Abdominal movements are a rare presentation of epileptic seizures. Previously reported patients were known to have epilepsy, were already receiving antiepileptic drugs, presented with focal status epilepticus, and/or their seizure motor phenomenology also included the eyes, face, limbs, and even loss of awareness. Localization of the epileptogenic and symptomatogenic zones has therefore been controversial (Table 1).1, 2, 3, 4, 5, 6, 7, 8
Table 1.
Summary of cases of abdominal motor seizures reported in the English literature
| Reference | Age and Sex | Phenomenology Description | Seizure Type | Lesion and/or Seizure Focus Location |
|---|---|---|---|---|
| Nathanson 197811 |
Varied (4 cases) |
Coma with brainstem signs and bilateral clonic axial contractions (face, tongue, palate, pharynx, diaphragm, abdomen) | Focal motor with or without loss of awareness versus status epilepticus | Brainstem (“lowest‐level seizures”) |
| Matsuo 19841 |
19‐year‐old woman | Right truncal spasms spreading to right limb muscles; postictal right hemiparesis described | Focal motor | Contralateral parietal (abscess, meningioma, idiopathic) |
| 66‐year‐old woman | Left lower abdominal spasms with postictal left hemiparesis | |||
| 42‐year‐old man | Right abdominal contractions spreading to right shoulder, neck, face, and eyes | |||
| Rosenbaum 19902 |
65‐year‐old man | Left hemiparesis followed by clonic left‐sided seizures that localized to the left abdomen after antiepileptic therapy; recurrent seizures involved left‐sided abdominal, limbs, and facial muscles | Focal motor status epilepticus | Right frontoparietal metastasis |
| Chalk 199112 |
66‐year‐old man | Short episodes of brief, rhythmic (2–3 Hz) left abdominal contractions with occasional proximal left leg involvement and subclinical facial contractions | Focal motor status epilepticus | Right parasagittal |
| Fernandez‐Torre 20043 |
77‐year‐old woman | Dysphasia, left‐sided hemiparesis, hemianesthesia, hemiasomatognosia, and clonic twitching with right‐sided gaze deviation that localized to the left abdomen after antiepileptic therapy (video available) |
Focal motor status epilepticus (bilateral cortical myoclonus) | Right frontal metastasis (also right temporal, left mesial frontal, and right cerebellar metastases) |
| Dafotakis 20065 |
62‐year‐old man | Clonic twitching of left abdominal muscles (video available) |
Focal motor status epilepticus | Unknown (transient right parietal MRI abnormalities during status) |
| Tezer 20086 |
25‐year‐old woman | Treated epileptic patient with increased seizure frequency including right abdominal and facial myoclonic twitching at 1 to 2 Hz (video available) | Focal motor status epilepticus | Left mesial parieto‐occipital (cortical displasia) |
| Oster 20114 |
59‐year‐old man | Right abdominal spasms followed by additional right limb clonic contractions and occasional tonic‐clonic generalization | Focal motor onset with and without secondary generalization | Left mesial frontoparietal (ictal single‐photon emission computed tomography) |
| Ribeiro 20157 |
69‐year‐old man | Treated epileptic patient with dysphasia, right hemiparesis and continuous right‐sided abdominal myoclonic jerks (video available) |
Focal motor status epilepticus | Left occipital (in the context of prrvious left frontal temporo‐occipital hemorrhage) |
| 75‐year‐old man | Left hemiparesis, left‐sided gaze deviation, and continuous left‐sided myoclonic movements that localized to left abdomen after antiepileptic treatment (video available) |
Right occipital (in the context of prior right occipital infarction) | ||
| Aljaafari 20188 |
26‐year‐old man | Treated epileptic patient with right neck and shoulder pulling, with or without arm contractions and right head deviation, followed by right abdominal clonic movements and occasional tonic‐clonic generalization (video available) |
Focal motor onset with and without secondary generalization | Left mesial parietal |
Here, we present a treatment‐naïve patient with focal seizures secondary to a mesial parietal hemorrhage whose epileptic motor phenomenology exclusively involved abdominal muscles. In this patient, the epileptogenic zone most likely corresponded to the mesial parietal region and the symptomatogenic zone to the primary sensorimotor representation of the trunk. Based on video‐EEG and MRI findings, we propose a seizure propagation pattern that could be applied to previously reported cases.
Case Report
A 77‐year‐old right‐handed man with a history of diabetes and hypertension presented with 3 days of intermittent, short‐lasting episodes of involuntary, painless abdominal movements. There was no loss of awareness, and he denied visual, sensory, visceral, or autonomic symptoms. Neurological examination revealed episodes of semicontinuous, right‐greater‐than‐left, clonic‐like abdominal contractions lasting for 10 to 20 seconds (Video S1, segment 1). These episodes did not vary with distraction, breath‐holding, speaking, tactile stimuli, or positional changes. They increased in frequency during drowsiness and were also present during sleep. Neurological examination between episodes was unremarkable. Brain MRI studies during one of these episodes showed an acute subcortical hemorrhage in the left mesial parietal region, with adjacent cortical cytotoxic edema extending cranially through the medial aspect of the left parietal lobe to reach the postcentral and central sulci near the vertex (Fig. 1). Further workup was consistent with a venous angioma.
Figure 1.
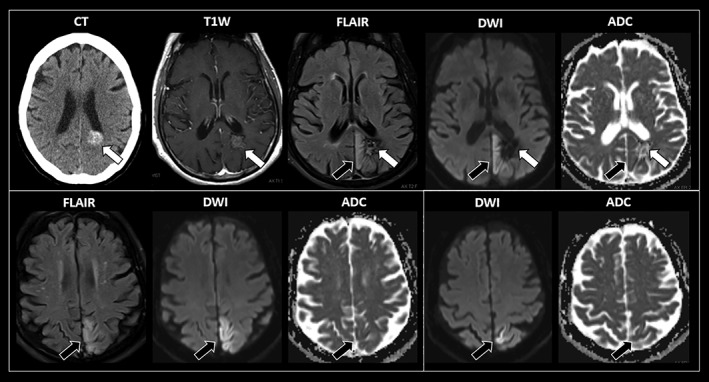
Sequential axial imaging studies of this patient with recurrent abdominal motor seizures. Images inside a white square correspond to the same axial cut. An acute subcortical hemorrhage is evidenced in the left mesial parietal region (white arrows). There is adjacent cortical cytotoxic edema extending cranially through the mesial aspect of the left parietal lobe to reach the postcentral and central sulci near the vertex. This region corresponds to the somatotopic localization of the sensorimotor areas corresponding to the trunk (black arrows). MRI sequences: T1W, T1‐weighted MR with intravenous contrast; FLAIR, fluid‐attenuated inversion recovery; DWI, diffusion‐weighted imaging; ADC, apparent diffusion coefficient.
Continuous video‐EEG monitoring demonstrated interictal epileptiform activity in the mesial parietal and left parieto‐occipital regions (electrodes Pz/P3/O1), propagating to mesial central and left fronto‐central regions (electrodes Cz/C3/Fz/F3), particularly during drowsiness. During the abdominal dyskinesia, this interictal epileptiform activity concurrently evolved into an ictal pattern consisting of 3.5‐ to 4‐Hz, rhythmic, and sharply contoured activity in the mesial parietal region and left posterior quadrant (electrodes Pz/P3/T5/O1), propagating anteriorly to mesial central and left frontocentral regions (electrodes Cz/C3/F3; Video S1, segment 2). Treatment with levetiracetam 500 mg twice‐daily, followed by additional phenytoin 100 mg three times daily, was necessary for complete remission of the abdominal motor seizures over 3 days.
Discussion
Abnormal abdominal movements have been illustratively described by the term “belly dancer's dyskinesia” (BDD), which originally referred to semicontinuous, slow, contorting movements displacing the umbilicus in a semicircular fashion after abdominal injury.9 Other peripherally induced dyskinesias present with unusual phenomenology similar to BDD, and several descriptive terms are used (“painful legs moving toes”; “jumpy stumps”). We have recently described peripherally induced dyskinesias involving the muscles of the back similar to BDD for which we used the term “dancing.”10 This term might be confusing, but it could be useful to describe the unique phenomenology of peripherally induced dyskinesias.9, 10
Several etiologies for BDD have been reported, mostly localizing to the corresponding thoracoabdominal spinal cord segments and/or peripheral nerve innervation. Nevertheless, belly dancers perform a broad variety of movements, including semirotatory, clonic‐like, and asymmetric movements. Thus, BDD has been inappropriately extended to describe irregular, jerky, and asymmetric movements that could be epileptic in origin (“epileptic belly dancing”). Even though EEG and brain imaging are normal in most cases, seizures have been consistently included in the differential diagnosis of BDD.
In this patient, the episodic, short‐lasting, clonic‐like, asymmetric dyskinesias suggested an epileptic etiology. Left‐sided movements might have been transmitted from right‐sided clonic seizures, and concurrent electromyographic recordings might have helped clarify this. The clinical, video‐EEG, and MRI findings in this case suggest that (1) the epileptogenic zone most likely corresponded to the cortical region medially overlying an acute subcortical hemorrhage in the left mesial parietal region, and that (2) the symptomatogenic zone extended cranially to reach the somatotopic primary sensorimotor representation of the trunk in the postcentral and central gyri near the vertex. Although most reported cases presented with focal status epilepticus, neither clinical nor EEG criteria were met in this case.
In patients with abdominal motor seizures, epileptic activity arising in mesial brain regions might therefore spread through the “noneloquent” mesial cortex to eventually reach the primary sensorimotor region corresponding to the trunk, without involving other primary sensorimotor areas. This propagation pattern could be applied to previously reported cases of abdominal motor seizures originating in parasagittal, mesial frontal, parietal, and occipital regions (Table 1).1, 2, 3, 4, 5, 6, 7, 8 In cases of abdominal motor seizures, scalp EEG recordings are not as helpful to accurately localize the epilepto‐ and symptomatogenic zones given their deep foci. Yet, source localization techniques utilized in a recently reported case revealed a similar propagation pattern as the one we propose.8 Multimodal assessments, including novel electrophysiological and molecular imaging techniques, could shed further light on these epileptic circuits.4, 8
Author Roles
Research Project: A. Conception, B. Organization, C. Execution; (2) Data Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
K.J.L.: 1A, 1B, 1C, 2A, 2B, 3A
E.A.S.: 1A, 2A, 2C, 3B
L.T.: 1A, 2A, 2C, 3B
A.E.L.: 1A, 2A, 2C, 3B
A.M.K.: 1A, 1B, 1C, 2A, 2B, 3B
Disclosures
Ethical Compliance Statement: We confirm that the approval of an institutional review board was not required for this work and that the appropriate written informed consent was obtained from the patient. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: The authors report no sources of funding and no conflicts of interest.
Financial Disclosures for previous 12 months: K.J.L. has received a fellowship grant from the Dystonia Medical Research Foundation Canada. A.E.L. has received consulting fees from AbbVie, Acorda, Biogen, Janssen, Jazz Pharma, Sun Pharma, Kallyope, Merck, Paladin, Theravance, and Corticobasal Degeneration Solutions; honoraria from Sun Pharma, Medichem, Medtronic, AbbVie, and Sunovion; grants from Brain Canada, Canadian Institutes of Health Research, Corticobasal Degeneration Solutions, Edmond J. Safra Philanthropic Foundation, Michael J. Fox Foundation, the Ontario Brain Institute, National Parkinson Foundation, Parkinson Society Canada, and W. Garfield Weston Foundation. A.M.K. has received consulting fees from Neuropace and publishing royalties from Lippincott Williams and Wilkins, Elsevier, and Wiley Blackwell.
Supporting information
Video S1. Abdominal motor seizures during continuous video‐EEG monitoring. Segment 1. Example of an abdominal motor seizure consisting of right‐greater‐than‐left clonic abdominal contractions lasting for approximately 15 seconds, without additional motor phenomenology or loss of awareness. Segment 2. Continuous video‐EEG monitoring demonstrating interictal epileptiform activity in the mesial parietal and left parieto‐occipital regions (electrodes Pz, P3, and O1) that propagates to mesial central and left frontocentral regions (electrodes Cz, C3, Fz, and F3). During another episode of abnormal abdominal movements, this interictal epileptiform activity evolves into an ictal pattern consisting of 3.5‐ to 4‐Hz rhythmic and sharply contoured activity in the mesial parietal and left posterior quadrant regions (electrodes Pz, P3, T5, and O1), propagating anteriorly to mesial central and left frontocentral regions (electrodes Cz, C3, and F3). This electrographic seizure lasted for approximately 30 seconds and gradually returned to the above‐described interictal activity. EEG activity was recorded with scalp electrodes positioned according to the 10‐20 international system. Depicted signals from average referential montage were recorded at a sample rate of 500 Hz with the high‐frequency filter at 70 Hz and the low‐frequency filter at 1 Hz. Concurrent electromyography was not obtained.
Acknowledgments
The authors thank the patient and his family.
Dr. Kanner and Dr. Lang contributed equally as senior authors of the manuscript.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Matsuo F. Partial epileptic seizures beginning in the truncal muscles. Acta Neurol Scand 1984;69:264–269. [DOI] [PubMed] [Google Scholar]
- 2. Rosenbaum DH, Rowan AJ. Unilateral truncal seizures: frontal origin. Epilepsia 1990;31:37–40. [DOI] [PubMed] [Google Scholar]
- 3. Fernandez‐Torre JL, Calleja J, Pascual J, Galdos P, De Pablos C, Berciano J. Epilepsia partialis continua of the abdominal muscles: a detailed electrophysiological study of a case. Mov Disord 2004;19:1375–1378. [DOI] [PubMed] [Google Scholar]
- 4. Oster JM, Aljumairi F, Cosgrove GR. Metabolic imaging correlate of truncal onset seizures. Arch Neurol 2011;68:251–253. [DOI] [PubMed] [Google Scholar]
- 5. Dafotakis M, Sparing R, Becker S, Fink GR. Epilepsia partialis continua of the abdominal muscles with transient MRI abnormalities. Neurology 2006;66:1099. [DOI] [PubMed] [Google Scholar]
- 6. Tezer FI, Celebi O, Ozgen B, Saygi S. A patient with two episodes of epilepsia partialis continua of the abdominal muscles caused by cortical dysplasia. Epileptic Disord 2008;10:306–311. [DOI] [PubMed] [Google Scholar]
- 7. Ribeiro JJ, Sousa M, Teotonio R, Bento C, Sales F. Epilepsia partialis continua of the abdominal muscles due to cerebrovascular disease. Epileptic Disord 2015;17:72–76. [DOI] [PubMed] [Google Scholar]
- 8. Aljaafari D, Nascimento FA, Abraham A, Andrade DM, Wennberg RA. Unilateral abdominal clonic seizures of parietal lobe origin: EEG findings. Epileptic Disord 2018;20:158–163. [DOI] [PubMed] [Google Scholar]
- 9. Iliceto G, Thompson PD, Day BL, Rothwell JC, Lees AJ, Marsden CD. Diaphragmatic flutter, the moving umbilicus syndrome, and “belly dancer's” dyskinesia. Mov Disord 1990;5:15–22. [DOI] [PubMed] [Google Scholar]
- 10. Lizarraga KJ, Thompson PD, Moore HP, Mizraji G, Gershanik OS, Singer C, Lang AE. Dancing dorsal quadrilaterals: a novel peripherally‐induced movement disorder. JAMA Neurol 2019;76:351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nathanson M, Krumholz A, Biddle D. Seizures of axial structures. Presumptive evidence for brain stem origin. Arch Neurol 1978;35:448–452. [DOI] [PubMed] [Google Scholar]
- 12. Chalk CH, McManis PG, Cascino GD. Cryptococcal meningitis manifesting as epilepsia partialis continua of the abdomen. Mayo Clin Proc 1991;66:926–929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Abdominal motor seizures during continuous video‐EEG monitoring. Segment 1. Example of an abdominal motor seizure consisting of right‐greater‐than‐left clonic abdominal contractions lasting for approximately 15 seconds, without additional motor phenomenology or loss of awareness. Segment 2. Continuous video‐EEG monitoring demonstrating interictal epileptiform activity in the mesial parietal and left parieto‐occipital regions (electrodes Pz, P3, and O1) that propagates to mesial central and left frontocentral regions (electrodes Cz, C3, Fz, and F3). During another episode of abnormal abdominal movements, this interictal epileptiform activity evolves into an ictal pattern consisting of 3.5‐ to 4‐Hz rhythmic and sharply contoured activity in the mesial parietal and left posterior quadrant regions (electrodes Pz, P3, T5, and O1), propagating anteriorly to mesial central and left frontocentral regions (electrodes Cz, C3, and F3). This electrographic seizure lasted for approximately 30 seconds and gradually returned to the above‐described interictal activity. EEG activity was recorded with scalp electrodes positioned according to the 10‐20 international system. Depicted signals from average referential montage were recorded at a sample rate of 500 Hz with the high‐frequency filter at 70 Hz and the low‐frequency filter at 1 Hz. Concurrent electromyography was not obtained.


