Abstract
Adrenoceptors play an important role in adipose tissue biology and physiology that includes regulating the synthesis and storage of triglycerides (lipogenesis), the breakdown of stored triglycerides (lipolysis), thermogenesis (heat production), glucose metabolism, and the secretion of adipocyte‐derived hormones that can control whole‐body energy homeostasis. These processes are regulated by the sympathetic nervous system through actions at different adrenoceptor subtypes expressed in adipose tissue depots. In this review, we have highlighted the role of adrenoceptor subtypes in white, brown, and brite adipocytes in both rodents and humans and have included detailed analysis of adrenoceptor expression in human adipose tissue and clonally derived adipocytes. We discuss important considerations when investigating adrenoceptor function in adipose tissue or adipocytes.
Linked Articles
This article is part of a themed section on Adrenoceptors—New Roles for Old Players. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.14/issuetoc
Abbreviations
- BAT
brown adipose tissue
- GLUT
glucose transporter
- KO
knockout
- UCP1
uncoupling protein 1
- WAT
white adipose tissue
1. INTRODUCTION
There are three types of adipocytes: white, brown, and brite (brown in white, also known as beige) adipocytes. Briefly, white adipose tissue (WAT) is the main tissue for storing energy. Under conditions of energy surplus, lipids are stored as triglycerides and can then be released as fuel under conditions of negative energy balance. Brown adipose tissue (BAT) is responsible for non‐shivering thermogenesis, essentially converting stored lipids into heat through fatty acid oxidation. The function of brite adipocytes is less well characterised, but they have a phenotype intermediate between white and brown adipocytes. They differ in their appearance, morphology, function, and gene expression profile, summarised in Figure 1, as well as expression of adrenoceptor subtypes and hence function when activated by endogenous catecholamines.
Figure 1.
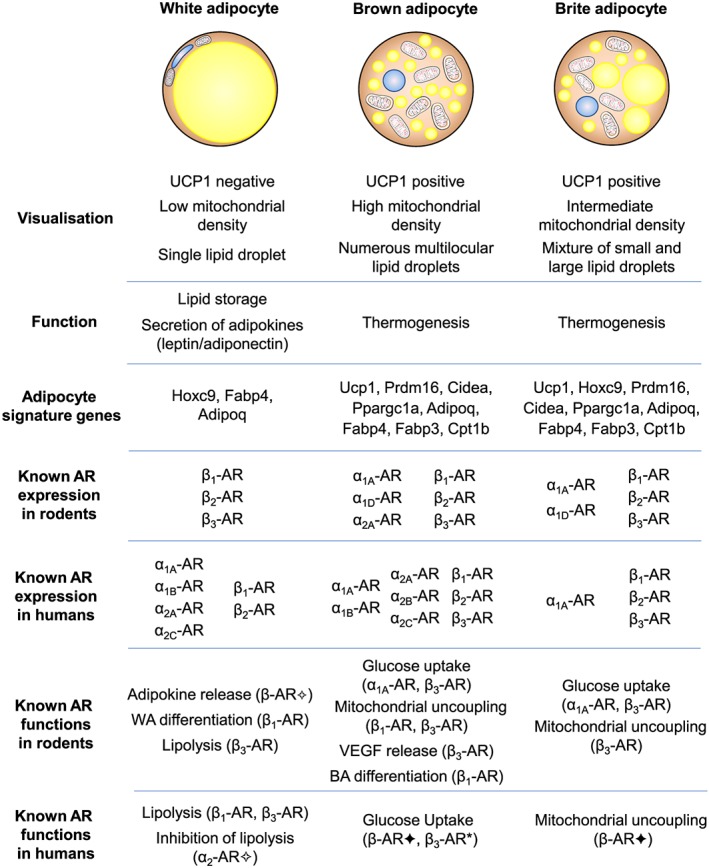
Differences in visualisation, function, and signature gene expression in white, brown, and brite adipocytes and the current understanding of adrenoceptor (AR) expression and function in rodents and humans. No mention of an adrenoceptor subtype indicates that there is no current evidence for receptor protein expression/function. In some cases, functional evidence is based on the use of non‐selective agonists (✦) including isoprenaline (Bartesaghi et al., 2015) and ephedrine (Carey et al., 2013) or antagonists (✧) including phentolamine (Stich et al., 1999) or a combination of propranolol and SR59230A to inhibit all β‐adrenoceptor‐mediated responses (Imai et al., 2006). *Uptake of 2‐[18F]fluoro‐2‐deoxyglucose was measured in response to the β3‐adrenoceptor‐selective agonist mirabegron in human brown adipose tissue (Cypess et al., 2015). BA: brown adipocyte; UCP1: uncoupling protein 1; WA: white adipocyte
Adrenoceptors belong to the Family A class of GPCRs and respond to the endogenous catecholamines noradrenaline (released from nerve terminals) and adrenaline (a hormone released from the adrenal gland). Noradrenaline and adrenaline mediate a diverse range of physiological processes by acting at nine different adrenoceptor subtypes (α1A‐, α1B‐, α1D‐, α2A‐, α2B‐, α2C‐, β1‐, β2‐, and β3‐adrenoceptors). Structurally, they consist of seven transmembrane domains, with an extracellular N‐terminus and an intracellular C‐terminal tail. Once activated by a ligand, adrenoceptors couple to different G proteins to mediate their cellular effects: α1‐adrenoceptors couple mainly to Gαq/11 proteins, resulting in increased DAG and inositol triphosphate (IP3) levels, which then activate PKC and IP3 receptors to increase calcium influx respectively; β‐adrenoceptors couple predominantly to Gαs proteins that activate adenylate cyclase to increase intracellular cAMP levels, whereas α2‐adrenoceptors couple primarily to Gαi/o proteins that inhibit adenylate cyclase activity. The sympathetic nervous system has a significant role in whole‐body energy metabolism and glucose homeostasis, through activation of different adrenoceptor subtypes located in different tissues and cells including the pancreas, skeletal muscle, heart, liver, brain, gastrointestinal tract, and adipose tissue. Since adipose tissue is vital for whole‐body energy homeostasis and has key roles in normal physiology and pathophysiology, it is important to understand how the tissue is regulated. In this review, we will be addressing the role and action of adrenoceptors in adipocytes.
2. ADRENOCEPTORS IN WAT
According to the World Health Organisation, more than 1.9 billion adults aged over 18 years of age are overweight, with 650 million of these adults classified as obese (http://www.who.int/news‐room/fact‐sheets/detail/obesity‐and‐overweight). This increases the risk for the development of many diseases including type 2 diabetes, cardiovascular disease, musculoskeletal disorders, and even some types of cancers. This global obesity epidemic has increased the awareness of the complexities of adipose tissue.
White adipocytes are the predominant adipocyte type in the body and are located in discrete WAT depots characterised as either intraabdominal (visceral fat surrounding the internal organs, namely, mesenteric, perirenal, and gonadal fat) or subcutaneous (such as inguinal fat). Visceral fat is considered to be more detrimental to overall health, while subcutaneous fat is considered protective (Gesta, Tseng, & Kahn, 2007). White adipocytes store fuel (glucose and fatty acids) as triglycerides within a single lipid droplet, and WAT also acts as an endocrine organ to release adipokines such as leptin and adiponectin that regulate whole‐body energy homeostasis (Galic, Oakhill, & Steinberg, 2010). While WAT is a heterogenous tissue that is comprised of several different cell types (adipocytes, adipocyte precursor cells, endothelial cells, fibroblasts, immune cells, etc.), we are focusing on adipocytes here.
WAT has an enormous ability to adapt to energy availability that is dependent upon two processes: hyperplasia (cell proliferation) and hypertrophy (cell enlargement). Unlike BAT, the stromal vascular fraction (SVF) of WAT is composed mainly of immature adipocytes that possess a similar phenotype to mature cells, in that despite morphological differences in size (20 μm compared to 200 μm for mature adipocytes), they are functionally still able to accumulate lipids. These immature adipocytes give WAT its plasticity, being capable of proliferation (and subsequent hypertrophy), whereas larger mature adipocytes are capable only of hypertrophy. Each WAT depot comprises different levels of immature and mature adipocytes, with adipocytes from subcutaneous depots dominated by the former and adipocytes from visceral depots generally dominated by the latter (Tchkonia et al., 2005). Consequently, in rodents fed a high‐fat diet, generally, visceral fat expands mainly via hypertrophy, while subcutaneous fat expands mainly via hyperplasia (Gawronska‐Kozak, Staszkiewicz, Gimble, & Kirk‐Ballard, 2014; Q. A. Wang, Tao, Gupta, & Scherer, 2013). Following storage of excess energy as triglycerides in white adipocytes, free fatty acids can be regenerated through lipolysis and released into the vasculature to meet whole‐body energy demands. The depot‐specific nature of WAT also extends to triglyceride turnover (lipogenesis and lipolysis) rates, with visceral depots exhibiting a higher triglyceride turnover rate as compared to subcutaneous depots (Caserta et al., 2001; Sackmann‐Sala, Berryman, Munn, Lubbers, & Kopchick, 2012).
WAT is sympathetically innervated, and noradrenaline release in WAT is key to controlling both lipolytic rates and regulation of hyperplasia (Bartness, Liu, Shrestha, & Ryu, 2014; Bartness, Shrestha, Vaughan, Schwartz, & Song, 2010; Bowers et al., 2004). Although other hormonal stimuli may play a role in lipolysis, sympathetic innervation is a central control point of lipolytic activity in WAT (Bartness et al., 2010; Bartness et al., 2014). Sympathetic innervation allows for noradrenaline output onto WAT in a depot‐specific manner: energy demand by cold exposure drives higher noradrenaline turnover in inguinal WAT and retroperitoneal WAT than epididymal WAT—inverse to the triglyceride turnover capacities of these cells (Brito, Brito, & Bartness, 2008; Garofalo, Kettelhut, Roselino, & Migliorini, 1996). Conversely, sympathetic denervation inhibits lipolysis and promotes uncontrolled accumulation of lipids and WAT proliferation (Youngstrom & Bartness, 1998).
In rodents, all three β‐adrenoceptor subtypes are expressed in a range of subcutaneous and visceral depots (Collins et al., 1994; Collins, Daniel, & Rohlfs, 1999; Germack, Starzec, Vassy, & Perret, 1997; Granneman, 1992; Hollenga & Zaagsma, 1989; Komai et al., 2016; Llado et al., 2002; Susulic et al., 1995), with the β3‐adrenoceptor being the main receptor responsible for β‐adrenoceptor‐mediated lipolysis in mature white adipocytes. β‐Adrenoceptor expression is also affected by the differentiation state of the white adipocyte. The general β‐adrenoceptor agonist isoprenaline, but not the highly selective β3‐adrenoceptor agonist CL316243, increases preadipocyte proliferation, suggesting a β1‐adrenoceptor‐mediated role, while both β1‐ and β3‐adrenoceptors mediate lipolysis in mature adipocytes (Germack et al., 1997; Klaus, Seivert, & Boeuf, 2001; Louis, Jackman, Nero, Iakovidis, & Louis, 2000; Susulic et al., 1995). A role for the β2‐adrenoceptor was excluded using receptor selective antagonists and agonists.
β3‐Adrenoceptor knockout (KO) mice show mildly increased adiposity (Revelli et al., 1997; Susulic et al., 1995), highlighting their importance in the control of body weight. In white adipocytes derived from β3‐adrenoceptor KO mice, there is severe impairment of β‐adrenoceptor function (cAMP and lipolysis), with a minor compensatory role presumably by β1‐adrenoceptors (Revelli et al., 1997; Susulic et al., 1995). In β‐less mice that do not express β1‐, β2‐, or β3‐adrenoceptors, WAT depot sizes are markedly increased, and a small residual lipolytic response to noradrenaline still exists, although the receptor responsible for this effect has not been resolved (Tavernier et al., 2005). These mice become mildly obese on a chow diet, which is exaggerated when they are placed on a high‐fat diet (Bachman et al., 2002; Jimenez et al., 2002). These studies collectively show that β‐adrenoceptors are essential for WAT function but that compensatory mechanisms exist when the β3‐adrenoceptor is lacking. There appears to be no role for the β2‐adrenoceptor despite its detection in WAT, and its functional role in WAT is still undetermined. Similarly, there is no convincing evidence of a functional contribution by α1‐ or α2‐adrenoceptors in authentic white adipocytes from rodents (Merlin, Sato, Nowell, et al., 2018).
WAT is also an endocrine organ since it releases adipokines in response to physiological stimuli. Two important adipokines with respect to energy metabolism are leptin and adiponectin. Leptin is secreted predominantly from white adipocytes, where it acts at receptors in the hypothalamus to regulate feeding. Its secretion in white adipocytes/WAT is regulated by the sympathetic nervous system. Acute cold exposure of mice leads to a reduction in leptin mRNA in WAT and reduces circulating leptin levels (Evans, Agar, & Summers, 1999; Trayhurn, Duncan, & Rayner, 1995). This can be mimicked by the treatment of animals with β‐adrenoceptor agonists (Evans et al., 1999; Trayhurn et al., 1995), with a predominant role for the β3‐adrenoceptor and a minor contribution from β1/β2‐adrenoceptors (Evans et al., 1999). This is thought to be due to a direct action on white adipocytes, as these effects are replicated in isolated white adipocytes (Gettys, Harkness, & Watson, 1996; Hardie, Guilhot, & Trayhurn, 1996). There is less known about the regulation of adiponectin by adrenoceptors. Adiponectin, a second adipokine secreted from white and brown adipocytes, regulates glucose uptake, lipogenesis, lipolysis, and fatty acid oxidation in several tissues, including WAT, in an autocrine fashion. Acute cold exposure of mice (that increases sympathetic activity) decreases adiponectin mRNA and protein levels in WAT (Imai et al., 2006; Jankovic et al., 2013), mediated by activation of the sympathetic nervous system and actions at β3‐adrenoceptors (Imai et al., 2006). These effects are replicated in vivo by β‐adrenoceptor ligand administration (Delporte, Funahashi, Takahashi, Matsuzawa, & Brichard, 2002). Hence, as well as the contribution of adrenoceptors to lipid metabolism, adrenoceptors expressed in WAT/white adipocytes also have important roles in regulating whole‐body energy homeostasis by modulating the actions of important adipokines.
One important caveat to the findings outlined above is that studies in rodents are usually performed at room temperature (18–24°C), which is well below the thermoneutral temperature of 30°C for mice. At this temperature, mice do not need to burn energy to keep warm (Cannon & Nedergaard, 2011). As such, it is unclear whether adrenoceptor expression in WAT/white adipocytes is altered at thermoneutrality compared to room temperature housing conditions, and this may have implications for adrenoceptor function in white adipocytes. This is important, as in animals housed at 30°C, WAT depots are practically devoid of uncoupling protein 1 (UCP1) mRNA and protein (Kalinovich, de Jong, Cannon, & Nedergaard, 2017), as observed for white adipocytes grown in culture in the absence of browning agents (Merlin, Sato, Nowell, et al., 2018; Petrovic et al., 2010). In mice housed at room temperature, there is appreciable UCP1 content in WAT. This raises the issue of whether WAT from animals at 18–24°C is indeed composed of white adipocytes or in fact brite adipocytes (detailed below). One recent study (de Jong et al., 2017) showed that β1‐ and β3‐adrenoceptor mRNA expression is increased in BAT, inguinal WAT, and epididymal WAT in mice acclimatised to 4°C compared to 30°C, but there is still appreciable expression of β1‐ and β3‐adrenoceptors in WAT in mice housed at 30°C. More research is needed to clarify whether adrenoceptors have an in vivo role in pure white adipocytes or whether their effects are only evident when mice are cold stressed at least to some degree.
3. ADRENOCEPTORS IN BAT
BAT has emerged as an attractive target for the treatment of obesity and metabolic disease. In mice, BAT is predominantly located dorsally within the scapular region, in interscapular, subscapular, and cervical depots, with smaller BAT depots located retroperitoneally, and in larger animals, in intercostal depots and the lining of the aortic arch (Cinti, 2005). Despite its name, only a small proportion of cells (5–50%) within each BAT depot represent true mature brown adipocytes. The SVF of BAT (the immature content of adipose tissue depots that includes endothelial and mesenchymal progenitors) is dominated by undifferentiated preadipocytes, which provide a means of thermogenic adaptability through proliferation and differentiation. A high vascular endothelial content reflects the importance of blood flow for BAT function, providing delivery of energy sources (such as lipids and glucose) to brown adipocytes, and for the dissipation of heat from BAT to core organs. The main function of BAT is non‐shivering thermogenesis, which has been extensively reviewed before (Cannon & Nedergaard, 2004) and is briefly summarised here.
Noradrenaline released from sympathetic nerve terminals in BAT exerts its actions predominantly through β‐adrenoceptors. Brown adipocytes have high expression of the mitochondrial protein UCP1, which is located on the inner mitochondrial membrane and exerts its effects through its ability when activated to uncouple aerobic respiration from mitochondrial ATP generation to dissipate heat, a process termed non‐shivering thermogenesis. In unstimulated adipocytes (such as in mice housed at thermoneutrality or adipocytes grown in culture), UCP1 is inactive, but in response to cold exposure or noradrenaline, UCP1 activity increases through two different mechanisms (Figure 2): acutely or through adaptive thermogenesis. Acutely, noradrenaline stimuli increase UCP1 activity through a well‐characterised pathway involving a Gαs‐cAMP‐PKA‐mediated mechanism, resulting in the liberation of fatty acids from intracellular stores that can then bind to and activate UCP1. In adaptive thermogenesis, cold exposure or noradrenaline increases the expression of UCP1 mRNA and protein via a Gαs‐cAMP‐PKA‐p38MAPK‐mediated pathway. This results in increased UCP1 protein that allows for greater mitochondrial uncoupling capacity. As well as its well‐recognised role in thermogenesis, BAT now has an accepted role in whole‐body glucose regulation. Analysis of the transcriptome during adaptive thermogenesis shows that cold exposure strongly up‐regulates genes associated with glucose metabolism (Hao et al., 2015) and acute activation drives the consumption of glucose and lipids (Peirce & Vidal‐Puig, 2013; Stanford et al., 2013).
Figure 2.
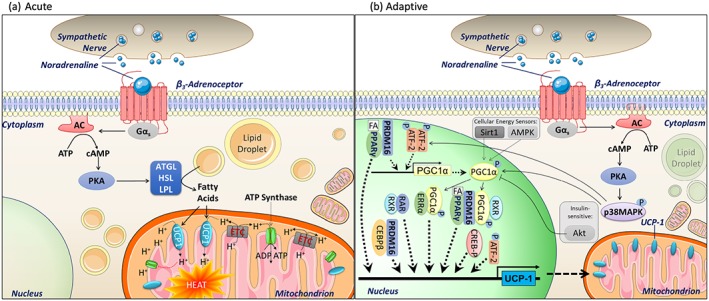
Mechanism of thermogenesis by noradrenaline in brown adipose tissue. (a) Acutely, noradrenaline increases mitochondrial uncoupling via activation of β3‐adrenoceptors. This occurs via a Gαs‐cAMP‐PKA‐mediated mechanism leading to activation of a series of lipases (adipose triglyceride lipase (ATGL), hormone sensitive lipase (HSL), and lipoprotein lipase (LPL)), increasing lipolysis of triglycerides stored in lipid droplets to fatty acids. These fatty acids can then activate uncoupling protein 1 (UCP1) located on the inner mitochondrial membrane, allowing protons into the inner mitochondrial membrane. This disrupts the proton gradient across the inner mitochondrial membrane, blocking ATP synthase and up‐regulating the electron transport chain activity in a futile attempt to redress the gradient, thereby generating heat. (b) Adaptive thermogenesis: PKA activation leads to phosphorylation of p38MAPK, which then exerts effects on UCP1 transcription via phosphorylation of PGC1α and ATF‐2 (which also promotes PGC1α transcription). Fatty acids also exert adaptive changes by activation of PPARγ, which combines with PRDM16 to promote PGC1α. PPARγ, PCG1α, PRDM16, and ATF‐2 play central roles in regulating and increasing UCP1 transcription, leading to increased UCP1 protein content and therefore greater mitochondrial uncoupling capacity
mRNA and protein for all three β‐adrenoceptor subtypes have been reported from rodent BAT, although the β3‐adrenoceptor is the most abundant, with the ratio of β1:β2:β3‐adrenoceptor mRNA levels reported to be 3:1:190 (Collins et al., 1994). In an elegant study, RNA sequencing (RNA‐Seq) was conducted exclusively on mRNAs undergoing active translation only in UCP1‐positive adipocytes derived from inguinal WAT, epididymal WAT, and BAT of cold‐exposed mice (Long et al., 2014). RNA‐Seq from BAT‐derived RNA showed robust β3‐adrenoceptor expression of 11.2 ± 0.27 fragments per kilobase per million reads, somewhat less β1‐adrenoceptor expression (3.1 ± 0.35), and very low β2‐adrenoceptor expression (0.87 ± 0.20). It is widely accepted that functionally, the predominant β‐adrenoceptor subtype in BAT is the β3‐adrenoceptor, supported by a vast array of studies using receptor selective agonists, including CL316243, that increase thermogenesis and UCP1 content in mice (this has been extensively reviewed previously; Arch & Kaumann, 1993; Cannon & Nedergaard, 2004). Despite the importance of β3‐adrenoceptors in BAT function, β3‐adrenoceptor KO mice display no significant impairment in basal metabolic rate or responses to cold exposure (de Jong et al., 2017; Mattsson et al., 2011; Susulic et al., 1995). This may in part be due to compensation by an increase in the levels and function of β1‐adrenoceptors in BAT/brown adipocytes, leading to intact responses to noradrenaline with respect to increased glucose uptake in vitro and non‐shivering thermogenesis in vivo (Chernogubova, Hutchinson, Nedergaard, & Bengtsson, 2005; Mattsson et al., 2011; Susulic et al., 1995). β1‐Adrenoceptors are expressed in both BAT and brown adipocytes (Chernogubova et al., 2005) but are not usually coupled to any significant functions in mature brown adipocytes. Instead, β1‐adrenoceptors play a role in preadipocytes to increase cAMP levels and promote cell proliferation (Bronnikov, Houstek, & Nedergaard, 1992), with β1‐adrenoceptor expression and function declining during brown adipocyte differentiation, resulting in a switch in coupling from β1‐adrenoceptors to β3‐adrenoceptors (Bronnikov et al., 1999). It is important to note that in brown preadipocytes from wild‐type mice, noradrenaline cannot increase UCP1 expression, ruling out a role for β1‐adrenoceptors in UCP1 function normally, but noradrenaline and isoprenaline can increase UCP1 levels in mature brown adipocytes derived from β3‐adrenoceptor KO mice (Chernogubova et al., 2005; Mattsson et al., 2011). However, as BAT itself is a mixture of both mature and preadipocytes, β1‐adrenoceptors will have roles at least in native BAT. Indeed, in isolated mouse BAT membranes, 23% of the adenylate cyclase response to adrenaline is mediated via β1/β2‐adrenoceptors (Collins et al., 1994). A role for β1‐adrenoceptors in BAT/brown adipocytes comes from studies using KO animals. As mentioned above, a compensatory role for β1‐adrenoceptors when β3‐adrenoceptors are lacking has been reported (Chernogubova et al., 2005), while mice with adipose tissue‐specific β1‐adrenoceptor overexpression are resistant to diet‐induced obesity (Soloveva, Graves, Rasenick, Spiegelman, & Ross, 1997), and β1‐adrenoceptor KO mice display impaired BAT function under cold stimuli (Ueta et al., 2012). Therefore, there appears to be some role for β1‐adrenoceptors in brown adipocytes/BAT which requires more investigation.
While β2‐adrenoceptor mRNA and protein can be detected in native BAT (Chernogubova et al., 2005; Levin & Sullivan, 1986; Rothwell, Stock, & Sudera, 1985), it is not believed that they are expressed in brown adipocytes (Bengtsson et al., 2000; Chernogubova et al., 2005) but rather most likely are localised to the vascular system, with activation of β2‐adrenoceptors promoting increased blood flow to BAT (Ernande et al., 2016) and β2‐adrenoceptor KO mice having intact cold and diet‐induced thermogenesis (Fernandes et al., 2014). Even in mice devoid of both β1‐ and β3‐adrenoceptors, BAT morphology resembles that of wild‐type mice, suggesting even that β2‐adrenoceptors can compensate for the lack of β1‐ and β3‐adrenoceptors (Bachman et al., 2002). However, there is a gross difference in mice lacking all three β‐adrenoceptors. Most β‐less mice do not survive upon exposure to the cold (Jimenez et al., 2002), presumably since they lack the adaptive response to increase UCP1 in BAT following cold exposure (Bachman et al., 2002; Jimenez et al., 2002). In these mice, BAT shows gross morphological changes and is light brown in colour, and brown adipocytes resemble white adipocytes (Bachman et al., 2002; Jimenez et al., 2002) and even express leptin, which is considered a white adipocyte marker. These studies highlight the critical role of β‐adrenoceptors in normal BAT development and physiology.
BAT and brown adipocytes also express α1‐adrenoceptors (Chernogubova et al., 2005; Kikuchi‐Utsumi, Kikuchi‐Utsumi, Cannon, & Nedergaard, 1997; Merlin, Sato, Nowell, et al., 2018) and α2‐adrenoceptors (Bronnikov et al., 1999). The α1‐adrenoceptor agonist cirazoline and the α2‐adrenoceptor agonist clonidine both promote substantial increases in ERK1/2 phosphorylation, comparable to the effect of CL316243 (Wang, Falting, et al., 2013). We have shown by RT‐qPCR that there is significant expression of the α2A‐adrenoceptor, α1A‐adrenoceptor, and the α1D‐adrenoceptor in mouse brown adipocytes and activation of α1A‐adrenoceptors in brown adipocytes with the highly selective agonist A61603 increases calcium mobilisation, glycolysis, and glucose uptake, with no effects on oxygen consumption rates (Merlin, Sato, Nowell, et al., 2018). When using the α1‐adrenoceptor agonist cirazoline, glucose uptake responses are also evident in brown adipocytes derived from β3‐adrenoceptor KO mice (Chernogubova et al., 2005). Overall, it is thought that increased calcium influx mediated by α1‐adrenoceptor activation may play a minor role in enhancing β3‐adrenoceptor‐stimulated lipolysis and mitochondrial uncoupling (Tavernier et al., 2005; Thonberg, Zhang, Tvrdik, Jacobsson, & Nedergaard, 1994) and may contribute to β3‐adrenoceptor‐mediated increases in UCP1 transcription (Thonberg, Fredriksson, Nedergaard, & Cannon, 2002), with α1A‐adrenoceptor expression increasing following cold exposure (Granneman, Zhai, & Lahners, 1997). α2‐Adrenoceptors are Gi/o coupled receptors, and their activation suppresses noradrenaline‐mediated increases in cAMP levels in brown adipocytes (Bronnikov et al., 1999). Although the functional relevance of adipocyte α2‐adrenoceptors is unclear, they have been postulated as a therapeutic target in reducing body temperature during fever. This however is an indirect effect, as administration of α2‐adrenoceptor agonists inhibits BAT sympathetic nerve activity and BAT thermogenesis through centrally mediated mechanisms (Madden, Tupone, Cano, & Morrison, 2013).
In addition to its effects on thermogenesis, there is increasing appreciation of the role of BAT in whole‐body glucose homeostasis (Bartelt et al., 2011; Cawthorne, 1989; Nedergaard, Bengtsson, & Cannon, 2011). This is highlighted in a study where BAT from healthy mice was transplanted into mice fed a high‐fat diet (Stanford et al., 2013). The recipient mice had improved glucose tolerance, increased insulin sensitivity, and reduced body weight and fat mass. This effect was postulated to be due to release of IL‐6 from the transplanted BAT (Stanford et al., 2013). The sympathetic nervous system plays an important role in BAT glucose uptake. BAT consumes a very high amount of glucose per gram of tissue from the blood (Liu, Perusse, & Bukowiecki, 1994; Shibata, Perusse, Vallerand, & Bukowiecki, 1989), especially under conditions such as cold exposure, that is, ~75% of glucose is disposed in BAT in cold‐exposed obese animals (Bartelt et al., 2011; Nedergaard et al., 2011). Cold exposure (Shibata et al., 1989; Shimizu, Nikami, & Saito, 1991) increases glucose uptake into BAT, an effect due to activation of the sympathetic nervous system (Shimizu et al., 1991), and is mimicked by administration of β‐adrenoceptor agonists in vivo (Cawthorne, 1989; Liu et al., 1994; Olsen et al., 2014) and in vitro (Chernogubova, Cannon, & Bengtsson, 2004; Chernogubova et al., 2005; Dallner, Chernogubova, Brolinson, & Bengtsson, 2006; Merlin, Sato, Nowell, et al., 2018; Olsen et al., 2014). The β3‐adrenoceptor appears to be the main adrenoceptor subtype mediating increased glucose uptake into brown adipocytes (Chernogubova et al., 2005; Merlin, Sato, Nowell, et al., 2018), with a minor role for β1‐ and α1‐adrenoceptors in β3‐adrenoceptor KO mice (Chernogubova et al., 2005). Importantly, the mechanism of β3‐adrenoceptor‐mediated glucose uptake differs from that of insulin. Whereas insulin causes rapid translocation of the glucose transporter GLUT4 from intracellular vesicles to the cell surface to facilitate glucose uptake, β3‐adrenoceptor stimulation increases the synthesis and translocation of GLUT1 (Dallner et al., 2006; Merlin, Sato, Nowell, et al., 2018; Olsen et al., 2014) via a pathway that is independent of key insulin signalling molecules that are typically down‐regulated in type 2 diabetes. Instead, a cAMP‐mammalian target of rapamycin complex 2 (mTORC2)‐mediated mechanism is employed (Olsen et al., 2017; Olsen et al., 2014) and is independent of UCP1 since β3‐adrenoceptor‐mediated glucose uptake in vivo is absent in UCP1 KO mice (Olsen et al., 2017). Hence, there is renewed interest in whether BAT can be targeted for the treatment of type 2 diabetes.
There is growing evidence for an endocrine role of BAT, with the release of several of the adipokines and secretory factors also being regulated by the sympathetic nervous system as reviewed elsewhere (Cannon & Nedergaard, 2004; Villarroya, Cereijo, & Villarroya, 2013; G. X. Wang, Zhao, & Lin, 2015). Upon cold exposure, BAT undergoes hyperplasia and hypertrophy, which involves angiogenesis (leading to increased vascularisation of BAT to allow for increased blood flow that the tissue requires; Bukowiecki, Collet, Follea, Guay, & Jahjah, 1982). This is due to an increase in VEGF expression and release from brown adipocytes following both cold exposure and stimulation of β3‐adrenoceptors (Fredriksson, Lindquist, Bronnikov, & Nedergaard, 2000; Tonello et al., 1999), independently of UCP1 (Fredriksson, Nikami, & Nedergaard, 2005). In brown preadipocytes, stimulation of β1‐adrenoceptors also results in increased VEGF expression (Fredriksson et al., 2000).
Collectively, in rodents, increased sympathetic output to BAT activates β3‐adrenoceptors located on mature adipocytes to acutely increase BAT mitochondrial uncoupling, whereas chronic stimulation of β3‐adrenoceptors increases UCP1 mRNA and protein levels (Figure 2). β3‐Adrenoceptors are also involved in increasing glucose uptake in BAT and affecting the secretion of several adipokines that include VEGF that promotes vascular remodelling. It is considered that the β3‐adrenoceptor is the predominant adrenoceptor subtype mediating effects in BAT/brown adipocytes, with a smaller role for β1‐adrenoceptors in brown preadipocytes.
4. EMERGING ROLE OF BRITE ADIPOCYTES
There is considerable interest in identifying new approaches to the treatment of obesity, with much recent interest focused on brite adipocytes. While early studies identified the presence of brown adipocytes (defined by UCP1 expression) in depots primarily considered to be white (Cousin et al., 1992; Himms‐Hagen et al., 2000; Young, Arch, & Ashwell, 1984), it has only been over the past decade through gene expression profiling that these adipocytes have been determined to differ from conventional brown adipocytes (de Jong, Larsson, Cannon, & Nedergaard, 2015; Petrovic et al., 2010; Pisani et al., 2011). These cells have been termed brite (brown in white) or beige adipocytes and are defined as brown‐like adipocytes located in WAT depots (reviewed in Merlin et al., 2016). There are other reviews (Sanchez‐Gurmaches, Hung, & Guertin, 2016) that eloquently outline the genetic differences in brite versus brown and white adipocytes and adipocyte development. Briefly, brite adipocytes develop from a white adipocyte precursor cell (Myf5−, Hoxc9+) and not a brown adipocyte precursor cell (Myf5+). They display visual characteristics that are intermediate between a brown and white adipocyte (mitochondrial content, lipid droplet size/number).
Much interest in the brite adipocyte field is aimed at identifying agents/targets that can increase the appearance of UCP1 in white adipocytes both in vivo and in vitro (reviewed in Merlin et al., 2016). The most potent in vivo browning agent identified to date is cold exposure. While cold exposure as discussed above is a key regulator of BAT (increasing the presence and function of UCP1 in this tissue; Cannon & Nedergaard, 2004), it also increases UCP1 content in adipose depots considered to be “white” in rodents (Cousin et al., 1992; Guerra, Koza, Yamashita, Walsh, & Kozak, 1998; Kalinovich et al., 2017; Vitali et al., 2012). Importantly, mitochondria isolated from brite adipocytes (obtained from inguinal WAT of mice exposed to 4°C) are thermogenically functional, displaying UCP1‐dependent oxygen consumption (Shabalina et al., 2013). This browning of white adipocytes following cold exposure is also thought to occur from activation of the sympathetic nervous system, with viral tracking techniques illustrating sympathetic innervation to both subcutaneous and visceral WAT depots (Bartness et al., 2014) and cold exposure increasing sympathetic drive to BAT and WAT resulting in increased noradrenaline release from nerve terminals within these depots (Brito et al., 2008). The effect of cold exposure on browning is thought to be mediated by the β3‐adrenoceptor since long‐term treatment of animals with β3‐adrenoceptor agonists also promotes the appearance of UCP1 in WAT depots (Cousin et al., 1992; Granneman, Li, Zhu, & Lu, 2005; Guerra et al., 1998; Pico, Bonet, & Palou, 1998). This is further supported by a study performed in β3‐adrenoceptor KO mice. β3‐Adrenoceptor KO mice exposed to the cold have blunted UCP1 mRNA and protein levels in WAT depots compared to wild‐type mice (Jimenez et al., 2003). However, in these cold exposed mice (10 days at 6°C), there is still some increased UCP1 mRNA/protein as compared to mice housed at 24°C (Jimenez et al., 2003), which may suggest that in addition to the β3‐adrenoceptor component, a very small component of cold‐induced browning via a non‐β3‐adrenoceptor is also present.
While catecholamines are typically formed in sympathetic nerves or the adrenal medulla, there is evidence that they can be generated in alternatively activated M2 macrophages that are recruited upon cold exposure, stimulating lipolysis (Nguyen et al., 2011) and promoting adipose tissue remodelling and browning of WAT (Y. H. Lee, Petkova, & Granneman, 2013; Qiu et al., 2014). However, this hypothesis was not supported by a recent study (Fischer et al., 2017) showing that these macrophages do not produce enough catecholamines to have an effect and are unlikely to have any direct roles in adipocyte metabolism or browning of WAT.
While most interest in the adipocyte field has been to identify agents/targets that increase the appearance of UCP1 mRNA/protein expression in WAT (browning), this does necessarily mean that the resultant brite adipocytes are functionally capable. They still must be able to respond to a thermogenic agent to increase thermogenesis. It appears that the β3‐adrenoceptor may be important in browning WAT, as in two studies using β3‐adrenoceptor KO mice, upon cold exposure, the formation of multilocular cells and the expression of cold inducible genes were impaired (Barbatelli et al., 2010; Jimenez et al., 2003); however, this was not replicated in another study (de Jong et al., 2017). There is limited information on the expression and function of adrenoceptors in brite adipocytes that have been browned using any other stimuli. In vitro, brite adipocytes (from rosiglitazone‐treated white adipocytes) express β1‐, β2‐, and β3‐adrenoceptor mRNA, but functionally, only the β3‐adrenoceptor appears to mediate responses (Merlin, Sato, Nowell, et al., 2018). These brite adipocytes display enhanced β3‐adrenoceptor mRNA levels and functional β3‐adrenoceptor‐mediated responses that include increases in UCP1 mRNA/protein levels leading to enhanced O2 consumption/thermogenesis, cAMP levels, glucose uptake and glycolysis, GLUT1 mRNA, and GLUT1 cell surface abundance (Merlin, Sato, Nowell, et al., 2018). These effects are more pronounced in brite adipocytes derived from white adipocytes from inguinal WAT than from epididymal WAT (Merlin, Sato, Nowell, et al., 2018), consistent with the notion that visceral WAT is harder to brown than subcutaneous WAT (Okamatsu‐Ogura et al., 2013; Walden, Hansen, Timmons, Cannon, & Nedergaard, 2012). These brite adipocytes also express the α1A‐ and α1D‐adrenoceptor, with acute activation of the α1A‐adrenoceptor in differentiated brite adipocytes increasing calcium mobilisation, glycolysis, and glucose uptake but having no effect on oxygen consumption (Merlin, Sato, Nowell, et al., 2018). One recent study (Klepac et al., 2016) suggested that activation of Gαq/11 coupled receptors (such as the endothelin ETA receptor) reduces brown and brite adipocyte differentiation and decreases UCP1 expression, suggesting that Gαq/11 receptor antagonists may be a promising target to increase browning. Hence, further studies aimed at investigating the acute and long‐term effects of Gαq/11 receptor activation or inhibition should be performed.
As indicated above, the housing temperature of animals is of importance, as ambient temperature has a drastic effect on UCP1 expression. This has been reviewed and highlighted elsewhere (Cannon & Nedergaard, 2011; Kalinovich et al., 2017). Given that mouse thermoneutral temperatures are close to 30°C, animals housed at ambient room temperatures of 20–24°C are already cold stressed. As a result, they have a higher metabolic rate and express significant near‐maximal levels of UCP1 mRNA and protein in BAT in comparison to animals housed at 30°C (Cannon & Nedergaard, 2011; Kalinovich et al., 2017). In comparison, there is negligible expression of UCP1 in inguinal WAT when mice are housed at 30°C, some expression at ambient temperature, but significant increases following cold exposure (Kalinovich et al., 2017). Hence, housing conditions of mice should be an important consideration when investigating potential browning agents.
5. ADRENOCEPTORS IN HUMAN ADIPOSE TISSUE
5.1. Expression of adrenoceptors in native human adipose tissue and cultured adipocytes
We have reanalysed publicly available RNA‐Seq data sets for adrenoceptor mRNA expression in human adipose tissue and adipocytes (accession numbers E‐MTAB‐2602 (Shinoda et al., 2015), E‐MTAB‐4031 (Chondronikola et al., 2016), and GSE 97205 (Ding et al., 2018)). We compared native adipose tissue with primary human adipocyte cultures and have also included expression levels for the mature adipocyte markers FABP4 (aP2) and ADIPOQ (adiponectin), the white adipocyte lineage marker HOXC9, and genes characteristically expressed in thermogenic brown or brite adipocytes (PPARGC1A, CPT1B, FABP3, and UCP1), as these provide important information regarding the predominant phenotype of cultured cells or in tissue samples. Full details of the methodology and rationale employed are provided in the Supporting Information.
Figure 3 shows that FABP4 and ADIPOQ levels are high in native BAT and WAT, as well as differentiated supraclavicular brown and subcutaneous white adipocytes, consistent with a high proportion of mature adipocytes. Conversely, both mRNAs are absent from all undifferentiated adipocyte cultures. HOXC9 is a marker of the white adipocyte lineage, and expression levels are highest in subcutaneous WAT samples, preadipocytes, and differentiated adipocytes. HOXC9 is also expressed in supraclavicular BAT and primary brown adipocytes, reflecting the accepted notion that human BAT is not classical BAT but is instead composed largely of brite adipocytes (P. Lee, Werner, Kebebew, & Celi, 2014; Sharp et al., 2012; Shinoda et al., 2015; Wu et al., 2012). Interestingly, HOXC9 mRNA is also present in fetal interscapular BAT, indicating that these human depots may also contain brite adipocytes. The genes characteristic of thermogenic brown or brite adipocytes, namely, PPARGC1A, CPT1B, FABP3, and UCP1, show substantial expression mainly in native BAT, and all four are markedly increased in the supraclavicular BAT of an individual subjected to cold exposure for 5 hr (Chondronikola et al., 2016). FABP3 is expressed at high levels in differentiated adipocytes from all fetal and adult depots, whereas the other three genes are expressed at very low levels even in mature adipocyte cultures.
Figure 3.
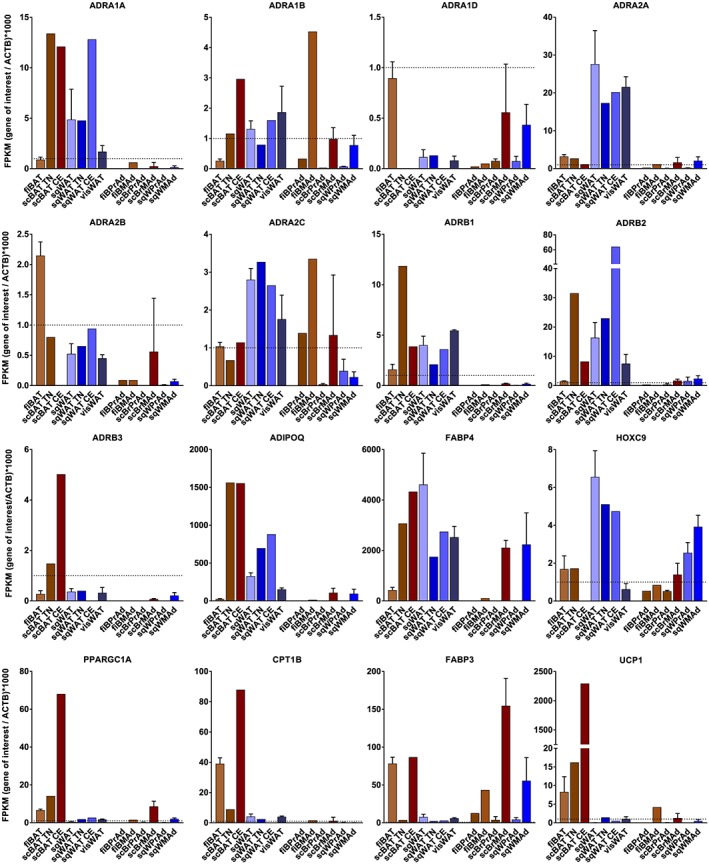
RNA sequencing‐derived expression of adrenoceptor and adipocyte marker genes relative to β‐actin, FPKM (gene of interest/ACTB) × 1,000 (see Supporting Information). RNA samples on the left of each panel are from native human fetal interscapular brown adipose tissue (fiBAT, n = 4), biopsies of supraclavicular BAT from an individual maintained at thermoneutrality (scBAT TN, n = 1) or subject to 5‐hr cold exposure (scBAT CE, n = 1), subcutaneous white adipose tissue (sqWAT, n = 3), biopsies of subcutaneous WAT from an individual maintained first thermoneutrality (scWAT TN, n = 1) or subject to 5‐hr cold exposure (scWAT CE, n = 1), and omental visceral WAT (visWAT, n = 3). RNA samples on the right of each panel are from human preadipocytes (PrAd) or differentiated mature adipocytes (MAd) cultured from fetal interscapular BAT (fiBrPrAd, n = 1 or fiBrMAd, n = 1), supraclavicular BAT (scBrPrAd, n = 3 or scBrMAd, n = 3), and subcutaneous WAT (sqWPrAd, n = 4 or sqWMAd, n = 4). Dotted horizontal lines denote a fragments per kilobase per million reads (FPKM) value of 1.0
The α1A‐adrenoceptor displays robust expression in all native adult samples but negligible expression in cultured adipocytes. In contrast, mRNA for the α1B‐adrenoceptor is observed in native tissues but also in differentiated adipocytes from all depots, while α1D‐adrenoceptor expression is extremely low both in tissues and in primary cultures. The α2A‐adrenoceptor displays robust expression in adult WAT depots, much lower expression in BAT, and low but significant expression in mature human adipocyte cultures. The α2B‐adrenoceptor is highest in fetal BAT, while α2C‐adrenoceptor expression is high in adult WAT and fetal BAT. Significant α2C‐adrenoceptor mRNA levels are also observed in cultured fetal interscapular brown adipocytes. As described above, there is extensive literature on the role of β‐adrenoceptors in adipose tissue in animals; thus, it is interesting that all three receptors are expressed in native human adipose depots. β1‐ and β2‐adrenoceptor mRNAs are present in all BAT and WAT depots, whereas β3‐adrenoceptor mRNA is expressed primarily in adult supraclavicular BAT. Like other thermogenic markers, the number of β3‐adrenoceptors is increased in supraclavicular BAT from a subject exposed to cold (Chondronikola et al., 2016).
One question arising from this RNA‐Seq data is whether β3‐adrenoceptor mRNA is present at a physiologically significant level in human WAT depots. Earlier reports used RT‐PCR to demonstrate β3‐adrenoceptor expression in WAT, although signals were consistently higher in infant BAT or in perirenal BAT (Krief et al., 1993; Lonnqvist et al., 1993; Tavernier et al., 1996). The consistent finding of very low WAT β3‐adrenoceptor expression from both RT‐PCR and RNA‐Seq suggests that there may be minor subpopulations of β3‐adrenoceptor‐positive cells in human WAT depots.
Adipocyte cultures derived from the SVF of human adipose depots display negligible expression of β3‐adrenoceptors, even after differentiation in the presence of highly adipogenic cocktails (Ding et al., 2018; Shinoda et al., 2015). β1‐Adrenoceptor mRNA is also negligible in primary human adipocytes, whereas the β2‐adrenoceptor is expressed in differentiated cultures with mean fragments per kilobase per million reads values of 1.8 (supraclavicular brown adipocytes) and 2.2 (subcutaneous white adipocytes). The lack of β3‐adrenoceptor expression occurs in parallel with low levels of mRNA for PPARGC1A, CPT1B, and UCP1, all central to the cellular control of thermogenesis. This suggests that the differentiation of thermogenic brown or brite adipocytes is difficult to achieve experimentally in primary human adipocytes derived from the SVF. Shinoda et al. (2015) noted that differentiation of clonally derived supraclavicular brown adipocyte cultures in the presence of 1 μM rosiglitazone, and/or treatment of mature adipocytes with 10 μM forskolin for 4 hr, was sufficient to induce UCP1 expression levels similar to those seen in native scBAT biopsies, as we observed in mouse brown and brite adipocyte cultures (Merlin, Sato, Chia, et al., 2018). This type of induction may be necessary to promote expression of β3‐adrenoceptors, PPARGC1A, and CPT1B.
In addition, low expression of these genes in human adipocyte cultures may reflect cellular heterogeneity in the biopsy specimens from which preadipocytes were isolated. In the study by Shinoda et al. (2015), immortalised adipocytes expressing SV40 T antigen were cloned by limiting dilution and screened for their capacity to undergo adipogenesis; thus, the final adipocyte lines represent a small subset of the starting preadipocyte population. The notion of adipocyte heterogeneity within native depots has been raised by a number of groups (Lee et al., 2013; Shinoda et al., 2015; Spaethling et al., 2016). Indeed, based on single‐cell RNA‐Seq data, even amongst individual mature brown adipocytes isolated from mouse interscapular BAT, there are substantial differences in expression of marker genes and key adrenoceptors, and there is no correlation between expression of the β3‐adrenoceptor and Ucp1 (Spaethling et al., 2016).
Alternative cell models, including human multipotent adipose‐derived stem cells (Elabd et al., 2009; Pisani et al., 2011), Simpson–Golabi–Behmel syndrome (SGBS) preadipocytes (Wabitsch et al., 2001), and immortalised PAZ6 brown adipocytes (Zilberfarb et al., 1997), have been utilised for the study of human adipocyte biology. Low‐level expression of β‐adrenoceptor subtypes has been detected by qPCR in human multipotent adipose‐derived stem cells, with a ratio of 3:12:1 for β1:β2:β3‐adrenoceptor (Mattsson et al., 2011), but only β1‐ and β3‐adrenoceptor agonists increase UCP1 mRNA and protein levels in these cells (Mattsson et al., 2011). Differentiated SGBS and PAZ6 cells have been analysed by RNA‐Seq (Guennoun et al., 2015). β3‐Adrenoceptor expression is undetectable in SGBS cells but is significant in differentiated PAZ6 cells (2.5 RPKM (reads per kilobase per million mapped reads); Guennoun et al., 2015). It is thus evident that expression levels of adrenoceptors and thermogenic markers must be considered in different model systems when investigating potential browning agents.
5.2. Adrenoceptor function in human WAT
In human WAT, multiple studies indicate little or no lipolytic or thermogenic response to β3‐adrenoceptor activation; rather, these responses are mediated by β1‐ and β2‐adrenoceptors (Bousquet‐Melou, Galitzky, Lafontan, & Berlan, 1995; Carpene et al., 1994; Carpene et al., 1999; Tavernier et al., 1996). These findings are consistent with the mRNA expression data; however, it should be noted that most of these studies were performed using β3‐adrenoceptor agonists such as CL316243, which have little effect at the human β3‐adrenoceptor. Studies using agonists such as mirabegron that are active at the human receptor will help elucidate whether there is any functional role of β3‐adrenoceptors in human white adipocytes.
Human and rodent WAT/white adipocytes differ significantly with respect to expression of α2‐adrenoceptors, with high expression of α2‐adrenoceptors in human WAT (Figure 3; Galitzky, Larrouy, Berlan, & Lafontan, 1990; Mauriege et al., 1991; Mauriege, Marette, et al., 1995; Mauriege, Prud'homme, Lemieux, Tremblay, & Despres, 1995), but little expression in rodent white adipocytes (Merlin, Sato, Nowell, et al., 2018; Valet et al., 2000). Activation of Gαi/o‐coupled α2‐adrenoceptors in human white adipocytes inhibits catecholamine‐stimulated increases in lipolysis, thereby counteracting β‐adrenoceptor‐mediated lipolysis (Stich et al., 1999), and white adipocytes from obese humans have increased α2‐adrenoceptor levels, increased α2:β‐adrenoceptor ratios, and increased α2‐adrenoceptor‐mediated responses (Galitzky et al., 1990; Mauriege et al., 1991; Mauriege, Marette, et al., 1995; Mauriege, Prud'homme, et al., 1995). When the human α2‐adrenoceptor is overexpressed in adipose tissue of β3‐adrenoceptor KO mice, catecholamine‐mediated lipolysis in white adipocytes is attenuated, and the mice develop increased obesity on a high‐fat diet (Valet et al., 2000). Despite significant expression of α1A‐ and α1B‐adrenoceptors in native human adipose tissue (Figure 3), there is no convincing functional evidence for direct activity in adipocytes based on characterised α1‐adrenoceptor selective ligands.
5.3. Adrenoceptor function in human BAT
Originally, BAT was thought to be present only in human newborn infants, with expression rapidly declining in the postnatal period, or in adult humans exposed to extreme cold environments. Approximately 10 years ago, the presence of active BAT in adult humans was demonstrated (Nedergaard, Bengtsson, & Cannon, 2007) and found to be inversely correlated with age, body mass index (BMI), and glycaemic status (Cypess et al., 2009; Ouellet et al., 2011; van Marken Lichtenbelt et al., 2009; Vijgen et al., 2011). This is important as studies in rodents show that increased BAT ameliorates the effects of a high‐fat diet (Stanford et al., 2013) while BAT‐deficient mice are prone to obesity (Lowell et al., 1993). Activation of human BAT increases glucose uptake as assessed by 2‐[18F]fluoro‐2‐deoxyglucose PET scans (Carey et al., 2013; Cypess et al., 2009; Cypess et al., 2015; Nedergaard et al., 2007; Ouellet et al., 2011; van Marken Lichtenbelt et al., 2009; Virtanen et al., 2009) and oxygen consumption as assessed by [15O]O2 PET scans (U Din et al., 2016). As such, agents that activate human BAT have been advocated as a potential strategy for the treatment of metabolic diseases.
While there are many studies demonstrating various pharmacological/environmental ways to activate rodent BAT in vivo, only cold exposure (Cypess et al., 2012; Orava et al., 2011; Saito et al., 2009; van Marken Lichtenbelt et al., 2009; Virtanen et al., 2009) and activation of the sympathetic nervous system (Carey et al., 2013; Cypess et al., 2015) have been conclusively shown to increase human BAT activity. This includes the non‐selective adrenergic agonist ephedrine (Carey et al., 2013) and the β3‐adrenoceptor agonist mirabegron (Baskin et al., 2018; Cypess et al., 2015) which is used clinically for overactive bladder (OAB; Dehvari, da Silva Junior, Bengtsson, & Hutchinson, 2018). Mirabegron also increases energy expenditure and supraclavicular skin temperature in humans (Baskin et al., 2018; Loh et al., 2019). Whether the effects of mirabegron are via direct actions on adipocytes or via centrally mediated mechanisms has not been determined but are likely to not be the latter since mirabegron does not cross the blood brain barrier (Department of Health Therapeutic Goods Adminstration: Australian public assessment report for mirabegron, 2014). Hence, targeting of the β3‐adrenoceptor in humans has been advocated to treat obesity/type 2 diabetes, despite the failure of previous β3‐adrenoceptor agonists in clinical trials (Arch, 2011). Again, this is thought to be due to earlier studies using ligands such as CL316243, which are rodent‐selective, with little activity at the human β3‐adrenoceptor, due to subtle differences in the structure of the mouse compared to the human receptor.
5.4. Brite adipocytes
Although BAT has been identified in adult humans, several studies have suggested that human BAT may comprise both brown and brite adipocytes (Sharp et al., 2012; Wu et al., 2012), with other groups suggesting that BAT depots from the deep neck and supraclavicular regions resemble brown adipocytes, with some brite adipocytes in regions closer to the skin (Cypess et al., 2013; Jespersen et al., 2013). The debate over whether humans have brown/brite adipocytes is complicated, since humans typically live under thermoneutral conditions, where a clear distinction between brown and brite adipocytes may be difficult to ascertain.
One attractive goal, however, is to identify treatments that promote recruitment and activation of brite adipocytes within WAT depots, especially subcutaneous WAT. Mildly obese humans (BMI 31) are estimated to have up to 27 kg of subcutaneous WAT (Virtanen et al., 2005). Even if rates of brite conversion and thermogenesis are low compared to classical BAT, there are likely to be substantial metabolic benefits, as seen, for example, in mice expressing the brown fat marker Prdm16 in subcutaneous WAT (Seale et al., 2011). In one study, human adipose‐derived stromal progenitor cells differentiated into brite adipocytes by rosiglitazone exhibited increased β‐adrenoceptor mRNA levels, and responsiveness (mitochondrial function; Bartesaghi et al., 2015), which was similar to that observed in mouse brite adipocytes (Merlin, Sato, Nowell, et al., 2018). One recent in vivo study showed that mirabegron (50 mg·day−1 for 10 weeks) increases UCP1 protein levels in subcutaneous WAT (Finlin et al., 2018); however, its effects on mitochondrial function were not examined. In a separate study, mitochondria from human abdominal subcutaneous WAT following cold exposure showed increased uncoupled and maximal respiration (Kern et al., 2014). There also appears to be a seasonal variation in UCP1 content in human subcutaneous WAT (Finlin et al., 2018; Kern et al., 2014), which also occurs in human adult BAT. Hence, there is some evidence of β‐adrenoceptor function in human brite adipocytes, but more research is warranted. This includes elucidation of the β‐adrenoceptor subtype involved, whether β‐adrenoceptor agonists can activate brite adipocytes in vivo in clinically relevant populations and whether brite adipocyte activation has beneficial effects on human obesity and diabetes status.
5.5. Perspectives on the use of β3‐adrenoceptor agonists in human obesity and diabetes
β3‐Adrenoceptor agonists failed in early clinical trials for the treatment of obesity and type 2 diabetes. The main reasons for these failures include poor activity at the human versus rodent β3‐adrenoceptor, and poor bioavailability and pharmacokinetic profiles (Arch, 2002). The development of mirabegron (Astellas Pharma Inc.), a β3‐adrenoceptor agonist used clinically for the treatment of OAB, has once again reinvigorated the field, as it acutely activates human BAT activity in healthy young males (Baskin et al., 2018; Cypess et al., 2015). However, there is a lack of published data from the numerous clinical trials by Astellas on whether mirabegron has any effects on obesity/diabetes endpoints such as body weight or plasma glucose levels. This could be interpreted as a lack of effect of mirabegron on these parameters, at least in OAB populations. Human BAT activity is inversely correlated with age, BMI, and diabetes status (Cypess et al., 2009; van Marken Lichtenbelt et al., 2009; Vijgen et al., 2011), and in mice, β3‐adrenoceptor expression and function is decreased in obese or diabetic animals (Collins et al., 1994). Hence, it is plausible that the patients who would benefit most from activation of BAT β3‐adrenoceptors by mirabegron may be less able to respond. This does not discount the possibility of mirabegron being useful for obesity/diabetes treatment in the future, if β3‐adrenoceptor expression could be increased in these patients. The notion of a dual therapy for activation of brown/brite adipocytes is gaining attention (Merlin et al., 2016). For example, the PPARγ agonist rosiglitazone promotes browning of white adipocytes, increases brite adipocyte β3‐adrenoceptor expression, and thereby facilitates responsiveness to β3‐adrenoceptor agonists (Merlin, Sato, Nowell, et al., 2018). In line with this notion of dual therapy, there is one clinical trial underway (NCT02919176) in patients with a BMI of 29–45. Patients will be treated with the PPARγ activator pioglitazone (30 mg·day−1), mirabegron (50 mg·day−1), or a combination of both drugs, for 10 weeks. Parameters to be measured include BAT activity, insulin sensitivity, and glucose tolerance, and WAT biopsies will be taken to assess browning. Outcomes for this trial are expected in 2020.
6. CONCLUSION
After extensive collection of data from diverse fields (pharmacology, physiology, cell biology, genetics, and metabolism), several important considerations were noted from a GPCR perspective that may not be evident to the wider field and should be highlighted when studying the function of adrenoceptors in adipose tissue or adipocytes.
It is very important to establish the adrenoceptor profile in the tissue/cells being investigated, using appropriate tools that take into account ligand selectivity and species differences. Particular care should be taken in choosing optimal ligands based on known receptor subtype activity. For example, early studies utilised BRL35135 or the active metabolite BRL37344 as a β3‐adrenoceptor specific ligand. It is now appreciated that this is a dual β2/β3‐adrenoceptor agonist, yet its actions at β2‐adrenoceptors are generally overlooked. Likewise, the β3‐adrenoceptor agonist CL316243 is relatively ineffective at the human β3‐adrenoceptor yet is still used in human studies despite the availability of mirabegron.
One important factor that researchers outside of the GPCR field do not consider is the notorious lack of selectivity and specificity of antibodies against GPCRs in general (Michel, Wieland, & Tsujimoto, 2009). Hence, care should be employed in interpreting results with GPCR antibodies unless the appropriate validation has been demonstrated, for example, showing that signals are absent from receptor KO mice.
Determining whether housing conditions, in particular temperature, are likely to affect adipose tissue function. This has implications in studies, for instance, in WAT tissue analysis in animals housed at room temperature, as WAT will consist of both white and brite adipocytes under these conditions of mild cold stress.
As highlighted in Figure 3, the expression of most adrenoceptors is dramatically reduced (or absent) in clonally‐derived human adipocytes compared to freshly dissected tissue. This has wide implications in using these cells for investigation of adrenoceptor function. Those working in the GPCR field may not find this surprising as there are many cases of a GPCR that is highly expressed in a tissue only to be absent in primary derived cells (particularly following subculturing), but our results here show that this is very evident for adrenoceptors and should be taken into consideration. We have also highlighted the known heterogeneity of adipocyte populations within native adipose depots.
In conclusion, there is no doubt that adrenoceptors play an important role in adipose tissue/adipocyte functions, regulating adipocytes as an energy store, an endocrine organ (through the release of adipokines), modulating glucose metabolism, and as a thermogenic organ.
6.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Christopoulos et al., 2017; Alexander, Fabbro et al., 2017; Alexander, Kelly et al., 2017; Alexander, Peters et al., 2017).
CONFLICT OF INTEREST
T.B. owns stocks in the following pharmaceutical companies: Sigrid Therapeutics AB, Atrogi AB, and Glucox Biotechnology AB. D.H. owns stocks in Glucox Biotechnology AB and is a scientific advisor for Atrogi AB.
Supporting information
Table S1.
Source of native human adipose tissue RNA samples
Table S2. Culture and differentiation of human adipocytes
TABLE S3. Original FPKM values in human adipose depot RNA samples
TABLE S4. Original FPKM values in human adipocyte RNA samples
Figure S1. Expression of endogenous control genes relative to β‐actin [FPKM (gene of interest/ACTB) × 1000] (see section on RNA‐Seq data analysis). RNA samples on the left of each panel are from native human foetal interscapular BAT (fiBAT, n = 4), biopsies of supraclavicular BAT from an individual maintained at thermoneutrality (scBAT TN, n = 1) or subject to 5 hours cold exposure (scBAT CE, n = 1), subcutaneous WAT (sqWAT, n = 3), biopsies of subcutaneous WAT from an individual maintained first thermoneutrality (scWAT TN, n = 1) or subject to 5 hours cold exposure (scWAT CE, n = 1), and omental visceral WAT (visWAT, n = 3). RNA samples on the right of each panel are from human pre‐adipocytes (PrAd) or differentiated mature adipocytes (MAd) cultured from foetal interscapular BAT (fiBrPrAd, n = 1 or fiBrMAd, n = 1), supraclavicular BAT (scBrPrAd, n = 3 or scBrMAd, n = 3), and subcutaneous WAT (sqWPrAd, n = 4 or sqWMAd, n = 4). Dotted horizontal lines denote an FPKM value of 1.0.
ACKNOWLEDGEMENT
Some of the artwork contained in this manuscript was obtained from Servier Medical Art (http://smart.servier.com/).
Evans BA, Merlin J, Bengtsson T, Hutchinson DS. Adrenoceptors in white, brown, and brite adipocytes. Br J Pharmacol. 2019;176:2416–2432. 10.1111/bph.14631
REFERENCES
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174(Suppl 1), S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174(S1), S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Transporters. British Journal of Pharmacology, 174(S1), S360–S446. 10.1111/bph.13883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Peters, J. A. , Kelly, E. , Marrion, N. V. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Ligand‐gated ion channels. British Journal of Pharmacology, 174(S1), S130–S159. 10.1111/bph.13879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arch, J. R. (2002). β3‐Adrenoceptor agonists: Potential, pitfalls and progress. European Journal of Pharmacology, 440, 99–107. 10.1016/S0014-2999(02)01421-8 [DOI] [PubMed] [Google Scholar]
- Arch, J. R. (2011). Challenges in β3‐adrenoceptor agonist drug development. Therapeutic Advances in Endocrinology and Metabolism, 2, 59–64. 10.1177/2042018811398517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arch, J. R. , & Kaumann, A. J. (1993). β3 and atypical β‐adrenoceptors. Medicinal Research Reviews, 13, 663–729. 10.1002/med.2610130604 [DOI] [PubMed] [Google Scholar]
- Bachman, E. S. , Dhillon, H. , Zhang, C. Y. , Cinti, S. , Bianco, A. C. , Kobilka, B. K. , & Lowell, B. B. (2002). βAR signaling required for diet‐induced thermogenesis and obesity resistance. Science, 297, 843–845. 10.1126/science.1073160 [DOI] [PubMed] [Google Scholar]
- Barbatelli, G. , Murano, I. , Madsen, L. , Hao, Q. , Jimenez, M. , Kristiansen, K. , … Cinti, S. (2010). The emergence of cold‐induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. American Journal of Physiology. Endocrinology and Metabolism, 298, E1244–E1253. 10.1152/ajpendo.00600.2009 [DOI] [PubMed] [Google Scholar]
- Bartelt, A. , Bruns, O. T. , Reimer, R. , Hohenberg, H. , Ittrich, H. , Peldschus, K. , … Heeren, J. (2011). Brown adipose tissue activity controls triglyceride clearance. Nature Medicine, 17, 200–205. 10.1038/nm.2297 [DOI] [PubMed] [Google Scholar]
- Bartesaghi, S. , Hallen, S. , Huang, L. , Svensson, P. A. , Momo, R. A. , Wallin, S. , … Peng, X. R. (2015). Thermogenic activity of UCP1 in human white fat‐derived beige adipocytes. Molecular Endocrinology, 29, 130–139. 10.1210/me.2014-1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness, T. J. , Liu, Y. , Shrestha, Y. B. , & Ryu, V. (2014). Neural innervation of white adipose tissue and the control of lipolysis. Frontiers in Neuroendocrinology, 35, 473–493. 10.1016/j.yfrne.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness, T. J. , Shrestha, Y. B. , Vaughan, C. H. , Schwartz, G. J. , & Song, C. K. (2010). Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Molecular and Cellular Endocrinology, 318, 34–43. 10.1016/j.mce.2009.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin, A. S. , Linderman, J. D. , Brychta, R. J. , McGehee, S. , Anflick‐Chames, E. , Cero, C. , … Cypess, A. M. (2018). Regulation of human adipose tissue activation, gallbladder size, and bile acid metabolism by a β3‐adrenergic receptor agonist. Diabetes, 67, 2113–2125. 10.2337/db18-0462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson, T. , Cannon, B. , Nedergaard, J. , Bronnikov, G. , Kramarova, L. , & Golozoubova, V. (2000). Differential adrenergic regulation of the gene expression of the β‐adrenoceptor subtypes β1, β2 and β3 in brown adipocytes. The Biochemical Journal, 347, 643–651. 10.1042/bj3470643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet‐Melou, A. , Galitzky, J. , Lafontan, M. , & Berlan, M. (1995). Control of lipolysis in intra‐abdominal fat cells of nonhuman primates: Comparison with humans. Journal of Lipid Research, 36, 451–461. [PubMed] [Google Scholar]
- Bowers, R. R. , Festuccia, W. T. , Song, C. K. , Shi, H. , Migliorini, R. H. , & Bartness, T. J. (2004). Sympathetic innervation of white adipose tissue and its regulation of fat cell number. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 286, R1167–R1175. [DOI] [PubMed] [Google Scholar]
- Brito, N. A. , Brito, M. N. , & Bartness, T. J. (2008). Differential sympathetic drive to adipose tissues after food deprivation, cold exposure or glucoprivation. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 294, R1445–R1452. 10.1152/ajpregu.00068.2008 [DOI] [PubMed] [Google Scholar]
- Bronnikov, G. , Bengtsson, T. , Kramarova, L. , Golozoubova, V. , Cannon, B. , & Nedergaard, J. (1999). β1‐ to β3‐ switch in control of cyclic adenosine monophosphate during brown adipocyte development explains distinct β‐adrenoceptor subtype mediation of proliferation and differentiation. Endocrinology, 140, 4185–4197. 10.1210/endo.140.9.6972 [DOI] [PubMed] [Google Scholar]
- Bronnikov, G. , Houstek, J. , & Nedergaard, J. (1992). β‐Adrenergic, cAMP‐mediated stimulation of proliferation of brown fat cells in primary culture. Mediation via β1 but not via β3 adrenoceptors. The Journal of Biological Chemistry, 267, 2006–2013. [PubMed] [Google Scholar]
- Bukowiecki, L. , Collet, A. J. , Follea, N. , Guay, G. , & Jahjah, L. (1982). Brown adipose tissue hyperplasia: A fundamental mechanism of adaptation to cold and hyperphagia. The American Journal of Physiology, 242, E353–E359. 10.1152/ajpendo.1982.242.6.E353 [DOI] [PubMed] [Google Scholar]
- Cannon, B. , & Nedergaard, J. (2004). Brown adipose tissue: Function and physiological significance. Physiological Reviews, 84, 277–359. [DOI] [PubMed] [Google Scholar]
- Cannon, B. , & Nedergaard, J. (2011). Nonshivering thermogenesis and its adequate measurement in metabolic studies. The Journal of Experimental Biology, 214, 242–253. 10.1242/jeb.050989 [DOI] [PubMed] [Google Scholar]
- Carey, A. L. , Formosa, M. F. , Van Every, B. , Bertovic, D. , Eikelis, N. , Lambert, G. W. , … Kingwell, B. A. (2013). Ephedrine activates brown adipose tissue in lean but not obese humans. Diabetologia, 56, 147–155. 10.1007/s00125-012-2748-1 [DOI] [PubMed] [Google Scholar]
- Carpene, C. , Castan, I. , Collon, P. , Galitzky, J. , Moratinos, J. , & Lafontan, M. (1994). Adrenergic lipolysis in guinea pig is not a β3‐adrenergic response: Comparison with human adipocytes. The American Journal of Physiology, 266, R905–R913. 10.1152/ajpregu.1994.266.3.R905 [DOI] [PubMed] [Google Scholar]
- Carpene, C. , Galitzky, J. , Fontana, E. , Atgie, C. , Lafontan, M. , & Berlan, M. (1999). Selective activation of β3‐adrenoceptors by octopamine: Comparative studies in mammalian fat cells. Naunyn‐Schmiedeberg's Archives of Pharmacology, 359, 310–321. 10.1007/PL00005357 [DOI] [PubMed] [Google Scholar]
- Caserta, F. , Tchkonia, T. , Civelek, V. N. , Prentki, M. , Brown, N. F. , McGarry, J. D. , … Kirkland, J. L. (2001). Fat depot origin affects fatty acid handling in cultured rat and human preadipocytes. American Journal of Physiology. Endocrinology and Metabolism, 280, E238–E247. 10.1152/ajpendo.2001.280.2.E238 [DOI] [PubMed] [Google Scholar]
- Cawthorne, M. A. (1989). Does brown adipose tissue have a role to play in glucose homeostasis? The Proceedings of the Nutrition Society, 48, 207–214. 10.1079/PNS19890031 [DOI] [PubMed] [Google Scholar]
- Chernogubova, E. , Cannon, B. , & Bengtsson, T. (2004). Norepinephrine increases glucose transport in brown adipocytes via β3‐adrenoceptors through a cAMP, PKA, and PI3‐kinase‐dependent pathway stimulating conventional and novel PKCs. Endocrinology, 145, 269–280. 10.1210/en.2003-0857 [DOI] [PubMed] [Google Scholar]
- Chernogubova, E. , Hutchinson, D. S. , Nedergaard, J. , & Bengtsson, T. (2005). α1‐ and β1‐Adrenoceptor signaling fully compensate for β3‐adrenoceptor deficiency in brown adipocyte norepinephrine‐stimulated glucose uptake. Endocrinology, 146, 2271–2284. 10.1210/en.2004-1104 [DOI] [PubMed] [Google Scholar]
- Chondronikola, M. , Volpi, E. , Borsheim, E. , Porter, C. , Saraf, M. K. , Annamalai, P. , … Sidossis, L. S. (2016). Brown adipose tissue activation is linked to distinct systemic effects on lipid metabolism in humans. Cell Metabolism, 23, 1200–1206. 10.1016/j.cmet.2016.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti, S. (2005). The adipose organ. Prostaglandins, Leukotrienes, and Essential Fatty Acids, 73, 9–15. 10.1016/j.plefa.2005.04.010 [DOI] [PubMed] [Google Scholar]
- Collins, S. , Daniel, K. W. , & Rohlfs, E. M. (1999). Depressed expression of adipocyte β‐adrenergic receptors is a common feature of congenital and diet‐induced obesity in rodents. International Journal of Obesity and Related Metabolic Disorders, 23, 669–677. 10.1038/sj.ijo.0800894 [DOI] [PubMed] [Google Scholar]
- Collins, S. , Daniel, K. W. , Rohlfs, E. M. , Ramkumar, V. , Taylor, I. L. , & Gettys, T. W. (1994). Impaired expression and functional activity of the β3‐ and β1‐adrenergic receptors in adipose tissue of congenitally obese (C57BL/6J ob/ob) mice. Molecular Endocrinology, 8, 518–527. [DOI] [PubMed] [Google Scholar]
- Cousin, B. , Cinti, S. , Morroni, M. , Raimbault, S. , Ricquier, D. , Penicaud, L. , & Casteilla, L. (1992). Occurrence of brown adipocytes in rat white adipose tissue: Molecular and morphological characterization. Journal of Cell Science, 103(Pt 4), 931–942. [DOI] [PubMed] [Google Scholar]
- Cypess, A. M. , Chen, Y. C. , Sze, C. , Wang, K. , English, J. , Chan, O. , … Kahn, C. R. (2012). Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proceedings of the National Academy of Sciences of the United States of America, 109, 10001–10005. 10.1073/pnas.1207911109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess, A. M. , Lehman, S. , Williams, G. , Tal, I. , Rodman, D. , Goldfine, A. B. , … Kahn, C. R. (2009). Identification and importance of brown adipose tissue in adult humans. The New England Journal of Medicine, 360, 1509–1517. 10.1056/NEJMoa0810780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess, A. M. , Weiner, L. S. , Roberts‐Toler, C. , Elia, E. F. , Kessler, S. H. , Kahn, P. A. , … Kolodny, G. M. (2015). Activation of human brown adipose tissue by a β3‐adrenergic receptor agonist. Cell Metabolism, 21, 33–38. 10.1016/j.cmet.2014.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess, A. M. , White, A. P. , Vernochet, C. , Schulz, T. J. , Xue, R. , Sass, C. A. , … Tseng, Y. H. (2013). Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nature Medicine, 19, 635–639. 10.1038/nm.3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallner, O. S. , Chernogubova, E. , Brolinson, K. A. , & Bengtsson, T. (2006). β3‐adrenergic receptors stimulate glucose uptake in brown adipocytes by two mechanisms independently of glucose transporter 4 translocation. Endocrinology, 147, 5730–5739. 10.1210/en.2006-0242 [DOI] [PubMed] [Google Scholar]
- de Jong, J. M. , Larsson, O. , Cannon, B. , & Nedergaard, J. (2015). A stringent validation of mouse adipose tissue identity markers. American Journal of Physiology. Endocrinology and Metabolism, 308, E1085–E1105. [DOI] [PubMed] [Google Scholar]
- de Jong, J. M. A. , Wouters, R. T. F. , Boulet, N. , Cannon, B. , Nedergaard, J. , & Petrovic, N. (2017). The β3‐adrenergic receptor is dispensable for browning of adipose tissues. American Journal of Physiology. Endocrinology and Metabolism, 312, E508–e518. 10.1152/ajpendo.00437.2016 [DOI] [PubMed] [Google Scholar]
- Dehvari, N. , da Silva Junior, E. D. , Bengtsson, T. , & Hutchinson, D. S. (2018). Mirabegron: Potential off target effects and uses beyond the bladder. British Journal of Pharmacology, 175, 4072–4082. 10.1111/bph.14121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delporte, M. L. , Funahashi, T. , Takahashi, M. , Matsuzawa, Y. , & Brichard, S. M. (2002). Pre‐ and post‐translational negative effect of beta‐adrenoceptor agonists on adiponectin secretion: In vitro and in vivo studies. The Biochemical Journal, 367, 677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health Therapeutic Goods Adminstration: Australian public assessment report for mirabegron (2014). [Online] Retrieved from https://www.tga.gov.au/auspar/auspar‐mirabegron. [Accessed: 12/07/2017].
- Ding, C. , Lim, Y. C. , Chia, S. Y. , Walet, A. C. E. , Xu, S. , Lo, K. A. , … Sun, L. (2018). De novo reconstruction of human adipose transcriptome reveals conserved lncRNAs as regulators of brown adipogenesis. Nature Communications, 9, 1329 10.1038/s41467-018-03754-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elabd, C. , Chiellini, C. , Carmona, M. , Galitzky, J. , Cochet, O. , Petersen, R. , … Amri, E. Z. (2009). Human multipotent adipose‐derived stem cells differentiate into functional brown adipocytes. Stem Cells, 27, 2753–2760. 10.1002/stem.200 [DOI] [PubMed] [Google Scholar]
- Ernande, L. , Stanford, K. I. , Thoonen, R. , Zhang, H. , Clerte, M. , Hirshman, M. F. , … Scherrer‐Crosbie, M. (2016). Relationship of brown adipose tissue perfusion and function: A study through β2‐adrenoreceptor stimulation. Journal of Applied Physiology (1985), 120, 825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, B. A. , Agar, L. , & Summers, R. J. (1999). The role of the sympathetic nervous system in the regulation of leptin synthesis in C57BL/6 mice. FEBS Letters, 444, 149–154. 10.1016/S0014-5793(99)00049-6 [DOI] [PubMed] [Google Scholar]
- Fernandes, G. W. , Ueta, C. B. , Fonseca, T. L. , Gouveia, C. H. , Lancellotti, C. L. , Brum, P. C. , … Ribeiro, M. O. (2014). Inactivation of the adrenergic receptor β2 disrupts glucose homeostasis in mice. The Journal of Endocrinology, 221, 381–390. 10.1530/JOE-13-0526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlin, B. S. , Memetimin, H. , Confides, A. L. , Kasza, I. , Zhu, B. , Vekaria, H. J. , et al. (2018). Human adipose beiging in response to cold and mirabegron. JCI Insight, 3, 121510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, K. , Ruiz, H. H. , Jhun, K. , Finan, B. , Oberlin, D. J. , van der Heide, V. , … Buettner, C. (2017). Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nature Medicine, 23, 623–630. 10.1038/nm.4316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson, J. M. , Lindquist, J. M. , Bronnikov, G. E. , & Nedergaard, J. (2000). Norepinephrine induces vascular endothelial growth factor gene expression in brown adipocytes through a β‐adrenoreceptor/cAMP/protein kinase A pathway involving Src but independently of Erk1/2. The Journal of Biological Chemistry, 275, 13802–13811. 10.1074/jbc.275.18.13802 [DOI] [PubMed] [Google Scholar]
- Fredriksson, J. M. , Nikami, H. , & Nedergaard, J. (2005). Cold‐induced expression of the VEGF gene in brown adipose tissue is independent of thermogenic oxygen consumption. FEBS Letters, 579, 5680–5684. [DOI] [PubMed] [Google Scholar]
- Galic, S. , Oakhill, J. S. , & Steinberg, G. R. (2010). Adipose tissue as an endocrine organ. Molecular and Cellular Endocrinology, 316, 129–139. 10.1016/j.mce.2009.08.018 [DOI] [PubMed] [Google Scholar]
- Galitzky, J. , Larrouy, D. , Berlan, M. , & Lafontan, M. (1990). New tools for human fat cell α2A adrenoceptor characterization. Identification on membranes and on intact cells using the new antagonist [3H]RX821002. The Journal of Pharmacology and Experimental Therapeutics, 252, 312–319. [PubMed] [Google Scholar]
- Garofalo, M. A. , Kettelhut, I. C. , Roselino, J. E. , & Migliorini, R. H. (1996). Effect of acute cold exposure on norepinephrine turnover rates in rat white adipose tissue. Journal of the Autonomic Nervous System, 60, 206–208. 10.1016/0165-1838(96)00037-9 [DOI] [PubMed] [Google Scholar]
- Gawronska‐Kozak, B. , Staszkiewicz, J. , Gimble, J. M. , & Kirk‐Ballard, H. (2014). Recruitment of fat cell precursors during high fat diet in C57BL/6J mice is fat depot specific. Obesity (Silver Spring), 22, 1091–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germack, R. , Starzec, A. B. , Vassy, R. , & Perret, G. Y. (1997). β‐adrenoceptor subtype expression and function in rat white adipocytes. British Journal of Pharmacology, 120, 201–210. 10.1038/sj.bjp.0700885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesta, S. , Tseng, Y. H. , & Kahn, C. R. (2007). Developmental origin of fat: Tracking obesity to its source. Cell, 131, 242–256. 10.1016/j.cell.2007.10.004 [DOI] [PubMed] [Google Scholar]
- Gettys, T. W. , Harkness, P. J. , & Watson, P. M. (1996). The β3‐adrenergic receptor inhibits insulin‐stimulated leptin secretion from isolated rat adipocytes. Endocrinology, 137, 4054–4057. 10.1210/endo.137.9.8756584 [DOI] [PubMed] [Google Scholar]
- Granneman, J. G. (1992). Effects of agonist exposure on the coupling of β1 and β3 adrenergic receptors to adenylyl cyclase in isolated adipocytes. The Journal of Pharmacology and Experimental Therapeutics, 261, 638–642. [PubMed] [Google Scholar]
- Granneman, J. G. , Li, P. , Zhu, Z. , & Lu, Y. (2005). Metabolic and cellular plasticity in white adipose tissue I: Effects of β3‐adrenergic receptor activation. American Journal of Physiology. Endocrinology and Metabolism, 289, E608–E616. 10.1152/ajpendo.00009.2005 [DOI] [PubMed] [Google Scholar]
- Granneman, J. G. , Zhai, Y. , & Lahners, K. N. (1997). Selective up‐regulation of α1A‐adrenergic receptor protein and mRNA in brown adipose tissue by neural and β3‐adrenergic stimulation. Molecular Pharmacology, 51, 644–650. 10.1124/mol.51.4.644 [DOI] [PubMed] [Google Scholar]
- Guennoun, A. , Kazantzis, M. , Thomas, R. , Wabitsch, M. , Tews, D. , Seetharama Sastry, K. , … Chouchane, L. (2015). Comprehensive molecular characterization of human adipocytes reveals a transient brown phenotype. Journal of Translational Medicine, 13, 135 10.1186/s12967-015-0480-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra, C. , Koza, R. A. , Yamashita, H. , Walsh, K. , & Kozak, L. P. (1998). Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. Journal of Clinical Investigation, 102, 412–420. 10.1172/JCI3155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao, Q. , Yadav, R. , Basse, A. L. , Petersen, S. , Sonne, S. B. , Rasmussen, S. , … Hansen, J. B. (2015). Transcriptome profiling of brown adipose tissue during cold exposure reveals extensive regulation of glucose metabolism. American Journal of Physiology. Endocrinology and Metabolism, 308, E380–E392. 10.1152/ajpendo.00277.2014 [DOI] [PubMed] [Google Scholar]
- Hardie, L. J. , Guilhot, N. , & Trayhurn, P. (1996). Regulation of leptin production in cultured mature white adipocytes. Hormone and Metabolic Research, 28, 685–689. 10.1055/s-2007-979878 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS guide to pharmacology in 2018: Updates and expansion to encompass the new Guide to immunopharmacology. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himms‐Hagen, J. , Melnyk, A. , Zingaretti, M. C. , Ceresi, E. , Barbatelli, G. , & Cinti, S. (2000). Multilocular fat cells in WAT of CL‐316243‐treated rats derive directly from white adipocytes. American Journal of Physiology. Cell Physiology, 279, C670–C681. 10.1152/ajpcell.2000.279.3.C670 [DOI] [PubMed] [Google Scholar]
- Hollenga, C. , & Zaagsma, J. (1989). Direct evidence for the atypical nature of functional β‐adrenoceptors in rat adipocytes. British Journal of Pharmacology, 98, 1420–1424. 10.1111/j.1476-5381.1989.tb12692.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai, J. , Katagiri, H. , Yamada, T. , Ishigaki, Y. , Ogihara, T. , Uno, K. , … Oka, Y. (2006). Cold exposure suppresses serum adiponectin levels through sympathetic nerve activation in mice. Obesity (Silver Spring), 14, 1132–1141. 10.1038/oby.2006.130 [DOI] [PubMed] [Google Scholar]
- Jankovic, A. , Korac, A. , Buzadzic, B. , Otasevic, V. , Stancic, A. , Vucetic, M. , … Korac, B. (2013). Endocrine and metabolic signaling in retroperitoneal white adipose tissue remodeling during cold acclimation. Journal of Obesity, 2013, 937572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen, N. Z. , Larsen, T. J. , Peijs, L. , Daugaard, S. , Homoe, P. , Loft, A. , … Scheele, C. (2013). A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metabolism, 17, 798–805. 10.1016/j.cmet.2013.04.011 [DOI] [PubMed] [Google Scholar]
- Jimenez, M. , Barbatelli, G. , Allevi, R. , Cinti, S. , Seydoux, J. , Giacobino, J. P. , … Preitner, F. (2003). β3‐adrenoceptor knockout in C57BL/6J mice depresses the occurrence of brown adipocytes in white fat. European Journal of Biochemistry, 270, 699–705. 10.1046/j.1432-1033.2003.03422.x [DOI] [PubMed] [Google Scholar]
- Jimenez, M. , Leger, B. , Canola, K. , Lehr, L. , Arboit, P. , Seydoux, J. , … Prietner, F. (2002). β1/β2/β3‐adrenoceptor knockout mice are obese and cold‐sensitive but have normal lipolytic responses to fasting. FEBS Letters, 530, 37–40. 10.1016/S0014-5793(02)03387-2 [DOI] [PubMed] [Google Scholar]
- Kalinovich, A. V. , de Jong, J. M. , Cannon, B. , & Nedergaard, J. (2017). UCP1 in adipose tissues: Two steps to full browning. Biochimie, 134, 127–137. 10.1016/j.biochi.2017.01.007 [DOI] [PubMed] [Google Scholar]
- Kern, P. A. , Finlin, B. S. , Zhu, B. , Rasouli, N. , McGehee, R. E. Jr. , Westgate, P. M. , et al. (2014). The effects of temperature and seasons on subcutaneous white adipose tissue in humans: Evidence for thermogenic gene induction. The Journal of Clinical Endocrinology and Metabolism, 99, E2772–E2779. 10.1210/jc.2014-2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi‐Utsumi, K. , Kikuchi‐Utsumi, M. , Cannon, B. , & Nedergaard, J. (1997). Differential regulation of the expression of α1‐adrenergic receptor subtype genes in brown adipose tissue. The Biochemical Journal, 322(Pt 2), 417–424. 10.1042/bj3220417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus, S. , Seivert, A. , & Boeuf, S. (2001). Effect of the β3‐adrenergic agonist Cl316,243 on functional differentiation of white and brown adipocytes in primary cell culture. Biochimica et Biophysica Acta, 1539, 85–92. 10.1016/S0167-4889(01)00093-3 [DOI] [PubMed] [Google Scholar]
- Klepac, K. , Kilic, A. , Gnad, T. , Brown, L. M. , Herrmann, B. , Wilderman, A. , … Pfeifer, A. (2016). The Gq signalling pathway inhibits brown and beige adipose tissue. Nature Communications, 7, 10895 10.1038/ncomms10895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komai, A. M. , Musovic, S. , Peris, E. , Alrifaiy, A. , El Hachmane, M. F. , Johansson, M. , … Olofsson, C. S. (2016). White adipocyte adiponectin exocytosis is stimulated via β3‐adrenergic signaling and activation of Epac1: Catecholamine resistance in obesity and type 2 diabetes. Diabetes, 65, 3301–3313. 10.2337/db15-1597 [DOI] [PubMed] [Google Scholar]
- Krief, S. , Lonnqvist, F. , Raimbault, S. , Baude, B. , Van Spronsen, A. , Arner, P. , … Emorine, L. J. (1993). Tissue distribution of β3‐adrenergic receptor mRNA in man. The Journal of Clinical Investigation, 91, 344–349. 10.1172/JCI116191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, P. , Werner, C. D. , Kebebew, E. , & Celi, F. S. (2014). Functional thermogenic beige adipogenesis is inducible in human neck fat. International Journal of Obesity, 38, 170–176. 10.1038/ijo.2013.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y. H. , Petkova, A. P. , & Granneman, J. G. (2013). Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metabolism, 18, 355–367. 10.1016/j.cmet.2013.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, B. E. , & Sullivan, A. C. (1986). β1‐Receptor is the predominant β‐adrenoreceptor on rat brown adipose tissue. The Journal of Pharmacology and Experimental Therapeutics, 236, 681–688. [PubMed] [Google Scholar]
- Liu, X. , Perusse, F. , & Bukowiecki, L. J. (1994). Chronic norepinephrine infusion stimulates glucose uptake in white and brown adipose tissues. The American Journal of Physiology, 266, R914–R920. 10.1152/ajpregu.1994.266.3.R914 [DOI] [PubMed] [Google Scholar]
- Llado, I. , Rodriguez‐Cuenca, S. , Pujol, E. , Monjo, M. , Estrany, M. E. , Roca, P. , & Palou, A. (2002). Gender effects on adrenergic receptor expression and lipolysis in white adipose tissue of rats. Obesity Research, 10, 296–305. 10.1038/oby.2002.41 [DOI] [PubMed] [Google Scholar]
- Loh, R. K. C. , Formosa, M. F. , La Gerche, A. , Reutens, A. T. , Kingwell, B. A. , & Carey, A. L. (2019). The acute metabolic and cardiovascular effects of mirabegron in healthy individuals. Diabetes, Obesity & Metabolism, 21, 276–284. [DOI] [PubMed] [Google Scholar]
- Long, J. Z. , Svensson, K. J. , Tsai, L. , Zeng, X. , Roh, H. C. , Kong, X. , … Spiegelman, B. M. (2014). A smooth muscle‐like origin for beige adipocytes. Cell Metabolism, 19, 810–820. 10.1016/j.cmet.2014.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonnqvist, F. , Krief, S. , Strosberg, A. D. , Nyberg, S. , Emorine, L. J. , & Arner, P. (1993). Evidence for a functional β3‐adrenoceptor in man. British Journal of Pharmacology, 110, 929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis, S. N. , Jackman, G. P. , Nero, T. L. , Iakovidis, D. , & Louis, W. J. (2000). Role of β‐adrenergic receptor subtypes in lipolysis. Cardiovascular Drugs and Therapy, 14, 565–577. 10.1023/A:1007838125152 [DOI] [PubMed] [Google Scholar]
- Lowell, B. B. , S‐Susulic, V. , Hamann, A. , Lawitts, J. A. , Himms‐Hagen, J. , Boyer, B. B. , … Flier, J. S. (1993). Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature, 366, 740–742. [DOI] [PubMed] [Google Scholar]
- Madden, C. J. , Tupone, D. , Cano, G. , & Morrison, S. F. (2013). α2 Adrenergic receptor‐mediated inhibition of thermogenesis. The Journal of Neuroscience, 33, 2017–2028. 10.1523/JNEUROSCI.4701-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson, C. L. , Csikasz, R. I. , Chernogubova, E. , Yamamoto, D. L. , Hogberg, H. T. , Amri, E. Z. , … Bengtsson, T. (2011). β1‐Adrenergic receptors increase UCP1 in human MADS brown adipocytes and rescue cold‐acclimated β3‐adrenergic receptor‐knockout mice via nonshivering thermogenesis. American Journal of Physiology. Endocrinology and Metabolism, 301, E1108–E1118. 10.1152/ajpendo.00085.2011 [DOI] [PubMed] [Google Scholar]
- Mauriege, P. , Despres, J. P. , Prud'homme, D. , Pouliot, M. C. , Marcotte, M. , Tremblay, A. , & Bouchard, C. (1991). Regional variation in adipose tissue lipolysis in lean and obese men. Journal of Lipid Research, 32, 1625–1633. [PubMed] [Google Scholar]
- Mauriege, P. , Marette, A. , Atgie, C. , Bouchard, C. , Theriault, G. , Bukowiecki, L. K. , … Després, J. P. (1995). Regional variation in adipose tissue metabolism of severely obese premenopausal women. Journal of Lipid Research, 36, 672–684. [PubMed] [Google Scholar]
- Mauriege, P. , Prud'homme, D. , Lemieux, S. , Tremblay, A. , & Despres, J. P. (1995). Regional differences in adipose tissue lipolysis from lean and obese women: Existence of postreceptor alterations. The American Journal of Physiology, 269, E341–E350. [DOI] [PubMed] [Google Scholar]
- Merlin, J. , Evans, B. A. , Dehvari, N. , Sato, M. , Bengtsson, T. , & Hutchinson, D. S. (2016). Could burning fat start with a brite spark? Pharmacological and nutritional ways to promote thermogenesis. Molecular Nutrition & Food Research, 60, 18–42. 10.1002/mnfr.201500251 [DOI] [PubMed] [Google Scholar]
- Merlin, J. , Sato, M. , Chia, L. Y. , Fahey, R. , Pakzad, M. , Nowell, C. J. , … Hutchinson, D. S. (2018). Rosiglitazone and a β3‐adrenoceptor agonist are both required for functional browning of white adipocytes in culture. Frontiers in Endocrinology (Lausanne), 9, 249 10.3389/fendo.2018.00249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin, J. , Sato, M. , Nowell, C. , Pakzad, M. , Fahey, R. , Gao, J. , … Hutchinson, D. S. (2018). The PPARγ agonist rosiglitazone promotes the induction of brite adipocytes, increasing β‐adrenoceptor‐mediated mitochondrial function and glucose uptake. Cellular Signalling, 42, 54–66. 10.1016/j.cellsig.2017.09.023 [DOI] [PubMed] [Google Scholar]
- Michel, M. C. , Wieland, T. , & Tsujimoto, G. (2009). How reliable are G‐protein‐coupled receptor antibodies? Naunyn‐Schmiedeberg's Archives of Pharmacology, 379, 385–388. 10.1007/s00210-009-0395-y [DOI] [PubMed] [Google Scholar]
- Nedergaard, J. , Bengtsson, T. , & Cannon, B. (2007). Unexpected evidence for active brown adipose tissue in adult humans. American Journal of Physiology. Endocrinology and Metabolism, 293, E444–E452. 10.1152/ajpendo.00691.2006 [DOI] [PubMed] [Google Scholar]
- Nedergaard, J. , Bengtsson, T. , & Cannon, B. (2011). New powers of brown fat: Fighting the metabolic syndrome. Cell Metabolism, 13, 238–240. [DOI] [PubMed] [Google Scholar]
- Nguyen, K. D. , Qiu, Y. , Cui, X. , Goh, Y. P. , Mwangi, J. , David, T. , … Chawla, A. (2011). Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature, 480, 104–108. 10.1038/nature10653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamatsu‐Ogura, Y. , Fukano, K. , Tsubota, A. , Uozumi, A. , Terao, A. , Kimura, K. , & Saito, M. (2013). Thermogenic ability of uncoupling protein 1 in beige adipocytes in mice. PLoS ONE, 8, e84229 10.1371/journal.pone.0084229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen, J. M. , Csikasz, R. I. , Dehvari, N. , Lu, L. , Sandstrom, A. , Oberg, A. I. , … Bengtsson, T. (2017). β3‐Adrenergically induced glucose uptake in brown adipose tissue is independent of UCP1 presence or activity: Mediation through the mTOR pathway. Molecular Metabolism, 6, 611–619. 10.1016/j.molmet.2017.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen, J. M. , Sato, M. , Dallner, O. S. , Sandstrom, A. L. , Pisani, D. F. , Chambard, J. C. , … Bengtsson, T. (2014). Glucose uptake in brown fat cells is dependent on mTOR complex 2‐promoted GLUT1 translocation. The Journal of Cell Biology, 207, 365–374. 10.1083/jcb.201403080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orava, J. , Nuutila, P. , Lidell, M. E. , Oikonen, V. , Noponen, T. , Viljanen, T. , … Virtanen, K. A. (2011). Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metabolism, 14, 272–279. 10.1016/j.cmet.2011.06.012 [DOI] [PubMed] [Google Scholar]
- Ouellet, V. , Routhier‐Labadie, A. , Bellemare, W. , Lakhal‐Chaieb, L. , Turcotte, E. , Carpentier, A. C. , & Richard, D. (2011). Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose‐uptake activity of 18F‐FDG‐detected BAT in humans. The Journal of Clinical Endocrinology and Metabolism, 96, 192–199. 10.1210/jc.2010-0989 [DOI] [PubMed] [Google Scholar]
- Peirce, V. , & Vidal‐Puig, A. (2013). Regulation of glucose homoeostasis by brown adipose tissue. The Lancet Diabetes and Endocrinology, 1, 353–360. 10.1016/S2213-8587(13)70055-X [DOI] [PubMed] [Google Scholar]
- Petrovic, N. , Walden, T. B. , Shabalina, I. G. , Timmons, J. A. , Cannon, B. , & Nedergaard, J. (2010). Chronic peroxisome proliferator‐activated receptor γ (PPARγ) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1‐containing adipocytes molecularly distinct from classic brown adipocytes. The Journal of Biological Chemistry, 285, 7153–7164. 10.1074/jbc.M109.053942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pico, C. , Bonet, M. L. , & Palou, A. (1998). Stimulation of uncoupling protein synthesis in white adipose tissue of mice treated with the β3‐adrenergic agonist CGP‐12177. Cellular and Molecular Life Sciences, 54, 191–195. 10.1007/s000180050142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani, D. F. , Djedaini, M. , Beranger, G. E. , Elabd, C. , Scheideler, M. , Ailhaud, G. , et al. (2011). Differentiation of human adipose‐derived stem cells into “brite” (brown‐in‐white) adipocytes. Frontiers in Endocrinology (Lausanne), 2, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, Y. , Nguyen, K. D. , Odegaard, J. I. , Cui, X. , Tian, X. , Locksley, R. M. , … Chawla, A. (2014). Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell, 157, 1292–1308. 10.1016/j.cell.2014.03.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revelli, J. P. , Preitner, F. , Samec, S. , Muniesa, P. , Kuehne, F. , Boss, O. , … Ody, C. (1997). Targeted gene disruption reveals a leptin‐independent role for the mouse β3‐adrenoceptor in the regulation of body composition. The Journal of Clinical Investigation, 100, 1098–1106. 10.1172/JCI119620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell, N. J. , Stock, M. J. , & Sudera, D. K. (1985). β‐adrenoreceptors in rat brown adipose tissue: Proportions of β1‐ and β2‐subtypes. The American Journal of Physiology, 248, E397–E402. 10.1152/ajpendo.1985.248.4.E397 [DOI] [PubMed] [Google Scholar]
- Sackmann‐Sala, L. , Berryman, D. E. , Munn, R. D. , Lubbers, E. R. , & Kopchick, J. J. (2012). Heterogeneity among white adipose tissue depots in male C57BL/6J mice. Obesity (Silver Spring), 20, 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito, M. , Okamatsu‐Ogura, Y. , Matsushita, M. , Watanabe, K. , Yoneshiro, T. , Nio‐Kobayashi, J. , … Tsujisaki, M. (2009). High incidence of metabolically active brown adipose tissue in healthy adult humans. Diabetes, 58, 1526–1531. 10.2337/db09-0530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez‐Gurmaches, J. , Hung, C. M. , & Guertin, D. A. (2016). Emerging complexities in adipocyte origins and identity. Trends in Cell Biology, 26, 313–326. 10.1016/j.tcb.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale, P. , Conroe, H. M. , Estall, J. , Kajimura, S. , Frontini, A. , Ishibashi, J. , … Spiegelman, B. M. (2011). Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. The Journal of Clinical Investigation, 121, 96–105. 10.1172/JCI44271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabalina, I. G. , Petrovic, N. , de Jong, J. M. , Kalinovich, A. V. , Cannon, B. , & Nedergaard, J. (2013). UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Reports, 5, 1196–1203. [DOI] [PubMed] [Google Scholar]
- Sharp, L. Z. , Shinoda, K. , Ohno, H. , Scheel, D. W. , Tomoda, E. , Ruiz, L. , … Kajimura, S. (2012). Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS ONE, 7, e49452 10.1371/journal.pone.0049452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata, H. , Perusse, F. , Vallerand, A. , & Bukowiecki, L. J. (1989). Cold exposure reverses inhibitory effects of fasting on peripheral glucose uptake in rats. The American Journal of Physiology, 257, R96–R101. 10.1152/ajpregu.1989.257.1.R96 [DOI] [PubMed] [Google Scholar]
- Shimizu, Y. , Nikami, H. , & Saito, M. (1991). Sympathetic activation of glucose utilization in brown adipose tissue in rats. Journal of Biochemistry (Tokyo), 110, 688–692. [DOI] [PubMed] [Google Scholar]
- Shinoda, K. , Luijten, I. H. , Hasegawa, Y. , Hong, H. , Sonne, S. B. , Kim, M. , … Kajimura, S. (2015). Genetic and functional characterization of clonally derived adult human brown adipocytes. Nature Medicine, 21, 389–394. 10.1038/nm.3819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloveva, V. , Graves, R. A. , Rasenick, M. M. , Spiegelman, B. M. , & Ross, S. R. (1997). Transgenic mice overexpressing the β1‐adrenergic receptor in adipose tissue are resistant to obesity. Molecular Endocrinology, 11, 27–38. 10.1210/mend.11.1.9870 [DOI] [PubMed] [Google Scholar]
- Spaethling, J. M. , Sanchez‐Alavez, M. , Lee, J. , Xia, F. C. , Dueck, H. , Wang, W. , … Eberwine, J. (2016). Single‐cell transcriptomics and functional target validation of brown adipocytes show their complex roles in metabolic homeostasis. The FASEB Journal, 30, 81–92. 10.1096/fj.15-273797 [DOI] [PubMed] [Google Scholar]
- Stanford, K. I. , Middelbeek, R. J. , Townsend, K. L. , An, D. , Nygaard, E. B. , Hitchcox, K. M. , … Goodyear, L. J. (2013). Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. The Journal of Clinical Investigation, 123, 215–223. 10.1172/JCI62308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stich, V. , de Glisezinski, I. , Crampes, F. , Suljkovicova, H. , Galitzky, J. , Riviere, D. , … Berlan, M. (1999). Activation of antilipolytic α2‐adrenergic receptors by epinephrine during exercise in human adipose tissue. The American Journal of Physiology, 277, R1076–R1083. [DOI] [PubMed] [Google Scholar]
- Susulic, V. S. , Frederich, R. C. , Lawitts, J. , Tozzo, E. , Kahn, B. B. , Harper, M. E. , … Lowell, B. B. (1995). Targeted disruption of the β3‐adrenergic receptor gene. The Journal of Biological Chemistry, 270, 29483–29492. 10.1074/jbc.270.49.29483 [DOI] [PubMed] [Google Scholar]
- Tavernier, G. , Barbe, P. , Galitzky, J. , Berlan, M. , Caput, D. , Lafontan, M. , & Langin, D. (1996). Expression of β3‐adrenoceptors with low lipolytic action in human subcutaneous white adipocytes. Journal of Lipid Research, 37, 87–97. [PubMed] [Google Scholar]
- Tavernier, G. , Jimenez, M. , Giacobino, J. P. , Hulo, N. , Lafontan, M. , Muzzin, P. , & Langin, D. (2005). Norepinephrine induces lipolysis in β1/β2/β3‐adrenoceptor knockout mice. Molecular Pharmacology, 6, 6. [DOI] [PubMed] [Google Scholar]
- Tchkonia, T. , Tchoukalova, Y. D. , Giorgadze, N. , Pirtskhalava, T. , Karagiannides, I. , Forse, R. A. , … Kirkland, J. L. (2005). Abundance of two human preadipocyte subtypes with distinct capacities for replication, adipogenesis, and apoptosis varies among fat depots. American Journal of Physiology. Endocrinology and Metabolism, 288, E267–E277. 10.1152/ajpendo.00265.2004 [DOI] [PubMed] [Google Scholar]
- Thonberg, H. , Fredriksson, J. M. , Nedergaard, J. , & Cannon, B. (2002). A novel pathway for adrenergic stimulation of cAMP‐response‐element‐binding protein (CREB) phosphorylation: Mediation via α1‐adrenoceptors and protein kinase C activation. The Biochemical Journal, 364, 73–79. 10.1042/bj3640073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thonberg, H. , Zhang, S. J. , Tvrdik, P. , Jacobsson, A. , & Nedergaard, J. (1994). Norepinephrine utilizes α1‐ and β‐adrenoreceptors synergistically to maximally induce c‐fos expression in brown adipocytes. The Journal of Biological Chemistry, 269, 33179–33186. [PubMed] [Google Scholar]
- Tonello, C. , Giordano, A. , Cozzi, V. , Cinti, S. , Stock, M. J. , Carruba, M. O. , & Nisoli, E. (1999). Role of sympathetic activity in controlling the expression of vascular endothelial growth factor in brown fat cells of lean and genetically obese rats. FEBS Letters, 442, 167–172. 10.1016/S0014-5793(98)01627-5 [DOI] [PubMed] [Google Scholar]
- Trayhurn, P. , Duncan, J. S. , & Rayner, D. V. (1995). Acute cold‐induced suppression of ob (obese) gene expression in white adipose tissue of mice: Mediation by the sympathetic system. The Biochemical Journal, 311(Pt 3), 729–733. 10.1042/bj3110729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U Din, M. , Raiko, J. , Saari, T. , Kudomi, N. , Tolvanen, T. , Oikonen, V. , … Virtanen, K. A. (2016). Human brown adipose tissue [15O]O2 PET imaging in the presence and absence of cold stimulus. European Journal of Nuclear Medicine and Molecular Imaging, 43, 1878–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueta, C. B. , Fernandes, G. W. , Capelo, L. P. , Fonseca, T. L. , Maculan, F. D. , Gouveia, C. H. , … Ribeiro, M. O. (2012). β1 Adrenergic receptor is key to cold‐ and diet‐induced thermogenesis in mice. The Journal of Endocrinology, 214, 359–365. 10.1530/JOE-12-0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valet, P. , Grujic, D. , Wade, J. , Ito, M. , Zingaretti, M. C. , Soloveva, V. , … Lowell, B. B. (2000). Expression of human α2‐adrenergic receptors in adipose tissue of β3‐adrenergic receptor‐deficient mice promotes diet‐induced obesity. The Journal of Biological Chemistry, 275, 34797–34802. 10.1074/jbc.M005210200 [DOI] [PubMed] [Google Scholar]
- van Marken Lichtenbelt, W. D. , Vanhommerig, J. W. , Smulders, N. M. , Drossaerts, J. M. , Kemerink, G. J. , Bouvy, N. D. , … Teule, G. J. (2009). Cold‐activated brown adipose tissue in healthy men. The New England Journal of Medicine, 360, 1500–1508. 10.1056/NEJMoa0808718 [DOI] [PubMed] [Google Scholar]
- Vijgen, G. H. , Bouvy, N. D. , Teule, G. J. , Brans, B. , Schrauwen, P. , & van Marken Lichtenbelt, W. D. (2011). Brown adipose tissue in morbidly obese subjects. PLoS ONE, 6, e17247 10.1371/journal.pone.0017247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya, J. , Cereijo, R. , & Villarroya, F. (2013). An endocrine role for brown adipose tissue? American Journal of Physiology. Endocrinology and Metabolism, 305, E567–E572. 10.1152/ajpendo.00250.2013 [DOI] [PubMed] [Google Scholar]
- Virtanen, K. A. , Iozzo, P. , Hallsten, K. , Huupponen, R. , Parkkola, R. , Janatuinen, T. , … Nuutila, P. (2005). Increased fat mass compensates for insulin resistance in abdominal obesity and type 2 diabetes: A positron‐emitting tomography study. Diabetes, 54, 2720–2726. 10.2337/diabetes.54.9.2720 [DOI] [PubMed] [Google Scholar]
- Virtanen, K. A. , Lidell, M. E. , Orava, J. , Heglind, M. , Westergren, R. , Niemi, T. , … Nuutila, P. (2009). Functional brown adipose tissue in healthy adults. The New England Journal of Medicine, 360, 1518–1525. 10.1056/NEJMoa0808949 [DOI] [PubMed] [Google Scholar]
- Vitali, A. , Murano, I. , Zingaretti, M. C. , Frontini, A. , Ricquier, D. , & Cinti, S. (2012). The adipose organ of obesity‐prone C57BL/6J mice is composed of mixed white and brown adipocytes. Journal of Lipid Research, 53, 619–629. 10.1194/jlr.M018846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wabitsch, M. , Brenner, R. E. , Melzner, I. , Braun, M. , Moller, P. , Heinze, E. , … Hauner, H. (2001). Characterization of a human preadipocyte cell strain with high capacity for adipose differentiation. International Journal of Obesity and Related Metabolic Disorders, 25, 8–15. 10.1038/sj.ijo.0801520 [DOI] [PubMed] [Google Scholar]
- Walden, T. B. , Hansen, I. R. , Timmons, J. A. , Cannon, B. , & Nedergaard, J. (2012). Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. American Journal of Physiology. Endocrinology and Metabolism, 302, E19–E31. 10.1152/ajpendo.00249.2011 [DOI] [PubMed] [Google Scholar]
- Wang, G. X. , Zhao, X. Y. , & Lin, J. D. (2015). The brown fat secretome: Metabolic functions beyond thermogenesis. Trends in Endocrinology and Metabolism, 26, 231–237. 10.1016/j.tem.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q. A. , Tao, C. , Gupta, R. K. , & Scherer, P. E. (2013). Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nature Medicine, 19, 1338–1344. 10.1038/nm.3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Falting, J. M. , Mattsson, C. L. , Holmstrom, T. E. , & Nedergaard, J. (2013). In brown adipocytes, adrenergically induced β1‐/β3‐Gs‐, α2‐Gi‐ and α1‐Gq‐signalling to Erk1/2 activation is not mediated via EGF receptor transactivation. Experimental Cell Research, 319, 2718–2727. 10.1016/j.yexcr.2013.08.007 [DOI] [PubMed] [Google Scholar]
- Wu, J. , Bostrom, P. , Sparks, L. M. , Ye, L. , Choi, J. H. , Giang, A. H. , … Spiegelman, B. M. (2012). Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell, 150, 366–376. 10.1016/j.cell.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, P. , Arch, J. R. , & Ashwell, M. (1984). Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Letters, 167, 10–14. 10.1016/0014-5793(84)80822-4 [DOI] [PubMed] [Google Scholar]
- Youngstrom, T. G. , & Bartness, T. J. (1998). White adipose tissue sympathetic nervous system denervation increases fat pad mass and fat cell number. The American Journal of Physiology, 275, R1488–R1493. [DOI] [PubMed] [Google Scholar]
- Zilberfarb, V. , Pietri‐Rouxel, F. , Jockers, R. , Krief, S. , Delouis, C. , Issad, T. , & Strosberg, A. D. (1997). Human immortalized brown adipocytes express functional β3‐adrenoceptor coupled to lipolysis. Journal of Cell Science, 110(Pt 7), 801–807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Source of native human adipose tissue RNA samples
Table S2. Culture and differentiation of human adipocytes
TABLE S3. Original FPKM values in human adipose depot RNA samples
TABLE S4. Original FPKM values in human adipocyte RNA samples
Figure S1. Expression of endogenous control genes relative to β‐actin [FPKM (gene of interest/ACTB) × 1000] (see section on RNA‐Seq data analysis). RNA samples on the left of each panel are from native human foetal interscapular BAT (fiBAT, n = 4), biopsies of supraclavicular BAT from an individual maintained at thermoneutrality (scBAT TN, n = 1) or subject to 5 hours cold exposure (scBAT CE, n = 1), subcutaneous WAT (sqWAT, n = 3), biopsies of subcutaneous WAT from an individual maintained first thermoneutrality (scWAT TN, n = 1) or subject to 5 hours cold exposure (scWAT CE, n = 1), and omental visceral WAT (visWAT, n = 3). RNA samples on the right of each panel are from human pre‐adipocytes (PrAd) or differentiated mature adipocytes (MAd) cultured from foetal interscapular BAT (fiBrPrAd, n = 1 or fiBrMAd, n = 1), supraclavicular BAT (scBrPrAd, n = 3 or scBrMAd, n = 3), and subcutaneous WAT (sqWPrAd, n = 4 or sqWMAd, n = 4). Dotted horizontal lines denote an FPKM value of 1.0.


