Abstract
Introduction:
Sound is integral to communication and connects us to the world through speech and music. Cochlear hair cells are essential for converting sounds into neural impulses. However, these cells are highly susceptible to damage from an array of factors, resulting in degeneration and ultimately irreversible hearing loss in humans. Since the discovery of hair cell regeneration in birds, there have been tremendous efforts to identify therapies that could promote hair cell regeneration in mammals.
Areas covered:
Here, we will review recent studies describing spontaneous hair cell regeneration and direct cellular reprograming as well as other factors that mediate mammalian hair cell regeneration.
Expert opinion:
Numerous combinatorial approaches have successfully reprogrammed non-sensory supporting cells to form hair cells, albeit with limited efficacy and maturation. Studies on epigenetic regulation and transcriptional network of hair cell progenitors may accelerate discovery of more promising reprogramming regimens.
Keywords: Cochlea, Hair cell, Hearing loss, Supporting cell, utricle
1. Introduction
The cochlea is an exquisite sensory organ dedicated to transducing sounds into neural impulses. The organ of Corti is critical for this function, precisely arranged with four rows of mechanosensory hair cells intercalated with non-sensory supporting cells. In mammals, lost hair cells are not regenerated, leading to irreversible hearing loss. Clinical therapies that are currently available include hearing aids and cochlear implants, the latter being a device, which aims to replace the role of the hair cells by providing electrical signals directly to the spiral ganglion neurons. To date, biological therapies have been unsuccessful in fully regenerating hair cells and restoring auditory function; however, recent advances have revealed the potential for native supporting cells to serve as an endogenous source for hair cell regeneration. In this review, we will discuss spontaneous hair cell regeneration in the cochlea and utricle, the competence of supporting cells to act as hair cell progenitors, and finally, direct cellular reprogramming approaches to coerce supporting cells to undergo cell fate change.
2. Hearing Loss
Over 6% of the world’s population (around half a billion people) suffer from disabling hearing impairment [1]. This prevalence is expected to continue to grow as the population ages, doubling the number of adults in the United States affected by hearing loss in the coming decades [2]. The social, emotional, and economic impact that hearing impairment places on individuals and society at large is significant. The exclusion from spoken communication, for example, as a result of hearing loss has adverse effects on day-to-day life, particularly for children who can experience a delay in language development [3]. Moreover, there is a growing body of evidence associating hearing loss with cognitive dysfunction in the elderly, including a connection between hearing loss and dementia [4, 5].
The most common pathological finding of hearing impaired patients postmortem is damage to the cochlear sensory epithelium, primarily the loss of sensory hair cells, as well as a reduction in the number of spiral ganglion neurons [6, 7]. This loss of hair cells can arise from a number of causes ranging from side effects of therapeutic agents (such as aminoglycoside antibiotics and chemotherapy drugs), noise trauma, genetic disorders, and aging. Currently, the only treatments available are hearing aids and cochlear implants. These devices can provide significant benefits to patients, particularly when implanted early, with children implanted before the age of 4 performing as well as post-lingual deaf adults on open-set tasks, allowing them to enter the mainstream education system with their normal hearing peers [8]. Despite their success, however there remains wide variations in patient outcomes and many of them lack adequate access to treatment. Importantly, the devices fail to reverse the underlying pathology of hair cell loss. To complement or move beyond a device-based treatment, studies have begun to examine potential biological approaches to regenerating the cochlear sensory epithelium after insult. It is important to note, however, that hair cell loss is not the only pathology causing hearing loss, and as such hair cell regeneration is only one of many possible therapeutic approaches. For example, studies have examined regeneration of other cell types such as the spiral ganglion neurons and supporting cells, and a growing body of literature has examined the promising approach of correcting genetic mutations within hair cells and supporting cells [9, 10, 11, 12].
3. Cochlear Sensory Epithelium
The mammalian cochlear sensory epithelium consists of an orderly arrangement of hair cells and surrounding supporting cells. Hair cells consist of one row of inner hair cells, the primary sound transducers, and three rows of outer hair cells, which act as amplifiers of low-level sounds [13]. The inner and outer hair cells are separated by the inner and outer pillar cells, supporting cell subtypes that together form the tunnel of Corti (Figure 1). Other subtypes of supporting cells include the Deiters’ cells and inner phalangeal cells that underlie and surround the outer hair cells and the inner hair cells, respectively. As transducers of the cochlea, sensory hair cells are sensitive to a wide variety of insults. After severe damage and extensive hair cell loss, dramatic remodeling of the sensory epithelium can occur [14, 15]. For example, after aminoglycoside-induced hair cell loss, supporting cells expand to form a scar-like, flattened epithelium composed of non-specialized cells [15]. The dramatic change in the milieu and architecture of the cochlear sensory epithelium after damage may in part limit its ability to regenerate hair cells, a topic that will be discussed in the following section.
Figure 1.
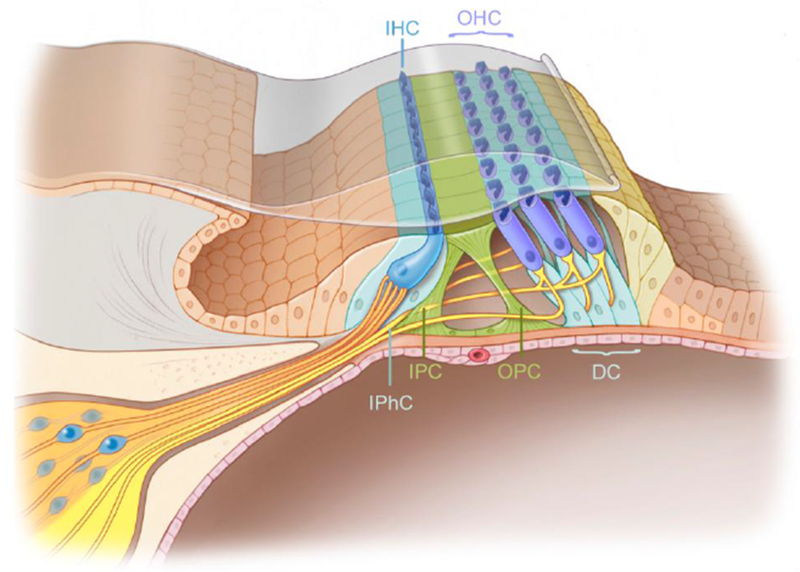
The organ of Corti, the sensory domain of the cochlea, houses one row of inner hair cells and three rows of outer hair cells. Inner hair cells are supported by inner phalangeal cells and outer hair cells are supported by Deiters’ cells, collectively referred to as supporting cells. The inner and outer hair cells are separated by the inner and outer pillar cells which form the tunnel of Corti. IHC: inner hair cell, OHC: outer hair cell, IPhC: Inner phalangeal cell, IP: Inner pillar cell, OP: Outer pillar cell, DC: Deiters’ cell. Modified and reprinted from [16] under a CC BY license, with permission from Springer Nature, Understanding the cochlea (2017).
4. Spontaneous Hair Cell Regeneration
A number of studies, beginning with two seminal papers from nearly 30 years ago showed that hair cells of the avian basilar papilla (analogous to the mammalian cochlea) and utricle can regenerate hair cells from underlying supporting cells [17, 18, 19, 20]. Supporting cells have since been labeled as hair cell progenitors, and the process of regeneration can be broadly characterized to occur via mitotic and non-mitotic mechanisms. Mitotic regeneration involves supporting cells undergoing cell division prior to differentiating into hair cells. Conversely, non-mitotic regeneration, also termed direct transdifferentiation, occurs when supporting cells differentiate into hair cells without an antecedent mitotic event [21].
In many non-mammalian sensory organs such as the avian vestibular organs and the zebrafish lateral line system, hair cells continuously turn over and regenerate, both of which are increased after damage until homeostasis is reached [22, 23, 24, 25, 26, 27]. On the other hand, the avian basilar papilla can regenerate lost hair cells even though it has no continuous turnover of cells [17, 18, 19]. The mature mammalian cochlea, however, lacks the ability to regenerate hair cells via either mitotic or non-mitotic mechanisms [6, 28]. In contrast, the mature mammalian vestibular system retains a limited degree of non-mitotic regenerative capacity [29, 30, 31, 32, 33].
The neonatal mammalian cochlea was first shown to harbor a population of cells capable of generating new hair cells in vitro [34, 35, 36, 37]. Using flow cytometry and the p27Kip1 transgenic reporter mouse line, White and colleagues found that isolated cochlear supporting cells were able to proliferate and differentiate into hair cells – both features of hair cell progenitors [38]. Oshima et al. also demonstrated that cells from the neonatal cochlear sensory epithelium display stem/progenitor cell behavior by forming spheres in vitro – clonal colonies formed from individual cells – and subsequently differentiate into new hair cells [37]. Importantly, this group found that cells from the sensory epithelia of both the neonatal and mature utricle exhibit stem/progenitor cell ability [37, 39].
Recent studies have built on these findings and revealed that cochlear supporting cells marked by Lgr5 – a marker for somatic stem cells in the skin and intestines – proliferate and differentiate into hair cells at a much greater propensity as compared to other Lgr5-negative supporting cells in vitro [40, 41]. To delineate the role of Lgr5-positive supporting cells in vivo, Cox et al. performed lineage tracing experiments in a Pou4f3DTR transgenic mouse line where hair cells, which express the human diphtheria toxin receptor, can be selectively ablated [42]. Following hair cell death in the neonatal cochlea, a modest degree of spontaneous hair cell regeneration and proliferation was observed, with Lgr5-positive supporting cells contributing to both processes in vivo [42]. Limited spontaneous hair cell regeneration was also observed after ototoxic aminoglycoside insult of the neonatal mouse cochlea in vitro [43]. By contrast, hair cell ablation in the neonatal utricle results in a much more robust regenerative response via both mitotic and non-mitotic pathways, with hair cell recovery up to approximately 60% one month after damage [44, 45]. Lastly, the mature mammalian utricle has been shown to display some degree of regenerative capacity after hair cell loss [29, 31, 32]. This capacity was further characterized in a recent study, whereby hair cells were specifically ablated in the adult utricle using a transgenic mouse model [30]. Fourteen days after ablation only 6% of hair cells remained, with hair cell numbers returning to ∼17% relative to controls by 60 days. These new hair cells displayed evidence of mechanotransduction, synaptic connections and were generated non-mitotically via direct transdifferentiation of supporting cells [30, 46].
Unfortunately, in the neonatal cochlea most regenerated hair cells degenerate in a delayed fashion for reasons not completely clear. Moreover, supporting cells rapidly lose their ability to regenerate hair cells after the first postnatal week within the mature cochlea. Collectively, these studies demonstrated that at least a subset of supporting cells in the neonatal cochlea, and the neonatal and mature utricle, can act as hair cell progenitors. We will next review studies examining mechanisms regulating mammalian hair cell progenitors.
5. Direct Cellular Reprogramming to Enhance Cellular Regeneration
As regeneration does not occur in the mature mammalian cochlea, there have been considerable efforts aimed at coercing supporting cells to regenerate lost hair cells (Figure 2), with cellular reprogramming being a major focus. The targeted manipulation of cell fate through the introduction of transcription factors is broadly termed cellular reprogramming. Over three decades ago, the introduction of a single transcription factor, MyoD, was shown to convert fibroblasts directly to myoblasts in vitro [47], shifting the notion that somatic cell fate is fixed. The plasticity of somatic cell fate was further highlighted by work carried out by Takahashi and colleagues, who successfully induced pluripotency with a cocktail of four transcription factors, the so-called “Yamanaka factors” [48]. Since these studies, many reprogramming approaches to induce pluripotency have been used prior to implementing guided differentiation protocols [49]. Moreover, new strategies to directly convert a cell’s identity (without a preceding dedifferentiation event) have been examined in a growing number of organ systems [50, 51, 52]. This new strategy, of “direct cellular reprogramming” will be the focus of the remainder of this review. For a comprehensive discussion of cellular reprogramming more broadly, we refer the avid reader to the following reviews [53, 54, 55].
Figure 2.
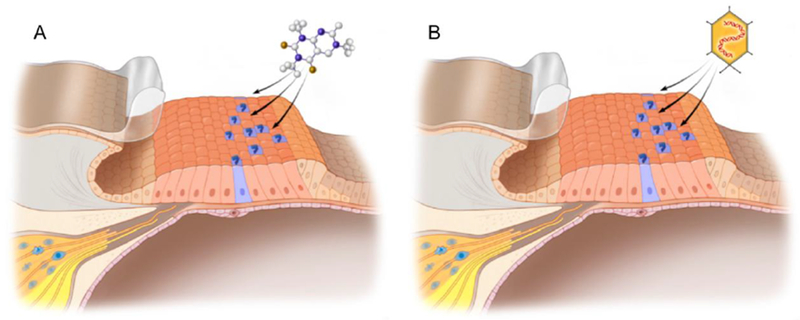
Schematics of cellular reprogramming in the damaged organ of Corti. A) Introduction of a small molecule or siRNA (A) or viral vectors (B) to induce hair cell regeneration.
In the inner ear, one transcription factor that plays a central in hair cell identity is called Atoh1 (previously Math1). Atoh1 is a basic helix-loop-helix transcription factor necessary for hair cell development [56]. Early after ototoxic insult in the avian cochlea and prior to proliferation or regeneration of mature hair cells, Atoh1 expression is upregulated in supporting cells in the damaged avian cochlea [57] and damaged mature mouse utricle [30]. This suggests that similar to development, Atoh1 may play a key role in the specification of hair cells during regeneration. One of the earliest reports of in vivo reprogramming introduced Atoh1 into the mature guinea pig cochlea damaged by aminoglycosides as a means to induce regeneration of hair cells from supporting cells [58]. Subsequently, numerous other studies further explored the potential of Atoh1 as a singular factor to convert supporting cells towards a hair cell fate [59, 60, 61, 62, 63, 64]. The results of Atoh1 preclinical animal studies, however, have been mixed, with generally low efficacy and only one report of limited functional recovery.
More recent studies have more thoroughly characterized components of regenerated hair cells such as the expression of numerous hair cell markers, the presence of stereociliary bundles, mechanotransduction, and synapse formation [59, 63, 65]. In the neonatal cochlea, supporting cell subtypes display different Atoh1-responsiveness, with those within the organ Corti (Deiters’ and pillar cells) being less competent than cells in the greater epithelial ridge [66]. In both the neonatal and juvenile cochlea, Atoh1 overexpression via a transgenic approach induces about 10% of pillar and Deiters’ cells towards a hair cell phenotype [63]. Ectopic hair cells formed from Atoh1 expression display many markers of nascent hair cells and synaptic proteins but failed to express terminal differentiation markers such as prestin and oncomodulin. They develop immature stereocilia, do not have the classic mature hair cell morphology, but are labeled with the styryl dye FM1-43, which permeates patent mechanotransduction channels. This incomplete maturation may have resulted from a constitutive expression of Atoh1 as it is normally downregulated during hair cell maturation. Moreover, the long-term survival of these newly regenerated cells has yet to be carefully examined, indeed a recent study has found that even subtle changes in endogenous Atoh1 can lead to hair cell degeneration, underscoring the importance of characterizing these regenerated cells induced via aberrant Atoh1 expression [67].
Independent studies have shown that the effectiveness of Atoh1 overexpression is rather limited in the adult cochlea, where little to no functional recovery was observed [58, 59, 62, 63, 68]. Similarly, Atoh1 overexpression induces ectopic hair cells in the neonatal utricle with varied results reported in the mature organ [69, 70]. Despite these mixed results in preclinical studies, they led to the opening of a clinical trial assessing the safety and potential benefits of Atoh1 transfection in hearing loss patients (NCT02132130). The results may shed lights firstly on the safety of inner ear viral delivery, and possibly also on the efficacy of a single-factor reprogramming approach, which has been found effective in some organ systems [71, 72, 73]. In a recent study in the visual system of mature mice, for example, forced expression of the transcription factor Ascl1 in combination with a histone deacetylase inhibitor, was able to stimulate functional retinal neurons from Müller glia [74]. Without the addition of the histone deacetylase inhibitor, however, no regeneration was observed. This suggests that epigenetic regulators can play an important role in governing cellular reprogramming, a topic which will be discussed in the following section.
A multi-factor approach has been used to successfully stimulate regeneration of different systems throughout the body. For instance, viral transfection of Pdx1 and MafA transcription factors resulted in the reprogramming of mouse pancreatic α-cells into β-cells in vivo, postponing the onset of diabetes in mice [75]. Direct reprogramming using three defined factors (Gata4, Mef21 and Tbx5), which converts mouse fibroblast into functional cardiomyocyte-like cells, improved cardiac function and reduced fibrosis after myocardial infarction [76].
In recent years, several groups have found the multi-factor direct reprogramming approach effective in generating hair cells in vitro. First of all, the combination of Atoh1, Gfi1 and Pou4f3, all transcription factors critical for hair cell development, is more effective than Atoh1 alone in converting embryonic stem cells into hair cells [77]. In the embryonic and neonatal cochlea, a different combination (Gata3, Ets2, Etv4, NMyc, Tcf3) in concert with Atoh1 induces more ectopic hair cells than Atoh1 alone [78, 79]. Moreover, the ectopic co-expression of Eya1 and Six1 is able to induce supernumerary hair cells in the embryonic mouse cochlea in the absence of Atoh1 [80].
Similar approaches have been attempted using transgenic mouse models, with promising results showing that the co-activation of Gata3 or Pou4f3 with Atoh1 successfully stimulating the conversion of supporting cells into hair cell-like cells in the adult cochlea. The combination of Pou4f3 and Atoh1 activation resulted in approximately 21% of recombined cells expressing the hair cell marker MyosinVIIa, an order of magnitude greater than with Pou4f3 alone. Interestingly, deletion of p27Kip1, a cell cycle inhibitor expressed in cochlear supporting cells, was also able to promote the conversion of supporting cells to hair cells in response to Atoh1 activation. In this model 12% of recombined cells expressed hair cell markers MyosinVIa/VIIa. Upon further examination, deletion of p27Kip1 upregulates Gata3, which is postulated to be the mechanism enabling transdifferentiation of supporting cells to hair cells in response to Atoh1 in the mature mouse cochlea [81]. However, no supporting cell proliferation was observed, implicating a cell-cycle independent role of p27Kip1 mediated by Gata3. The interplays of these multiple transcription factors are only beginning to be explored and may help rekindle the lost plasticity of supporting cells in the mature cochlea and in doing so enable regeneration of the damaged organ.
6. Alternative approaches
A notable effector of hair cell regeneration is the developmental stage of the cochlea, where the plasticity of the supporting cells is reduced with maturation [59, 61, 63, 81, 82, 83, 84]. One candidate mechanism is changes in the epigenetic status of cochlear supporting cells as the organ matures, which is an area of active investigation. Epigenetic changes are known to play a key role in regulating cellular reprogramming and regeneration in a wide range of systems including during the generation of induced pluripotent stem cells [85], and regeneration of limbs [86], axons [87], and retinal neurons [74]. In the inner ear for example, the degree of methylation of Sox2 enhancers (NOP1 and NOP2) correlates with the dedifferentiation potential of post-mitotic supporting cells into otic stem cells [88]. Repressive complexes such as NuRD and PRC2 have also been reported in the neonatal cochlea, and the presence of these repressive complexes correlates with transcriptional silencing of known target genes of their cofactors such as Atoh1 [89]. Moreover, epigenetic modifications have been shown to regulate the effects of Atoh1 during development [90], and they are postulated to govern the efficacy of Atoh1 overexpression or other regenerative approaches in the postnatal and mature cochlea. In support of this concept, when combined with histone deacetylase inhibitors, Wnt activation dramatically increases Lgr5-positive supporting cells in cultured neonatal cochlea and the number of clonal colonies formed by cochlear supporting cells, although the effects on supporting cells from the mature cochlea are significantly reduced [91].
Other combinatorial manipulations have also been found to modulate hair cell regeneration. For instance, in the neonatal cochlea, Sox2 haploinsufficiency and damage enhances the effects of Wnt activation via stabilization of β-catenin on proliferation and hair cell formation from supporting cells, possibly via the downregulating of Notch signaling [84]. When Li and colleagues inhibited Notch signaling, they observed that active Wnt signaling induces more robust proliferation and hair cell formation in the neonatal cochlea [92]. Interestingly, in addition to its effects on hair cell formation, Atoh1 overexpression has been observed to induce proliferation in the neonatal cochlea [61]. When combined with active Wnt signaling and/or Notch inhibition, both cell division and ectopic hair cell formation increase in the neonatal cochlea [82, 83]. It is a common notion that supporting cell proliferation is important for cochlear regeneration as it can help restore the hair cell-supporting cell ratio thought to be important for function. While these studies indicate that both proliferation and hair cell formation can be modulated with different approaches in the neonatal, immature cochlea, their effectiveness in the mature cochlea is rather limited [81, 84]. In addition to epigenetic changes mentioned above, a decrease in Notch signaling has been proposed as one mechanism leading to the inability of the mature mammalian cochlea to regenerate [93]. Moreover, the severely damaged cochlea with a complete loss of hair cells appears as a scar-like, flat epithelium occupied by cuboidal cells, which may be rather different from native supporting cells [15, 68]. It would be of interest for future studies to compare the gene expression of the neonatal and mature cochlear supporting cells, particularly their changes in response to damage.
7. Delivery of therapeutic agents
Many studies have probed strategies that most effectively introduce viral vectors or pharmaceutical compounds into the cochlea. When determining the suitability of each approach, a number of elements should be considered, including the intended biological effect, the target cell population, the longevity of the expression needed, and any possible off-target effects. Two of the most commonly used viruses have been adeno-associated virus (AAV) and adenovirus, each of which has its own advantages and shortcomings. AAV in particular has been an appealing candidate vehicle for gene transfer because it has a long-lived transgene expression, is not linked with human diseases, and has a relatively wide expression pattern within the cochlea [94]. However, it has a relatively small genetic capacity, limiting the types of genes that can be delivered. In contrast, adenovirus has a larger packing capacity, allowing for a larger array of possible genetic insertions, but the transgene expression is transient.
Multiple studies examining Atoh1 and hair cell regeneration have used adenovirus as a means to target supporting cells, such as the pillar cells, Deiters’ cells, as well as Hensen cells [58, 59, 95]. Specifically, adenovirus serotype 5 has been shown to be efficacious in transducing supporting cells [96]. Adenovirus-mediated Atoh1 studies, however, only found small increases in hair cell number and limited to no recorded functional improvement. In the mature utricle, one study using adenovirus reported hair cell conversion and improved vestibular function by behavioral measures [97]. These studies show that adenovirus can transduce a wide range of supporting cells, highlighting its potential use in future therapies. For a list of information on viral and non-viral approaches, please see Table 1.
Table 1.
Viral vectors for inner ear gene therapy
| Viral vector | Transgene | Animal | Route | Tropism | Side Effects | Reference |
|---|---|---|---|---|---|---|
| Adenovirus | ||||||
| Ad5 | GFP | Mouse (adult) | RW | Cochlear and vestibular hair cells | Inflammatory response | [98] |
| Ad5 (E1A-, E1B-) | lacZ | Guinea pig (adult) | RW | Spiral ganglion neurons, cochlear supporting cells and connective tissue | Mild inflammatory response | [99] |
| Ad (E1-) | β-gal | Rat (neonatal) | In vitro | Organ of Corti, spiral ganglion neurons | No signs of cellular damage reported | [100] |
| Ad (E1-, E3-, CMV) | GFP | Rat (adult) | Peri- and endolymphatic perfusion | Mesothelial cells of scala media (i.e. Reissner’s membrane); Hensen’s, Deiters’, pillar and phalangeal cells; satellite cells surrounding SGN | Increased compound action potential (CAP) threshold | [101] |
| Coch | Cochlear inner and outer hair cells (in 2/6 animals) | Increased CAP threshold | ||||
| Ad (E1-, E3-, pol-) | GFP | Guinea pig (adult) | RW | Cochlear inner hair cell and pillar cells | Loss of DPOAE | [102] |
| Ad (E1-, E3-, pol-) | GFP-BDNF GFP-NT3 | Guinea pig (adult) | Coch | Inner and outer pillar cells, Deiters’ cells, Hansen’s cell, inner sulcus cells and interdental cells | Mild to moderate fibrosis and new bone growth | [103] |
| Ad | GFP-Kir2.1 | Mouse (neonate) | In vitro | Vestibular hair cells | No evidence of toxicity | [104] |
| AAV | ||||||
| AAV2/1 | GFP | Mouse (Embryonic) | Trans-uterine | Cochlear progenitor cells, which gave rise to inner and outer hair cells and supporting cells | No adverse effects found | [105] |
| AAV1 | GFP | Mouse (neonate) | Coch | Inner hair cells, cochlear supporting cells and lateral wall | Hearing loss reported when injected into scala media | [106] |
| AAV2 | dXIAP | Rat (adult) | RW | Spiral ganglion and stria vascularis | None reported | [107] |
| AAV1 | VGLUT3 | Mouse (juvenile) | RW or Coch | Inner hair cells | None reported | [108] |
| AAV8 | Whirlin | Mouse (neonatal) | RW | Inner hair cells and some outer hair cells | None reported | [109] |
| Mouse (adult) | RW | Inner hair cells (low efficacy) | ||||
| AAV8 | Whirlin | Mouse (neonatal) | PSSC | Vestibular hair cells and cochlear inner hair cells | None reported | [110] |
| AAV8 | Neurotrophin-3 | Guinea pig (adult) | Coch | Inner hair cells | None reported | [111] |
| Anc80L65 | GFP | Mouse (neonatal) | RW | Inner and outer hair cells, spiral ganglion neurons, cochlear supporting cells | No adverse effects on hair cell or hearing function | [112] |
| Human | In vitro | Vestibular hair cells and supporting cells | ||||
| AAV8 | Sans-IRES-GFP | Mouse (neonate) | RW | Vestibular and cochlear hair cells | None reported | [113] |
| AAV2/Anc80L65 | GFP | Mouse (adult) | PSSC | Cochlear hair cells, interdental cells, Reissner’s membrane, spiral limbus. Vestibular hair cells and supporting cells and vestibular ganglion cells | No adverse effects on hair cell or hearing function | [114] |
| Herpes Simplex virus | lacZ | Guinea pig (adult) | RW | Supporting cells, epithelial and connective tissues of cochlea. | Inflammatory response | [115] |
| NT-3myc | Mouse (neonatal) | In vitro | Spiral ganglion | None reported | [116] | |
RW-round window
PSSC-posterior semicircular canal
Coch-Cochleostomy
AAV - adeno-associated virus
8. Conclusion
The mature mammalian cochlea does not regenerate lost hair cells, resulting in a permanent hearing deficit. Recent studies have found that supporting cells in the neonatal cochlea spontaneously regenerate hair cells, and that multiple approaches including direct cellular reprogramming can modulate regeneration. However, inducing robust hair cell regeneration in the mature cochlea remains challenging. Recent advances in multi-factor direct cellular reprogramming approaches and viral and non-viral delivery methods into the inner ear should open the door for novel biological therapies for hearing loss.
9. Expert Opinion
The organ of Corti is a highly delicate structure containing a multitude of cell types that are precisely interwoven and innervated, enabling it to convey the smallest whisper to the cacophony of an orchestra with high fidelity. Damage causing loss of sensory hair cells results in permanent hearing loss, for which there is currently no biological treatment. Unlike non-mammalian sensory organs, which are able to repopulate hair cells, the mature mammalian cochlea has no spontaneous regeneration. Re-engineering the mature mammalian cochlea to regenerate hair cells as a means to restore hearing is therefore of immense interest and could provide remarkable benefit.
Studies of the neonatal cochlea and utricle have provided significant insights into how to tackle this problem. First, supporting cells isolated from the neonatal cochlea are able to proliferate and develop new hair cells, indicating their progenitor potential. A subset of supporting cells – particularly those marked by the Wnt responsive gene Lgr5 – had a greater propensity to proliferate and differentiate into hair cells, suggesting that Lgr5 may be an enrichment marker. Second, in vivo studies have shown that supporting cells in the neonatal cochlea acted as hair cell progenitors after damage and regenerated new hair cells. However, regeneration is limited both in degree and to the apical region of the cochlea. Moreover, fate mapping demonstrated that at least a subset of these regenerated hair cells were derived from Lgr5-positive supporting cells. These findings indicate that within their native environment, supporting cells are capable of proliferating and converting into hair cells after injury. Unfortunately, this capacity is rapidly lost after the first postnatal week for reasons that are not completely clear.
Numerous studies have attempted to coerce hair cell regeneration via the upregulation of Atoh1 since it can successfully force supporting cells to differentiate into hair cells in the neonatal cochlea. Another approach has been to activate Wnt signaling as a means to induce proliferation of quiescent supporting cells. These single factor approaches, however, have mostly been unsuccessful in inducing regeneration in the mature cochlea. These findings highlight two important points: firstly, while the neonatal cochlea provides a conducive environment for manipulations and can provide proof-of-principle results, it is necessary to validate the findings in the mature cochlea; and secondly, a single factor approach is unlikely able to promote the necessary cell proliferation and cell conversion in the mature cochlea. It remains to be tested whether a multifactor approach can induce supporting cell proliferation and hair cell regeneration. Also why cochlear supporting cells become rapidly unresponsive to manipulation after birth has not yet been fully elucidated. As such, the field would benefit from a greater understanding of the differential gene expression and epigenetic changes of supporting cells in the neonatal and mature cochlea.
During development, hair cells display dynamic changes of many genes that are beginning to be revealed, including those specifying hair cell subtypes and regulating subcellular structures critical for hair cell function. It is highly plausible that the milieu of the mature cochlea will require a novel set of genes to allow proper hair cell differentiation and integration, all unexplored and challenging questions needing answers. As new technologies evolve to better examine the damaged mammalian cochlea and novel techniques develop to deliver therapeutic agents, the possibility of hair cell regeneration as a biological therapy for hearing loss may be realized.
Article Highlights.
Limited spontaneous hair cell regeneration occurs in the neonatal mouse cochlea and in the neonatal and adult mouse utricle.
As in non-mammalian species, supporting cells in mammalian sensory organs can act as hair cell progenitors.
Cochlear supporting cells rapidly lose the ability to regenerate hair cells as the organ matures.
Direct cellular reprogramming is efficacious in inducing ectopic hair cells in the neonatal cochlea; however, results have been mixed in the mature organ.
The overall efficacy of a combination approach to induce hair cell regeneration in the mature cochlea remains low.
Acknowledgements:
We thank Z. Sayyid and T. Jan for critical reading and C. Gralapp for graphical illustration.
Funding
This work has been funded by Garnett Passe and Rodney Williams Memorial Foundation, Lucile Packard Foundation for Children’s Health Stanford, the NIH National Center for Translation Sciences – Clinical and Translational Sciences Awards program UL1TR001085 and NIH/NIDCD RO1DC013910, California Institute in Regenerative Medicine RN3-06529.
Footnotes
Declaration of Interests
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
References:
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Wilson BS, Tucci DL, Merson MH, et al. Global hearing health care: new findings and perspectives. Lancet. 2017. December 2;390(10111):2503–2515. doi: 10.1016/S0140-6736(17)31073-5. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 2.Goman AM, Reed NS, Lin FR. Addressing Estimated Hearing Loss in Adults in 2060. JAMA Otolaryngol Head Neck Surg. 2017. July 1;143(7):733–734. doi: 10.1001/jamaoto.2016.4642. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomblin JB, Harrison M, Ambrose SE, et al. Language Outcomes in Young Children with Mild to Severe Hearing Loss. Ear Hear. 2015. Nov-Dec;36 Suppl 1:76S–91S. doi: 10.1097/AUD.0000000000000219. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrison Bush AL, Lister JJ, Lin FR, et al. Peripheral Hearing and Cognition: Evidence From the Staying Keen in Later Life (SKILL) Study. Ear Hear. 2015. Jul-Aug;36(4):395–407. doi: 10.1097/AUD.0000000000000142. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin FR, Metter EJ, O’Brien RJ, et al. Hearing loss and incident dementia. Arch Neurol. 2011. February;68(2):214–20. doi: 10.1001/archneurol.2010.362. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGill TJ, Schuknecht HF. Human cochlear changes in noise induced hearing loss. Laryngoscope. 1976. September;86(9):1293–1302. doi: 10.1288/00005537-197609000-00001. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 7.Nadol JB, Jr., Shiao JY, Burgess BJ, et al. Histopathology of cochlear implants in humans. Ann Otol Rhinol Laryngol. 2001. September;110(9):883–91. doi: 10.1177/000348940111000914. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 8.Dowell RC, Dettman SJ, Blamey PJ, et al. Speech perception in children using cochlear implants: prediction of long-term outcomes. Cochlear implants international. 2002. March;3(1):1–18. doi: 10.1179/cim.2002.3.1.1. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 9.Wise AK, Hume CR, Flynn BO, et al. Effects of localized neurotrophin gene expression on spiral ganglion neuron resprouting in the deafened cochlea. Mol Ther. 2010. June;18(6):1111–22. doi: 10.1038/mt.2010.28. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan B, Askew C, Galvin A, et al. Gene therapy restores auditory and vestibular function in a mouse model of Usher syndrome type 1c. Nat Biotechnol. 2017. March;35(3):264–272. doi: 10.1038/nbt.3801. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]; **A recent study which demonstrated the utility of gene therapy targeting hair cells as a means to restore function.
- 11.Mellado Lagarde MM, Wan G, Zhang L, et al. Spontaneous regeneration of cochlear supporting cells after neonatal ablation ensures hearing in the adult mouse. Proc Natl Acad Sci U S A. 2014. November 25;111(47):16919–24. doi: 10.1073/pnas.1408064111. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu Q, Wang Y, Chang Q, et al. Virally expressed connexin26 restores gap junction function in the cochlea of conditional Gjb2 knockout mice. Gene Ther. 2014. January;21(1):71–80. doi: 10.1038/gt.2013.59. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goutman JD, Elgoyhen AB, Gomez-Casati ME. Cochlear hair cells: The sound-sensing machines. FEBS Lett. 2015. November 14;589(22):3354–61. doi: 10.1016/j.febslet.2015.08.030. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raphael Y, Kim YH, Osumi Y, et al. Non-sensory cells in the deafened organ of Corti: approaches for repair. Int J Dev Biol. 2007;51(6–7):649–54. doi: 10.1387/ijdb.072370yr. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 15.Taylor RR, Jagger DJ, Forge A. Defining the cellular environment in the organ of Corti following extensive hair cell loss: a basis for future sensory cell replacement in the Cochlea. PLoS One. 2012;7(1):e30577. doi: 10.1371/journal.pone.0030577. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schnee ME, Ricci A. Hair Cells and Their Synapses In: Manley GA, Gummer AW, Popper AN, et al. , editors. Understanding the Cochlea. Cham: Springer International Publishing; 2017. p. 183–213. [Google Scholar]
- 17.Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988. June 24;240(4860):1772–4. PubMed PMID: . [DOI] [PubMed] [Google Scholar]; **Part of a collection of papers to demonstrate hair cell regeneration after trauma in the avian cochlea
- 18.Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988. June 24;240(4860):1774–6. PubMed PMID: . [DOI] [PubMed] [Google Scholar]; **Part of a collection of papers to demonstrate hair cell regeneration after trauma in the avian cochlea
- 19.Cruz RM, Lambert PR, Rubel EW. Light microscopic evidence of hair cell regeneration after gentamicin toxicity in chick cochlea. Arch Otolaryngol Head Neck Surg. 1987. October;113(10):1058–62. PubMed PMID: . [DOI] [PubMed] [Google Scholar]; **First paper to show hair cell regeneration occurs in the chicken cochlea after insult.
- 20.Weisleder P, Rubel EW. Hair cell regeneration in the avian vestibular epithelium. Experimental neurology. 1992. January;115(1):2–6. PubMed PMID: ; [DOI] [PubMed] [Google Scholar]
- 21.Stone JS, Cotanche DA. Hair cell regeneration in the avian auditory epithelium. Int J Dev Biol. 2007;51(6–7):633–47. doi: 10.1387/ijdb.072408js. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 22.Cruz IA, Kappedal R, Mackenzie SM, et al. Robust regeneration of adult zebrafish lateral line hair cells reflects continued precursor pool maintenance. Dev Biol. 2015. June 15;402(2):229–38. doi: 10.1016/j.ydbio.2015.03.019. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinto-Teixeira F, Viader-Llargues O, Torres-Mejia E, et al. Inexhaustible hair-cell regeneration in young and aged zebrafish. Biol Open. 2015. May 22;4(7):903–9. doi: 10.1242/bio.012112. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romero-Carvajal A, Navajas Acedo J, Jiang L, et al. Regeneration of Sensory Hair Cells Requires Localized Interactions between the Notch and Wnt Pathways. Dev Cell. 2015. August 10;34(3):267–82. doi: 10.1016/j.devcel.2015.05.025. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]; *An interesting paper which demonstrated a balance between the self-renewal and differentiation is tightly controlled by Notch and Wnt-signaling in the zebrafish lateral line.
- 25.Sun H, Lin CH, Smith ME. Growth hormone promotes hair cell regeneration in the zebrafish (Danio rerio) inner ear following acoustic trauma. PLoS One. 2011;6(11):e28372. doi: 10.1371/journal.pone.0028372. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris JA, Cheng AG, Cunningham LL, et al. Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio). J Assoc Res Otolaryngol. 2003. June;4(2):219–34. doi: 10.1007/s10162-002-3022-x. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsui JI, Oesterle EC, Stone JS, et al. Characterization of damage and regeneration in cultured avian utricles. J Assoc Res Otolaryngol. 2000. August;1(1):46–63. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soucek S, Michaels L, Frohlich A. Evidence for hair cell degeneration as the primary lesion in hearing loss of the elderly. J Otolaryngol. 1986. June;15(3):175–83. PubMed PMID: . [PubMed] [Google Scholar]
- 29.Forge A, Li L, Nevill G. Hair cell recovery in the vestibular sensory epithelia of mature guinea pigs. J Comp Neurol. 1998. July 20;397(1):69–88. PubMed PMID: . [PubMed] [Google Scholar]; **An important paper, which discovered that the mature mammalian vestibular sensory epithelia was capable of hair cell regeneration, albeit limited, after damage.
- 30.Golub JS, Tong L, Ngyuen TB, et al. Hair cell replacement in adult mouse utricles after targeted ablation of hair cells with diphtheria toxin. J Neurosci. 2012. October 24;32(43):15093–105. doi: 10.1523/JNEUROSCI.1709-12.2012. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawamoto K, Izumikawa M, Beyer LA, et al. Spontaneous hair cell regeneration in the mouse utricle following gentamicin ototoxicity. Hear Res. 2009. January;247(1):17–26. doi: 10.1016/j.heares.2008.08.010. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin V, Golub JS, Nguyen TB, et al. Inhibition of Notch activity promotes nonmitotic regeneration of hair cells in the adult mouse utricles. J Neurosci. 2011. October 26;31(43):15329–39. doi: 10.1523/JNEUROSCI.2057-11.2011. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanyeri H, Lopez I, Honrubia V. Histological evidence for hair cell regeneration after ototoxic cell destruction with local application of gentamicin in the chinchilla crista ampullaris. Hear Res. 1995. September;89(1–2):194–202. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 34.Sinkkonen ST, Chai R, Jan TA, et al. Intrinsic regenerative potential of murine cochlear supporting cells. Sci Rep. 2011;1:26. doi: 10.1038/srep00026. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malgrange B, Belachew S, Thiry M, et al. Proliferative generation of mammalian auditory hair cells in culture. Mech Dev. 2002. March;112(1–2):79–88. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 36.Oshima K, Grimm CM, Corrales CE, et al. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J Assoc Res Otolaryngol. 2007. March;8(1):18–31. doi: 10.1007/s10162-006-0058-3. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oshima K, Senn P, Heller S. Isolation of sphere-forming stem cells from the mouse inner ear. Methods Mol Biol. 2009;493:141–62. doi: 10.1007/978-1-59745-523-7_9. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White PM, Doetzlhofer A, Lee YS, et al. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006. June 22;441(7096):984–7. doi: 10.1038/nature04849. PubMed PMID: . [DOI] [PubMed] [Google Scholar]; **A seminal study, which first showed that mammalian cochlear supporting are able to divide and transdifferentiaton into hair cells upon isolation, indicating that the cochlea environment likely plays a key role in prohibiting hair cell regeneration.
- 39.Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat Med. 2003. October;9(10):1293–9. doi: 10.1038/nm925. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 40.Chai R, Kuo B, Wang T, et al. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc Natl Acad Sci U S A. 2012. May 22;109(21):8167–72. doi: 10.1073/pnas.1202774109. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi F, Kempfle JS, Edge AS. Wnt-responsive Lgr5-expressing stem cells are hair cell progenitors in the cochlea. J Neurosci. 2012. July 11;32(28):9639–48. doi: 10.1523/JNEUROSCI.1064-12.2012. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]; *An interesting study, highlighting the differing capacities of supporting cell subtypes to act as hair cell progenitors when isolated.
- 42.Cox BC, Chai R, Lenoir A, et al. Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development. 2014. February;141(4):816–29. doi: 10.1242/dev.103036. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]; * A novel study, which was the first to show that the neonatal mammalian cochlea retained the ability to mitotically regenerate hair cells after damage.
- 43.Bramhall NF, Shi F, Arnold K, et al. Lgr5-positive supporting cells generate new hair cells in the postnatal cochlea. Stem Cell Reports. 2014. March 11;2(3):311–22. doi: 10.1016/j.stemcr.2014.01.008. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang T, Chai R, Kim GS, et al. Lgr5+ cells regenerate hair cells via proliferation and direct transdifferentiation in damaged neonatal mouse utricle. Nat Commun. 2015. April 7;6:6613. doi: 10.1038/ncomms7613. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burns JC, Cox BC, Thiede BR, et al. In vivo proliferative regeneration of balance hair cells in newborn mice. J Neurosci. 2012. May 9;32(19):6570–7. doi: 10.1523/JNEUROSCI.6274-11.2012. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bucks SA, Cox BC, Vlosich BA, et al. Supporting cells remove and replace sensory receptor hair cells in a balance organ of adult mice. Elife. 2017. March 6;6. doi: 10.7554/eLife.18128. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987. December 24;51(6):987–1000. PubMed PMID: . [DOI] [PubMed] [Google Scholar]; **A groundbreaking which for the first time demonstrated that somatic cell fate is not immutable but rather can be altered, in this case, with the expression of a single transcription factor.
- 48.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006. August 25;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. PubMed PMID: . [DOI] [PubMed] [Google Scholar]; **A paradigm shifting paper, which discovered that pluripotent stem cells could be generated from both embryonic and adult fibroblasts with a simple cocktail of transcription factors. This study has provided a powerful tool for the study of human disease and furthered translational research.
- 49.Cobaleda C, Jochum W, Busslinger M. Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors. Nature. 2007. September 27;449(7161):473–7. doi: 10.1038/nature06159. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 50.Guo Z, Zhang L, Wu Z, et al. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell. 2014. February 6;14(2):188–202. doi: 10.1016/j.stem.2013.12.001. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]; **A fascinating paper which found that the expression of a single neural transcription factor, NeuoD1, had the ability to directly reprogram reactive glial cells in the cortex into functional neurons in vivo. This may open the door to alternative approaches for the repair in the injured brain.
- 51.Qian L, Huang Y, Spencer CI, et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012. May 31;485(7400):593–8. doi: 10.1038/nature11044. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]; **A novel study using lineage tracing to show that non-myocytes in the mouse heart can be reprogrammed into cardiomyocyte-like cells in vivo by the local delivery of a cocktail of transcription factors. Importantly, this experimental treatment was shown to decrease infarct size and attenuation cardiac dysfunction.
- 52.Song G, Pacher M, Balakrishnan A, et al. Direct Reprogramming of Hepatic Myofibroblasts into Hepatocytes In Vivo Attenuates Liver Fibrosis. Cell Stem Cell. 2016. June 2;18(6):797–808. doi: 10.1016/j.stem.2016.01.010. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 53.Smith ZD, Sindhu C, Meissner A. Molecular features of cellular reprogramming and development. Nat Rev Mol Cell Biol. 2016. March;17(3):139–54. doi: 10.1038/nrm.2016.6. PubMed PMID: . [DOI] [PubMed] [Google Scholar]; *An interesting review covering the control and maintenance of cell identity using direct reprogramming with an emphasis on epigenetic and transcriptional regulation.
- 54.Srivastava D, DeWitt N. In Vivo Cellular Reprogramming: The Next Generation. Cell. 2016. September 8;166(6):1386–1396. doi: 10.1016/j.cell.2016.08.055. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]; *A very good review of the current state of play in cellular reprogramming covering both reprogramming to pluripotency and direct conversion across a number of different organ systems.
- 55.Takahashi K Cellular reprogramming--lowering gravity on Waddington’s epigenetic landscape. J Cell Sci. 2012. June 1;125(Pt 11):2553–60. doi: 10.1242/jcs.084822. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 56.Bermingham NA, Hassan BA, Price SD, et al. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999. June 11;284(5421):1837–41. PubMed PMID: ; [DOI] [PubMed] [Google Scholar]; **The first study to demonstrate using a knockout mouse to show the necessity of Atoh1, then known as Math1, for inner ear hair cell development.
- 57.Cafaro J, Lee GS, Stone JS. Atoh1 expression defines activated progenitors and differentiating hair cells during avian hair cell regeneration. Dev Dyn. 2007. January;236(1):156–70. doi: 10.1002/dvdy.21023. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 58.Izumikawa M, Minoda R, Kawamoto K, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005. March;11(3):271–6. doi: 10.1038/nm1193. PubMed PMID: . [DOI] [PubMed] [Google Scholar]; *An important study, which found that the transcription factor Atoh1 is able to induce hair cell regeneration in the mature mammalian cochlea after damage and that this regeneration lead to a functional improvement. Follow-up studies have also demonstrated hair cell regeneration however functional improvement has been more elusive.
- 59.Atkinson PJ, Wise AK, Flynn BO, et al. Hair cell regeneration after ATOH1 gene therapy in the cochlea of profoundly deaf adult guinea pigs. PLoS One. 2014;9(7):e102077. doi: 10.1371/journal.pone.0102077. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Y, Yu H, Zhang Y, et al. Cotransfection of Pax2 and Math1 promote in situ cochlear hair cell regeneration after neomycin insult. Sci Rep. 2013. October 21;3:2996. doi: 10.1038/srep02996. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kelly MC, Chang Q, Pan A, et al. Atoh1 directs the formation of sensory mosaics and induces cell proliferation in the postnatal mammalian cochlea in vivo. J Neurosci. 2012. May 9;32(19):6699–710. doi: 10.1523/JNEUROSCI.5420-11.2012. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kraft S, Hsu C, Brough DE, et al. Atoh1 induces auditory hair cell recovery in mice after ototoxic injury. Laryngoscope. 2013. April;123(4):992–9. doi: 10.1002/lary.22171. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Z, Dearman JA, Cox BC, et al. Age-dependent in vivo conversion of mouse cochlear pillar and Deiters’ cells to immature hair cells by Atoh1 ectopic expression. J Neurosci. 2012. May 9;32(19):6600–10. doi: 10.1523/JNEUROSCI.0818-12.2012. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pan S, Wan J, Liu S, et al. Lentivirus carrying the Atoh1 gene infects normal rat cochlea. Neural Regen Res. 2013. June 15;8(17):1551–9. doi: 10.3969/j.issn.1673-5374.2013.17.002. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Z, Fang J, Dearman J, et al. In vivo generation of immature inner hair cells in neonatal mouse cochleae by ectopic Atoh1 expression. PLoS One. 2014;9(2):e89377. doi: 10.1371/journal.pone.0089377. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000. June;3(6):580–6. doi: 10.1038/75753. PubMed PMID: [DOI] [PubMed] [Google Scholar]
- 67.Xie WR, Jen HI, Seymour ML, et al. An Atoh1-S193A Phospho-Mutant Allele Causes Hearing Deficits and Motor Impairment. J Neurosci. 2017. September 6;37(36):8583–8594. doi: 10.1523/JNEUROSCI.0295-17.2017. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Izumikawa M, Batts SA, Miyazawa T, et al. Response of the flat cochlear epithelium to forced expression of Atoh1. Hear Res. 2008. June;240(1–2):52–6. doi: 10.1016/j.heares.2008.02.007. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao Z, Kelly MC, Yu D, et al. Spatial and Age-Dependent Hair Cell Generation in the Postnatal Mammalian Utricle. Mol Neurobiol. 2016. April;53(3):1601–1612. doi: 10.1007/s12035-015-9119-0. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 70.Schlecker C, Praetorius M, Brough DE, et al. Selective atonal gene delivery improves balance function in a mouse model of vestibular disease. Gene Ther. 2011. September;18(9):884–90. doi: 10.1038/gt.2011.33. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ueki Y, Wilken MS, Cox KE, et al. Transgenic expression of the proneural transcription factor Ascl1 in Muller glia stimulates retinal regeneration in young mice. Proc Natl Acad Sci U S A. 2015. November 3;112(44):13717–22. doi: 10.1073/pnas.1510595112. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li W, Nakanishi M, Zumsteg A, et al. In vivo reprogramming of pancreatic acinar cells to three islet endocrine subtypes. Elife. 2014. January 1;3:e01846. doi: 10.7554/eLife.01846. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Niu W, Zang T, Zou Y, et al. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat Cell Biol. 2013. October;15(10):1164–75. doi: 10.1038/ncb2843. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]; *An excellent paper, describing a single transcription factor approach to cellular reprogramming. This study highlighted the plasticity of certain cell types in vivo and harnessing that to generate potential therapeutic outcomes.
- 74.Jorstad NL, Wilken MS, Grimes WN, et al. Stimulation of functional neuronal regeneration from Muller glia in adult mice. Nature. 2017. August 3;548(7665):103–107. doi: 10.1038/nature23283. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]; *An important paper, which found that histone modifications can play a key role in the ability of certain factors to drive cellular reprogramming.
- 75.Xiao X, Guo P, Shiota C, et al. Endogenous Reprogramming of Alpha Cells into Beta Cells, Induced by Viral Gene Therapy, Reverses Autoimmune Diabetes. Cell Stem Cell. 2018. January 4;22(1):78–90 e4. doi: 10.1016/j.stem.2017.11.020. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]; *A fascinating paper, highlighting the power of cellular reprogramming to induce cell type conversion and importantly functional improvement.
- 76.Miyamoto K, Akiyama M, Tamura F, et al. Direct In Vivo Reprogramming with Sendai Virus Vectors Improves Cardiac Function after Myocardial Infarction. Cell Stem Cell. 2018. January 4;22(1):91–103 e5. doi: 10.1016/j.stem.2017.11.010. PubMed PMID: . [DOI] [PubMed] [Google Scholar]; *A similarly interesting study that showed that viral-mediated gene delivery was a robust and effective way to deliver a combination of reprogramming factors and that these reprogrammed cells lead to functional improvement.
- 77.Costa A, Sanchez-Guardado L, Juniat S, et al. Generation of sensory hair cells by genetic programming with a combination of transcription factors. Development. 2015. June 1;142(11):1948–59. doi: 10.1242/dev.119149. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 78.Ikeda R, Pak K, Chavez E, et al. Transcription factors with conserved binding sites near ATOH1 on the POU4F3 gene enhance the induction of cochlear hair cells. Mol Neurobiol. 2015. April;51(2):672–84. doi: 10.1007/s12035-014-8801-y. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Masuda M, Pak K, Chavez E, et al. TFE2 and GATA3 enhance induction of POU4F3 and myosin VIIa positive cells in nonsensory cochlear epithelium by ATOH1. Dev Biol. 2012. December 1;372(1):68–80. doi: 10.1016/j.ydbio.2012.09.002. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ahmed M, Wong EY, Sun J, et al. Eya1-Six1 interaction is sufficient to induce hair cell fate in the cochlea by activating Atoh1 expression in cooperation with Sox2. Dev Cell. 2012. February 14;22(2):377–90. doi: 10.1016/j.devcel.2011.12.006. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Walters BJ, Coak E, Dearman J, et al. In Vivo Interplay between p27(Kip1), GATA3, ATOH1, and POU4F3 Converts Non-sensory Cells to Hair Cells in Adult Mice. Cell Rep. 2017. April 11;19(2):307–320. doi: 10.1016/j.celrep.2017.03.044. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]; *A recent and important study that discovered that a multifactor approach to induce hair cell regeneration in the mature mammalian cochlea. This has the potential to provide a new avenue for exploration in an array of different damage paradigms.
- 82.Kuo BR, Baldwin EM, Layman WS, et al. In Vivo Cochlear Hair Cell Generation and Survival by Coactivation of beta-Catenin and Atoh1. J Neurosci. 2015. July 29;35(30):10786–98. doi: 10.1523/JNEUROSCI.0967-15.2015. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ni W, Lin C, Guo L, et al. Extensive Supporting Cell Proliferation and Mitotic Hair Cell Generation by In Vivo Genetic Reprogramming in the Neonatal Mouse Cochlea. J Neurosci. 2016. August 17;36(33):8734–45. doi: 10.1523/JNEUROSCI.0060-16.2016. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Atkinson PJ, Dong Y, Gu S, et al. Sox2 haploinsufficiency primes regeneration and Wnt responsiveness in the mouse cochlea. J Clin Invest. 2018. April 2;128(4):1641–1656. doi: 10.1172/JCI97248. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Doi A, Park IH, Wen B, et al. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nat Genet. 2009. December;41(12):1350–3. doi: 10.1038/ng.471. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yakushiji N, Suzuki M, Satoh A, et al. Correlation between Shh expression and DNA methylation status of the limb-specific Shh enhancer region during limb regeneration in amphibians. Dev Biol. 2007. December 1;312(1):171–82. doi: 10.1016/j.ydbio.2007.09.022. PubMed PMID: [DOI] [PubMed] [Google Scholar]
- 87.Cho Y, Sloutsky R, Naegle KM, et al. Injury-induced HDAC5 nuclear export is essential for axon regeneration. Cell. 2013. November 7;155(4):894–908. doi: 10.1016/j.cell.2013.10.004. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]; **A fascinating paper that begins to delve into the mechanisms by which the reactivation of silent transcriptional programs can be reactivated after injury and the key role that histone modifications plays in this process.
- 88.Waldhaus J, Cimerman J, Gohlke H, et al. Stemness of the organ of Corti relates to the epigenetic status of Sox2 enhancers. PLoS One. 2012;7(5):e36066. doi: 10.1371/journal.pone.0036066. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Layman WS, Sauceda MA, Zuo J. Epigenetic alterations by NuRD and PRC2 in the neonatal mouse cochlea. Hear Res. 2013. October;304:167–78. doi: 10.1016/j.heares.2013.07.017. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stojanova ZP, Kwan T, Segil N. Epigenetic regulation of Atoh1 guides hair cell development in the mammalian cochlea. Development. 2015. October 15;142(20):3529–36. doi: 10.1242/dev.126763. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]; *An important study, which explored the epigenetic regulation and silencing of Atoh1 within the cochlea during the first postnatal week. This timeframe is notable, as it has been shown by others that after the first postnatal week no spontaneous hair cell regeneration occurs in the mouse cochlea.
- 91.McLean WJ, Yin X, Lu L, et al. Clonal Expansion of Lgr5-Positive Cells from Mammalian Cochlea and High-Purity Generation of Sensory Hair Cells. Cell Rep. 2017. February 21;18(8):1917–1929. doi: 10.1016/j.celrep.2017.01.066. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li W, Wu J, Yang J, et al. Notch inhibition induces mitotically generated hair cells in mammalian cochleae via activating the Wnt pathway. Proc Natl Acad Sci U S A. 2015. January 6;112(1):166–71. doi: 10.1073/pnas.1415901112. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maass JC, Gu R, Basch ML, et al. Changes in the regulation of the Notch signaling pathway are temporally correlated with regenerative failure in the mouse cochlea. Frontiers in cellular neuroscience. 2015;9:110. doi: 10.3389/fncel.2015.00110. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hildebrand MS, Newton SS, Gubbels SP, et al. Advances in molecular and cellular therapies for hearing loss. Mol Ther. 2008. February;16(2):224–36. doi: 10.1038/sj.mt.6300351. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 95.Kawamoto K, Ishimoto S, Minoda R, et al. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003. June 1;23(11):4395–400. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]; *This study importantly observed that mature non-sensory cells in the mammalian cochlea retain the competence to differentiate, at least partially, to hair cells when coerced using specific transcription factors, in this case Atoh1.
- 96.Sheffield AM, Gubbels SP, Hildebrand MS, et al. Viral vector tropism for supporting cells in the developing murine cochlea. Hear Res. 2011. July;277(1–2):28–36. doi: 10.1016/j.heares.2011.03.016. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Staecker H, Praetorius M, Baker K, et al. Vestibular hair cell regeneration and restoration of balance function induced by math1 gene transfer. Otol Neurotol. 2007. February;28(2):223–31. doi: 10.1097/MAO.0b013e31802b3225. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 98.Staecker H, Li D, O’Malley BW, Jr., et al. Gene expression in the mammalian cochlea: a study of multiple vector systems. Acta Otolaryngol. 2001. January;121(2):157–63. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 99.Raphael Y, Frisancho JC, Roessler BJ. Adenoviral-mediated gene transfer into guinea pig cochlear cells in vivo. Neurosci Lett. 1996. March 29;207(2):137–41. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 100.Dazert S, Battaglia A, Ryan AF. Transfection of neonatal rat cochlear cells in vitro with an adenovirus vector. Int J Dev Neurosci. 1997. July;15(4–5):595–600. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 101.Venail F, Wang J, Ruel J, et al. Coxsackie adenovirus receptor and alpha nu beta3/alpha nu beta5 integrins in adenovirus gene transfer of rat cochlea. Gene Ther. 2007. January;14(1):30–7. doi: 10.1038/sj.gt.3302826. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 102.Luebke AE, Steiger JD, Hodges BL, et al. A modified adenovirus can transfect cochlear hair cells in vivo without compromising cochlear function. Gene Ther. 2001. May;8(10):789–94. doi: 10.1038/sj.gt.3301445. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 103.Atkinson PJ, Wise AK, Flynn BO, et al. Neurotrophin gene therapy for sustained neural preservation after deafness. PLoS One. 2012;7(12):e52338. doi: 10.1371/journal.pone.0052338. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Holt JR, Johns DC, Wang S, et al. Functional expression of exogenous proteins in mammalian sensory hair cells infected with adenoviral vectors. J Neurophysiol. 1999. April;81(4):1881–8. doi: 10.1152/jn.1999.81.4.1881. PubMed PMID: [DOI] [PubMed] [Google Scholar]
- 105.Bedrosian JC, Gratton MA, Brigande JV, et al. In vivo delivery of recombinant viruses to the fetal murine cochlea: transduction characteristics and long-term effects on auditory function. Mol Ther. 2006. September;14(3):328–35. doi: 10.1016/j.ymthe.2006.04.003. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iizuka T, Kanzaki S, Mochizuki H, et al. Noninvasive in vivo delivery of transgene via adeno-associated virus into supporting cells of the neonatal mouse cochlea. Hum Gene Ther. 2008. April;19(4):384–90. doi: 10.1089/hum.2007.167. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 107.Cooper LB, Chan DK, Roediger FC, et al. AAV-mediated delivery of the caspase inhibitor XIAP protects against cisplatin ototoxicity. Otol Neurotol. 2006. June;27(4):484–90. doi: 10.1097/01.mao.0000202647.19355.6a. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 108.Akil O, Seal RP, Burke K, et al. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron. 2012. July 26;75(2):283–93. doi: 10.1016/j.neuron.2012.05.019. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]; *An interesting and thorough paper, which demonstrated the feasibility of gene replacement into cochlea hair cells as a means to restore function. Importantly, this study found functional improvement after this gene therapy approach targeting hair cells.
- 109.Chien WW, Isgrig K, Roy S, et al. Gene Therapy Restores Hair Cell Stereocilia Morphology in Inner Ears of Deaf Whirler Mice. Mol Ther. 2016. February;24(1):17–25. doi: 10.1038/mt.2015.150. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Isgrig K, Shteamer JW, Belyantseva IA, et al. Gene Therapy Restores Balance and Auditory Functions in a Mouse Model of Usher Syndrome. Mol Ther. 2017. March 1;25(3):780–791. doi: 10.1016/j.ymthe.2017.01.007. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen H, Xing Y, Xia L, et al. AAV-mediated NT-3 overexpression protects cochleae against noise-induced synaptopathy. Gene Ther. 2018. July;25(4):251–259. doi: 10.1038/s41434-018-0012-0. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Landegger LD, Pan B, Askew C, et al. A synthetic AAV vector enables safe and efficient gene transfer to the mammalian inner ear. Nat Biotechnol. 2017. March;35(3):280–284. doi: 10.1038/nbt.3781. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Emptoz A, Michel V, Lelli A, et al. Local gene therapy durably restores vestibular function in a mouse model of Usher syndrome type 1G. Proc Natl Acad Sci U S A. 2017. September 5;114(36):9695–9700. doi: 10.1073/pnas.1708894114. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Suzuki J, Hashimoto K, Xiao R, et al. Cochlear gene therapy with ancestral AAV in adult mice: complete transduction of inner hair cells without cochlear dysfunction. Sci Rep. 2017. April 3;7:45524. doi: 10.1038/srep45524. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Derby ML, Sena-Esteves M, Breakefield XO, et al. Gene transfer into the mammalian inner ear using HSV-1 and vaccinia virus vectors. Hear Res. 1999. August;134(1–2):1–8. PubMed PMID: [DOI] [PubMed] [Google Scholar]
- 116.Chen X, Frisina RD, Bowers WJ, et al. HSV amplicon-mediated neurotrophin-3 expression protects murine spiral ganglion neurons from cisplatin-induced damage. Mol Ther. 2001. June;3(6):958–63. doi: 10.1006/mthe.2001.0334. PubMed PMID: . [DOI] [PubMed] [Google Scholar]


