Abstract
Background and Purpose
Bradykinin may induce vasoconstriction in selected vessels or under specific experimental conditions. We hypothesized that inflammatory stimuli, such as endotoxin challenge, may induce the dimerization of AT1/B2 receptors, altering the vascular effects of bradykinin.
Experimental Approach
Wistar rats received LPS (1 mg·kg−1, i.p.) and were anaesthetized for assessment of BP. Mesenteric resistance arteries were used in organ baths and subjected to co‐immunoprecipitation and Western blot analyses.
Key Results
At 24 and 48 hr after LPS, bradykinin‐induced hypotension was followed by a sustained increase in BP, which was not found in non‐endotoxemic animals. The B2 receptor antagonist Hoe‐140 fully blocked the responses to bradykinin. The pressor effect of bradykinin was not prevented by prazosin, an α1‐adrenoceptor antagonist, but it was inhibited by the AT1 receptor antagonist losartan or the Rho‐kinase inhibitor Y‐27632. Endotoxemic rats also displayed enhanced pressor responses to angiotensin II, which were blocked by Hoe‐140. Co‐immunoprecipitation isolated using anti‐B2 or anti‐AT1 receptor antibodies showed that resistance arteries presented augmented levels of the AT1/B2 receptor complexes at 24 hr after LPS injection. The presence of AT1/B2 receptor heterodimers did correlate with the development of losartan‐sensitive contractile responses to bradykinin and potentiation of angiotensin II‐induced contraction, which was prevented by Hoe‐140.
Conclusions and Implications
Endotoxin challenge is a stimulus for AT1/B2 receptor heterodimerization in native vessels and shifts the B2 receptor‐dependent vascular effect of bradykinin to a more complex pathway, which also depends on AT1 receptors and their intracellular signalling pathways.
Abbreviations
- Co‐IP
co‐immunoprecipitation
- DABK
des‐Arg9‐bradykinin
- DALBK
des‐Arg9‐[Leu8]‐bradykinin
- DAP
diastolic arterial pressure
- HR
heart rate
- MAP
mean arterial pressure
- PSS
physiological saline solution
- SAP
systolic arterial pressure
What is already known
Bradykinin is a well‐known vasodilator involved in the physiological control of BP.
Systemic activation of bradykinin B2 receptors results in reduction of BP.
What this study adds
After LPS challenge, bradykinin generates a secondary pressor effect in rats.
B2 receptor and angiotensin II AT1 receptor heterodimerization accounts for this pressor effect.
What is the clinical significance
Bradykinin presents a differential role in healthy and diseased vessels.
Targeting AT1/B2 receptor heterodimers may be a useful pharmacological strategy in inflammation‐associated vascular diseases.
1. INTRODUCTION
Bradykinin is the major product of the kallikrein‐kinin system, a multiprotein complex formed by kinins, including Lys‐bradykinin (or kallidin) and their active metabolites des‐Arg9‐bradykinin and Lys‐des‐Arg9‐bradykinin, kininogenases (kinin‐forming enzymes), kininogens, degradative enzymes, and kinin receptors. The very first biological actions described for bradykinin were the contraction of guinea pig ileum and a rapid decrease in rabbit BP after intravenous administration (Rocha e Silva, Beraldo, & Rosenfeld, 1949). Bradykinin is now recognized as an essential mediator of several physiological processes, including, but not restricted to, regulation of vascular tone (Mombouli & Vanhoutte, 1995), vascular permeability, pain, and inflammation (Marceau & Regoli, 2004), and has been shown to be involved in angioedema, ischaemia–reperfusion injury, diabetes, atherosclerosis, and sepsis (Maurer et al., 2011), among other conditions.
The effects triggered by bradykinin occur through binding at B1 and B2 receptors located at the cell surface. These receptors are members of the GPCR family and differ in their pharmacological profiles and expression patterns (Leeb‐Lundberg, Marceau, Muller‐Esterl, Pettibone, & Zuraw, 2005 ; Regoli, Barabé, & Park, 1977). The bradykinin B2 receptor is preformed (constitutive) and widely distributed in tissues. In contrast, the bradykinin B1 receptor is an inducible GPCR, highly regulated by proinflammatory cytokines, and presenting low expression in healthy tissues (Marceau & Regoli, 2004; Regoli & Barabé, 1980). It is generally considered that the B2 receptor plays a role in acute inflammatory responses, whereas the function of B1 receptors is mainly related to chronic inflammatory processes (McLean, Perretti, & Ahluwalia, 2000). The native kinins, bradykinin and kallidin (generated by plasma and tissue kallikreins, respectively), can activate both B1 and B2 receptors , with some selectivity for the B2 receptors. On the other hand, both Lys‐des‐Arg9‐bradykinin and des‐Arg9‐bradykinin are highly selective for the B1 receptor (Charignon, Späth, Martin, & Drouet, 2012; Leeb‐Lundberg et al., 2005).
Under physiological conditions, bradykinin‐induced vasorelaxation results from activation of B2 receptors in endothelial cells, which are coupled to Gαq proteins and stimulate phosphoinositide hydrolysis by PLC (Lambert, Kent, & Whorton, 1986), with subsequent elevation of intracellular Ca2+ and release of endothelial factors with relaxation properties, such as hyperpolarizing mediators, including NO (Palmer, Ferrige, & Moncada, 1987), and arachidonic acid‐derived eicosanoids (Burch & Axelrod, 1987). Although the central intracellular mediator may vary with the vessel studied, and endothelium‐independent vasodilatory effects have been described for specific vessels and species (Sai, Okamura, Amakata, & Toda, 1995), the role and some mechanisms of bradykinin‐induced vasodilation are well recognized.
Despite the presumed vasodilatory effect attributed to the kallikrein‐kinin system, it has been noted that bradykinin contracts brachial, femoral, and carotid arteries of guinea pig (Starr & West, 1966) and rabbit aortic strip preparations (Garrett & Brown, 1972; Regoli et al., 1977), and generates a biphasic effect characterized by endothelium‐dependent vasodilation followed by endothelium‐independent vasoconstriction in isolated perfused rat mesenteric arteries precontracted by noradrenaline (Fasciolo, Vargas, Lama, & Nolly, 1990). Besides, stimulation of both B1 and B2 receptors by kinins induces concentration‐dependent contractions in the rat portal and human umbilical veins (Campos & Calixto, 1994; Sardi, Pérez, Antúnez, & Rothlin, 1997). Contractile responses were also found when porcine basilar arteries were stimulated by high concentrations of bradykinin (Miyamoto, Ishiguro, & Nishio, 1999). Notably, endothelium‐denuded porcine coronary arteries developed contractile response to bradykinin after exposure to endotoxin, which appears to be mediated by both B1 and B2 receptors (More, Kim, Khang, et al., 2014; More, Kim, Zhao, et al., 2014). Moreover, experimental hypertension resulted in a leftward shift and enhancement of the endothelium‐dependent vasoconstriction induced by bradykinin in the porcine basilar artery (Islam et al., 2017).
Bradykinin has a very short half‐life (30 s or less; Saameli & Eskes, 1962), mainly because it is rapidly cleaved by ACE and other plasma peptidases. Notably, the kallikrein‐kinin and the renin‐angiotensin systems show various interactions, which orchestrate their effects on vascular functionality preventing conflicting stimuli and are putatively important for cardiovascular diseases. For instance, inhibition of ACE reduces the formation of angiotensin II, the main mediator of the renin‐angiotensin system, but it is commonly accepted that the resulting bradykinin accumulation contributes at several levels to the beneficial effects of ACE inhibitors (Regoli, Plante, & Gobeil, 2012). Nevertheless, the role of bradykinin in cardiovascular conditions associated with overwhelming inflammation and consequent augmented activation of the kallikrein‐kinin system, such as sepsis, remains poorly understood. For instance, experiments using B2 receptor antagonists or double knockout mice for B1 and B2 receptors revealed either protective (Cayla et al., 2007; Ridings, Sugerman, Blocher, Fisher, & Fowler, 1995; Wilson et al., 1989) or non‐protective (Barratt‐Due et al., 2011) effects when the animals were challenged by bacteria or endotoxins, while clinical studies were inconclusive (Fein et al., 1997).
Notwithstanding the robust evidence that counter regulatory effects between the kallikrein‐kinin and renin‐angiotensin systems play a pivotal role in the physiological regulation of both systems, heterodimerization between the angiotensin II AT1 receptors and the B2 receptors in HEK‐293 cells increased the efficacy and potency of angiotensin II (AbdAlla, Lother, & Quitterer, 2000). Further, AT1/B2 receptor heterodimers displaying enhanced sensitivity to angiotensin II were found in renal mesangial cells from spontaneously hypertensive rats (AbdAlla, Abdel‐Baset, Lother, el Massiery, & Quitterer, 2005) and omental vessels from pre‐eclamptic women (AbdAlla, Lother, el Massiery, & Quitterer, 2001). Given the ability of bradykinin to induce vasoconstriction in selected vessels, or under experimental conditions such as endotoxemia, and the potential ability of AT1 receptors and B2 receptors to form heterodimers, this study was designed to explore the hypothesis that inflammatory stimulus such as that induced by endotoxin challenge may modulate the dimerization of AT1/B2 receptors, regulating the vascular effects of bradykinin during pathogenic processes.
2. METHODS
2.1. Animals and experimental groups
All animal care and experimental procedures were approved by our Institutional Animal Care and Use Committee. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology. We used female and male Wistar rats (3–4 months) weighing 200–250 g and 300–350 g, respectively, supplied by the animal facilities of Universidade Federal de Santa Catarina (Florianópolis, SC, Brazil). The rats were maintained in individually ventilated cages under controlled temperature (22 ± 2°C), light/dark cycle (12/12 hr), and free access to water and standard chow. All experimental groups used in this study were divided randomly, compliant with Curtis et al. (2018). Different groups of animals were treated with bacterial LPS (1 mg·kg−1, i.p.) or sterile PBS (1 ml·kg−1, i.p.). The dose of LPS adopted in this study was 10 times smaller than the treatment able to consistently induce cardiovascular hallmarks of septic shock in our animals, as previously demonstrated for high doses of LPS (Gonçalves, Guarido, Assreuy, & da Silva‐Santos, 2014; Guarido, Gonçalves, Júnior, & da Silva‐Santos, 2014). The animals were subjected to direct measurement of BP and evaluation of the cardiovascular effects of bradykinin, which were first assessed at 6, 24, 48, or 72 hr after either LPS or PBS administration. Pharmacological and molecular approaches were conducted 24 hr after LPS or PBS administration, as detailed in the following items. No blinding of the experiments was possible because there were marked differences between the pressor effect of bradykinin recorded in the control and LPS groups.
2.2. Direct measurement of BP in anaesthetized rats
The animals were anaesthetized with ketamine and xylazine (90 and 15 mg·kg−1, respectively), administered by intramuscular route and supplemented as needed with 30% of the initial dose. The animals maintained spontaneous breathing throughout the entire experiments. After confirming the complete lack of peripheral reflex responses and reaction for nociceptive stimuli, the animals were fixed on a heated platform (37°C) in the supine position, and the left femoral vein was dissected for insertion of a polyethylene catheter (PE 20) used for intravenous administrations. All animals received a single dose of heparin (3.5 IU) to avoid clots. The carotid artery was carefully isolated from the vagus nerve and surrounding tissues for insertion of another polyethylene catheter, connected with a pressure transducer coupled to a data acquisition system and its application software (MLT0699, PowerLab and LabChart version 7.3.7, respectively; all from AD Instruments, Castle Hill, Australia). In selected experiments, besides the left femoral vein, the carotid artery was dissected for insertion of a polyethylene catheter (PE 50) and used for intra‐arterial administrations. In this approach, the right femoral artery was dissected and cannulated for the assessment of BP values. The mean, systolic, and diastolic arterial pressures (MAP, SAP, and DAP, respectively; in mmHg) and the heart rate (HR, in beats·min−1) were continuously measured. For stabilization of BP, an interval of 20 min was allowed between the end of animal manipulation and the beginning of the experimental protocols described ahead. All intravenous and intra‐arterial bolus injections were made at intervals of 10 min in a total volume of 50 μl, followed by 150 μl of sterile PBS for catheter flushing. At the end of these experiments, the animals were killed by anaesthetic overdose.
2.3. Assessment of systemic effects of bradykinin on the cardiovascular system of endotoxemic rats
At 6, 24, 48, or 72 hr after administration of LPS or PBS (n = 6 per group), the animals were prepared for BP recordings and received intravenous bolus injections of bradykinin (6, 20, and 60 nmol·kg−1) and ACh (60 nmol·kg−1). In these experiments, the three doses of bradykinin were administered randomly.
2.4. Blocking of bradykinin, α1‐adrenoceptors and AT1 receptors, and inhibition of COX and Rho‐kinase enzymes
These experiments were conducted only at 24 hr after administration of LPS or PBS (n = 6 per group). After recording the dose–response curve of effects of bradykinin (6, 20, and 60 nmol·kg−1, i.v.) on BP, different groups of animals (n = 6 per group) were treated with (a) Hoe‐140 (a selective B2 receptor antagonist; 27 mg·kg−1, s.c.), (b) des‐Arg9‐[Leu8]‐bradykinin (DALBK, a selective B1 receptor antagonist; 87 mg·kg−1, i.v.), (c) prazosin (an α1‐adrenoceptor antagonist; 0.5 mg·kg−1, i.v.), (d) losartan (an AT1 receptor antagonist; 15 mg·kg−1, i.v.), (e) indomethacin (a COX inhibitor; 10 mg·kg−1, s.c.), or (f) Y‐27632 (a selective inhibitor of Rho‐kinase; 0.1 mg·kg−1; i.v.). Twenty minutes after Hoe‐140 or 10 min after all other treatments, the animals received a second sequence of non‐cumulative doses of bradykinin (6, 20, and 60 nmol·kg−1, i.v.). In experiments in which prazosin and losartan were used, an additional dose of either phenylephrine (60 nmol·kg−1, i.v.) or angiotensin II (60 pmol·kg−1, i.v.) was administered before and after the administration of the respective antagonist to ascertain the effectiveness of receptor blockade. A schematic drawing of this protocol and a trace recording showing the SAP measured in one control (PBS treated) animal are presented in Figure 1.
Figure 1.
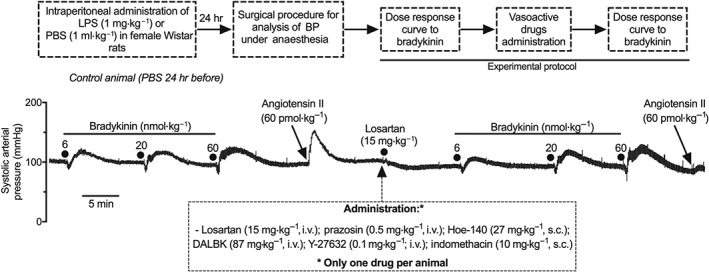
Schematic drawing and trace recording of a typical experiment showing the systolic arterial pressure (SAP) of an anaesthetized control rat. Bradykinin and angiotensin II intravenously administered before and after treatment with losartan (15 mg·kg−1, i.v.). The protocol and experiment showed here were performed in anaesthetized rats at 24 hr after intraperitoneal administration of LPS (1 mg·kg−1) or PBS (1 ml·kg−1). The box below the figure presents the antagonists and inhibitors used in these experiments. For additional details about the protocols, see Section 2
Additionally, the effects of bradykinin (6, 20, and 60 nmol·kg−1, i.v.) were evaluated in both LPS‐ and PBS‐treated animals before and after the administration of sub‐effective doses of both Hoe‐140 (1.35 mg·kg−1, s.c.) and losartan (5 mg·kg−1, i.v.), as illustrated by Figure S4.
2.5. Evaluation of the effects of a bradykinin B1 receptor agonist on BP and development of paw oedema
In a separate set of experiments, the effects of the selective B1 receptor agonist des‐Arg9‐bradykinin (DABK; 20, 60, and 200 nmol·kg−1, i.v.) on BP were assessed in both LPS and PBS 24‐hr groups (n = 6 per group).
Because LPS was previously associated with induction and up‐regulation of bradykinin B1 receptors (Regoli, Marceau, & Lavigne, 1981), but our experiments failed to demonstrate any significant effect of DABK on BP, we did evaluate the effects of DABK on the development of paw oedema, which was previously described in the LPS‐treated rats (Campos, Souza, & Calixto, 1996). These experiments were conducted in the same animals used for assessment of BP (control and LPS 24‐hr groups). After cannulation of vessels and before any intravenous administration of drugs, the animals received intraplantar injections (100 μl) containing sterile PBS (right hind paw) and DABK (100 nmol, left hind paw). The diameter of each paw and the development of oedema was measured by use of a digital pachymeter (Vonder, Itajaí, SC, Brazil) before and 5, 10, 15, and 30 min after intraplantar injections.
2.6. Evaluation of the influence of intra‐arterial administration in the effects of bradykinin, DABK, and DALBK on BP
To circumvent the putative influence of pulmonary first passage metabolism of bradykinin, DABK, and DALBK on our findings, an additional set of experiments using the intra‐arterial route was conducted. In this protocol, the animal received injections of bradykinin (6 and 20 nmol·kg−1), which were randomly administered through both intravenous and intra‐arterial pathways, followed by DABK (100 nmol·kg−1) and DALBK (87 mg·kg−1), both by intra‐arterial route only, followed by re‐administration of bradykinin (6 and 20 nmol·kg−1, i.a.).
2.7. Analysis of the influence of bradykinin B2 receptor antagonism on the pressor effect of angiotensin II
After stabilization of BP, a dose–response curve of the effect of angiotensin II (6, 20, and 60 pmol·kg−1, i.v.) on the systemic arterial pressure was obtained in rats from the control and LPS 24‐hr groups. At the end of the pressor response to the last dose of angiotensin II, the animals received a single treatment of Hoe‐140 (27 mg·kg−1, s.c.), and 20 min later, a second dose–response curve to angiotensin II was recorded, followed by a single dose of bradykinin (60 nmol·kg−1, i.v.). Using the same protocol in different groups of animals, we also analysed the pressor effects of phenylephrine (6, 20, and 60 nmol·kg−1, i.v.) before and after subcutaneous administration of Hoe‐140 (27 mg·kg−1, s.c.).
2.8. Haematological analysis and measurement of serum levels of nitrate and nitrite (NOx −)
For this, 500 μl of blood was randomly collected through the carotid artery from anaesthetized rats from both the control and LPS 24‐hr groups, before the beginning of protocols for evaluation of the systemic arterial pressure. For assessment of haematological parameters, the blood was collected in tubes containing EDTA (final concentration 6.8 mmol·L−1), and 30 μl of this blood was immediately analysed using an automatized blood counter (HORIBA ABX®, Micros 60, Montpellier, France). The remaining blood was centrifuged (750x g for 15 min) for plasma separation, which was frozen at −20°C until it was deproteinized by zinc sulfate (20%, 1:10), exposed to nitrate reduction using the nitrate reductase expressed in Escherichia coli (ATCC 25922), and mixed with Griess reagents for nitrite detection in a 96‐well plate, as detailed before (Da Silva‐Santos & Assreuy, 1999). The absorbance of the reaction was measured at 540 nm, and plasma nitrite concentrations were calculated by linear regression using standard curves of nitrate and nitrite (0–150 μmol·L−1).
2.9. Western blot analysis
The antibody‐based procedures used in this study comply with the recommendations made by the British Journal of Pharmacology. Animals from the control and LPS 24‐hr groups (n = 6 per group) were anaesthetized by intraperitoneal injection of ketamine and xylazine (90 and 15 mg·kg−1, respectively), subjected to a thoracic‐abdominal incision, and had a butterfly‐type catheter connected to a perfusion system inserted into their left ventricle. The animals were perfused with cold (4°C) isotonic saline solution before their thoracic aorta and mesenteric arterial bed (except the superior mesenteric artery) were collected. The vessels were cleaned from surrounding tissues, frozen in liquid nitrogen, and kept at −80°C until preparation for electrophoretic assays. For this, each sample was pulverized and incubated at 4°C in a protein extraction buffer containing 100‐mmol·L−1 PMSF, 100‐mmol·L−1 sodium orthovanadate, and 10 μl·ml−1 of protease cocktail inhibitor (composition in mmol·L−1: 104, AEBSF; 0.08, aprotinin; 4, bestatin; 1.4, E‐64; 2, leupeptin; and 1.5, pepstatin A). After 1 hr, the tubes containing the lysates were centrifuged (19,000x g, 30 min at 4°C), and the supernatants were collected. Protein concentrations were determined using the bicinchoninic acid (BCA) assay (Thermo Scientific, CA, USA; catalogue number 23227). The samples for use were diluted in Laemmli buffer (600 μl·ml−1 of 10% SDS, 200 μl·ml−1 of glycerol, 100 μl·ml−1 of 2‐mercaptoethanol, 250 μl·ml−1 of 1‐mol·L−1 Tris–HCl, and 0.3 mmol·L−1 of bromophenol blue) and heated at 95°C for 8 min. We used 40 and 60 μg of protein obtained from thoracic aorta and mesenteric vascular bed, respectively, for electrophoretic separation in 7% or 10% SDS‐PAGE, running in a Mini‐PROTEAN® Tetra cell apparatus under a PowerPac™ HC power supply (both from Bio‐Rad, CA, USA). The proteins were transferred to nitrocellulose membranes (0.2‐ or 0.45‐μm pore sizes; Bio‐Rad), which were stained with Ponceau solution for verification of band transfer. The membranes were washed three times (10 min each) with TBS‐T buffer (pH 7.4; 20‐mmol·L−1 Tris–HCl, 137‐mmol·L−1 NaCl, and 0.1% Tween 20) and blocked in 5% non‐fat dry milk prepared in TBS‐T. The membranes were incubated overnight at 4°C with primary antibodies against angiotensin II AT1 receptor (dilution 1:100) or B2 receptors (1:500). Peroxidase‐conjugated monoclonal antibody against β‐actin (1:25,000) was used as a loading control for all experiments. After incubation with primary antibodies, the membranes were washed three times (10 min each) with TBS‐T buffer and incubated with a secondary antibody conjugated to HRP (1:3,000 or 1:5,000) for 1 hr at room temperature. The membranes were washed another three times (10 min each) with TBS‐T buffer and exposed to HRP substrate. The proteins were detected and analysed by densitometry using a charge‐coupled device camera‐based digital imaging system (ChemiDoc MP) and the Image Lab software v. 5.2.1 (RRID:SCR_014210; both from Bio‐Rad, Hercules, CA, USA).
2.10. Co‐immunoprecipitation
The mesenteric vascular bed from the control and LPS 24‐hr animals (n = 8 per group) was collected and prepared as described for Western blot analysis. The pulverized tissue was homogenized in ice‐cold protein extraction lysis buffer (pH 7.4; composition: 4‐mmol·L−1 PMSF, 0.2‐mmol·L−1 sodium orthovanadate, 150‐mmol·L−1 NaCl, 10‐mmol·L−1 Tris–HCl, 1‐mmol·L−1 EDTA, 1‐mmol·L−1 EGTA, 1% Triton X‐100, and 1 μl·ml−1 of protease cocktail inhibitor). After 1 hr of incubation, the samples were centrifuged (19,000x g, 30 min at 4°C), and the supernatant was collected for protein determination. We kept 100 μl for use as the loading control in immunoelectrophoresis, and the remaining supernatant was adjusted to 1 mg·ml−1. In separate samples, the primary antibody against either AT1 receptors or B2 receptors (1 μl·ml−1) was incubated overnight at 4°C, under continuous orbital rotation. The protein A/G plus agarose (20 μl) was added into the samples, and the incubation was continued for an additional 3 hr, followed by centrifugation (750x g, 5 min at 4°C). The supernatant was removed, and the bead pellets were washed five times in ice‐cold lysis buffer. The remaining pellet was resuspended in 10 μl of lysis buffer containing 5‐μl Laemmli solution and heated at 95°C for 8 min. The total volume of each sample containing the immunocomplexes was processed for Western blot analysis using primary antibodies against either bradykinin B2 receptor (dilution 1:500) or angiotensin II AT1 receptor (dilution 1:100) and the appropriate secondary antibody conjugated to HRP (1:10,000).
2.11. Isolation of small mesenteric arteries
Animals from both the control and LPS 24‐hr groups (n = 6 per group) were killed by anaesthetic overdose (ketamine/xylazine, greater than 140/40 mg·kg−1, administered by intraperitoneal and intravenous routes). The entire small intestine and the attached mesenteric vascular bed were removed and placed in Petri dishes containing cold (~4°C) physiological saline solution (PSS; composition in mmol·L−1: 131.25, NaCl; 4.82, KCl; 1.59, CaCl2•2H2O; 1.18, KH2PO4; 1.17, MgSO4; 5.55, D‐glucose; 14.89, NaHCO3; and 0.03, EDTA). Branches of first‐ and second‐order arteries were carefully cleaned from veins, connective tissues, and surrounding fat. We used a dissecting microscope to insert two stainless steel wires (40 μm in diameter) into the arteries, which were cut into rings (~1 mm in length) and mounted in wire myograph chambers (model 620 M, Danish Myo Technology, Aarhus, Denmark), containing 5‐ml PSS at 37°C, and continuously bubbled with 95% O2/5% CO2. The development of force was recorded by the application software LabChart (version 8.15, AD Instruments). In all experiments, the vessels were maintained in the chamber for 15 min without any manipulation, before being stretched to a resting force of 5.5 mN. After an equilibration period of 45 min, all preparations were subjected to a modified PSS containing 120‐mmol·L−1 KCl (composition in mmol·L−1: 14.37, NaCl; 119.91, KCl; 1.59, CaCl2•2H2O; 1.18, KH2PO4; 1.17, MgSO4; 14.89, NaHCO3; 0.038, EDTA; and 5.55, D‐glucose), in order to evaluate functional integrity and the maximum contractile response evoked. The vessels were washed with regular PSS, and after stabilization for 30 min, the integrity of the endothelial layer was verified by the ability of 10‐μmol·L−1 ACh to induce relaxation during the tonic increase in force generated by 10‐μmol·L−1 phenylephrine. After an additional interval of 30 min, the arteries were subjected to vasoactive agents according to the experimental protocols described ahead.
Both endothelium‐intact and endothelium‐denuded arteries, subjected to mechanical removal of the endothelial layer, were used in this study. Only those vessels in which the ACh‐induced relaxation reached over 80% were considered as endothelium‐intact arteries, while the lack of responses to ACh was used as the criterion of endothelium removal.
2.12. Characterization of the effects of bradykinin and angiotensin II in resistance arteries of endotoxemic rats
After stabilization, endothelium‐intact and ‐denuded small mesenteric arteries from the control or LPS 24‐hr groups (n = 6 per group) were superfused with a single concentration of bradykinin (10 μmol·L−1), before and 30 min after incubation with losartan (10 μmol·L−1) or vehicle (5 μl of PSS). We also measured the ability of angiotensin II (10 μmol·L−1) to increase the vascular force in vessels previously incubated for 30 min with Hoe‐140 (1 μmol·L−1). Importantly, because repeated stimulation of AT1 receptors results in tachyphylaxis, each preparation of small mesenteric artery was subjected for a single incubation with angiotensin II.
2.13. Evaluation of the influence of COX inhibitors on the effects of bradykinin in resistance arteries of endotoxemic rats
After stabilization, endothelium‐intact small mesenteric arteries from the LPS 24‐hr group (n = 6 per group) were superfused with a single concentration of bradykinin (10 μmol·L−1), before and after incubation during 30 min with indomethacin (10 μmol·L−1) or NS‐398 (10 μmol·L−1, a selective inhibitor of COX‐2).
2.14. Materials
Phenylephrine hydrochloride, bradykinin acetate, ACh chloride, angiotensin II acetate, prazosin, indomethacin, des‐Arg9‐bradykinin (DABK) acetate salt, des‐Arg9‐[Leu8]‐bradykinin (DALBK) acetate salt, N‐[2‐(cyclohexyloxy)‐4‐nitrophenyl]methanesulfonamide (NS‐398), LPS from E. coli (serotype 1 O111:B4), sodium orthovanadate, PMSF, the protease inhibitor cocktail, EGTA, polyethylene glycol tert‐octylphenyl ether (Triton X‐100), the Ponceau S solution, and all salts used to prepare the PBS (concentration in mmol·L−1: 137, NaCl; 2.7, KCl; 1.5, KH2PO4; and 8.1, NaHPO4) and PSS were purchased from Sigma‐Aldrich (Saint Louis, MO, USA). Losartan was donated by Lucifarma Pharmacy (União da Vitória, PR, Brazil). Hoe‐140 and Y‐27632 dihydrochloride were acquired from Tocris Bioscience (Ellisville, MO, USA). Heparin was obtained from Hipolabor Farmacêutica (Belo Horizonte, MG, Brazil). Syntec (São Paulo, SP, Brazil) was the supplier of both ketamine and xylazine. All reagents used to prepare the SDS‐PAGE and Tris–HCl were acquired from Bio‐Rad (Hercules, CA, USA). The tissue protein extraction reagent (T‐PER™) and the enhanced chemiluminescent substrates (SuperSignal®) were from Thermo Scientific (Rockford, IL, USA). Tween‐20 was purchased from Vetec (Rio de Janeiro, RJ, Brazil). Protein A/G plus agarose was acquired from Santa Cruz (Dallas, TX, USA; catalogue number 2003; RRID:AB_10201400). The following antibodies were used (produced by; catalogue number): anti‐bradykinin B2 receptor monoclonal antibody (Abcam, Cambridge, UK; ab73625; RRID:AB_2064187), anti‐angiotensin II AT1 receptor polyclonal antibody (Santa Cruz, Dallas, TX, USA; sc‐1173; RRID:AB_2305402), anti‐β‐actin monoclonal antibody (Sigma‐Aldrich; A5441; RRID:AB_476744), and rabbit (7074; RRID:AB_2099233) and mouse (7076; RRID:AB_330924) secondary antibodies (Cell Signaling Technology, Beverly, MA, USA).
2.15. Data and statistical analysis
The data presented for BP measurements are expressed as dot plots or the mean ± SEM of basal values, or of the effect of the vasoactive agents on the MAP, SAP, and DAP (in mmHg), and on the HR (bpm), as measured in at least six animals per group. The results obtained in in vitro vascular preparations are presented as dot plot or the mean ± SEM of the increased force (in mN) generated by vasoactive substances (n = 6 preparations from different animals per group). The AUC of effects induced by vasoactive drugs was analysed from the beginning to the end of the changes in the SAP or vascular tone using LabChart 7.3.7 (AD Instruments). The results showed for Western blot assays were obtained in samples from at least five different animals per group. Co‐immunoprecipitation (Co‐IP) was performed in homogenates from resistance mesenteric arteries from eight animals per group. Data and statistical analyses comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2018). The statistical significance was determined using one‐ or two‐way ANOVA, followed by Bonferroni's post‐test only if F was significant and there was no variance in homogeneity, or Student's t test. A value of P ≤ .05 was considered statistically significant. The graphs were drawn, and the statistical analyses were performed using GraphPad Prism version 7.00 (RRID:SCR_002798; GraphPad Software, La Jolla, CA, USA).
2.16. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Christopoulos et al., 2017; Alexander, Fabbro et al., 2017).
3. RESULTS
3.1. General effects of endotoxemia on cardiovascular parameters, blood cell counts, and NOx − levels
Administration of LPS resulted in leucocytosis, accompanied by unchanged Hb and haematocrit, granulocytosis, monocytosis, and reduced counts of platelets and lymphocytes, as found in female rats from the LPS 24‐hr group. In addition, these animals also presented increased NO x − levels (Table S1). Nonetheless, administration of 1‐mg·kg−1 LPS did not decrease the BP in any of our experimental groups. For instance, the basal MAP and HR recorded in female rats from the control (non‐endotoxemic) and LPS 24‐hr groups were 84.1 ± 4.8 mmHg and 226 ± 6.8 bpm, and 83.2 ± 6.2 mmHg and 224 ± 9.2 bpm, respectively (Figure 2b,d).
Figure 2.
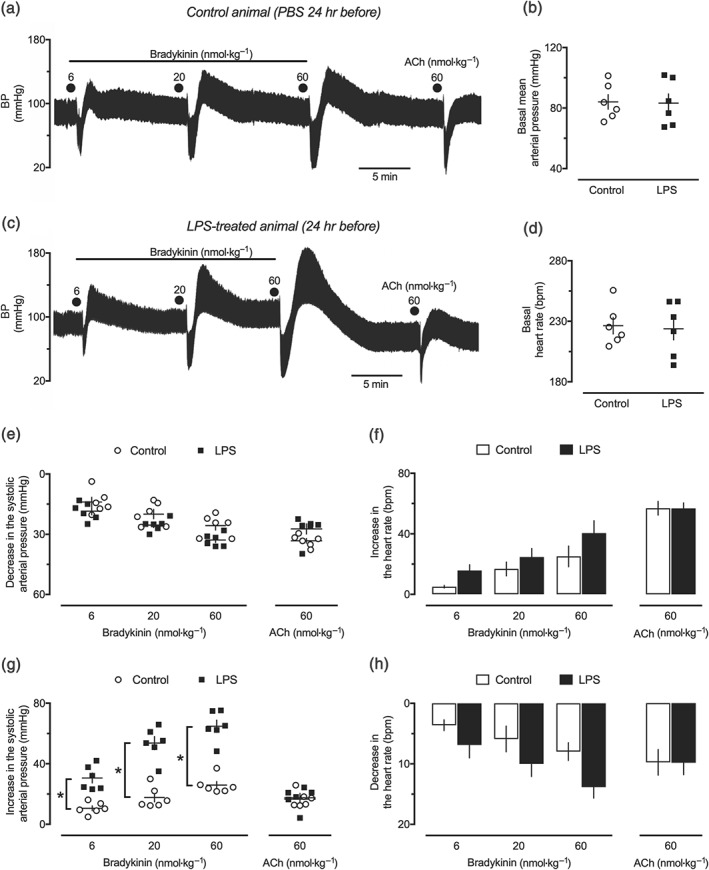
Effects of bradykinin in the cardiovascular system of endotoxemic female rats. Trace records of the systemic arterial pressure showing the effects of intravenous bradykinin and ACh in anaesthetized female rats 24 hr after intraperitoneal administration of (a) PBS (1 ml·kg−1, control group) and (c) LPS (1 mg·kg−1). The basal MAP (b) and the basal heart rate (d), as well the maximal hypotensive (e) and the secondary pressor responses (g) effects of bradykinin and ACh, were evaluated in the same experimental groups. The maximal changes in the heart rate obtained during the hypotensive and pressor effects of bradykinin and ACh are shown in panels (f) and (h), respectively. The results are presented as dot plot or the mean ± SEM of values obtained from six animals per group. *P < .05, significantly different from the control group; Student's t test (b, d) or two‐way ANOVA followed by Bonferroni's post‐test (e–h)
3.2. Biphasic effect of bradykinin in the BP of endotoxemic rats
As illustrated in Figure 2a,c, the intravenous administration of bradykinin resulted in strikingly similar dose‐dependent reductions in the BP of the control and LPS 24‐hr groups (Figure 2e), as well as for all periods of evaluation (Table S2). Bradykinin‐induced hypotension was followed by an augmentation of BP, as shown in the typical traces presented in Figure 2a,c. The analysis of DAP revealed that this secondary pressor response varied from peaks of 7 ± 1.2, 16 ± 2.6, and 21 ± 0.9 mmHg in the control animals to 25 ± 2.7, 33 ± 2.1, and 38 ± 2.7 mmHg in the LPS 24‐hr group, after administration of 6‐, 20‐, and 60‐nmol·kg−1 bradykinin, respectively. This enhancement in the increase in BP found in the LPS 24‐hr group following bradykinin administration was notably higher for the SAP (Figure 2g and Table S3) and was also found in the LPS 48‐hr groups, but not in earlier (6 hr) or later (72 hr) periods of endotoxemia (Tables S3 and S4). The effects of bradykinin in the systemic arterial pressure were accompanied by an increase followed by a reduction in the cardiac frequency, which were comparable for animals from the control and LPS 24‐hr groups (Figure 2f,h). Although an elevation in BP also followed the hypotensive effect of ACh, we did not find any difference in the magnitude of such effect in the LPS‐treated animals, compared with the control groups (Figure 2e–h). Importantly, male rats treated with LPS 24 hr before the assessment of BP also presented enhanced pressor responses to bradykinin, compared with the control group (Figure S1).
3.3. Bradykinin B2 receptors but not B1 receptors mediate the pressor effect of bradykinin in endotoxemic rats
Administration of the selective B2 receptor antagonist Hoe‐140 abolished bradykinin‐induced hypotension in both the control and LPS 24‐hr groups (data not shown). The blockade of B2 receptors also reduced the pressor effect generated after bradykinin injection to a transient peak similar to that found in the control animals (Figure 3a,c). On the other hand, treatment with the bradykinin B1 receptor antagonist DALBK did not influence the vasodilator (data not shown) or the pressor responses to bradykinin, neither in the control nor in the endotoxemic animals (Figure 3a,c). Evaluation of the systemic arterial pressure revealed that despite the lack of effects of DALBK on arterial pressure, non‐endotoxemic animals that received subcutaneous injection of Hoe‐140 developed a sustained decrease in their BP (~9.1–13.8 mmHg, 95% confidence interval), which was markedly reduced in the LPS 24‐hr group (Figure 3b). In contrast to the effects of bradykinin, the B2 receptor agonist DABK did not present any hypotensive (data not shown) or pressor effects on the systemic arterial pressure of animals from the control or LPS 24‐hr groups (Figure 3a,c), although an appreciable increase in the paw volume was noted after its intraplantar injection in animals from the LPS 24‐hr group (Figure 3d). Despite the potentiation found in both hypotensive and pressor effects of intra‐arterially injected bradykinin, the intra‐arterial administration of DABK and DALBK did not affect BP or the responses to bradykinin in the LPS‐treated animals (Figure 4).
Figure 3.
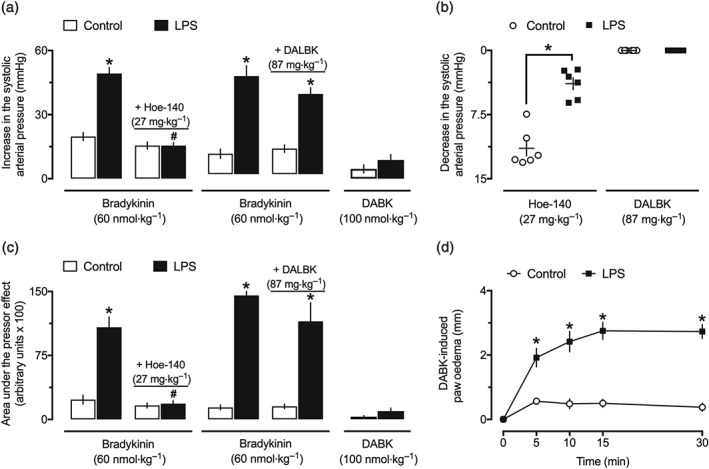
Activation of bradykinin B2 receptors accounts for the pressor effect of bradykinin in endotoxemic female rats. Secondary pressor responses to intravenous bradykinin and des‐Arg9‐bradykinin (DABK) and influence of the selective inhibition of B2 receptors by Hoe‐140 (27 mg·kg−1, s.c.) or B1 receptors by des‐Arg9‐[Leu8]‐bradykinin (DALBK; 87 mg·kg−1, i.v.) on the peak (a) and AUC (c) of the pressor effect of bradykinin in the control (PBS treated) and endotoxemic (LPS treated) rats. (b) Effects of Hoe‐140 and DALBK in the systolic arterial pressure of the control and endotoxemic animals. (d) Induction of paw oedema by the intraplantar injection of DABK (100 nmol) in the control and LPS groups. These experiments were performed in anaesthetized female rats at 24 hr after intraperitoneal administration of LPS (1 mg·kg−1) or PBS (1 ml·kg−1, control group). The results are presented as the mean ± SEM or the dot plot of values obtained from six animals per group. *P < .05, significantly different from the control group; # P < .05, significantly different from the respective group before administration of Hoe‐140 or DALBK; Student's t test (b) or two‐way ANOVA followed by Bonferroni's post‐test (a, c, d)
Figure 4.
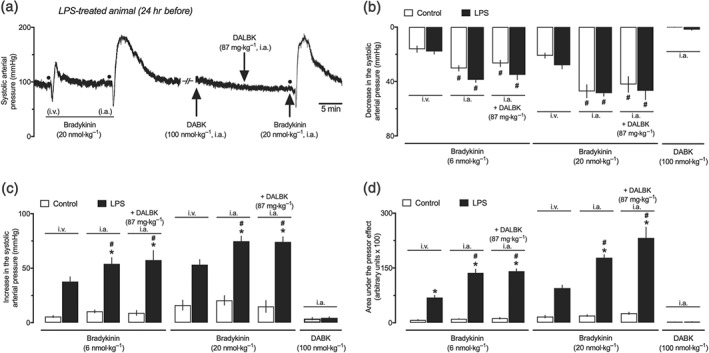
Enhanced pressor effect of bradykinin by intra‐arterial route in endotoxemic female rats. (a) Trace records of the systemic arterial pressure showing the effects of intravenous and intra‐arterial administration of bradykinin (20 nmol·kg−1), des‐Arg9‐bradykinin (DABK; 100 nmol·kg−1, i.a. only), and bradykinin after administration of des‐Arg9‐[Leu8]‐bradykinin (DALBK; 87 mg·kg−1, i.a.). (b) Primary hypotensive effects induced by bradykinin, administered by intravenous and intra‐arterial pathways, before and after intra‐arterial administration of DALBK. Lack of influence of intra‐arterially administered DALBK against the peak (c) and AUC (d) of the pressor effect induced by bradykinin. The last set of bars in panels (b) to (d) shows the inability of DABK (by intra‐arterial route) to induce any change in the systemic arterial pressure of both the control and LPS groups. These experiments were performed in anaesthetized female rats at 24 hr after intraperitoneal administration of LPS (1 mg·kg−1) or PBS (1 ml·kg−1, control group). The results are presented as the mean ± SEM obtained from six animals per group. # P < .05, significantly different from effects recorded after intravenous administration in the same experimental group; *P < .05, significantly different from the respective control group; one‐way ANOVA followed by Bonferroni's post‐test
3.4. Antagonism of α1‐adrenoceptors did not prevent the pressor effect of bradykinin in endotoxemic rats
Intravenous administration of 0.5‐mg·kg−1 prazosin was enough to abolish phenylephrine‐increased BP (Figure S2A) and caused a similar reduction in the SAP from both the control and LPS 24‐hr groups (Figure 5a). There was no influence of prazosin on the vasodilator effect of bradykinin in any of our experimental groups (data not shown). Endotoxemic animals treated with prazosin displayed partly reduced pressor responses to the initial (6 and 20 nmol·kg−1), but not to the highest (60 nmol·kg−1), doses of bradykinin (Figure 5b). Moreover, this α‐adrenoceptor antagonist did not alter the AUC of the pressor effect of bradykinin (Figure 5c).
Figure 5.
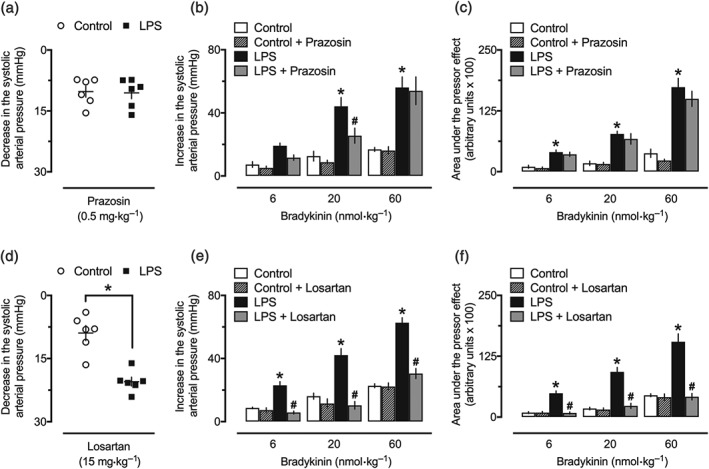
Blockade of angiotensin II AT1 receptors in the pressor effect of bradykinin in endotoxemic female rats. (a) Reduction of BP after intravenous administration of prazosin. Treatment with prazosin (0.5 mg kg, i.v.) failed to reduce the peak (b) and AUC (c) of the secondary pressor effect generated by bradykinin in endotoxemic animals. Reduction of BP (d) and inhibitory influence of losartan (15 mg·kg−1, i.v.) against the peak (e) and AUC (f) of the pressor effect induced by bradykinin in the LPS‐treated animals. These experiments were performed in anaesthetized female rats 24 hr after intraperitoneal administration of LPS (1 mg·kg−1) or PBS (1 ml·kg−1, control group). The results are presented as a dot plot or as the mean ± SEM of values obtained from six animals per group. *P < .05, significantly different from the control group; # P < .05, significantly different from the respective group before administration of prazosin or losartan; Student's t test (a, d) or two‐way ANOVA followed by Bonferroni's post‐test (b, c, e, f)
3.5. Blockade of angiotensin II AT1 receptors prevents the pressor effect of bradykinin in endotoxemic rats
The hypotensive effect achieved after a single administration of losartan was significantly higher in animals from the LPS 24‐hr group, compared with the effects recorded in the control animals (Figure 5d). The injection of losartan resulted in full inhibition of angiotensin II‐increased BP (Figure S2B), without any influence in the hypotensive action of bradykinin (data not shown; the traces of an experiment showing the effects of bradykinin, losartan, and angiotensin II in a control animal are shown in Figure 1). Moreover, treatment with losartan resulted in a marked inhibition of the pressor effect of bradykinin, as shown for SAP (Figure 5e) and AUC (Figure 5f) of the effects measured before and after losartan administration (see Figure S3 for trace recordings in an LPS 24‐hr‐treated animal).
3.6. Influence of COX and Rho‐kinase inhibitors in the pressor effect of bradykinin in endotoxemic rats
As used in our experiments, both indomethacin and Y‐27632 induced a similar drop in the systemic arterial pressure of rats, which did not differ between the control and LPS 24‐hr groups (Figure 6a,c, respectively). Neither of these inhibitors changed the hypotensive effects of bradykinin (data not shown). The pressor effects of all bradykinin doses on the SAP of the LPS 24‐hr group were similar before and after administration of indomethacin (Figures 6b and S5A). On the other hand, injection of Y‐27632 suppressed the pressor responses to bradykinin in endotoxemic animals, as shown for the peak of increase in the SAP (Figure 6d) and the AUC of the pressor effect of bradykinin (Figure S5B).
Figure 6.
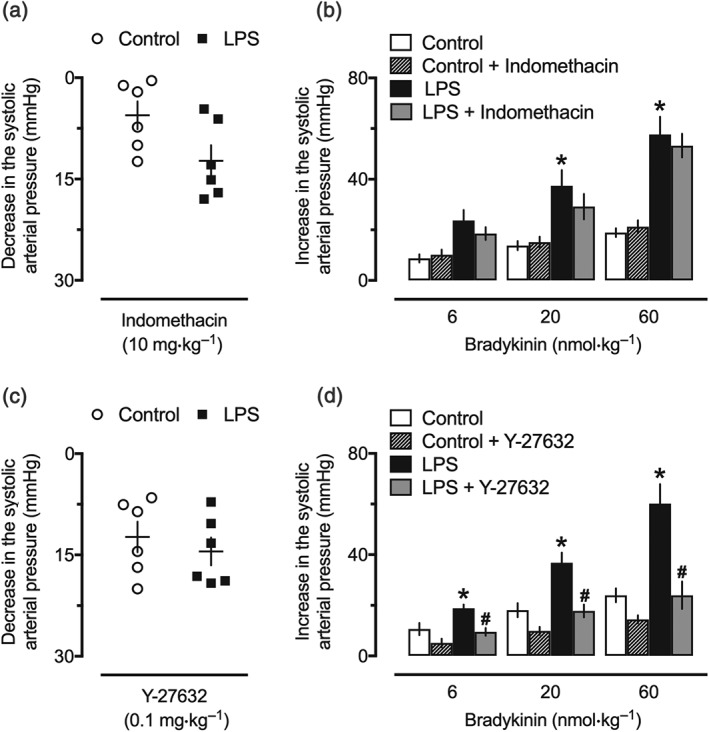
Involvement of the Rho‐A/Rho‐kinase (ROCK) pathway in the pressor effect of bradykinin in endotoxemic rats. Reduction of BP after intravenous administration of indomethacin (a) or Y‐27632 (c). Lack of influence of indomethacin (b) and inhibitory effect of Y‐27632 (d) against the pressor effect of bradykinin endotoxemic rats. These experiments were performed in anaesthetized female rats 24 hr after intraperitoneal administration of LPS (1 mg·kg−1) or PBS (1 ml·kg−1, control group). The results are presented as a dot plot or as the mean ± SEM of values obtained from six animals per group. *P < .05, significantly different from the control group; # P < .05, significantly different from the respective group before administration of Y‐27632; Student's t test (a, c) or two‐way ANOVA followed by Bonferroni's post‐test (b, d)
3.7. Levels of B2 receptors and AT1 receptors in mesenteric resistance arteries and aorta from endotoxemic rats
Western blotting assays revealed that both B2 receptors and AT1 receptors were increased in homogenates of the mesenteric vascular bed obtained from endotoxemic animals at 24 hr after LPS injection (Figure 7a,c, respectively). Nonetheless, there were no differences in the levels of these receptors in the aorta of animals from the LPS 24‐hr group compared with samples from the control animals (Figure 7b,d).
Figure 7.
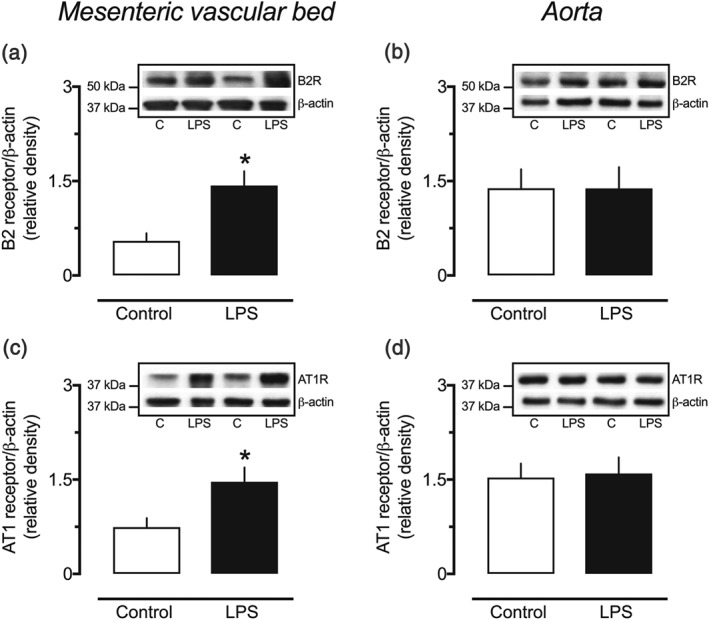
Increased expression of bradykinin B2 receptors and angiotensin II AT1 receptors in resistance arteries of endotoxemic female rats. Densitometric analyses and representative Western blots of the B2 receptors (a, b) and the AT1 receptors (c, d) in small mesenteric arteries and aortas obtained from female rats 24 hr after intraperitoneal administration of LPS (1 mg·kg−1) or PBS (1 ml·kg−1, control group). The values are mean ± SEM of results obtained from five (a) or six (b–d) different animals per group. * P < .05, significantly different from control; Student's t test
3.8. Detection of B2 receptor and AT1 receptor interaction in resistance mesenteric arteries from endotoxemic rats
Homogenates of small mesenteric arteries from the LPS 24‐hr group‐containing complexes precipitated with the anti‐AT1 receptor antibody were detected by the anti‐B2 receptor antibody when subjected to Western blotting analysis (Figure 8a). Similarly, Co‐IP isolated using the anti‐B2 receptor antibody revealed higher levels of the AT1/B2 receptor complex in samples from the LPS 24‐hr group, compared with homogenates from the control animals (Figure 8b).
Figure 8.
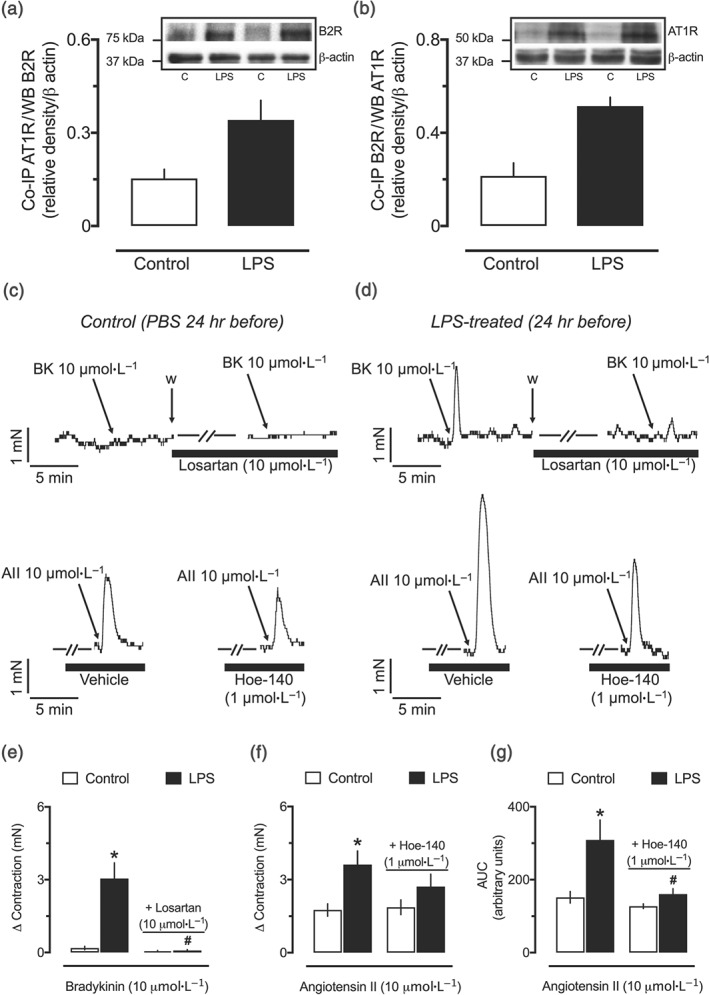
Biochemical and functional evidence of interaction between B2 and AT1 receptors in resistance arteries from endotoxemic female rats. Densitometric analyses and representative Western blots of lysates of resistance mesenteric arteries subjected for co‐immunoprecipitation (Co‐IP) using anti‐AT1 receptor antibodies and detected by an anti‐B2 receptor antibody (a) or Co‐IP using anti‐B2 receptor antibody and detected by an anti‐AT1 receptor antibody (b). Representative tracings showing the effect of 10‐μmol·L−1 bradykinin without or with incubation with 10‐μmol·L−1 losartan, upper traces in (c) and (d), or 10‐μmol·L−1 angiotensin II without or with incubation of 1‐μmol·L−1 Hoe‐140, lower traces in (c) and (d), in endothelium‐intact small mesenteric arteries obtained from the control (c) and LPS‐treated (d) rats. Losartan‐sensitive contractile effects of bradykinin (e) and Hoe‐140‐sensitive enhancement of angiotensin II‐induced vasoconstriction (f, g) in small mesenteric arteries from endotoxemic rats. Small mesenteric arteries were obtained from female rats 24 hr after intraperitoneal administration of LPS (1 mg·kg−1) or PBS (1 ml·kg−1, control group). Co‐IP was performed in samples from eight different animals per group: four used with the anti‐ AT1 receptor antibody (a) and four with the B2 receptor antibody (b). The experiments in organ baths (e–g) were conducted with resistance arteries obtained from six different animals per group. All panels show the mean ± SEM. *P < .05, significantly different from the control group; # P < .05, significantly different from the respective group without incubation with losartan or Hoe‐140; one‐way ANOVA followed by Bonferroni's post‐test (e–g)
3.9. Evidence of functional interaction between bradykinin‐ and angiotensin II‐induced contractile responses in mesenteric arteries from endotoxemic rats
Addition of bradykinin did not generate any change in the vascular tone of unstimulated endothelium‐intact small mesenteric arteries obtained from the control animals (Figure 8c, upper traces) but resulted in a very clear augmentation of tone in arteries from the LPS 24‐hr group (Figure 8d, upper traces), an effect fully inhibited by losartan (Figure 8e). Further, angiotensin II‐induced contraction was significantly higher in small arteries from endotoxemic rats (Figure 8c,d, lower traces), and regardless of the lack of significant inhibition in the maximum contractile response (Figure 8f), the AUC of the effect of angiotensin II was clearly reduced in preparations incubated with Hoe‐140 (Figure 8g). Similar results were found in endothelium‐denuded arteries and vessels from male rats (Figures S6 and S7, respectively).
3.10. Blockade of B2 receptors reduces the pressor effect of angiotensin II in endotoxemic rats
Animals subjected to injection of 1‐mg·kg−1 LPS presented increased reactivity to angiotensin II, as found in the initial experiments developed in this study, which were designed to explore the influence of the AT1 receptor antagonist losartan on the pressor effect of bradykinin (Figure S2B). Because the augmented reactivity to angiotensin II was also reproduced and prevented by Hoe‐140 using in vitro small mesenteric arteries from the LPS 24‐hr group (Figure 8f,g), this set of experiments aimed to investigate whether or not the administration of Hoe‐140 would reduce the in vivo effect of angiotensin II. As illustrated in typical traces of the SAP of the control and LPS‐treated animals presented in Figure 9a,b, respectively, the pressor effect of angiotensin II was significantly higher in animals from the LPS 24‐hr group. Notably, the responses to angiotensin II, including the peak and the AUC, returned to values found in the control animals after administration of Hoe‐140 (Figure 9c,d, respectively). On the other hand, antagonism of B2 receptors showed no influence on the hypertensive effect of phenylephrine in the LPS 24‐hr group, which did not differ from the control animals (Figure 9e).
Figure 9.
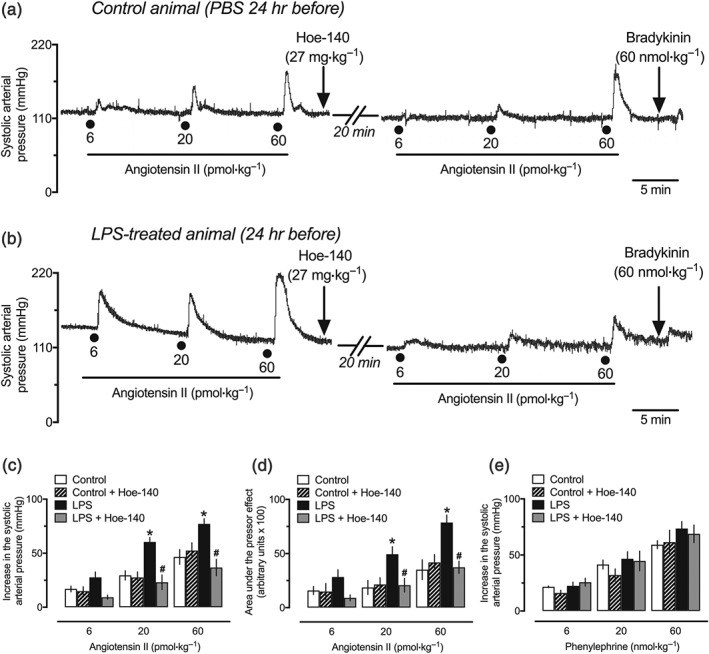
The potentiation of the pressor effect of angiotensin II is prevented by the bradykinin B2 receptor antagonist Hoe‐140. Trace recordings of experiments showing the pressor effects of angiotensin II before and after administration of Hoe‐140 in the control (a) and LPS‐treated (b) rats. Treatment with Hoe‐140 that reduced the peak (c) and the AUC (d) of the pressor effect of angiotensin II did not affect the hypertensive action of phenylephrine (e). These experiments were performed in anaesthetized female rats 24 hr after intraperitoneal administration of LPS (1 mg·kg−1) or PBS (1 ml·kg−1, control group). The results are presented as the mean ± SEM of values obtained from six animals per group. Statistical analyses were performed using *P < .05, significantly different from the control group; # P < .05, significantly different from the respective group before administration of Hoe‐140; two‐way ANOVA followed by Bonferroni's post‐test
4. DISCUSSION
The first important finding disclosed by our study was that rats pretreated with 1‐mg·kg−1 LPS presented a different profile of systemic vascular responses to bradykinin. In these animals, the classic drop in BP generated by the intravenous administration of bradykinin remained unaltered, but it was followed by an increase that resembled the pressor effect obtained after administration of vasoconstrictor agents.
In the field of cardiovascular research, moderate to high doses of bacterial LPS are widely used to study hypotension, hyporeactivity to vasoconstrictors, and other cardiovascular complications found in sepsis and septic shock. However, reduced amounts of LPS have also been adopted for the investigation of several conditions associated with inflammatory responses, such as neurodegeneration, and liver, lung, and prostate disease (dos Santos Gomes et al., 2018; Peng et al., 2004; Qin et al., 2007). Notably, the LPS dose chosen in this research did not induce any of the classical signs associated with endotoxic or septic shock, such as piloerection, prostration, and decreased mobility, hypotension, or vasoplegia. Nonetheless, it was enough to elevate the plasma levels of NO x −, an indication of increasing quantities of NO, and generated several haematological changes including leucocytosis and thrombocytopenia (Table S1), which are associated with inflammatory conditions. Thus, if on the one hand the development of the pressor effect of bradykinin after LPS administration cannot be related to advanced stages of sepsis and septic shock, on the other, it is conceivable to suggest that endotoxin‐induced inflammation, a condition associated with several pathological states, impairs the vascular sensitivity to bradykinin. This secondary response to bradykinin takes place several hours after LPS‐induced inflammation and is transitory, being reduced at 48 hr and completely absent at 72 hr after endotoxin administration.
According to previous studies, both bradykinin B1 and B2 receptors can be involved in the kinin‐mediated control of vascular tone, depending on the experimental environment and vessel evaluated (Mombouli & Vanhoutte, 1995). For instance, the contractile effects mediated by B1 receptors in the rat portal vein were associated with increased thromboxane A2 levels derived from phospholipase A2 and COX‐2 activities in endothelial cells (Basei et al., 2012). Moreover, endothelium‐denuded porcine coronary arteries display contractile responses mediated by B1 and B2 receptors after in vitro incubation with LPS (More, Kim, Khang, et al., 2014; More, Kim, Zhao, et al., 2014). Indeed, both pharmacological and molecular approaches did show that LPS is a trigger for up‐regulation of B1 receptors in the cardiovascular system of rabbits, pigs, mice, and rats (McLean et al., 2000; Regoli et al., 1981; Schremmer‐Danninger et al., 1996). However, considering that the B2 receptor antagonist Hoe‐140, but not the B1 receptor antagonist DALBK, abolished the secondary pressor effect of bradykinin, and the complete absence of cardiovascular responses to the B1 receptor agonist DABK, even after its intra‐arterial administration, an experimental strategy that avoids extensive first‐pass metabolism by peptidases in lungs, our study showed that B2 receptors did account, not only for the hypotensive, but also for the pressor effects induced by bradykinin in the endotoxemic rats used in our experiments.
Although in vitro approaches have shown the ability of bradykinin to increase the tone of selected arteries and veins instead of inducing vasodilation, there are only a very few reports describing experimental evidence of pressor actions of bradykinin following its intravenous administration. For instance, in the 1960s, a secondary hypertensive response to bradykinin was described in both nephrectomized and pentolinium‐treated rats (Croxatto & Belmar, 1961), as well as in anaesthetized cats and dogs (Lang & Pearson, 1968; Pearson & Lang, 1967). This pressor response to bradykinin was characterized as more intense under drug‐ or haemorrhage‐induced hypotension and was assigned to activation of the sympathetic nervous system, a peripheral release of catecholamines (Croxatto & Belmar, 1961; Lang & Pearson, 1968) and an unexplored activation of Rho‐associated kinase (ROCK; Zhang et al., 2015). In our experiments, bradykinin‐increased BP was not accompanied by any rise in the cardiac frequency, and it was not blocked by the α1‐adrenoceptor antagonist prazosin. Besides, this finding does not appear to be dependent on hypotension, and it was fully reproduced in organ baths using resistance arteries, with and without functional endothelium. Thus, we suggest that in endotoxemic animals, it is unlikely that the pressor effects generated by bradykinin are dependent on increased sympathetic activity.
Different from prazosin, the AT1 receptor antagonist losartan prevented the pressor action of bradykinin in endotoxemic animals, giving us the first experimental clue suggesting that a receptor–receptor interaction between bradykinin B2 receptors and angiotensin II AT1 receptors might be occurring in the vascular system after LPS administration. Importantly, using a combination of sub‐effective doses of losartan and Hoe‐140, we found a synergistic effect between these drugs against the pressor effect of bradykinin (see Figure S4), reinforcing this hypothesis. Activation of B2 receptors is one of the most studied effects of bradykinin in the vascular system. Although multiple intracellular pathways may be involved, accumulation of NO‐ and COX‐derived compounds is often found to be the primary mediator of the vascular activity of bradykinin (Burch & Axelrod, 1987; Campos & Calixto, 1994; Fasciolo et al., 1990; Garrett & Brown, 1972; Miyamoto et al., 1999; More, Kim, Zhao, et al., 2014; Palmer et al., 1987; Regoli et al., 1977; Sai et al., 1995; Starr & West, 1966). Using indomethacin, we were able to show that products of COX did not play any detectable role in bradykinin‐increased BP following exposure to LPS. Indeed, the enhanced effect of bradykinin found after incubation with NS‐398 (see Figure S8) suggests that vasodilatory products of COX‐2 may counteract the contractile responses to bradykinin in small mesenteric arteries from the LPS‐treated animals. On the other hand, the pressor effect of bradykinin was notably dependent on the activity of ROCK. It is well known that angiotensin II‐induced vasoconstriction is associated with simultaneous activation of intracellular components that lead to both calcium mobilization, mainly mediated by Gq/11 and inositol trisphosphate, and calcium sensitization, by activation of the RhoA/ROCK pathway (Kimura et al., 1996). Taken together, these findings indicate that endotoxin‐induced inflammation shifts the B2 receptor‐dependent vascular effects of bradykinin to a more complex pathway, which also involves both AT1 receptors and ROCK, resulting in the subsequent increase of BP seen in the LPS‐treated animals, after bradykinin administration. Nevertheless, the development of further rearrangements in the downstream mechanisms associated with calcium store and mobilization, and the pressor responses to bradykinin after LPS challenge requires additional investigation.
Previous evidence suggested that augmented amounts of bradykinin receptors facilitate the heterodimerization between B2 and AT1 receptors (AbdAlla et al., 2005; AbdAlla et al., 2001; AbdAlla et al., 2000). Despite the lack of differences in aortas, Western blot and Co‐IP assays disclosed the presence of higher levels of both B2 and AT1 receptors and the augmented occurrence of the AT1/B2 receptor complex in mesenteric resistance arteries obtained 24 hr after LPS administration. The presence of AT1/B2 receptor heterodimers did correlate with the development of losartan‐sensitive contractile responses to bradykinin, as well as with the potentiation of angiotensin II‐induced contraction, which was prevented by Hoe‐140. Indeed, the enhancement of the effects mediated by angiotensin II after stimulation of cells expressing AT1/B2 receptor heterodimers was described in previous studies reporting the physical interaction between these receptors (AbdAlla et al., 2005; AbdAlla et al., 2001; AbdAlla et al., 2000). The results presented in our research extend the biological relevance of AT1/B2 receptor heterodimers, disclosing that in an environment subjected to bacterial LPS, the interaction between these two receptors can also shift the B2 receptor‐mediated vascular effects from the classic vasodilatory to a hypertensive action. What our results do not allow us to predict is the nature of cellular stimuli that are necessary to determine whether or not the AT1/B2 receptor heterodimers will respond in a divergent way to their natural ligands, comparing with monomeric units, and how synthetic drugs or physiological ligands can differentially modulate their intracellular effects.
The results obtained in this study did provide functional and biochemical evidence suggesting that endotoxin challenge increases the expression levels and heterodimerization of AT1 and B2 receptors in native tissues. The consequences for the cardiovascular system included the development of secondary pressor responses to bradykinin, which appears to be derived from a direct, endothelium‐independent, contractile effect in resistance vessels such as small mesenteric arteries. In addition, the AT1/B2 receptor heterodimerization also potentiated the hypertensive effect of angiotensin II (see the schematic representation proposed for these findings in Figure 10). Even though the results presented in this study are from female rats only, the main findings (i.e., the secondary pressor effect of bradykinin and bradykinin‐induced vasoconstriction) were fully reproducible in male rats using both in vivo and in vitro approaches, suggesting the lack of gender‐related differences in this phenomenon. The physiological purposes of this phenomenon in healthy or diseased vessels, including its effects on the balance between the kallikrein‐kinin and the renin‐angiotensin systems, deserve further investigation. Recognizing the growing evidence that AT1/B2 receptor heterodimers may have a profound influence on vascular functionality, mainly during inflammation‐associated cardiovascular conditions, can provide new insights for the development of pharmacological agents able to properly modulate their activity.
Figure 10.
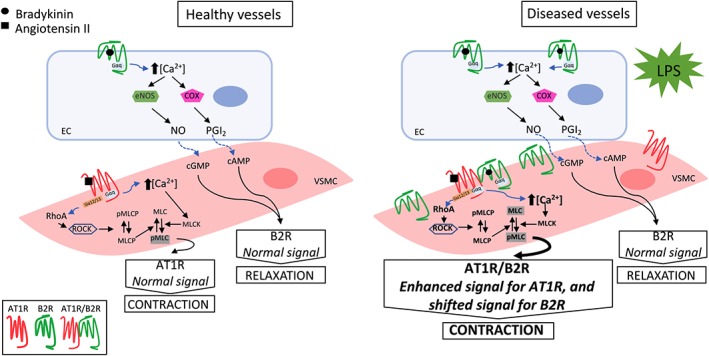
Schematic proposal of the mechanisms involved in the development of secondary pressor responses to bradykinin after endotoxin challenge. Increased levels of B2 receptors and occurrence of the AT1 / B2 receptor complexes (heterodimers) disclose a novel “biochemical fingerprint” in resistance arteries from endotoxemic rats (right), compared with healthy arteries obtained from non‐endotoxemic animals (left). The enlarged arrow indicates the potentiation of angiotensin II‐induced contraction. This hypothetical model of AT1 / B2 receptor heterodimerization is supported by in vivo and in vitro results obtained in this study, using both pharmacological and biochemical approaches. AT1R, angiotensin II AT1 receptor; B2R, bradykinin B2 receptor; EC, endothelial cell; eNOS, endothelial NOS; MLC, myosin light chain; MLCK, myosin light chain kinase; MLCP, myosin light chain phosphatase; PGI2, prostacyclin; pMLC, phosphorylated myosin light chain; pMLCP, phosphorylated myosin light chain phosphatase; RhoA, small G protein; ROCK, Rho‐associated kinase; VSMC, vascular smooth muscle cell
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
E.L.A. conducted the experiments, performed data analysis, and drafted the manuscript. J.A. was actively involved in the initial stages of the study and contributed in experimental design. D.F. and J.E.S.S. performed independent experiments which reproduced the findings showing the pressor effects of bradykinin in endotoxemic rats. J.E.S.S. conceived experiments and advised E.L.A. in experimental approaches and manuscript writing. All authors reviewed the final version of the manuscript.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis, Immunoblotting and Immunochemistry, and Animal Experimentation, and as recommended by funding agencies, publishers, and other organizations engaged with supporting research.
Supporting information
Table S1. Effects of endotoxemia on haematological and biochemistry parameters.
Table S2. Hypotensive effect of bradykinin and acetylcholine in animals pretreated with LPS (1 mg/kg, i.p.) or PBS (1 mL/kg i.p.) at 6, 24, 48 and 72 h before blood pressure evaluation.
Table S3. Area under curve of the pressor effect of bradykinin in animals pretreated with lipopolysaccharide (LPS, 1 mg kg‐1, i.p.) or phosphate buffered saline (PBS, 1 ml kg‐1, i.p.) at 6, 24, 48 and 72 h before blood pressure evaluation.
Table S4. Pressor effect of bradykinin in animals pretreated with LPS (1 mg kg‐1, i.p.) or PBS (1 mL kg‐1, i.p.) at 6, 24, 48 and 72 h before blood pressure evaluation.
Figure S1. Secondary pressor effect of bradykinin in endotoxemic male rats. The peak (A) and area under curve (B) of the secondary pressor effect of intravenously injected bradykinin were evaluated in anesthetized male rats 24 h after administration of lipopolysaccharide (LPS, 1 mg kg‐1, i.p.), phosphate buffered saline (1 mL kg‐1, i.p.; control group). The results are presented as dot plot or as the mean ± SEM of values obtained from 6 animals per group. Statistical analyses were performed using two‐way analysis of variance followed by Bonferroni's post‐test. * indicates P < 0.05 compared with the control group.
Figure S2. The effectiveness of the α‐adrenergic and AT 1 receptor antagonists prazosin and losartan. The increase in the systolic arterial pressure induced by the intravenous administration of phenylephrine (A) and angiotensin II (B) was fully inhibited by prazosin (0.5 mg kg‐1, i.v.) and losartan (15 mg kg‐1, i.v.) in both control (non‐endotoxemic) and LPS‐treated rats. These experiments were performed in anesthetized female rats 24 h after intraperitoneal administration of lipopolysaccharide (LPS, 1 mg kg‐1) or phosphate buffered saline (PBS, 1 mL kg‐1; control group). The results are presented as the mean ± SEM of values obtained from 6 animals per group. Statistical analyses were performed using twoway analysis of variance followed by Bonferroni's post‐test. * indicates P < 0.05 compared with the control group; # indicates P < 0.05 compared with the respective group before administration of prazosin or losartan.
Figure S3. Trace recording of a typical experiment showing the influence of losartan in the pressor effect of bradykinin in an endotoxemic rat. Intravenous (i.v.) bradykinin (6, 20 and 60 nmol kg‐1.) and angiotensin II (60 pmol kg‐1) were administered before and after the treatment with losartan (15 mg kg‐1, i.v.). The blood pressure was assessed in an anesthetized female rat 24 h after intraperitoneal administration of lipopolysaccharide (LPS, 1 mg kg‐1).
Figure S4. Inhibitory effect of sub‐effective doses of B2R and AT1R antagonists against the pressor effect of bradykinin in endotoxemic female rats. The treatment with losartan (5 mg kg‐1, i.v., A) or Hoe‐140 (1.35 mg kg‐1, s.c.; B) failed to reduce the peak of the secondary pressor effect generated by bradykinin in endotoxemic animals. The combined administration of sub‐effective doses of losartan (5 mg kg‐1, i.v.) and Hoe‐140 (1.35 mg kg‐1, s.c.) avoided the pressor effect induced by bradykinin in LPS‐treated animals (C). These experiments were performed in anesthetized female rats 24 h after intraperitoneal administration of lipopolysaccharide (LPS, 1 mg kg‐1) or phosphate buffered saline (PBS, 1 mL kg‐1, control group). The results are presented as the mean ± SEM of values obtained from 6 animals per group. Statistical analyses were performed using two‐way ANOVA followed by Bonferroni's post‐test (A, B, and C). * indicates P < 0.05 compared with the control group; # indicates P < 0.05 compared with the respective group before administration of Losartan + Hoe‐140.
Figure S5. Involvement of the Rho‐A/Rho‐kinase (ROCK) pathway in the pressor effect of bradykinin in female rats subjected to endotoxemia. Lack of influence of indomethacin (A) and ability of Y‐27632 (B) to reduce the area under curve of the pressor effect of bradykinin in endotoxemic rats. These experiments were performed in anesthetized female rats 24 h after intraperitoneal administration of lipopolysaccharide (LPS, 1 mg kg‐1) or phosphate buffered saline (PBS, 1 mL kg‐1; control group). The results are presented as the mean ± SEM of values obtained from 6 animals per group. Statistical analyses were performed using two‐way analysis of variance followed by Bonferroni's post‐test. * indicates P < 0.05 compared with the control group; # indicates P < 0.05 compared with the respective group before administration of Y‐27632.
Figure S6. Functional evidence of endothelium‐independent interaction between B2 and AT1 receptors in small mesenteric arteries from female rats subjected to endotoxemia. Losartan‐sensitive contractile effects of bradykinin (A and B) and Hoe‐140‐sensitive enhancement of angiotensin II‐induced vasoconstriction (C and D) in endothelium‐denuded small mesenteric arteries from female rats treated with lipopolysaccharide (LPS, 1 mg kg1; i.p.) 24 h before the experiment. Control (non‐endotoxemic) animals received phosphate buffered saline (PBS, 1 mL kg1; i.p.). The results are presented as the mean ± SEM of values obtained from 6 animals per group. Statistical analyses were performed using one‐way analysis of variance followed by Bonferroni's post‐test. * indicates P < 0.05 compared with the control group; # indicates P < 0.05 compared with the respective group before administration of losartan or Hoe‐140.
Figure S7. Functional evidence of interaction between B2 and AT1 receptors in resistance arteries from male rats subjected to endotoxemia. Losartan‐sensitive contractile effects of bradykinin (A and B) and Hoe‐140‐sensitive enhancement of angiotensin II‐induced vasoconstriction (C and D) in small mesenteric arteries from male rats treated with lipopolysaccharide (LPS, 1 mg kg1; i.p.) 24 h before the experiment. Control (non‐endotoxemic) animals received phosphate buffered saline (PBS, 1 mL kg1; i.p.). The results are presented as the mean ± SEM of values obtained from 6 animals per group, excepted for those experiments using Hoe‐140 (n = 4 per group, not subjected for statistical analyses). Statistical analyses were performed using one‐way analysis of variance followed by Bonferroni's post‐test. * indicates P < 0.05 compared with the control group; # indicates P < 0.05 compared with the respective group before administration of losartan.
Figure S8. Lack of influence of cyclooxygenase inhibitors on the effects of bradykinin in resistance arteries of endotoxemic rats. Inability of indomethacin (non‐selective cyclooxygenase inhibitor, 10 μmol L‐1) and NS‐398 (selective cyclooxygenase‐2 inhibitor, 10 μmol L‐1) to prevent peak (A and C) and the area under curve (B and D) of the contractile effect of bradykinin (10 μmol L‐1) in small mesenteric arteries from female rats treated with lipopolysaccharide (LPS, 1 mg kg1; i.p.) 24 h before the experiment. The results are presented as the mean ± SEM of values obtained from 6 animals per group. Statistical analyses were performed using one‐way analysis of variance followed by Bonferroni's post‐test. * indicates P < 0.05 compared with the control group (C); # indicates P < 0.05 compared with the respective group before administration of NS‐398.
ACKNOWLEDGEMENTS
The technical assistance of Adriane Madeira is gratefully acknowledged. The authors thank the staff of Multiuser Laboratory of the Center of Biological Sciences (LAMEB/CCB) of Universidade Federal de Santa Catarina for their technical support. This research was supported in part by grants from Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC, Brazil; TR2012000367), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES, 001), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil; 448738/2014; and a PhD fellowship to Elaine L. Anton).
Anton EL, Fernandes D, Assreuy J, da Silva‐Santos JE. Bradykinin increases BP in endotoxemic rats: functional and biochemical evidence of angiotensin II AT1/bradykinin B2 receptor heterodimerization. Br J Pharmacol. 2019;176:2608–2626. 10.1111/bph.14685
REFERENCES
- AbdAlla, S. , Abdel‐Baset, A. , Lother, H. , el Massiery, A. , & Quitterer, U. (2005). Mesangial AT1/B2 receptor heterodimers contribute to angiotensin II hyperresponsiveness in experimental hypertension. Journal of Molecular Neuroscience, 26, 209–217. [DOI] [PubMed] [Google Scholar]
- AbdAlla, S. , Lother, H. , el Massiery, A. , & Quitterer, U. (2001). Increased AT1receptor heterodimers in preeclampsia mediate enhanced angiotensin II responsiveness. Nature Medicine, 7, 1003–1009. 10.1038/nm0901-1003 [DOI] [PubMed] [Google Scholar]
- AbdAlla, S. , Lother, H. , & Quitterer, U. (2000). AT1‐receptor heterodimers show enhanced G‐protein activation and altered receptor sequestration. Nature, 407, 94–98. 10.1038/35024095 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174, S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174, S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt‐Due, A. , Johansen, H. T. , Sokolov, A. , Thorgersen, E. B. , Hellerud, B. C. , Reubsaet, J. L. , … Nielsen, E. W. (2011). The role of bradykinin and the effect of the bradykinin receptor antagonist icatibant in porcine sepsis. Shock, 36, 517–523. 10.1097/SHK.0b013e3182336a34 [DOI] [PubMed] [Google Scholar]
- Basei, F. L. , Cabrini, D. A. , Figueiredo, C. P. , Forner, S. , Hara, D. B. , Nascimento, A. F. , … Calixto, J. B. (2012). Endothelium dependent expression and underlying mechanisms of des‐Arg 9‐bradykinin‐induced B1R‐mediated vasoconstriction in rat portal vein. Peptides, 37, 216–224. 10.1016/j.peptides.2012.07.020 [DOI] [PubMed] [Google Scholar]
- Burch, R. M. , & Axelrod, J. (1987). Dissociation of bradykinin‐induced prostaglandin formation from phosphatidylinositol turnover in Swiss 3T3 fibroblasts: Evidence for G protein regulation of phospholipase A2. Proceedings of the National Academy of Sciences of the United States of America, 84, 6374–6378. 10.1073/pnas.84.18.6374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos, A. H. , & Calixto, J. B. (1994). Mechanisms involved in the contractile responses of kinins in rat portal vein rings: Mediation by B1 and B2 receptors. The Journal of Pharmacology and Experimental Therapeutics, 268, 902–909. [PubMed] [Google Scholar]
- Campos, M. M. , Souza, G. E. P. , & Calixto, J. B. (1996). Upregulation of B1 receptor mediating des‐Arg9‐BK‐induced rat paw oedema by systemic treatment with bacterial endotoxin. British Journal of Pharmacology, 117, 793–798. 10.1111/j.1476-5381.1996.tb15262.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayla, C. , Todiras, M. , Iliescu, R. , Saul, V. V. , Gross, V. , Pilz, B. , … Bader, M. (2007). Mice deficient for both kinin receptors are normotensive and protected from endotoxin‐induced hypotension. The FASEB Journal, 21, 1689–1698. 10.1096/fj.06-7175com [DOI] [PubMed] [Google Scholar]
- Charignon, D. , Späth, P. , Martin, L. , & Drouet, C. (2012). Icatibant, the bradykinin B2 receptor antagonist with target to the interconnected kinin systems. Expert Opinion on Pharmacotherapy, 13, 2233–2247. 10.1517/14656566.2012.723692 [DOI] [PubMed] [Google Scholar]
- Croxatto, H. , & Belmar, J. (1961). Hypertensive effects of bradykinin in rats. Nature, 192, 879–880. 10.1038/192879a0 [DOI] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva‐Santos, J. E. , & Assreuy, J. (1999). Long‐lasting changes of rat blood pressure to vasoconstrictors and vasodilators induced by nitric oxide donor infusion: Involvement of potassium channels. The Journal of Pharmacology and Experimental Therapeutics, 290, 380–387. [PubMed] [Google Scholar]
- dos Santos Gomes, F.O. , Oliveira, A.C. , Ribeiro, E.L. , da Silva, B.S. , dos Santos, L.A.M. , de Lima, I.T. , … Peixoto, C.A. (2018). Intraurethral injection with LPS: An effective experimental model of prostatic inflammation. Inflammation Research, 67, 43–55, 10.1007/s00011-017-1094-7 [DOI] [PubMed] [Google Scholar]
- Fasciolo, J. C. , Vargas, L. , Lama, M. C. , & Nolly, H. (1990). Bradykinin‐induced vasoconstriction of rat mesenteric arteries precontracted with noradrenaline. British Journal of Pharmacology, 101, 344–348. 10.1111/j.1476-5381.1990.tb12712.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein, A. M. , Bernard, G. R. , Criner, G. J. , Fletcher, E. C. , Good, J. T. , Knaus, W. A. , … Rodell, T. C. (1997). Treatment of severe systemic inflammatory response syndrome and sepsis with a novel bradykinin antagonist, deltibant (CP‐0127). Results of a randomized, double‐blind, placebo‐controlled trial. JAMA, 277, 482–487. 10.1001/jama.1997.03540300050033 [DOI] [PubMed] [Google Scholar]
- Garrett, R. L. , & Brown, J. H. (1972). Bradykinin interaction with 5‐hydroxytryptamine, norepinephrine, and potassium chloride in rabbit aorta. Proceedings of the Society for Experimental Biology and Medicine, 139, 1344–1348. 10.3181/00379727-139-36359 [DOI] [PubMed] [Google Scholar]
- Gonçalves, R. P. , Guarido, K. L. , Assreuy, J. , & da Silva‐Santos, J. E. (2014). Gender‐specific differences in the in situ cardiac function of endotoxemic rats detected by pressure‐volume catheter. Shock, 42, 415–423. 10.1097/SHK.0000000000000226 [DOI] [PubMed] [Google Scholar]
- Guarido, K. L. , Gonçalves, R. P. , Júnior, A. G. , & da Silva‐Santos, J. E. (2014). Increased activation of the Rho‐A/Rho‐kinase pathway in the renal vascular system is responsible for the enhanced reactivity to exogenous vasopressin in endotoxemic rats. Critical Care Medicine, 42, e461–e471. 10.1097/CCM.0000000000000313 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam, Z. , Kawaguchi, H. , Miura, N. , Miyoshi, N. , Yamazaki‐Himeno, E. , Shiraishi, M. , … Tanimoto, A. (2017). Hypertension alters the endothelial‐dependent biphasic response of bradykinin in isolated Microminipig basilar artery. Microvascular Research, 114, 52–57. 10.1016/j.mvr.2017.06.001 [DOI] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. J. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Improving bioscience research reporting: The arrive guidelines for reporting animal research. PLoS Biology, 8, e1000412 10.1371/journal.pbio.1000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, K. , Ito, M. , Amano, M. , Chihara, K. , Fukata, Y. , Nakafuku, M. , … Kaibuchi, K. (1996). Regulation of myosin phosphatase by Rho and Rho‐associated kinase (Rho‐kinase). Science, 273, 245–248. 10.1126/science.273.5272.245 [DOI] [PubMed] [Google Scholar]
- Lambert, T. L. , Kent, R. S. , & Whorton, A. R. (1986). Bradykinin stimulation of inositol polyphosphate production in porcine aortic endothelial cells. The Journal of Biological Chemistry, 261, 15288–15293. [PubMed] [Google Scholar]
- Lang, W. J. , & Pearson, L. (1968). Studies on the pressor responses produced by bradykinin and kallidin. British Journal of Pharmacology and Chemotherapy, 32, 330–338. 10.1111/j.1476-5381.1968.tb00976.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeb‐Lundberg, L. M. , Marceau, F. , Muller‐Esterl, W. , Pettibone, D. J. , & Zuraw, B. L. (2005). International union of pharmacology. XLV. Classification of the kinin receptor family: From molecular mechanisms to pathophysiological consequences. Pharmacological Reviews, 57, 27–77. 10.1124/pr.57.1.2 [DOI] [PubMed] [Google Scholar]
- Marceau, F. , & Regoli, D. (2004). Bradykinin receptor ligands: Therapeutic perspectives. Nature Reviews. Drug Discovery, 3, 845–852. 10.1038/nrd1522 [DOI] [PubMed] [Google Scholar]
- Maurer, M. , Bader, M. , Bas, M. , Bossi, F. , Cicardi, M. , Cugno, M. , … Magerl, M. (2011). New topics in bradykinin research. Allergy, 66, 1397–1406. 10.1111/j.1398-9995.2011.02686.x [DOI] [PubMed] [Google Scholar]
- McLean, P. G. , Perretti, M. , & Ahluwalia, A. (2000). Kinin B1 receptors and the cardiovascular system: Regulation of expression and function. Cardiovascular Research, 48, 194–210. 10.1016/S0008-6363(00)00184-X [DOI] [PubMed] [Google Scholar]
- Miyamoto, A. , Ishiguro, S. , & Nishio, A. (1999). Stimulation of bradykinin B2‐receptors on endothelial cells induces relaxation and contraction in porcine basilar artery in vitro. British Journal of Pharmacology, 128, 241–247. 10.1038/sj.bjp.0702783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombouli, J. V. , & Vanhoutte, P. M. (1995). Kinins and endothelial control of vascular smooth muscle. Annual Review of Pharmacology and Toxicology, 35, 679–705. 10.1146/annurev.pa.35.040195.003335 [DOI] [PubMed] [Google Scholar]
- More, A. S. , Kim, H. M. , Khang, G. , Hildebrandt, T. , Bernlöhr, C. , Doods, H. , … Wu, D. (2014). Des‐Arg9‐bradykinin causes kinin B1 receptor mediated endothelium‐independent contractions in endotoxin‐treated porcine coronary arteries. Pharmacological Research, 90, 18–24. 10.1016/j.phrs.2014.09.001 [DOI] [PubMed] [Google Scholar]
- More, A. S. , Kim, H. M. , Zhao, R. , Khang, G. , Hildebrandt, T. , Bernlöhr, C. , … Wu, D. (2014). COX‐2 mediated induction of endothelium‐independent contraction to bradykinin in endotoxin‐treated porcine coronary artery. Journal of Cardiovascular Pharmacology, 64, 209–217. 10.1097/FJC.0000000000000105 [DOI] [PubMed] [Google Scholar]
- Palmer, R. M. , Ferrige, A. G. , & Moncada, S. (1987). Nitric oxide release accounts for the biological activity of endothelium‐derived relaxing factor. Nature, 327, 524–526. 10.1038/327524a0 [DOI] [PubMed] [Google Scholar]
- Pearson, L. , & Lang, W. J. (1967). A comparison in conscious and anaesthetized dogs of the effect on blood pressure of bradykinin, kallidin, eledoisin and kallikrein. European Journal of Pharmacology, 2, 83–87. 10.1016/0014-2999(67)90029-5 [DOI] [PubMed] [Google Scholar]
- Peng, X. , Hassoun, P. M. , Sammani, S. , McVerry, B. J. , Burne, M. J. , Rabb, H. , … Garcia, J. G. (2004). Protective effects of sphingosine 1‐phosphate in murine endotoxin‐induced inflammatory lung injury. American Journal of Respiratory and Critical Care Medicine, 169, 1245–1251. 10.1164/rccm.200309-1258OC [DOI] [PubMed] [Google Scholar]
- Qin, L. , Wu, X. , Block, M. L. , Liu, Y. , Breese, G. R. , Hong, J. S. , … Crews, F. (2007). Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia, 55, 1416–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regoli, D. , & Barabé, J. (1980). Pharmacology of bradykinin and related kinins. Pharmacological Reviews, 32, 1–46. [PubMed] [Google Scholar]
- Regoli, D. , Barabé, J. , & Park, W. K. (1977). Receptors for bradykinin in rabbit aortae. Canadian Journal of Physiology and Pharmacology, 55, 855–867. 10.1139/y77-115 [DOI] [PubMed] [Google Scholar]
- Regoli, D. , Marceau, F. , & Lavigne, J. (1981). Induction of B1‐receptors for kinins in the rabbit by a bacterial lipopolysaccharide. European Journal of Pharmacology, 71, 105–115. 10.1016/0014-2999(81)90391-5 [DOI] [PubMed] [Google Scholar]
- Regoli, D. , Plante, G. E. , & Gobeil, F. (2012). Impact of kinins in the treatment of cardiovascular diseases. Pharmacology & Therapeutics, 135, 94–111. 10.1016/j.pharmthera.2012.04.002 [DOI] [PubMed] [Google Scholar]
- Ridings, P. C. , Sugerman, H. J. , Blocher, C. R. , Fisher, B. J. , & Fowler, A. A. (1995). Hemodynamic effects of bradykinin antagonism in porcine gram‐negative sepsis. Journal of Investigative Surgery, 8, 115–122. 10.3109/08941939509016514 [DOI] [PubMed] [Google Scholar]
- Rocha e Silva, M. , Beraldo, W. T. , & Rosenfeld, G. (1949). Bradykinin, a hypotensive and smooth muscle stimulating factor released from plasma globulin by snake venoms and by trypsin. The American Journal of Physiology, 156, 261–273. 10.1152/ajplegacy.1949.156.2.261 [DOI] [PubMed] [Google Scholar]
- Saameli, K. , & Eskes, T. K. (1962). Bradykinin and cardiovascular system: Estimation of half‐life. The American Journal of Physiology, 203, 261–265. 10.1152/ajplegacy.1962.203.2.261 [DOI] [PubMed] [Google Scholar]
- Sai, Y. , Okamura, T. , Amakata, Y. , & Toda, N. (1995). Comparison of responses of canine pulmonary artery and vein to angiotensin II, bradykinin and vasopressin. European Journal of Pharmacology, 282, 235–241. 10.1016/0014-2999(95)00343-J [DOI] [PubMed] [Google Scholar]
- Sardi, S. P. , Pérez, H. , Antúnez, P. , & Rothlin, R. P. (1997). Bradykinin B1 receptors in human umbilical vein. European Journal of Pharmacology, 321, 33–38. 10.1016/S0014-2999(96)00927-2 [DOI] [PubMed] [Google Scholar]
- Schremmer‐Danninger, E. , Offner, A. , Siebeck, M. , Heinz‐Erian, P. , Gais, P. , & Roscher, A. (1996). Autoradiographic visualization of B1 bradykinin receptors in porcine vascular tissues in the presence or absence of inflammation. Immunopharmacoly, 33, 95–100. 10.1016/0162-3109(96)00019-7 [DOI] [PubMed] [Google Scholar]
- Starr, M. S. , & West, G. B. (1966). The effect of bradykinin and anti‐inflammatory agents on isolated arteries. The Journal of Pharmacy and Pharmacology, 18, 838–840. 10.1111/j.2042-7158.1966.tb07827.x [DOI] [PubMed] [Google Scholar]
- Wilson, D. D. , de Garavilla, L. , Kuhn, W. , Togo, J. , Burch, R. M. , & Steranka, L. R. (1989). D‐Arg‐[Hyp3‐D‐Phe7]‐bradykinin, a bradykinin antagonist, reduces mortality in a rat model of endotoxic shock. Circulatory Shock, 27, 93–101. [PubMed] [Google Scholar]
- Zhang, J. , Yang, G. M. , Zhu, Y. , Peng, X. Y. , Liu, L. M. , & Li, T. (2015). Bradykinin induces vascular contraction after hemorrhagic shock in rats. The Journal of Surgical Research, 193, 334–343. 10.1016/j.jss.2014.06.033 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Effects of endotoxemia on haematological and biochemistry parameters.
Table S2. Hypotensive effect of bradykinin and acetylcholine in animals pretreated with LPS (1 mg/kg, i.p.) or PBS (1 mL/kg i.p.) at 6, 24, 48 and 72 h before blood pressure evaluation.
Table S3. Area under curve of the pressor effect of bradykinin in animals pretreated with lipopolysaccharide (LPS, 1 mg kg‐1, i.p.) or phosphate buffered saline (PBS, 1 ml kg‐1, i.p.) at 6, 24, 48 and 72 h before blood pressure evaluation.
Table S4. Pressor effect of bradykinin in animals pretreated with LPS (1 mg kg‐1, i.p.) or PBS (1 mL kg‐1, i.p.) at 6, 24, 48 and 72 h before blood pressure evaluation.
Figure S1. Secondary pressor effect of bradykinin in endotoxemic male rats. The peak (A) and area under curve (B) of the secondary pressor effect of intravenously injected bradykinin were evaluated in anesthetized male rats 24 h after administration of lipopolysaccharide (LPS, 1 mg kg‐1, i.p.), phosphate buffered saline (1 mL kg‐1, i.p.; control group). The results are presented as dot plot or as the mean ± SEM of values obtained from 6 animals per group. Statistical analyses were performed using two‐way analysis of variance followed by Bonferroni's post‐test. * indicates P < 0.05 compared with the control group.
Figure S2. The effectiveness of the α‐adrenergic and AT 1 receptor antagonists prazosin and losartan. The increase in the systolic arterial pressure induced by the intravenous administration of phenylephrine (A) and angiotensin II (B) was fully inhibited by prazosin (0.5 mg kg‐1, i.v.) and losartan (15 mg kg‐1, i.v.) in both control (non‐endotoxemic) and LPS‐treated rats. These experiments were performed in anesthetized female rats 24 h after intraperitoneal administration of lipopolysaccharide (LPS, 1 mg kg‐1) or phosphate buffered saline (PBS, 1 mL kg‐1; control group). The results are presented as the mean ± SEM of values obtained from 6 animals per group. Statistical analyses were performed using twoway analysis of variance followed by Bonferroni's post‐test. * indicates P < 0.05 compared with the control group; # indicates P < 0.05 compared with the respective group before administration of prazosin or losartan.
Figure S3. Trace recording of a typical experiment showing the influence of losartan in the pressor effect of bradykinin in an endotoxemic rat. Intravenous (i.v.) bradykinin (6, 20 and 60 nmol kg‐1.) and angiotensin II (60 pmol kg‐1) were administered before and after the treatment with losartan (15 mg kg‐1, i.v.). The blood pressure was assessed in an anesthetized female rat 24 h after intraperitoneal administration of lipopolysaccharide (LPS, 1 mg kg‐1).
Figure S4. Inhibitory effect of sub‐effective doses of B2R and AT1R antagonists against the pressor effect of bradykinin in endotoxemic female rats. The treatment with losartan (5 mg kg‐1, i.v., A) or Hoe‐140 (1.35 mg kg‐1, s.c.; B) failed to reduce the peak of the secondary pressor effect generated by bradykinin in endotoxemic animals. The combined administration of sub‐effective doses of losartan (5 mg kg‐1, i.v.) and Hoe‐140 (1.35 mg kg‐1, s.c.) avoided the pressor effect induced by bradykinin in LPS‐treated animals (C). These experiments were performed in anesthetized female rats 24 h after intraperitoneal administration of lipopolysaccharide (LPS, 1 mg kg‐1) or phosphate buffered saline (PBS, 1 mL kg‐1, control group). The results are presented as the mean ± SEM of values obtained from 6 animals per group. Statistical analyses were performed using two‐way ANOVA followed by Bonferroni's post‐test (A, B, and C). * indicates P < 0.05 compared with the control group; # indicates P < 0.05 compared with the respective group before administration of Losartan + Hoe‐140.
Figure S5. Involvement of the Rho‐A/Rho‐kinase (ROCK) pathway in the pressor effect of bradykinin in female rats subjected to endotoxemia. Lack of influence of indomethacin (A) and ability of Y‐27632 (B) to reduce the area under curve of the pressor effect of bradykinin in endotoxemic rats. These experiments were performed in anesthetized female rats 24 h after intraperitoneal administration of lipopolysaccharide (LPS, 1 mg kg‐1) or phosphate buffered saline (PBS, 1 mL kg‐1; control group). The results are presented as the mean ± SEM of values obtained from 6 animals per group. Statistical analyses were performed using two‐way analysis of variance followed by Bonferroni's post‐test. * indicates P < 0.05 compared with the control group; # indicates P < 0.05 compared with the respective group before administration of Y‐27632.
Figure S6. Functional evidence of endothelium‐independent interaction between B2 and AT1 receptors in small mesenteric arteries from female rats subjected to endotoxemia. Losartan‐sensitive contractile effects of bradykinin (A and B) and Hoe‐140‐sensitive enhancement of angiotensin II‐induced vasoconstriction (C and D) in endothelium‐denuded small mesenteric arteries from female rats treated with lipopolysaccharide (LPS, 1 mg kg1; i.p.) 24 h before the experiment. Control (non‐endotoxemic) animals received phosphate buffered saline (PBS, 1 mL kg1; i.p.). The results are presented as the mean ± SEM of values obtained from 6 animals per group. Statistical analyses were performed using one‐way analysis of variance followed by Bonferroni's post‐test. * indicates P < 0.05 compared with the control group; # indicates P < 0.05 compared with the respective group before administration of losartan or Hoe‐140.
Figure S7. Functional evidence of interaction between B2 and AT1 receptors in resistance arteries from male rats subjected to endotoxemia. Losartan‐sensitive contractile effects of bradykinin (A and B) and Hoe‐140‐sensitive enhancement of angiotensin II‐induced vasoconstriction (C and D) in small mesenteric arteries from male rats treated with lipopolysaccharide (LPS, 1 mg kg1; i.p.) 24 h before the experiment. Control (non‐endotoxemic) animals received phosphate buffered saline (PBS, 1 mL kg1; i.p.). The results are presented as the mean ± SEM of values obtained from 6 animals per group, excepted for those experiments using Hoe‐140 (n = 4 per group, not subjected for statistical analyses). Statistical analyses were performed using one‐way analysis of variance followed by Bonferroni's post‐test. * indicates P < 0.05 compared with the control group; # indicates P < 0.05 compared with the respective group before administration of losartan.
Figure S8. Lack of influence of cyclooxygenase inhibitors on the effects of bradykinin in resistance arteries of endotoxemic rats. Inability of indomethacin (non‐selective cyclooxygenase inhibitor, 10 μmol L‐1) and NS‐398 (selective cyclooxygenase‐2 inhibitor, 10 μmol L‐1) to prevent peak (A and C) and the area under curve (B and D) of the contractile effect of bradykinin (10 μmol L‐1) in small mesenteric arteries from female rats treated with lipopolysaccharide (LPS, 1 mg kg1; i.p.) 24 h before the experiment. The results are presented as the mean ± SEM of values obtained from 6 animals per group. Statistical analyses were performed using one‐way analysis of variance followed by Bonferroni's post‐test. * indicates P < 0.05 compared with the control group (C); # indicates P < 0.05 compared with the respective group before administration of NS‐398.


