Abstract
The liver plays a central role in glucose homeostasis in the whole-body by responding to environmental factors including nutrients, hormones, and oxygen. In conditions of metabolic overload such as diabetes mellitus and obesity, coordinated regulation between oxygen supply and consumption has been reported to be disrupted and subsequently cause tissue hypoxia, although pathological significance of the disease-related hypoxia remains elusive. To investigate the role of tissue hypoxia in the liver on systemic glucose homeostasis, mice lacking HIF-1α gene, a critical component of a master regulator of hypoxic response, in hepatocytes were exposed to high fat/sucrose diet (HFSD). Exposure to HFSD for 5 weeks elicited liver hypoxia with a transient increase in HIF-1α protein expression in the liver of control mice. Glucose disposal was marginally impaired in control mice when challenged oral glucose tolerance test, but such impairment was enhanced in the mutant mice. This alteration was accompanied by a complete inhibition of glucokinase induction with a significant reduction of hepatic glucose uptake. Mice fed HFSD for 20 weeks exhibited fasting hyperglycemia and glucose intolerance, whereas these metabolic phenotypes deteriorated considerably with severe insulin resistance in skeletal muscles and adipose tissues in the mutant mice. These findings suggest that HIF-1 in hepatocytes plays protective roles against the progression of diabetes mellitus.
Keywords: HIF-1, Hypoxia Glucokinase, Carbohydrate metabolism, Liver, Diabetes
1. Introduction
The liver plays central roles in the regulation of whole-body glucose homeostasis, since it changes the metabolic flow to produce or consume glucose. In fasting state, liver provides 90% of glucose to other tissues by activating gluconeogenesis, whereas it uptakes a large amounts of blood glucose to metabolize in TCA cycles completely or to store as glycogen after meals. In diabetic conditions, liver becomes insulin insensitive to stimulate aberrant glucose production concomitantly with decreased glycolytic activities, resulting in sustained hyperglycemia [1]. The drastic metabolic alterations occur under the tight and complicated control of multiple transcription factors and coregulators, which sense and respond to changes in its surroundings of the liver such as nutrients, hormones, and oxygen [2,3].
HIF-1 is a key transcription factor to adapt to hypoxic conditions, and activates many genes involved in angiogenesis, glucose metabolism, cellular growth, and apoptosis in response to hypoxia [4]. HIF-1 consists of an oxygen-sensitive α subunit and a constitutively expressed β subunit. Under normoxic conditions, HIF-1α is hydroxylated at specific proline residues in an oxygen-dependent manner, by which VHL binds to and targets HIF-1α for proteasomal degradation [5]. Upon hypoxia, HIF-1α escapes from the oxygen-mediated degradation and is subsequently stabilized to form a dimer with HIF-1β in nucleus, resulting in transcriptional activation of its target genes. In the liver, HIF-1α is constitutively expressed in pericentral hepatocytes [6], and its expression has been believed to be prerequisite for zone-specific expressions of carbohydrate metabolic enzymes to maintain blood glucose levels in a narrow range [7], since expressions of most glycolytic enzymes including PGK-1 and GK are induced by hypoxia exclusively in a HIF-1-dependent manner [8]. HIF-1β expression has been reported to be markedly reduced in liver of diabetic patients, and mice lacking hepatic HIF-1β exhibits increased gluconeogenesis with mild insulin resistance [9]. Constitutive activation of HIFαs by deleting VHL gene causes hypoglycemia due to decreased hepatic glucose production [10], although contribution of HIF-1 to these metabolic alterations remains elusive. In additions, we have previously reported that HIF-1 is prerequisite for gluconeogenesis in regenerated liver [11]. Although these reports strongly suggest importance of hepatic HIF-1 activity in the regulation of glucose homeostasis, not much has been done to clarify effects of loss of hepatic HIF-1α on the development and progression of diabetes in vivo.
To elucidate roles of HIF-1α in the control of glucose metabolism in the liver and whole-body glucose homeostasis directly, we have subjected mice lacking HIF-1α gene in the liver to HFSD to produce diabetic conditions in vivo. The mutant mice revealed no apparent alterations in glucose homeostasis compared to control mice on normal diet. Upon exposure to HFSD for 5 weeks, HIF-1α-deficient mice exhibited modest, but significant decreased hepatic glucose disposal with reduced expressions of GK. These metabolic alterations were further aggravated to promote insulin resistance in skeletal muscles and adipose tissues by 20 weeks-HFS challenge in the mutant mice. Our present results suggest that HIF-1α in the liver plays a protective role against the progression of diabetes, and may provide new therapeutic intervention.
2. Material and method
2.1. Animals and treatments
All experiments were conducted in accordance with Keio University Animal Welfare Guidelines. HIF-1αloxP/loxP mice (control) were bred with Alb-Cre mice to generate mice with hepatocyte-specific deletion of the HIF-1α gene (H-HIFKO), as described previously [11,12]. Five weeks old male mice were fed either a control normal diet (CE-2) (CLEA Japan Inc, Tokyo) or a HFSD (D12327; 20% protein, 40% carbohydrate, and 40% fat) (Research Diets Inc., New Brunswick, NJ).
2.2. Oral glucose tolerance test and insulin tolerance test
OGTT and ITT were performed on mice fasted for 16 h. Blood glucose levels of whole venous blood from mice were measured using an automatic glucose monitor (Accu-Chek, Roche Diagnostics). Serum insulin levels were determined by enzyme-linked immunosorbent assay (Rat/Mouse insulin ELISA kit, Millipore, Tokyo). For OGTT, mice were given a single dose (2 mg/g body weight) of d-glucose orally. For ITT, mice were given a single dose (0.75 IU/g body weight) of human regular insulin (Novo Nordisk, Tokyo) intraperitoneally.
2.3. Determination of hepatic glucose uptake
Mice fasted for 16 h were given a single dose (2 mg/g body weight) of DG (Wako Pure Chemical Industries, Tokyo) orally. Livers were removed quickly at 15 min after DG administration, and contents of 2-deoxy-glucose 6-phosphate (DG6P) in the liver were determined using contrast-enhanced time of flight/mass spectrometry, as described previously [11].
2.4. Histology
Immunohistochemical analysis for HIF-1α and insulin was performed, as described previously. Area of β cells stained with antiporcine insulin antibody (Dako Japan, Tokyo) was quantified from digital microscopic images with ImageJ. To detect tissue hypoxia, Hypoxyprobe-1 (60 mg/kg; Chemicon International, Temecula, CA) was injected into mice intraperitoneally at 1 h prior to sacrifice. Frozen tissues were stained with a monoclonal antibody specific for protein adducts of reductively-activated pimonidazole.
2.5. Western blotting
Immunoblotting analyses were carried out using 50–100 μg of either liver nuclear extract or whole liver extract, as described previously. Expressions of GK, Akt, phosphor-Akt, and β-actin were detected using specific antibodies against respective antigens, as follows: GK (Santa Cruz Biotechnology, Santa Cruz, CA), Akt and phosphor-Akt (Ser473) (Cell Signaling Technology, Beverly, MA), and β-actin (Sigma, St. Louis, MO).
2.6. RNA isolation and quantitative PCR
Reverse transcription of extracted liver total RNA to cDNA was performed using a SuperScript III First Strand Synthesis Kit (Invitrogen, Tokyo, Japan). Quantitative PCR was carried out in a 7300 Real-Time PCR system (Applied Biosystems Japan, Tokyo, Japan). TaqMan gene expression assays (Applied Biosystems) and sequences of primers and probe are listed in Supplementary Table 1. Genes are normalized to 36B4 as an internal control.
2.7. Statistical analyses
Statistical analyses of differences among groups were carried out by Mann–Whitney’s U-test or Student’s t-test. P values less than 0.05 was considered significant.
3. Results
3.1. HFSD induces a transient HIF-1α expression in liver
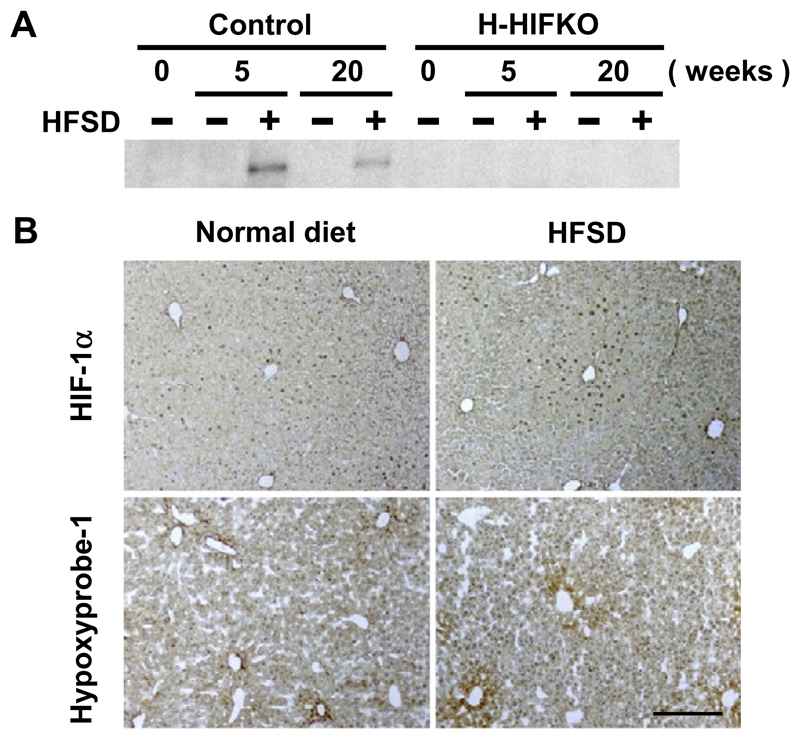
To examine whether exposure of mice to HFSD could induce HIF-1α expression in the liver, western blot analysis was performed. HIF1-α expression increased to a great extent by HFSD for 5 weeks, but the induction was substantially attenuated in mice fed HFSD for 20 weeks (Fig. 1A). Immunohistochemical analysis revealed that HIF-1α was detected in nuclei of hepatocytes around central venules of HFSD-treated liver, and the numbers of HIF-1α-positive cells greatly increased when compared to those in liver from mice fed normal chow (Fig. 1B). We next examined whether such induction of HIF-1α by HFSD is associated with liver hypoxia by injecting Hypoxyprobe-1. Consistent with other reports [13], adducts associated with the probe were limited to relatively small numbers of cells in liver of mice fed normal chow. HFSD challenge for 5 weeks markedly increased the numbers and signal intensity of Hypoxyprobe-1-positive hepatocytes around hepatic central venules (Fig. 1B). In H-HIFKO mice, expression of HIF-1α protein was hardly detectable even after exposure to HFSD (Fig. 1A), suggesting that hepatocytes are responsible for the diet-evoked induction of the protein.
Fig. 1. Exposure to HFSD induces HIF-1α expression transiently in mouse liver.
(A) Expression of HIF-1α protein in livers of control and H-HIFKO mice exposed to HFSD. (B) Representative images of control liver sections treated with either HFSD (right) or control (left) diets for 5 weeks stained with anti-HIF-1α (upper) and pimonidazol (lower) antibody, respectively. Scale bar: 500 μm.
3.2. Loss of HIF-1α in liver modestly impairs glucose disposal in response to HFSD
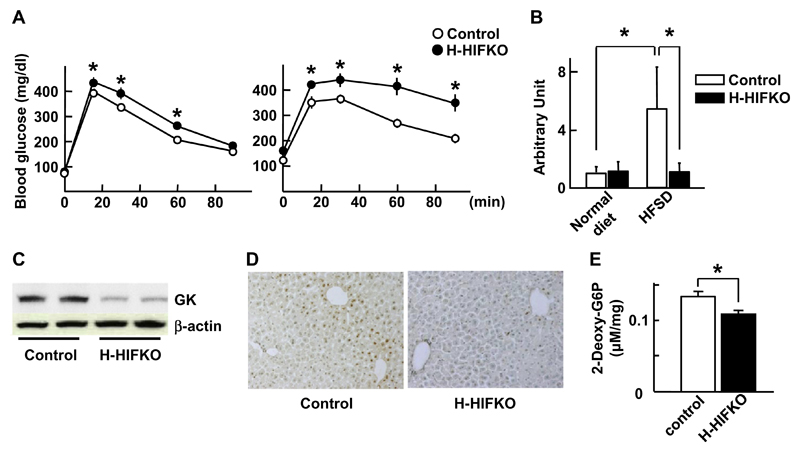
To explore the effect of HIF-1α induction on the whole-body glucose utilization in HFSD-treated mice, OGTT was performed. OGTT in H-HIFKO mice fed HFSD for 5 weeks revealed modest, but significant increase in glucose levels at 15, 30, and 60 min time points compared to those in control mice (left, Fig. 2A). However, sustained higher serum insulin levels were observed after 60 min of OGTT in H-HIFKO mice (upper left, Supplementary Fig. 1A). Consistent with these findings, phosphorylated form of Akt in liver of H-HIFKO mice was much greater at 90 min after glucose challenge (Supplementary Fig. 1B).
Fig. 2. Deletion of HIF-1α gene evokes glucose intolerance in mice treated with HFSD.
(A) Oral glucose tolerance test. Mice were administered orally glucose at a dose of 2 mg/g body weight after being treated with HFSD for either 5 (left) or 20 (right) weeks. Values are mean ± SEM. for at least 6 different experiments. *P < 0.05 compared to control mice. (B) Quantitative PCR analysis of GK in liver of 5 weeks-HFSD-treated mice. Values are expressed as mean ± SEM. *P < 0.05 compared to control mice for at least 6 different experiments. (C) Western blot analysis of GK in liver from mice treated with HFSD for 5 weeks. (D) Histochemical analysis of hepatic GK in control and H-HIFKO mice exposed to 5 weeks-HFSD. (E) Uptake of 2-deoxy-glucose by liver treated with HFSD for 5 weeks. Values are mean ± SEM. for at least 6 different experiments. P < 0.05 compared to control mice.
Next, we attempted to disclose molecular mechanism involved in impaired glucose disposal in H-HIFKO mice fed HFSD. In control mice exposed to HFSD for 5 weeks, relative amounts of GK, PGK and PK were elevated to varied degree, while those of PEPCK were greatly reduced (Fig. 2B and Supplementary Fig. 1C). The diet-evoked gene induction of GK was completely abolished in H-HIFKO mice (Fig. 2B), but loss of HIF-1α did not influence the HFSD-induced alterations in mRNA levels of other genes (Supplementary Fig. 1C). Consistent with these findings, western blot analysis of GK revealed that HFSD-fed H-HIFKO mice showed a drastic decrease in the protein levels compared to control mice (Fig. 2C). The attenuated expression of GK was further confirmed by immunohistochemical analysis in liver of HFSD-fed HIFKO mice (Fig. 2D). Since expression of GK was attenuated in liver of H-HIFKO mice fed HFSD, we speculated that the impaired glucose disposal upon acute glucose administration would be caused by reduced uptake of blood glucose by liver. As expected, influx of DG into liver assessed by measuring amounts of hepatic DG6P was significantly attenuated in H-HIFKO mice fed HFSD compared to that in control mice (Fig. 2E). Collectively, these findings suggest that reduction in hepatic glucose uptake causes impaired glucose disposal during OGTT in H-HIFKO mice fed HFSD.
3.3. Long-term HFSD feeding aggravates insulin resistance in H-HIFKO mice
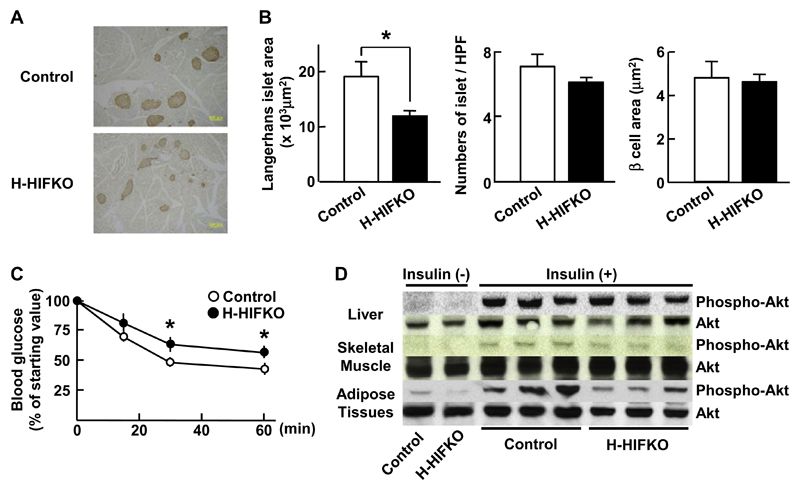
To examine impact of HIF-1α deficiency on glucose homeostasis in H-HIFKO mice fed HFSD for 20 weeks, we analyzed concentrations of blood glucose and insulin in the mutant mice. Long-term HFSD feeding raised fasted levels of blood glucose greatly in control and H-HIFKO mice relative to those in mice fed HFSD for 5 weeks, but the increase was comparable between the two groups (Supplementary Table 2). Fed glucose levels were much in H-HIFKO mice (Supplementary Table 2). Although both fasted and fed insulin levels increased several folds by long-term HFSD feeding in both mice, differences in the insulin levels between the two groups were not evident (Supplementary Table 2). Acute glucose administration raised blood glucose levels significantly at any time point tested in H-HIFKO mice compared to those in control mice (right, Fig. 2A). Although total area of pancreatic Langerhans islets in H-HIFKO mice was significantly smaller than that of control mice (Fig. 3A and B), H-HIFKO mice still have potential to secrete insulin comparable to control mice (right, Supplementary Fig. 1A). However, insulin resistance as assessed by ITT was greatly elevated in H-HIFKO mice but not in control mice by long-term HFSD (Fig. 3C). These observations are in good agreement with findings that Akt was less phosphorylated in both fat tissue and skeletal muscle of H-HIFKO mice in response to insulin injection compared to that in control mice (Fig. 3D). These findings suggested that loss of hepatic HIF-1α promote peripheral insulin resistance and β-cell dysfunction under long-term HFSD conditions.
Fig. 3. Inactivation of HIF-1 aggravates insulin resistance in response to long term exposure to HFSD.
(A) Representative images of β cells in pancreas exposed to HFSD. Pancreatic β cells (brown color) were detected by staining with antibody against insulin. (B) Histochemical analysis of area size and numbers of Langerhans islets and area size of pancreatic β cells in mice treated with HFSD. Values are mean ± SEM. for at least 6 different experiments. *P < 0.05 compared to control mice. (C) Insulin tolerance test. Insulin (0.75 IU/kg) was injected to mice fasted for 16 h, and blood glucose levels were measured. Values are mean ± SEM for at least 6 different experiments.*P < 0.05 compared to control mice. (D) Western blot analysis of phosphorylated Akt in liver, skeletal muscle, and adipose tissues of mice fed HFSD. Organs were harvested at 15 min after insulin (0.75 IU/kg) injection, and were subjected to Western blot analysis.
4. Discussion
In this study, we investigated whether inactivation of HIF-1α in the liver alters whole-body glucose homeostasis in vivo. Although mice lacking HIF-1α gene in hepatocytes did not show any alterations in glucose homeostasis under normal diet conditions, short-term treatment with HFSD evoked mild glucose intolerance due to impaired GK induction and subsequent decreased glucose disposal in the liver. When exposed to the diet for 20 weeks, the mutant mice have revealed features of type 2 diabetes, such as fasting hyperglycemia, severe glucose intolerance, and insulin resistance in adipose tissues and skeletal muscles. Our present findings provide genetic evidence for the first times that HIF-1α in hepatocytes plays a protective role against the progression of type 2 diabetes.
We have found that HIF-1α expression increases in liver of mice fed HFSD. Exposure of mice to high fat diet has been reported to induce ‘‘hypermetabolic state’’ in liver, in which continuous flux of free fatty acids stimulates oxygen consumption in mitochondriarich periportal hepatocytes, limiting supply of oxygen to down-stream pericentral hepatocytes [14]. Consistent with this idea, we observed that HIF-1α expression was limited to pericentral hepatocytes of liver treated with HFSD, where pimonidazole adducts, a marker of tissue hypoxia, were exclusively accumulated. Besides hypoxia, increase in insulin levels is attributable to HFSD-elicited HIF-1α expression, considering the fact that insulin can activate Akt-GSK-3β signaling by which inhibits phosphorylation and subsequent degradation of HIF-1α [15]. On the other hand, our present findings clearly displayed a drastic reduction of HIF-1α protein by exposure to HFSD for 20 weeks. This is in a good agreement with a report by Chavez et al. showing a transient increase in HIF-1α in nerve tissues from streptozotocin-evoked diabetic rats [16]. Islet HIF-1α expression has been recently reported to decrease in both human and mice of type 2 diabetes [17]. In addition, relative high levels of glucose themselves suppress HIF-1α induction even under hypoxia in vitro [18], suggesting an importance of sustained hyperglycemia in the regulation of HIF-1α expression. Consistent with this hypothesis, we observed a substantial increase in blood glucose levels of mice exposed to HFSD for 20 weeks. Thus, HIF-1α expression in the liver appears to be regulated in complicated manners as diabetes progress.
Loss of HIF-1α gene in the liver aggravates glucose intolerance in response to HFSD. However, effects of hepatic HIF-1α deficiency on whole-body glucose homeostasis appear to vary depending on exposure period of HFSD; in the early stage of the treatment, complete inhibition of GK induction reduces blood glucose uptakes of the liver, resulting in sustained higher blood glucose levels compared to those in control mice. On the other hand, long-term exposure to HFSD causes insulin resistance in skeletal muscles and adipose tissues, but appears not to show significant effects on hepatic insulin signaling and β cell functions. Given that GK catalyzes over 75% of glucose taken up by hepatocytes and, unlike other hexokinases, has unique properties with no negative feedback inhibition by glucose 6-phosphate [19,20], reduced expression of GK can decrease hepatic glucose disposal. In fact, we observed significant decreases in accumulation of 2-deoxy-glucose 6-phosphate in H-HIFKO liver fed HFSD, and found that such a small, but significant decrease in glucose uptake can account for over 70% of difference in peak values of serum glucose levels during OGTT, suggesting the importance of hepatic GK induction in the regulation of glucose disposal system. Liver-specific GK knockout mice have been reported to show mild hyperglycemia with impaired insulin secretion, supporting our hypothesis that HIF-1α in the liver serves as a protective mechanism to reduce post-prandial blood glucose levels by inducing GK expression. Considering that most of glycolytic enzymes including GK are under the control of HIF-1 under hypoxia [8], it is worthwhile to address the question as to why HIF-1α deficiency in hepatocytes selectively affects GK expression when exposed to HFSD.
Prolonged exposure to HFSD evokes multiple dysfunctions in whole organs including β cells, skeletal muscles, adipose tissues, and liver. We found that glucose tolerance was markedly impaired with higher fasting glucose levels in control mice fed HFSD for 20 weeks. Deletion of HIF-1α further deteriorates whole-body glucose disposal during OGTT with modestly, but significantly elevated fasting glucose levels compared to those in control mice. These metabolic alterations are inconsistent with those seen in mice lacking hepatic HIF-1β, which exhibit normal glucose tolerance presumably by compensatory increases in insulin secretion under high fat-diet conditions [9]. Although total numbers of β cells are greatly reduced in the pancreas of the mutant mice, the impaired glucose tolerance is not likely to be caused by β cell dysfunctions, because basal serum insulin levels and the ability of β cell to secrete insulin during OGTT are comparable between H-HIF-KO and control mice. Instead, our present findings showing a smaller decrease in glucose levels in response to exogenous insulin suggest that severe glucose intolerance in HIF-1α-deficient mice is due, in part, to accelerated insulin resistance in peripheral tissues. Consistent with this idea, Akt is less phosphorylated in skeletal muscles and adipose tissues during ITT. Considering that chronic, but even mild hyperglycemia itself has been proposed to cause insulin resistance predominantly in peripheral tissues [21], our present findings suggest an importance of liver HIF-1α in the regulation of whole-body glucose homeostasis. Further investigations are needed to elucidate detailed molecular mechanism(s) as to how hepatic deletion of HIF-1α disrupts peripheral insulin sensitivity under diabetic conditions.
In summary, our present data provide genetic evidence for the role of HIF-1 in the liver in the regulation of whole-body glucose homeostasis. A transient increase in HIF-1α expression induces hepatic GK gene expression and consequently prevents progression of type 2 diabetes when exposed to HFSD. Our mice model presented in this study could serve as a useful tool to explore molecular mechanism(s) of diabetes, and provide new therapeutic intervention in type 2 diabetes.
Supplementary Material
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bbrc.2011.10.089.
Acknowledgments
The authors thank Junko Amano from the Keio University animal care facility for assistance with breeding of H-HIFKO mice. Grant Support: D.O. was a research associate supported by Global COE Program for Human Metabolomic Systems Biology by MEXT, Japan. N.G. was supported in part by the ‘‘High-Tech Research Center’’ Project for Private Universities, with a matching fund subsidy from MEXT. Harvesting animals was supported by Research and Development of the Next-Generation Integrated Simulation of Living Matter, a part of the Development and Use of the Next-Generation Supercomputer Project of MEXT.
Abbreviations
- DG
2-deoxy-d-glucose
- DG6P
2-deoxy-glucose 6-phosphate
- GK
glucokinase
- G6Pase
glucose-6-phosphatase
- HFSD
high fat/sucrose diet
- H-HIFKO
hepatocyte-specific HIF-1α-null
- HIF
hypoxia inducible factor
- ITT
insulin tolerance test
- OGTT
oral glucose tolerance test
- PCR
polymerase chain reaction
- PEPCK
phosphoenolpyruvate carboxykinase
- PGK
phosphoglycerate kinase-1
- PK
pyruvate kinase L
- VHL
von Hipple–Lindau
References
- [1].Miethke H, Wittig B, Nath A, Jungermann K. Gluconeogenic-glycolytic capacities and metabolic zonation in liver of rats with streptozotocin, nonketotic as compared to alloxan, ketotic diabetes. Histochemistry. 1986;85:483–489. doi: 10.1007/BF00508430. [DOI] [PubMed] [Google Scholar]
- [2].Im SS, Kim SY, Kim HI, Ahn YH. Transcriptional regulation of glucose sensors in pancreatic beta cells and liver. Curr Diabetes Rev. 2006;2:11–18. doi: 10.2174/157339906775473581. [DOI] [PubMed] [Google Scholar]
- [3].Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- [5].Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- [6].Kietzmann T, Cornesse Y, Brechtel K, Modaressi S, Jungermann K. Perivenous expression of the mRNA of the three hypoxia-inducible factor alpha-subunits, HIF1alpha, HIF2alpha and HIF3alpha, in rat liver. Biochem J. 2001;354:531–537. doi: 10.1042/0264-6021:3540531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kietzmann T, Dimova EY, Flugel D, Scharf JG. Oxygen: modulator of physiological and pathophysiological processes in the liver. Z Gastroenterol. 2006;44:67–76. doi: 10.1055/s-2005-858987. [DOI] [PubMed] [Google Scholar]
- [8].Roth U, Jungermann K, Kietzmann T. Modulation of glucokinase expression by hypoxia-inducible factor 1 and upstream stimulatory factor 2 in primary rat hepatocytes. Biol Chem. 2004;385:239–247. doi: 10.1515/BC.2004.018. [DOI] [PubMed] [Google Scholar]
- [9].Wang XL, Suzuki R, Lee K, Tran T, Gunton JE, Saha AK, Patti ME, Goldfine A, Ruderman NB, Gonzalez FJ, Kahn CR. Ablation of ARNT/HIF1beta in liver alters gluconeogenesis, lipogenic gene expression, and serum ketones. Cell Metab. 2009;9:428–439. doi: 10.1016/j.cmet.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kucejova B, Sunny NE, Nguyen AD, Hallac R, Fu X, Pena-Llopis S, Mason RP, Deberardinis RJ, Xie XJ, Debose-Boyd R, Kodibagkar VD, Burgess SC, Brugarolas J. Uncoupling hypoxia signaling from oxygen sensing in the liver results in hypoketotic hypoglycemic death. Oncogene. 2011;30:2147–2160. doi: 10.1038/onc.2010.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tajima T, Goda N, Fujiki N, Hishiki T, Nishiyama Y, Senoo-Matsuda N, Shimazu M, Soga T, Yoshimura Y, Johnson RS, Suematsu M. HIF-1alpha is necessary to support gluconeogenesis during liver regeneration. Biochem Biophys Res Commun. 2009;387:789–794. doi: 10.1016/j.bbrc.2009.07.115. [DOI] [PubMed] [Google Scholar]
- [12].Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM, Johnson RS. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 2000;60:4010–4015. [PubMed] [Google Scholar]
- [13].Arteel GE, Thurman RG, Yates JM, Raleigh JA. Evidence that hypoxia markers detect oxygen gradients in liver: pimonidazole and retrograde perfusion of rat liver. Br J Cancer. 1995;72:889–895. doi: 10.1038/bjc.1995.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mantena SK, Vaughn DP, Andringa KK, Eccleston HB, King AL, Abrams GA, Doeller JE, Kraus DW, Darley-Usmar VM, Bailey SM. High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem J. 2009;417:183–193. doi: 10.1042/BJ20080868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Flugel D, Gorlach A, Michiels C, Kietzmann T. Glycogen synthase kinase 3 phosphorylates hypoxia-inducible factor 1alpha and mediates its destabilization in a VHL-independent manner. Mol Cell Biol. 2007;27:3253–3265. doi: 10.1128/MCB.00015-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chavez JC, Almhanna K, Berti-Mattera LN. Transient expression of hypoxia-inducible factor-1 alpha and target genes in peripheral nerves from diabetic rats. Neurosci Lett. 2005;374:179–182. doi: 10.1016/j.neulet.2004.10.050. [DOI] [PubMed] [Google Scholar]
- [17].Cheng K, Ho K, Stokes R, Scott C, Lau SM, Hawthorne WJ, O’Connell PJ, Loudovaris T, Kay TW, Kulkarni RN, Okada T, et al. Hypoxia-inducible factor-1alpha regulates beta cell function in mouse and human islets. J Clin Invest. 2010;120:2171–2183. doi: 10.1172/JCI35846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Catrina SB, Okamoto K, Pereira T, Brismar K, Poellinger L. Hyperglycemia regulates hypoxia-inducible factor-1alpha protein stability and function. Diabetes. 2004;53:3226–3232. doi: 10.2337/diabetes.53.12.3226. [DOI] [PubMed] [Google Scholar]
- [19].Ferre T, Riu E, Bosch F, Valera A. Evidence from transgenic mice that glucokinase is rate limiting for glucose utilization in the liver. FASEB J. 1996;10:1213–1218. doi: 10.1096/fasebj.10.10.8751724. [DOI] [PubMed] [Google Scholar]
- [20].O’Doherty RM, Lehman DL, Seoane J, Gomez-Foix AM, Guinovart JJ, Newgard CB. Differential metabolic effects of adenovirus-mediated glucokinase and hexokinase I overexpression in rat primary hepatocytes. J Biol Chem. 1996;271:20524–20530. doi: 10.1074/jbc.271.34.20524. [DOI] [PubMed] [Google Scholar]
- [21].Tomas E, Lin YS, Dagher Z, Saha A, Luo Z, Ido Y, Ruderman NB. Hyperglycemia and insulin resistance: possible mechanisms. Ann N Y Acad Sci. 2002;967:43–51. doi: 10.1111/j.1749-6632.2002.tb04262.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.