2014). Importantly, this self-administration procedure produces, “escalating cocaine intake [that] is paralleled by an increase in the motivational properties of the drug in the absence of apparent signs of tolerance to the reinforcing or incentive effects of cocaine” (p. 2731), based on multiple tests (Deroche et al. 1999; also see Deroche-Gamonet et al. 2004; Belin et al. 2009).
We suggest that the development of addiction-like behavior with IntA experience reflects the process of incentive-sensitization, by which the incentive motivational effects of drugs and drug cues increase with increasing drug experience (Robinson and Berridge 1993; Berridge and Robinson 2016). Consistent with this interpretation, IntA self-administration produces a progressive increase in the psychomotor activating effects of cocaine, and the degree of this psychomotor sensitization (unlike the degree of escalation) predicts subsequent motivation for cocaine assessed by breakpoint on a progressive ratio schedule (Allain et al. 2017). This is reminiscent of studies using intermittent exposure to cocaine via experimenter-administered i.p. injections, and showing that this promotes psychomotor sensitization, while continuous infusion promotes tolerance to this effect (Downs and Eddy 1932; Post and Rose 1976; Post 1980; Robinson and Becker 1986; Stewart and Badiani 1993). Very recent work also shows that increasing intermittency between IntA sessions by giving sessions every 1–4 days rather than every day further enhances behavioral symptoms of cocaine addiction, including escalation of intake, high economic demand for the drug and responding for cocaine in spite of punishment (James et al. 2018b).
Qo.
Interestingly, IntA does not alter the preferred level of drug consumption when cost is nil, as measured by the metric, Qo (Kawa et al. 2016, 2018; Singer et al. 2018; James et al. 2018a), whereas LgA experience produces an increase in Qo (Oleson and Roberts 2009; Bentzley et al. 2014; Kawa et al. 2018; James et al. 2018a) (Fig. 1), which can be very persistent (James et al. 2018a). Some have suggested Qo reflects a “hedonic set point” (Bentzley et al. 2013), although a “settling point” may be more appropriate (see Berridge 2004 for discussion). Whatever the case, the dissociation between measures of cocaine demand (motivation) and Qo prompts the speculation that IntA experience may increase drug “wanting”, without an associated change in drug “liking” (Berridge and Robinson 2016). Finally, incentive-sensitization is also evident when IntA procedures are used that preclude the development of S-R habits, such that instead of having to make the same operant response to gain access to cocaine every day, rats had to solve new puzzles daily (Singer et al. 2018). This suggests that following a history of IntA cocaine self-administration, habitual drug-seeking is not necessary for the emergence of addiction-like behavior.
Neurobiology.
Very little is known regarding the neurobiological consequences of IntA experience, however, the available evidence suggests that not only do IntA and LgA have very different effects, but in some instances produce opposite effects. Table 2 summarizes the neurobiological changes associated with IntA vs. LgA cocaine experience. As mentioned above, LgA is reported to produce marked tolerance in dopamine neurotransmission, including a decrease in cocaine-induced inhibition of dopamine uptake, at least when measured soon after the discontinuation of cocaine self-administration (e.g., Calipari et al. 2013). In contrast, IntA experience does the opposite, it increases cocaine-induced inhibition of dopamine uptake (Calipari et al. 2013). The different effects of LgA vs IntA on the DAT likely involves the temporal pattern of cocaine intake rather than differences in total intake because intake was similar in IntA and ShA rats, but only IntA rats showed altered DAT function (Calipari et al. 2013). This effect of IntA is even greater after a period of abstinence and it is also associated with increased motivation for drug (Calipari et al. 2015). Furthermore, IntA (but not LgA or ShA) increases electrically-evoked dopamine release (Calipari et al. 2013). Finally, IntA (but not LgA) cocaine self-administration experience produces cross-sensitization, increasing both methylphenidate- and amphetamine-induced inhibition of the DAT in the nucleus accumbens core (Calipari et al. 2014b).
Table 2.
A summary of the neurobiological effects of Long Access (LgA) and Intermittent Access (IntA) cocaine self-administration. The studies presented have generally compared LgA or IntA rats to drug-naïve rats or rats with Short Access cocaine self-administration experience. Horizontal lines with a double arrowhead indicate no effect, and the downward and upward facing arrowheads indicate a decrease and increase in the effect, respectively. DA, dopamine. DAT, dopamine transporter.
| LgA | IntA | ||
|---|---|---|---|
| Neurobiological adaptations |
METABOTROPIC GLUTAMATE GROUP II RECEPTOR FUNCTION (Hao et al. 2010, Allain et al. 2017) |
↑ | ↑ |
|
LATERAL HYPOTHALAMUS OREXIN NEURON ACTIVITY (James et al. 2018a) |
↔ | ↑ | |
|
ELECTRICALLY-EVOKED DA RELEASE ex vivo (Calipari et al. 2013, 2014a) |
↔/↓ | ↑ | |
|
COCAINE-INDUCED DA OVERFLOW in vivo (Ahmed et al. 2003, Calipari et al. 2014a, Kawa et al. 2018) |
↔/↓ | ↑ | |
|
COCAINE-INDUCED DAT INHIBITION ex vivo (Ferris et al. 2011, Calipari et al. 2013, 2015) |
↓ | ↑ | |
|
METHYLPHENIDATE- AND AMPHETAMINEINDUCED DAT INHIBITION ex vivo (Calipari et al. 2014b) |
↔ | ↑ | |
Most of the studies above on the effects of LgA vs IntA experience on DA function were conducted ex vivo, using tissue slices. However, Kawa et al. (2018) recently showed that a single self-administered injection of cocaine increased DA overflow in the nucleus accumbens core in vivo to a greater extent in rats with IntA than LgA or ShA experience, and the latter two groups did not differ. This is consistent with Ahmed et al. (2003), who also reported that LgA experience had no effect on cocaine-evoked DA overflow in vivo, relative to ShA. Interestingly, after IntA experience the magnitude of the DA response predicted motivation for cocaine (Kawa et al. 2018). Finally, Kawa et al. (2018) reported no effect of IntA, LgA or ShA experience on basal levels of DA in dialysate, consistent with others (Ahmed et al. 2003; Calipari et al. 2014a; but see Ferris et al. 2011).
LgA (but not ShA) cocaine experience also upregulates presynaptic metabotropic glutamate group II receptors (mGluR2/3s) in mesocorticolimbic regions (Hao et al. 2010), and this is thought to contribute to the expression of addiction-like behavior. Interestingly, this effect does not require the large amounts of drug intake associated with LgA, because even IntA-Limited experience, which involves very low levels of intake, and does not produce escalation of intake (see above), increases mGluR2/3 receptor function (Allain et al. 2017). Finally, IntA (but not LgA or ShA) experience persistently increases lateral hypothalamic orexin cell function in ways that promote addiction-like behavior, and this can be reversed by knockdown of orexin neurons (James et al. 2018a).
While additional parametric work is needed, the available evidence suggests that in terms of dopamine function, IntA experience produces sensitization and LgA experience produces tolerance, or no change (Table 2). The contrasting effects of IntA and LgA cocaine self-administration experience are consistent with a large literature involving the use of experimenter-administered psychomotor stimulant drugs. These studies show that intermittent injections produce psychomotor, incentive and dopamine sensitization, whereas treatments that result in continuously high brain levels of drug do not (Post 1980; Robinson and Becker 1986; Robinson and Berridge 1993).
Given that pathological motivation for drug is a defining feature of addiction (Table 1), we have focused primarily on drug-induced changes in motivational processes, and on neural systems thought to mediate these psychological functions. However, many other psychological processes are thought to contribute to the development of addiction. These include the ability of drug cues to bias attention towards them (Field et al. 2014; although see Christiansen et al. 2015) and poor attentional (cognitive) control over behavior (Wiers et al. 2013; Sarter and Phillips 2018). This can result, of course, in poor inhibitory control, especially when faced with temptations to use (Jentsch and Taylor 1999), and this may be both a cause and a consequence of drug use. Preclinical studies suggest that LgA cocaine self-administration experience can produce persistent deficits in attentional control (e.g., Briand et al. 2008). However, essentially nothing is known about the effects of IntA experience on this or other cognitive faculties relevant to addiction (Pitchers et al. 2017b, 2018).
Outstanding Questions
As should be obvious from the above, there is much to be learned about how pharmacokinetic factors, and IntA experience specifically, influence brain and behavior in ways that may promote the transition to addiction, and there are many important outstanding questions. For example, there is a dearth of quantitative, non-anecdotal evidence about exact patterns of drug-taking behavior in women and men, both between and within bouts of use, especially during the transition to addiction. Such information will be critical for the further refinement of animal models.
We also know that there are sex differences in the transition to addiction, so it will be important to determine if there are sex differences in the behavioral and neurobiological response to IntA drug self-administration experience. In this regard, a recent study mentioned above shows that when given IntA to cocaine, female rats take more cocaine than male rats, and females also show a faster and greater increase in motivation for the drug, as measured by behavioral economics indices (Kawa and Robinson 2018). This suggests that, under IntA conditions, females might be more vulnerable to cocaine-induced incentive-sensitization, an effect thought to facilitate the transition to addiction (Robinson and Berridge 1993; Kawa and Robinson 2018). This is reminiscent of the more robust psychomotor sensitization seen in female versus male rats following experimenter-administered drug (Glick and Hinds 1984; Robinson and Becker 1986).
Most people with a substance use disorder also typically use multiple drugs, and we do not know how this might influence the effects of IntA experience. Human drug users sometimes simultaneously take more than one substance, and sometimes they take different drugs sequentially, at different times (Ellinwood et al. 1976; Leri et al. 2003). The long-term behavioral, psychological and neurobiological effects of such practices can be studied using the IntA model (Leri and Stewart 2001; Calipari et al. 2014b). For example, does drug co-use produce cross-sensitization, as has been described with experimenter-administered drugs (Robinson and Becker 1986)?
Further, most studies using intermittent access procedures have been conducted in rats, and with cocaine, so it will be important to incorporate studies with other species and other drugs. For comparison, the LgA procedure has been used across drug classes and it can also induce behaviors relevant to addiction to heroin (Ahmed et al. 2000; Lenoir and Ahmed 2008), methamphetamine (Adhikary et al. 2017), and nicotine (Paterson and Markou 2004). This being said, there is work suggesting that intermittent access promotes symptoms of addiction to drugs of abuse other than cocaine. For instance, relative to continuous access, intermittent access to alcohol promotes the escalation of alcohol intake (e.g., Simms et al. 2008; also see Ahmed et al. 2018). New work also shows that compared to short-access fentanyl self-administration, intermittent access increases incentive motivation for fentanyl [decreased demand elasticity without changing preferred consumption (Qo)], and that an orexin-1 receptor antagonist decreases both incentive motivation for fentanyl and cue-induced relapse behavior in IntA rats but not in ShA rats (Fragale et al. 2018). In this regard, it will be important to determine whether IntA procedures prove useful in identifying and developing treatments for substance use disorders, especially relative to more traditional self-administration procedures using FR schedules (Haney and Spealman 2008; Czoty et al. 2016).
Concluding Remarks: Implications for Preclinical Models of Addiction
The studies summarized above clearly establish that the temporal pattern by which self-administered cocaine impinges on the brain has an enormous influence on its ability to change brain and psychological function in ways that are relevant for the transition to addiction. Indeed, studies using IntA self-administration procedures challenge fundamental assumptions underlying the most widely accepted rodent model for studying the transition to addiction – the LgA model. Most importantly, the development of addiction-like behavior – including high motivation for drug, continued drug use in the face of an adverse consequence, robust cue-induced reinstatement of drug-seeking, and even escalation of intake – does not require the ingestion of the large amounts of cocaine associated with LgA self-administration (Fig. 1).
IntA self-administration experience is especially effective in producing psychomotor sensitization, incentive-sensitization, dopamine sensitization, as well as addiction-like behavior. Some researchers have interpreted the hypodopaminergic state often associated with LgA or other high dose cocaine experience as support for the hypothesis that drug-seeking behavior in addiction is motivated by a desire to alleviate this dopamine deficiency (Volkow et al. 2016; Koob and Volkow 2016). However, studies using IntA cocaine self-administration procedures suggest the opposite – that cocaine use produces incentive-sensitization and a hyper-responsive dopaminergic state, and this leads to pathological drug “wanting” and the transition to addiction (Robinson and Berridge 1993). It is clearly problematic for the field if two purported animal models of addiction produce such different, and even opposite effects. As put by the statistician George Box (Box et al. 2005), “All models are wrong but some models are useful”. The most useful model(s) for studying drug-induced changes in brain and behavior that contribute to the transition to addiction would be the model(s) that best reflects the human condition. By our perspective, self-administration procedures that reflect the intermittent patterns characteristic of human cocaine use—both within and between bouts of drug taking—are best suited to model the transition to addiction in the laboratory.
Acknowledgements
Supported by NIDA grants PO1 DA031656 and T32 DA007281 to TER, and CIHR grant 157572 and FRQ-S salary grant 28988 to ANS. We thank David C Roberts for generous advice on implementation of the IntA procedure in our laboratories. We thank other members of the Robinson and Samaha laboratories who contributed to some of the studies cited here, especially Kyle Pitchers, Bryan Singer, Hajer Algallal and Karim Bouayad-Gervais.
Footnotes
Conflicts of Interest
ANS is a consultant for H. Lundbeck A/S. This had no influence on the manuscript’s content. TER, ABK and FA declare no conflicts of interest.
References
- Adhikary S, Caprioli D, Venniro M, et al. (2017) Incubation of extinction responding and cue-induced reinstatement, but not context- or drug priming-induced reinstatement, after withdrawal from methamphetamine. Addict Biol 22:977–990. doi: 10.1111/adb.12386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SH (2012) The science of making drug-addicted animals. Neuroscience 211:107–25. doi: 10.1016/j.neuroscience.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Badiani A, Miczek KA, Muller CP (2018) Non-pharmacological factors that determine drug use and addiction. Neurosci Biobehav Rev In Press. doi: 10.1016/j.neubiorev.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF (1998) Transition from moderate to excessive drug intake: change in hedonic set point. Science 282:298–300. doi: 10.1126/science.282.5387.298 [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Lin D, Koob GF, Parsons LH (2003) Escalation of cocaine self-administration does not depend on altered cocaine-induced nucleus accumbens dopamine levels. J Neurochem 86:102–13. doi: 10.1046/j.1471-4159.2003.01833.x [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, Koob GF (2000) Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology 22:413–21. doi: 10.1016/S0893-133X(99)00133-5 [DOI] [PubMed] [Google Scholar]
- Allain F, Bouayad-Gervais K, Samaha A-N (2018) High and escalating levels of cocaine intake are dissociable from subsequent incentive motivation for the drug in rats. Psychopharmacology (Berl) 235:317–328. doi: 10.1007/s00213-017-4773-8 [DOI] [PubMed] [Google Scholar]
- Allain F, Minogianis E-A, Roberts DCS, Samaha A-N (2015) How fast and how often: The pharmacokinetics of drug use are decisive in addiction. Neurosci Biobehav Rev 56:166–79. doi: 10.1016/j.neubiorev.2015.06.012 [DOI] [PubMed] [Google Scholar]
- Allain F, Roberts DCS, Lévesque D, Samaha A-N (2017) Intermittent intake of rapid cocaine injections promotes robust psychomotor sensitization, increased incentive motivation for the drug and mGlu2/3 receptor dysregulation. Neuropharmacology 117:227–237. doi: 10.1016/j.neuropharm.2017.01.026 [DOI] [PubMed] [Google Scholar]
- Allain F, Samaha A-N (2018) Revisiting long-access versus short-access cocaine self-administration in rats: intermittent intake promotes addiction symptoms independent of session length. Addict Biol. in press: doi: 10.1111/adb.12629 [DOI] [PubMed] [Google Scholar]
- Allen CP, Park K, Li A, et al. (2018) Enhanced neuronal and blunted hemodynamic reactivity to cocaine in the prefrontal cortex following extended cocaine access: optical imaging study in anesthetized rats. Addict Biol. in press: doi: 10.1111/adb.12615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony JC, Warner LA, Kessler RC (1994) Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the National Comorbidity Survey. Exp. Clin. Psychopharmacol 2:244–268 [Google Scholar]
- Badiani A (2013) Substance-specific environmental influences on drug use and drug preference in animals and humans. Curr Opin Neurobiol 23:588–96. doi: 10.1016/j.conb.2013.03.010 [DOI] [PubMed] [Google Scholar]
- Beckmann JS, Gipson CD, Marusich J a, Bardo MT (2012) Escalation of cocaine intake with extended access in rats: dysregulated addiction or regulated acquisition? Psychopharmacology (Berl) 222:257–67. doi: 10.1007/s00213-012-2641-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Balado E, Piazza PV, Deroche-Gamonet V (2009) Pattern of intake and drug craving predict the development of cocaine addiction-like behavior in rats. Biol Psychiatry 65:863–8. doi: 10.1016/j.biopsych.2008.05.031 [DOI] [PubMed] [Google Scholar]
- Bentzley BS, Fender KM, Aston-Jones G (2013) The behavioral economics of drug self-administration: A review and new analytical approach for within-session procedures. Psychopharmacology (Berl) 226:113–125. doi: 10.1007/s00213-012-2899-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Jhou TC, Aston-Jones G (2014) Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proc Natl Acad Sci 111:11822–7. doi: 10.1073/pnas.1406324111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC (2004) Motivation concepts in behavioral neuroscience. Physiol Behav 81:179–209. doi: 10.1016/j.physbeh.2004.02.004 [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE (2016) Liking, wanting, and the incentive-sensitization theory of addiction. Am Psychol 71:670–679. doi: 10.1037/amp0000059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T, Wray P, Brewer A, et al. (2012) Analyzing Human Cocaine Use Patterns to Inform Animal Addiction Model Development. In: College on Problems of Drug Dependence Annual Meeting; Palm Springs, CA [Google Scholar]
- Box GEP, Hunter WG, Hunter JS (2005) Statistics for Experimenters: An Introduction to Design, Data Analysis, and Model Building. Wiley Ser Probab Math Stat 2:528–529 [Google Scholar]
- Briand LA, Flagel SB, Garcia-Fuster MJ, et al. (2008) Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine. Neuropsychopharmacology 33:2969–80. doi: 10.1038/npp.2008.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Jones SR (2014a) Extended access of cocaine self-administration results in tolerance to the dopamine-elevating and locomotor-stimulating effects of cocaine. J Neurochem 128:224–232. doi: 10.1111/jnc.12452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Siciliano C a, et al. (2014b) Intermittent cocaine self-administration produces sensitization of stimulant effects at the dopamine transporter. J Pharmacol Exp Ther 349:192–8. doi: 10.1124/jpet.114.212993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Zimmer BA, et al. (2013) Temporal Pattern of Cocaine Intake Determines Tolerance vs Sensitization of Cocaine Effects at the Dopamine Transporter. Neuropsychopharmacology 38:2385–92. doi: 10.1038/npp.2013.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Siciliano C a, Zimmer B a, Jones SR (2015) Brief intermittent cocaine self-administration and abstinence sensitizes cocaine effects on the dopamine transporter and increases drug seeking. Neuropsychopharmacology 40:728–35. doi: 10.1038/npp.2014.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D, Celentano M, Dubla A, et al. (2009) Ambience and Drug Choice: Cocaine- and Heroin-Taking as a Function of Environmental Context in Humans and Rats. Biol Psychiatry 65:893–899. doi: 10.1016/j.biopsych.2008.12.009 [DOI] [PubMed] [Google Scholar]
- Childs E, de Wit H (2013) Contextual conditioning enhances the psychostimulant and incentive properties of d -amphetamine in humans. Addict Biol 18:985–992. doi: 10.1111/j.1369-1600.2011.00416.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen P, Schoenmakers TM, Field M (2015) Less than meets the eye: Reappraising the clinical relevance of attentional bias in addiction. Addict Behav 44:43–50. doi: 10.1016/j.addbeh.2014.10.005 [DOI] [PubMed] [Google Scholar]
- Cohen P, Sas A (1994) Cocaine use in Amsterdam in non Deviant Subcultures. Addict Res Theory 2:71–94. doi: 10.3109/16066359409005547 [DOI] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, et al. (2008) Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454:118–121. doi: 10.1038/nature06995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Stoops WW, Rush CR (2016) Evaluation of the “Pipeline” for Development of Medications for Cocaine Use Disorder: A Review of Translational Preclinical, Human Laboratory, and Clinical Trial Research. Pharmacol Rev 68:533–62. doi: 10.1124/pr.115.011668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, Gold MS (1985) New concepts in cocaine addiction: the dopamine depletion hypothesis. Neurosci Biobehav Rev 9:469–77. doi: 10.1016/0149-7634(85)90022-3 [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV (2004) Evidence for addiction-like behavior in the rat. Science 305:1014–1017. doi: 10.1126/science.1099020 [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Piazza PV (2014) Psychobiology of cocaine addiction: Contribution of a multi-symptomatic animal model of loss of control. Neuropharmacology 76 Pt B:437–49. doi: 10.1016/j.neuropharm.2013.07.014 [DOI] [PubMed] [Google Scholar]
- Deroche V, Le Moal M, Piazza PV (1999) Cocaine self-administration increases the incentive motivational properties of the drug in rats. Eur J Neurosci 11:2731–2736. doi: 10.1046/j.1460-9568.1999.00696.x [DOI] [PubMed] [Google Scholar]
- Downs A, Eddy N (1932) The effect of repeated doses of cocaine on the dog. J Pharmacol Exp Ther. 46(2): 199–200 [Google Scholar]
- Edwards S, Koob GF (2013) Escalation of drug self-administration as a hallmark of persistent addiction liability. Behav Pharmacol 24:356–62. doi: 10.1097/FBP.0b013e3283644d15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinwood EH, Eibergen RD, Kilbey MM (1976) Stimulants: interaction with clinically relevant drugs. Ann N Y Acad Sci 281:393–408. doi: 10.1111/j.1749-6632.1976.tb27948.x [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Giuliano C, Belin D (2018) Addictive behaviour in experimental animals: prospects for translation. Philos Trans R Soc Lond B Biol Sci 373:20170027. doi: 10.1098/rstb.2017.0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, et al. (2005) Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry 58:751–759. doi: 10.1016/j.biopsych.2005.04.046 [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Mateo Y, Roberts DCS, Jones SR (2011) Cocaine-insensitive dopamine transporters with intact substrate transport produced by self-administration. Biol Psychiatry 69:201–207. doi: 10.1016/j.biopsych.2010.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Marhe R, Franken IHA (2014) The clinical relevance of attentional bias in substance use disorders. CNS Spectr 19:225–30. doi: 10.1017/S1092852913000321 [DOI] [PubMed] [Google Scholar]
- Fragale JE, James MH, Behman V, et al. (2018) Intermittent access to fentanyl increases economic demand through an orexin/hypocretin mechanism Society for Neuroscience Abstracts. San Diego [Google Scholar]
- Glick SD, Hinds PA (1984) Sex differences in sensitization to cocaine-induced rotation. Eur J Pharmacol 99:119–121. doi: 10.1016/0014-2999(84)90442-4 [DOI] [PubMed] [Google Scholar]
- Gratton A, Wise R (1994) Drug- and behavior-associated changes in dopamine-related electrochemical signals during intravenous cocaine self-administration in rats. J Neurosci 14:4130–4146. doi: 10.1523/JNEUROSCI.14-07-04130.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin ML, Weiss RD, Mirin SM, Lange U (1989) A comparison of male and female cocaine abusers. Arch Gen Psychiatry 46:122–6. doi: 10.1001/archpsyc.1989.01810020024005 [DOI] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, et al. (2003) Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci 23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Spealman R (2008) Controversies in translational research: drug self-administration. Psychopharmacology (Berl) 199:403–19. doi: 10.1007/s00213-008-1079-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Martin-Fardon R, Weiss F (2010) Behavioral and functional evidence of metabotropic glutamate receptor 2/3 and metabotropic glutamate receptor 5 dysregulation in cocaine-escalated rats: factor in the transition to dependence. Biol Psychiatry 68:240–8. doi: 10.1016/j.biopsych.2010.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyne A, Wolffgramm J (1998) The development of addiction to d-amphetamine in an animal model: same principles as for alcohol and opiate. Psychopharmacology (Berl) 140:510–8. doi: 10.1007/s002130050796 [DOI] [PubMed] [Google Scholar]
- James MH, Stopper CM, Zimmer BA, et al. (2018a) Increased Number and Activity of a Lateral Subpopulation of Hypothalamic Orexin/Hypocretin Neurons Underlies the Expression of an Addicted State in Rats. Biol Psychiatry. in press: doi: 10.1016/j.biopsych.2018.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Bowrey HE, Fragale JE, Aston-Jones G (2018b) Variable episodic self-administration enhances economic demand for cocaine and sensitivity to the orexin/hypocretin receptor antagonist SB-334867 Society for Neuroscience Abstracts. San Diego [Google Scholar]
- Jentsch JD, Taylor JR (1999) Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 146:373–390. doi: 10.1007/PL00005483 [DOI] [PubMed] [Google Scholar]
- Johanson CE, Balster RL, Bonese K (1976) Self-administration of psychomotor stimulant drugs: the effects of unlimited access. Pharmacol Biochem Behav 4:45–51. doi: 10.1016/0091-3057(76)90174-X [DOI] [PubMed] [Google Scholar]
- Kawa AB, Bentzley BS, Robinson TE (2016) Less is more: prolonged intermittent access cocaine self-administration produces incentive-sensitization and addiction-like behavior. Psychopharmacology (Berl) 233:3587–602. doi: 10.1007/s00213-016-4393-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa AB, Robinson TE (2018) Sex differences in incentive-sensitization produced by intermittent access cocaine self-administration. Psychopharmacology (Berl). in press: doi: 10.1007/s00213-018-5091-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa AB, Valenta AC, Kennedy RT, Robinson TE (2018) Incentive and dopamine sensitization produced by intermittent but not long access cocaine self-administration. bioRxiv 499475. doi: 10.1111/ejn.14418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keramati M, Durand A, Girardeau P, et al. (2017) Cocaine addiction as a homeostatic reinforcement learning disorder. Psychol Rev 124:130–153. doi: 10.1037/rev0000046 [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis. The lancet Psychiatry 3:760–73. doi: 10.1016/S2215-0366(16)00104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ (1993) Gender differences in cocaine use and treatment response. J Subst Abuse Treat 10:63–6. doi: 10.1016/0740-5472(93)90100-G [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Ghazanfar AA, Gomez-Marin A, et al. (2017) Neuroscience Needs Behavior: Correcting a Reductionist Bias. Neuron 93:480–490. doi: 10.1016/j.neuron.2016.12.041 [DOI] [PubMed] [Google Scholar]
- Lenoir M, Ahmed SH (2008) Supply of a Nondrug Substitute Reduces Escalated Heroin Consumption. Neuropsychopharmacology 33:2272–2282. doi: 10.1038/sj.npp.1301602 [DOI] [PubMed] [Google Scholar]
- Leri F, Bruneau J, Stewart J (2003) Understanding polydrug use: review of heroin and cocaine co-use. Addiction 98:7–22. doi: 10.1046/j.1360-0443.2003.00236.x [DOI] [PubMed] [Google Scholar]
- Leri F, Stewart J (2001) Drug-induced reinstatement to heroin and cocaine seeking: A rodent model of relapse in polydrug use. Exp Clin Psychopharmacol 9:297–306. doi: 10.1037/1064-1297.9.3.297 [DOI] [PubMed] [Google Scholar]
- Leyton M, Vezina P (2014) Dopamine ups and downs in vulnerability to addictions: a neurodevelopmental model. Trends Pharmacol Sci 35:268–76. doi: 10.1016/j.tips.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y (2004) Incubation of cocaine craving after withdrawal: A review of preclinical data. Neuropharmacology 47:214–226 [DOI] [PubMed] [Google Scholar]
- Lynch WJ (2018) Modeling the development of drug addiction in male and female animals. Pharmacol Biochem Behav 164:50–61. doi: 10.1016/j.pbb.2017.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-García E, Courtin J, Renault P, et al. (2014) Frequency of Cocaine Self-Administration Influences Drug Seeking in the Rat: Optogenetic Evidence for a Role of the Prelimbic Cortex. Neuropsychopharmacology. doi: 10.1038/npp.2014.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance-Katz EF, Carroll KM, Rounsaville BJ (1999) Gender differences in treatment-seeking cocaine abusers--implications for treatment and prognosis. Am J Addict 8:300–11. doi: 10.1080/105504999305703 [DOI] [PubMed] [Google Scholar]
- Oleson EB, Roberts DCS (2009) Behavioral economic assessment of price and cocaine consumption following self-administration histories that produce escalation of either final ratios or intake. Neuropsychopharmacology 34:796–804. doi: 10.1038/npp.2008.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Yasar S, Nemeth-Coslett R, et al. (2005) Human Cocaine-Seeking Behavior and its Control by Drug-Associated Stimuli in the Laboratory. Neuropsychopharmacology 30:433–443. doi: 10.1038/sj.npp.1300599 [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A (2003) Increased motivation for self-administered cocaine after escalated cocaine intake. Neuroreport 14:2229–2232. doi: 10.1097/01.wnr.0000091685.94870.ba [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A (2004) Prolonged nicotine dependence associated with extended access to nicotine self-administration in rats. Psychopharmacology (Berl) 173:64–72. doi: 10.1007/s00213-003-1692-7 [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonet V (2013) A multistep general theory of transition to addiction. Psychopharmacology (Berl) 229:387–413. doi: 10.1007/s00213-013-3224-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Kane LF, Kim Y, et al. (2017a) ‘Hot’ vs. ‘cold’ behavioural-cognitive styles: motivational-dopaminergic vs. cognitive-cholinergic processing of a Pavlovian cocaine cue in sign- and goal-tracking rats. Eur J Neurosci 46:2768–2781. doi: 10.1111/ejn.13741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Phillips KB, Jones JL, et al. (2017b) Diverse Roads to Relapse: A Discriminative Cue Signaling Cocaine Availability Is More Effective in Renewing Cocaine Seeking in Goal Trackers Than Sign Trackers and Depends on Basal Forebrain Cholinergic Activity. J Neurosci 37:7198–7208. doi: 10.1523/JNEUROSCI.0990-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Sarter M, Robinson TE (2018) The hot ‘n’ cold of cue-induced drug relapse. Learn Mem 25:474–480. doi: 10.1101/lm.046995.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Wood TR, Skrzynski CJ, et al. (2017c) The ability for cocaine and cocaine-associated cues to compete for attention. Behav Brain Res 320:302–315. doi: 10.1016/j.bbr.2016.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM (1980) Intermittent versus continuous stimulation: effect of time interval on the development of sensitization or tolerance. Life Sci 26:1275–82. doi: 10.1016/0024-3205(80)90085-5 [DOI] [PubMed] [Google Scholar]
- Post RM, Rose H (1976) Increasing effects of repetitive cocaine administration in the rat. Nature 260:731–2. doi: 10.1038/260731a0 [DOI] [PubMed] [Google Scholar]
- Roberts DC., Brebner K, Vincler M, Lynch WJ (2002) Patterns of cocaine self-administration in rats produced by various access conditions under a discrete trials procedure. Drug Alcohol Depend 67:291–299. doi: 10.1016/S0376-8716(02)00083-2 [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB (1986) Enduring changes in brain and behavior produced by chronic amphetamine administration: A review and evaluation of animal models of amphetamine psychosis. Brain Res. Rev 11:157–198 [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18:247–91. doi: 10.1016/0165-0173(93)90013-P [DOI] [PubMed] [Google Scholar]
- Sarter M, Phillips KB (2018) The neuroscience of cognitive-motivational styles: Sign- and goal-trackers as animal models. Behav Neurosci 132:1–12. doi: 10.1037/bne0000226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster CR, Thompson T (1969) Self administration of and behavioral dependence on drugs. Annu Rev Pharmacol 9:483–502. doi: 10.1146/annurev.pa.09.040169.002411 [DOI] [PubMed] [Google Scholar]
- Shaham Y, Rodaros D, Stewart J (1994) Reinstatement of heroin-reinforced behavior following long-term extinction. Behav Pharmacol 5:360–364. doi: 10.1097/00008877-199406000-00015 [DOI] [PubMed] [Google Scholar]
- Siciliano CA, Fordahl SC, Jones SR (2016) Cocaine Self-Administration Produces Long-Lasting Alterations in Dopamine Transporter Responses to Cocaine. J Neurosci 36:7807–16. doi: 10.1523/JNEUROSCI.4652-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, et al. (2008) Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res 32:1816–23. doi: 10.1111/j.1530-0277.2008.00753.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SL, Richardson K, Dacey J, et al. (2002) A comparison of patterns of methamphetamine and cocaine use. J Addict Dis 21:35–44. doi: 10.1300/J069v21n01 [DOI] [PubMed] [Google Scholar]
- Singer BF, Fadanelli M, Kawa AB, Robinson TE (2018) Are Cocaine-Seeking “Habits” Necessary for the Development of Addiction-Like Behavior in Rats? J Neurosci 38:60–73. doi: 10.1523/JNEUROSCI.2458-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J, Badiani A (1993) Tolerance and sensitization to the behavioral effects of drugs. Behav. Pharmacol 4:289–312 [PubMed] [Google Scholar]
- Stewart J, Eikelboom R (1987) Conditioned Drug Effects. In: Handbook of Psychopharmacology. Springer US, Boston, MA, pp 1–57 [Google Scholar]
- Tornatzky W, Miczek KA (2000) Cocaine self-administration “binges”: transition from behavioral and autonomic regulation toward homeostatic dysregulation in rats. Psychopharmacology (Berl) 148:289–298. doi: 10.1007/s002130050053 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (2016) Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health [PubMed]
- Venniro M, Caprioli D, Shaham Y (2016) Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res 224:25–52. doi: 10.1016/bs.pbr.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Venniro M, Zhang M, Caprioli D, et al. (2018) Volitional social interaction prevents drug addiction in rat models. Nat Neurosci 21:1520–1529. doi: 10.1038/s41593-018-0246-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT (2016) Neurobiologic Advances from the Brain Disease Model of Addiction. N Engl J Med 374:363–71. doi: 10.1056/NEJMra1511480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AS, Haney M, Fischman MW, Foltin RW (1997) Binge cocaine self-administration in humans: intravenous cocaine. Psychopharmacology (Berl) 132:375–381. doi: 10.1007/s002130050358 [DOI] [PubMed] [Google Scholar]
- Weeks JR (1962) Experimental morphine addiction: Method for automatic intravenous injections in unrestrained rats. Science 138:143–144. doi: 10.1126/science.138.3537.143 [DOI] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, et al. (2000) Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci U S A 97:4321–6. doi: 10.1073/pnas.97.8.4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Markou A, Lorang MT, Koob GF (1992) Basal extracellular dopamine levels in the nucleus accumbens are decreased during cocaine withdrawal after unlimited-access self-administration. Brain Res 593:314–318. doi: 10.1016/0006-8993(92)91327-B [DOI] [PubMed] [Google Scholar]
- Wiers RW, Gladwin TE, Hofmann W, et al. (2013) Cognitive bias modification and cognitive control training in addiction and related psychopathology: Mechanisms, clinical perspectives, and ways forward. Clin Psychol Sci 1:192–212. doi: 10.1177/2167702612466547 [DOI] [Google Scholar]
- Willuhn I, Burgeno LM, Groblewski PA, Phillips PEM (2014) Excessive cocaine use results from decreased phasic dopamine signaling in the striatum. Nat Neurosci 17:704–709. doi: 10.1038/nn.3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME (2016) Synaptic mechanisms underlying persistent cocaine craving. Nat. Rev. Neurosci 17:351–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer BA, Dobrin CV, Roberts DCS (2011) Brain-Cocaine Concentrations Determine the Dose Self-Administered by Rats on a Novel Behaviorally Dependent Dosing Schedule. Neuropsychopharmacology 36:2741–2749. doi: 10.1038/npp.2011.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer BA, Oleson EB, Roberts DC (2012) The motivation to self-administer is increased after a history of spiking brain levels of cocaine. Neuropsychopharmacology 37:1901–10. doi: 10.1038/npp.2012.37 [DOI] [PMC free article] [PubMed] [Google Scholar]