Abstract
Hypoxia-inducible transcription factors (HIFs) control cellular adaptation to low oxygen. In the kidney, activation of HIF is beneficial during injury; however, the specific contribution of HIF-1α in renal endothelial cells (EC) remains elusive. Since EC display tissue-specific heterogeneity, we investigated how HIF-1α affects key functions of glomerular EC in vitro and its contribution to renal development and pathophysiological adaptation to acute or chronic renal injury in vivo. Loss of HIF-1α in glomerular EC induces hypoxic cell death and reduces hypoxic adhesion of macrophages in vitro. In vivo, HIF-1α expression in EC in mouse kidneys is detectable but limited. Accordingly, EC-specific ablation of HIF-1α does not lead to developmental or phenotypical abnormalities in the kidney. Renal function and expression of adhesion molecules during acute ischemic kidney injury is independent of HIF-1α in EC. Likewise, inflammation and development of fibrosis after unilateral ureteric obstruction is not influenced by endothelial HIF-1α. Taken together, although HIF-1α exerts effects on glomerular EC in vitro, endothelial HIF-1α does not influence renal development and pathophysiological adaptation to kidney injury in vivo. This implies a profound difference of the hypoxic response of the renal vascular bed compared to other organs, such as the heart. This has implications for the development of pharmacological strategies targeting the endothelial hypoxic response pathways.
Keywords: Oxygen, Hypoxia response, Transcription factor, Fibrosis, Ischemia
Introduction
Chronic kidney disease (CKD) is a global health problem with increasing prevalence [1]. Progression of CKD to end-stage renal disease (ESRD) is often accelerated by acute kidney injury (AKI), which itself bears considerable morbidity and mortality. In both instances, hypoxia is believed to play a critical role: renal oxygen tensions are generally low [2] and the metabolic demand especially in the proximal tubular segment is high. Thus, reduced blood oxygen supply by renal hypoperfusion, e.g., during AKI, can easily lead to critical hypoxia. In CKD, the final common pathway of renal destruction involves interstitial fibrosis, tubular atrophy, and capillary rarefaction. Interstitial hypoxia as a consequence of these abnormalities has been suggested as one of the major contributors to disease progression [3].
The renal endothelium is involved in various processes such as vasomotor control, regulation of inflammation, and coagulation and thus plays a pivotal role in renal (patho-)physiology. Endothelial dysfunction contributes to the no-reflow phenomenon after AKI and may also impair renal autoregulation, which aggravates tubular injury. Nitric oxide (NO) is a major contributor to endothelial dysfunction in AKI [4]. In CKD, on the other hand, endothelial cells (EC) eventually disappear due to apoptosis and a reduction of angiogenic factors which in turn aggravates inflammatory cell adhesion and vascular occlusion by coagulation. Blocking NO aggravates capillary loss in a model of chronic kidney disease (CKD) [5], and endothelial injury due to deficiency of NO production accelerates CKD [6]. The hypoxia response pathway and the NO pathway are closely linked [7]. Until now, however, the impact of hypoxia response mechanisms in the renal endothelium on disease progression is incompletely understood.
Hypoxia triggers an adaptive tissue response to ameliorate damage and promote survival under low oxygen conditions. The central regulator of the hypoxic response in mammalian cells is the Hypoxia-Inducible Factor (HIF). It consists of two subunits, the constitutive HIF-1β and one of two oxygen-regulated α-subunits, HIF-1α or HIF-2α. In normoxia, HIFα is rapidly hydroxylated at specific prolyl and asparagyl residues and targeted for proteasomal degradation by the von Hippel Lindau Protein. In hypoxia, HIFα dimerizes with HIF-1β and can activate target genes involved in metabolic adaptation, angiogenesis, and erythropoiesis (for review see [8]). Expression profiling has revealed that both HIFα-isoforms play unique, tissue-specific, and sometimes opposing roles in physiological processes [9].
HIF-2α was originally identified in EC and is also known as endothelial PAS domain protein 1 (EPAS1) [10]. In agreement with a robust endothelial expression, HIF-2α is detectable in the glomerulus and in peritubular capillaries in hypoxia [11]. Surprisingly, endothelial deletion of HIF-2α in mice does not lead to systemic developmental defects, and also the renal vasculature is intact [12]. The presence and functional relevance of HIF-1α in EC is less clear. Global deficiency of HIF-1α is embryonic lethal because of the absence of cephalic or somitic vascularization, which suggests that HIF-1α is critical for capillary network formation [13, 14]. EC require HIF-1α for proliferation, chemotaxis, extracellular matrix penetration, and wound healing during neovascularization. Moreover, NO-homeostasis in EC is critically dependent on HIF-1α and impacts metastasis formation [7]. Studies in mice revealed a protective role of HIF-1α in EC during ischemic preconditioning and pressure overload in the heart [15, 16]. Additionally, HIF-1α in EC has been found to be a critical mediator of glucose uptake in the heart and brain [17]. In the kidney, however, no HIF-1α has been detected so far in renal EC in adult rats or mice [11, 18], although HIF-1 has been suggested to contribute to glomerulogenesis [19]. In line with the notion of a potential organotypic HIF-1 functionality, there is increasing evidence of distinct tissue-specific regulation of transcription factors in EC [20].
Taken together, the functional role of HIF-1α in EC for renal physiology and pathophysiology is still unclear. Here, we demonstrate that in a murine renal glomerular microvascular EC line, HIF-1α is critical for survival under hypoxic conditions and for the interaction with macrophages. In mouse kidneys, we find limited endothelial HIF-1α protein expression during systemic hypoxia, after PHD inhibition or during sepsis, mostly localized to medullary EC. In mice with endothelial-specific deletion of HIF-1α, renal development, renal function, and the renal vasculature appear normal. Moreover, HIF-1α in EC does not affect the outcome of acute ischemic or chronic obstructive kidney injury. Thus, our data suggest that HIF-1α in EC is dispensable for renal vascular development, physiology, and pathology. This implies differential control of HIF-1α functionality in EC in different vascular beds.
Materials and methods
Cell culture
The original murine glomerular microvascular endothelial cell line (glEND.2) was kindly provided by R. Hallmann (Muenster, Germany). Cells were cultured at 37 °C and 7.5 % CO2 in Dulbecco’s modified Eagle’s medium (DMEM) containing 10 % fetal calf serum (FCS) and routinely split in a 1:5 ratio. The stable knockdown of HIF-1α was induced by lentiviral transduction with shRNA producing constructs as described previously [21]. Cells with stable knockdown of HIF-1α (shHIF-1α) were compared to cells with stable transfection of an irrelevant shRNA (shGFP). Hypoxic conditions were 1 % O2, 5 % CO2, and balance nitrogen. For Intercellular Adhesion Molecule 1 (ICAM1) expression analysis, cells were incubated for 18 h.
Cell cycle analysis
After incubation for the indicated times and conditions, glEND.2 cells were washed and fixed in ice-cold 70 % ethanol and stored at 4 °C overnight. For staining, cells were resuspended in cold PBS buffer, washed two times, and then resuspended in staining solution containing PBS buffer, 20 μg/ml propidium iodide (PI; Sigma-Aldrich), 0.1 % Triton X (Sigma-Aldrich), and 200 μg/ml DNAse-free RNAse A (AppliChem). Stained cells were incubated at 37 °C for 15 min and data was acquired using FACSCanto II (Becton Dickinson). Cell cycle analysis was performed using ModFit software (Verity Software House).
Apoptosis experiments
Apoptosis analysis was performed by Annexin V and PI double staining. After incubation for the indicated times and conditions, cells were washed and then resuspended in staining solution containing 5 % Annexin V FITC (Biolegend) in HBSS buffer (GE Healthcare). After 10 min of incubation, PI was added to the cell suspension to a final concentration of 2 μg/ml. Double-stained cells were incubated on ice for 15 min and data was immediately acquired using FACSCanto II. Samples were analyzed using FlowJo software (Tree Star).
Adhesion experiments
Bone marrow derived macrophages (BMDMs) were generated as described before [22]. glEND.2 cells were harvested and plated onto glass slides in 24-well plates and settled for 24 h and incubated with 5 % CO2. The cultured BMDMs were labeled with Vybrant® DiI Cell-Labeling Solution (Life Technologies) according to the manufacturer’s protocol. Labeled macrophages were added to the glEND.2 cells and were cocultured for 2 h at 37 °C in normoxic and hypoxic (1 % O2, 5 % CO2) conditions. After incubation, cells were washed with PBS to remove nonadherent macrophages and then fixed with 4 % paraformaldehyde (PFA). Nuclei were counterstained with Hoechst stain (Sigma-Aldrich). Images of adherent macrophages were captured under Nikon fluorescence microscope (five randomly selected optical fields). The number of adherent macrophages was counted with the ImageJ software (US National Institutes of Health) in a blinded fashion.
MTT assays
MTT assays were performed essentially as previously described [23]. Briefly, glEND.2 cells were seeded on 96-well plates and kept under normoxic (7.5 % CO2) and hypoxic conditions (5%CO2) for the indicated times. MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (Sigma-Aldrich) was added to a final concentration of 5 mg/ml. After incubation for 3 h at 37 °C in normoxic and hypoxic conditions, 100 μl of medium was replaced by DMSO to dissolve the formazan product. Optical density was measured at 570 nm in a multiwell plate reader (SpectraMax, Molecular Devices). Results are the mean of five independent experiments with three replicates for each condition and genotype in each experiment.
Animal experiments
All animal experiments were approved by the Regierung von Mittelfranken (Az.54-2532.1-8/11, Az.54-2532.1-9/11, Az.54-2532.1-06/11). Mice with conditional deletion of HIF-1α were generated by crossing mice with a floxed allele of HIF-1α+f/+f [14] with the endothelial cell-specific cre-expressing mouse line Tie2-Cre [24]. Animals were housed on a 12:12 h light–dark cycle under constant temperature (24 ± 1 °C) in standard cages, fed a standard diet (Ssniff), and had free access to tap water. To measure water intake and collect urine samples, mice were kept in metabolic cages for 24 h. For all experiments, cre-negative littermates were used as control mice. Mice we exposed to 6 % hypoxia for 6 h or treated with the PHD inhibitor 2-(1-chloro-4-hydroxyisoquinoline-3-carboxamido) acetate (ICA) as described previously [25].
Renal ischemia-reperfusion injury
Renal ischemia-reperfusion injury (IRI) was essentially performed as described [25]. Briefly, under isoflurane anesthesia (Baxter), both renal pedicles were clamped for 25 min. In the sham group, clamps were applied but immediately removed. For renal functional and morphological analyses, mice were sacrificed 3 days after reperfusion. Kidney tissues were either snap frozen for subsequent mRNA extraction or fixed in 4 % PBS-buffered PFA for immunohistochemistry.
Unilateral ureter obstruction
Unilateral ureter obstruction was performed as follows: after abdominal midline incision under isoflurane anesthesia, the right ureter was ligated twice and subsequently cut. Mice were sacrificed after 5 or 7 days. The contralateral non-obstructed kidney served as control. Tissues were processed as described above.
Cecal ligation and puncture
Sepsis was induced by cecal ligation and puncture (CLP) essentially as described before [26]. Briefly, under isoflurane anesthesia, skin midline incision was performed, and after entry into the peritoneal cavity, the cecum was located. The cecum was ligated 1 cm from the cecal tip, punctured twice with an 18G needle and gently squeezed to express a small amount of fecal material, and then relocated into the abdominal cavity. After surgery, mice were resuscitated by injecting 1 ml per 25 g body weight of pre-warmed saline. Eighteen hours after surgery, mice were sacrificed. For hypoxia studies, mice were injected i.p. with 60 mg/kg of pimonidazole hydrochloride 30 min prior to sacrifice. PFA-fixed paraffin-embedded kidney sections were stained using the Hypoxyprobe plus kit (Natural Pharmacia International).
Genotyping and determination of deletion efficiency
Primers for genotyping of tail biopsies are listed in Supplemental Table 1. Deletion efficiency was determined by quantitative PCR of the HIF-1 floxed allele with genomic DNA isolated from whole kidney of 9-week-old mice (performed with DNeasy Kit (Qiagen) according to the manufacturer’s recommendation). Quantification was calculated with the delta-ct method, where the housekeeping gene was a nonfloxed gene, in this case VHL. These primers and primers to visualize the Hif1a deletion on agarose gels (3 %, Sigma) are all listed in Supplemental Table 1.
Quantitative RT-PCR
RT-PCR was essentially performed as described [27]. Total RNA was isolated from snap-frozen kidneys with Trizol Reagent (Invitrogen) according to the manufacturer’s protocol. First-strand synthesis was performed with 1 μg of total RNA with the Maxima™ First Strand cDNA synthesis kit (Fermentas). cDNAs were amplified in SYBR Green Maxima™ Master Mix (Fermentas) with a StepOne Plus™ Real-Time PCR system (Applied Biosystems). Expression levels were related to 18s using the delta-ct method. Primer sequences are listed in Supplemental Table 2.
Histology, immunohistochemistry, immunofluorescence, and immunoblotting
Immunohistochemistry was essentially performed as described [27]. Kidneys were fixed by immersion in 4 % PFA in PBS (pH 7.4) for 24 h, dehydrated in a graded ethanol series, and finally embedded in paraffin. Paraffin sections (5 μm) were stained with hematoxylin and eosin (H&E) or periodic acid-Schiff reagent (PAS) according to standard routine protocols. For immunohistochemistry, paraffin sections were deparaffinized and boiled in Target Retrieval Solution (Dako, #S169984-2), blocked with either 1 % bovine serum albumin in PBS or Protein Block (Dako, #X090930-2), and incubated with the following primary antibodies: polyclonal rabbit anti-mouse HIF-1α (Cayman #10006421 1:25,000), HIF-2α (#PM9; 1:20, 000 [28]), monoclonal rat anti-mouse F4/80 (clone CI:A3-1, AbD Serotec, 1:500), polyclonal rat anti-human CD3 (Dako, A0452, 1:300), monoclonal mouse anti-human PCNA (clone PC10, Dako, 1:50), and monoclonal mouse MECA-32, IgG1 (1:5) specific for endothelial cells [29]. Biotinylated polyclonal rabbit anti-rat IgG and rabbit anti-goat IgG antibodies (Dako) were detected using the Vectastain Elite ABC Kit (Vector Laboratories). For signal amplification, a catalyzed signal amplification system (Dako) was used. Anti-PCNA immunostaining was performed using MOM staining kit (Vector Laboratories). For immunofluorescence staining, undiluted monoclonal mouse MECA-32 and secondary antibody Alexa 594 (1:250; Life Technologies) were used. Sections were counterstained with DAPI (1:5000; Life Technologies). Immunoblots were performed as described [21].
Morphometric analyses
H&E- and PAS-stained kidney sections were evaluated by a nephropathologist in a blinded manner, as described before [25]. Fibrosis was assessed by Sirius Red staining counterstained with aniline blue. Digital images of Sirius Red-, F4/80-, and CD3-stained sections at 200-fold magnification (10 images per slide) were analyzed by Metavue-Software (Molecular Devices). The number of PCNA- and HIF-1α-positive nuclei per section was quantified using ImageJ software.
Statistical analyses
Data are presented as means + SEM of n >3 animals or independent experiments as detailed in the figures and legends. To compare two samples, Student’s t test was used with Prism (GraphPad Software). A p value <0.05 was considered significant.
Results
Deletion of HIF-1α in glomerular endothelial cell line impairs survival in hypoxia and reduces adhesion of macrophages
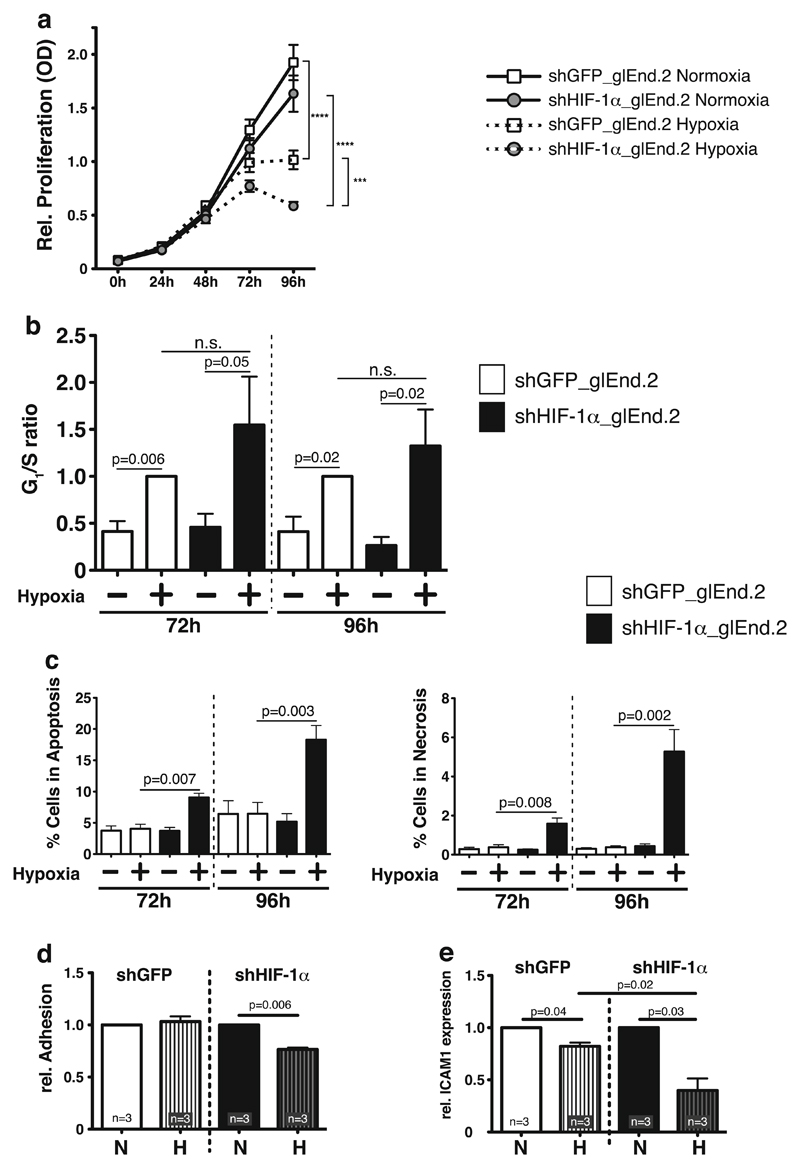
HIF-1α has been demonstrated to be essentially involved in the hypoxic response of EC [30, 31]. To investigate renal EC responses to prolonged hypoxia, which occurs, e.g., due to vascular rarefaction in kidney fibrosis, we generated a stable knockdown of HIF-1α in a glomerular microvascular endothelial cell line (glEND.2) utilizing shRNA as previously described [21]. Successful knockdown was demonstrated by complete ablation of hypoxic induction of HIF-1α protein (Supplemental Fig. 1a). In line with previous reports, prolonged hypoxia (72 and 96 h) itself leads to downregulation of HIF-1α protein [32], but the induction of the HIF target gene phosphoglycerate kinase (PGK) displays complete HIF-1α dependency even after prolonged hypoxia (Supplemental Fig. 1b). To assess HIF-1α-dependent effects on proliferation of renal EC in hypoxia, MTT assays were performed. Normoxic proliferation is not impaired by loss of HIF-1α in renal EC, irrespective of the CO2 concentration used in the culture (5 vs. 7.5 %) (Fig. 1a and not shown). After 72 h of hypoxia with 5 % CO2, proliferation is significantly reduced in both wild-type and HIF-1α knockout cells. In contrast to previous reports [33], loss of HIF-1α further slows EC proliferation in hypoxia, an effect which is observed at 72 and 96 h (Fig. 1a). To determine the underlying mechanism for the observed effect, cell cycle analysis was performed. The G1/S ratio as an indicator for cell cycle arrest is increased in both hypoxic wild-type and HIF-1α knockout cells, which indicates that HIF-1α is dispensable for hypoxic cell cycle control (Fig. 1b). In contrast, HIF-1α significantly impacts survival of renal EC in hypoxia; we observe a significant increase of both apoptosis and necrosis (Fig. 1c) in cells with deletion of HIF-1α. Adhesion of inflammatory cells such as monocytes/macrophages to the endothelial lining is critical for subsequent transmigration and inflammation. We therefore assessed adhesion of primary bone marrow derived macrophages (BMDM) on glEND.2 cells in relation to their HIF-1α-status; deletion of HIF-1α in EC significantly reduces macrophage adhesion in hypoxia (Fig. 1d). Accordingly, the adhesion molecule ICAM1 is significantly downregulated by hypoxia, which has been demonstrated before [34]. Deletion of HIF-1α further reduces ICAM1 expression, which indicates that hypoxia-activated HIF-1α is essentially involved in preventing further reduction of ICAM1 expression levels (Fig. 1e). Taken together, our results demonstrate that HIF-1α is critically important for hypoxic survival and leukocyte adhesion in a glomerular EC line.
Fig. 1. Effects of HIF-1α deletion in glomerular microvascular endothelial cells (glEND.2).
a Deletion of HIF-1α in glEND.2 does not affect normoxic proliferation. Hypoxia significantly reduces proliferation starting at 72 h and continuing until 96 h. Deletion of HIF-1α further reduces hypoxic proliferation (n=9–11 per group). b In hypoxic wild-type glEND.2 cells (open bars) G1/S ratio, which indicates cell cycle arrest, is significantly induced compared to normoxia after 72 and 96 h. Deletion of HIF-1α (closed bars) does not significantly change the hypoxic G1/S ratio, thus hypoxic cell cycle arrest is independent of HIF-1α (n >3 per group). c Deletion of HIF-1α (closed bars) significantly induces apoptosis and necrosis in glEnd.2 cells during prolonged hypoxia (n=4–5). d Adhesion of primary macrophages to glEND.2 cells is significantly reduced in hypoxia after deletion of HIF-1α (n =3). e mRNA expression of ICAM1 in glEND.2 cells is significantly reduced after 18 h of hypoxia. Deletion of HIF-1α further reduces hypoxic ICAM1 expression, which indicates that HIF-1α stabilization prevents ICMA1 downregulation in hypoxia (n=3)
HIF-1α expression is detectable but limited to endothelial cells in the kidney
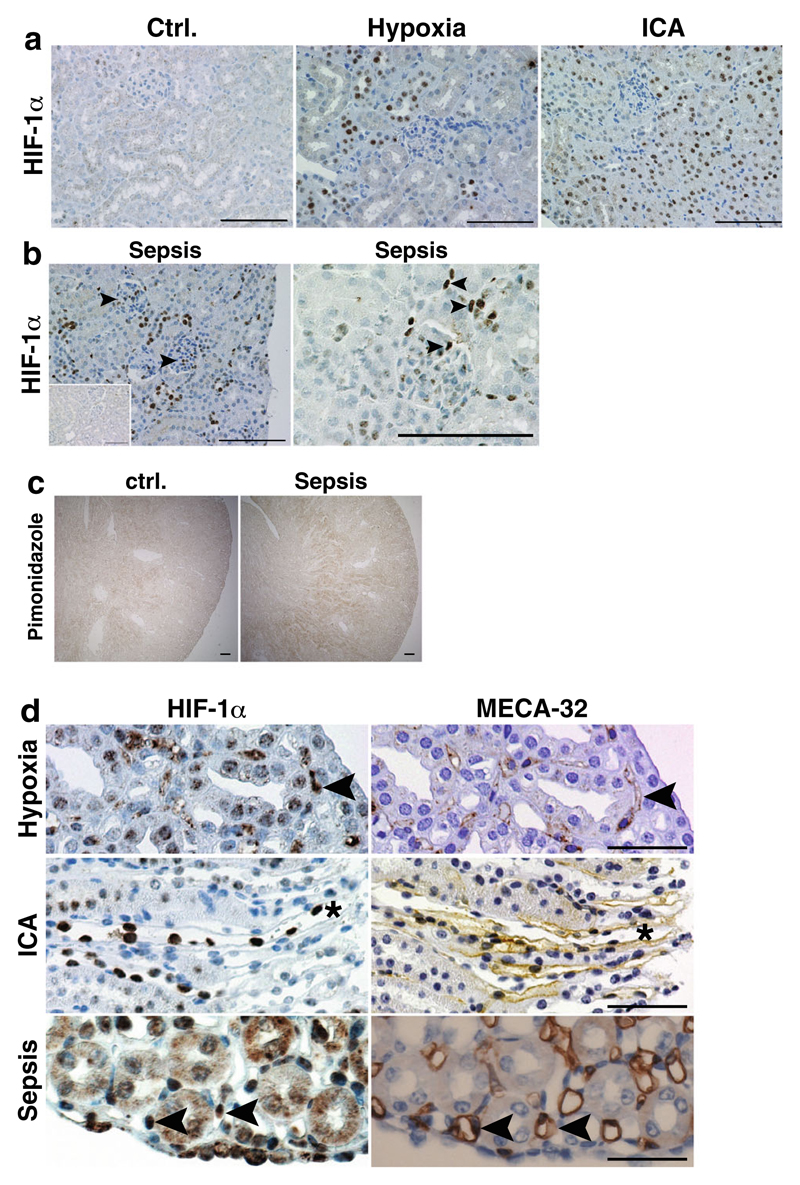
There are conflicting reports on the expression and functional relevance of HIF-1α protein in endothelial cells; although not detectable in some studies, functionality of endothelial HIF-1α was demonstrated in various models, tissues, or cells. Our data indicate that HIF-1α is vital for various aspects of EC physiology in glomerular EC in vitro. Therefore, we tested HIF-1α activation in kidneys using various stimuli such as hypoxia, the PHD inhibitor ICA [25], and severe sepsis by cecal ligation and puncture (CLP), which leads to renal hypoxia [35]. No HIF-1α is detected in kidney cortex in control conditions (Fig. 2a). Tubular cell HIF-1α is stabilized in hypoxia and after administration of ICA, but expression is absent in EC in the kidney cortex (glomerular or peritubular capillary EC; Fig. 2a). In the systemic sepsis model of CLP, HIF-1α is stabilized in tubular, glomerular, and also interstitial and peritubular cells of the kidney cortex (Fig. 2b). The presence of hypoxia after CLP is demonstrated by staining for the hypoxia marker pimonidazole (Fig. 2c) confirming observations by Yasuda et al. [34]. We next evaluated whether EC in the medulla or papilla express HIF-1α. To this end, we stained consecutive sections for HIF-1α and the endothelial marker MECA-32. Here, we do see HIF-1α expression in renal medullary EC in hypoxia after PHD inhibition and in severe sepsis (Fig. 2d). However, the total number of positive EC is low compared to tubular HIF-1α expression. These data demonstrate that strong stimuli such as hypoxia, PHD inhibition, or sepsis are sufficient to induce endothelial HIF-1α expression in wild-type animals, although overall expression levels are low and mostly confined to the renal medulla.
Fig. 2. HIF-1α activation in wild-type kidneys after different stimuli.
a No HIF-1α protein is observed in control conditions. Global HIF-1α activation is more profound after PHD inhibition with ICA than in hypoxia. The majority of HIF-1α protein is detectable in tubular epithelial cells. No glomerular or interstitial HIF-1α staining is visible in hypoxic and ICA-treated kidneys (×400, scale bar 100 μm). b In septic kidneys, tubular, glomerular, and interstitial HIF-1α positivity is observed (arrowheads; ×400, scale bar 100 μm; inset—sham kidney). c Pimonidazole staining confirms hypoxic areas in kidneys of mice treated with CLP-induced sepsis compared to control kidneys (×40, scale bar 100 μm). d Serial sections stained for HIF-1α (left) or the endothelial marker MECA-32 (right) shows specific HIF-1α protein expression (arrowheads) in medullary/papillary EC after stimulation with hypoxia or PHD inhibitors (asterisk denotes the same vessel) or after CLP-induced sepsis (representative images, ×400, scale bar 50 μm)
Deletion of HIF-1α in endothelial cells does not impair renal development
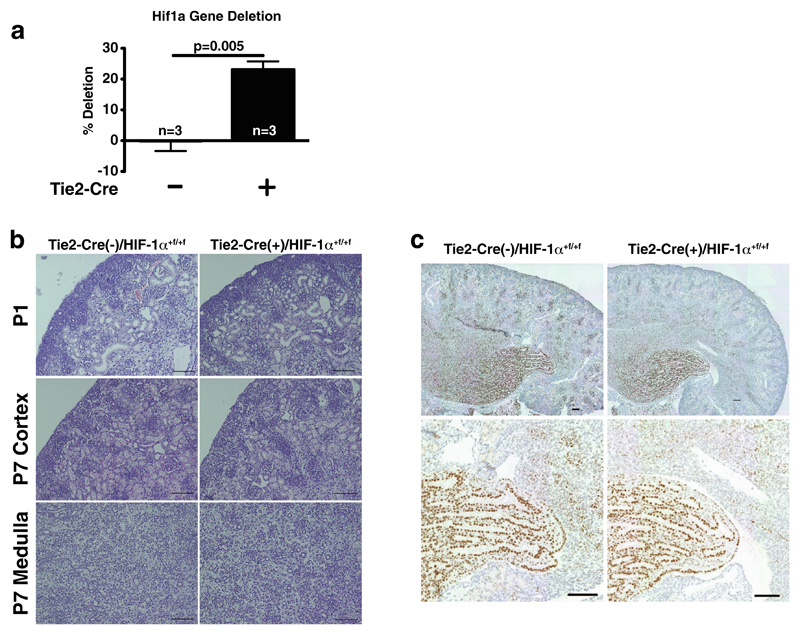
To examine whether HIF-1α in renal EC also affects survival and inflammation in vivo, we conditionally deleted HIF-1α in EC by crossing Tie2-Cre-expressing mice [24] with floxed HIF-1α animals [31]. Tie2-Cre drives the deletion of floxed alleles in EC as well as in bone marrow cells. Tie2-Cre(+)/HIF-1α+f/+f animals develop normally and are indistinguishable from their wild-type littermates, as described before [16]. Successful deletion of the Hif1a gene in the kidney was determined by quantitative PCR on whole kidney DNA samples (Fig. 3a) and also visualized with specific primers amplifying the recombined 1-lox allele (Supplemental Fig. 1c). HIFs are detectable during renal development with HIF-1α being found during glomerulogenesis [19]. Thus, we investigated renal development at days P1 and P7 post partum in wild-type and EC HIF-1α knockouts; comma- and s-shaped bodies are comparable in size and numbers on P1 (Fig. 3b, upper panel). Also on P7, cortex and medulla of kidneys look identical (Fig. 3b, lower panel). HIF-1α protein is abundant mostly in tubular cells in the medulla at P7 but not at P1 in both genotypes (Fig. 3c and data not shown). No HIF-1α staining is observed in EC at these stages as assessed by serial sections stained for the endothelial-specific marker MECA-32 (not shown). Taken together, deletion of HIF-1α in EC does not affect renal development and glomerulogenesis in mice.
Fig. 3. Deletion efficiency, renal development, and HIF-1α protein expression in Tie2-Cre/HIF-1α+f/+f mice.
a Quantification of Hif1a gene in whole kidney DNA shows 23±2.7 % deletion in 9-week-old cre(+) mice (n = 3). b Representative H&E stainings of postnatal kidneys of both genotypes (P1 and P7; ×200, scale bar 100 μm). c Representative HIF-1α staining of P7 postnatal kidneys shows prominent tubular positivity mostly in the papilla in both genotypes (×200, scale bar 100 μm)
Deletion of HIF-1α in endothelial cells does not affect vessel number and renal function in adult mice
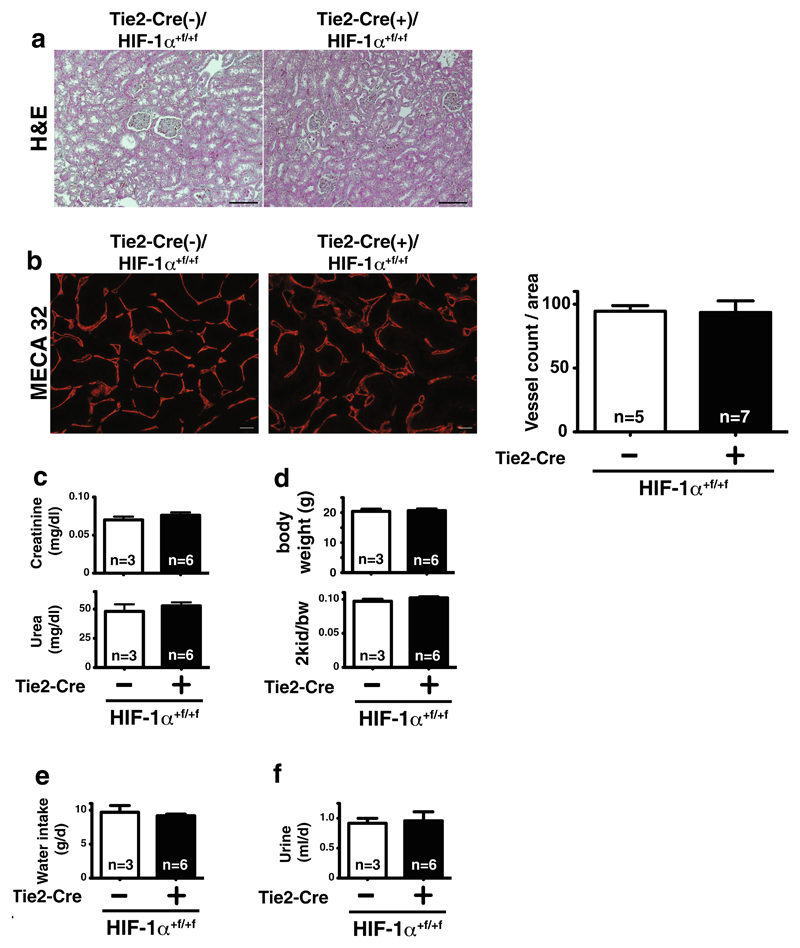
Renal morphology, glomerular size, and number are not different in adult wild-type and conditional knockout animals (Fig. 4a, upper panel). Quantification of EC staining by MECA-32 demonstrates no difference in vessel numbers (Fig. 4b). In line with the normal renal development and morphology, renal function at 12 weeks of age, as assessed by serum levels of creatinine and urea, is independent of endothelial HIF-1α (Fig. 4c). Body weight and kidney-to-body weight ratio are also unaltered (Fig. 4d), as is the absolute kidney weight (not shown). Water intake and urine production over 24 h, assessed in metabolic cages, are also not different between genotypes (Fig. 4e, f). Taken together, in vivo, EC-specific deletion of HIF-1α does not affect renal function in adults.
Fig. 4. Renal morphology and functional parameters in adult mice with deletion of HIF-1α in EC.
a H&E-stained kidney sections show no morphological difference in wild-type (Tie2-cre(−)) and endothelial HIF-1α knockout (Tie2-cre(+)) mice (×200, scale bar 100μm). b Immunofluorescent MECA32 stainings (×400) and quantification of renal sections show no differences in renal vessel density (scale bar 20 μm; n=5–7). c Renal function (plasma creatinine (upper panel) and urea (lower panel)) is not significantly different between genotypes. d Body weight (upper panel) and two kidney-to-body weight (bw) ratio (lower panel) as well as water intake (e) and urine production per 24 h (f) in metabolic cages are not affected by deletion of HIF-1α in EC in mice (for all metabolic measurements—WT, n=3; KO, n=6)
Loss of HIF-1α in endothelial cells does not influence renal function after ischemia reperfusion injury
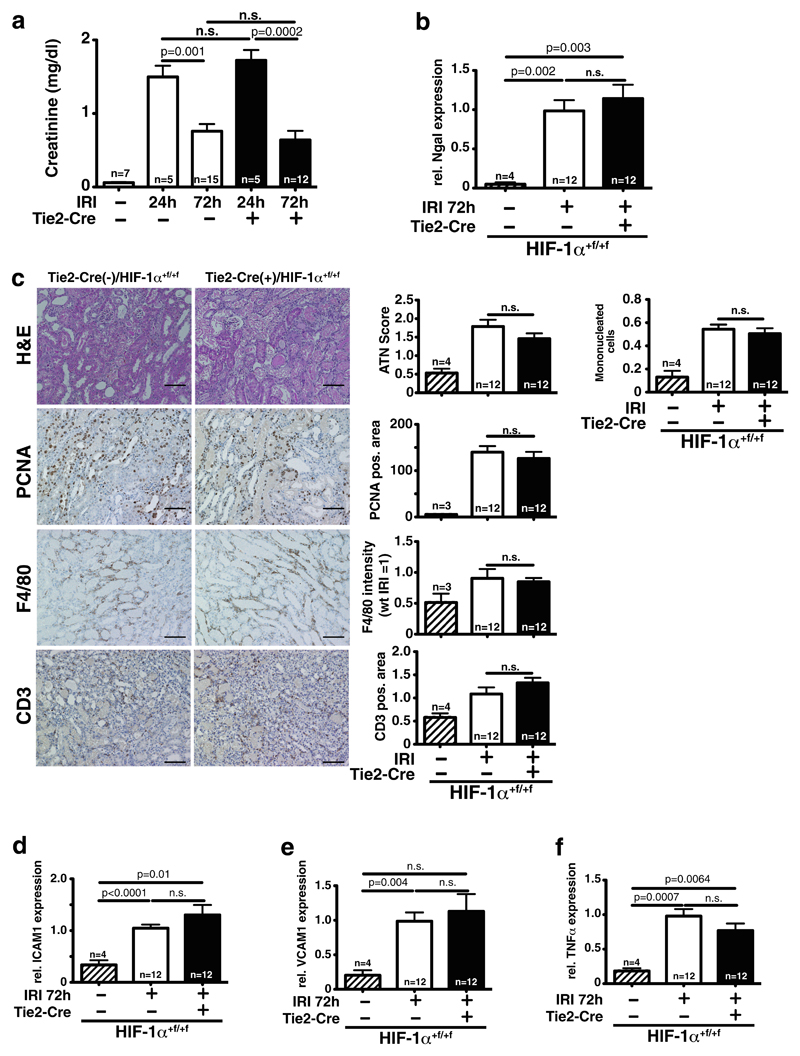
HIF-1α is detected in renal EC during sepsis (Fig. 2b); therefore, we investigated whether this model is suitable to specifically investigate renal injury. In septic wild-type mice, creatinine levels are increased compared to controls (Supplemental Fig. 1d). In contrast to the findings of Yasuda et al. [35], no specific renal damage as assessed by semiquantitative scoring of acute tubular necrosis (ATN) is observed (Supplemental Fig. 1e). However, Yasuda et al. assessed tubular vacuolar degeneration, used older mice (38 to 44 weeks), and in contrast to us administered antibiotic therapy. Sepsis affects multiple organs and renal impairment during sepsis can be caused by multiple, often secondary effects. Since we specifically wanted to investigate HIF-1α in renal EC, we utilized renal ischemia-reperfusion injury. Moreover, 24 and 72 h after ischemia and reperfusion, renal function is significantly impaired compared to sham-operated animals. Conditional knockout of HIF-1α in EC does not induce significant changes as compared to wild-type animals, neither with respect to the maximum impairment nor in the degree of recovery (Fig. 5a). Accordingly, mRNA expression of the kidney injury marker Ngal in whole kidney lysates is significantly increased independent of HIF-1α in EC 72 h after IRI (Fig. 5b). Renal sections were analyzed for tubular injury, tubular regeneration, and infiltration with cells of the innate and adaptive immunity. Blinded analyses of H&E-stained sections demonstrate that deletion of HIF-1α in EC does not affect severity of tubular damage (ATN score; Fig. 5c, upper panel) and the capacity of tubular cells to proliferate (PCNA staining, Fig. 5c). Furthermore, infiltration of F4/80-positive myeloid cells and CD3-positive T cells is not affected by loss of HIF-1α in EC (Fig. 5c, lower panels). On the mRNA level, the inflammatory cytokine TNFα and adhesion molecules ICAM1 and VCAM1 are upregulated after renal IRI, but all genes are not influenced by HIF-1α in EC, corroborating the renal functional data (Fig. 5d–f). Endothelial HIF-1α is negatively regulating TGFβ in a model of cardiac overload [16], but in renal IRI, TGFβ mRNA in whole kidney lysates is not affected by deletion of HIF-1α in EC (Supplemental Fig. 2a). Taken together, renal damage, recovery, and inflammatory reaction after acute ischemic kidney injury are not affected by HIF-1α in EC.
Fig. 5. Loss of endothelial HIF-1α does not affect kidney damage and recovery after ischemia-reperfusion injury (IRI).
a 24 and 72 h after bilateral IRI, renal function of both wild-type and conditional HIF-1α knockout mice is not significantly different (sham, n=7; IRI 24 h, n=5; IRI 72 h, n=12). b 72 h after reperfusion, mRNA of the kidney injury marker Ngal in whole kidney lysates is significantly upregulated compared to sham, but without significant differences between genotypes (sham, n=4; IRI, n=12). c Blinded quantification of tubular injury (ATN score) and infiltrating mononuclear cells on H&E sections shows no difference between wild-type and conditional HIF-1α knockouts 72 h after renal IRI (upper panel). Tubular regeneration (PCNA-positive tubular cells, below) and infiltration of myeloid cells (F4/80 positive) and T cells (CD3 positive) are also not affected by loss of HIF-1α in EC (quantitative analysis of 10 HPF/section; for all histological analyses—sham, n=4; IRI, n=12; shown are representative sections, ×200, scale bar 100 μm). d–f Accordingly, mRNA expression of the adhesion molecules ICAM1 and VCAM1 as well as the proinflammatory cytokine TNFα is significantly upregulated by renal IRI, but deletion of HIF-1α in EC does not change expression (for all mRNA analyses—sham, n=4; IRI, n=12)
Progression of renal fibrosis is independent of HIF-1α in endothelial cells
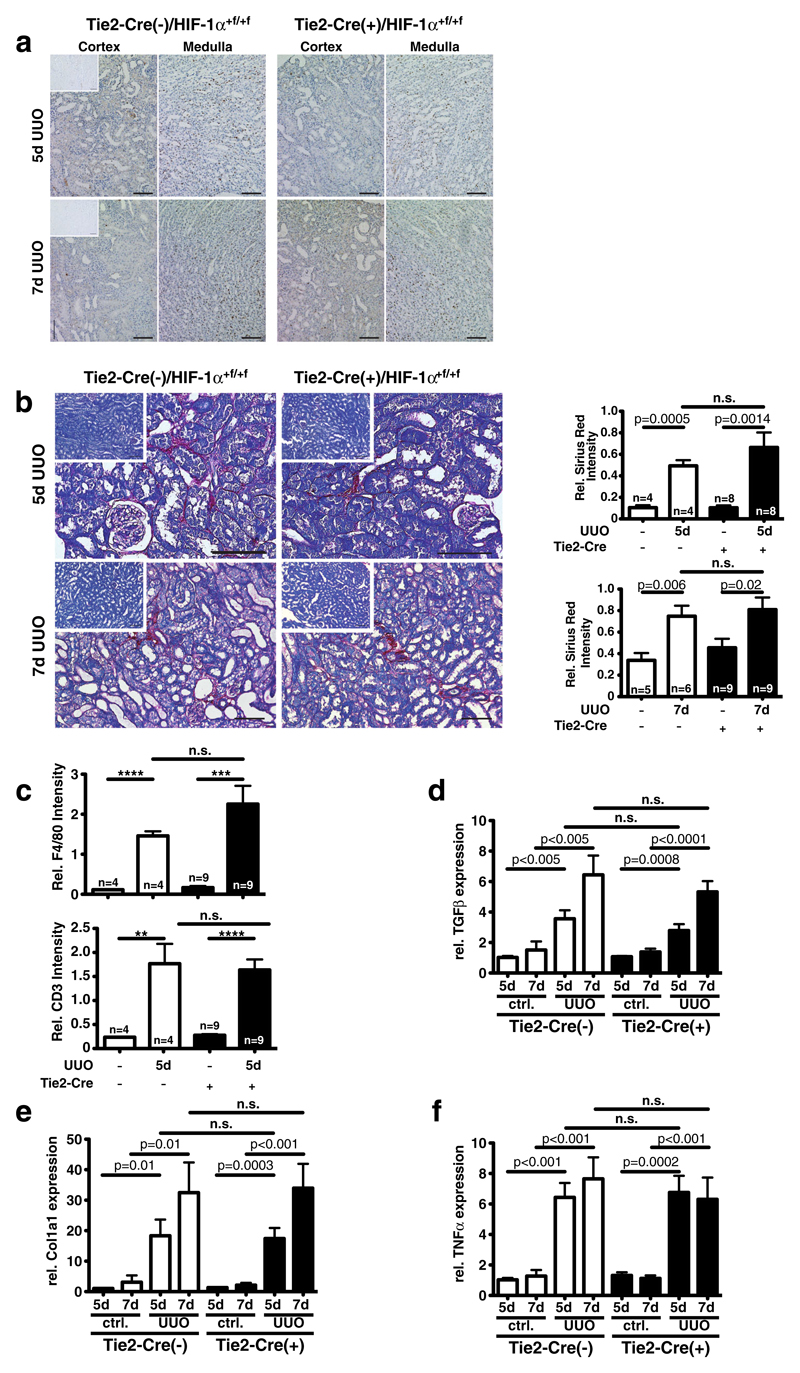
Renal fibrosis in the course of CKD results in vascular rarefaction which is associated with interstitial hypoxia [36]. We therefore investigated whether HIF-1α signaling in EC influences kidney fibrosis using the unilateral ureter obstruction (UUO) model. After 5 days of obstruction, tubular HIF-1α protein is detectable mostly in the outer medulla (Fig. 6a, upper panel). Progression of fibrosis appears to reduce HIF-1α protein after 7 days of obstruction (Fig. 6a, lower panel). Mostly tubular HIF-1α is stabilized during UUO, and thus a non-significant trend towards a reduced global HIF-1α (tubular and EC) expression is observed in EC-knockout animals (Supplemental Fig. 2b). Renal function deteriorates over time from day 5 to 7 after UUO, with no significant difference between wild-type animals and mice with conditional HIF-1α knockout in EC (Supplemental Fig. 2c). Progression of fibrosis in the obstructed kidneys as determined by Sirius Red staining is independent of HIF-1α in EC (Fig. 6b). Histologic analysis of infiltrating inflammatory cells shows an influx of F4/80-positive monocytes and CD3-positive T cells into obstructed kidneys. No differences are observed between genotypes (Fig. 6c). In line with progressive fibrosis, expression of the pro-fibrotic gene TGFβ gradually increases over time independently of HIF-1α in EC (Fig. 6d). Moreover, fibrosis-associated genes such as Col1a1 (Fig. 6e), Col1a2, and Pai1 (Supplemental Fig. 2d and e, respectively) are upregulated in the obstructed kidneys irrespective of the genotype. Seven-day obstruction significantly induces expression of α-smooth muscle actin (αSMA), independent of HIF-1α in EC (Supplemental Fig 2f). The extent of inflammation, determined by the expression of TNFα mRNA (Fig. 6f), is also not significantly influenced by HIF-1α in EC. Thus, deterioration of renal function and development of renal fibrosis in a model of obstructive kidney disease is independent of HIF-1α signaling in EC. Collectively, our results demonstrate that endothelial HIF-1α is dispensable during kidney development for normal renal function and for pathophysiological adaptation to acute and chronic renal injury.
Fig. 6. Loss of endothelial HIF-1α does not affect renal fibrosis in unilateral ureteral obstruction (UUO).
a HIF-1α protein is predominantly activated in tubular cells in the obstructed kidney. Five days after obstruction (top), more HIF-1α expression is visible than after 7 days. No difference in HIF-1α activation is visible between wild-type and endothelial deletion of HIF-1α (representative pictures; ×200, scale bar 100 μm; insets—contralateral control kidney). b Quantification of renal fibrosis by Sirius Red staining with anillin blue counterstain, representative pictures of both genotypes 5 and 7 days after UUO. Insets: representative pictures of contralateral unobstructed kidneys. Five days after UUO (upper panel), fibrotic area increases significantly in both genotypes over the unobstructed kidney without differences after deletion of HIF-1α in EC. Seven days after UUO (lower panel), fibrosis is also significantly increased compared to the unobstructed kidney, without genotype-specific differences (WT, n=4–6; KO, n=8–9; representative pictures; ×200, scale bar 100 μm; insets—contralateral control kidney). c Influx of F4/80-positive cells (upper panel) and CD3-positive T cells (lower panel) is increased after 5 days in the obstructed kidney independent of HIF-1α in EC (WT, n=4; KO, n=9). d Expression of the pro-fibrotic gene TGFβ in total kidney RNA is induced in a time-dependent fashion by UUO, but without influence of endothelial deletion of HIF-1α. e Col1a1 gene expression is also significantly induced in the obstructed kidney in a time-dependent fashion. No genotype-specific difference of expression levels is observed. f mRNA of the proinflammatory cytokine TNFα is induced by UUO independent of HIF-1α in EC (all mRNA analyses—WT, n=4–6; KO, n=9)
Discussion
During kidney injury, hypoxia occurs either acutely as a direct consequence of reduced arterial blood supply or develops chronically secondary to vascular rarefaction. As a result, hypoxia-inducible factors HIF-1α and HIF-2α, master regulators of the adaptive response to tissue hypoxia, are induced in renal parenchymal, interstitial, and endothelial cells. Although HIF activation has been shown to be protective, tissue-specific HIFα function during responses to renal injury is largely unclear. Therefore, we analyzed HIF-1α in endothelial cells (EC) in vitro and during renal injury in vivo. Our data show that HIF-1α affects hypoxic survival and leukocyte adhesion in glomerular microvascular endothelial cells in vitro. In contrast, deletion of HIF-1α in EC in mice does not affect renal development, renal vascular architecture, and renal function. Moreover, acute and chronic kidney injury is independent of HIF-1α in EC. Taken together, our data imply that endothelial HIF-1α does not play a significant role in renal development and disease, which is contrary to other organs such as the heart [15], lung [37], or skin [38] and strongly implies organ-specific regulatory mechanisms.
Previous data on expression and functional relevance of HIF-1α in EC in vivo is complex. In early reports in rats and mice, no HIF-1α expression was detected by immunohistochemistry in hypoxic renal, cerebral, hepatic, or pulmonal EC [11, 18]. In contrast, genetic studies with the same animal model as in the present work attributed significant functionality to HIF-1α in EC; HIF-1α promoted repair in the tracheal microvasculature in an orthotropic tracheal transplantation model [37, 39]. Moreover, HIF-1α in EC is required for glucose uptake in the brain [17]. Thus, even low or—by means of immunohistochemistry—undetectable expression of HIF-1α does not preclude functionality in a pathophysiological setting. In extension of the previous genetic studies [16, 17], our current data add important information on the organspecific modulation of HIF-1α function in EC: in vivo HIF-1α appears to be dispensable in renal EC, whereas it is needed for functional integrity in the lung, heart, and the brain. Of note, the reduction of metastatic success in the lung by loss of HIF-1α further corroborates this tissue-specific structural attributes [7]. Future studies are clearly warranted of how organ-specific HIF-1α responses are controlled and maintained.
Interestingly, cardiac EC can upregulate both HIFα isoforms in hypoxia and after ischemia [40]. In line with this, endothelial HIF-1α has been demonstrated to be critically important for ischemic preconditioning and also for protection from pressure overload in the heart [15, 16]. A possible explanation for this might be a dose effect; in contrast to cardiac or pulmonary EC, HIF-1α protein expression in renal EC is limited. A recent study demonstrated that molecular signatures of microvascular endothelial cells are heterogeneous and give rise to structural and functional diversity [20]. In light of our data and published literature, it seems reasonable to speculate that HIF expression is also part of those different and unique repertoires of pulmonal, cardiac, and renal EC. Analysis of the transcript abundance of Hif1a gene products, however, shows that it exhibits a rather constant level throughout different organs (D. Nolan, personal communication), which implies that regulation takes place on the protein level, potentially by expression of the HIF-prolyl hydroxylases [20].
In a very recent report, Kapitsinou and co-workers convincingly demonstrated that HIF-2α is the dominant HIFα isoform in renal EC which attenuates inflammatory cell influx by controlling VCAM1 expression [41]. While our study focused on HIF-1α and utilized a different cre-expressing mouse strain, we confirm their findings that endothelial HIF-1α lacks a significant impact on renal pathophysiologic responses. Of note, expression of the EPAS1 gene appears to be more regulated between organs than Hif1a, with higher levels in the lungs than in glomeruli ([20] and personal communication with D. Nolan). Accordingly, Kapitsinou et al. confirmed that vascular permeability after deletion of both HIFα isoforms was increased in the lung but not in the kidney [41]. Again, this argues for organ-specific modulation of endothelial HIF responses, which have to be taken into account when pharmacological manipulations of the HIF system are developed.
One weakness of our animal model is that deletion of the floxed allele is not limited to EC but also affects hematopoietic cells. Thus, we cannot rule out that an HIF-1α-dependent effect in EC is offset by simultaneous deletion in bone marrow cells. However, in light of the limited expression of HIF-1α in renal EC and the findings of Kapitsinou et al. using a different deleter strain [41], our results seem plausible. Tie2 is expressed in glomerular and peritubular EC [42], and deletion efficiency on the genomic level appears adequate to the estimated extend of the renal endothelial compartment [43]. Thus, it is unlikely that a potential phenotype is missed due to poor deletion efficiency of HIF-1α. The limited protein expression and functionality of renal endothelial HIF-1α is further supported by the mRNA levels of the classical HIF-1α target glucose transporter-1 (Glut-1). Upon pharmacological activation of HIF by PHD inhibitor administration, Glut-1 upregulation in wild-type and conditional knockout mice is not different (Supplemental Fig. 2g).
HIF-1α influences survival of microvascular glomerular endothelial cells and leukocyte adhesion in vitro; however, we do not observe a difference between EC wild-type and HIF-1α knockout in kidney development and two different renal failure models in vivo. As argued above, this might be due to the limited HIF-1α protein expression in renal EC. Thus, subtle changes might be missed by analyses involving whole kidneys. Moreover, for in vitro studies, endothelial cells were cultured in 1 % oxygen for at least 24 h, in specific experiments up to 96 h. These conditions are difficult to translate to the pathophysiological changes in vivo induced by 25 min of ischemia or ureteral obstruction of the kidney. Metabolic byproducts or cell death in vivo are different from a pure cultured cell line. Shear stress upon reperfusion is adding another injury upon reoxygenation, which is not accounted for in cell culture models. Moreover, metabolic adaptations such as the shift towards anaerobic glycolysis can differ considerably in cultured cells. Taken together, although in vitro experiments apparently do not predict the renal phenotype of HIF-1α deletion in EC in vivo, they still add valuable mechanistic information which need to be tested eventually in refined models. In organs with more pronounced HIF-1α expression in EC such as the lungs or tumors, mechanistic analyses in vitro proved more valuable in determining the molecular basis of the observed phenotypes [7, 31].
Taken together, our study provides a comprehensive analysis of endothelial HIF-1α in vitro and during kidney development and injury in vivo. In contrast to the heart and the lung, endothelial HIF-1α exhibits a limited expression in renal EC and its deletion does not affect tissue damage, inflammatory responses, and fibrosis development. This implies that within the same cell type, functionality of HIF-1α is modulated in an organ-specific fashion. In light of emerging therapeutical means to influence HIFα signaling, our findings add an important new facet to understand the complexity and the isoform specificity of the hypoxic response in the kidney.
Supplementary Material
Electronic supplementary material The online version of this article (doi:10.1007/s00109-015-1264-4) contains supplementary material, which is available to authorized users.
Key message.
HIF-1α controls hypoxic survival and adhesion on endothelial cells (EC) in vitro.
In vivo, HIF-1α expression in renal EC is low.
Deletion of HIF-1α in EC does not affect kidney development and function in mice.
Renal function after acute and chronic kidney injury is independent of HIF-1α in EC.
Data suggest organ-specific regulation of HIF-1α function in EC.
Acknowledgments
AW is supported by the Deutsche Forschungsgemeinschaft (WE4275) and the Dr. Robert Pfleger-Stiftung. JK, GS, and AW are supported by the ELAN-Fond of the University of Erlangen-Nürnberg. We thank Dr. N. Takeda (Tokyo, Japan), Dr. M. Waldner, and Dr. J. Jantsch for helpful discussions and Prof. M. Goppelt-Struebe (Erlangen, Germany) for providing glEND.2 cells.
Footnotes
Author contribution JK and GS performed experiments and wrote the manuscript; AG and BK performed experiments and evaluated data; SO and JB performed experiments; KA and JV analyzed kidney histologies; RSJ, CW, and KUE provided support, generated mice, evaluated data, and wrote the manuscript; AW designed the study, performed experiments, evaluated results, and wrote the manuscript.
Disclosure The authors have nothing to disclose.
Contributor Information
Jasmin Baumgartl, Department of Nephrology and Hypertension, Friedrich-Alexander-University Erlangen-Nürnberg (FAU), Ulmenweg 18, 91054 Erlangen, Germany.
Kerstin Amann, Department of Nephropathology, Friedrich-Alexander-University Erlangen-Nürnberg (FAU), Erlangen, Germany.
Carsten Willam, Department of Nephrology and Hypertension, Friedrich-Alexander-University Erlangen-Nürnberg (FAU), Ulmenweg 18, 91054 Erlangen, Germany.
Randall S. Johnson, Department of Physiology, Development and Neuroscience, University of Cambridge, CB2 3EG Cambridge, UK
References
- 1.U.S. Renal Data System. USRDS 2013 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2013. [Google Scholar]
- 2.Schurek HJ, Jost U, Baumgartl H, Bertram H, Heckmann U. Evidence for a preglomerular oxygen diffusion shunt in rat renal cortex. Am J Physiol. 1990;259:F910–F915. doi: 10.1152/ajprenal.1990.259.6.F910. [DOI] [PubMed] [Google Scholar]
- 3.Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int. 2008;74:867–872. doi: 10.1038/ki.2008.350. [DOI] [PubMed] [Google Scholar]
- 4.Goligorsky MS, Brodsky SV, Noiri E. Nitric oxide in acute renal failure: NOS versus NOS. Kidney Int. 2002;61:855–861. doi: 10.1046/j.1523-1755.2002.00233.x. [DOI] [PubMed] [Google Scholar]
- 5.Sun D, Wang Y, Liu C, Zhou X, Li X, Xiao A. Effects of nitric oxide on renal interstitial fibrosis in rats with unilateral ureteral obstruction. Life Sci. 2012;90:900–909. doi: 10.1016/j.lfs.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 6.Nakayama T, Sato W, Kosugi T, Zhang L, Campbell-Thompson M, Yoshimura A, Croker BP, Johnson RJ, Nakagawa T. Endothelial injury due to eNOS deficiency accelerates the progression of chronic renal disease in the mouse. Am J Physiol Renal Physiol. 2009;296:F317–F327. doi: 10.1152/ajprenal.90450.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Branco-Price C, Zhang N, Schnelle M, Evans C, Katschinski DM, Liao D, Ellies L, Johnson RS. Endothelial cell HIF-1alpha and HIF-2alpha differentially regulate metastatic success. Cancer Cell. 2012;21:52–65. doi: 10.1016/j.ccr.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ. 2008;15:621–627. doi: 10.1038/cdd.2008.12. [DOI] [PubMed] [Google Scholar]
- 9.Warnecke C, Weidemann A, Volke M, Schietke R, Wu X, Knaup KX, Hackenbeck T, Bernhardt W, Willam C, Eckardt KU, et al. The specific contribution of hypoxia-inducible factor-2alpha to hypoxic gene expression in vitro is limited and modulated by cell type-specific and exogenous factors. Exp Cell Res. 2008;314:2016–2027. doi: 10.1016/j.yexcr.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberger C, Mandriota S, Jurgensen JS, Wiesener MS, Horstrup JH, Frei U, Ratcliffe PJ, Maxwell PH, Bachmann S, Eckardt KU. Expression of hypoxia-inducible factor-1alpha and -2alpha in hypoxic and ischemic rat kidneys. J Am Soc Nephrol. 2002;13:1721–1732. doi: 10.1097/01.asn.0000017223.49823.2a. [DOI] [PubMed] [Google Scholar]
- 12.Skuli N, Liu L, Runge A, Wang T, Yuan L, Patel S, Iruela-Arispe L, Simon MC, Keith B. Endothelial deletion of hypoxiainducible factor-2alpha (HIF-2alpha) alters vascular function and tumor angiogenesis. Blood. 2009;114:469–477. doi: 10.1182/blood-2008-12-193581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. Embo J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarkar K, Cai Z, Gupta R, Parajuli N, Fox-Talbot K, Darshan MS, Gonzalez FJ, Semenza GL. Hypoxia-inducible factor 1 transcriptional activity in endothelial cells is required for acute phase cardioprotection induced by ischemic preconditioning. Proc Natl Acad Sci U S A. 2012;109:10504–10509. doi: 10.1073/pnas.1208314109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei H, Bedja D, Koitabashi N, Xing D, Chen J, Fox-Talbot K, Rouf R, Chen S, Steenbergen C, Harmon JW, et al. Endothelial expression of hypoxia-inducible factor 1 protects the murine heart and aorta from pressure overload by suppression of TGF-beta signaling. Proc Natl Acad Sci U S A. 2012;109:E841–E850. doi: 10.1073/pnas.1202081109. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Huang Y, Lei L, Liu D, Jovin I, Russell R, Johnson RS, Di Lorenzo A, Giordano FJ. Normal glucose uptake in the brain and heart requires an endothelial cell-specific HIF-1alpha-dependent function. Proc Natl Acad Sci U S A. 2012;109:17478–17483. doi: 10.1073/pnas.1209281109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stroka DM, Burkhardt T, Desbaillets I, Wenger RH, Neil DA, Bauer C, Gassmann M, Candinas D. HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J. 2001;15:2445–2453. doi: 10.1096/fj.01-0125com. [DOI] [PubMed] [Google Scholar]
- 19.Bernhardt WM, Schmitt R, Rosenberger C, Munchenhagen PM, Grone HJ, Frei U, Warnecke C, Bachmann S, Wiesener MS, Willam C, et al. Expression of hypoxia-inducible transcription factors in developing human and rat kidneys. Kidney Int. 2006;69:114–122. doi: 10.1038/sj.ki.5000062. [DOI] [PubMed] [Google Scholar]
- 20.Nolan DJ, Ginsberg M, Israely E, Palikuqi B, Poulos MG, James D, Ding BS, Schachterle W, Liu Y, Rosenwaks Z, et al. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell. 2013;26:204–219. doi: 10.1016/j.devcel.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weidemann A, Breyer J, Rehm M, Eckardt KU, Daniel C, Cicha I, Giehl K, Goppelt-Struebe M. HIF-1alpha activation results in actin cytoskeleton reorganization and modulation of Rac-1 signaling in endothelial cells. Cell Commun Signal. 2013;11:80. doi: 10.1186/1478-811X-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeda N, O’Dea EL, Doedens A, Kim JW, Weidemann A, Stockmann C, Asagiri M, Simon MC, Hoffmann A, Johnson RS. Differential activation and antagonistic function of HIF-{alpha} isoforms in macrophages are essential for NO homeostasis. Genes Dev. 2010;24:491–501. doi: 10.1101/gad.1881410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hackenbeck T, Knaup KX, Schietke R, Schodel J, Willam C, Wu X, Warnecke C, Eckardt KU, Wiesener MS. HIF-1 or HIF-2 induction is sufficient to achieve cell cycle arrest in NIH3T3 mouse fibroblasts independent from hypoxia. Cell Cycle. 2009;8:1386–1395. doi: 10.4161/cc.8.9.8306. [DOI] [PubMed] [Google Scholar]
- 24.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 25.Schley G, Klanke B, Schodel J, Kroning S, Turkoglu G, Beyer A, Hagos Y, Amann K, Burckhardt BC, Burzlaff N, et al. Selective stabilization of HIF-1alpha in renal tubular cells by 2-oxoglutarate analogues. Am J Pathol. 2012;181:1595–1606. doi: 10.1016/j.ajpath.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weidemann A, Kerdiles YM, Knaup KX, Rafie CA, Boutin AT, Stockmann C, Takeda N, Scadeng M, Shih AY, Haase VH, et al. The glial cell response is an essential component of hypoxia-induced erythropoiesis in mice. J Clin Invest. 2009;119:3373–3383. doi: 10.1172/JCI39378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiesener MS, Jurgensen JS, Rosenberger C, Scholze CK, Horstrup JH, Warnecke C, Mandriota S, Bechmann I, Frei UA, Pugh CW, et al. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J. 2003;17:271–273. doi: 10.1096/fj.02-0445fje. [DOI] [PubMed] [Google Scholar]
- 29.Daniel C, Amann K, Hohenstein B, Bornstein P, Hugo C. Thrombospondin 2 functions as an endogenous regulator of angiogenesis and inflammation in experimental glomerulonephritis in mice. J Am Soc Nephrol. 2007;18:788–798. doi: 10.1681/ASN.2006080873. [DOI] [PubMed] [Google Scholar]
- 30.Samarin J, Wessel J, Cicha I, Kroening S, Warnecke C, Goppelt-Struebe M. FoxO proteins mediate hypoxic induction of connective tissue growth factor in endothelial cells. J Biol Chem. 2010;285:4328–4336. doi: 10.1074/jbc.M109.049650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang N, Wang L, Esko J, Giordano FJ, Huang Y, Gerber HP, Ferrara N, Johnson RS. Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell. 2004;6:485–495. doi: 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 32.Uchida T, Rossignol F, Matthay MA, Mounier R, Couette S, Clottes E, Clerici C. Prolonged hypoxia differentially regulates hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression in lung epithelial cells: implication of natural antisense HIF-1alpha. J Biol Chem. 2004;279:14871–14878. doi: 10.1074/jbc.M400461200. [DOI] [PubMed] [Google Scholar]
- 33.Goda N, Ryan HE, Khadivi B, McNulty W, Rickert RC, Johnson RS. Hypoxia-inducible factor 1alpha is essential for cell cycle arrest during hypoxia. Mol Cell Biol. 2003;23:359–369. doi: 10.1128/MCB.23.1.359-369.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willam C, Schindler R, Frei U, Eckardt KU. Increases in oxygen tension stimulate expression of ICAM-1 and VCAM-1 on human endothelial cells. Am J Physiol. 1999;276:H2044–H2052. doi: 10.1152/ajpheart.1999.276.6.H2044. [DOI] [PubMed] [Google Scholar]
- 35.Yasuda H, Yuen PS, Hu X, Zhou H, Star RA. Simvastatin improves sepsis-induced mortality and acute kidney injury via renal vascular effects. Kidney Int. 2006;69:1535–1542. doi: 10.1038/sj.ki.5000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mimura I, Nangaku M. The suffocating kidney: tubulointerstitial hypoxia in end-stage renal disease. Nat Rev Nephrol. 2010;6:667–678. doi: 10.1038/nrneph.2010.124. [DOI] [PubMed] [Google Scholar]
- 37.Jiang X, Hsu JL, Tian W, Yuan K, Olcholski M, Perez Vde J, Semenza GL, Nicolls MR. Tie2-dependent VHL knockdown promotes airway microvascular regeneration and attenuates invasive growth of Aspergillus fumigatus. J Mol Med (Berl) 2013;91:1081–1093. doi: 10.1007/s00109-013-1063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarkar K, Rey S, Zhang X, Sebastian R, Marti GP, Fox-Talbot K, Cardona AV, Du J, Tan YS, Liu L, et al. Tie2-dependent knock-out of HIF-1 impairs burn wound vascularization and homing of bone marrow-derived angiogenic cells. Cardiovasc Res. 2012;93:162–169. doi: 10.1093/cvr/cvr282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang X, Khan MA, Tian W, Beilke J, Natarajan R, Kosek J, Yoder MC, Semenza GL, Nicolls MR. Adenovirus-mediated HIF-1alpha gene transfer promotes repair of mouse airway allograft microvasculature and attenuates chronic rejection. J Clin Invest. 2011;121:2336–2349. doi: 10.1172/JCI46192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jurgensen JS, Rosenberger C, Wiesener MS, Warnecke C, Horstrup JH, Grafe M, Philipp S, Griethe W, Maxwell PH, Frei U, et al. Persistent induction of HIF-1alpha and -2alpha in cardiomyocytes and stromal cells of ischemic myocardium. FASEB J. 2004;18:1415–1417. doi: 10.1096/fj.04-1605fje. [DOI] [PubMed] [Google Scholar]
- 41.Kapitsinou PP, Sano H, Michael M, Kobayashi H, Davidoff O, Bian A, Yao B, Zhang MZ, Harris RC, Duffy KJ, et al. Endothelial HIF-2 mediates protection and recovery from ischemic kidney injury. J Clin Invest. 2014 doi: 10.1172/JCI69073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woolf AS, Yuan HT. Angiopoietin growth factors and Tie receptor tyrosine kinases in renal vascular development. Pediatr Nephrol. 2001;16:177–184. doi: 10.1007/s004670000509. [DOI] [PubMed] [Google Scholar]
- 43.Bentley MD, Ortiz MC, Ritman EL, Romero JC. The use of microcomputed tomography to study microvasculature in small rodents. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1267–R1279. doi: 10.1152/ajpregu.00560.2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.