Abstract
In asthma, airway nerve dysfunction leads to excessive bronchoconstriction and cough. It is well established that eosinophils alter nerve function and that airway eosinophilia is present in 50 to 60% of asthmatics. However, the effects of eosinophils on airway nerve structure have not been established. We tested whether eosinophils alter airway nerve structure and measured the physiological consequences of those changes. Our results in humans with and without eosinophilic asthma showed that airway innervation and substance P expression were increased in moderate persistent asthmatics compared to mild intermittent asthmatics and healthy subjects. Increased innervation was associated with a lack of bronchodilator responsiveness and increased irritant sensitivity. In a mouse model of eosinophilic airway inflammation, the increase in nerve density and airway hyperresponsiveness were mediated by eosinophils. Our results implicate airway nerve remodeling as a key mechanism for increased irritant sensitivity and exaggerated airway responsiveness in eosinophilic asthma.
INTRODUCTION
Asthma is an inflammatory airway disease characterized by periods of increased bronchoconstriction and heightened sensitivity to inhaled irritants (1). These responses are controlled by an integrated network of sensory and parasympathetic airway nerves (2). Sensory nerves detect chemical and mechanical stimuli and induce bronchoconstriction by activating parasympathetic nerves through a central neuronal reflex pathway (3, 4). Reflex bronchoconstriction is clearly relevant in asthma because muscarinic antagonists that block neuronally mediated bronchoconstriction, such as tiotropium, improve lung function and asthma symptoms (5, 6). Eosinophils, which are a defining feature of the most common asthma phenotype, termed type 2-high asthma (7), cause excessive bronchoconstriction in part by altering parasympathetic nerve function (8–10). This mechanism is well established. However, whether eosinophils also affect airway sensory nerves comprising the afferent limb of the reflex arc is not fully known.
In type 2-high asthma, interleukin-5 (IL-5) promotes eosinophil maturation, recruitment, and survival (11). Accordingly, therapies that block IL-5 rapidly reduce blood eosinophils and decrease the frequency of asthma exacerbations. However, anti-IL-5 therapies only modestly reduce eosinophils within airway parenchyma (12). These discordant responses suggest that mechanisms regulating eosinophils in airway parenchyma are more complex than those governing eosinophils in the blood. For example, airway nerves control eosinophil recruitment by releasing a variety of mediators (13–15). This phenomenon is starkly apparent in airways of severe asthmatics who died from asthma exacerbations, where more eosinophils are found clustered around airway nerves than in any other airway compartment, such as around blood vessels or within the airway parenchyma (9).
Studying airway sensory nerves has historically been challenging because of the morphologic complexity of sensory nerve structure. Sensory nerves are arranged in three-dimensional (3D) networks that only cover 1 to 3% of the airway epithelium yet span tens to hundreds of 5- to 15-μm-thick histologic tissue sections (16). Consequently, features such as nerve branching and axonal length cannot be captured in individual slices. Previous studies that attempted to quantify nerve architecture in asthma using conventional methodology and semiquantitative manual analyses were prone to sampling error and yielded conflicting results (17–20). These studies may also have been confounded by inadequate asthma phenotyping and comparing samples from disparate airway locations that have different nerve structures at baseline. To overcome these limitations, we developed an imaging method that quantifies 3D nerve structure (nerve length and branch points) in intact, whole-mount human airway specimens obtained by endobronchial biopsy using tissue optical clearing and 3D reconstruction of confocal microscopy images (21, 22). Whole-mount microscopy has the advantage of more fully capturing 3D epithelial nerve structure.
Using this technique, we measured airway innervation, substance P expression, and eosinophilia in bronchial biopsies from humans with and without asthma and found that airway innervation and substance P expression were increased in moderate persistent asthmatics compared to mild intermittent asthmatics and healthy subjects. The increase was most evident in patients with airway and peripheral blood eosinophilia and was associated with a lack of bronchodilator responsiveness and an increased sensitivity to inhaled irritants. Airway innervation was also increased in Il5-overexpressing transgenic mice with airway eosinophilia, and these mice had airway hyperresponsiveness that was mediated by increased airway innervation. Crossing Il5-over expressing mice with mice congenitally deficient in eosinophils yielded mice with high airway Il5 but no eosinophils. These mice had neither increased airway innervation nor hyperresponsiveness, indicating that eosinophils, not Il5, are responsible for these changes. These results suggest that eosinophil-induced airway nerve remodeling might play a key role in lung function impairment in eosinophilic asthma.
RESULTS
Moderate persistent asthma is associated with worse lung function and lower quality of life
Sixty-three patients were included in the final analysis and were grouped by asthma treatment status per Expert Panel Report 3 Asthma Management criteria (23). Mild intermittent asthmatics were treated only with short-acting bronchodilators as needed. Moderate persistent asthmatics were patients who required long-acting inhaled controller medications, including an inhaled corticosteroid. Nonatopic, nonsmoking subjects free of existing lung disease served as healthy controls. Age, sex, and body mass index were similar between groups (Table 1). Moderate persistent asthmatics had higher blood eosinophil counts, lower forced expiratory volume in 1 s (FEV1), and lower FEV1/forced vital capacity (FVC) ratios than mild intermittent asthmatics and healthy controls. Moderate persistent asthmatics also reported more asthma-related symptoms, which were reflected in lower Asthma Quality of Life Questionnaire (AQLQ) scores compared to mild intermittent asthma (Table 1).
Table 1. Clinical characteristics of study subjects.
Values are means ± SD unless otherwise stated. NA, not applicable.
| Asthma severity | |||
|---|---|---|---|
| Control | Intermittent | Persistent | |
| Characteristic | n = 19 | n = 13 | n = 31 |
| Age (range) | 56.1 (21–76) | 58.9 (40–75) | 55.9 (28–78) |
| Female sex—no. (%) | 14 (73) | 8 (62) | 17 (55) |
| Body mass index* | 26.9 ± 4.9 | 27.3 ± 4.5 | 28.4 ± 5.5 |
| Former smoker—no. (%) | 1 (5) | 1 (7) | 7 (23) |
| FEV1 before bronchodilation | |||
| Mean (liters) | 2.78 ± 0.7 | 2.43 ± 0.8 | 2.11 ± 0.9† |
| Percent predicted value | 106.4 ± 15.1 | 87.5 ± 11.1 | 72.9 ± 22.5 |
| FEV1/FVC ratio before bronchodilation | 77.5 ± 4.0 | 67.7 ± 6.5 | 61.1 ± 13.9± |
| FEV1 after bronchodilation | |||
| Mean (liters) | NA | 2.45 ± 0.6 | 2.41 ± 1.0 |
| Percent predicted value | NA | 88.9 ± 11.2 | 80.9 ± 22.7 |
| FEV1/FVC ratio after bronchodilation | NA | 67.7 ± 6.5 | 70.3 ± 15.6 |
| Blood eosinophil count (cells/ml) | 182 ± 93 | 277±289 | 301±225 |
| Use of inhaled corticosteroid—no. (%) | 0 (0) | 0 (0) | 31 (100) |
| Use of long-acting β2 agonist (%) | 0 (0) | 0 (0) | 28 (90) |
| Use of >2 controller medications (%) | 0 (0) | 0 (0) | 21 (68) |
| Use of daily systemic steroids—no. (%) | 0 (0) | 0 (0) | 4 (11) |
| AQLQ score§ | NA | 5.4 ± 1.1 | 4.1 ± 1.4 |
| Symptoms§ | NA | 4.1 ± 1.7 | 3.1 ± 1.6 |
| Emotional function§ | NA | 4.0 ± 1.8 | 2.9 ± 1.9 |
| Environmental stimuli§ | NA | 4.5 ± 2.1 | 3.4 ± 1.9 |
| Activity limitation§ | NA | 4.4 ± 1.4 | 3.8 ± 2.4 |
The body mass index is the weight in kilograms divided by the height squared.
P < 0.05 compared to control.
P < 0.05 compared to all other groups.
Minimally important differences are reflected by differences in scores >0.5 points.
Airway innervation is increased in moderate persistent asthma
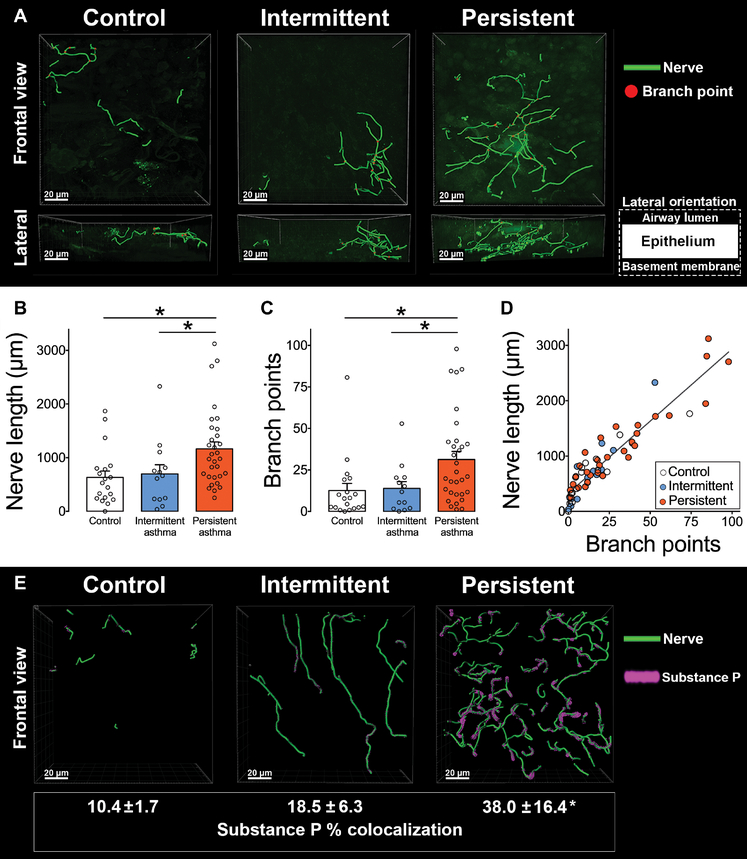
Airway nerve length and the number of nerve branch points in human lungs were quantified using immunofluorescence and 3D nerve modeling in biopsies from the right middle lobe bronchus (Fig. 1A and movies S1 and S2). Moderate persistent asthmatics had significantly increased nerve length (Fig. 1B) and increased nerve branching (Fig. 1C) compared to mild intermittent asthmatics and healthy nonasthmatic controls (*P < 0.05). Nerve length and branching were similar between mild intermittent asthmatics and control subjects (Fig. 1, B and C). Individuals with longer nerves tended to have more branch points, indicating that neuronal growth and arborization occur concurrently (r2 = 0.87, *P < 0.0001; Fig. 1D). Sensory nerve substance P expression was also increased in moderate persistent asthma compared to mild intermittent asthma and controls (*P < 0.05; Fig. 1E and movie S3).
Fig. 1. Airway sensory innervation and substance P expression are increased in moderate persistent asthma.
(A) Representative 3D nerve models generated from bronchoscopic human airway biopsies immunolabeled with antibody to the pan-neuronal protein PGP9.5. (B and C) Bar graphs showing nerve length (B) and branch points (C) in samples derived from healthy subjects (control) and patients with mild intermittent asthma (intermittent) and moderate persistent asthma (persistent). (D) Correlation between nerve length and branch points in control and asthmatic patients (r2 = 0.87, P < 0.0001). (E) Representative 3D nerve models generated from bronchoscopic human airway biopsies obtained from healthy subjects (control) and patients with mild intermittent asthma (intermittent) and moderate persistent asthma (persistent), immunolabeled with antibody to neuronal substance P, and the pan-neuronal protein PGP9.5. Movies of nerve modeling are available in the online supplement (movies S1 to S3). Data are presented as means ± SEM. Asterisk (*) indicates P < 0.05 compared to all other groups. Statistical significance was determined using one-way analysis of variance (ANOVA) with a Bonferroni post hoc test (B, C, and E) and linear regression (D). In total, 63 subjects were included in the final analysis. Three biopsy specimens were analyzed per subject with 10 randomized z-stack images obtained per specimen.
Airway and blood eosinophils are associated with increased airway innervation
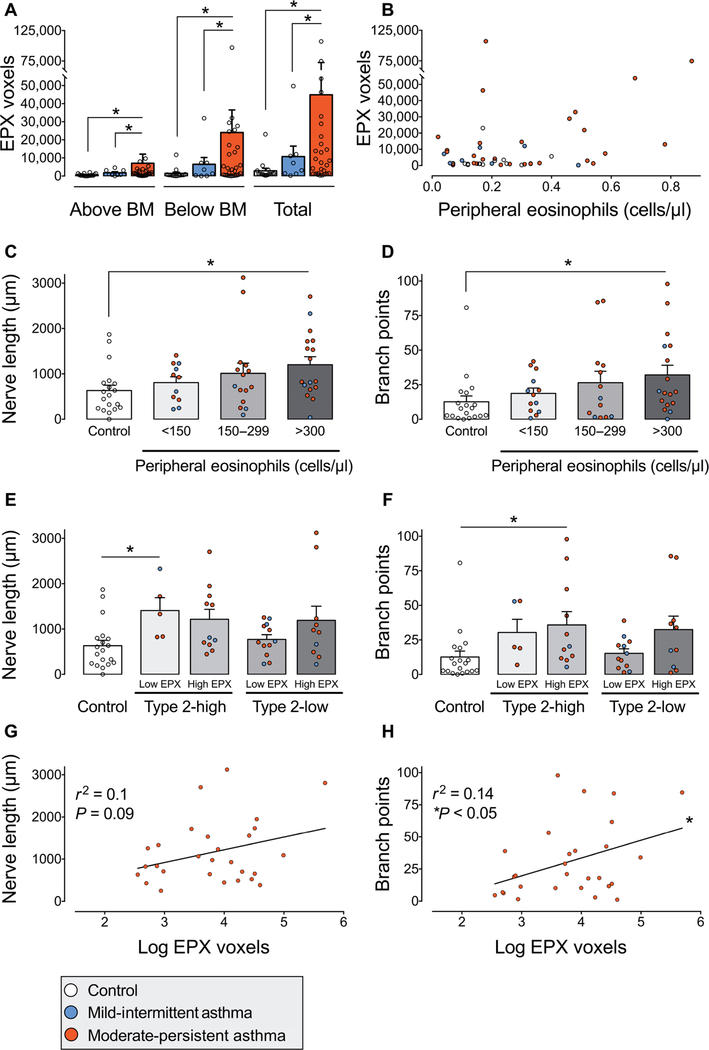
Airway eosinophils were labeled with an antibody against eosinophil peroxidase. Total eosinophil peroxidase-positive voxels were quantified 20 μm above and 20 μm below the airway epithelial basement membrane. Moderate persistent asthmatics had increased eosinophil peroxidase both above and below the epithelial basement membrane compared to mild intermittent asthmatics and control subjects (*P < 0.05; Fig. 2A). Within individual subjects, the number of blood eosinophils measured at the time of airway sampling did not correlate with the amount of airway eosinophil peroxidase (r2 = 0.01, P = 0.8; Fig. 2B).
Fig. 2. Airway and peripheral blood eosinophils are associated with increased airway innervation in asthma.
(A) Eosinophil peroxidase (EPX) in human airway biopsies from healthy subjects (control, white bars) and from patients with mild intermittent (blue bars) and moderate persistent asthma (red bars). EPX was quantified above and below the epithelial basement membrane (BM). Data points represent the average of three biopsies per subject. A total of 57 subjects were evaluated. (B) Correlation between blood eosinophils and airway EPX for each subject. r2 = 0.01, P = 0.8. Colored dots correspond with mild intermittent asthma (blue), moderate persistent asthma (red), or control (white). (C and D) Nerve length and branch points in patients stratified into terciles by peripheral blood eosinophil count. n = 62 subjects. (E and F) Nerve length and branch points in subgroups stratified by type 2 asthma phenotype and airway EPX. Type 2-low versus type 2-high asthma was defined by blood eosinophils less than or greater than 300 cells/μl. High EPX was defined as greater than 5500 positive voxels. n = 57 subjects. (G and H) Correlation of nerve length (G) and branch points (H) with airway EPX in patients with moderate persistent asthma. Linear regression for length r2 = 0.1 and P = 0.09 and for branch points r2 = 0.14 and P < 0.05. n = 28. Bar graphs represent means ± SEM. Asterisk (*) indicates P < 0.05. Statistical significance was determined using one-way ANOVA with a Bonferroni post hoc test (A and C to F) and linear regression.
Subjects with a type 2-high asthma phenotype, defined as blood eosinophils greater than 300 cells/μl (24), had longer airway nerves (Fig. 2C) and increased nerve branch points (Fig. 2D) compared to control airways (*P < 0.05). In contrast, nerves in type 2-low asthmatics with blood eosinophils less than 300 cells/μl were not significantly different from healthy subjects (Fig. 2, C and D). Type 2-high and type 2-low asthmatics were further stratified on the basis of the amount of eosinophil peroxidase present within airway parenchyma. Nerve length (Fig. 2E) and branch points (Fig. 2F) were similar between type 2-low asthmatics and control subjects irrespective of their amount of airway eosinophil peroxidase. However, a portion of type 2-low asthmatics were found to have increased airway eosinophil peroxidase. Moreover, in patients with moderate persistent asthma, the amount of eosinophil peroxidase positively correlated with nerve branch points but not with nerve length (r2 = 0.14, *P < 0.05 and r2 = 0.1, P = 0.09, respectively; Fig. 2, G and H). In mild intermittent asthmatics, eosinophil peroxidase did not correlate with nerve length (P = 0.45) or branch points (P = 0.77).
Airway innervation is associated with irritant sensitivity and worse quality of life
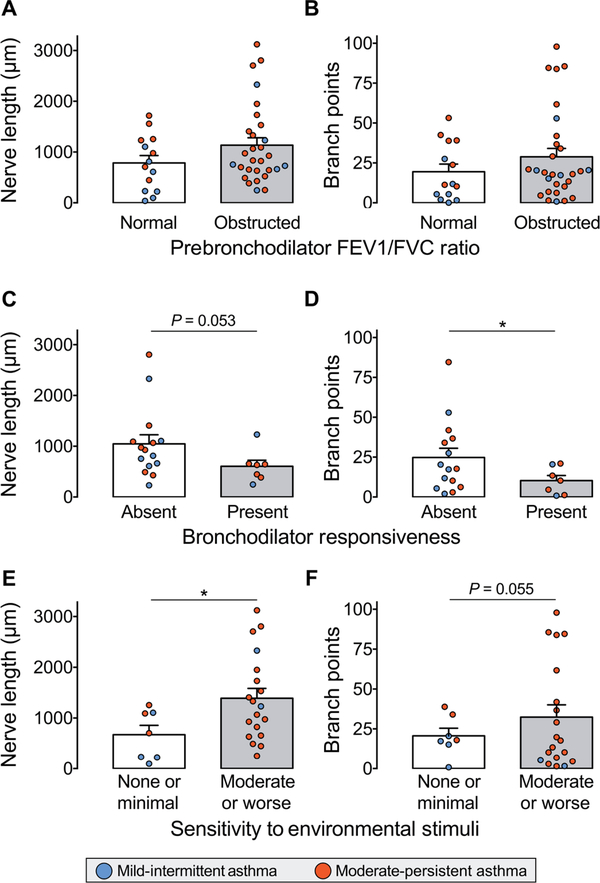
We next tested the association between airway nerve structure and pulmonary function. Nerve length and number of branch points were not different between asthmatics with and without airflow obstruction (Fig. 3, A and B). A subgroup of patients received an inhaled bronchodilator (short-acting β agonist and/or muscarinic antagonist) before repeating spirometry to assess for bronchodilator responsiveness. A “positive bronchodilator response” was defined as a greater than 200 ml increase in expiratory volume that represented a >12% improvement from their prebronchodilator result. Increased nerve branching, but not nerve length, was associated with a lack of bronchodilator responsiveness (*P < 0.05 and P = 0.053, respectively; Fig. 3, C and D). Increased sensitivity to inhaled irritants, defined as moderate or worse sensitivity (score 5 or less) on the Asthma Quality of Life Questionnaire, was associated with longer nerve length (*P < 0.05; Fig. 3E) but not with increased branching (P = 0.055; Fig. 3F).
Fig. 3. Increased airway nerve density is associated with lack of bronchodilator responsiveness and increased sensitivity to environmental stimuli.
(A and B) Nerve length and branch points in patients with asthma stratified by the presence or absence of airflow obstruction on pulmonary function testing (defined as a ratio of FEV1/FVC less than 0.7). n = 43. (C and D) Nerve length and branch points in patients with asthma stratified by the presence or absence of bronchodilator responsiveness to inhaled albuterol. Bronchodilator responsiveness was defined as an increase in postbronchodilator FEV1 and/or FVC > 200 ml and >12%. n = 22. (E and F) Nerve length and branch points in patients with asthma stratified by their sensitivity to environmental stimuli, determined by the Asthma Quality of Life Questionnaire. Moderate or worse sensitivity to environmental triggers was defined as an environmental stimuli domain score of ≤5, whereas scores >5 indicated minimal or no sensitivity. n = 26. Colored dots indicate mild intermittent (blue) and moderate persistent (red) asthma. Data are presented as means ± SEM. Asterisk (*) indicates P < 0.05. Statistical significance was determined by t test (unpaired, two-tailed).
Airway innervation is not associated with a patient’s sex, age, or body habitus
Airway nerve length and branching were evaluated in asthma patients stratified by sex, body mass index, and age to determine whether nerve morphology was associated with a patient’s demographics or body habitus. Nerve length and branching were similar between men and women within each asthma cohort (fig. S1, A and B) and were unrelated to body mass index (fig. S1, C and D). Age also did not correlate with airway innervation (branching and length r2 = 0.003, P = 0.23 and r2 = <0.005, P = 0.7, respectively; fig. S1, E and F).
Eosinophils increase epithelial innervation and neuronal reflex bronchoconstriction in mice
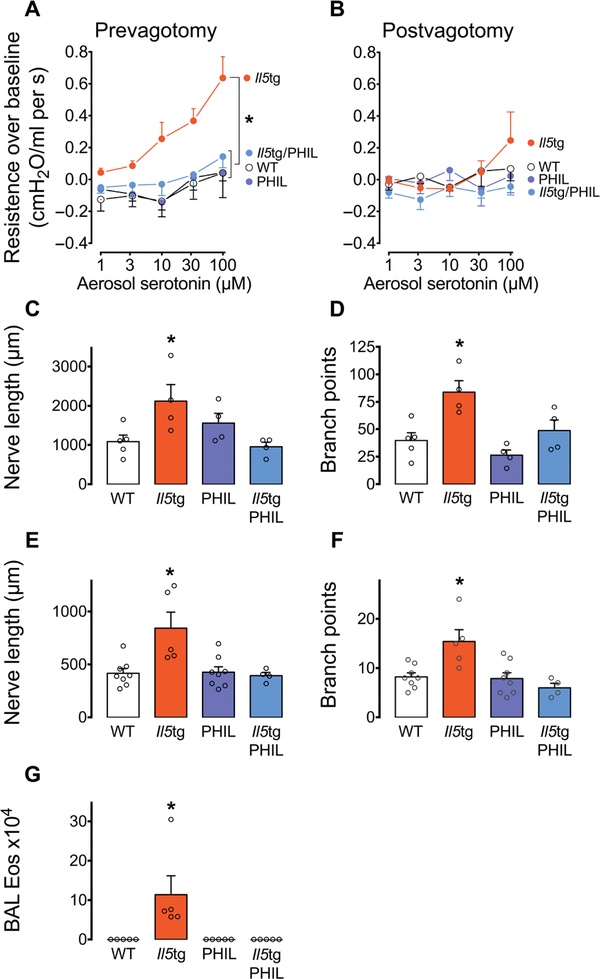
To test whether eosinophils alter airway nerve structure and to define the consequences of those changes, we evaluated nerve morphology and airway responsiveness using transgenic mice with eosinophilic airway inflammation induced by Il5 overexpression in respiratory epithelium (Il5tg) (25). These mice were compared with wild-type (WT) mice, with eosinophil-deficient (PHIL) mice (26) and with crossed Il5tg/PHIL mice (with elevated Il5 and eosinophil deficiency) to discriminate the effects of eosinophils from those of Il5. Increases in airway resistance in response to aerosolized serotonin were recorded with intact vagus nerves to measure neuronal reflex-mediated bronchoconstriction. In WT mice, serotonin induced minimal bronchoconstriction. In contrast, Il5tg mice had significantly increased bronchoconstriction in response to aerosolized serotonin (*P < 0.05 compared to all other groups; Fig. 4A). Serotonin-induced bronchoconstriction in Il5tg mice was eliminated by vagotomy, demonstrating that bronchoconstriction was mediated by a neuronal reflex (Fig. 4B). Eosinophil-deficient PHIL mice and Il5tg/PHIL mice had similar airway responsiveness compared to WT mice (Fig. 4, A and B), indicating that eosinophils, not Il5, mediate airway hyperresponsiveness.
Fig. 4. Airway eosinophils increase sensory innervation and cause neuronally mediated airway hyperresponsiveness in mice.
(A) Airway responsiveness to aerosolized serotonin in WT mice, transgenic mice with airway eosinophilia driven by airway-specific Il5 overproduction (Il5tg), eosinophil-deficient mice (PHIL) and eosinophil-deficient mice over-expressing airway Il5 (Il5tg/PHIL). (B) Airway responsiveness to aerosolized serotonin after vagotomy. n = 6 to 9 per group. (C and D) Nerve length and branch points in the proximal trachea of WT, Il5tg, PHIL, and Il5tg/PHIL mice. n = 4 to 5 per group. (E and F) Nerve length and branch points at the carina in WT, Il5tg, PHIL, and Il5tg/PHIL mice. n = 4 to 8 per group. (G) Bronchoalveolar lavage (BAL) eosinophils (Eos). n = 4 to 5 per group. Data are presented as means ± SEM. Asterisk (*) indicates P < 0.05 compared to all other groups. Statistical significance was determined using two-way repeated measures ANOVA (A and B) and one-way ANOVA with a Bonferroni post hoc test (C to G).
Il5tg mice with airway eosinophilia also showed increased epithelial nerve length and branching in both the proximal trachea (*P < 0.05 compared to all other groups; Fig. 4, C and D, and table S1) and at the carina (*P < 0.05 compared to all other groups; Fig. 4, E and F). Airway nerve length and branching in eosinophil-deficient PHIL mice and Il5tg/PHIL mice were similar to controls (Fig. 4, C to F). Bronchoalveolar lavage was used to confirm the presence or absence of airway eosinophils. As expected, Il5tg mice had significantly more eosinophils compared to all other groups (Fig. 4G).
DISCUSSION
Here, we report that airway epithelial sensory nerves undergo substantial structural remodeling in eosinophilic asthma that manifests as increased airway nerve density. We also show that airway eosinophils increased epithelial sensory innervation and neuronally mediated airway responsiveness in a transgenic mouse model. These findings define a critical link between eosinophilic inflammation and airway nerve growth and suggest that sensory neuroplasticity may contribute to heightened symptoms and worse lung function in asthma.
Distinguishing asthmatics with increased innervation may be clinically relevant given that this phenotype is associated with increased sensitivity to environmental stimuli and may preferentially benefit from therapies targeting neuronal reflex bronchoconstriction, such as muscarinic antagonists (27). This subgroup’s potentially unique response to therapies targeting eosinophil-nerve interactions should also be considered when designing future clinical trials. To this point, our data show that airway eosinophilia correlates with increased airway innervation and that some type 2-low asthmatics (defined by low blood eosinophils) have elevated airway eosinophils. Thus, phenotyping eosinophilic asthma patients using blood eosinophil counts, as was common in many recent asthma trials, may overlook this population of patients who could benefit from eosinophiltargeting therapies.
Nerve morphology is not currently used to stratify asthma phenotypes. However, quantitative measurement of sensory nerve density and morphology in skin biopsies has diagnostic and prognostic utility for diseases involving aberrant nerve function, such as pain hypersensitivity (28). A similar approach using tissue optical clearing to characterize airway epithelial sensory neuroplasticity may be useful in the lungs. 3D nerve modeling has been used to describe airway nerve subtypes in healthy human lungs (16) and demonstrated that airway innervation increased in response to allergen and ozone exposure in rhesus monkeys (29). Our study further supports the feasibility of this technique. Our analysis intentionally limited evaluation of innervation to endobronchial samples from the bifurcation of the right middle lobe bronchus to target a location enriched for airway sensory nerves and to limit confounding from natural variations in nerve density at different airway locations (21, 22).
Airway nerve growth primarily involved axons expressing substance P. Substance P is a neuropeptide that lies at the nexus of neuroimmune interactions in the airways given its ability to recruit and activate eosinophils (30), increase nerve sensitivity (31), induce phenotypic switching of sensory nerve subpopulations (32), and stimulate airway smooth muscle contraction (33). Previous studies reported increased substance P in airway epithelium and bronchoalveolar lavage from asthmatics (34, 35). However, drugs designed to block substance P in asthma have had limited efficacy in clinical trials (36–38), possibly due to inadequate patient phenotyping or because unanticipated changes in substance P signaling occur in asthma, such as alterations in the sensitivity of substance P receptors neurokinin 1 (NK1) and neurokinin 2 (NK2), or changes in substance P metabolism by neutral endopeptidase.
Substance P is one of several neuropeptides [neurokinin A and calcitonin gene-related peptide (39, 40)] and chemokines [eotaxin (41)] that actively recruit eosinophils to the lungs and specifically to airway nerves. As a result, eosinophils are found clustered around nerve fibers and parasympathetic ganglia in histologic sections of airways from animals after antigen or ozone exposure (9, 41, 42) and in airways of humans who died of asthma (9). When eosinophils physically interact with nerves, granule proteins are released, which alter nerve function and promote airway hyperresponsiveness (43–47). For example, eosinophil major basic protein stimulates transient receptor potential V1 (TRPV1) expression in sensory nerves in vitro (48), which may explain why asthmatics have increased sensitivity to TRPV1 agonists (49). Major basic protein is also an allosteric antagonist of M2 muscarinic receptors on parasympathetic nerves (50). Loss of M2 function results in excessive parasympathetic nerve acetylcholine release and potentiation of bronchoconstriction. This mechanism is observed after virus infection (51), ozone inhalation (52), organophosphorus pesticide exposure (53), and after antigen sensitization and challenge in experimental models (8, 54). Loss of neuronal M2 receptor function has been documented in asthmatic humans as well (55, 56).
Although our data show a positive correlation between airway eosinophil peroxidase and increased innervation, the specific mediator(s) driving nerve growth in human airways remains unknown. Other eosinophil granule proteins (major basic protein and eosinophil cationic protein) and/or eosinophil-derived neurotrophins may be responsible for sensory nerve growth (57, 58). In a previous study, blocking the neurotrophin nerve growth factor was insufficient to prevent eosinophil-induced nerve growth in cutaneous sensory nerves (59), suggesting that other neurotrophins such as brain-derived neurotrophic factor or neurotrophin-3 may be involved, or that considerable redundancy exists in this system. Eosinophil peroxidase and major basic protein have also been linked to nerve proliferation and survival, albeit in a neuroblastoma-based model of cholinergic neurons (60–63). However, in contrast to our findings in sensory nerves, granule proteins reduced cholinergic nerve length in this in vitro model (64). It is also possible that eosinophils exert their effects by altering cytokine or neurotrophin release from neighboring cells close to nerves, such as respiratory epithelium or satellite glial cells.
Close proximity of eosinophils to nerves is critical for the development of neuronally mediated airway hyperreactivity. Accordingly, treatments that prevent eosinophil recruitment to airway nerves, such as antibodies against eosinophil surface receptors C-C chemokine receptor type 3 (41) and very late antigen-4 (65), and corticosteroids (66, 67), preserve nerve function and prevent neuronally mediated airway hyperreactivity in animal models. However, the long-term effects from preventing eosinophil-nerve interactions in the airways have yet to be studied. Thus, the critical question remains whether neuronal remodeling is a reversible process. In skin, topical application of capsaicin or lidocaine decreases epidermal nerve density (68, 69), suggesting that changes in nerve density are dynamic and might be targeted therapeutically. A similar approach targeting structural changes in airway sensory nerves, in combination with blockade of nerve function, may be beneficial in asthma.
In this regard, newer therapies that block the IL-5 pathway might be effective in preventing eosinophil-nerve interactions. Antibodies against IL-5 (mepolizumab and reslizumab) and IL-5 receptors (benralizumab) produce rapid and profound reductions in circulating eosinophils and reduce the frequency of exacerbations in type 2-high asthma (11). Anti-IL-5 antibodies also reduce the number of airway tissue eosinophils and eosinophils physically associated with airway nerves in animals (42), although in the case of mepolizumab, a more modest reduction in airway tissue eosinophils was seen in an early human trial (12). The newest of these agents, benralizumab, produces the largest reduction in circulating eosinophils in this drug class and, unlike other anti-IL-5 agents, improves expired airflow on spirometry, possibly indicating that benralizumab improves neuronal dysfunction as well.
Our data indicate that airway innervation does not correlate with body habitus (obesity) or female gender, which are both characteristics associated with specific asthma phenotypes (70). However, our study could not assess all demographic and environmental variables that are potentially related to increased airway innervation. Furthermore, because our study assessed innervation at a single time point, our data are unable to illuminate when pathologic nerve growth begins. That said, our data do suggest that nerve growth is not a function of normal aging in adults, which contrasts with lung development during childhood when airway innervation increases between infancy and adolescence (71).
In sum, our data indicate that airway nerves contribute to asthma pathology. We have shown that moderate persistent asthmatics have increased airway sensory innervation that is especially marked in asthmatics with accompanying eosinophilia. The role of eosinophils in neuronal remodeling and the effects of remodeling on airway hyperreactivity are confirmed in mice. It is possible that increased sensory innervation represents a unique asthma phenotype that may respond to drugs targeting eosinophils or their proteins, or drugs that interrupt the neuronal reflex arc such as anticholinergics.
MATERIALS AND METHODS
Study design
This study was designed to evaluate airway sensory innervation and eosinophilia in humans with and without asthma and to characterize the physiologic consequences of eosinophil and airway nerve interactions using transgenic mice. To this end, confocal microscopy and 3D nerve modeling were used to quantify nerve morphology (nerve length and branching), neuropeptide expression, and eosinophils in immunofluorescently labeled, optically cleared airway specimens obtained by endobronchial biopsy. In total, 63 subjects were included in the final analysis. Three biopsy specimens were analyzed per subject with 10 randomized z-stack images obtained per specimen. Sample sizes were chosen on the basis of nerve morphology pilot data.
Airway sensory nerve morphology and airway responsiveness to aerosolized serotonin were measured in mice that overexpressed Il5 in airway epithelium (Il5tg), in eosinophil-deficient mice (PHIL), in eosinophil-deficient mice with Il5 overexpressed in airway epithelium (Il5tg/PHIL), and in WT mice before and after vagotomy to define neuronally mediated bronchoconstriction. Researchers were blinded to experimental conditions when conducting experiments and throughout the analysis of results.
Patient recruitment
Eligible patients were ≥18 years old. Subjects provided written informed consent. The protocol was approved by the Institutional Review Board of Royal College of Surgeons (Dublin, Ireland). Medication use, pulmonary function testing, blood eosinophil counts, serum immunoglobulin E (IgE) levels, and smoking history were obtained. Subjects with asthma completed the Asthma Quality of Life Questionnaire to assess asthma burden across four domains (symptoms, activity limitation, emotional function, and environmental exposure) using a seven-point Likert scale with higher scores indicating better quality of life (72). Minimally important differences were indicated by a greater than 0.5 difference in overall score and within each individual domain.
Animals
C57BL/6 mice (8 to 12 weeks old, male and female; Jackson Laboratory), transgenic mice overexpressing Il5 driven by an airway epithelium-specific promoter (Il5tg; NJ.1726 line) (25), and eosinophil-deficient mice (PHIL line) (26) were maintained by backcrossing on a C57BL/6 background, housed in a pathogen-free environment, and handled in accordance with the U.S. Animal Welfare Act as set forth in National Institutes of Health guidelines. Eosinophil-deficient mice with elevated airway Il5 were also generated by crossing Il5tg mice with PHIL mice (Il5tg/PHIL line). Protocols were approved by the Institutional Animal Care and Use Committee of Oregon Health and Science University.
Airway sampling
Human bronchial biopsies (three to five per subject) were taken from the bifurcation of the right middle lobe and immediately fixed in formalin overnight. Mouse lungs were removed en bloc and fixed in Zamboni’s fixative overnight. After fixation, samples were washed in phosphate-buffered saline and stored in 70% ethanol.
Immunohistochemistry
Tissues were immunostained at 4°C on a shaker. Tissues were washed with pH 7.4 tris-buffered saline (TBS) and then blocked overnight with 4% normal goat serum, 1% Triton X-100, and 5% powdered milk in TBS. Airway nerves were labeled with rabbit polyclonal antibody against pan-neuronal marker PGP9.5 (protein gene product 9.5) (Millipore) and with rat polyclonal antibody against substance P (BD Pharmingen), followed by Alexa Fluor anti-rabbit 488 and anti-rat 555 secondary antibodies (Life Technologies). Eosinophils were labeled with an antibody against eosinophil peroxidase, followed by Alexa Fluor anti-mouse 647 secondary antibody (Life Technologies). No primary, rat IgG, and rabbit IgG controls were run in parallel. Tissues were dehydrated with methanol and then rendered transparent via optical clearing using benzyl alcohol and benzyl benzoate [1:2 (v/v)]. Samples were mounted in medium containing the nuclear counterstain DAPI (4′6-diamidino-2-phenylindole, dilactate; Molecular Probes) on well slides (1 mm thick) and sealed with Permount (Thermo Fisher Scientific) underneath a glass coverslip (60 mm × 22 mm × 0.15 mm).
Image acquisition and processing
Images were acquired using a Zeiss LSM780 confocal microscope and 63×/0.45 PlanApo objective with a 2-mm working distance (Carl Zeiss). The in-plane (x and y) and out-of-plane (z) resolution was 0.264 μm × 0.264 μm × 1.00 μm. Ten randomized images were taken of each human biopsy using DAPI to locate the epithelium. The total in-plane field of view and scan depth for a single human biopsy z-stack image acquisition was ~135 μm × 135 μm × 80 μm.
3D mapping and quantification of airway epithelial nerves
Airway nerves were modeled in 3D with commercially available image processing software that uses adaptive contour fiber tracking to generate best-fit tubular nerve models between user-defined control points (Imaris). Users were blinded to study group at the time of nerve modeling. Nerve length, nerve branch points, and eosinophil peroxidase-positive voxels were quantified within 20 μm of the airway epithelial basement membrane. Elevated airway eosinophil peroxidase was defined as greater than 5500 arbitrary units (1.5 times the interquartile range of control eosinophil peroxidase added to control mean). Neuronal substance P expression was quantified on the basis of colocalization of substance P-positive voxels within PGP9.5-positive nerve axons.
Airway physiology
Mice were anesthetized with ketamine (100 mg/kg) and xylazine (5 mg/kg) intraperitoneally, paralyzed with succinylcholine (5 mg/kg), and ventilated via a tracheal cannula (125 breaths/min; tidal volume, 0.3 ml; positive end-expiratory pressure, 2 mmH2O). Airway peak pressures during ventilation and airway plateau pressures obtained at the end of a 0.2-s inspiratory pause were measured via an in-line pressure transducer at baseline and after escalating doses of aerosolized serotonin (5-HT; 10 μl at 1 to 100 mM; Aeroneb). Airway resistance was calculated as the difference between peak pressure and plateau pressure divided by the inspiratory flow. In separate experiments, the vagus nerves were surgically ligated to eliminate neuronal reflex bronchoconstriction, and a serotonin dose response was performed.
Statistical analysis
Nerve length and branching were analyzed using a one-way ANOVA with a Bonferroni post hoc test and by linear regression. Dose responses were analyzed using two-way ANOVA for repeated measures. Baseline characteristics and cell counts were analyzed using one-way ANOVA with a Bonferroni post hoc test. All statistics were analyzed using GraphPad Prism 7 (GraphPad), and data are presented as means ± SEM.
Supplementary Material
Fig. S1. Airway innervation was not associated with sex, body mass index, or age.
Table S1. Raw data of airway nerve modeling and eosinophilia in mice.
Movie S1. Quantitative 3D model of airway epithelial sensory nerves from a healthy nonasthmatic subject.
Movie S2. Quantitative 3D model of airway epithelial sensory nerves from a subject with moderate persistent asthma.
Movie S3. Quantitative 3D model of airway epithelial sensory nerves costained for substance P from a subject with moderate persistent asthma.
Acknowledgments
We thank J. Wagner (BS, MCR) and the Advanced Light Microscopy Core at Oregon Health and Science University for their assistance with this work.
Funding: This work was supported by NIH National Heart, Lung, and Blood Institute grant nos. HL124165 (to D.B.J.), AR061567 (to D.B.J. and J.J.L.), HL131525 (to A.D.F. and Z.N.), HL121254 (to M.G.D.), and UL1GM118964 (to M.G.D.), by the American Thoracic Society Foundation grant no. 1012827 (to M.G.D.), by the Health Research Board of Ireland Clinician Scientist Award (to R.W.C.), and by the Health Effects Institute award no. 4905 RFPA10–3/11–6 (to A.D.F.).
Footnotes
Competing interests: D.B.J. formerly consulted for GlaxoSmithKline on the development of mepolizumab. A.D.F. is a consultant in lung toxicology for the fragrance industry. R.W.C. is funded by GlaxoSmithKline and is a consultant for GlaxoSmithKline, TEVA, Genentech, and Novartis on technologies related to medication adherence. All other authors declare that they have no competing interests.
Data and materials availability: All data are included in the paper or in its Supplementary Materials.
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/10/457/eaar8477/DC1
REFERENCES AND NOTES
- 1.Olin JT, Wechsler ME, Asthma: Pathogenesis and novel drugs for treatment. BMJ 349, g5517 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Belvisi MG, Airway sensory innervation as a target for novel therapies: An outdated concept? Curr. Opin. Pharmacol 3, 239–243 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Taylor-Clark T, Undem BJ, Transduction mechanisms in airway sensory nerves. J. Appl. Physiol 101, 950–959 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Costello RW, Evans CM, Yost BL, Belmonte KE, Gleich GJ, Jacoby DB, Fryer AD, Antigen-induced hyperreactivity to histamine: Role of the vagus nerves and eosinophils. Am. J. Physiol 276, L709–L714 (1999). [DOI] [PubMed] [Google Scholar]
- 5.Peters SP, Kunselman SJ, Icitovic N, Moore WC, Pascual R, Ameredes BT, Boushey HA, Calhoun WJ, Castro M, Cherniack RM, Craig T, Denlinger L, Engle LL, Dimango EA, Fahy JV, Israel E, Jarjour N, Kazani SD, Kraft M, Lazarus SC, Lemanske RF Jr., Lugogo N, Martin RJ, Meyers DA, Ramsdell J, Sorkness CA, Sutherland ER, Szefler SJ, Wasserman SI, Walter MJ, Wechsler ME, Chinchilli VM, Bleecker ER; National Heart, Lung, and Blood Institute Asthma Clinical Research Network, Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N. Engl. J. Med 363, 1715–1726 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerstjens HAM, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, Sigmund R, Seibold W, Moroni-Zentgraf P, Bateman ED, Tiotropium in asthma poorly controlled with standard combination therapy. N. Engl. J.Med 367, 1198–1207 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Levy BD, Noel PJ, Freemer MM, Cloutier MM, Georas SN, Jarjour NN, Ober C, Woodruff PG, Barnes KC, Bender BG, Camargo CA Jr., Chupp GL, Denlinger LC, Fahy JV, Fitzpatrick AM, Fuhlbrigge A, Gaston BM, Hartert TV, Kolls JK, Lynch SV, Moore WC, Morgan WJ, Nadeau KC, Ownby DR, Solway J, Szefler SJ, Wenzel SE, Wright RJ, Smith RA, Erzurum SC, Future research directions in asthma. An NHLBI working group report. Am. J. Respir. Crit. Care Med 192, 1366–1372 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fryer AD, Wills-Karp M, Dysfunction of M2-muscarinic receptors in pulmonary parasympathetic nerves after antigen challenge. J. Appl. Physiol 71, 2255–2261 (1991). [DOI] [PubMed] [Google Scholar]
- 9.Costello RW, Schofield BH, Kephart GM, Gleich GJ, Jacoby DB, Fryer AD, Localization of eosinophils to airway nerves and effect on neuronal M2 muscarinic receptor function. Am. J. Physiol 273, L93–L103 (1997). [DOI] [PubMed] [Google Scholar]
- 10.Kingham PJ, McLean WG, Sawatzky DA, Walsh MT, Costello RW, Adhesion-dependent interactions between eosinophils and cholinergic nerves. Am. J. Physiol. Lung Cell. Mol. Physiol 282, L1229–L1238 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Ray A, Raundhal M, Oriss TB, Ray P, Wenzel SE, Current concepts of severe asthma. J. Clin. Invest 126, 2394–2403 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS, Eosinophil’s role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am. J. Respir. Crit. Care Med 167, 199–204 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Costello RW, Jacoby DB, Gleich GJ, Fryer AD, Eosinophils and airway nerves in asthma. Histol. Histopathol 15, 861–868 (2000). [DOI] [PubMed] [Google Scholar]
- 14.Kingham PJ, Costello RW, McLean WG, Eosinophil and airway nerve interactions. Pulm. Pharmacol. Ther 16, 9–13 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Drake MG, Lebold KM, Roth-Carter QR, Pincus AB, Blum ED, Proskocil BJ, Jacoby DB, Fryer AD, Nie Z, Eosinophil and airway nerve interactions in asthma. J. Leukoc. Biol 104, 61–67 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.West PW, Canning BJ, Merlo-Pich E, Woodcock AA, Smith JA, Morphologic characterization of nerves in whole-mount airway biopsies. Am. J. Respir. Crit. Care Med 192, 30–39 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ollerenshaw SL, Jarvis D, Sullivan CE, Woolcock AJ, Substance P immunoreactive nerves in airways from asthmatics and nonasthmatics. Eur. Respir. J 4, 673–682 (1991). [PubMed] [Google Scholar]
- 18.Howarth PH, Djukanovic R, Wilson JW, Holgate ST, Springall DR, Polak JM, Mucosal nerves in endobronchial biopsies in asthma and non-asthma. Int. Arch. Allergy Appl. Immunol 94, 330–333 (1991). [DOI] [PubMed] [Google Scholar]
- 19.Chanez P, Springall D, Vignola AM, Moradoghi-Hattvani A, Polak JM, Godard P, Bousquet J, Bronchial mucosal immunoreactivity of sensory neuropeptides in severe airway diseases. Am. J. Respir. Crit. Care Med 158, 985–990 (1998). [DOI] [PubMed] [Google Scholar]
- 20.Howarth PH, Springall DR, Redington AE, Djukanovic R, Holgate ST, Polak JM, Neuropeptide-containing nerves in endobronchial biopsies from asthmatic and nonasthmatic subjects. Am. J. Respir. Cell Mol. Biol 13, 288–296 (1995). [DOI] [PubMed] [Google Scholar]
- 21.Scott GD, Fryer AD, Jacoby DB, Quantifying nerve architecture in murine and human airways using three-dimensional computational mapping. Am. J. Respir. Cell Mol. Biol 48, 10–16 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott GD, Blum ED, Fryer AD, Jacoby DB, Tissue optical clearing, three-dimensional imaging, and computer morphometry in whole mouse lungs and human airways. Am. J. Respir. Cell Mol. Biol 51, 43–55 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.U.S. Department of Health and Human Services, National Institutes of Health (NIH), “Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma” (EPR-3, U.S. Department of Health and Human Services, NIH, 2007); http://www.nhlbi.nih.gov/health-pro/guidelines/current/asthma-guidelines.
- 24.McCracken JL, Veeranki SP, Ameredes BT, Calhoun WJ, Diagnosis and management of asthma in adults: A review. JAMA 318, 279–290 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Lee JJ, McGarry MP, Farmer SC, Denzler KL, Larson KA, Carrigan PE, Brenneise IE, Horton MA, Haczku A, Gelfand EW, Leikauf GD, Lee NA, Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J. Exp. Med 185, 2143–2156 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O’Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, Lenkiewicz E, Colbert D, Rinaldi L, Ackerman SJ, Irvin CG, Lee NA, Defining a link with asthma in mice congenitally deficient in eosinophils. Science 305, 1773–1776 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Peters SP, Bleecker ER, Kunselman SJ, Icitovic N, Moore WC, Pascual R, Ameredes BT, Boushey HA, Calhoun WJ, Castro M, Cherniack RM, Craig T, Denlinger LC, Engle LL, DiMango EA, Israel E, Kraft M, Lazarus SC, Lemanske RF Jr., Lugogo N, Martin RJ, Meyers DA, Ramsdell J, Sorkness CA, Sutherland ER, Wasserman SI, Walter MJ, Wechsler ME, Chinchilli VM, Szefler SJ; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network, Predictors of response to tiotropium versus salmeterol in asthmatic adults. J. Allergy Clin. Immunol 132, 1068–1074.e1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ebenezer GJ, Hauer P, Gibbons C, McArthur JC, Polydefkis M, Assessment of epidermal nerve fibers: A new diagnostic and predictive tool for peripheral neuropathies. J. Neuropathol. Exp. Neurol 66, 1059–1073 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Kajekar R, Pieczarka EM, Smiley-Jewell SM, Schelegle ES, Fanucchi MV, Plopper CG, Early postnatal exposure to allergen and ozone leads to hyperinnervation of the pulmonary epithelium. Respir. Physiol. Neurobiol 155, 55–63 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Fajac I, Braunstein G, Ickovic M-R, Lacronique J, Frossard N, Selective recruitment of eosinophils by substance P after repeated allergen exposure in allergic rhinitis. Allergy 50, 970–975 (1995). [DOI] [PubMed] [Google Scholar]
- 31.Pan J, Rhode HK, Undem BJ, Myers AC, Neurotransmitters in airway parasympathetic neurons altered by neurotrophin-3 and repeated allergen challenge. Am. J. Respir. Cell Mol. Biol 43, 452–457 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lieu TM, Myers AC, Meeker S, Undem BJ, TRPV1 induction in airway vagal low-threshold mechanosensory neurons by allergen challenge and neurotrophic factors. Am. J. Physiol. Lung Cell. Mol. Physiol 302, L941–L948 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheung D, van der Veen H, den Hartigh J, Dijkman JH, Sterk PJ, Effects of inhaled substance P on airway responsiveness to methacholine in asthmatic subjects in vivo. J. Appl. Physiol 77, 1325–1332 (1994). [DOI] [PubMed] [Google Scholar]
- 34.Chu HW, Kraft M, Krause JE, Rex MD, Martin RJ, Substance P and its receptor neurokinin 1 expression in asthmatic airways. J. Allergy Clin. Immunol 106, 713–722 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Nieber K, Baumgarten C, Rathsack R, Furkert J, Laake E, Müller S, Kunkel G, Effect of azelastine on substance P content in bronchoalveolar and nasal lavage fluids of patients with allergic asthma. Clin. Exp. Allergy 23, 69–71 (1993). [DOI] [PubMed] [Google Scholar]
- 36.Ichinose M, Miura M, Yamauchi H, Kageyama N, Tomaki M, Oyake T, Ohuchi Y, Hida W, Miki H, Tamura G, Shirato K, A neurokinin 1-receptor antagonist improves exercise-induced airway narrowing in asthmatic patients. Am. J. Respir. Crit. Care Med 153, 936–941 (1996). [DOI] [PubMed] [Google Scholar]
- 37.Boot JD, de Haas S, Tarasevych S, Roy C, Wang L, Amin D, Cohen J, Sterk PJ, Miller B, Paccaly A, Burggraaf J, Cohen AF, Diamant Z, Effect of an NK1/NK2 receptor antagonist on airway responses and inflammation to allergen in asthma. Am. J. Respir. Crit. Care Med 175, 450–457 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Kraan J, Vink-Klooster H, Postma DS, The NK-2 receptor antagonist SR 48968C does not improve adenosine hyperresponsiveness and airway obstruction in allergic asthma. Clin. Exp. Allergy 31, 274–278 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Numao T, Agrawal DK, Neuropeptides modulate human eosinophil chemotaxis. J. Immunol 149, 3309–3315 (1992). [PubMed] [Google Scholar]
- 40.Dunzendorfer S, Wiedermann CJ, Neuropeptide-induced chemotaxis of eosinophils in pulmonary diseases. Ann. Med 32, 429–439 (2000). [DOI] [PubMed] [Google Scholar]
- 41.Fryer AD, Stein LH, Nie Z, Curtis DE, Evans CM, Hodgson ST, Jose PJ, Belmonte KE, Fitch E, Jacoby DB, Neuronal eotaxin and the effects of CCR3 antagonist on airway hyperreactivity and M2 receptor dysfunction. J. Clin. Invest 116, 228–236 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yost BL, Gleich GJ, Jacoby DB, Fryer AD, The changing role of eosinophils in long-term hyperreactivity following a single ozone exposure. Am. J. Physiol. Lung Cell. Mol. Physiol 289, L627–L635 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Durcan N, Costello RW, McLean WG, Blusztajn J, Madziar B, Fenech AG, Hall IP, Gleich GJ, McGarvey L, Walsh M-T, Eosinophil-mediated cholinergic nerve remodeling. Am. J. Respir. Cell Mol. Biol 34, 775–786 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Akasheh N, Walsh M-T, Costello RW, Eosinophil peroxidase induces expression of cholinergic genes via cell surface neural interactions. Mol. Immunol 62, 37–45 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Gundel RH, Letts LG, Gleich GJ, Human eosinophil major basic protein induces airway constriction and airway hyperresponsiveness in primates. J. Clin. Invest 87, 1470–1473 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coyle AJ, Ackerman SJ, Burch R, Proud D, Irvin CG, Human eosinophil-granule major basic protein and synthetic polycations induce airway hyperresponsiveness in vivo dependent on bradykinin generation. J. Clin. Invest 95, 1735–1740 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee L-Y, Gu Q, Gleich GJ, Effects of human eosinophil granule-derived cationic proteins on C-fiber afferents in the rat lung. J. Appl. Physiol 91, 1318–1326 (2001). [DOI] [PubMed] [Google Scholar]
- 48.Gu Q, Wiggers ME, Gleich GJ, Lee L-Y, Sensitization of isolated rat vagal pulmonary sensory neurons by eosinophil-derived cationic proteins. Am. J. Physiol. Lung Cell. Mol. Physiol 294, L544–L552 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Belvisi MG, Birrell MA, Khalid S, Wortley MA, Dockry R, Coote J, Holt K, Dubuis E, Kelsall A, Maher SA, Bonvini S, Woodcock A, Smith JA, Neurophenotypes in airway diseases. Insights from translational cough studies. Am. J. Respir. Crit. Care Med 193, 1364–1372 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacoby DB, Gleich GJ, Fryer AD, Human eosinophil major basic protein is an endogenous allosteric antagonist at the inhibitory muscarinic M2 receptor. J. Clin. Invest 91, 1314–1318 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adamko DJ, Yost BL, Gleich GJ, Fryer AD, Jacoby DB, Ovalbumin sensitization changes the inflammatory response to subsequent parainfluenza infection. Eosinophils mediate airway hyperresponsiveness, m2 muscarinic receptor dysfunction, and antiviral effects. J. Exp. Med 190, 1465–1478 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schultheis AH, Bassett DJ, Fryer AD, Ozone-induced airway hyperresponsiveness and loss of neuronal M2 muscarinic receptor function. J. Appl. Physiol 76, 1088–1097 (1994). [DOI] [PubMed] [Google Scholar]
- 53.Proskocil BJ, Bruun DA, Garg JA, Villagomez CC, Jacoby DB, Lein PJ, Fryer AD, The influence of sensitization on mechanisms of organophosphorus pesticide-induced airway hyperreactivity. Am. J. Respir. Cell Mol. Biol 53, 738–747 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fryer AD, Jacoby DB, Function of pulmonary M2 muscarinic receptors in antigen-challenged guinea pigs is restored by heparin and poly-L-glutamate. J. Clin. Invest 90, 2292–2298 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ayala LE, Ahmed T, Is there loss of protective muscarinic receptor mechanism in asthma? Chest 96, 1285–1291 (1989). [DOI] [PubMed] [Google Scholar]
- 56.Minette PA, Lammers JW, Dixon CM, McCusker MT, Barnes PJ, A muscarinic agonist inhibits reflex bronchoconstriction in normal but not in asthmatic subjects. J. Appl. Physiol 67, 2461–2465 (1989). [DOI] [PubMed] [Google Scholar]
- 57.Kobayashi H, Gleich GJ, Butterfield JH, Kita H, Human eosinophils produce neurotrophins and secrete nerve growth factor on immunologic stimuli. Blood 99, 2214–2220 (2002). [DOI] [PubMed] [Google Scholar]
- 58.Noga O, Englmann C, Hanf G, Grutzkau A, Seybold J, Kunkel G, The production, storage and release of the neurotrophins nerve growth factor, brain-derived neurotrophic factor and neurotrophin-3 by human peripheral eosinophils in allergics and non-allergics. Clin. Exp. Allergy 33, 649–654 (2003). [DOI] [PubMed] [Google Scholar]
- 59.Foster EL, Simpson EL, Fredrikson LJ, Lee JJ, Lee NA, Fryer AD, Jacoby DB, Eosinophils increase neuron branching in human and murine skin and in vitro. PLOS ONE 6, e22029 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walsh M-T, Connell K, Sheahan AM, Gleich GJ, Costello RW, Eosinophil peroxidase signals via epidermal growth factor-2 to induce cell proliferation. Am. J. Respir. Cell Mol. Biol 45, 946–952 (2011). [DOI] [PubMed] [Google Scholar]
- 61.Hennigan K, Conroy PJ, Walsh M-T, Amin M, O’Kennedy R, Ramasamy P, Gleich GJ, Siddiqui Z, Glynn S, McCabe O, Mooney C, Harvey BJ, Costello RW, McBryan J, Eosinophil peroxidase activates cells by HER2 receptor engagement and (β1-integrin clustering with downstream MAPK cell signaling. Clin. Immunol 171, 1–11 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morgan RK, Costello RW, Durcan N, Kingham PJ, Gleich GJ, McLean WG, Walsh M-T, Diverse effects of eosinophil cationic granule proteins on IMR-32 nerve cell signaling and survival. Am. J. Respir. Cell Mol. Biol 33, 169–177 (2005). [DOI] [PubMed] [Google Scholar]
- 63.Walsh M-T, Curran DR, Kingham PJ, Morgan RK, Durcan N, Gleich GJ, McLean WG, Costello RW, Effect of eosinophil adhesion on intracellular signaling in cholinergic nerve cells. Am. J. Respir. Cell Mol. Biol 30, 333–341 (2004). [DOI] [PubMed] [Google Scholar]
- 64.Kingham PJ, McLean WG, Walsh M-T, Fryer AD, Gleich GJ, Costello RW, Effects of eosinophils on nerve cell morphology and development: The role of reactive oxygen species and p38 MAP kinase. Am. J. Physiol. Lung Cell. Mol. Physiol 285, L915–L924 (2003). [DOI] [PubMed] [Google Scholar]
- 65.Fryer AD, Costello RW, Yost BL, Lobb RR, Tedder TF, Steeber DA, Bochner BS, Antibody to VLA-4, but not to L-selectin, protects neuronal M2 muscarinic receptors in antigen-challenged guinea pig airways. J. Clin. Invest 99, 2036–2044 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Evans CM, Jacoby DB, Fryer AD, Effects of dexamethasone on antigen-induced airway eosinophilia and M2 receptor dysfunction. Am. J. Respir. Crit. Care Med 163, 1484–1492 (2001). [DOI] [PubMed] [Google Scholar]
- 67.Moreno L, Jacoby DB, Fryer AD, Dexamethasone prevents virus-induced hyperresponsiveness via multiple mechanisms. Am. J. Physiol. Lung Cell. Mol. Physiol 285, L451–L455 (2003). [DOI] [PubMed] [Google Scholar]
- 68.Gibbons CH, Wang N, Freeman R, Capsaicin induces degeneration of cutaneous autonomic nerve fibers. Ann. Neurol 68, 888–898 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wehrfritz A, Namer B, Ihmsen H, Mueller C, Filitz J, Koppert W, Leffler A, Differential effects on sensory functions and measures of epidermal nerve fiber density after application of a lidocaine patch (5%) on healthy human skin. Eur. J. Pain 15, 907–912 (2011). [DOI] [PubMed] [Google Scholar]
- 70.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D’Agostino R Jr., Castro M, Curran-Everett D, Fitzpatrick AM, Gaston B, Jarjour NN, Sorkness R, Calhoun WJ, Chung KF, Comhair SAA, Dweik RA, Israel E, Peters SP, Busse WW, Erzurum SC, Bleecker ER; National Heart, Lung, and Blood Institute’s Severe Asthma Research Program, Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med 181, 315–323 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hislop AA, Wharton J, Allen KM, Polak JM, Haworth SG, Immunohistochemical localization of peptide-containing nerves in human airways: Age-related changes. Am. J. Respir. Cell Mol. Biol 3, 191–198 (1990). [PubMed] [Google Scholar]
- 72.Juniper EF, Guyatt GH, Epstein RS, Ferrie PJ, Jaeschke R, Hiller TK, Evaluation of impairment of health related quality of life in asthma: Development of a questionnaire for use in clinical trials. Thorax 47, 76–83 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Airway innervation was not associated with sex, body mass index, or age.
Table S1. Raw data of airway nerve modeling and eosinophilia in mice.
Movie S1. Quantitative 3D model of airway epithelial sensory nerves from a healthy nonasthmatic subject.
Movie S2. Quantitative 3D model of airway epithelial sensory nerves from a subject with moderate persistent asthma.
Movie S3. Quantitative 3D model of airway epithelial sensory nerves costained for substance P from a subject with moderate persistent asthma.