We read with interest the article by Seier and colleagues,1 reporting a positive effect of alternating stimulation settings on habituation in essential tremor (ET) patients with DBS in the ventral intermediate nucleus (VIM). In their randomized, placebo‐controlled trial, 16 patients were either assigned to an experimental treatment arm (N = 7) with alternating stimulation parameters on a weekly basis or a standard care arm (N = 9) with continuous stimulation. In contrast to a continuous stimulation, alternating stimulation settings sustained tremor control after 12 weeks. This is in line with the experiences in our center.2 However, the optimal alternation interval remains to be explored.
We conducted a similar, rater‐blinded, placebo‐controlled, block‐randomized trial including ET patients who underwent bilateral DBS implantation in the VIM at least 3 months before study entry. Stimulation parameters were optimized at baseline, and two equally effective stimulation settings were determined per subject (setting A, setting B). Settings differed by at least two of the following parameters: electrode configuration, amplitude, frequency, or pulse width. Patients in the experimental treatment arm received setting A and setting B, and the standard care group received setting A in duplicate. Patients were instructed to switch between the two programmed settings on a daily basis, but were neither allowed to change their ET medication nor DBS parameters. Tremor control was assessed by a blinded rater using the Tremor Rating Scale (TRS) at baseline before optimization (T1), at baseline after optimization (T2) in setting A, and after 10 weeks (T3) again in setting A. TRS scores at all time points were compared using the Friedman test and post‐hoc Wilcoxon signed‐rank test. The Wilcoxon rank‐sum test was used to compare TRS change scores from T2 to T3 between treatment groups.
Nineteen patients were enrolled. Two patients dropped (1 DBS reprogramming during study; 1 missing data), resulting in 17 patients analyzed (experimental treatment: N = 11; standard care: N = 6). The Friedman test was significant for differences between time points (P < 0.001). Mean TRS scores improved at T2 (T1, 24.71 [±11.82]; T2, 17.47 [±10.79]; P = 0.001), but worsened again at T3 (T3, 21.35 [±12.73]; P = 0.004). As illustrated in Figure 1, no difference was observed between T1 and T3 (P = 0.991). At T3, TRS change scores did not differ significantly between groups (experimental treatment: 4.0 [±5.97]; standard care: 3.67 [±4.55]; P = 0.980).
Figure 1.
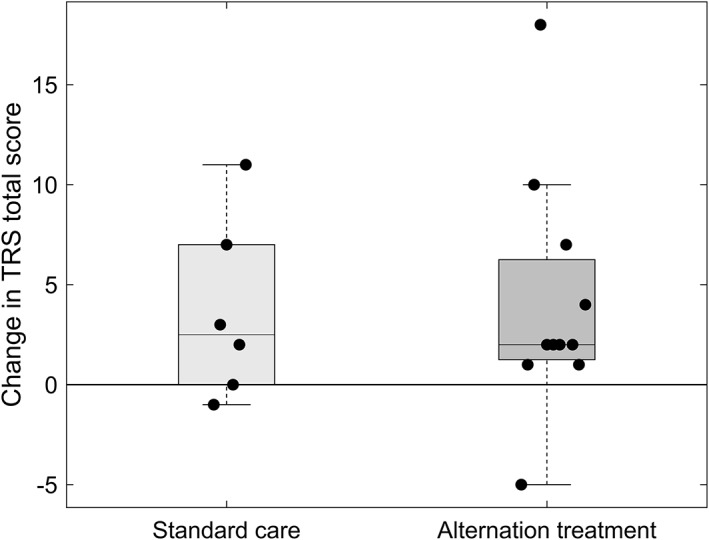
Change in TRS total score at follow‐up. After 10 weeks, mean TRS total score increased in both groups without significant difference in change scores (P = 0.98).
The loss of stimulation effect 10 weeks after DBS optimization has been described previously in ET.3 Although Seier and colleagues were able to reduce this so‐called habituation by switching parameters on a weekly basis,1 a daily parameter change was not able to prevent it in our cohort. However, a limitation of our study is the difference in group sizes, which was attributed to an early study termination after interim analysis, where no prevention of habituation was observed. Considering the evidence available so far, it seems that more‐prolonged stimulation cycles should be chosen to avoid, or at least reduce, habituation. Further research into the adaption of the pathological tremor network after DBS, the neuroanatomical background, and the clinical features associated with habituation is needed.4, 5
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
J.N.P.S.: 2A, 2B, 3A, 3B
P.R.: 1C, 2C, 3B
J.P.: 1A, 1B, 1C, 2C, 3B
V.V.V: 1C, 2C, 3B
T.A.D.: 2A,2C, 3B
M.T.B.: 1A, 2C, 3B
Disclosures
Ethical Compliance Statement: The study was approved by the ethics committee of the University of Cologne (vote 13‐082), conducted in accordance with the Declaration of Helsinki, and all patients gave written informed consent. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: J.P. was funded by the Koln Fortune program. The authors report no conflicts of interest.
Financial Disclosures for previous 12 months: Veerle Visser‐Vandewalle is a member of the advisory boards and reports consultancies for Medtronic, Boston Scientific, and Abbott and received a grant from SAPIENS Steering Brain Stimulation (now Medtronic Eindhoven Design Center). Till A. Dembek reports speaker honoraria from Boston Scientific. Michael T. Barbe reports speaker honoraria from Medtronic, Abbott, GE Medical, UCB, and Bial and research grants from Medtronic.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Seier M, Hiller A, Quinn J, Murchison C, Brodsky M, Anderson S. Alternating thalamic deep brain stimulation for essential tremor: a trial to reduce habituation. Mov Disord Clin Pract 2018;5:620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barbe MT, Pochmann J, Lewis CJ, et al. Utilization of predefined stimulation groups by essential tremor patients treated with VIM‐DBS. Parkinsonism Relat Disord 2014;20:1415–1418. [DOI] [PubMed] [Google Scholar]
- 3. Barbe MT, Liebhart L, Runge M, et al. Deep brain stimulation in the nucleus ventralis intermedius in patients with essential tremor: habituation of tremor suppression. J Neurol 2011;258:434–439. [DOI] [PubMed] [Google Scholar]
- 4. Anthofer JM, Steib K, Lange M, et al. Distance between active electrode contacts and dentatorubrothalamic tract in patients with habituation of stimulation effect of deep brain stimulation in essential tremor. J Neurol Surg A Cent Eur Neurosurg 2017;78:350–357. [DOI] [PubMed] [Google Scholar]
- 5. Merchant SH, Kuo SH, Qiping Y, et al. Objective predictors of ‘early tolerance’ to ventral intermediate nucleus of thalamus deep brain stimulation in essential tremor patients. Clin Neurophysiol 2018;129:1628–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]


