Abstract
Objective
Hypoxia-inducible factor is a hypoxia-responsive transcriptional factor that controls the expression of proteins contributing to homeostatic responses to hypoxia. Spatial heterogeneity of tissue oxygenation has been postulated as a determinant of structure and function of hepatic lobules, although its molecular mechanisms remain unknown. This study aimed to examine the role of HIF-1 expressed in hepatocytes in regulation of hepatic microcirculation.
Methods
We have generated mice harboring a floxed HIF-1α allele, and employed the albumin-Cre transgenic line to inactivate the gene site-specifically in hepatocytes.
Results
Intravital observation of the hepatic microcirculation revealed extension of hepatic lobules in HIF-1α-deficient mice. Measurement of microvascular diameter, velocity, and local oxygen tension by laser-assisted phosphorimetry showed that the oxygen consumption in the lobules of HIF-1α-deficient mice was greater than that in those of control mice. Isolated hepatocytes from HIF-1α-deficient mice also stimulated oxygen consumptions with increased contents of mtDNA. Overexpression of HIF-1α decreased the expression of PGC-1α mRNA, whereas the knockdown of the HIF-1α gene increased it, suggesting that HIF-1 regulates cellular respiration through mitochondrial biogenesis.
Conclusions
Our results suggest that constitutive expression of HIF-1α in hepatocytes acts as a determinant of hepatic lobular structure and oxygen consumption by changing mitochondrial contents.
Keywords: HIF-1α, liver, oxygen consumption, mitochondrial biogenesis
Introduction
Hypoxia-inducible factor is a hypoxia-responsive transcriptional factor that transactivates genes encoding proteins contributing to homeostatic responses to hypoxia [21,22]. Under normoxic conditions the protein HIF-1α is degraded in an oxygen-dependent manner mediated by prolyl hydroxylases targeting it for ubiquitination [10], and HIF-1α is known as a master switch in hypoxic emergency. During inflammation HIF-1α transcription is activated, even in normoxic conditions, by cytokines, nitric oxide, and reactive oxygen species [4].
The hepatic blood supply is supported by both arterial and venous systems; approximately 30% of blood flow to the liver is well-oxygenated blood provided by the hepatic artery, and the other 70% is poorly oxygenated blood derived from the portal vein. The normal range of pO2 in the hepatic artery is 90–100 mmHg, whereas that in the portal vein pO2 is 45–55 mmHg [8]. Consequently, the tissue oxygenation in liver is lower than that in other tissues.
Thin sinusoidal endothelial cell cytoplasm, open fenestrae, and lack of an organized basement membrane of the liver microcirculation reduce the distance from the sinusoids to the hepatocytes and thereby optimize oxygen delivery. Delivery of oxygen, hormones, and nutrients via sinusoidal blood flow and their consumption and metabolism in the liver create spatial gradients of these biological components between the periportal and pericentral regions of the hepatic lobule. Tissue responses to these gradients are likely to contribute to development in differences of metabolic characterization between hepatocytes in periportal regions and those in pericentral regions. Among these differences include relative enrichment of ureagenesis, gluconeogenesis, and oxidative metabolism in periportal hepatocytes and that of xenobiotic detoxification and reductive biosynthesis in pericentral hepatocytes [11]. The oxygen gradient seems a crucial factor determining such a heterogeneity in metabolism in the hepatic lobules, although the relationship between HIF-1 and metabolic features of this kind remains unknown.
In aerobic respiration, cytochrome c oxidase in mitochondria uses approximately 95% of molecular oxygen consumed in the body to generate water through tetravalent reduction. The oxygen gradient in the microcirculation occurs as a result of oxygen consumption that appears to depend on mitochondrial distribution in parenchyma surrounding the microvascular units. Recent observations have discussed the relationship between HIF-1 and mitochondrial biogenesis [17,18,30], but most studies among them inquired roles of inducible HIF-1 under hypoxic conditions. In other words, the role of HIF-1 expressed in parenchymal cells in determining structure and function of microvascular units has not fully been investigated under aerobic physiological conditions.
This study aims to investigate the role of HIF-1α expressed in the liver parenchyma, that is, hepatocytes, in structure and function of hepatic microvascular systems under normoxic physiological conditions. To this end, we evaluated differences in oxygen consumption and regional hemodynamics in the hepatic microcirculation between wild-type and hepatocyte-specific conditional HIF-1α knockout mice. We have herein determined the microcirculatory blood flow by using fluorescence-labeled erythrocytes, and measured the pO2 in the hepatic lobules by microscopic phosphorescence photometry. We also investigated cellular respiration by HIF-1 through mitochondrial biogenesis. To do that, we used in vitro HIF-1α overexpression and knockdown experiments to examine the relation between mitochondrial biogenesis and PGC-1, a transcriptional coactivator that regulates genes involved in energy metabolism.
Materials and Methods
Animal Preparation
All the experimental protocols we used were approved by the Animal Care Committee of Keio University School of Medicine. Using the Cre-loxP recombination system, we crossed mice harboring a floxed HIF-1α allele with the albumin-Cre transgenic line to inactivate the gene in hepatocytes [16,20,25]. Eight- to 12-week-old male mice (20–25 g) lacking HIF-1α in hepatocytes (HIF−/−)and littermate controls were fasted overnight before experiments.
Southern Blot Analysis
Hepatocyte-specific deletion of the HIF-1α gene was confirmed by Southern blot analysis. In brief, genomic DNA isolated from mouse organs and hepatocytes was digested with EcoRI and PstI, and was separated on 1% agarose. After the DNA was transferred onto a Hybond-N+ membrane, the membrane was hybridized with a 32P-labeled DNA probe containing an upstream region (0.7 kb) of HIF-1α exon 2 and then autoradiographed on X-ray film.
Measurement of RBC Velocity and Sinusoid Length
Intravital video microscopy was carried out according to our previous methods with specific modifications described below [15,28]. Under light anesthesia (sodium pentobarbital; 40 mg/kg, i.p.), a femoral vein was cannulated for drug injection. After midline and pericostal incisions exposed the left lobe of the liver, the mouse was given 0.7% of isoflurane through a respiration mask while the left lobe was set on a cover glass mounted in the plastic plate on an inverted-type fluorescence microscope. This anesthesia regimen assured the stability of systemic blood pressure during four-hour observation of the exteriorized portion of the liver surface. Small slips of plastic film were tiled around the edge of the hepatic lobe to minimize moving with respiration and to keep it from drying.
The RBC flow in hepatic microvessels was visualized by perfusing RBCs labeled with FITC isomer I (Sigma-Aldrich, St. Louis, MO, USA) [9]. Blood was withdrawn from a donor wild-type mouse, and the RBCs were centrifuged and washed. The FITC bound to the erythrocyte membrane was excited by irradiating it, at an energy density of 5 mW/cm2, with mercury lamp light that had passed through a band-pass filter (450–490 nm). Each mouse received 20 μL of FITC-labeled RBC suspension via a cannulated femoral vein, and high-magnification (×40 lens and ×2 digital zoom) video images were recorded—after waiting 15 minutes to ensure that the hepatic blood flow was stable—by using a cooled-CCD camera (C6653; Hamamatsu Photonics, Hama-matsu, Japan) mounted on the inverted-type microscope. Erythrocyte velocities in central venules and sinusoids were calculated off-line from the frame rate and the distance FITC-labeled erythrocytes moved between one frame and the next. Sinusoid length from a portal venule to a central venule was measured by using ImageJ software to trace the sinusoids (n = 251 in HIF+/+ mice and n = 239 in HIF−/− mice) in the fluorescence images.
Oxygen Consumption Measurement in Hepatic Lobule
Oxygen consumption in hepatic lobules was estimated from the measured RBC velocity and oxygen tension in central venules as shown in the formula
| (1) |
where rCV and vCV are, respectively, the radius and the RBC velocity in a central venule, pO2 (PV) and pO2 (CV) are, respectively, the oxygen tension in a portal venule and a central venule. L+/+ and L−/− indicate the average sinusoid lengths of control and HIF−/− mice, and the triplicate ratio of them normalizes the difference of lobular size. The oxygen tension in each central venule was measured by evaluating the oxygen-dependent quenching of phosphorescence as described in a previous paper [27]. Briefly, 30 mg/kg of Pd-TCPP (Frontier Scientific, Logan, UT, USA) was given by slow intravenous injection, and the targeted single hepatic venule was irradiated with the second harmonic of a Q-switched Nd:YAG pulse laser (532-nm wavelength, six nano second pulse width at half maximum, 1-Hz pulse recurrence frequency, 200-nJ/pulse irradiation energy) through the objective lens of a microscope. The phosphorescence lifetime was obtained by least-squares fitting of the remaining data to a single exponential curve. The oxygen tension (pO2) was calculated from the Stern-Volmer equation
| (2) |
where I0 and τ0 are the phosphorescence intensity and lifetime in the absence of oxygen, I and τ are the intensity and lifetime at a given oxygen tension, and kq is the rate constant of oxygen quenching. At 37°C and a pH of 7.4 the kq and τ0 values for our system were 374/Torr/sec and 0.74 ms.
Hepatocyte Isolation
Hepatocytes were isolated from control and HIF−/− mice by digesting the liver with type IV collagenase as described previously [6]. Briefly, all perfusions were carried out at flow rates of 4 mL/min and all buffers were maintained at 37°C. The first buffer consisted of Hank’s-HEPES solution containing 1 mM EGTA, and the second buffer consisted of Hank’s-HEPES containing collagenase I (0.5 mg/mL; Wako Pure Chemical Industries, Osaka, Japan). Hepatocytes were purified by washing twice with ice-cold Hank’s-HEPES buffer solution followed by Percoll gradient separation. Hepatocytes were suspended in William’s medium E containing 10% fetal bovine serum, 0.5 nM insulin, 100 nM dexamethasone, and EGF, 20 ng/mL (complete culture medium). The cell number and viability were determined using a hemocytometer and the trypan blue exclusion technique.
Mitochondria Isolation and mtDNA Extraction
The removal of the mouse livers was followed by perfusion of Hank’s-HEPES buffer at a rate of 4 mL/min. Weighed liver tissue was homogenized in ice-cold mitochondria isolation buffer containing 650 mM manitol, 6 mM succinate, 10 mM K2HPO4, 10 mM KCl, 0.1 mM EDTA, and 0.1% (w/v) BSA supplemented with the protease inhibitors leupeptin (10 μM) and PMSF (0.1 mM). Unbroken cells and nuclei were pelleted two times by centrifugation at 600 g for five minutes at 4°C. The supernatants were further centrifuged at 10,000 g for 10 minutes at 4°C to pellet the mitochondria fraction. The mitochondria pellets were separated by a density gradient that consisted of 16% (v/v) Percoll (GE Healthcare Life Sciences, UK), 10 mM HEPES, and 0.5 M sucrose. The suspension/gradient was centrifuged at 60,000 g for one hour. The mitochondrial fractions were washed twice and resuspended in isolation buffer and then stored before use for respiration measurement. For measurement of mtDNA, liver tissue was homogenized and mtDNA was extracted using mtDNA Extractor (R) CT Kit (Wako Pure Chemical Industries, Osaka, Japan). The concentration of mtDNA was measured using a spectrophotometer.
Measurement of the Oxygen Consumption of Isolated Hepatocytes and Mitochondria
Isolated hepatocytes (5 × 104) were suspended in William’s medium E containing 10% fetal bovine serum, 0.5 nM insulin, 100 nM dexamethasone, and EGF, 20 ng/mL in a 20-mL gas-tight vial to prevent the entry of atmospheric oxygen. In a similar manner, isolated mitochondria were suspended in respiration buffer (isolation buffer, previously described, added 200 μM ADP) at a concentration of 0.25 mg protein/mL The vial was placed in a circulating water bath maintained at 37°C with continuous mixing by a magnetic stirrer. The oxygen tension of the suspension was measured with a needle-type optical oxygen sensor (Oxy-Mini; WPI, Sarasota, FL, USA). The signal was digitized with an AD converter and stored in a PC. Oxygen consumption was calculated from the rate at which the oxygen tension in the sealed vial fell.
Transfection Assays for Overexpression and Knockdown of HIF-1α
HEK293 cells were cultured in low-glucose DMEM supplemented with 10% (v/v) fetal bovine serum (Biochrom, Berlin, Germany), 100 units/mL penicillin, and 50 μg/mL streptomycin, and were kept at 37°C in a humidified atmosphere containing 5% CO2 in air. To knockdown the HIF-1α gene, cells were transfected with MISSION shRNA Plasmid DNA (Sigma-Aldrich, St. Louis, MO, USA). MISSION™ pLKO.1-puro control vector (Sigma-Aldrich) was used as a control. For overexpression of the HIF-1α gene, mouse HIF-1α cDNA cloned into pcDNA3.1/myc-His vector was used. Transfection was carried out using a Lipofectamine LTX kit (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s specifications. To normalize variance of transfection efficiency, Renilla luciferase DNA in the pRL-TK vector (40 ng; Promega, Leiden, the Netherlands) was used as an internal control. At 48 hours after the transfection, cells were harvested to obtain total RNA. In some experiments, transfected cells were incubated under 8% oxygen for 24 hours to evaluate the efficiency of the overexpression or knockdown of the HIF-1α gene.
Total RNA Isolation and Quantitative PCR
Total RNA from frozen liver tissue and cultured cells were extracted using an RNeasy kit (Qiagen, Valencia, CA, USA). cDNA was synthesized from total RNA using SuperScript II RNase H reverse transcriptase (Life Technologies), and the resultant first-strand cDNA was stored at −80°C until analysis. Quantitative real-time RT-PCR was performed using the ABI Prism 7,300 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) with a standard temperature protocol and 2× SYBR Green PCR Master Mix reagent (Applied Biosystems) in a 25-μL volume in duplicate. As an internal control, β-actin was used for normalizing, and data were presented as the relative mRNA variation. The primer sequences were as follows: human PGC-1α, forward 5′-AACC ACACCCACAGGATCAGA-3′, reverse 5′-TCTTCGCTTTATTGCTCCATGA-3′; mouse PGC-1α, forward 5′-CTGCGA ACATATTTGAG-3′, reverse 5′-GGGTCAGAGGAAGAGAT AAAGTTGT-3′; human β-actin, forward 5′-CTCTTCCAG CCTTCCTTCCT-3′, reverse 5′-AGCACTGTGTTGGCGTAC AG-3′; and mouse β-actin, forward 5′-AGAGGGAAATCGT GCGTGAC-3′, reverse 5′-CAATAGTGATGACCTGGCCGT-3′.
Western Blotting
Cultured cells were lysed in 200 μL of lysis buffer (Cell Signaling Technology, Danvers, MA, USA) with 1 mM PMSF. Protein concentration was determined by the Bradford method using bovine serum albumin as a standard. Samples were boiled for five minutes with SDS sample buffer, and 20 μg of protein per sample was loaded onto a 10% SDS-polyacrylamide gel. Electroblots were blocked at room temperature in Tris buffer NaCl-Tween (TBST) containing 5% skim milk powder. Western blot analysis was carried out with a specific antibody against HIF-1α (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and β-actin antibodies (Sigma Aldrich). The antibodies were diluted in TBST buffer + 5% skim milk and left overnight at 4°C. After a TBST washing procedure, the blots were incubated with a 2nd antibody for one hour at room temperature. The immune reaction was detected by enhanced chemiluminescence.
Statistical Analysis
All data are presented as means ± SD. Mean values were analyzed by a one-way ANOVA and Student’s t-test where appropriate. A value of p < 0.05 was considered statistically significant.
Results
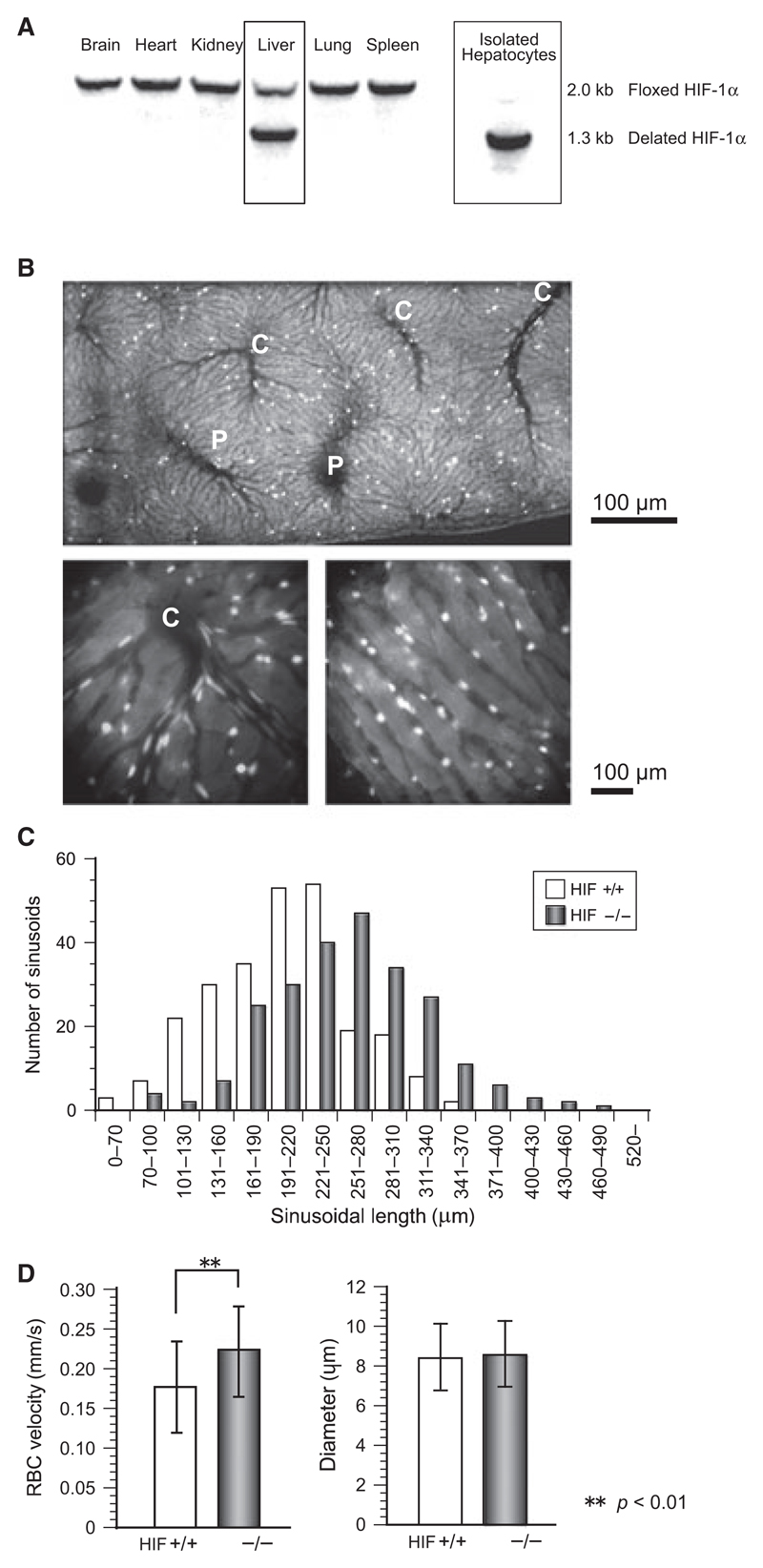
Southern blotting analysis indicates hepatocytes-specific deletion of the HIF-1α gene as shown in Figure 1A. To quantify the extents of deletion of floxed HIF-1α genomic fragments in vivo, Southern blot analysis was performed using genomic DNA collected from various organs of HIF−/− mice. In the liver but not in other organs from the adult mutant mice, the deletion of floxed gene occurred in ~70% of the tissue constituents. The intact floxed gene in the liver appeared to result from the presence of the HIF-1α gene in cells other than parenchymal cells, since primary cultured hepatocytes from the mutant mice exhibited the Cremediated gene deletion almost completely. Hepatic micro-circulation was visualized with FITC-labeled erythrocytes as shown in Figure 1B. Hepatic blood flow was maintained at a physiologically steady state for at least two hours, and the portal veins and central venules were identified from the blood flow directions in the video images. A histogram of sinusoid length is shown in Figure 1C. Although wet liver weights did not differ between control and HIF−/− mice (data not shown), the histogram as a whole shows a right shift for the knockout mice, and the averages were 257.7 ± 67.3 μm in HIF−/− mice and 232.4 ± 60.2 μm in control mice (p < 0.01). This suggests that a lack of HIF-1α in hepatocytes increases sinusoid length and consequently expands the hepatic lobules. The sinusoid diameter in control mice (8.43 ± 1.68 μm, n = 80) did not differ significantly from that in control mice (8.60 ± 1.65 μm, n = 88), but as shown in Figure 1D the velocity of sinusoidal blood flow in the HIF−/− mice (0.22 ± 0.06 mm/sec) was significantly greater than that in the control mice (0.18 ± 0.06 mm/sec).
Figure 1.
(A) Southern blotting results of tissue samples from various organs. Only hepatocytes showed deletion of HIF-1α gene. (B) Hepatic microcirculation was visualized using erythrocytes fluorescently labeled with FITC. Flow direction in video helped to identify portal veins, sinusoids, and central venules. “P” and “C” indicate portal veins and central venules. Lower panels are magnified images of central venules (left) and sinusoids (right). (C) Histogram showing elongation of the hepatic sinusoids in knockout mice. The average length (± SD) was 257.7 ± 67.3 μm in HIF−/− mice (n = 239) and 232.4 ± 60.2 in HIF-1α+/+ mice (n = 251). (D) RBC velocity in sinusoids and the sinusoid diameter were measured under a high-powered microscope, and the flow velocity in HIF−/− (0.22 ± 0.06 mm/sec) was significantly greater than that in control mice (0.18 ± 0.06 mm/sec) (p < 0.01).
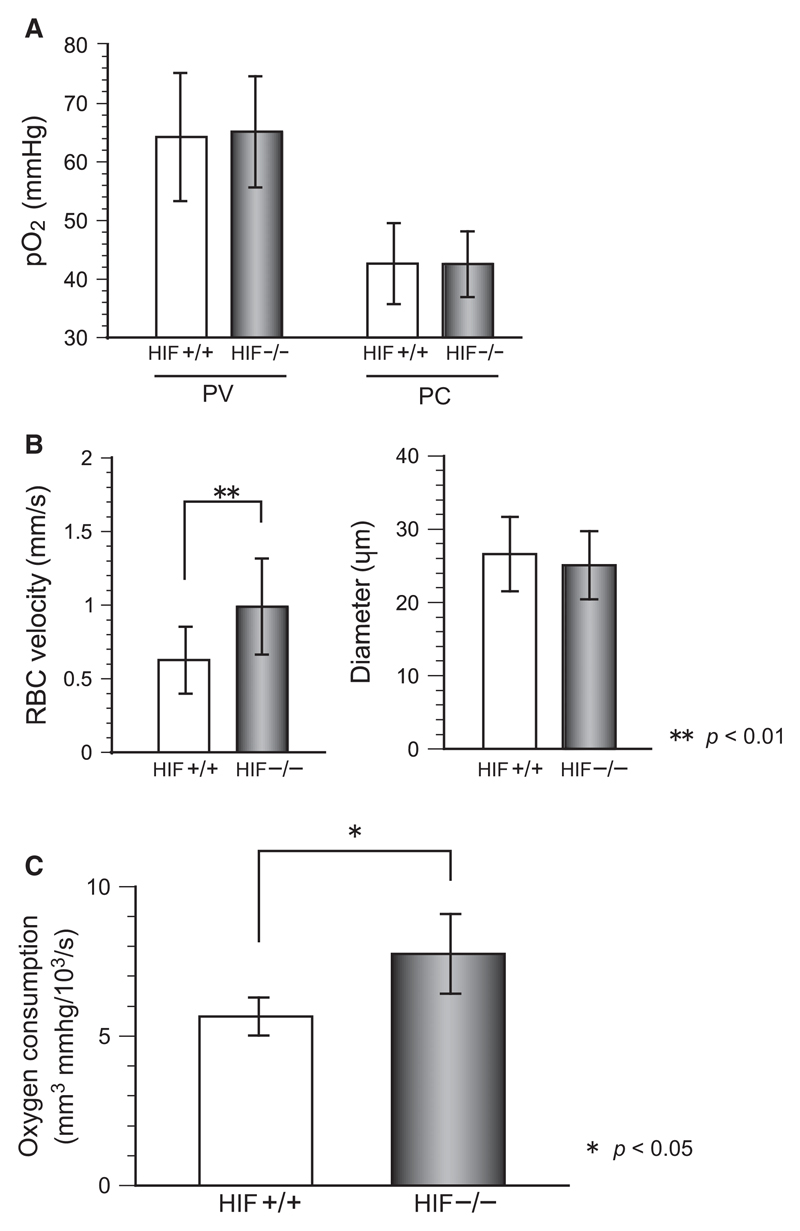
To analyze the influence of HIF-1α on oxygen delivery quantitatively, we used phosphorescence-based pO2 measurement with a short -pulse laser that irradiated an approximately 10-μm spot on the target vessel through a ×100 objective lens. As shown in Figure 2A, the pO2 values in the portal and central venules did not differ between control and HIF−/− mice. As shown in Figure 2B, however, the flow velocity in central venules was greater in HIF−/− mice even though the venule diameter did not differ between the HIF−/− mice and the control mice. The oxygen consumption estimated from formula (1) was significantly greater in HIF−/− mice (Figure 2C).
Figure 2.
Blood flow distribution in hepatic microcirculation and oxygen consumption were measured. (A) 10-μm spots on portal venules (PV) and central venules (CV) were irradiated by a short-pulse laser, and the pO2 was measured with the oxygen-quenching method. (B) RBC velocity in central venules and the diameter were measured off-line in fluorescent images, and the velocity in HIF−/− mice was greater than that in control mice (p < 0.01). (C) The hepatic lobule oxygen consumption estimated with formula (1) was greater in HIF−/− mice than that in control mice.
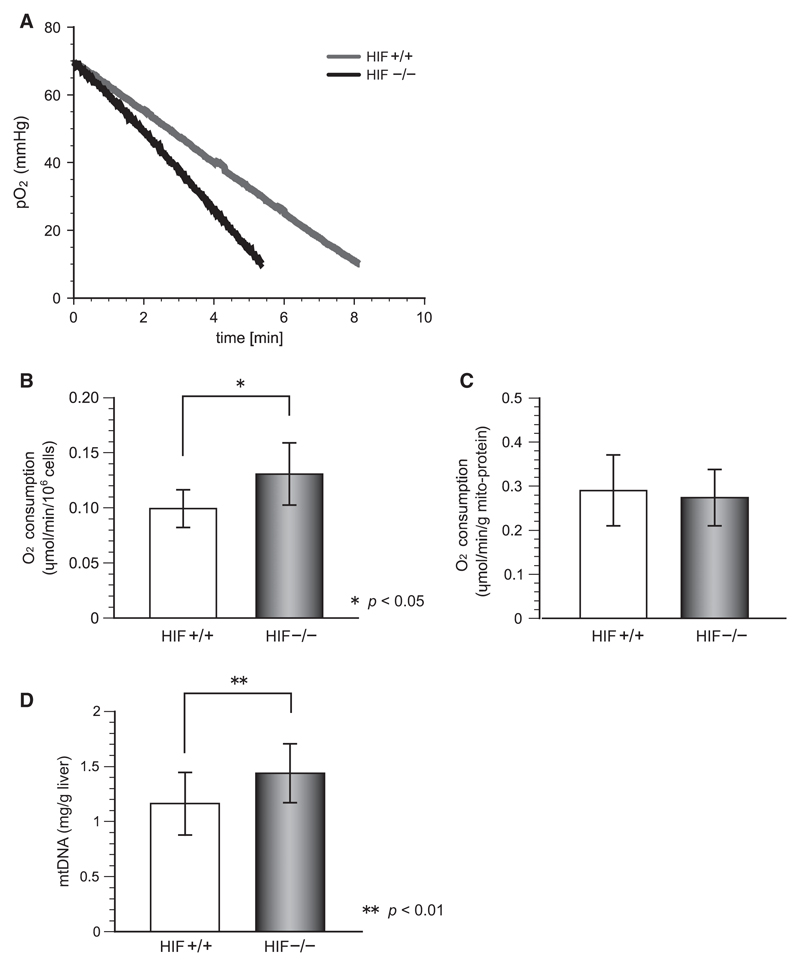
To examine whether the increased oxygen consumption in HIF−/− liver is caused by the increased oxygen consumption of hepatocytes, we measured the oxygen consumption of isolated hepatocytes in vitro. Figure 3A shows representative trends of the oxygen consumption rates of isolated hepatocytes. Oxygen concentrations declined at roughly constant rates in cells irrespective of HIF-1α gene status, but the oxygen consumption rates of HIF-1α-deficient hepatocytes were greater than those of control cells. As shown in Figure 3B, the oxygen consumption rates calculated from the slopes shown in Figure 3A were 0.099 μmol/min/106 cells for control cells and 0.131 μmol/min/106 cells for HIF−/− cells; i.e., 32% greater in the HIF−/− hepatocytes. The results suggest some modification regulating oxygen consumption in mitochondria, major consumers of oxygen in cells, so we measured oxygen consumption of mitochondria isolated from both control and HIF−/− hepatocytes. As shown in Figure 3C, mitochondrial respiration activity did not differ significantly between them, suggesting that HIF-1α does not change the enzymatic activity or components of mitochondria. The increase in cellular respiration of the knockout mice that is shown in Figure 3B indicates a possibility of a greater mitochondrial content in HIF−/− liver. As mtDNA content reflects mitochondrial quantity, mtDNA was isolated from liver tissue and it concentration was measured by UV spectrophotometry. The mtDNA contents in liver tissues were 1.16 ± 0.28 mg/g liver in controls and 1.44 ± 0.27 mg/g liver in HIF−/− mice, indicating that HIF-1 might be related to mitochondrial biogenesis in liver.
Figure 3.
Oxygen consumption in isolated hepatocytes. (A) Slopes fitted to cellular respiration rates of 5 × 104 hepatocytes measured in a gas-tight vial. (B) The oxygen consumption in HIF−/− hepatocytes was significantly greater than that in control hepatocytes (p < 0.05). (C) There was no difference between the respiration rates of mitochondria isolated from HIF−/− and control hepatocytes. (D) Significantly more mitochondrial DNA was isolated from the liver tissue of HIF−/− mice (p < 0.01), indicating that HIF-1 controls cellular respiration by regulating mitochondrial biogenesis.
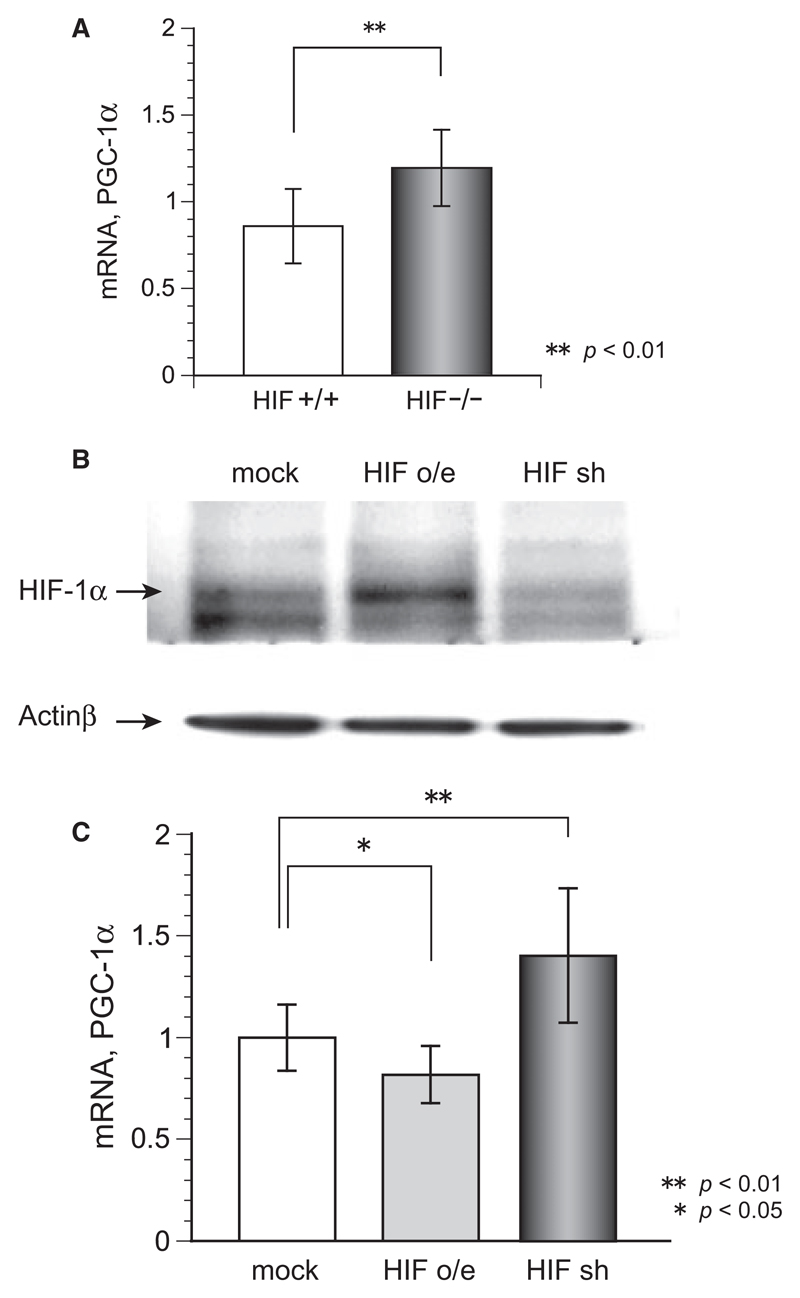
To determine the relation between HIF-1 and mitochondrial biogenesis, we used qRT-PCR to quantify the expression of PGC-1α mRNA. Figure 4A shows that the liver tissue of HIF−/− mice contains significantly more PGC-1α mRNA than the liver tissue of control mice does, suggesting that PGC-1 is an intermediate in HIF-1 regulated mitochondrial biogenesis. Western blot analysis showed that transfection of vectors for overexpression and knockdown of HIF-1α in HEK cells worked effectively (Figure 4B). The overexpression of HIF-1α decreased PGC-1α mRNA, and the knockdown of HIF-1α increased it. These results indicate that under normoxic conditions HIF-1 regulates mitochondrial biogenesis via the PGC-1α pathway.
Figure 4.
(A) Quantitative PCR analysis results showing significantly more PGC-1α mRNA in HIF−/− liver than in control liver (p < 0.01). (B) Western blotting for evaluating transfection efficiency showed HIF-1α expression compared to actin β increased in overexpressed cells (HIF o/e) and decreased in knockdown cells (HIF sh), respectively, (C) PGC-1α mRNA expression in HEK293 cells was significantly increased by overexpression of HIF-1α (HIF o/e, p < 0.05) and decreased by knockdown of HIF-1α (HIF sh, p < 0.01).
Discussion
The roles of HIF-1α have been mainly investigated under hypoxic conditions, especially in tumor and ischemiareperfusion models, but little is known about its roles under physiological conditions. The results of our microvascular experiments showed that hepatic sinusoids in hepatocyte-specific HIF−/− mice are longer than those in the wild-type mice. Furthermore, the blood flow in central venules of the HIF−/− mice is significantly faster than that of the wild-type mice. As a result, the estimated oxygen consumption in the hepatic lobules of HIF−/− mice becomes greater than that of the wild-type mice.
Under normoxic conditions, HIF-1α regulates mitochondrial biogenesis through PGC-1α. Other experimental results showed that isolated hepatocytes obtained from HIF−/− mice consume 32% more oxygen than do isolated hepatocytes obtained from control mice even though mitochondrial respiration is comparable between isolated hepatocytes collected from HIF−/− and those from wild-type mice. This increased oxygen consumption was almost equivalent to that measured in vivo.
The sinusoids of the HIF−/− mice were approximately 25 μm longer than those of the control mice, which is a distance almost equal to the size of a single hepatocyte, being a 10.9% increase in the average sinusoid length. Such an increase in the length corresponds to a 37% increase in the volume of a single hepatic lobule. Flow cytometry experiments showed no difference in individual cellar volume between the HIF−/− and wild-type hepatocytes (data not shown), indicating that the expansion of hepatic lobules does not result from swelling of hepatocytes. It might instead result from an increased numbers of hepatocytes residing along individual sinusoids, although this possibility should be examined by statistical analysis of the number of hepatocytes between portal and central venules.
Hepatic zonation relates sinusoidal hemodynamics—which forms gradients of oxygen, metabolites, and cytokines beneficial for hepatocytes and other cells—to the parenchymal matrix [7]. Hepatocytes and other cells at the afferent and efferent ends in hepatic lobules are subjected to different micro-environmental conditions. Oxygen levels in the blood at afferent and efferent ends of sinusoids differ greatly because oxygen is efficiently extracted by hepatocytes at the afferent ends of hepatic plates, exposing downstream hepatocytes to relatively hypoxic conditions. This oxygen gradient alone can explain much of the heterogeneity of hepatocyte function related to position in lobules [11]. The liver is very adaptable in this respect and balances an increased or decreased oxygen supply by decreasing or increasing oxygen extraction from the portal and arterial blood [1,5,14]. Actually, as shown in Figures 1D and 2B, the blood flow in sinusoids and central venules was greater in HIF−/− liver. The oxygen gradient seemed to be compensated by an increased blood flow in the HIF−/− microcirculation although oxygen consumption was accelerated as shown in Figure 2C. The portal vascular resistance is the sum of the resistances of hepatic portal venules, sinusoids, central venules, and hepatic veins. The regulation of the blood flow in the sinusoids is influenced in different ways. Neural factors, anatomical mechanisms, and vasoactive substances (e.g., endothelin, CO, NO, and adenosine) are related [2,12,23]. In our model, however, no significant HIF-related differences in the diameters of sinusoids and central venules were observed and no HIF-related change of endothelin and systemic blood pressure was observed; 70.5 ± 5.1 mmHg in control mice (n = 13) and 74.1 ± 9.3 mmHg in HIF−/− mice (n = 14). It seems that the increase in the resistance conflicts with the increase in blood flow in central venules. It may be that there are more sinusoids in a lobule in HIF−/− mice. An increased number of sinusoids to a central venule decreases flow resistance in the lobule, and thus blood flow in the central venule is increased even if blood pressure is constant. This increased blood flow is analogous to increased current flow through parallel resistances in an electric circuit. The details of the mechanism are not known, but a lack of HIF-1α in hepatocytes leads to an increase of oxygen consumption in the liver either directly or indirectly. Further studies at cellular and molecular levels and regarding cytokines or metabolites that control hepatic blood flow or regarding Kupffer cells or leukocytes that cause physical stress in blood flow or regarding gaseous molecules mediating vascular tone will be required.
Oxygen consumption in a hepatic lobule was estimated by using equation (1), which as shown in Figure 2C indicated a 32% increase in HIF−/− mice. It was essential that we added a correction term with the cube of the sinusoid length ratio to normalize the oxygen consumption because the oxygen gradient depends on the size of the hepatic lobule. Figure 2C showed that the estimated oxygen consumption of the HIF−/− liver exceeded that of the control lever even with the normalization of hepatic lobule size, indicating that the oxygen consumption of individual hepatocytes in the HIF−/− liver is increased.
Isolated hepatocytes from HIF−/− mice consumed 32% more oxygen consumption than did isolated hepatocytes from control mice (Figure 3A,B), and this increase is almost equal to that estimated by formula (1) in the in vivo experiments, suggesting that hepatocytes cause the increase of oxygen consumption. Because isolated mitochondria did not show any difference in the oxygen consumption per unit weight of mitochondrial protein (Figure 3C), it seems that HIF-1α does not modify mitochondrial respiration, protein composition, or enzymatic activity but instead regulates mitochondrial biogenesis. PGC-1α and PGC-1β are known to be key regulators of energy metabolism and control mitochondrial biogenesis and respiratory function. Recent studies showed that HIF-1α regulates C-Myc activity through MXI-1 and inhibits mitochondrial biogenesis by reducing PGC-1β [30]. Conversely, inactivation of HIF-1α promotes mitochondrial biogenesis, and this is consistent with our data. In our model, however, PGC-1α mRNA increased in HIF−/− hepatocytes (Figure 4A), but PCG-1β mRNA showed no change (data not shown), suggesting that there other mechanisms relating HIF-1α and the regulation of respiration through mitochondrial biogenesis. And PCG-1α couples HIF-1α signaling and promotes mitochondrial biogenesis [17]. Genetical experiments showed that overexpression of HIF-1α decreased PGC-1α and knock-down of HIF-1α increased it (Figure 4C). HIF-1α therefore controls the hepatic microcirculation by modifying cellular respiration through mitochondrial biogenesis. The detailed mechanisms of this control, however, especially the relations between HIF-1α and hepatic mitochondria biogenesis and the expansion of hepatic lobules, should be further investigated.
There is no established standard method for measuring oxygen metabolism in the hepatic microcirculation. Measurement with microlight guides and miniature oxygen electrodes showed oxygen gradients within the liver lobule, with periportal regions more highly oxygenated than pericentral regions [13]. The hepatic microcirculation is, however, vulnerable to physical invasion. In the work reported here, Pd-porphyrin, with relatively low toxicity and longlasting emission, was injected intravenously, each targeted microvessel was irradiated with a short-pulse laser beam, and the decay of phosphorescence due to a photochemical reaction with oxygen was evaluated to quantify the pO2 in the vessel [24,26,29]. This method let us measure the absolute value of pO2, and pinpoint measurement of pO2 is available by focusing the laser light on individual microvessels under microscopic fields. Indeed, to estimate oxygen consumption in hepatic lobules by using equation (1), each portal and central venules were measured selectively. Singlet oxygen is generated in photochemical reactions [28] and can trigger HIF-1α expression [3], but this optical measurement for temporal measurement is a powerful tool for quantifying oxygen metabolism in vivo.
Perspective.
Visualization of the hepatic microcirculation and laser-assisted pO2 measurement reveals that HIF-1α expressed in hepatocytes is a determinant of hepatic lobular structure and regional blood flow distribution under physiological conditions. It will be of interest to elucidate for understanding hepatic hemodynamics and oxygen metabolisms under pathological conditions.
Acknowledgments
Harvesting of conditional HIF-1α-null mice was supported by Development of the Next-Generation Integrated Simulation of Living Matter, A Part of Development and Use of the Next-Generation Supercomputer Project of MEXT, Japan.
Grants
Ministry of Education, Science, Sports and Culture, Grantin-Aid for Young Scientists (B), 2010, 22700476 (to K.T.). Suzuken Memorial Foundation 2010 (to K.T.). Research and Development of the Next-Generation Integrated Simulation of Living Matter, a part of the Development and Use of the Next-Generation Supercomputer Project of MEXT (to M.S.). Japan Science and Technology Agency, Exploratory Research for Advanced Technology (ERATO), Suematsu Gas Biology Project.
Abbreviations used
- EGF
epidermal growth factor
- FITC
fluorescent isothiocyanate
- HIF
hypoxia-inducible factor
- mtDNA
mitochondrial DNA
- Pd-TCPP
Pd-meso-tetra-(4-carboxyphenyl)-porphyrin
- PGC
peroxisome proliferator-activated receptor gamma coactivator
- pO2
partial oxygen pressure
References
- 1.Alvarez D, Mastai R, Lennie A, Soifer G, Levi D, Terg R. Noninvasive measurement of portal venous blood flow in patients with cirrhosis: effects of physiological and pharmacological stimuli. Dig Dis Sci. 1991;36:82–86. doi: 10.1007/BF01300092. [DOI] [PubMed] [Google Scholar]
- 2.Brenner BM, Troy JL, Ballermann BJ. Endothelium-dependent vascular responses. Mediators and mechanisms. J Clin Invest. 1989;84:1373–1378. doi: 10.1172/JCI114309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dehne N, Brüne B. HIF-1 in the inflammatory microenvironment. Exp Cell Res. 2009;315:1791–1797. doi: 10.1016/j.yexcr.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Eipel C, Abshagen K, Vollmar B. Regulation of hepatic blood flow: the hepatic arterial buffer response revisited. World J Gastroenterol. 2010;16:6046–6057. doi: 10.3748/wjg.v16.i48.6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goda N, Suzuki K, Naito M, Takeoka S, Tsuchida E, Ishimura Y, Tamatani T, Suematsu M. Distribution of heme oxygenase isoforms in rat liver. Topographic basis for carbon monoxide-mediated microvascular relaxation. J Clin Invest. 1998;101:604–612. doi: 10.1172/JCI1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goresky CA. A linear method for determining the liver sinusoidal and extravascular volumes. J Physiol. 1963;204:626–640. doi: 10.1152/ajplegacy.1963.204.4.626. [DOI] [PubMed] [Google Scholar]
- 8.Grote J. Physiologie der Menschen. 20th edn. New York: Springer-Verlag; 1980. p. 560. [Google Scholar]
- 9.Ishikawa M, Sekizuka E, Shimizu K, Yam-aguchi N, Kawase T. Measurement of RBC velocities in the rat pial arteries with an image-intensified high-speed video camera system. Microvasc Res. 1998;56:166–172. doi: 10.1006/mvre.1998.2100. [DOI] [PubMed] [Google Scholar]
- 10.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 11.Jungermann K, Kietzmann T. Oxygen: modular of metabolic zonation and disease of the liver. Hepatology. 2000;31:255–260. doi: 10.1002/hep.510310201. [DOI] [PubMed] [Google Scholar]
- 12.Lautt WW, Legare DJ, d’Almeida MS. Adenosine as putative regulator of hepatic arterial flow (the buffer response) Am J Physiol. 1985;248:H331–H338. doi: 10.1152/ajpheart.1985.248.3.H331. [DOI] [PubMed] [Google Scholar]
- 13.Lemasters JJ, Ji S, Thurman RG. New micromethods for studying sublobular structure and function in the isolated, perfused rat liver. In: Thurman RG, Kauffman F, Jungermann K, editors. Regulation of Hepatic Metabolism. New York: Plenum; 1986. pp. 159–184. [Google Scholar]
- 14.McCormick PA, Burroughs AK. Hemodynamic evaluation of portal hypertension. Hepatogastroenterology. 1990;37:546–550. [PubMed] [Google Scholar]
- 15.Morikawa T, Kajimura M, Nakamura T, Hishiki T, Nakanishi T, Yukutake Y, Nagahata Y, Ishikawa M, Hattori K, Takenouchi T, Takahashi T, et al. Hypoxic regulation of the cerebral microcirculation is mediated by a carbon monoxide-sensitive hydrogen sulfide pathway. Proc Natl Acad Sci U S A. 2012;109:1293–1298. doi: 10.1073/pnas.1119658109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishiyama Y, Goda N, Kanai M, Niwa D, Osanai K, Yamamoto Y, Senoo-Matsuda N, Johnson RS, Miura S, Kabe Y, Suematsu M. HIF-1α induction suppresses excessive lipid accumulation in alcoholic fatty liver in mice. J Hepatol. 2012;56:441–447. doi: 10.1016/j.jhep.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 17.O’Hagan KA, Cocchiglia S, Zhdanov AV, Tambuwala MM, Cummins EP, Monfared M, Agbor TA, Garvey JF, Papkovsky DB, Taylor CT, Allan BB. PGC-1alpha is coupled to HIF-1alpha-dependent gene expression by increasing mitochondrial oxygen consumption in skeletal muscle cells. Proc Natl Acad Sci U S A. 2009;106:2188–2193. doi: 10.1073/pnas.0808801106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Rasband WS. ImageJ. U. S. National Institutes of Health, Bethesda; Maryland, USA: 1997–2005. http://rsb.info.nih.gov/ij/ [Google Scholar]
- 20.Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM, Johnson RS. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 2000;60:4010–4015. [PubMed] [Google Scholar]
- 21.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 22.Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- 23.Suematsu M, Ishimura Y. The heme oxygenase-carbon monoxide system: a regulator of hepatobiliary function. Hepatology. 2000;31:3–6. doi: 10.1002/hep.510310102. [DOI] [PubMed] [Google Scholar]
- 24.Suganuma K, Tsukada K, Kashiba M, Tsuneshige A, Furukawa T, Kubota T, Goda N, Kitajima M, Yonetani T, Suematsu M. Erythrocytes with T-state-stabilized hemoglobin as a therapeutic tool for postischemic liver dysfunction. Antioxid Redox Signal. 2006;8:1847–1855. doi: 10.1089/ars.2006.8.1847. [DOI] [PubMed] [Google Scholar]
- 25.Tajima T, Goda N, Fujiki N, Hishiki T, Nishiyama Y, Senoo-Matsuda N, Shimazu M, Soga T, Yoshimura Y, Johnson RS, Suematsu M. HIF-1alpha is necessary to support gluconeogenesis during liver regeneration. Biochem Biophys Res Commun. 2009;387:789–794. doi: 10.1016/j.bbrc.2009.07.115. [DOI] [PubMed] [Google Scholar]
- 26.Torres Filho IP, Kerger H, Intaglietta M. pO2 measurements in arteriolar networks. Microvasc Res. 1996;51:202–212. doi: 10.1006/mvre.1996.0021. [DOI] [PubMed] [Google Scholar]
- 27.Tsukada K, Sekizuka E, Oshio C, Tsujioka K, Minamitani H. Red blood cell velocity and oxygen tension measurement in cerebral microvessels by double-wavelength photoexcitation. J Appl Physiol. 2004;96:1561–1568. doi: 10.1152/japplphysiol.00764.2003. [DOI] [PubMed] [Google Scholar]
- 28.Tsukada K, Suematsu M. Visualization and analysis of blood flow and oxygen consumption in hepatic microcirculation: application to an acute hepatitis model. J Vis Exp. 2012;66:e3996. doi: 10.3791/3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanderkooi JM, Maniara G, Green TJ, Wilson DF. An optical method for measurement of dioxygen concentration based upon quenching of phosphorescence. J Biol Chem. 1987;262:5476–5482. [PubMed] [Google Scholar]
- 30.Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, Dang CV, Semenza GL. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11:407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]