Abstract
Background and Purpose
Stress‐related catecholamines have a role in cancer and β‐adrenoceptors; specifically, β2‐adrenoceptors have been identified as new targets in treating melanoma. Recently, β3‐adrenoceptors have shown a pleiotropic effect on melanoma micro‐environment leading to cancer progression. However, the mechanisms by which β3‐adrenoceptors promote this progression remain poorly understood. Catecholamines affect the immune system by modulating several factors that can alter immune cell sub‐population homeostasis. Understanding the mechanisms of cancer immune‐tolerance is one of the most intriguing challenges in modern research. This study investigates the potential role of β3‐adrenoceptors in immune‐tolerance regulation.
Experimental Approach
A mouse model of melanoma in which syngeneic B16‐F10 cells were injected in C57BL‐6 mice was used to evaluate the effect of β‐adrenoceptor blockade on the number and activity of immune cell sub‐populations (Treg, NK, CD8, MDSC, macrophages, and neutrophils). Pharmacological and molecular approaches with β‐blockers (propranolol and SR59230A) and specific β‐adrenoceptor siRNAs targeting β2‐ or β3‐adrenoceptors were used.
Key Results
Only β3‐, but not β2‐adrenoceptors, were up‐regulated under hypoxia in peripheral blood mononuclear cells and selectively expressed in immune cell sub‐populations including Treg, MDSC, and NK. SR59230A and β3‐adrenoceptor siRNAs increased NK and CD8 number and cytotoxicity, while they attenuated Treg and MDSC sub‐populations in the tumour mass, blood, and spleen. SR59230A and β3‐adrenoceptor siRNAs increased the ratio of M1/M2 macrophages and N1 granulocytes.
Conclusions and Implications
Our data suggest that β3‐adrenoceptors are involved in immune‐tolerance, which opens the way for new strategic therapies to overcome melanoma growth.
Linked Articles
This article is part of a themed section on Adrenoceptors—New Roles for Old Players. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.14/issuetoc
Abbreviations
- CD8
CD8 T cells
- Ad
adrenaline
- MDSC
myeloid‐derived suppressor cells
- NA
noradrenaline
- PBMC
peripheral blood mononuclear cells
- Treg
regulatory T cells
What is already known about this subject
β‐adrenoceptors have been identified as new targets in treating melanoma.
β‐adrenergic system is one of the major players in the regulation of the immune system.
What this study adds
β3‐adrenoceptors are involved in mediating the switch from an immunocompetent to an immunosuppressive tumor microenvironment.
β3‐adrenoceptors blockade reduces the growth of melanoma inducing a reversion of immune‐tolerance.
What is the clinical significance
β3‐adrenoceptors blockade could represent a new strategy to overcome melanoma immune‐editing.
1. INTRODUCTION
Several studies demonstrate that tumour neurogenesis or stress‐related catecholamines, noradrenaline (NA) and adrenaline (Ad), accelerate cancer progression and reduce the overall survival of patients (Cole & Sood, 2012; Magnon et al., 2013). The increased secretion of catecholamines usually promotes favourable environment for tumour cells to grow and metastasize predominantly by acting at β‐adrenoceptors (Entschladen, Drell, Lang, Joseph, & Zaenker, 2004). Signalling activated by β‐adrenoceptors regulates tumour growth, progression, and metastasis by influencing a number of cellular and molecular processes (Armaiz‐Pena et al., 2013; Cheng et al., 2018).
There is evidence that stress‐related catecholamines enhance tumour growth mainly through β2‐adrenoceptors and that non‐selective β‐adrenoceptor blockers (acting at β1‐ and β2‐adrenoceptors) provide protection against different types of cancer (Childers, Hollenbeak, & Cheriyath, 2015; Yazawa et al., 2016). Melanoma, like other tumours, shows a surprisingly positive response to propranolol, a β‐adrenoceptor blocker targeting β1‐ and β2‐adrenoceptors (Barbieri et al., 2012; Glasner et al., 2010), although the role of β1‐adrenoceptors in stimulating melanoma growth, and tumour growth in general, seems to be questionable (Armaiz‐Pena et al., 2013; Dal Monte et al., 2013; Thaker et al., 2006). In addition, propranolol reduces cell proliferation in human and murine melanoma cell lines (Moretti et al., 2013; Wrobel & Le Gal, 2015; Yang et al., 2009). Finally, clinical studies demonstrate the positive impact of β1‐ and/or β2‐adrenoceptor blockade in the overall survival of melanoma patients (De Giorgi et al., 2011; De Giorgi et al., 2017; Kokolus et al., 2017; Lemeshow et al., 2011), although these findings have been recently called into question (Livingstone et al., 2013; McCourt et al., 2014).
A role for β3‐adrenoceptors in melanoma has been proposed and recently reviewed (Dal Monte et al., 2018). In fact, the use of two different β3‐adrenoceptor blockers, SR59230A and L‐748337, is effective in reducing tumour growth in a mouse model of melanoma (Dal Monte et al., 2013; Sereni, Dal Monte, Filippi, & Bagnoli, 2015). In addition, SR59230A and L‐748337 as well as selective β3‐adrenoceptor siRNAs reduce the proliferation and induce apoptosis of human and mouse melanoma cells (Calvani et al., 2015; Dal Monte et al., 2013; Dal Monte et al., 2014), while β3‐adrenoceptor agonism stimulates melanoma cell proliferation and reduces apoptosis (Dal Monte et al., 2014). However, the mechanisms by which β3‐adrenoceptors promote melanoma growth are not yet fully elucidated.
There is evidence that mechanisms of immune‐tolerance, which are known to prevent autoimmune diseases, may be used by tumours to bypass the development of an effective immune response (King, Sharma, Davis, & Jimeno, 2018). Immune cells in cancer exhibit functional plasticity and undergo a dramatic phenotypic change, leading to an alternative activation promoting tumour progression by inducing immune‐tolerance (Granot & Fridlender, 2015). Currently, the mechanisms of cancer immune‐tolerance have not been completely clarified. Established tumours with higher mutation rates use various escape mechanisms to bypass immune‐surveillance including decreasing the number of cytotoxic immune cells such as NK and CD8 T cells (CD8) and/or increasing immune‐suppressive cells, the so‐called myeloid‐derived suppressor cells (MDSC) and regulatory T cells (Treg; Vinay et al., 2015). In addition, the phenotype of myeloid cells (macrophages and neutrophils), which plays a key role within the immunosuppressive network, may be altered by the tumour micro‐environment: an environment rich in M2 macrophages and N2 neutrophils enhances immune‐escape and supports tumour growth (Schouppe, De Baetselier, Van Ginderachter, & Sarukhan, 2012).
The β‐adrenergic system has been identified as one of the major players in the regulation of the immune system. In this context, catecholamines bind to specific receptors, in particular β2‐adrenoceptors (Nance & Sanders, 2007), on white blood cells and have diverse regulatory effects on the distribution and function of these cells that mainly result in immunosuppression. For instance, the stimulation of β2‐adrenoceptors inhibits lymphocyte responses, NK cytotoxicity, and dendritic cell functions (Marino & Cosentino, 2013). In addition, β‐adrenoceptor signalling significantly suppresses the proliferation and the cytolytic killing ability of cytotoxic CD8 cells as well as their capability to produce IFN‐γ (Nissen, Sloan, & Mattarollo, 2018). Moreover, MDSC are increased in patients with breast cancer characterized by high levels of stress (Mundy‐Bosse, Thornton, Yang, Andersen, & Carson, 2011). Finally, studies using rodent models of different tumours have shown that catecholamines or stress suppress NK cell activity leading to tumour metastasis, likely through β2‐adrenoceptor stimulation (Inbar et al., 2011).
Melanoma is one of the most immunogenic tumours, and it is highly sensitive to immune therapeutic agents (Tawbi et al., 2018). Of note, the melanoma micro‐environment is enriched in tumour‐associated M2 macrophages, Treg and MDSC, which promote the defective cytotoxicity of T cells (Fujimura, Kambayashi, & Aiba, 2012). In addition, melanoma cells inhibit the activity of CD8 and NK cells through the production of negative modulators such as VEGF, IL‐8, and IL‐10 (Passarelli, Mannavola, Stucci, Tucci, & Silvestris, 2017). Little is known about a role of β‐adrenoceptors in regulating the immune environment in melanoma, the only evidence being limited to the findings that β‐adrenergic stimulation recruits and polarizes macrophages, thus promoting tumour progression (Cole, Nagaraja, Lutgendorf, Green, & Sood, 2015) and that β2‐adrenoceptor blockade improves the anti‐tumour efficacy of immunotherapy (Kokolus et al., 2017).
This study evaluates the potential role of β3‐adrenoceptors in the regulation of melanoma immune‐tolerance by investigating the effects of its antagonism on cytotoxic and suppressive immune cell sub‐populations. In addition, the regulatory role of β3‐adrenoceptors was compared with that of β2‐adrenoceptors by the use of pharmacological and molecular approaches. This research suggests the possibility that β3‐adrenoceptor antagonism could reduce melanoma growth in vivo by increasing the number of NK and CD8 cells as well as their cytotoxicity and by attenuating Treg and MDSC sub‐populations in the tumour micro‐environment. A shift in macrophage and neutrophil phenotypes from both M2 to M1 and N2 to N1 was also observed after β3‐adrenoceptor blockade.
2. METHODS
2.1. Cell cultures
Murine B16‐F10 melanoma cell lines were obtained from American Type Culture Collection (ATCC, Cat# CRL‐6475, RRID:CVCL_0159). Cells were maintained in DMEM containing 10% fetal calf serum (Euroclone, Milan, Italy), 2 mM l‐glutamine, 100 U·ml−1 penicillin and 100 μg·ml−1 streptomycin at 37°C in 5% CO2. The cell lines have been mycoplasma tested (Euroclone, Milan, Italy).
2.2. In vivo transfection assay
β2‐siRNA (SASI_Mm01 00154297) and β3‐siRNA (SASI_Mm01_00145466) were complexed with 200 μl of Invivofectamine reagent‐plasmid duplex (reagent for in vivo plasmid delivery, Invitrogen, Carlsbad, CA, USA) and were injected into the tail vein when a palpable tumour was formed (as described below). In vitro transfection assay with β2‐ and β3‐siRNA was performed by using Polyplus INTERFERin siRNA Transfection Reagent. The efficiency of β2‐ and β3‐siRNA was assessed by cytofluorymetric analysis of protein expression (Figure S1).
2.3. Western blot
The expression of β‐adrenoceptors was evaluated in murine lymphocytes, isolated from mice and cultured in normoxia (24 hr at 21% O2), hypoxia (24 hr at 1% O2), and 1 hr of normoxic re‐exposure after 24 hr of hypoxia. Cell lysates were prepared in an appropriate volume of RIPA. After protein quantification (Bradford, Biorad, Hercules, CA, USA), 20 μg of proteins from total lysates were subjected to SDS‐PAGE and western blot analysis as reported in previous work. Rabbit polyclonal antibodies directed to β2‐adrenoceptors (Santa Cruz Biotechnology Cat# sc‐569, RRID:AB_630926) and β3‐adrenoceptors (Santa Cruz Biotechnology Cat# sc‐50436, RRID:AB_781613) have been recently validated (Sereni et al., 2015).
The immuno‐related procedures used comply with the recommendations made by the British Journal of Pharmacology.
2.4. Co‐cultures and MTT assay
Tumour cells were seeded in MW24 and pretreated with propranolol or SR59230A (10 μM) for 24 hr. Subsequently, the tumour cells were washed with PBS and co‐cultured with peripheral blood mononuclear cells (PBMC; pretreated or not with propranolol or SR59230A [10 μM] for 24 hr) in a seeding ratio of 1:3 for the next 24 hr. At the end of the total incubation period of 48 hr, PBMC were withdrawn from the medium of the co‐culture and analysed by FACS, while tumour cells were tested for viability. Viability of tumour cells, in all conditions, was evaluated using an MTT ((3‐[4,5‐dimethylthiazol‐2‐yl]‐2,5‐diphenyltetrazolium bromide; thiazolyl blue) assay (Sigma Aldrich, Saint Louis, MO, USA) following the manufacturer's instructions. The intensity of the absorbance at 550 nm was evaluated using a spectrophotometer (FlexStation3, Molecular Devices). The same experiment was performed in each tumour cell line silenced or not with Scr‐siRNA, β2‐siRNA, and β3‐siRNA.
2.5. Mice
In vivo experiments and tissue collection were carried out according to the European Union (EU) guidelines for animal care procedures and the Italian legislation (DLgs 26/2014) application of the EU Directive 2010/63/EU. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010) and with the recommendations made by the British Journal of Pharmacology. Studies were conducted under University of Florence and Italian Health Minister research permits No. 401/2015‐PR. C57BL/6 mice (male, 20–25 g, 5–6 weeks‐old; IMSR Cat# JAX:000664, RRID:IMSR_JAX:000664) were used. Animals were housed in a temperature and humidity‐controlled vivarium (12 hr dark/light cycle, free access to food and water, maximum 10 animals per cage). All the experiments were performed in a quiet, temperature‐controlled room (20–22°C). Animals were killed by the use of inhaled CO2 plus 10–50% O2.
2.6. Mouse B16‐F10 syngeneic model and treatments
B16‐F10 were implanted in C57BL/6 recipient mice by injecting 1 × 106 cells in 200 μl PBS s.c. in the right flank of the mice. Mice were monitored daily. After 8–10 days, when B16‐F10 cells formed a palpable tumour, the treatment was started. The treatments were administered twice a day with a window time of 4–6 hr between each treatment. Diluted DMSO (vehicle), propranolol (20 mg·kg−1·day−1, Saint Louis, MO, USA), and SR59230A, CAS: 174689–39‐5 (20 mg·kg−1·day−1, Sigma Aldrich, Saint Louis, MO, USA) were injected i.p. Mice were killed on Days 7 and 14 of treatment; peripheral blood was collected; tumour and spleen were weighed and measured. B16‐F10‐GFP cell lines (Creative Biogene Biotechnology Cat# CSC‐RR0109, RRID:CVCL_QZ86) were injected in C57BL/6 mice to precisely discriminate the tumour cells from tumour stroma.
2.7. In vivo MRI
For the in vivo MRI, C57BL/6 mice were fully anaesthetized with Avertin (2,2,2‐tribromoethanol; 0.3 mg·g−1 of body weight) intraperitoneal somministration, for the acquisition procedure. Anesthetic depth in each group was assessed with noxious stimuli using atraumatic forceps. T2‐weighted scans of the mice were performed using 7 T Bruker PharmaScan MRI scanner. T2‐weighted images were acquired by spin‐echo sequences with TR = 1642 ms, TE = 25 ms, FOV = 4.0 × 4.0 cm, matrix size = 256 × 256, 15 slices and slice thickness of 1 mm.
2.8. Isolation of tumour cells and preparation of spleen and blood cells
Mouse tumour tissues were minced with scissors and incubated in C‐Tubes (Miltenyi Biotec, Gladbach, Germany) containing a storage tissue solution (Miltenyi Biotec, Gladbach, Germany). Tumour samples were then homogenized using a Tumour Dissociation Kit (Miltenyi Biotec, Gladbach, Germany) and the heating function of the gentle MACS Octo Dissociator (Miltenyi Biotec, Gladbach, Germany) with an appropriate heater programme. After homogenization, the samples were filtered with pre‐separation filters to remove cell aggregates or large particles. The lymphocyte cells were then separated by tumour sample, using anti‐CD45 beads (Miltenyi Biotec, Gladbach, Germany) and AutoMACS separator Pro (Miltenyi Biotec, Gladbach, Germany), according to the manufacturer's instructions. Mouse spleens were homogenized in PBS using gentleMACS Octo Dissociator and then filtered with pre‐separation filters. Mouse blood was diluted in Red Blood Cell Lysis Solution 10× (Miltenyi Biotec, Gladbach, Germany), for optimal lysis of erythrocytes, according to the manufacturer's instructions.
2.9. Flow cytometry and morphological analysis
Cells isolated from mouse tumours, spleens, and blood were incubated and stained with appropriate dilutions of various combinations of the following fluorochrome‐conjugated antibodies: anti‐CD 45‐VioBlue (Miltenyi Biotec Cat# 130‐092‐880, RRID:AB_1103220) or VioGreen (Miltenyi Biotec Cat# 130‐096‐906, RRID:AB_2660419), anti‐NKp46‐FITC (Miltenyi Biotec Cat# 130‐102‐300, RRID:AB_2661345), anti‐CD8a‐APC Vio 770 (Miltenyi Biotec Cat# 130‐102‐305, RRID:AB_2659897), anti‐CD3e (17A2)‐PE Vio 770 (Miltenyi Biotec Cat# 130‐105‐461, RRID:AB_2657921), anti‐CD107‐PE (Miltenyi Biotec Cat# 130‐111‐318, RRID:AB_2654464), anti‐CD161(NK1.1)‐PerCP Vio700 (Miltenyi Biotec Cat# 130‐117‐773, RRID:AB_2728038), anti‐CD25‐PE (Miltenyi Biotec Cat# 130‐102‐593, RRID:AB_2660259), anti‐CD4‐FITC (Miltenyi Biotec Cat# 130‐102‐541, RRID:AB_2659902), anti‐CD127‐APC (Miltenyi Biotec Cat# 130‐110‐274, RRID:AB_2654842), anti‐CD11b‐PerCP Vio700 (Miltenyi Biotec Cat# 130‐109‐289, RRID:AB_2654659), anti‐Gr1‐PE (Miltenyi Biotec Cat# 130‐102‐426, RRID:AB_2659861), anti‐CD95 (Fas)‐APC (Miltenyi Biotec Cat# 130‐106‐907, RRID:AB_2659651), anti‐F4/80‐PerCP Vio700 (Miltenyi Biotec Cat# 130‐102‐161, RRID:AB_2651711), anti‐CD16/32‐VioBright FITC (Miltenyi Biotec Cat# 130‐108‐364, RRID:AB_2660221), anti‐CD11c (integrin, α X subunit)‐APC Vio770 (Miltenyi Biotec Cat# 130‐107‐461, RRID:AB_2660162), anti‐IL‐10‐APC (Miltenyi Biotec Cat# 130‐102‐349, RRID:AB_2660626), anti‐integrin α 7‐APC (Miltenyi Biotec Cat# 130‐102‐717, RRID:AB_2652466), anti‐iNOS‐APC (Santa Cruz Biotechnology Cat# sc‐7271, RRID:AB_627810), anti‐Arg1‐FITC (R and D Systems Cat# IC5868F, RRID:AB_10718118), anti‐β2‐FITC (Biorbyt Cat# orb15065, RRID:AB_10735676), anti‐β3‐PE (Biorbyt Cat# orb124479, RRID:AB_2783863), or PerCP Vio700 (Biorbyt Cat# orb123003, RRID:AB_2783864). For intracellular staining, the cells were further permeabilized using Inside Stain Kit (Miltenyi Biotec, Gladbach, Germany) and then stained for iNOS, IL‐10, β2‐adrenoceptors, and β3‐adrenoceptors. The stained cells were acquired on a MACSQuant Analyzer 10 Flow Cytometer (Miltenyi Biotec, Gladbach, Germany), and the data were processed using Flowlogic software (Miltenyi Biotec, Gladbach, Germany).
2.10. Statistical analysis
Statistical analysis was performed using the SAS 9.2 software. Values are presented as mean ± SD. Differences with P < 0.05 were considered significant. For a t test with Bonferroni correction for multiple comparison, an expected tumour growing difference of 2.5 cm3 between groups (8, 5.5, and 3 cm3 in vehicle, propranolol, and SR group, respectively), an SD for each group of 2 cm3 (Sereni et al., 2015), and a first type error set to 1.7%, six mice per group were needed to guarantee a power of 80%. Allocation concealment was performed using a randomization procedure (http://www.randomizer.org/). To asses normal distribution and homoscedasticity for each quantitative outcome in each group, Kolmogorov–Smirnov's test and Bartlett's test were used, respectively. In order to evaluate the difference in quantitative outcomes between groups, according to normality and homoscedasticity tests results, ANOVA and post hoc t test with Bonferroni correction for multiple comparison or Welch ANOVA and post hoc Satterthwait t test with Bonferroni correction for multiple comparison or Kruskal–Wallis and Dwass, Steel, Critchlow–Fligner method for multiple comparison were used. A post hoc test was performed only if ANOVA, Welch ANOVA, or Kruskal–Wallis analysis were statistically significant. The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2018).
2.11. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Fabbro et al., 2017a,b; Alexander, Christopoulos, et al., 2017; Alexander, Kelly et al., 2017).
3. RESULTS
3.1. β‐adrenoceptors expressed in immune cells influence melanoma cell viability through an effect on immune cells
We first analysed the expression of both receptors in PBMC and in immune cell sub‐populations to assess whether β2‐ and β3‐adrenoceptors could be implicated in regulating melanoma cell viability by affecting the immune system. Previous studies demonstrated β2‐adrenoceptor expression in immune cell sub‐populations such as NK (Maisel, Fowler, Rearden, Motulsky, & Michel, 1989), CD8 (Estrada, Ağaç, & Farrar, 2016), and Treg (Guereschi et al., 2013) cells, without exploring the presence of β3‐adrenoceptors. In this study, we have observed that both β2‐ and β3‐adrenoceptors were expressed in mouse PBMC, but only β3‐adrenoceptors were up‐regulated under hypoxic conditions, used to mimic the tumour micro‐environment, and fast down‐regulated after oxygen re‐exposure (Figure 1a). We then performed a cytofluorimetric analysis to evaluate the expression of β2‐ and β3‐adrenoceptors in different PBMC sub‐populations isolated from either blood or tumour tissue, including NK, Treg cells, and MDSC. The expression of β2‐adrenoceptors in NK, Treg cells, and MDSC infiltrating the tumour did not differ from that in circulating cells, while β3‐adrenoceptors were up‐regulated in the immune cell sub‐populations infiltrating the tumour (Figure 1b).
Figure 1.
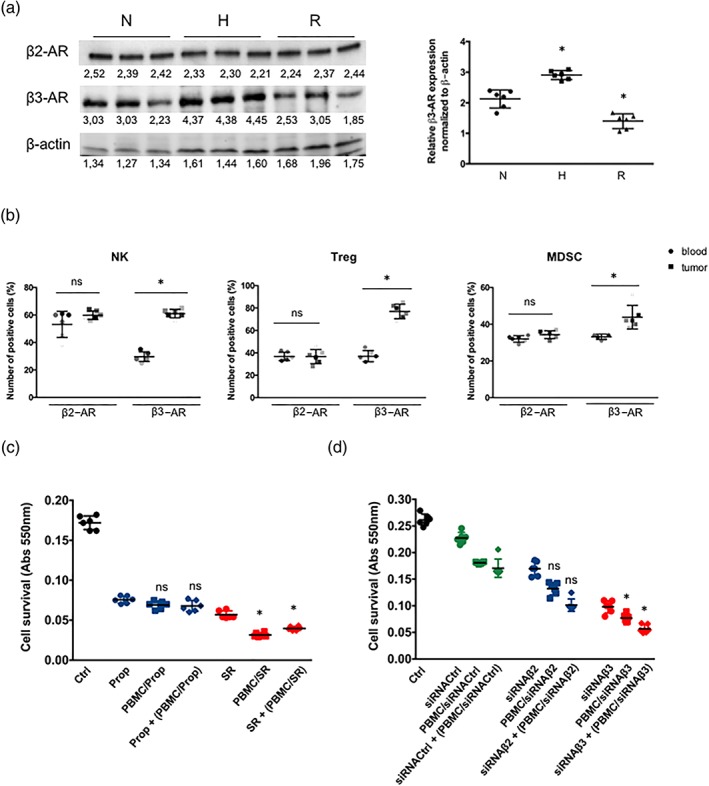
(a) Representative WB of β3‐adrenoceptors in murine lymphocytes after 24 hr of normoxic (N) or 24 hr hypoxic conditions (H) and 1 hr of normoxic re‐exposure (R) and relative densitometric quantification (n = 6). Results are reported as mean ± SD of relative expression normalized to β‐actin. *P < 0.05 hypoxic (H) and re‐exposure conditions compared with normoxic (N). (b) FACS quantification of β2‐ and β3‐adrenoceptors expression in blood and tumour infiltrating NK and Treg cells, and MDSC. *P < 0.05 β3‐adrenoceptor in tumour compared with β3‐adrenoceptor in blood (n = 6). (c) MTT cell viability assay in B16‐F10 cells untreated or treated with propranolol (Prop) or SR59230A (SR) and co‐cultured for 48 hr under hypoxic conditions with PBMC untreated or pretreated with Prop or SR. Dot plots show the changes of each treatment compared with Ctrl (n = 6). ns: not significant, *P < 0.05 PBMC/SR or SR + (PBMC/SR) compared with SR. (d) MTT cell viability assay in B16‐F10 cells silenced with siRNA‐Ctrl, siRNA‐β2, or siRNA‐β3 and co‐cultured for 48 hr under hypoxic conditions with PBMC untreated or pretreated with Ctrl‐siRNA, siRNA‐β2, or siRNA‐β3. Dot plots show the changes of each treatment compared with Ctrl (n = 6). ns: not significant, *P < 0.05 PBMC/siRNA‐β3 or siRNA‐β3 + (PBMC/siRNA‐β3) compared with siRNA‐β3
We evaluated the hypothesis that β3‐adrenoceptors expressed in PBMC, and in particular in tumour infiltrating lymphocytes, could affect tumour cell viability. We co‐cultured under hypoxic conditions B16‐F10 cells with mouse PBMC pre‐exposed to SR59230A, propranolol, or selective siRNAs targeting β2‐ or β3‐adrenoceptors to assess this hypothesis. The results show that co‐culturing B16‐F10 cells with PBMC pretreated with SR59230A induced an increase in cell death compared to melanoma cells treated with SR59230A only or cultured exclusively with PBMC. Pretreatment of PBMC with propranolol had no significant effects on cell viability. The silencing approach substantially confirmed that the effects obtained with SR59230A were due to the blockade of β3‐adrenoceptors (Figure 1c,d). The enhanced efficacy of SR59230A pretreatment in PBMC suggests that β3‐adrenoceptor antagonism, by acting on immune cells, might pilot cancer cell death.
3.2. Targeting β2‐ or β3‐adrenoceptors reduces tumour growth in a mouse model of melanoma
In line with previous findings (Dal Monte et al., 2013), additional investigation performed in this study allowed us to confirm that propranolol or SR59230A administered for 14 days significantly reduced tumour volume (Figure 2a), with a major effect of β3‐ over β2‐adrenoceptor blockade, as also confirmed by the greater efficacy of SR59230A and β3‐adrenoceptor silencing approach (Figure 2b). Moreover, the administration of the β2‐adrenoceptor agonist terbutaline to mice treated with β3‐adrenoceptor siRNA showed no growth rebound, thus confirming the predominant role of β3‐adrenoceptor subtype in controlling tumour growth. At the same time, the administration of the β3‐adrenoceptor agonist BRL37344 in β3‐adrenoceptor‐silenced mice did not affect tumour growth compared to siRNA‐β3‐adrenoceptor condition alone (Figure S2). The efficacy of propranolol and SR59230A in reducing the tumour volume was also demonstrated here by NMR (Figure 2c). The rate of tumour cell death in the tumour mass was analysed by cytofluorimetric analysis, injecting into C57BL/6 mice a stable B16‐F10 cell line expressing the GFP (B16‐F10‐GFP), to precisely discriminate the tumour cells from tumour stroma. Both propranolol and SR59230A induced an increased early apoptotic rate after 7 days of treatment, with an effect of SR59230A that was significantly higher than that of propranolol (Figure 2d). In addition, in the tumour mass, both propranolol and SR59230A, with a major effect of SR59230A, increased the apoptotic marker FAS (Figure 2e), which is known to bind to its ligand in NK cells or neutrophils, thus leading to apoptosis of tumour cells (Abrahams, Kamsteeg, & Mor, 2003). The early apoptotic rate after SR59230A treatment decreased after 14 days in favour of an extensive cell death in the tumour mass, as indicated by large areas of tissue necrosis (Figure 2f).
Figure 2.
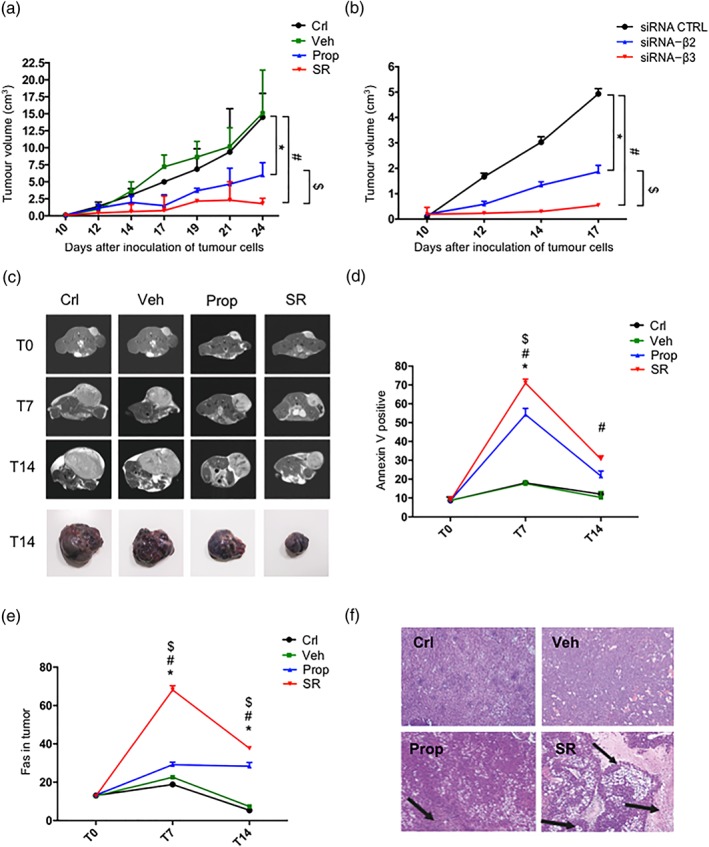
(a) Tumour growth rate in control‐ (Crl), vehicle‐ (Veh), propranolol (Prop)‐, and SR59230A (SR)‐ treated mice (n = 6). (b) Tumour growth rate in siRNA‐CTRL, siRNA‐β2, and siRNA‐β3 treated mice (n = 6). (c) MR images of mouse ventral section in control, vehicle‐, propranolol‐, and SR59230A‐treated mice (n = 6). (d) FACS analysis of AnnexinV positive cells in control, vehicle‐, propranolol‐, and SR59230A‐treated mice (n = 6). (e) FACS analysis of Fas marker expression in tumours of control, vehicle‐, propranolol ‐, and SR59230A‐treated mice (n = 6). (f) Representative fields of haematoxylin–eosin (H&E) staining at T14 in control, vehicle‐, propranolol‐, and SR59230A‐treated mice (n = 6). *P < 0.05 Prop‐ (or siRNA‐β2) compared with Veh‐; # P < 0.05 SR‐ (or siRNA‐β3) compared with Veh‐; $ P < 0.05 SR‐ (or siRNA‐β3) compared with Prop‐ (or siRNA‐β2)
3.3. Targeting β3‐adrenoceptors induces immune‐competent sub‐populations in the tumour micro‐environment
The possible role of immune cells expressing β‐adrenoceptors in counteracting melanoma growth was also investigated in the in vivo model treated with propranolol or SR59230A or with β2‐ or β3‐adrenoceptor siRNA. As shown in Figure 3a, the spleen weight, used as an indirect indicator of activation of the immune system, was significantly higher in SR59230A‐treated than in vehicle‐treated mice. In contrast, propranolol did not affect the spleen weight. The maximal effect of SR59230A was detected after 7 days of treatment. As shown in Figure 3b,c, the cytofluorimetric analysis of cells from the tumour mass of SR59230A‐treated mice showed an increased number of NK and CD8 cells, which was already detectable after 7 days of treatment. SR59230A also led to the activation of NK and CD8 cytotoxic activity as evidenced by the increased expression of the activation markers perforin (van den Broek & Hengartner, 2000) and CD107A (Aktas, Kucuksezer, Bilgic, Erten, & Deniz, 2009) respectively (Figure 3d,e). Propranolol did not affect the number of NK or CD8 cells or induce the expression of perforin or CD107A. The silencing approach confirmed the prevalent role played by β3‐adrenoceptors (Figure 3f,g).
Figure 3.
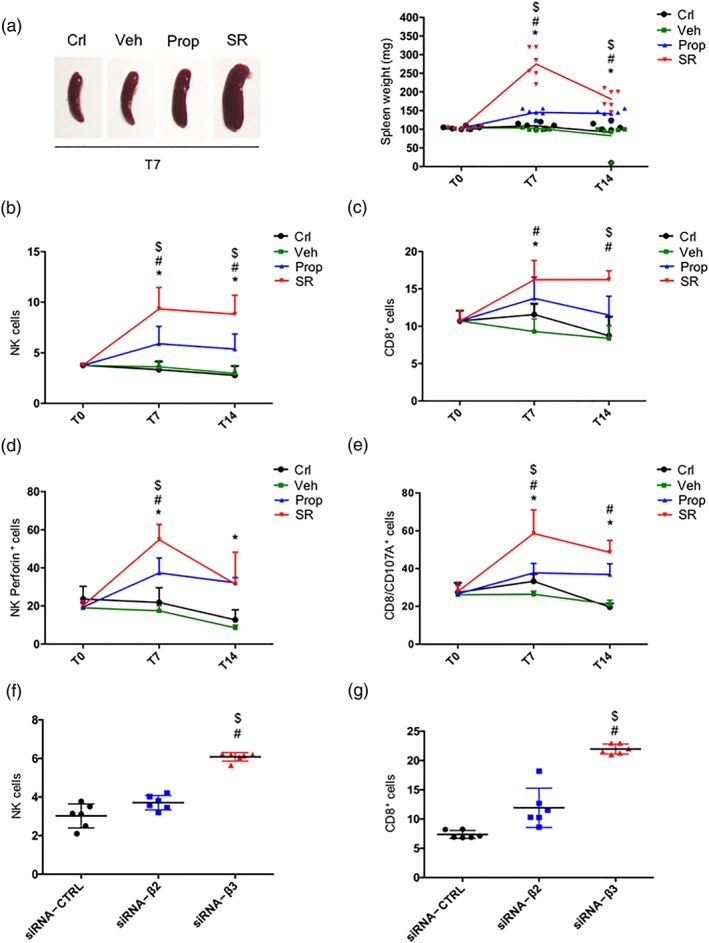
(a) Representative images of mouse spleens at T7 (n = 6; left) and mean weight of mouse spleens (right). (b) FACS analysis and quantification at T7 and T14 of NK (NKp46+/NK1.1+ gated on CD3−/CD45+). (c) FACS analysis and quantification at T7 and T14 of CD8+ (gated on CD45+). (d) FACS analysis and quantification at T7 and T14 of perforin expression on NKp46+/NK1.1+ cells. Two‐way ANOVA analysis was performed. (e) FACS analysis and quantification at T7 and T14 of CD8+ cytotoxic (CD107+ gated on CD8+). (f) FACS analysis and quantification at T14 of NK (NKp46+/NK1.1+ gated on CD3−/CD45+) cells in siRNA‐CTRL, siRNA‐β2, and siRNA‐β3 treated mice (n = 6). (g) FACS analysis and quantification at T14 of CD8+ (gated on CD45+) cells in siRNA‐CTRL, siRNA‐β2, and siRNA‐β3 treated mice (n = 6). *P < 0.05 Prop‐ (or siRNA‐β2) compared with Veh‐; # P < 0.05 SR‐ (or siRNA‐β3) compared with Veh‐; $ P < 0.05 SR‐ (or siRNA‐β3) compared with Prop‐ (or siRNA‐β2)
3.4. Prevalent role of β3‐adrenoceptors in reducing immune‐suppressive sub‐populations in the tumour micro‐environment
We also analysed the number of Treg and MDSC sub‐populations in excised tumour mass, as shown in Figure 4a,b. Treatments with either propranolol or SR59230A reduced Treg and MDSC immune‐suppressive sub‐populations with the effect of SR59230A being significantly higher than that of propranolol. In addition, the CD8/Treg ratio was significantly increased by SR59230A, while it was not affected by propranolol (Figure 4c). Furthermore, the expression of the inducible form of NOS (iNOS), a landmark of the immune‐suppressed phenotype in MDSC (Mazzoni et al., 2002), was reduced by both propranolol and, more effectively, by SR59230A (Figure 4d). The silencing approach substantially confirmed the immune‐suppressive action of β3‐adrenoceptors (Figure 4e,f).
Figure 4.
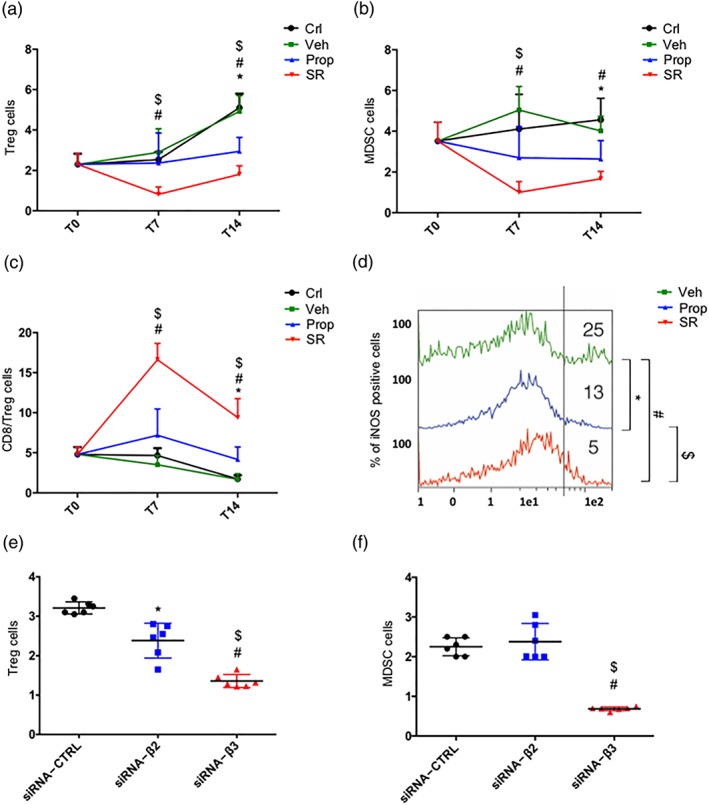
(a) FACS analysis and quantification at T7 and T14 of Treg (CD25+/CD127− gated on CD45+/CD4+). (b) FACS analysis and quantification at T7 and T14 of MDSC (in CD11b+, GR1+ gated on CD45+). (c) FACS analysis and quantification at T7 and T14 of CD8+/Treg ratio. (d) iNOS expression in MDSC. (e) FACS analysis and quantification at T14 of Treg (CD25+/CD127− gated on CD45+/CD4+) in siRNA‐CTRL, siRNA‐β2, and siRNA‐β3 treated mice (n = 6). (f) FACS analysis and quantification at T14 of MDSC (in CD11b+, GR1+ gated on CD45+) in siRNA‐CTRL, siRNA‐β2, and siRNA‐β3 treated mice (n = 6). *P < 0.05 propranolol (Prop)‐ (or siRNA‐β2) compared with Veh‐; # P < 0.05 SR59230A (SR)‐ (or siRNA‐β3) compared with Veh‐; $ P < 0.05 SR‐ (or siRNA‐β3) compared with Prop‐ (or siRNA‐β2)
3.5. Prevalent role of β3‐adrenoceptors in inducing macrophage M1 and neutrophil N1 phenotypes in the tumour micro‐environment
An immune‐suppressive micro‐environment is characterized by a high density of M2 macrophages and N2 neutrophils, while an immune‐competent micro‐environment is characterized by the presence of M1 macrophages and N1 neutrophils (Fridlender et al., 2009; Mantovani & Locati, 2013). We observed that SR59230A, but not propranolol, increased the M1/M2 ratio in the tumour micro‐environment (Figure 5a), likely by reducing the M2 sub‐population, as evidenced by the reduced levels of IL‐10, a marker of M2 macrophages (Figure 5b). In addition, SR59230A, but not propranolol, increased the number of N1 cells and decreased the expression of arginase‐1, a marker of immune suppression in immune cells (Munder, 2009), in neutrophils (Figure 5c,d). The silencing approach substantially confirmed that the polarization of neutrophils and macrophages was influenced by both β2‐ and β3‐adrenoceptors but with β3‐adrenoceptors having a greater effect (Figure 5e,f).
Figure 5.
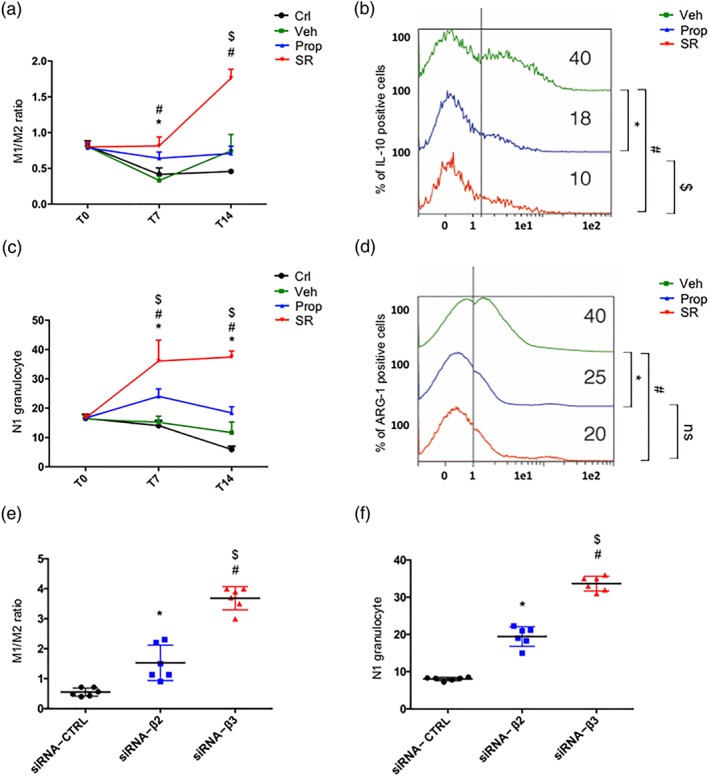
(a) FACS analysis and quantification at T7 and T14 of M1/M2 ratio on CD45+ cells. (b) IL‐10 expression in M2 macrophages. (c) FACS analysis and quantification at T7 and T14 of N1 granulocytes (CD54+, CD95+, and CD11b+). (d) Arg1 expression in N1 granulocytes. (e) FACS analysis and quantification at T14 of M1/M2 ratio on CD45+ cells in siRNA‐CTRL, siRNA‐β2, and siRNA‐β3 treated mice (n = 6). (f) FACS analysis and quantification at T14 of N1 granulocytes (CD54+, CD95+, and CD11b+) in siRNA‐CTRL, siRNA‐β2, and siRNA‐β3 treated mice (n = 6). *P < 0.05 propranolol (Prop)‐ (or siRNA‐β2) compared with Veh‐; # P < 0.05 SR59230A (SR)‐ (or siRNA‐β3) compared with Veh‐; $ P < 0.05 SR‐ (or siRNA‐β3) compared with Prop‐ (or siRNA‐β2)
3.6. Targeting β2‐ or β3‐adrenoceptors affects immune sub‐populations in the spleen and the blood
Results from the analysis of immune‐competent NK and CD8 sub‐populations in the spleen and the blood from mice treated with propranolol, SR59230A, or siRNAs were in line with those obtained in tumour infiltrating cells and demonstrated a major effect of β3‐adrenoceptor blockade (Figure 6a–d). However, propranolol and SR59230A were equally effective at reducing the immune‐suppressive Treg and MDSC sub‐populations in the spleen and the blood (Figure 7a,b). The silencing approach confirmed these similar effects of β2‐ or β3‐adrenoceptor antagonism on Treg reduction. In contrast, the silencing of β3‐adrenoceptors was more effective than the silencing of β2‐adrenoceptors at reducing the MDSC sub‐population (Figure 7c,d).
Figure 6.
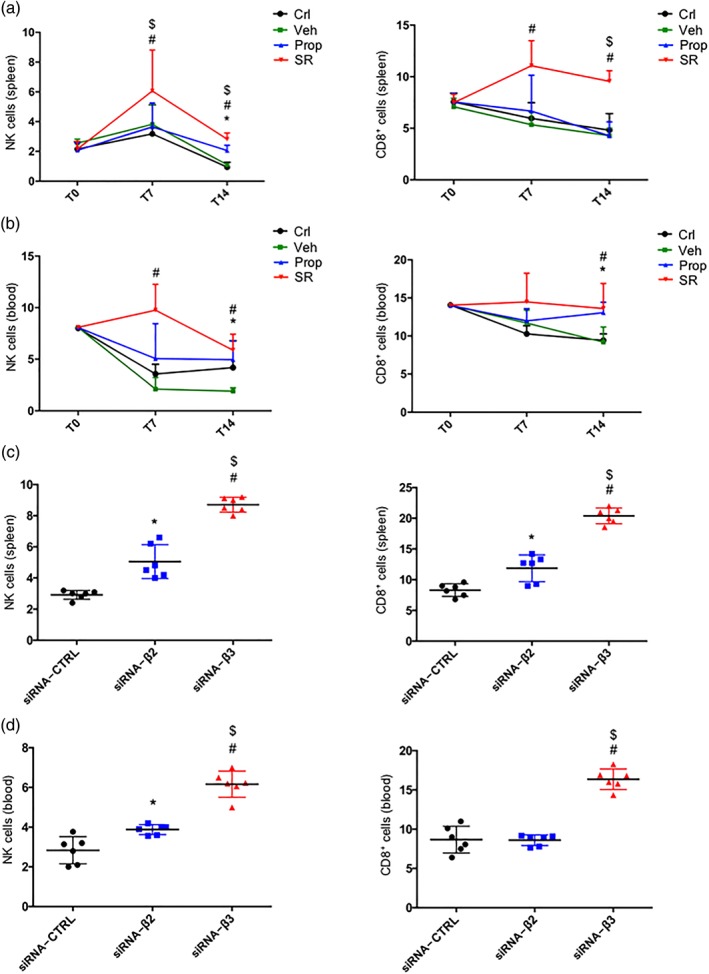
(a) FACS analysis and quantification at T7 and T14 of NK (NKp46+/NK1.1+ gated on CD3−/CD45+) cells (left) and CD8+ (gated on CD45+) cells (right) in spleen of control (Crl), vehicle (Veh)‐, propranolol (Prop)‐, and SR59230A (SR)‐treated mice (n = 6). (b) FACS analysis and quantification at T7 and T14 of NK (NKp46+/NK1.1+ gated on CD3−/CD45+) cells (left) and CD8+ (gated on CD45+) cells (right) in blood of control, vehicle‐, propranolol‐, and SR59230A‐treated mice (n = 6). (c) FACS analysis and quantification at T7 and T14 of NK (NKp46+/NK1.1+ gated on CD3−/CD45+) cells (left) and CD8+ (gated on CD45+) cells (right) in spleen of siRNA‐CTRL, siRNA‐β2, and siRNA‐β3 treated mice (n = 6). (d) FACS analysis and quantification at T7 and T14 of NK (NKp46+/NK1.1+ gated on CD3−/CD45+) cells (left) and CD8+ (gated on CD45+) cells (right) in blood of siRNA‐CTRL, siRNA‐β2, and siRNA‐β3 treated mice (n = 6). *P < 0.05 Prop‐ (or siRNA‐β2) compared with Veh‐; # P < 0.05 SR‐ (or siRNA‐β3) compared with Veh‐; $ P < 0.05 SR‐ (or siRNA‐β3) compared with Prop‐ (or siRNA‐β2)
Figure 7.
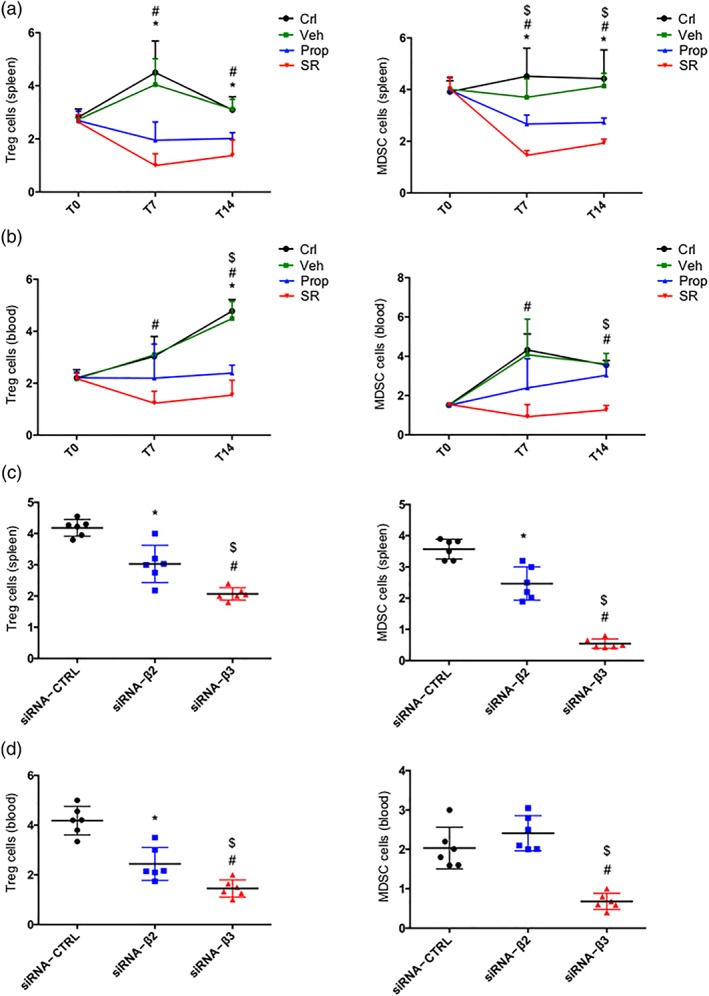
(a) FACS analysis and quantification at T7 and T14 of Treg (CD25+/CD127− gated on CD45+/CD4+) cells (left) and MDSC (in CD11b+, GR1+ gated on CD45+) (right) in spleen of control (Crl), vehicle (Veh)‐, propranolol (Prop)‐, and SR59230A (SR)‐treated mice (n = 6). (b) FACS analysis and quantification at T7 and T14 of Treg (CD25+/CD127− gated on CD45+/CD4+) cells (left) and MDSC (in CD11b+, GR1+ gated on CD45+) cells (right) in blood of control, vehicle‐, propranolol‐, and SR59230A‐treated mice (n = 6). (c) FACS analysis and quantification at T7 and T14 of Treg (CD25+/CD127− gated on CD45+/CD4+) cells (left) and MDSC (in CD11b+, GR1+ gated on CD45+) (right) in spleen of siRNA‐CTRL, siRNA‐β2, and siRNA‐β3 treated mice (n = 6). (d) FACS analysis and quantification at T7 and T14 of Treg (CD25+/CD127− gated on CD45+/CD4+) cells (left) and MDSC (in CD11b+, GR1+ gated on CD45+) (right) in blood of siRNA‐CTRL, siRNA‐β2, and siRNA‐β3 treated mice (n = 6). *P < 0.05 Prop‐ (or siRNA‐β2) compared with Veh‐; # P < 0.05 SR‐ (or siRNA‐β3) compared with Veh‐; $ P < 0.05 SR‐ (or siRNA‐β3) compared with Prop‐ (or siRNA‐β2)
4. DISCUSSION
The fine control of immune cell sub‐populations is completely reversed in cancer, favouring an immune‐tolerant phenotype. Understanding the mechanisms promoting this immunological shift is currently one of the most important challenges in oncological research.
Although conflicting results have been reported on the regulation of the immune system by catecholamines in humans, the majority of reports agree that Ad and NA act as immunosuppressants. In early studies, elevated NK cell activity was observed after Ad infusion, open‐heart surgery, or physical exercise (Pedersen et al., 1988). However, subsequent findings have suggested that the observed increase in NK cell activity is due to a marked, but transitory, increase in the number of circulating NK cells, rather than to an increase in activity per NK cell (Palmø et al., 1995). This increase in the number of circulating NK cells occurs during the time of elevated catecholamine levels and dissipates shortly after catecholamines decline (Benschop, Rodriguez‐Feuerhahn, & Schedlowski, 1996). The additional finding that NA impairs the cytotoxicity of NK cells and that β2‐adrenoceptor activation suppresses CD8 cytotoxicity further supports the immune‐suppressive role of the β‐adrenergic system (Gan, Zhang, Solomon, & Bonavida, 2002). While the role of β2‐adrenoceptors on immune cell function has been widely studied, both the expression and the possible role of β3‐adrenoceptors have not yet been clarified. Here, we provide some evidence for a possible new role for β3‐adrenoceptors in the regulation of melanoma immune‐tolerance in tumour‐bearing mice.
In this study, propranolol has been used as non‐selective β1‐/β2‐adrenoceptor antagonist, and SR59230A has been chosen as a β3‐adrenoceptor antagonist and preferred to a different antagonist, L‐748,337, because of its higher affinity for β3‐adrenoceptors in rodents (Candelore et al., 1999). However, even though SR592390A has been previously indicated as a selective β3‐adrenoceptor antagonist, a similar affinity has been demonstrated for all the three subtypes (Baker, 2005; Hoffmann, Leitz, Oberdorf‐Maass, Lohse, & Klotz, 2004; Niclauss, Michel‐Reher, Alewijnse, & Michel, 2006). A second issue is related to the fact that SR59230A can act as a partial agonist, with the degree of partial agonism strongly depending on the model system. In addition, in some systems, SR59230A acts as a full agonist (Sato, Horinouchi, Hutchinson, Evans, & Summers, 2007; Vrydag & Michel, 2007). In this context, the siRNA molecular approach represents a useful tool to clarify the real mechanism of action of SR59230A and, as previously observed, the silencing approach has demonstrated that in B16‐F10 cells, SR59230A actually acts at β3‐adrenoceptors (Dal Monte et al., 2013). According to this study, the in vivo use of selective β3‐adrenoceptor siRNAs provides results overlapping with those obtained with SR59230A, thus confirming the role of SR59230A as a β3‐adrenoceptor antagonist in this scenario. Moreover, the administration of the β2‐adrenoceptor agonist terbutaline to pre‐silenced β3‐adrenoceptor melanoma‐bearing mice showed no rebound in tumour growth demonstrating the predominant role of the β3‐adrenoceptor subtype in controlling tumour development (Figure S2). In addition, the difference between the effects of SR59230A and propranolol treatment observed in the present study could depend on the β3‐adrenoceptor component, because both drugs saturate the receptors, as we can extrapolate from previous studies in the same mouse model used here and from previous data on the relative affinity of SR59230A and propranolol for β‐adrenoceptors (Dal Monte et al., 2013; Hoffmann et al., 2004). In addition, the fact that, based on the difference in MW between SR59230A and propranolol, the molar doses of propranolol used in the present study are higher than those of SR59230A should also be considered. Assuming these premises, this study supports the hypothesis that the blockade of β2‐ or β3‐adrenoceptors may promote immune‐competence in tumour infiltrating lymphocytes of melanoma‐bearing mice and that β3‐adrenoceptors rather than β2‐adrenoceptors play a major role in the ability of melanoma to evade the immune system.
The observation that the expression of β3‐adrenoceptors, but not β2‐adrenoceptors, in mouse lymphocytes was up‐regulated in a hypoxic environment and down‐regulated after oxygen re‐exposure suggests a fine post‐translational regulation of β3‐adrenoceptor protein under the control of oxygen. This is intriguing, considering that hypoxia is indicated as one of the most important regulators of cancer immune‐tolerance (Facciabene et al., 2011) and supports the hypothesis that β3‐adrenoceptors might participate, in a hypoxic environment, in the acquisition of an immune‐tolerant phenotype.
T‐lymphocytes are known to express both β2‐ and β3‐adrenoceptors, of which β3‐adrenoceptors were primarily up‐regulated in response to stress (Laukova et al., 2012). In addition, the present finding that β3‐adrenoceptors are localized to the sub‐populations of immune cells involved in both immune‐suppression (Treg and MDSC) and immune‐toxicity (CD8 and NK) suggests a possible role for β3‐adrenoceptors in the immune response. As shown by our results, both SR59230A and propranolol counteract melanoma growth in vivo, and their effect is associated with a significant increase in NK and CD8 cells and a strong reduction in Treg cells and MDSC within the tumour mass. More precisely, SR59230A appears to act with greater effectiveness when compared with propranolol. The additional finding that the effects of SR59230A are mimicked by β3‐adrenoceptor silencing supports a role for β3‐adrenoceptors in mediating the switch from an immunosuppressive to an immunocompetent tumour micro‐environment. This shift is better documented at T7 than at T14, suggesting that after 14 days of treatment, the immune reactivity is in regression and mouse melanoma in resolution, as demonstrated here by the high rate of necrosis at T14.
Although our data suggest that β3‐adrenoceptors have a significant role in regulating the immune system, one of the main limitations of the present study is the inability to precisely discriminate if the effect observed on immune phenotype is directly related to β3‐adrenoceptor blockade in immune cells or is a consequence of the reduction in tumour growth. This study, indeed, did not evaluate the cause–effect relationships between tumour cell death and immune modulation in vivo, which should be investigated in the future by selective direct manipulations of immune cell sub‐populations. However, the results obtained with pretreatment of PBMC with SR59230A or selective β3‐adrenoceptor siRNAs suggest a direct effect of β3‐adrenoceptor blockade on immune cells, independent of an action on tumour cells.
The loss of the cytotoxicity of tumour‐infiltrating M1 macrophages and N1 neutrophils represents a substantial barrier to immune clearance of solid tumours (Jaiswal, Chao, Majeti, & Weissman, 2010; Nicolás‐Ávila, Adrover, & Hidalgo, 2017). As shown here, SR59230A and β3‐adrenoceptor siRNAs, but not propranolol or β2‐adrenoceptor siRNAs, induce a strong increase in M1 macrophage and N1 neutrophil populations within the tumour micro‐environment, suggesting that the phenotypic shift in macrophages and neutrophils is mainly mediated by β3‐adrenoceptors. This finding is consistent with the recent revelation that β3‐adrenoceptor agonism inhibits the pro‐inflammatory (M1) activity of macrophages (Hadi et al., 2017) and with previous studies demonstrating that there is a phenotypic plasticity of macrophages and neutrophils in the tumour micro‐environment (Schouppe et al., 2012).
In conclusion, this study supports the hypothesis that β3‐adrenoceptors play a role in the promotion of immune‐tolerance of melanoma. If future experiments confirm this beneficial effect of β3‐adrenoceptor blockade on immune system editing and tumour resolution, β3‐adrenoceptor blockade could represent a new strategy to overcome cancer immune‐editing and an effective therapy against melanoma. Unfortunately, the poor pharmacological profile of the β3‐adrenoceptor blockers currently available may limit the development of future therapies. Nevertheless, the fact that the results from the siRNA approach fit well with those from the pharmacological study supports the possibility that selective β3‐adrenoceptor antagonists (when available) could be metamorphosed from experimental tools into therapeutic drugs.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
L.F. and M.C. developed the concept and experiment and wrote the manuscript. G.B., F.F., and L.C. performed and analysed the animal model and functional assays. M.C., A.C., and M.B. performed flow cytometry experiments. R.N. and F.D. performed immunohistochemical analysis. F.B. and A.P. performed RMI analysis. M.D.M., G.F., G.l.M., L.C., P.B., P.C., C.A., P.G., and C.F. revised the experiments and the manuscript.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis, Immunoblotting and Immunochemistry, and Animal Experimentation, and as recommended by funding agencies, publishers and other organizations engaged with supporting research.
Supporting information
Figure S1. A) β2‐adrenoceptors expression (left) and β3‐adrenoceptors expression (right) in B16‐F10 cells silenced with siRNA‐CTRL, siRNA‐β2 or siRNA‐β3 (and used for MTT cell viability assay (Figure 1D)). B) β2‐adrenoceptors expression (left) and β3‐adrenoceptors expression (right) in B16‐F10 cells silenced with siRNA‐CTRL, siRNA‐β2 or siRNA‐β3 (and used for the in vivo experiments). **** P<0.0001 siRNA‐β2 compared with siRNA‐CTRL, #### P<0.0001 siRNA‐β3 compared with siRNA‐CTRL
Figure S2. A) Tumor growth rate (left) in Vehicle, siRNA‐β3, siRNA‐β3 + Terbutaline (β2‐adrenoceptor agonist) (5 mg/kg), siRNA‐β3 + BRL37344 (β3‐adrenoceptor agonist) (5 mg/kg) treated mice (n=6). β3‐adrenoceptors expression (right) in B16‐F10 cells in Vehicle and siRNA‐β3 conditions (used for the in vivo experiment). B) Tumor weight in Vehicle, siRNA‐β3, siRNA‐β3 + Terbutaline (β2‐adrenoceptor agonist), siRNA‐β3 + BRL37344 (β3‐adrenoceptor agonist) treated mice (n=6). (ns = not significative, ## P<0,01 siRNA‐β3 compared with Vehicle, * P<0,05 siRNA‐β3 + Terbutaline compared with Vehicle, $$$ P<0,001 siRNA‐β3 + BRL37344 compared with Vehicle).
ACKNOWLEDGEMENTS
This work was supported in part by a grant from the Italian Ministry of Health (RF‐2011‐02351158). This study was supported by Meyer Foundation, A. Meyer Children's University Hospital, Florence, Italy. We would like to thank Prof. Antonino Morabito for the kind revision of the manuscript.
Calvani M, Bruno G, Dal Monte M, et al. β3‐Adrenoceptor as a potential immuno‐suppressor agent in melanoma. Br J Pharmacol. 2019;176:2509–2524. 10.1111/bph.14660
REFERENCES
- Abrahams, V. M. , Kamsteeg, M. , & Mor, G. (2003). The Fas/Fas ligand system and cancer: Immune privilege and apoptosis. Molecular Biotechnology, 25, 19–30. 10.1385/MB:25:1:19 [DOI] [PubMed] [Google Scholar]
- Aktas, E. , Kucuksezer, U. C. , Bilgic, S. , Erten, G. , & Deniz, G. (2009). Relationship between CD107a expression and cytotoxic activity. Cellular Immunology, 254, 149–154. 10.1016/j.cellimm.2008.08.007 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators (2017). The concise guide to pharmacology 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174, S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators . (2017a). The concise guide to pharmacology 2017/18: Enzymes. Br J Pharmacol, 174, S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators . (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Catalytic receptors. British Journal of Pharmacology, 174(S1), S225–S271. 10.1111/bph.13876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Overview. British Journal of Pharmacology, 174, S1–S16. 10.1111/bph.13882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armaiz‐Pena, G. N. , Allen, J. K. , Cruz, A. , Stone, R. L. , Nick, A. M. , Lin, Y. G. , … Sood, A. K. (2013). Src activation by β‐adrenoreceptors is a key switch for tumour metastasis. Nature Communications, 4, 1403 10.1038/ncomms2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, J. G. (2005). The selectivity of β‐adrenoceptor antagonists at the human β1, β2 and β3 adrenoceptors. British Journal of Pharmacology, 144, 317–322. 10.1038/sj.bjp.0706048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri, A. , Palma, G. , Rosati, A. , Giudice, A. , Falco, A. , Petrillo, A. , … Arra, C. (2012). Role of endothelial nitric oxide synthase (eNOS) in chronic stress‐promoted tumour growth. Journal of Cellular and Molecular Medicine, 16, 920–926. 10.1111/j.1582-4934.2011.01375.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benschop, R. J. , Rodriguez‐Feuerhahn, M. , & Schedlowski, M. (1996). Catecholamine‐induced leukocytosis: early observations, current research, and future directions. Brain, Behavior, and Immunity, 10, 77–91. 10.1006/brbi.1996.0009 [DOI] [PubMed] [Google Scholar]
- Calvani, M. , Pelon, F. , Comito, G. , Taddei, M. L. , Moretti, S. , Innocenti, S. , … Chiarugi, P. (2015). Norepinephrine promotes tumor microenvironment reactivity through β3‐adrenoreceptors during melanoma progression. Oncotarget, 6, 4615–4632. 10.18632/oncotarget.2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candelore, M. R. , Deng, L. , Tota, L. , Guan, X. M. , Amend, A. , Liu, Y. , … Weber, A. E. (1999). Potent and selective human β(3)‐adrenergic receptor antagonists. The Journal of Pharmacology and Experimental Therapeutics, 290, 649–655. [PubMed] [Google Scholar]
- Cheng, Y. , Gao, X. H. , Li, X. J. , Cao, Q. H. , Zhao, D. D. , Zhou, J. R. , … Yang, Y. (2018). Depression promotes prostate cancer invasion and metastasis via a sympathetic‐cAMP‐FAK signaling pathway. Oncogene, 37, 2953–2966. 10.1038/s41388-018-0177-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers, W. K. , Hollenbeak, C. S. , & Cheriyath, P. (2015). β‐Blockers reduce breast cancer recurrence and breast cancer death: A meta‐analysis. Clinical Breast Cancer, 15, 426–431. 10.1016/j.clbc.2015.07.001 [DOI] [PubMed] [Google Scholar]
- Cole, S. W. , Nagaraja, A. S. , Lutgendorf, S. K. , Green, P. A. , & Sood, A. K. (2015). Sympathetic nervous system regulation of the tumour microenvironment. Nature Reviews. Cancer, 15, 563–572. 10.1038/nrc3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, S. W. , & Sood, A. K. (2012). Molecular pathways: β‐Adrenergic signaling in cancer. Clinical Cancer Research, 18, 1201–1206. 10.1158/1078-0432.CCR-11-0641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte, M. , Calvani, M. , Cammalleri, M. , Favre, C. , Filippi, L. , & Bagnoli, P. (2018). β‐Adrenoceptors as drug targets in melanoma: Novel preclinical evidence for a role of β3‐adrenoceptors. British Journal of Pharmacology. 10.1111/bph.14552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte, M. , Casini, G. , Filippi, L. , Nicchia, G. P. , Svelto, M. , & Bagnoli, P. (2013). Functional involvement of β3‐adrenergic receptors in melanoma growth and vascularization. J Mol Med (Berl), 91, 1407–1419. 10.1007/s00109-013-1073-6 [DOI] [PubMed] [Google Scholar]
- Dal Monte, M. , Fornaciari, I. , Nicchia, G. P. , Svelto, M. , Casini, G. , & Bagnoli, P. (2014). β3‐Adrenergic receptor activity modulates melanoma cell proliferation and survival through nitric oxide signaling. Naunyn‐Schmiedeberg's Archives of Pharmacology, 387, 533–543. 10.1007/s00210-014-0969-1 [DOI] [PubMed] [Google Scholar]
- De Giorgi, V. , Grazzini, M. , Benemei, S. , Marchionni, N. , Geppetti, P. , & Gandini, S. (2017). β‐Blocker use and reduced disease progression in patients with thick melanoma: 8 years of follow‐up. Melanoma Research, 27, 268–270. 10.1097/CMR.0000000000000317 [DOI] [PubMed] [Google Scholar]
- De Giorgi, V. , Grazzini, M. , Gandini, S. , Benemei, S. , Lotti, T. , Marchionni, N. , & Geppetti, P. (2011). Treatment with β‐blockers and reduced disease progression in patients with thick melanoma. Archives of Internal Medicine, 171, 779–781. [DOI] [PubMed] [Google Scholar]
- Entschladen, F. , Drell, T. L. , Lang, K. , Joseph, J. , & Zaenker, K. S. (2004). Tumour‐cell migration, invasion, and metastasis: Navigation by neurotransmitters. The Lancet Oncology, 5, 254–258. 10.1016/S1470-2045(04)01431-7 [DOI] [PubMed] [Google Scholar]
- Estrada, L. D. , Ağaç, D. , & Farrar, J. D. (2016). Sympathetic neural signaling via the β2‐adrenergic receptor suppresses T‐cell receptor‐mediated human and mouse CD8+ T‐cell effector function. European Journal of Immunology, 46, 1948–1958. 10.1002/eji.201646395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facciabene, A. , Peng, X. , Hagemann, I. S. , Balint, K. , Barchetti, A. , Wang, L. P. , … Coukos, G. (2011). Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T (reg) cells. Nature, 475, 226–230. 10.1038/nature10169 [DOI] [PubMed] [Google Scholar]
- Fridlender, Z. G. , Sun, J. , Kim, S. , Kapoor, V. , Cheng, G. , Ling, L. , … Albelda, S. M. (2009). Polarization of tumor‐associated neutrophil phenotype by TGF‐β: “N1” versus “N2” TAN. Cancer Cell, 16, 183–194. 10.1016/j.ccr.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura, T. , Kambayashi, Y. , & Aiba, S. (2012). Crosstalk between regulatory T cells (Tregs) and myeloid derived suppressor cells (MDSCs) during melanoma growth. Oncoimmunology, 1, 1433–1434. 10.4161/onci.21176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan, X. , Zhang, L. , Solomon, G. F. , & Bonavida, B. (2002). Mechanism of norepinephrine‐mediated inhibition of human NK cytotoxic functions: Inhibition of cytokine secretion, target binding, and programming for cytotoxicity. Brain, Behavior, and Immunity, 16, 227–246. 10.1006/brbi.2000.0615 [DOI] [PubMed] [Google Scholar]
- Glasner, A. , Avraham, R. , Rosenne, E. , Benish, M. , Zmora, O. , Shemer, S. , … Ben‐Eliyahu, S. (2010). Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a β‐adrenergic antagonist and a cyclooxygenase‐2 inhibitor. Journal of Immunology, 184, 2449–2457. 10.4049/jimmunol.0903301 [DOI] [PubMed] [Google Scholar]
- Granot, Z. , & Fridlender, Z. G. (2015). Plasticity beyond cancer cells and the “immunosuppressive switch”. Cancer Research, 75, 4441–4445. 10.1158/0008-5472.CAN-15-1502 [DOI] [PubMed] [Google Scholar]
- Guereschi, M. G. , Araujo, L. P. , Maricato, J. T. , Takenaka, M. C. , Nascimento, V. M. , Vivanco, B. C. , … Basso, A. S. (2013). β2‐adrenergic receptor signaling in CD4+ Foxp3+ regulatory T cells enhances their suppressive function in a PKA‐dependent manner. European Journal of Immunology, 43, 1001–1012. 10.1002/eji.201243005 [DOI] [PubMed] [Google Scholar]
- Hadi, T. , Douhard, R. , Dias, A. M. , Wendremaire, M. , Pezzè, M. , Bardou, M. , … Lirussi, F. (2017). β3 adrenergic receptor stimulation in human macrophages inhibits NADPH oxidase activity and induces catalase expression via PPARγ activation. Biochimica et Biophysica Acta, 1864, 1769–1784. 10.1016/j.bbamcr.2017.07.003 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucl Acids Res, 46(D1), D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, C. , Leitz, M. R. , Oberdorf‐Maass, S. , Lohse, M. J. , & Klotz, K. N. (2004). Comparative pharmacology of human β‐adrenergic receptor subtypes—Characterization of stably transfected receptors in CHO cells. Naunyn‐Schmiedeberg's Archives of Pharmacology, 369, 151–159. 10.1007/s00210-003-0860-y [DOI] [PubMed] [Google Scholar]
- Inbar, S. , Neeman, E. , Avraham, R. , Benish, M. , Rosenne, E. , & Ben‐Eliyahu, S. (2011). Do stress responses promote leukemia progression? An animal study suggesting a role for epinephrine and prostaglandin‐E2 through reduced NK activity. PLoS ONE, 6, e19246 10.1371/journal.pone.0019246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal, S. , Chao, M. P. , Majeti, R. , & Weissman, I. L. (2010). Macrophages as mediators of tumor immunosurveillance. Trends in Immunology, 31, 212–219. 10.1016/j.it.2010.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, G. T. , Sharma, P. , Davis, S. L. , & Jimeno, A. (2018). Immune and autoimmune‐related adverse events associated with immune checkpoint inhibitors in cancer therapy. Drugs Today (Barc), 54, 103–122. 10.1358/dot.2018.54.2.2776626 [DOI] [PubMed] [Google Scholar]
- Kokolus, K. M. , Zhang, Y. , Sivik, J. M. , Schmeck, C. , Zhu, J. , Repasky, E. A. , … Schell, T. D. (2017). β blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology, 7, e1405205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukova, M. , Vargovic, P. , Csaderova, L. , Chovanova, L. , Vlcek, M. , Imrich, R. , … Kvetnansky, R. (2012). Acute stress differently modulates β1, β2 and β3 adrenoceptors in T cells, but not in B cells, from the rat spleen. Neuroimmunomodulation, 19, 69–78. 10.1159/000329002 [DOI] [PubMed] [Google Scholar]
- Lemeshow, S. , Sørensen, H. T. , Phillips, G. , Yang, E. V. , Antonsen, S. , Riis, A. H. , … Glaser, R. (2011). β‐Blockers and survival among Danish patients with malignant melanoma: A population‐based cohort study. Cancer Epidemiology, Biomarkers & Prevention, 20, 2273–2279. 10.1158/1055-9965.EPI-11-0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone, E. , Hollestein, L. M. , van Herk‐Sukel, M. P. , van de Poll‐Franse, L. , Nijsten, T. , Schadendorf, D. , & de Vries, E. (2013). β‐Blocker use and all‐cause mortality of melanoma patients: Results from a population‐based Dutch cohort study. European Journal of Cancer, 49, 3863–3871. 10.1016/j.ejca.2013.07.141 [DOI] [PubMed] [Google Scholar]
- Magnon, C. , Hall, S. J. , Lin, J. , Xue, X. , Gerber, L. , Freedland, S. J. , & Frenette, P. S. (2013). Autonomic nerve development contributes to prostate cancer progression. Science, 341, 1236361 10.1126/science.1236361 [DOI] [PubMed] [Google Scholar]
- Maisel, A. S. , Fowler, P. , Rearden, A. , Motulsky, H. J. , & Michel, M. C. (1989). A new method for isolation of human lymphocyte subsets reveals differential regulation of β‐adrenergic receptors by terbutaline treatment. Clinical Pharmacology and Therapeutics, 46, 429–439. 10.1038/clpt.1989.161 [DOI] [PubMed] [Google Scholar]
- Mantovani, A. , & Locati, M. (2013). Tumor‐associated macrophages as a paradigm of macrophage plasticity, diversity, and polarization: Lessons and open questions. Arteriosclerosis, Thrombosis, and Vascular Biology, 33, 1478–1483. 10.1161/ATVBAHA.113.300168 [DOI] [PubMed] [Google Scholar]
- Marino, F. , & Cosentino, M. (2013). Adrenergic modulation of immune cells: An update. Amino Acids, 45, 55–71. 10.1007/s00726-011-1186-6 [DOI] [PubMed] [Google Scholar]
- Mazzoni, A. , Bronte, V. , Visintin, A. , Spitzer, J. H. , Apolloni, E. , Serafini, P. , … Segal, D. M. (2002). Myeloid suppressor lines inhibit T cell responses by an NO‐dependent mechanism. Journal of Immunology, 168, 689–695. 10.4049/jimmunol.168.2.689 [DOI] [PubMed] [Google Scholar]
- McCourt, C. , Coleman, H. G. , Murray, L. J. , Cantwell, M. M. , Dolan, O. , Powe, D. G. , & Cardwell, C. R. (2014). β‐Blocker usage after malignant melanoma diagnosis and survival: A population‐based nested case‐control study. The British Journal of Dermatology, 170, 930–938. 10.1111/bjd.12894 [DOI] [PubMed] [Google Scholar]
- Moretti, S. , Massi, D. , Farini, V. , Baroni, G. , Parri, M. , Innocenti, S. , … Chiarugi, P. (2013). β‐Adrenoceptors are upregulated in human melanoma and their activation releases pro‐tumorigenic cytokines and metalloproteases in melanoma cell lines. Laboratory Investigation, 93, 279–290. 10.1038/labinvest.2012.175 [DOI] [PubMed] [Google Scholar]
- Munder, M. (2009). Arginase: An emerging key player in the mammalian immune system. British Journal of Pharmacology, 158, 638–651. 10.1111/j.1476-5381.2009.00291.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy‐Bosse, B. L. , Thornton, L. M. , Yang, H. C. , Andersen, B. L. , & Carson, W. E. (2011). Psychological stress is associated with altered levels of myeloid‐derived suppressor cells in breast cancer patients. Cellular Immunology, 270, 80–87. 10.1016/j.cellimm.2011.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance, D. M. , & Sanders, V. M. (2007). Autonomic innervation and regulation of the immune system (1987‐2007). Brain, Behavior, and Immunity, 21, 736–745. 10.1016/j.bbi.2007.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niclauss, N. , Michel‐Reher, M. B. , Alewijnse, A. E. , & Michel, M. C. (2006). Comparison of three radioligands for the labelling of human β‐adrenoceptor subtypes. Naunyn‐Schmiedeberg's Archives of Pharmacology, 374, 99–105. 10.1007/s00210-006-0104-z [DOI] [PubMed] [Google Scholar]
- Nicolás‐Ávila, J. Á. , Adrover, J. M. , & Hidalgo, A. (2017). Neutrophils in homeostasis, immunity, and cancer. Immunity, 46, 15–28. 10.1016/j.immuni.2016.12.012 [DOI] [PubMed] [Google Scholar]
- Nissen, M. D. , Sloan, E. K. , & Mattarollo, S. R. (2018). β‐Adrenergic signaling impairs antitumor CD8+ T‐cell responses to B‐cell lymphoma immunotherapy. Cancer Immunology Research, 6, 98–109. 10.1158/2326-6066.CIR-17-0401 [DOI] [PubMed] [Google Scholar]
- Palmø, J. , Asp, S. , Daugaard, J. R. , Richter, E. A. , Klokker, M. , & Pedersen, B. K. (1995). Effect of eccentric exercise on natural killer cell activity. Journal of Applied Physiology (1985), 78, 1442–1446. [DOI] [PubMed] [Google Scholar]
- Passarelli, A. , Mannavola, F. , Stucci, L. S. , Tucci, M. , & Silvestris, F. (2017). Immune system and melanoma biology: A balance between immunosurveillance and immune escape. Oncotarget, 8, 106132–106142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen, B. K. , Tvede, N. , Hansen, F. R. , Andersen, V. , Bendix, T. , Bendixen, G. , … Klarlund, K. (1988). Modulation of natural killer cell activity in peripheral blood by physical exercise. Scandinavian Journal of Immunology, 27, 673–678. 10.1111/j.1365-3083.1988.tb02400.x [DOI] [PubMed] [Google Scholar]
- Sato, M. , Horinouchi, T. , Hutchinson, D. S. , Evans, B. A. , & Summers, R. J. (2007). Ligand‐directed signaling at the ß3‐adrenoceptor produced by SR59230A relative to receptor agonists. Molecular Pharmacology, 74, 1359–1368. [DOI] [PubMed] [Google Scholar]
- Schouppe, E. , De Baetselier, P. , Van Ginderachter, J. A. , & Sarukhan, A. (2012). Instruction of myeloid cells by the tumor microenvironment: Open questions on the dynamics and plasticity of different tumor‐associated myeloid cell populations. Oncoimmunology, 1, 1135–1145. 10.4161/onci.21566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereni, F. , Dal Monte, M. , Filippi, L. , & Bagnoli, P. (2015). Role of host β1‐ and β2‐adrenergic receptors in a murine model of B16 melanoma: Functional involvement of β3‐adrenergic receptors. Naunyn‐Schmiedeberg's Archives of Pharmacology, 388, 1317–1331. 10.1007/s00210-015-1165-7 [DOI] [PubMed] [Google Scholar]
- Tawbi, H. A. , Forsyth, P. A. , Algazi, A. , Hamid, O. , Hodi, F. S. , Moschos, S. J. , … Margolin, K. (2018). Combined nivolumab and ipilimumab in melanoma metastatic to the brain. The New England Journal of Medicine, 379, 722–730. 10.1056/NEJMoa1805453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaker, P. H. , Han, L. Y. , Kamat, A. A. , Arevalo, J. M. , Takahashi, R. , Lu, C. , … Sood, A. K. (2006). Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nature Medicine, 12, 939–944. 10.1038/nm1447 [DOI] [PubMed] [Google Scholar]
- van den Broek, M. F. , & Hengartner, H. (2000). The role of perforin in infections and tumour surveillance. Experimental Physiology, 85, 681–685. 10.1017/S0958067000020972 [DOI] [PubMed] [Google Scholar]
- Vinay, D. S. , Ryan, E. P. , Pawelec, G. , Talib, W. H. , Stagg, J. , Elkord, E. , … Kwon, B. S. (2015). Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Seminars in Cancer Biology, 35(Suppl), S185–S198. 10.1016/j.semcancer.2015.03.004 [DOI] [PubMed] [Google Scholar]
- Vrydag, W. , & Michel, M. C. (2007). Tools to study β3‐adrenoceptors. Naunyn‐Schmiedeberg's Archives of Pharmacology, 374, 385–398. 10.1007/s00210-006-0127-5 [DOI] [PubMed] [Google Scholar]
- Wrobel, L. J. , & Le Gal, F. A. (2015). Inhibition of human melanoma growth by a non‐cardioselective β‐blocker. The Journal of Investigative Dermatology, 135, 525–531. 10.1038/jid.2014.373 [DOI] [PubMed] [Google Scholar]
- Yang, E. V. , Kim, S. J. , Donovan, E. L. , Chen, M. , Gross, A. C. , Webster Marketon, J. I. , … Glaser, R. (2009). Norepinephrine upregulates VEGF, IL‐8, and IL‐6 expression in human melanoma tumor cell lines: Implications for stress‐related enhancement of tumor progression. Brain, Behavior, and Immunity, 23, 267–275. 10.1016/j.bbi.2008.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa, T. , Kaira, K. , Shimizu, K. , Shimizu, A. , Mori, K. , Nagashima, T. , … Kuwano, H. (2016). Prognostic significance of β2‐adrenergic receptor expression in non‐small cell lung cancer. American Journal of Translational Research, 8, 5059–5070. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. A) β2‐adrenoceptors expression (left) and β3‐adrenoceptors expression (right) in B16‐F10 cells silenced with siRNA‐CTRL, siRNA‐β2 or siRNA‐β3 (and used for MTT cell viability assay (Figure 1D)). B) β2‐adrenoceptors expression (left) and β3‐adrenoceptors expression (right) in B16‐F10 cells silenced with siRNA‐CTRL, siRNA‐β2 or siRNA‐β3 (and used for the in vivo experiments). **** P<0.0001 siRNA‐β2 compared with siRNA‐CTRL, #### P<0.0001 siRNA‐β3 compared with siRNA‐CTRL
Figure S2. A) Tumor growth rate (left) in Vehicle, siRNA‐β3, siRNA‐β3 + Terbutaline (β2‐adrenoceptor agonist) (5 mg/kg), siRNA‐β3 + BRL37344 (β3‐adrenoceptor agonist) (5 mg/kg) treated mice (n=6). β3‐adrenoceptors expression (right) in B16‐F10 cells in Vehicle and siRNA‐β3 conditions (used for the in vivo experiment). B) Tumor weight in Vehicle, siRNA‐β3, siRNA‐β3 + Terbutaline (β2‐adrenoceptor agonist), siRNA‐β3 + BRL37344 (β3‐adrenoceptor agonist) treated mice (n=6). (ns = not significative, ## P<0,01 siRNA‐β3 compared with Vehicle, * P<0,05 siRNA‐β3 + Terbutaline compared with Vehicle, $$$ P<0,001 siRNA‐β3 + BRL37344 compared with Vehicle).


