Abstract
A vital role of adrenoceptors in metabolism and energy balance has been well documented in the heart, skeletal muscle, and adipose tissue. It has been only recently demonstrated, however, that activation of the mechanistic target of rapamycin (mTOR) makes a significant contribution to various metabolic and physiological responses to adrenoceptor agonists. mTOR exists as two distinct complexes named mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) and has been shown to play a critical role in protein synthesis, cell proliferation, hypertrophy, mitochondrial function, and glucose uptake. This review will describe the physiological significance of mTORC1 and 2 as a novel paradigm of adrenoceptor signalling in the heart, skeletal muscle, and adipose tissue. Understanding the detailed signalling cascades of adrenoceptors and how they regulate physiological responses is important for identifying new therapeutic targets and identifying novel therapeutic interventions.
Linked Articles
This article is part of a themed section on Adrenoceptors—New Roles for Old Players. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.14/issuetoc
Abbreviations
- 4E‐BP1
4E binding protein‐1
- AMPK
5′AMP‐activated protein kinase
- AS160
Akt substrate of 160 kDa
- BAT
brown adipose tissue
- DEPTOR
DEP domain‐containing mTOR‐interacting protein
- eIF4E
eukaryotic translation initiation factor 4E
- ERRα
oestrogen‐related receptor α
- GLUT4
glucose transporter 4
- GRK
G protein receptor kinases
- hMADS
human multipotent adipose‐derived stem
- LARP1
La‐related protein 1
- MLST8
mammalian lethal with SEC13 protein 8
- Mst1
macrophage stimulating 1
- mTOR
mechanistic target of rapamycin
- mTORC1
mTOR complex 1
- mTORC2
mTOR complex 2
- NRVM
neonatal rat ventricular myocytes
- PDK1
phosphoinositide‐dependent kinase 1
- PIP3
phosphatidylinositol 3,4,5‐trisphosphate
- Raptor
regulatory‐associated protein of mTOR
- REPTOR
repressed by TOR
- Rictor
rapamycin‐insensitive companion of mTOR
- S6K1/2
ribosomal protein S6 kinase 1 and 2
- SGK1
serum and glucocorticoid‐responsive kinase‐1
- Tel2
telomere maintenance 2
- TSC1/2
tuberous sclerosis complex
- UCP1
uncoupling protein 1
- WAT
white adipose tissue
1. INTRODUCTION
Adrenoceptors belong to the GPCR family, a conserved family of seven transmembrane receptors that is one of the largest protein classes to be targeted for drug therapy (Sriram & Insel, 2018). These receptors are classified as α‐ or β‐adrenoceptors, based on differences in responses to various catecholamines such as adrenaline, noradrenaline and isoprenaline. The α‐adrenoceptors have been classified into two major families: α1 and α2 and the β‐adrenoceptors are subdivided into β1‐, β2‐, and β3‐subtypes . All adrenoceptor subtypes have common primary structures comprising one extracellular N‐terminal domain, seven α‐helical transmembrane spanning regions, and one intracellular C‐terminal tail. Recent studies have shown that α1‐ and β1‐adrenoceptors in the heart, β2‐adrenoceptors in skeletal muscle, and β3‐ adrenoceptors in brown adipose tissue (BAT) can link to a protein called mechanistic target of rapamycin (mTOR), which plays a significant role in physiological and metabolic responses.
mTOR is an atypical serine/threonine kinase with a molecular weight of ~289 kDa, belonging to the PI3K‐related kinase family. mTOR interacts with other molecular components to form two physically and functionally distinct complexes, namely, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). In mTORC1, the mTOR protein interacts with regulatory‐associated protein of mTOR (Raptor; Kim et al., 2002), proline‐rich PKB (Akt) substrate of 40 kDa (PRAS40; Sancak et al., 2007), mammalian lethal with SEC13 protein 8 (MLST8; Kim et al., 2003), DEP domain‐containing mTOR‐interacting protein (DEPTOR; Peterson et al., 2009), Tel two interacting protein 1 (Tti1), and telomere maintenance 2 (Tel2; Kaizuka et al., 2010). On the other hand, mTORC2 comprises mTOR; the scaffold protein rapamycin‐insensitive companion of mTOR (Rictor; Sarbassov et al., 2004); mammalian stress‐activated protein kinase interacting protein 1 (mSIN1, Jacinto et al., 2006); protein observed with Rictor 1 and 2 (Protor1/2, Pearce et al., 2007); MLST8 (Kim et al., 2003); DEPTOR (Peterson et al., 2009); inhibitor of nuclear factor κ‐B kinase (IKK, Xu et al., 2013); Sestrin 3 (Tao, Xiong, Liangpunsakul, & Dong, 2015); exchange factor found in platelets, leukemic, and neuronal tissues (Xpln, Khanna, Fang, Yoon, & Chen, 2013); tuberous sclerosis complex 2 (TSC2; Huang, Dibble, Matsuzaki, & Manning, 2008); and Tel2 and Tti1 (Kaizuka et al., 2010).
While remarkable progress has been made in understanding the role of mTORC1, the contributions of mTORC2 are less well understood. Collectively, many studies have demonstrated that mTORC1 plays a vital role in the regulation of cellular homeostasis, growth and response to stress. mTORC1 activated under nutrient‐replete conditions promotes protein synthesis by several complementary mechanisms. First, mTORC1 activates the ribosomal protein S6 kinase 1 and ribosomal protein S6 kinase 2 (S6K1/2), which in turn activates the protein translation process (Laplante & Sabatini, 2013; Saxton & Sabatini, 2017). In parallel, mTORC1 inhibits eukaryotic translation initiation factor 4E (eIF4E)‐binding protein‐1 (4E‐BP1) and thus allows the formation of the eIF4F complex that triggers cap‐dependent translation (Kennedy & Lamming, 2016; Laplante & Sabatini, 2013; Saxton & Sabatini, 2017). Finally, mTORC1 boosts translation by phosphorylation and consequent inactivation of the target La‐related protein 1 (LARP1; Fonseca et al., 2015). When active, LARP1 represses translation of terminal oligopyrimidine mRNAs that encode ribosomal proteins and positive regulators of translation.
mTORC1 modulates cell metabolism, as it increases glycolysis by promoting transcription and translation of hypoxia‐induced factor 1α (TNFα, Hudson et al., 2002). It also activates the transcription factor sterol regulatory element‐binding proteins 1 and 2 (SREBP1/2), which promote lipogenesis (Kennedy & Lamming, 2016; Laplante & Sabatini, 2013; Saxton & Sabatini, 2017). Further, mTORC1 plays a role in mitochondrial biogenesis through PPAR‐γ‐mediated activation of the transcription factor Ying‐Yang 1 (Cunningham et al., 2007; Laplante & Sabatini, 2012). When cells are subjected to stress or nutrient starvation, they undergo a regulated catabolic process termed autophagy. Another well‐characterized role of mTORC1 is the inhibition of autophagy under nutrient replete conditions. mTORC1 phosphorylates Unc‐51 like autophagy activating kinase (ULK1), preventing its activation via 5′AMP‐activated protein kinase (AMPK), which in turn inhibits autophagy (Kim, Kundu, Viollet, & Guan, 2011). The phosphorylation and nuclear translocation of the transcription factor EB (TFEB), which regulates the expression of proteins governing autophagy and lysosomal biogenesis, is inhibited by mTORC1 (Settembre et al., 2012). Recent studies have demonstrated that mTORC1 also contributes to protein turnover via the ubiquitin–proteasome system. Acute inhibition of mTORC1 increases proteasome‐dependent proteolysis (Rousseau & Bertolotti, 2016; Zhao et al., 2015a). Interestingly, long‐term activation of mTORC1 in mouse embryonic fibroblasts due to deletion of inhibitory Tsc2 also increases proteasome activity (Zhang et al., 2014). This finding was replicated in a mouse model of neuronal Tsc2 deletion and in the liver of a wild‐type mice subject to fasting then 6 hr refeeding. The authors suggest that longer term activation of proteasomal pathways by mTORC1 is an adaptive response that supports protein synthesis by replenishing the cellular amino acid pool (Zhang et al., 2014). Two further mTORC1 targets have been identified: (a) The γ isoform of phosphatidylinositol‐5‐phosphate 4‐kinase (PIP4K2C) maintains basal mTORC1 signalling during starvation (Mackey, Sarkes, Bettencourt, Asara, & Rameh, 2014); and (b) repressed by TOR (REPTOR) is a downstream effector of TORC1 in Drosophila melanogaster (Tiebe et al., 2015). When TORC1 phosphorylates REPTOR, it leads to cytoplasmic retention; in contrast, upon inhibition of TORC1, REPTOR is dephosphorylated, translocates into the nucleus, and activates transcription of target genes involved in energy homeostasis and cellular survival under conditions of nutrient starvation (Tiebe et al., 2015).
Compared to mTORC1, studies and knowledge of mTORC2 regulation and function have lagged behind. One well‐characterized role of mTORC2 is its response to growth factors and insulin via PI3K‐dependent mechanisms (Gan, Wang, Su, & Wu, 2011). mTORC2 directly phosphorylates Akt at Ser473, which is facilitated by prior phosphorylation of Thr308 by phosphoinositide‐dependent kinase 1 (PDK1), as part of the insulin cascade (Sarbassov, Guertin, Ali, & Sabatini, 2005). mTORC2 can modulate PKCα activity and thereby play a role in remodelling of the actin cytoskeleton (Sarbassov et al., 2004). Similarly, a study by Jacinto et al. (2004) demonstrated that mTORC2 regulates cell polarity and cytoskeletal organization through the regulation of PKCα and Ras homolog gene family member A. mTORC2 has also been demonstrated to regulate other PKC family members, including PKCδ (Gan et al., 2012) and PKCζ (Li & Gao, 2014). Hydrophobic motif phosphorylation and activation of PKCδ plays a vital role in fibroblast migration and pulmonary fibrosis development (Gan et al., 2012) whereas mTORC2 modulation of PKCζ activity is involved in organization of the actin cytoskeleton (Li & Gao, 2014). Sciarretta et al. (2015) conducted a study showing that mTORC2 negatively regulates the activity of macrophage stimulating 1 (MST1), as disruption of Rictor/mTORC2 leads to a significant activation of MST1. This marked MST1 activation promotes cardiac dilation, cardiac dysfunction, and impaired cardiac growth and adaptation in response to pressure overload.
While mTORC1 and mTORC2 both have distinct functions, there is evidence that these two complexes are interconnected. S6K1, downstream of mTORC1, directly phosphorylates rictor of mTORC2 and promotes a negative regulatory effect on the mTORC2‐dependent phosphorylation of Akt‐Ser473 (Dibble, Asara, & Manning, 2009). mTORC2‐activated Akt, in contrast, enhances mTORC1 activity through the inactivation of tuberous sclerosis complex (TSC1/2), a complex that inhibits mTORC1 via GTPase‐activating protein activity towards Ras homologue enriched in the brain (Dibble et al., 2012).
We will discuss the manner in which current knowledge of mTOR relates to recent studies demonstrating that adrenoceptor agonists increase activation of mTORC1‐mediated cell growth and also mTORC2‐mediated glucose uptake and cell survival in vivo and in vitro (Olsen et al., 2014; Sato et al., 2014; Sato et al., 2018). We have focused this review on the interplay between adrenoceptors and mTOR in skeletal and cardiac muscle as well as adipose tissue, in light of our own expertise and the need to assimilate considerable information that is now available for these tissues. However, given the ubiquitous expression of mTOR and its partner proteins, as well as widespread expression of different adrenoceptor subtypes, it is highly likely that adrenoceptor–mTOR pathways are important in additional cell types. For example, there are a number of studies linking activation of hippocampal β‐adrenoceptors with mTOR‐dependent increases in protein translation (Connor, Wang, & Nguyen, 2011; Gelinas et al., 2007). These mechanisms are critical for long‐term potentiation and memory consolidation.
1.1. Role of β2‐adrenoceptor‐mediated mTOR activation in skeletal muscle
Skeletal muscle comprises up to 50% of total body mass, consumes a significant proportion of metabolic fuel, and has a major role in whole‐body metabolic homeostasis, being responsible for 75% of insulin‐mediated glucose uptake and utilization in the fed state. There is evidence showing that the sympathetic nervous system promotes glucose uptake in active skeletal muscle (e.g., during exercise and fight‐or‐flight responses), which results primarily from noradrenaline release from adrenergic nerve terminals, acting on β‐adrenoceptors at the cell surface (Nonogaki, 2000). Skeletal muscle expresses abundant β‐adrenoceptors that are predominantly β2‐adrenoceptors, with 7–10% β1‐adrenoceptors and no detectable β3‐adrenoceptors (Nevzorova, Bengtsson, Evans, & Summers, 2002). Stimulation with the β‐adrenoceptor agonist isoprenaline promotes glucose uptake in L6 myoblasts and myotubes, and intact skeletal muscle in vitro and in vivo (Nevzorova, Evans, Bengtsson, & Summers, 2006; Sato et al., 2014). Notably, isoprenaline increases glucose uptake to a greater extent than insulin in vivo in wild‐type mice, but not in β1/β2‐adrenoceptor knockout mice (Sato, Dehvari, Oberg, Dallner, et al., 2014), consistent with another study showing that mice lacking all three β‐adrenoceptors display glucose intolerance (Asensio, Jimenez, Kuhne, Rohner‐Jeanrenaud, & Muzzin, 2005).
Insulin stimulates skeletal muscle glucose uptake by activating signalling steps that increase the translocation of glucose transporter 4 (GLUT4) from intracellular vesicles to the cell surface. Following insulin‐mediated increases in PI3K activity, phosphatidylinositol 3,4,5‐trisphosphate (PIP3) recruits PDK1 and inactive Akt to the plasma membrane via N‐terminal PH domains, facilitating Akt phosphorylation at Thr308 by PDK1. In parallel, mTORC2 is phosphorylated via unknown mechanisms. A conformational change in Akt associated with phosphorylation of Thr308 enables mTORC2 to phosphorylate Akt at Ser473, leading to full activation. Akt promotes subsequent phosphorylation of the Rab GTPase‐activating protein Akt substrate of 160 kDa (AS160) at Thr642, which is critical for insulin‐increased GLUT4 translocation (Figure 1). Our previous studies showed that isoprenaline‐stimulated glucose uptake in L6 muscle cells was markedly reduced by the PI3K inhibitors PI‐103, wortmannin, and LY294002 (Sato, Dehvari, Oberg, Dallner, et al., 2014), suggesting that insulin receptor and β2‐adrenoceptor‐mediated glucose uptake may share a common signalling pathway. Unlike responses, we observed to insulin; however, there was no Akt phosphorylation at Thr308 or Ser473, or AS160 phosphorylation at Thr642 upon isoprenaline treatment, nor any increase in PIP3 levels, and glucose uptake was not inhibited by Akt inhibitor X (Nevzorova et al., 2002; Nevzorova et al., 2006; Sato, Dehvari, Oberg, Dallner, et al., 2014). Earlier studies demonstrated that PI‐103 and other widely used PI3K inhibitors including wortmannin and LY294002 have substantial affinity for related kinases including mTOR (Brunn et al., 1996; Knight et al., 2006). It is thus clearly important to consider the involvement of mTOR as well as PI3K when interpreting inhibitory effects of LY294002, wortmannin, or PI‐103 on downstream signalling outputs. In light of this, we found that the highly specific mTOR inhibitor KU0063794 (Sato, Dehvari, Oberg, Dallner, et al., 2014) inhibited both isoprenaline and insulin‐stimulated glucose uptake indicating that mTOR is involved in adrenoceptor‐stimulated glucose uptake. The combined results show that the pathways shared by insulin and isoprenaline overlap at a more downstream point leading to mTOR activation, and the β2‐adrenoceptor‐associated pathway does not include PI3K or Akt. siRNA knockdown of mTORC2 (rictor), but not mTORC1 (raptor), markedly inhibits both insulin‐mediated and β2‐adrenoceptor‐mediated glucose uptake (Sato, Dehvari, Oberg, Dallner, et al., 2014). In addition, in muscle lacking rictor, insulin‐stimulated Akt phosphorylation at Ser473 and AS160 at Thr642 are dramatically decreased, and muscle‐specific rictor knockout mice display glucose intolerance and decreased insulin‐stimulated glucose uptake (Kumar et al., 2008). This confirms mTORC2 as a key regulator of glucose uptake in skeletal muscle. Confirming this, we found that KU0063794 also inhibits β2‐adrenoceptor‐mediated skeletal muscle glucose uptake ex vivo and in vivo (Sato, Dehvari, Oberg, Dallner, et al., 2014).
Figure 1.
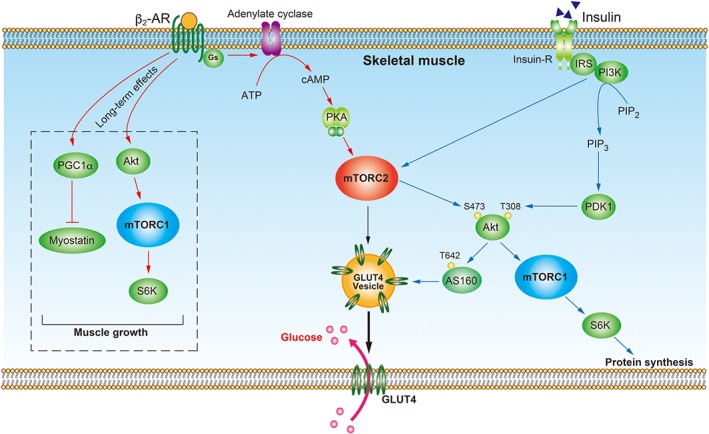
Proposed mechanisms for β2‐adrenoceptor‐mediated mTOR signalling in skeletal muscle. In skeletal muscle, activation of the β2‐adrenoceptor (β2‐AR) coupled to Gαs stimulates adenylate cyclase, leading to cAMP accumulation. cAMP activates PKA, which then phosphorylates mTORC2. The phosphorylated mTORC2 and GRK2 stimulates translocation of GLUT4 vesicles from the cytosol to the plasma membrane, leading to increased glucose uptake. The long‐term stimulation of β2‐adrenoceptors causes activation of the Akt–mTORC1–S6K pathway and inhibition of myostatin production by PGC1α, which contributes to muscle growth. Insulin binds to the insulin receptor (Insulin‐R), resulting in activation of PI3K. PI3K then increases levels of PIP3, which activates PDK1 to phosphorylate Akt at Thr308. PI3K also activates mTORC2, which phosphorylates Akt at Ser473. Fully activated Akt phosphorylates AS160 to promote GLUT4 translocation to the plasma membrane leading to increased glucose uptake. Akt also phosphorylates mTORC1 and thereby promotes protein synthesis
The β2‐adrenoceptor couples primarily to Gαs proteins, activating adenylyl cyclase to increase intracellular cAMP levels, resulting in PKA activation. β2‐Adrenoceptor stimulation can also cause cellular effects independently of this classical cAMP–PKA pathway. After agonist stimulation, β2‐adrenoceptors are rapidly phosphorylated by G protein receptor kinases (GRKs), allowing recruitment of β‐arrestins (which uncouple the receptor from its Gα protein partners), receptor internalization, and activation of β‐arrestin‐mediated signalling pathways (Tobin, Butcher, & Kong, 2008). The signalling effectors linking the β2‐adrenoceptor with activation of mTORC2 are still unknown but may be downstream of PKA as the selective PKA inhibitor PKI decreases isoprenaline‐induced mTORC2 phosphorylation, and 8‐bromo‐cAMP increases mTORC2 phosphorylation (Sato, Dehvari, Oberg, Dallner, et al., 2014). Interestingly, β2‐adrenoceptor‐stimulated glucose uptake is only partly dependent on cAMP (Nevzorova et al., 2002; Nevzorova et al., 2006; Sato, Dehvari, Oberg, Dallner, et al., 2014), suggesting contributions from alternative effectors that are cAMP‐independent. Involvement of GRK2 in β2‐adrenoceptor‐mediated glucose homeostasis has been suggested as one possible mechanism (Dehvari et al., 2012). CHO‐K1 cells stably expressing the human GLUT4 carrying an exofacial c‐Myc epitope (CHO‐GLUT4myc) were transfected with wild type or a truncated β2‐adrenoceptor lacking the entire C‐terminal tail, or co‐transfected with wild‐type β2‐adrenoceptor and βARKct, which sequesters Gβγ subunits required for GRK2 recruitment to the plasma membrane. Cells expressing wild‐type β2‐adrenoceptor plus βARKct, or the truncated receptor alone, showed markedly reduced isoprenaline‐stimulated glucose uptake compared with cells expressing the wild‐type β2‐adrenoceptor only. In addition, CHO‐GLUT4myc cells expressing a kinase‐dead GRK2 K220R mutant displayed significantly decreased GLUT4 translocation to the cell surface (Dehvari et al., 2012). Collectively, our studies indicate the potential role of GRK2 and PKA as upstream kinases of mTORC2 following activation of β2‐adrenoceptors.
Glucose uptake mediated by β2‐adrenoceptors is blocked by GLUT inhibitors and by pretreatment with GLUT4 siRNA (Sato, Dehvari, Oberg, Dallner, et al., 2014). Type 2 diabetes is closely associated with defects in insulin signalling mechanisms involving insulin receptor substrates (Chakrabarti et al., 2013), PI3K activity, and Akt phosphorylation (Cusi et al., 2000), but β2‐adrenoceptors expressed in skeletal muscle could bypass these defects through mTORC2‐mediated regulation of GLUT4 trafficking, providing a compensatory pathway following loss of insulin sensitivity (Sato et al., 2014; Sato, Dehvari, Oberg, Dallner, et al., 2014). This is of particular interest considering that β2‐adrenoceptor expression is unaltered in skeletal muscle from diabetic patients (Frederiksen et al., 2008).
Apart from being important for glucose uptake, β2‐adrenoceptor‐stimulated cAMP accumulation can have long‐term effects on muscle phenotype (Pearen, Ryall, Lynch, & Muscat, 2009). Chronic stimulation of skeletal muscle β2‐adrenoceptors utilizing agonists such as clenbuterol, fenoterol, and formoterol can activate anabolic signalling pathways, leading to increased muscle mass and force‐producing capacity (Lynch & Ryall, 2008) The anabolic and anti‐catabolic processes in response to β2‐adrenoceptor agonists occur via protein translation and synthesis mediated by the Akt–mTOR–S6 kinase signalling axis (Hagg et al., 2016; Figure 1). Chronic stimulation of β2‐adrenoceptors increases the transcription of PPAR‐γ coactivator 1‐α, which is associated with the suppression of myostatin, and these effects are blocked by ICI‐118,551, a highly selective β2‐adrenoceptor antagonist (Jesinkey, Korrapati, Rasbach, Beeson, & Schnellmann, 2014). Treatment of mice with formoterol stimulates small but significant increases in the phosphorylation of Akt and mTOR in gastrocnemius muscle after 8 hr, differing in time frame from more acute measurements of Akt/mTOR phosphorylation and glucose uptake (10 min to 2 hr; Sato, Dehvari, Oberg, Dallner, et al., 2014). Dexamethasone‐induced muscular atrophy and slow‐to‐fast myosin heavy chain isoform transition is antagonized by the β2‐adrenoceptor agonist clenbuterol, which stimulates Akt and mTORC1 activity, and insulin‐like growth factor 1 expression (Jesinkey et al., 2014). These findings could potentially provide a new basis for a pharmacological approach to target mTOR for the treatment of conditions involving muscle loss.
1.2. The role of adrenoceptor‐mediated mTOR activation in the heart
1.2.1. α1‐adrenoceptors and mTOR in the heart
Cardiac function is tightly regulated via both α1‐ and β‐adrenoceptors, due to release of noradrenaline from sympathetic nerve terminals innervating the heart and by circulating adrenaline released from the adrenal gland in response to danger or stress. The β‐adrenoceptors comprise roughly 90% of total cardiac adrenoceptors, and the α1‐ adrenoceptors account for the remaining 10%. In heart failure, unlike β1‐adrenoceptors, α1‐ adrenoceptors are not down‐regulated and may therefore play an enhanced role in regulating cardiac contractility (Skomedal, Borthne, Aass, Geiran, & Osnes, 1997). Although mRNAs for all three α1‐ adrenoceptor subtypes are detected in the heart of mice and rats, cardiomyocytes express only the α1A‐ and α1B‐subtypes (O'Connell et al., 2003) while α1D‐adrenoceptors are confined to the coronary vasculature (McCloskey et al., 2003; O'Connell et al., 2003). Due to the putative enhanced role in heart failure, α1‐ adrenoceptor function and signalling are therefore of particular interest.
A series of knockout mouse studies indicate that neither α1A‐ nor α1B‐ adrenoceptors are required for basal contractile function (O'Connell et al., 2003; Vecchione et al., 2002). However, cardiomyocyte‐specific overexpression of the α1A‐ adrenoceptor enhances basal contractile function (Lin et al., 2001) and reduces adverse remodelling following pressure overload (Du et al., 2004; Du et al., 2006). These results are consistent with an in vitro study by Mohl et al. (2011), identifying an α1A‐ adrenoceptor‐mediated signalling pathway that increases calcium entry and cardiomyocyte contractility. In contrast, overexpression of the α1B‐adrenoceptor causes depressed contractile function and pathological remodelling in the heart (Lemire et al., 2001; Wang, Du, Autelitano, Milano, & Woodcock, 2000). The capacity of α1A‐ adrenoceptors to increase contractile function may have important compensatory roles in the failing heart.
In addition to maintaining myocyte contractility, activation of α1‐adrenoceptors promotes glucose uptake (Shi, Papay, & Perez, 2016), receptor‐mediated preconditioning, cardiac hypertrophy, and inhibition of cardiomyocyte apoptosis (Jensen, O'Connell, & Simpson, 2011; O'Connell, Jensen, Baker, & Simpson, 2014). α1‐adrenoceptors are expressed in human myocardium and are not down‐regulated in heart failure (Jensen, Swigart, De Marco, Hoopes, & Simpson, 2009), and blockade of α1‐ adrenoceptors worsens heart failure (Dhaliwal et al., 2009; Jensen et al., 2011). In murine cardiac myocytes that express endogenous α1A‐ and α1B‐ adrenoceptors, long‐term agonist treatment increases the abundance of α1A‐ adrenoceptors without desensitization of inotropic effects, while increased stimulation or expression of the α1A‐ but not the α1B‐ adrenoceptor in vivo limits global cardiac remodelling and reduces mortality from heart failure (Du et al., 2006; Rorabaugh et al., 2005). In a transgenic rat model that overexpresses the cardiomyocyte α1A‐adrenoceptors, animals are protected from heart failure by increased angiogenesis associated with secretion of VEGF from cardiomyocytes (Zhao, Zhai, Gygi, & Goldberg, 2015).
In vitro and in vivo studies have indicated that stimulation of α1‐adrenoceptors reduces cardiomyocyte cell death. Hypoxia‐, serum starvation‐, and isoprenaline‐induced apoptosis can be inhibited by exposure of cardiomyocytes to phenylephrine, a non‐selective α1‐adrenoceptor agonist. This phenylephrine cytoprotective effect was blocked by phentolamine and prazosin (Iwai‐Kanai et al., 1999). Cardiomyocytes from α1A‐/α1B‐adrenoceptor knockout mice display significantly increased necrosis and apoptosis when subject to toxic stimuli such as doxorubicin or H2O2 (Huang et al., 2007; O'Connell et al., 2006), and this sensitivity can be reduced by re‐expression of α1A‐ adrenoceptors but not α1B‐adrenoceptors (Huang et al., 2007). The chemotherapeutic agent doxorubicin produces cardiotoxic effects in patients and in animal models. In mice, long‐term in vivo infusion of the α1A‐ adrenoceptor agonist A61603 protects cardiomyocytes against apoptosis and reduces adverse ventricular remodelling and myocardial fibrosis following doxorubicin treatment, thereby improving cardiac function (Chan, Dash, & Simpson, 2008; Montgomery et al., 2017). These protective effects of A61603 are not observed in α1A‐ adrenoceptor knockout mice. Another study showed that dabuzalgron, an orally available, selective α1A‐ adrenoceptor agonist also increases survival and preserves fractional shortening in wild‐type but not in α1A‐ adrenoceptor knockout mice (Beak et al., 2017). All of these studies indicate that α1‐ adrenoceptors could be an important target in the failing heart.
The non‐selective α1‐ adrenoceptor agonist phenylephrine is a well‐known hypertrophic agent in the heart and has been linked to activation of the mTORC1 target S6K1 (Boluyt et al., 1997). Treatment of neonatal rat ventricular myocytes (NRVMs) with phenylephrine stimulated the activity of S6K1, increased protein synthesis, and produced a 50% increase in cardiomyocyte area. Phenylephrine‐induced S6K1 activity and hypertrophy were significantly reduced by the mTORC1 inhibitor rapamycin and by the PI3K inhibitor LY294002; however, the authors acknowledge that compounds such as LY294002 affect other PI3K‐related kinases (Boluyt et al., 1997). As outlined in the skeletal muscle section of this review, PI3K inhibitors including wortmannin and LY294002 have substantial activity at mTOR (Brunn et al., 1996; Knight et al., 2006). Thus, studies in which LY294002 is used as a sole PI3K inhibitor should be regarded with caution. Taken together, these results suggest that phenylephrine activates S6K1 and promotes cardiomyocyte hypertrophy via mTORC1 and possibly PI3K. We have shown recently that treatment of NRVMs with the highly selective α1A‐AR agonist A61603 increases phosphorylation of S6 ribosomal protein, a downstream target of mTORC1 and S6K1, and this is inhibited by rapamycin. NRVM hypertrophy observed in response to A61603 was prevented by the mTOR inhibitor KU0063794, which blocks the phosphorylation and activation of both mTORC1 and mTORC2 (Sato et al., 2018). It is thus clear that α1‐adrenoceptors stimulate mTORC1 and that this could be an important player in the ability of α1‐adrenoceptors to protect the heart.
Phenylephrine stimulates activation of S6K1 and phosphorylation of 4E‐BP1 in adult cardiomyocytes (Wang & Proud, 2002). The latter protein interacts with eIF4E and represses translation. Phosphorylation of 4E‐BP results in its dissociation from eIF4E and activation of mRNA translation. The response to phenylephrine was blocked by MEK inhibitors, and adenoviral expression of constitutively active MEK caused activation of S6K1, phosphorylation of 4E‐BP1, and activation of protein synthesis in a rapamycin‐sensitive manner. This study provides insight into a signalling pathway involving Ras, MEK, and mTOR (Wang & Proud, 2002). Phenylephrine also activates S6K2 in adult rat ventricular cardiomyocytes. Both MEK1/2 inhibitors and rapamycin abolished phenylephrine‐induced activation of S6K2, and the expression of constitutively active MEK1 activated S6K2. This indicates that MEK/ERK1/2 in combination with mTOR signalling plays a role in regulating phenylephrine‐induced S6K2 activation (Wang, Gout, & Proud, 2001).
Although the classic α1‐ adrenoceptor signalling pathway includes Ca2+‐dependent PKC, phenylephrine also regulates S6K1/2 and 4E‐BP1 (downstream substrates of mTORC1) leading to protein synthesis in a Ca2+‐independent PKC manner in adult cardiomyocytes (Wang, Rolfe, & Proud, 2003). The classical Ca2+‐dependent PKCα and the Ca2+‐independent PKCδ and PKCε are readily detected in adult cardiomyocytes (Puceat, Hilal‐Dandan, Strulovici, Brunton, & Brown, 1994; Steinberg, Goldberg, & Rybin, 1995). In addition, Ca2+‐independent PKC is also required for the phenylephrine‐induced ERK1/2 activation demonstrated by the significantly reduced ERK1/2 activation in the presence of the broad‐spectrum PKC inhibitor BIM I (Toullec et al., 1991). Rottlerin (Gschwendt et al., 1994), a selective inhibitor of PKCδ, almost completely inhibited the phenylephrine‐induced ERK1/2 phosphorylation, while Gö6979 (Martiny‐Baron et al., 1993), an inhibitor of Ca2+‐dependent PKC has no obvious effect on ERK1/2 activation. Furthermore, Rottlerin prevented phenylephrine‐induced S6K activation whereas Gö6979 had no apparent effects. Phosphorylation of 4E‐BP1 was also inhibited by rottlerin in a similar manner (Wang et al., 2003). These data suggest Ca2+‐independent PKC isoforms play a vital role in α1‐adrenoceptor‐mediated mTOR signalling in adult cardiomyocytes.
While mTORC1 plays an important role in cardiomyocyte hypertrophy, there is convincing evidence that mTORC2 promotes cardiomyocyte development and survival (Gonzalez‐Teran et al., 2016; Shende et al., 2016; Xu & Brink, 2016). For example, mice with cardiomyocyte‐specific knockdown of rictor and thus disruption of mTORC2 display abnormalities by the age of 6 months, including cardiac dilation, fibrosis, and exacerbated heart failure in response to pressure overload (Sciarretta et al., 2015; Yano et al., 2014). Following ischaemic preconditioning, activation of mTORC2 promotes cardiomyocyte survival in part by suppressing activity of the kinase Mst1 (large tumour suppressor kinase 2), a key component of the Hippo pathway that promotes apoptosis and inhibits cell growth (Sciarretta et al., 2015; Yano et al., 2014). Importantly, cardiomyocytes that are rictor‐deficient or overexpress Mst1 display increased cell death. In the study by Shende et al. (2016), tamoxifen‐inducible cardiomyocyte‐specific rictor knockdown was used to allow normal cardiac development. Mice in which Cre recombinase expression was induced at 4 or 10 weeks of age displayed normal cardiac size and echocardiography up to 44 weeks after tamoxifen treatment, but transverse aortic constriction and resultant pressure overload caused more pronounced cardiac dysfunction than in wild‐type mice, indicating the importance of mTORC2 in the failing heart (Shende et al., 2016; Volkers et al., 2013).
Cardiac α1‐adrenoceptorshave been linked with mTOR in exerting cardioprotective effects. Serum and glucocorticoid‐responsive kinase‐1 (SGK1) is a downstream substrate of mTORC2 (Garcia‐Martinez & Alessi, 2008) that regulates cardiomyocyte survival and hypertrophy in response to the non‐selective α1‐adrenoceptor agonist phenylephrine, both in vivo and in vitro (Aoyama et al., 2005). Cardiomyocytes infected with an adenoviral vector encoding constitutively active SGK1 show reduced apoptosis after serum‐ or oxygen‐deprivation and increased [3H]‐leucine incorporation in response to phenylephrine, while expression of kinase‐dead SGK1 increases apoptosis. SGK1 has also been placed downstream of PI3K (Park et al., 1999), although again inhibition of mTOR may have confounded the interpretation of these experiments involving the use of LY294002 as a PI3K inhibitor.
We have demonstrated that noradrenaline and the α1A‐adrenoceptor agonist A61603 increase glucose uptake in NRVMs by parallel activation of AMPK and mTORC2 but do not promote phosphorylation of Akt at Thr308 or Ser473 (Sato et al., 2018). The lack of Akt phosphorylation mirrors similar findings by Wang et al. (2001), who demonstrated using adult cardiomyocytes that phenylephrine does not produce Akt phosphorylation at Ser473 and that adenoviral expression of a dominant‐negative Akt mutant fails to block activation of S6K2 by phenylephrine. We found that the mTORC1/2 inhibitor KU0063794 partly reduced α1A‐ adrenoceptor and insulin‐stimulated glucose uptake in cardiomyocytes, whereas the mTORC1 inhibitor rapamycin had no effect. A61603 stimulated the phosphorylation of mTOR at Ser2448 and Ser2481. Overall, the data suggest that α1A‐adrenoceptors stimulate mTORC2 to increase glucose uptake and mTORC1 to promote protein synthesis and hypertrophy in NRVMs (Sato et al., 2018; Figure 2), but the detailed mechanism whereby α1A‐adrenoceptors activate mTORC2 is still not known.
Figure 2.
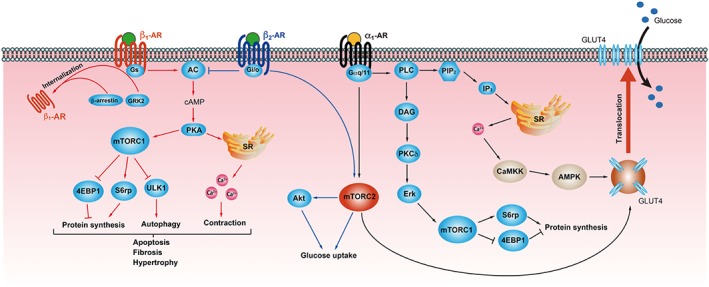
Proposed mechanisms for mTOR signalling in the heart, mediated by α1‐ and β‐adrenoceptors. Activation of α1‐adrenoceptors (α1‐AR) results in increased levels of cytosolic Ca2+ through a classical pathway involving Gαq/11, PLC, phosphatidylinositol bisphosphate (PIP2), and inositol trisphosphate (IP3). Release of intracellular Ca2+ activates CaMKK and AMPK pathways. α1‐Adrenoceptors also activate mTORC2 via unknown mechanisms. AMPK and mTORC2 both play significant roles in GLUT4 translocation to the plasma membrane, resulting in increased glucose uptake. α1‐Adrenoceptors promote mTORC1 activation via diacyl glycerol (DAG), PKCδ, and Erk1/2, leading to increased activation of S6rp and 4EBP1, which promote protein translation. β1‐ and β2‐adrenoceptors (β1‐ AR, β2‐AR) both couple to Gαs, whereas β2‐adrenoceptors switch coupling to Gαi/o in pathological states such as heart failure. Overstimulation of β1‐adrenoceptors increases Ca2+ mobilization and mTORC1 activation leading to increased protein synthesis and inhibition of autophagy, resulting in apoptosis, fibrosis, and hypertrophy. In the later stages of heart failure, GRK2 overexpression results in adrenoceptor phosphorylation and interaction with β‐arrestin, thereby promoting receptor internalization. Activation of the β2‐adrenoceptor–Gαi/o pathway inhibits cAMP production and counteracts the pro‐apoptotic effects of excessive β1‐ adrenoceptor stimulation. The β2‐adrenoceptors stimulate glucose uptake through mTORC2‐Akt activation
1.2.2. β‐ adrenoceptors and mTOR in the heart
Both β1‐ and β2‐adrenoceptors are expressed in the mammalian heart, although in isolated cardiomyocytes, the β1‐adrenoceptor is the predominant subtype (Buxton & Brunton, 1985). In the human heart, the abundance of β1‐adrenoceptor protein in cardiomyocytes is 68–80 fmol·mg−1 protein, and it decreases to 30–41 fmol·mg−1 protein in failing hearts (Bristow et al., 1993; Morisco et al., 2008). Both β1‐ and β2‐ adrenoceptor subtypes are present in large coronary arteries (Young, Vatner, & Vatner, 1990), while the primary subtype found in fibroblasts and on the small vessel endothelium is the β2‐adrenoceptor (Freissmuth, Hausleithner, Nees, Bock, & Schutz, 1986; Zhou & Pu, 2016).
Activation of β‐adrenoceptors plays an important role in the regulation of cardiovascular function, including positive inotropic and chronotropic effects (Bristow et al., 1993; Brodde, 1991). Noradrenaline exerts its effects on the heart nearly exclusively via β1‐adrenoceptors (Kaumann, Hall, Murray, Wells, & Brown, 1989). Thus, under normal physiological conditions β1‐adrenoceptors are the predominant cardiac adrenoceptors responsible for regulation of heart rate and contractility. The β1‐adrenoceptors activate the canonical Gαs‐adenylate cyclase‐cAMP‐PKA signalling cascade. In cardiomyocytes, the activation of PKA promotes phosphorylation of multiple proteins that increase calcium mobilization primarily from the sarcoplasmic reticulum and, to a lesser extent, from the extracellular milieu, leading to increased rates of contraction and relaxation and to increased force of contraction (Sirenko et al., 2014; Figure 2). In the early stages of heart failure, cardiac output is increased via overstimulation of β1‐adrenoceptors as a compensatory mechanism for the insufficient blood and oxygen supply (Brodde, 1993), but this leads to longer term structural damage, including ventricular remodelling, cardiomyocyte apoptosis and fibrosis, and cardiac hypertrophy (Engelhardt, Hein, Wiesmann, & Lohse, 1999; O'Connor et al., 1999). In addition, recent evidence has shown that β1‐adrenoceptors decrease myocardial autophagy that maintains cellular homeostasis (Wang et al., 2013; Wang et al., 2015). Inhibition of autophagy causes the accumulation of denatured proteins and damaged organelles, contributing to cardiac dysfunction (Magnusson, Wallukat, Waagstein, Hjalmarson, & Hoebeke, 1994), and up‐regulation of autophagy by the mTORC1 inhibitor rapamycin can improve impaired cardiac function (Wang et al., 2015). The β1‐adrenoceptor‐mediated inhibition of autophagy occurs via PKA phosphorylation of Ser12 in the autophagy‐related protein LC3 (Kroemer, Zamzami, & Susin, 1997). mTORC1 is overactive in the early stages of heart failure and plays a role in the β1‐adrenoceptor‐mediated inhibition of autophagy (Wang et al., 2015; Figure 2).
Cardiac β1‐adrenoceptors become desensitized and down‐regulated as heart failure progresses to end‐stage dilated cardiomyopathy (Bohm et al., 1988). Desensitization is related in part to an increased abundance and activity of GRK2, the predominant GRK subtype in the heart (Cannavo, Liccardo, & Koch, 2013). Phosphorylation of β1‐adrenoceptors by GRK2 leads to increased interaction with β‐arrestin, thereby promoting receptor internalization and degradation (Rockman, Koch, & Lefkowitz, 2002; Figure 2). The β2‐adrenoceptors, on the other hand, are pleiotropic receptors that couple to Gαs, Gαi/o, and Gβγ (Evans, Sato, Sarwar, Hutchinson, & Summers, 2010; Xiao, Cheng, Zhou, Kuschel, & Lakatta, 1999). In the healthy human heart, β2‐adrenoceptors preferentially couple to Gαs proteins, whereas in pathological states involving high circulating catecholamine levels and high expression levels of cardiac Gαi/o proteins during congestive heart failure, the β2‐adrenoceptors switch to Gαi/o signalling (Brown & Harding, 1992; Woo, Song, Xiao, & Zhu, 2015). Activation of the β2‐adrenoceptor–Gαi/o pathway inhibits cAMP production and protects cardiomyocytes from the pro‐apoptotic effects of excessive β1‐adrenoceptor stimulation (Chesley et al., 2000; Zhu et al., 2001). β2‐adrenoceptor‐Gαi/o signalling also activates Akt, which is known to be activated by PI3K and mTORC2 (Figure 2). The Akt signalling cascade is known to promote protein synthesis and glucose uptake in cardiomyocytes (Chesley et al., 2000).
1.3. The role of β‐adrenoceptors and mTOR in adipose tissue
There are two types of adipose tissue with distinct physiological functions: white adipose tissue (WAT) that stores chemical energy as triacylglycerol; and BAT that releases chemical energy as heat (thermogenesis). BAT is responsible for sympathetically mediated non‐shivering thermogenesis in mammals and is activated by members of the adrenoceptor family (Cannon & Nedergaard, 2004). In addition, many groups have described the existence of brown adipocytes in depots thought to be primarily WAT, both in animal models and in humans (Petrovic et al., 2010; Wu et al., 2012). These cells differ from prototypical BAT found in rodents or human infants and have been termed “brite” (brown in white) or “beige” adipocytes (Petrovic et al., 2010; Wu et al., 2012). The appearance of brite adipocytes per se is insufficient to promote increased energy expenditure, as these cells must also be activated by environmental, hormonal, or pharmacological stimuli such as drugs acting at GPCRs (Merlin et al., 2016). The expression of adrenoceptors in brown, white, and brite adipocytes and their contribution to adipocyte function is described in detail in an accompanying review (Evans, Merlin, Bengtsson, & Hutchinson, 2019). We will focus here on the interplay between adrenoceptor signalling and the role of mTOR complexes in adipocyte browning and glucose metabolism.
1.3.1. β‐adrenoceptors and mTOR in WAT
When nutrients are plentiful, insulin is released from the pancreas and stimulates the uptake of glucose and fatty acids by adipose tissue, where they are stored as triacylglycerol forming lipid droplets. Insulin signalling in adipocytes is mediated by the PI3K–Akt–mTOR pathway, producing anabolic effects including cell growth and inhibition of lipolysis (Chakrabarti et al., 2013). During periods of fasting or stress, catecholamines are released by the sympathetic nervous system to activate β‐adrenoceptors. Stimulation of the β3‐adrenoceptors in WAT activates adenylyl cyclase, leading to increased cAMP levels and PKA activity. PKA phosphorylates and regulates several important targets in adipocytes, including hormone‐sensitive lipase and the lipid droplet‐associated perilipins, which collectively promote triglyceride hydrolysis and liberation of free fatty acids (Granneman & Moore, 2008; Figure 3).
Figure 3.
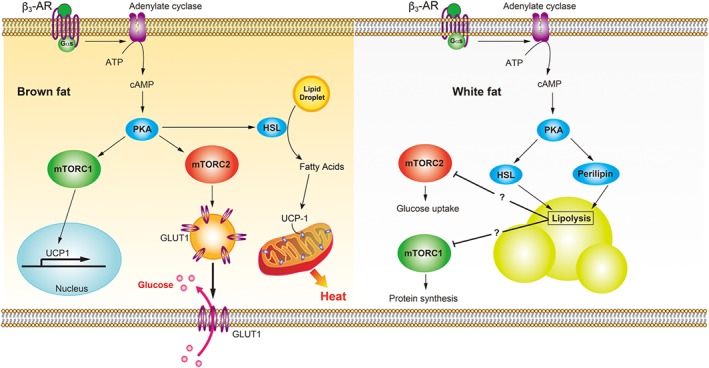
mTOR signalling pathways stimulated by β3‐adrenoceptors (β3‐AR) in BAT and WAT. Stimulation of these receptors increases the production of cAMP via Gαs‐adenylate cyclase and enhances activation of PKA in BAT and WAT. In brown adipocytes, PKA promotes the transcription and translation of UCP1 via mTORC1. The increased abundance and activation of UCP1 in mitochondria by free fatty acids promotes thermogenesis. GLUT1 translocation is increased due to mTORC2 activation, leading to increased glucose uptake. In white adipocytes, β3‐adrenoceptor‐mediated PKA activation leads to phosphorylation of hormone‐sensitive lipase (HSL) and perilipin. Phosphorylated perilipin undergoes a conformational change and interacts with lipid droplet HSL, which then hydrolyses the stored triglycerides into fatty acids. Lipolytic products may inhibit mTORC1‐mediated protein synthesis and mTORC2‐mediated glucose uptake in white adipocytes
Two studies have suggested that adrenoceptor‐stimulated lipolysis inactivates mTOR in WAT (Mullins et al., 2014; Scott & Lawrence, 1998). Mullins et al. (2014) demonstrated that β‐adrenoceptor‐mediated lipolysis suppresses glucose uptake because lipolysis causes both mTORC1 and mTORC2 complexes to dissociate (Figure 3). This is in agreement with the proposal that in white adipocytes, cAMP indirectly prevents activation of mTOR, since there is a decrease in p70S6K, a downstream target of mTORC1 (Scott & Lawrence, 1998). Conversely, there are new studies indicating that stimulation of β3‐adrenoceptors in WAT does not inhibit mTOR complexes but instead activates mTORC1 through PKA (Liu et al., 2016), resulting in browning of WAT depots. This variance in results might be due to the fact that β‐adrenoceptor stimulation interacts differently with mTOR in different WAT depots. Nonetheless, these results suggest that β‐adrenoceptor regulation of mTOR could have an important role in WAT function.
1.3.2. β‐adrenoceptors and mTOR in BAT
Binding of noradrenaline to BAT β‐adrenoceptors activates intracellular signalling cascades leading to increased expression of uncoupling protein 1 (UCP1) and breakdown of triglycerides to free fatty acids that activate UCP1 in the inner mitochondrial membrane (Figure 3). Activated UCP1 collapses the proton gradient that drives ATP synthesis and energy storage; thus, β‐adrenoceptor signalling increases mitochondrial respiration and non‐shivering thermogenesis (Cannon & Nedergaard, 2004). The metabolic capacity of BAT potentially allows it to influence whole‐body energy homeostasis. For instance, BAT has been shown to play an important role in the regulation of glucose homeostasis and insulin secretion (Guerra et al., 2001). Cold exposure of animals increases glucose uptake into BAT due to activation of the sympathetic nervous system (Shibata, Perusse, Vallerand, & Bukowiecki, 1989; Shimizu, Nikami, & Saito, 1991), and this response is mimicked by administration of β‐adrenoceptor agonists in vivo (Liu, Perusse, & Bukowiecki, 1994; Olsen et al., 2014). Mouse brown adipocytes cultured in vitro also display increased glucose uptake upon treatment with β‐adrenoceptor agonists (Chernogubova, Hutchinson, Nedergaard, & Bengtsson, 2005; Dallner, Chernogubova, Brolinson, & Bengtsson, 2006; Merlin et al., 2018; Olsen et al., 2014). While a role for β3‐adrenoceptor‐mediated glucose uptake in rodents is well established, the contribution of these receptors in human adipose tissue is less clear. It has been demonstrated, however, that cold exposure increases 18F‐2‐deoxyglucose uptake in human BAT depots, and this effect can be mimicked by administration of the β3‐adrenoceptor agonist mirabegron that is used clinically for overactive bladder (Baskin et al., 2018; Cypess et al., 2015).
There is strong evidence that glucose uptake in response to β3‐adrenoceptor agonists occurs via a Gαs–cAMP–PKA pathway, based on the use of pharmacological inhibitors (Chernogubova, Cannon, & Bengtsson, 2004; Olsen et al., 2014). In addition, 8‐bromo‐cAMP and upstream activation of Gαs by cholera toxin both increase glucose uptake in primary brown adipocytes (Chernogubova et al., 2004; Olsen et al., 2014). Other mechanisms involved in β3‐adrenoceptor‐mediated glucose uptake include localization of the β3‐adrenoceptors in lipid‐rich microenvironments in the plasma membrane (Sato et al., 2012), conventional and novel PKC isoforms (Chernogubova et al., 2004), and AMPK (Hutchinson, Chernogubova, Dallner, Cannon, & Bengtsson, 2005; Inokuma et al., 2005). As demonstrated in skeletal muscle, mTORC2 plays a pivotal role in adipocyte glucose uptake stimulated by β‐adrenoceptor agonists, as well as insulin.
The contributions of mTORC1 and mTORC2 have been examined in mice with specific ablation of raptor or rictor in all adipocytes, as these cells express Cre recombinase under control of the adiponectin promoter (Kumar et al., 2010; Polak et al., 2008). Ablation of raptor (mTORC1) in adipose tissue increases mitochondrial uncoupling but has no effect on insulin‐mediated Akt phosphorylation or glucose tolerance profiles in chow‐fed mice (Polak et al., 2008). In contrast, adipocytes isolated from mice with fat‐specific ablation of rictor (mTORC2) display reduced insulin‐stimulated Akt‐Ser473 phosphorylation, GLUT4 translocation to the cell surface, and glucose uptake, and these mice have impaired glucose tolerance profiles in vivo (Kumar et al., 2010). These studies indicate that like in skeletal muscle, the mTORC2 complex is involved in glucose homeostasis in adipocytes.
We have demonstrated using brown adipocytes that mTORC2 is involved in β3‐adrenoceptor‐mediated glucose uptake (Olsen et al., 2014). Overall inhibition of mTOR by Torin‐1 or KU0063794 reduces glucose uptake, but two lines of evidence demonstrate the involvement of mTORC2 rather than mTORC1: (a) 24‐hr, but not 2‐hr, rapamycin treatment attenuates β3‐adrenoceptor‐mediated glucose uptake (rapamycin acutely inhibits mTORC1, whereas long‐term treatment prevents mTORC2 assembly), and (b) siRNA against rictor, but not raptor, reduces glucose uptake by β3‐adrenoceptors (Mohl et al., 2011; Olsen et al., 2014). In brown adipocytes, β3‐adrenoceptor‐mediated glucose uptake depends on de novo synthesis and translocation of GLUT1 (Dallner et al., 2006), which are both cAMP‐dependent (Figure 3). mTORC2 is specifically involved in the translocation of newly synthesized GLUT1 to the plasma membrane, but is not required for de novo synthesis of GLUT1 (Olsen et al., 2014). In brown adipocyte cultures, inhibition of PI3K by compound 15e, or of Akt by inhibitor X, reduced insulin‐ but not isoprenaline‐stimulated glucose uptake. Akt was phosphorylated at Thr308 and Ser473 in response to insulin but not isoprenaline (Olsen et al., 2014).
A recent study has also shown that mice lacking rictor in adipose tissue are hypothermic, show increased susceptibility to cold, and have impairment of cold‐induced glucose uptake and glycolysis (Albert et al., 2016). This study indicates that mTORC2 plays a central role in adipose tissue metabolism and translocation of GLUT 1/4 in vitro and in vivo. Interestingly, the GLUT 1/4 content in the plasma membrane of brown adipocytes was not altered by cold exposure in that study. Also in contrast to our previous findings (Olsen et al., 2014), both immortalized mouse brown adipocytes treated with noradrenaline (1 μM) for 5 min and native BAT from wild‐type mice treated for 30 min in vivo with noradrenaline (1 mg·kg−1) showed phosphorylation of Akt at Ser473 , known to be downstream of mTORC2. There is no clear explanation for the disparity with our brown adipocytes; however, an emerging view is that adipose depots display considerable heterogeneity in cell composition (Shinoda et al., 2015). This would account for differences between in vivo and in vitro data and may also be consistent with phenotypic differences between primary brown adipocyte cultures that are representative of the starting population of stromal vascular pre‐adipocytes and immortalized adipocytes that have been selected for the presence of plasmid encoding SV40 T antigen (Klein, Fasshauer, Klein, Benito, & Kahn, 2002) and therefore represent only a small subset of the starting cell population. In addition, noradrenaline may activate the α2‐adrenoceptors present in BAT or brown adipocytes, promoting signalling via a Gαi/o‐Gβγ‐PI3K‐PDK1‐Akt axis. In immortalized human multipotent adipose‐derived stem (hMADS) brown adipocytes treated with low concentrations of isoprenaline, glucose uptake is blocked by the mTOR inhibitor KU0063794, as seen in mouse brown adipocyte primary cultures (Olsen et al., 2014). It would be interesting to determine whether hMADS cells display Akt phosphorylation at Ser473 in response to isoprenaline treatment.
1.3.3. mTORC1 mediates browning of brite adipocytes
In addition to BAT, there is increasing evidence for the existence of brown adipocytes in depots thought to be primarily WAT, both in animal models and in humans (Petrovic et al., 2010). These cells differ from prototypical BAT found in rodents or human infants and have been termed “brite” (brown in white) or “beige” adipocytes. Two studies indicate that brite adipocytes contribute significantly to whole‐body energy expenditure: Mouse models that have increased brite adipocytes in WAT are protected from diet‐induced obesity (Seale et al., 2011), and browning of WAT contributes to non‐shivering adaptive thermogenesis in the absence of classical brown adipocytes (Schulz et al., 2013). Our in vitro results show that stimulation of the β3‐adrenoceptors increases glucose uptake in brown and brite adipocytes, but not white adipocytes, in contrast to insulin, which increases glucose uptake in all three adipocyte cultures (Merlin et al., 2018).
Separate studies have shown that the β‐adrenoceptor–cAMP–PKA pathway can lead to mTORC1 activation (Figure 3) and is necessary for the induction of adipose tissue browning and BAT development (Liu et al., 2016). In addition, wild‐type mice treated with the mTORC1 inhibitor rapamycin or mice with adipocyte‐specific deletion of raptor are cold‐intolerant and show impaired expression of UCP1 and other mitochondrial components in inguinal WAT, suggesting that there may be a role for mTORC1 even in the early development of inguinal WAT brite adipocytes (Liu et al., 2016; Tran et al., 2016). Several downstream target genes of PPAR‐α and oestrogen‐related receptor α (ERRα) are similarly under the control of mTORC1. PPAR‐α is a master nuclear receptor for fatty acid β‐oxidation and has been shown to participate in UCP1 expression either directly or indirectly through ERRα (Morganstein et al., 2010). Therefore, mTORC1 appears to have an important role in the catabolic process of adipose tissue browning and the dissipation of chemical energy by thermogenesis.
2. CONCLUSIONS
This review has summarized the evidence for metabolic and survival roles of adrenoceptor‐mTOR signalling in the heart, skeletal muscle, and brown/brite adipocytes. The α1A‐ adrenoceptors mediate glucose uptake and cardioprotection via mTOR in the failing heart. In skeletal muscle, β2‐adrenoceptors facilitate protein synthesis and glucose uptake via mTORC1 and mTORC2 respectively. Type 2 diabetes is associated with defects in insulin signalling components including insulin receptor substrate, PI3K, and Akt, causing impaired glucose uptake. These defects can be bypassed by the β2‐adrenoceptor–mTORC2 pathway in skeletal muscle, which is independent of insulin signalling. Likewise, adipose β‐adrenoceptors play a significant role in lipolysis in WAT and increase glucose uptake in BAT, which can contribute significantly to whole‐body energy expenditure. mTORC1 also plays a role in browning of brite adipocytes. The capacity of key GPCRs to modulate physiological responses through mTOR activation represents a novel paradigm that holds great potential in the identification of drug targets for treating a range of metabolic disorders.
2.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander, Christopoulos et al., 2017; Alexander, Cidlowski et al., 2017; Alexander, Fabbro et al., 2017; Alexander, Kelly et al. 2017).
CONFLICT OF INTEREST
T.B. owns stocks in the following pharmaceutical companies: Sigrid Therapeutics AB, Atrogi AB, and Glucox Biotechnology AB. D.S.H. owns stocks in Glucox Biotechnology AB and is a scientific advisor for Atrogi AB.
Chia LY, Evans BA, Mukaida S, Bengtsson T, Hutchinson DS, Sato M. Adrenoceptor regulation of the mechanistic target of rapamycin in muscle and adipose tissue. Br J Pharmacol. 2019;176:2433–2448. 10.1111/bph.14616
REFERENCES
- Albert, V. , Svensson, K. , Shimobayashi, M. , Colombi, M. , Munoz, S. , Jimenez, V. , … Hall, M. N. (2016). mTORC2 sustains thermogenesis via Akt‐induced glucose uptake and glycolysis in brown adipose tissue. EMBO Molecular Medicine, 8, 232–246. 10.15252/emmm.201505610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174 Suppl, 1, S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Cidlowski, J. A. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Nuclear hormone receptors. British Journal of Pharmacology, 174, S208–S224. 10.1111/bph.13880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174, S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Transporters. British Journal of Pharmacology, 174, S360–S446. 10.1111/bph.13883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama, T. , Matsui, T. , Novikov, M. , Park, J. , Hemmings, B. , & Rosenzweig, A. (2005). Serum and glucocorticoid‐responsive kinase‐1 regulates cardiomyocyte survival and hypertrophic response. Circulation, 111, 1652–1659. 10.1161/01.CIR.0000160352.58142.06 [DOI] [PubMed] [Google Scholar]
- Asensio, C. , Jimenez, M. , Kuhne, F. , Rohner‐Jeanrenaud, F. , & Muzzin, P. (2005). The lack of beta‐adrenoceptors results in enhanced insulin sensitivity in mice exhibiting increased adiposity and glucose intolerance. Diabetes, 54, 3490–3495. [DOI] [PubMed] [Google Scholar]
- Baskin, A. S. , Linderman, J. D. , Brychta, R. J. , McGehee, S. , Anflick‐Chames, E. , Cero, C. , … Cypess, A. M. (2018). Regulation of Human Adipose Tissue Activation, Gallbladder Size, and Bile Acid Metabolism by a beta3‐Adrenergic Receptor Agonist. Diabetes, 67, 2113–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beak, J. , Huang, W. , Parker, J. S. , Hicks, S. T. , Patterson, C. , Simpson, P. C. , … Jensen, B. C. (2017). An oral selective alpha‐1A adrenergic receptor agonist prevents doxorubicin cardiotoxicity. JACC Basic Transl Sci, 2, 39–53. 10.1016/j.jacbts.2016.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm, M. , Beuckelmann, D. , Brown, L. , Feiler, G. , Lorenz, B. , Nabauer, M. , … Erdmann, E. (1988). Reduction of beta‐adrenoceptor density and evaluation of positive inotropic responses in isolated, diseased human myocardium. European Heart Journal, 9, 844–852. [DOI] [PubMed] [Google Scholar]
- Boluyt, M. O. , Zheng, J. S. , Younes, A. , Long, X. , O'Neill, L. , Silverman, H. , … Crow, M. T. (1997). Rapamycin inhibits alpha 1‐adrenergic receptor‐stimulated cardiac myocyte hypertrophy but not activation of hypertrophy‐associated genes. Evidence for involvement of p70 S6 kinase. Circulation Research, 81, 176–186. [DOI] [PubMed] [Google Scholar]
- Bristow, M. R. , Minobe, W. A. , Raynolds, M. V. , Port, J. D. , Rasmussen, R. , Ray, P. E. , & Feldman, A. M. (1993). Reduced beta 1 receptor messenger RNA abundance in the failing human heart. The Journal of Clinical Investigation, 92, 2737–2745. 10.1172/JCI116891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodde, O. E. (1991). Beta 1‐ and beta 2‐adrenoceptors in the human heart: Properties, function, and alterations in chronic heart failure. Pharmacological Reviews, 43, 203–242. [PubMed] [Google Scholar]
- Brodde, O. E. (1993). Beta‐adrenoceptors in cardiac disease. Pharmacology & Therapeutics, 60, 405–430. 10.1016/0163-7258(93)90030-H [DOI] [PubMed] [Google Scholar]
- Brown, L. A. , & Harding, S. E. (1992). The effect of pertussis toxin on beta‐adrenoceptor responses in isolated cardiac myocytes from noradrenaline‐treated guinea‐pigs and patients with cardiac failure. British Journal of Pharmacology, 106, 115–122. 10.1111/j.1476-5381.1992.tb14302.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunn, G. J. , Williams, J. , Sabers, C. , Wiederrecht, G. , Lawrence, J. C. Jr. , & Abraham, R. T. (1996). Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3‐kinase inhibitors, wortmannin and LY294002. The EMBO Journal, 15, 5256–5267. [PMC free article] [PubMed] [Google Scholar]
- Buxton, I. L. , & Brunton, L. L. (1985). Direct analysis of beta‐adrenergic receptor subtypes on intact adult ventricular myocytes of the rat. Circulation Research, 56, 126–132. 10.1161/01.RES.56.1.126 [DOI] [PubMed] [Google Scholar]
- Cannavo, A. , Liccardo, D. , & Koch, W. J. (2013). Targeting cardiac beta‐adrenergic signaling via GRK2 inhibition for heart failure therapy. Frontiers in Physiology, 4, 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon, B. , & Nedergaard, J. (2004). Brown adipose tissue: Function and physiological significance. Physiological Reviews, 84, 277–359. [DOI] [PubMed] [Google Scholar]
- Chakrabarti, P. , Kim, J. Y. , Singh, M. , Shin, Y. K. , Kim, J. , Kumbrink, J. , … Kandror, K. V. (2013). Insulin inhibits lipolysis in adipocytes via the evolutionarily conserved mTORC1‐Egr1‐ATGL‐mediated pathway. Molecular and Cellular Biology, 33, 3659–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, T. , Dash, R. , & Simpson, P. C. (2008). An alpha‐1A‐adrenergic receptor subtype agonist prevents cardiomyopathy without increasing blood pressure. Circulation, 118(Suppl 18). [Google Scholar]
- Chernogubova, E. , Cannon, B. , & Bengtsson, T. (2004). Norepinephrine increases glucose transport in brown adipocytes via beta3‐adrenoceptors through a cAMP, PKA, and PI3‐kinase‐dependent pathway stimulating conventional and novel PKCs. Endocrinology, 145, 269–280. [DOI] [PubMed] [Google Scholar]
- Chernogubova, E. , Hutchinson, D. S. , Nedergaard, J. , & Bengtsson, T. (2005). Alpha1‐ and beta1‐adrenoceptor signaling fully compensates for beta3‐adrenoceptor deficiency in brown adipocyte norepinephrine‐stimulated glucose uptake. Endocrinology, 146, 2271–2284. [DOI] [PubMed] [Google Scholar]
- Chesley, A. , Lundberg, M. S. , Asai, T. , Xiao, R. P. , Ohtani, S. , Lakatta, E. G. , & Crow, M. T. (2000). The beta(2)‐adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)‐dependent coupling to phosphatidylinositol 3′‐kinase. Circulation Research, 87, 1172–1179. 10.1161/01.RES.87.12.1172 [DOI] [PubMed] [Google Scholar]
- Connor, S. A. , Wang, Y. T. , & Nguyen, P. V. (2011). Activation of {beta}‐adrenergic receptors facilitates heterosynaptic translation‐dependent long‐term potentiation. The Journal of Physiology, 589, 4321–4340. 10.1113/jphysiol.2011.209379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, J. T. , Rodgers, J. T. , Arlow, D. H. , Vazquez, F. , Mootha, V. K. , & Puigserver, P. (2007). mTOR controls mitochondrial oxidative function through a YY1‐PGC‐1alpha transcriptional complex. Nature, 450, 736–740. 10.1038/nature06322 [DOI] [PubMed] [Google Scholar]
- Cusi, K. , Maezono, K. , Osman, A. , Pendergrass, M. , Patti, M. E. , Pratipanawatr, T. , … Mandarino, L. J. (2000). Insulin resistance differentially affects the PI 3‐kinase‐ and MAP kinase‐mediated signaling in human muscle. The Journal of Clinical Investigation, 105, 311–320. 10.1172/JCI7535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess, A. M. , Weiner, L. S. , Roberts‐Toler, C. , Franquet, E. E. , Kessler, S. H. , Kahn, P. A. , … Kolodny, G. M. (2015). Activation of human brown adipose tissue by a beta3‐adrenergic receptor agonist. Cell Metab, 21, 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallner, O. S. , Chernogubova, E. , Brolinson, K. A. , & Bengtsson, T. (2006). Beta3‐adrenergic receptors stimulate glucose uptake in brown adipocytes by two mechanisms independently of glucose transporter 4 translocation. Endocrinology, 147, 5730–5739. [DOI] [PubMed] [Google Scholar]
- Dehvari, N. , Hutchinson, D. S. , Nevzorova, J. , Dallner, O. S. , Sato, M. , Kocan, M. , … Bengtsson, T. (2012). beta(2)‐Adrenoceptors increase translocation of GLUT4 via GPCR kinase sites in the receptor C‐terminal tail. British Journal of Pharmacology, 165, 1442–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaliwal, A. S. , Habib, G. , Deswal, A. , Verduzco, M. , Souchek, J. , Ramasubbu, K. , … Bozkurt, B. (2009). Impact of alpha 1‐adrenergic antagonist use for benign prostatic hypertrophy on outcomes in patients with heart failure. The American Journal of Cardiology, 104, 270–275. [DOI] [PubMed] [Google Scholar]
- Dibble, C. C. , Asara, J. M. , & Manning, B. D. (2009). Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Molecular and Cellular Biology, 29, 5657–5670. 10.1128/MCB.00735-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibble, C. C. , Elis, W. , Menon, S. , Qin, W. , Klekota, J. , Asara, J. M. , … Manning, B. D. (2012). TBC1D7 is a third subunit of the TSC1‐TSC2 complex upstream of mTORC1. Molecular Cell, 47, 535–546. 10.1016/j.molcel.2012.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, X. J. , Fang, L. , Gao, X. M. , Kiriazis, H. , Feng, X. , Hotchkin, E. , … Graham, R. (2004). Genetic enhancement of ventricular contractility protects against pressure‐overload‐induced cardiac dysfunction. Journal of Molecular and Cellular Cardiology, 37, 979–987. [DOI] [PubMed] [Google Scholar]
- Du, X. J. , Gao, X. M. , Kiriazis, H. , Moore, X. L. , Ming, Z. , Su, Y. , … Graham, R. (2006). Transgenic alpha1A‐adrenergic activation limits post‐infarct ventricular remodeling and dysfunction and improves survival. Cardiovascular Research, 71, 735–743. 10.1016/j.cardiores.2006.06.015 [DOI] [PubMed] [Google Scholar]
- Engelhardt, S. , Hein, L. , Wiesmann, F. , & Lohse, M. J. (1999). Progressive hypertrophy and heart failure in beta1‐adrenergic receptor transgenic mice. Proceedings of the National Academy of Sciences of the United States of America, 96, 7059–7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, B. A. , Sato, M. , Sarwar, M. , Hutchinson, D. S. , & Summers, R. J. (2010). Ligand‐directed signalling at beta‐adrenoceptors. British Journal of Pharmacology, 159, 1022–1038. 10.1111/j.1476-5381.2009.00602.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, B. A. , Merlin, J. , Bengtsson, T. , & Hutchinson, D. S. (2019). Adrenoceptors in white, brown, and brite adipocytes. British Journal of Pharmacology. 10.1111/bph.14631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca, B. D. , Zakaria, C. , Jia, J. J. , Graber, T. E. , Svitkin, Y. , Tahmasebi, S. , … Damgaard, C. K. (2015). La‐related protein 1 (LARP1) represses terminal oligopyrimidine (TOP) mRNA translation downstream of mTOR complex 1 (mTORC1). The Journal of Biological Chemistry, 290, 15996–16020. 10.1074/jbc.M114.621730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen, C. M. , Hojlund, K. , Hansen, L. , Oakeley, E. J. , Hemmings, B. , Abdallah, B. M. , … Gaster, M. (2008). Transcriptional profiling of myotubes from patients with type 2 diabetes: No evidence for a primary defect in oxidative phosphorylation genes. Diabetologia, 51, 2068–2077. 10.1007/s00125-008-1122-9 [DOI] [PubMed] [Google Scholar]
- Freissmuth, M. , Hausleithner, V. , Nees, S. , Bock, M. , & Schutz, W. (1986). Cardiac ventricular beta 2‐adrenoceptors in guinea‐pigs and rats are localized on the coronary endothelium. Naunyn‐Schmiedeberg's Archives of Pharmacology, 334, 56–62. 10.1007/BF00498740 [DOI] [PubMed] [Google Scholar]
- Gan, X. , Wang, J. , Su, B. , & Wu, D. (2011). Evidence for direct activation of mTORC2 kinase activity by phosphatidylinositol 3,4,5‐trisphosphate. The Journal of Biological Chemistry, 286, 10998–11002. 10.1074/jbc.M110.195016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan, X. , Wang, J. , Wang, C. , Sommer, E. , Kozasa, T. , Srinivasula, S. , … Wu, D. (2012). PRR5L degradation promotes mTORC2‐mediated PKC‐delta phosphorylation and cell migration downstream of Galpha12. Nature Cell Biology, 14, 686–696. 10.1038/ncb2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Martinez, J. M. , & Alessi, D. R. (2008). mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum‐ and glucocorticoid‐induced protein kinase 1 (SGK1). The Biochemical Journal, 416, 375–385. 10.1042/BJ20081668 [DOI] [PubMed] [Google Scholar]
- Gelinas, J. N. , Banko, J. L. , Hou, L. , Sonenberg, N. , Weeber, E. J. , Klann, E. , & Nguyen, P. V. (2007). ERK and mTOR signaling couple beta‐adrenergic receptors to translation initiation machinery to gate induction of protein synthesis‐dependent long‐term potentiation. The Journal of Biological Chemistry, 282, 27527–27535. 10.1074/jbc.M701077200 [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Teran, B. , Lopez, J. A. , Rodriguez, E. , Leiva, L. , Martinez‐Martinez, S. , Bernal, J. A. , … Sabio, G. (2016). p38gamma and delta promote heart hypertrophy by targeting the mTOR‐inhibitory protein DEPTOR for degradation. Nature Communications, 7, 10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granneman, J. G. , & Moore, H. P. (2008). Location, location: Protein trafficking and lipolysis in adipocytes. Trends in Endocrinology and Metabolism, 19, 3–9. 10.1016/j.tem.2007.10.006 [DOI] [PubMed] [Google Scholar]
- Gschwendt, M. , Muller, H. J. , Kielbassa, K. , Zang, R. , Kittstein, W. , Rincke, G. , & Marks, F. (1994). Rottlerin, a novel protein kinase inhibitor. Biochemical and Biophysical Research Communications, 199, 93–98. 10.1006/bbrc.1994.1199 [DOI] [PubMed] [Google Scholar]
- Guerra, C. , Navarro, P. , Valverde, A. M. , Arribas, M. , Bruning, J. , Kozak, L. P. , … Benito, M. (2001). Brown adipose tissue‐specific insulin receptor knockout shows diabetic phenotype without insulin resistance. The Journal of Clinical Investigation, 108, 1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagg, A. , Colgan, T. D. , Thomson, R. E. , Qian, H. , Lynch, G. S. , & Gregorevic, P. (2016). Using AAV vectors expressing the beta2‐adrenoceptor or associated Galpha proteins to modulate skeletal muscle mass and muscle fibre size. Scientific Reports, 6, 23042 10.1038/srep23042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J. , Dibble, C. C. , Matsuzaki, M. , & Manning, B. D. (2008). The TSC1‐TSC2 complex is required for proper activation of mTOR complex 2. Molecular and Cellular Biology, 28, 4104–4115. 10.1128/MCB.00289-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y. , Wright, C. D. , Merkwan, C. L. , Baye, N. L. , Liang, Q. , Simpson, P. C. , & O'Connell, T. D. (2007). An alpha1A‐adrenergic‐extracellular signal‐regulated kinase survival signaling pathway in cardiac myocytes. Circulation, 115, 763–772. 10.1161/CIRCULATIONAHA.106.664862 [DOI] [PubMed] [Google Scholar]
- Hudson, C. C. , Liu, M. , Chiang, G. G. , Otterness, D. M. , Loomis, D. C. , Kaper, F. , … Abraham, R. T. (2002). Regulation of hypoxia‐inducible factor 1alpha expression and function by the mammalian target of rapamycin. Molecular and Cellular Biology, 22, 7004–7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson, D. S. , Chernogubova, E. , Dallner, O. S. , Cannon, B. , & Bengtsson, T. (2005). Beta‐adrenoceptors, but not alpha‐adrenoceptors, stimulate AMP‐activated protein kinase in brown adipocytes independently of uncoupling protein‐1. Diabetologia, 48, 2386–2395. 10.1007/s00125-005-1936-7 [DOI] [PubMed] [Google Scholar]
- Inokuma, K. , Ogura‐Okamatsu, Y. , Toda, C. , Kimura, K. , Yamashita, H. , & Saito, M. (2005). Uncoupling protein 1 is necessary for norepinephrine‐induced glucose utilization in brown adipose tissue. Diabetes, 54, 1385–1391. 10.2337/diabetes.54.5.1385 [DOI] [PubMed] [Google Scholar]
- Iwai‐Kanai, E. , Hasegawa, K. , Araki, M. , Kakita, T. , Morimoto, T. , & Sasayama, S. (1999). alpha‐ and beta‐adrenergic pathways differentially regulate cell type‐specific apoptosis in rat cardiac myocytes. Circulation, 100, 305–311. [DOI] [PubMed] [Google Scholar]
- Jacinto, E. , Facchinetti, V. , Liu, D. , Soto, N. , Wei, S. , Jung, S. Y. , … Su, B. (2006). SIN1/MIP1 maintains rictor‐mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell, 127, 125–137. 10.1016/j.cell.2006.08.033 [DOI] [PubMed] [Google Scholar]
- Jacinto, E. , Loewith, R. , Schmidt, A. , Lin, S. , Ruegg, M. A. , Hall, A. , & Hall, M. N. (2004). Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nature Cell Biology, 6, 1122–1128. 10.1038/ncb1183 [DOI] [PubMed] [Google Scholar]
- Jensen, B. C. , O'Connell, T. D. , & Simpson, P. C. (2011). Alpha‐1‐adrenergic receptors: Targets for agonist drugs to treat heart failure. Journal of Molecular and Cellular Cardiology, 51, 518–528. 10.1016/j.yjmcc.2010.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, B. C. , Swigart, P. M. , De Marco, T. , Hoopes, C. , & Simpson, P. C. (2009). {alpha}1‐Adrenergic receptor subtypes in nonfailing and failing human myocardium. Circulation. Heart Failure, 2, 654–663. 10.1161/CIRCHEARTFAILURE.108.846212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesinkey, S. R. , Korrapati, M. C. , Rasbach, K. A. , Beeson, C. C. , & Schnellmann, R. G. (2014). Atomoxetine prevents dexamethasone‐induced skeletal muscle atrophy in mice. The Journal of Pharmacology and Experimental Therapeutics, 351, 663–673. 10.1124/jpet.114.217380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaizuka, T. , Hara, T. , Oshiro, N. , Kikkawa, U. , Yonezawa, K. , Takehana, K. , … Mizushima, N. (2010). Tti1 and Tel2 are critical factors in mammalian target of rapamycin complex assembly. The Journal of Biological Chemistry, 285, 20109–20116. 10.1074/jbc.M110.121699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaumann, A. J. , Hall, J. A. , Murray, K. J. , Wells, F. C. , & Brown, M. J. (1989). A comparison of the effects of adrenaline and noradrenaline on human heart: The role of beta 1‐ and beta 2‐adrenoceptors in the stimulation of adenylate cyclase and contractile force. European Heart Journal, 10(Suppl B), 29–37. 10.1093/eurheartj/10.suppl_B.29 [DOI] [PubMed] [Google Scholar]
- Kennedy, B. K. , & Lamming, D. W. (2016). The mechanistic target of rapamycin: The grand conducTOR of metabolism and aging. Cell Metabolism, 23, 990–1003. 10.1016/j.cmet.2016.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna, N. , Fang, Y. , Yoon, M. S. , & Chen, J. (2013). XPLN is an endogenous inhibitor of mTORC2. Proceedings of the National Academy of Sciences of the United States of America, 110, 15979–15984. 10.1073/pnas.1310434110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D. H. , Sarbassov, D. D. , Ali, S. M. , King, J. E. , Latek, R. R. , Erdjument‐Bromage, H. , … Sabatini, D. M. (2002). mTOR interacts with raptor to form a nutrient‐sensitive complex that signals to the cell growth machinery. Cell, 110, 163–175. 10.1016/S0092-8674(02)00808-5 [DOI] [PubMed] [Google Scholar]
- Kim, D. H. , Sarbassov, D. D. , Ali, S. M. , Latek, R. R. , Guntur, K. V. , Erdjument‐Bromage, H. , … Sabatini, D. M. (2003). GbetaL, a positive regulator of the rapamycin‐sensitive pathway required for the nutrient‐sensitive interaction between raptor and mTOR. Molecular Cell, 11, 895–904. 10.1016/S1097-2765(03)00114-X [DOI] [PubMed] [Google Scholar]
- Kim, J. , Kundu, M. , Viollet, B. , & Guan, K. L. (2011). AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nature Cell Biology, 13, 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, J. , Fasshauer, M. , Klein, H. H. , Benito, M. , & Kahn, C. R. (2002). Novel adipocyte lines from brown fat: A model system for the study of differentiation, energy metabolism, and insulin action. BioEssays, 24, 382–388. 10.1002/bies.10058 [DOI] [PubMed] [Google Scholar]
- Knight, Z. A. , Gonzalez, B. , Feldman, M. E. , Zunder, E. R. , Goldenberg, D. D. , Williams, O. , … Shokat, K. M. (2006). A pharmacological map of the PI3‐K family defines a role for p110alpha in insulin signaling. Cell, 125, 733–747. 10.1016/j.cell.2006.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer, G. , Zamzami, N. , & Susin, S. A. (1997). Mitochondrial control of apoptosis. Immunology Today, 18, 44–51. 10.1016/S0167-5699(97)80014-X [DOI] [PubMed] [Google Scholar]
- Kumar, A. , Harris, T. E. , Keller, S. R. , Choi, K. M. , Magnuson, M. A. , & Lawrence, J. C. Jr. (2008). Muscle‐specific deletion of rictor impairs insulin‐stimulated glucose transport and enhances Basal glycogen synthase activity. Molecular and Cellular Biology, 28, 61–70. 10.1128/MCB.01405-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, A. , Lawrence, J. C. Jr. , Jung, D. Y. , Ko, H. J. , Keller, S. R. , Kim, J. K. , … Harris, T. E. (2010). Fat cell‐specific ablation of rictor in mice impairs insulin‐regulated fat cell and whole‐body glucose and lipid metabolism. Diabetes, 59, 1397–1406. 10.2337/db09-1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante, M. , & Sabatini, D. M. (2012). mTOR signaling in growth control and disease. Cell, 149, 274–293. 10.1016/j.cell.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante, M. , & Sabatini, D. M. (2013). Regulation of mTORC1 and its impact on gene expression at a glance. Journal of Cell Science, 126, 1713–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire, I. , Ducharme, A. , Tardif, J. C. , Poulin, F. , Jones, L. R. , Allen, B. G. , … Rindt, H. (2001). Cardiac‐directed overexpression of wild‐type alpha1B‐adrenergic receptor induces dilated cardiomyopathy. American Journal of Physiology. Heart and Circulatory Physiology, 281, H931–H938. [DOI] [PubMed] [Google Scholar]
- Li, X. , & Gao, T. (2014). mTORC2 phosphorylates protein kinase Czeta to regulate its stability and activity. EMBO Reports, 15, 191–198. 10.1002/embr.201338119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, F. , Owens, W. A. , Chen, S. , Stevens, M. E. , Kesteven, S. , Arthur, J. F. , … Graham, R. M. (2001). Targeted alpha(1A)‐adrenergic receptor overexpression induces enhanced cardiac contractility but not hypertrophy. Circulation Research, 89, 343–350. [DOI] [PubMed] [Google Scholar]
- Liu, D. , Bordicchia, M. , Zhang, C. , Fang, H. , Wei, W. , Li, J. L. , … Collins, S. (2016). Activation of mTORC1 is essential for beta‐adrenergic stimulation of adipose browning. The Journal of Clinical Investigation, 126, 1704–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Perusse, F. , & Bukowiecki, L. J. (1994). Chronic norepinephrine infusion stimulates glucose uptake in white and brown adipose tissues. The American Journal of Physiology, 266, R914–R920. [DOI] [PubMed] [Google Scholar]
- Lynch, G. S. , & Ryall, J. G. (2008). Role of beta‐adrenoceptor signaling in skeletal muscle: Implications for muscle wasting and disease. Physiological Reviews, 88, 729–767. 10.1152/physrev.00028.2007 [DOI] [PubMed] [Google Scholar]
- Mackey, A. M. , Sarkes, D. A. , Bettencourt, I. , Asara, J. M. , & Rameh, L. E. (2014). PIP4kgamma is a substrate for mTORC1 that maintains basal mTORC1 signaling during starvation. Science Signaling, 7, ra104 10.1126/scisignal.2005191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson, Y. , Wallukat, G. , Waagstein, F. , Hjalmarson, A. , & Hoebeke, J. (1994). Autoimmunity in idiopathic dilated cardiomyopathy. Characterization of antibodies against the beta 1‐adrenoceptor with positive chronotropic effect. Circulation, 89, 2760–2767. 10.1161/01.CIR.89.6.2760 [DOI] [PubMed] [Google Scholar]
- Martiny‐Baron, G. , Kazanietz, M. G. , Mischak, H. , Blumberg, P. M. , Kochs, G. , Hug, H. , … Schächtele, C. (1993). Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. The Journal of Biological Chemistry, 268, 9194–9197. [PubMed] [Google Scholar]
- McCloskey, D. T. , Turnbull, L. , Swigart, P. , O'Connell, T. D. , Simpson, P. C. , & Baker, A. J. (2003). Abnormal myocardial contraction in alpha(1A)‐ and alpha(1B)‐adrenoceptor double‐knockout mice. Journal of Molecular and Cellular Cardiology, 35, 1207–1216. 10.1016/S0022-2828(03)00227-X [DOI] [PubMed] [Google Scholar]
- Merlin, J. , Evans, B. A. , Dehvari, N. , Sato, M. , Bengtsson, T. , & Hutchinson, D. S. (2016). Could burning fat start with a brite spark? Pharmacological and nutritional ways to promote thermogenesis. Molecular Nutrition & Food Research, 60, 18–42. 10.1002/mnfr.201500251 [DOI] [PubMed] [Google Scholar]
- Merlin, J. , Sato, M. , Nowell, C. , Pakzad, M. , Fahey, R. , Gao, J. , … Hutchinson, D. S. (2018). The PPARgamma agonist rosiglitazone promotes the induction of brite adipocytes, increasing beta‐adrenoceptor‐mediated mitochondrial function and glucose uptake. Cellular Signalling, 42, 54–66. 10.1016/j.cellsig.2017.09.023 [DOI] [PubMed] [Google Scholar]
- Mohl, M. C. , Iismaa, S. E. , Xiao, X. H. , Friedrich, O. , Wagner, S. , Nikolova‐Krstevski, V. , … Graham, R. M. (2011). Regulation of murine cardiac contractility by activation of alpha(1A)‐adrenergic receptor‐operated Ca(2+) entry. Cardiovascular Research, 91, 310–319. [DOI] [PubMed] [Google Scholar]
- Montgomery, M. D. , Chan, T. , Swigart, P. M. , Myagmar, B. E. , Dash, R. , & Simpson, P. C. (2017). An alpha‐1A adrenergic receptor agonist prevents acute doxorubicin cardiomyopathy in male mice. PLoS ONE, 12, e0168409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganstein, D. L. , Wu, P. , Mane, M. R. , Fisk, N. M. , White, R. , & Parker, M. G. (2010). Human fetal mesenchymal stem cells differentiate into brown and white adipocytes: A role for ERRalpha in human UCP1 expression. Cell Research, 20, 434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisco, C. , Marrone, C. , Galeotti, J. , Shao, D. , Vatner, D. E. , Vatner, S. F. , … Sadoshima, J. (2008). Endocytosis machinery is required for beta1‐adrenergic receptor‐induced hypertrophy in neonatal rat cardiac myocytes. Cardiovascular Research, 78, 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins, G. R. , Wang, L. , Raje, V. , Sherwood, S. G. , Grande, R. C. , Boroda, S. , … Harris, T. E. (2014). Catecholamine‐induced lipolysis causes mTOR complex dissociation and inhibits glucose uptake in adipocytes. Proceedings of the National Academy of Sciences of the United States of America, 111, 17450–17455. 10.1073/pnas.1410530111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevzorova, J. , Bengtsson, T. , Evans, B. A. , & Summers, R. J. (2002). Characterization of the beta‐adrenoceptor subtype involved in mediation of glucose transport in L6 cells. British Journal of Pharmacology, 137, 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevzorova, J. , Evans, B. A. , Bengtsson, T. , & Summers, R. J. (2006). Multiple signalling pathways involved in beta2‐adrenoceptor‐mediated glucose uptake in rat skeletal muscle cells. British Journal of Pharmacology, 147, 446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonogaki, K. (2000). New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia, 43, 533–549. 10.1007/s001250051341 [DOI] [PubMed] [Google Scholar]
- O'Connell, T. D. , Ishizaka, S. , Nakamura, A. , Swigart, P. M. , Rodrigo, M. C. , Simpson, G. L. , … Simpson, P. C. (2003). The alpha(1A/C)‐ and alpha(1B)‐adrenergic receptors are required for physiological cardiac hypertrophy in the double‐knockout mouse. The Journal of Clinical Investigation, 111, 1783–1791. 10.1172/JCI200316100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell, T. D. , Jensen, B. C. , Baker, A. J. , & Simpson, P. C. (2014). Cardiac alpha1‐adrenergic receptors: Novel aspects of expression, signaling mechanisms, physiologic function, and clinical importance. Pharmacological Reviews, 66, 308–333. 10.1124/pr.112.007203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell, T. D. , Swigart, P. M. , Rodrigo, M. C. , Ishizaka, S. , Joho, S. , Turnbull, L. , … Simpson, P. C. (2006). Alpha1‐adrenergic receptors prevent a maladaptive cardiac response to pressure overload. The Journal of Clinical Investigation, 116, 1005–1015. 10.1172/JCI22811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor, C. M. , Gattis, W. A. , Zannad, F. , McNulty, S. E. , Gheorghiade, M. , Adams, K. F. , … Swedberg, K. (1999). Beta‐blocker therapy in advanced heart failure: Clinical characteristics and long‐term outcomes. European Journal of Heart Failure, 1, 81–88. 10.1016/S1388-9842(98)00004-X [DOI] [PubMed] [Google Scholar]
- Olsen, J. M. , Sato, M. , Dallner, O. S. , Sandstrom, A. L. , Pisani, D. F. , Chambard, J. C. , … Bengtsson, T. (2014). Glucose uptake in brown fat cells is dependent on mTOR complex 2‐promoted GLUT1 translocation. The Journal of Cell Biology, 207, 365–374. 10.1083/jcb.201403080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. , Leong, M. L. , Buse, P. , Maiyar, A. C. , Firestone, G. L. , & Hemmings, B. A. (1999). Serum and glucocorticoid‐inducible kinase (SGK) is a target of the PI 3‐kinase‐stimulated signaling pathway. The EMBO Journal, 18, 3024–3033. 10.1093/emboj/18.11.3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce, L. R. , Huang, X. , Boudeau, J. , Pawlowski, R. , Wullschleger, S. , Deak, M. , … Alessi, D. R. (2007). Identification of Protor as a novel Rictor‐binding component of mTOR complex‐2. The Biochemical Journal, 405, 513–522. 10.1042/BJ20070540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearen, M. A. , Ryall, J. G. , Lynch, G. S. , & Muscat, G. E. (2009). Expression profiling of skeletal muscle following acute and chronic beta2‐adrenergic stimulation: Implications for hypertrophy, metabolism and circadian rhythm. BMC Genomics, 10, 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, T. R. , Laplante, M. , Thoreen, C. C. , Sancak, Y. , Kang, S. A. , Kuehl, W. M. , … Sabatini, D. M. (2009). DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell, 137, 873–886. 10.1016/j.cell.2009.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic, N. , Walden, T. B. , Shabalina, I. G. , Timmons, J. A. , Cannon, B. , & Nedergaard, J. (2010). Chronic peroxisome proliferator‐activated receptor g (PPARg) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1‐containing adipocytes molecularly distinct from classic brown adipocytes. The Journal of Biological Chemistry, 285, 7153–7164. 10.1074/jbc.M109.053942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak, P. , Cybulski, N. , Feige, J. N. , Auwerx, J. , Ruegg, M. A. , & Hall, M. N. (2008). Adipose‐specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metabolism, 8, 399–410. 10.1016/j.cmet.2008.09.003 [DOI] [PubMed] [Google Scholar]
- Puceat, M. , Hilal‐Dandan, R. , Strulovici, B. , Brunton, L. L. , & Brown, J. H. (1994). Differential regulation of protein kinase C isoforms in isolated neonatal and adult rat cardiomyocytes. The Journal of Biological Chemistry, 269, 16938–16944. [PubMed] [Google Scholar]
- Rockman, H. A. , Koch, W. J. , & Lefkowitz, R. J. (2002). Seven‐transmembrane‐spanning receptors and heart function. Nature, 415, 206–212. 10.1038/415206a [DOI] [PubMed] [Google Scholar]
- Rorabaugh, B. R. , Ross, S. A. , Gaivin, R. J. , Papay, R. S. , McCune, D. F. , Simpson, P. C. , & Perez, D. (2005). alpha1A‐ but not alpha1B‐adrenergic receptors precondition the ischemic heart by a staurosporine‐sensitive, chelerythrine‐insensitive mechanism. Cardiovascular Research, 65, 436–445. 10.1016/j.cardiores.2004.10.009 [DOI] [PubMed] [Google Scholar]
- Rousseau, A. , & Bertolotti, A. (2016). An evolutionarily conserved pathway controls proteasome homeostasis. Nature, 536, 184–189. 10.1038/nature18943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak, Y. , Thoreen, C. C. , Peterson, T. R. , Lindquist, R. A. , Kang, S. A. , Spooner, E. , … Sabatini, D. M. (2007). PRAS40 is an insulin‐regulated inhibitor of the mTORC1 protein kinase. Molecular Cell, 25, 903–915. 10.1016/j.molcel.2007.03.003 [DOI] [PubMed] [Google Scholar]
- Sarbassov, D. D. , Ali, S. M. , Kim, D. H. , Guertin, D. A. , Latek, R. R. , Erdjument‐Bromage, H. , … Sabatini, D. M. (2004). Rictor, a novel binding partner of mTOR, defines a rapamycin‐insensitive and raptor‐independent pathway that regulates the cytoskeleton. Current Biology, 14, 1296–1302. 10.1016/j.cub.2004.06.054 [DOI] [PubMed] [Google Scholar]
- Sarbassov, D. D. , Guertin, D. A. , Ali, S. M. , & Sabatini, D. M. (2005). Phosphorylation and regulation of Akt/PKB by the rictor‐mTOR complex. Science, 307, 1098–1101. 10.1126/science.1106148 [DOI] [PubMed] [Google Scholar]
- Sato, M. , Dehvari, N. , Oberg, A. I. , Dallner, O. S. , Sandstrom, A. L. , Olsen, J. M. , … Bengtsson, T. (2014). Improving type 2 diabetes through a distinct adrenergic signaling pathway involving mTORC2 that mediates glucose uptake in skeletal muscle. Diabetes, 63, 4115–4129. 10.2337/db13-1860 [DOI] [PubMed] [Google Scholar]
- Sato, M. , Dehvari, N. , Oberg, A. I. , Summers, R. J. , Hutchinson, D. S. , & Bengtsson, T. (2014). Response to comment on Sato et al. Improving type 2 diabetes through a distinct adrenergic signaling pathway involving mTORC2 that mediates glucose uptake in skeletal muscle. Diabetes 2014;63:4115‐4129. Diabetes, 63, e22–e23. [DOI] [PubMed] [Google Scholar]
- Sato, M. , Evans, B. A. , Sandstrom, A. L. , Chia, L. Y. , Mukaida, S. , Thai, B. S. , … Bengtsson, T. (2018). Alpha1A‐adrenoceptors activate mTOR signalling and glucose uptake in cardiomyocytes. Biochemical Pharmacology, 148, 27–40. 10.1016/j.bcp.2017.11.016 [DOI] [PubMed] [Google Scholar]
- Sato, M. , Hutchinson, D. S. , Halls, M. L. , Furness, S. G. , Bengtsson, T. , Evans, B. A. , & Summers, R. J. (2012). Interaction with caveolin‐1 modulates G protein coupling of mouse beta3‐adrenoceptor. The Journal of Biological Chemistry, 287, 20674–20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton, R. A. , & Sabatini, D. M. (2017). mTOR signaling in growth, metabolism, and disease. Cell, 169, 361–371. 10.1016/j.cell.2017.03.035 [DOI] [PubMed] [Google Scholar]
- Schulz, T. J. , Huang, P. , Huang, T. L. , Xue, R. , McDougall, L. E. , Townsend, K. L. , … Tseng, Y.‐H. (2013). Brown‐fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature, 495, 379–383. 10.1038/nature11943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciarretta, S. , Zhai, P. , Maejima, Y. , Del Re, D. P. , Nagarajan, N. , Yee, D. , … Sadoshima, J. (2015). mTORC2 regulates cardiac response to stress by inhibiting MST1. Cell Reports, 11, 125–136. 10.1016/j.celrep.2015.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, P. H. , & Lawrence, J. C. Jr. (1998). Attenuation of mammalian target of rapamycin activity by increased cAMP in 3T3‐L1 adipocytes. The Journal of Biological Chemistry, 273, 34496–34501. [DOI] [PubMed] [Google Scholar]
- Seale, P. , Conroe, H. M. , Estall, J. , Kajimura, S. , Frontini, A. , Ishibashi, J. , … Spiegelman, B. M. (2011). Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. The Journal of Clinical Investigation, 121, 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre, C. , Zoncu, R. , Medina, D. L. , Vetrini, F. , Erdin, S. , Erdin, S. , … Ballabio, A. (2012). A lysosome‐to‐nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. The EMBO Journal, 31, 1095–1108. 10.1038/emboj.2012.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shende, P. , Xu, L. , Morandi, C. , Pentassuglia, L. , Heim, P. , Lebboukh, S. , … Brink, M. (2016). Cardiac mTOR complex 2 preserves ventricular function in pressure‐overload hypertrophy. Cardiovascular Research, 109, 103–114. 10.1093/cvr/cvv252 [DOI] [PubMed] [Google Scholar]
- Shi, T. , Papay, R. S. , & Perez, D. M. (2016). alpha1A‐Adrenergic receptor prevents cardiac ischemic damage through PKCdelta/GLUT1/4‐mediated glucose uptake. Journal of Receptor and Signal Transduction Research, 36, 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata, H. , Perusse, F. , Vallerand, A. , & Bukowiecki, L. J. (1989). Cold exposure reverses inhibitory effects of fasting on peripheral glucose uptake in rats. The American Journal of Physiology, 257, R96–R101. 10.1152/ajpregu.1989.257.1.R96 [DOI] [PubMed] [Google Scholar]
- Shimizu, Y. , Nikami, H. , & Saito, M. (1991). Sympathetic activation of glucose utilization in brown adipose tissue in rats. Journal of Biochemistry, 110, 688–692. 10.1093/oxfordjournals.jbchem.a123642 [DOI] [PubMed] [Google Scholar]
- Shinoda, K. , Luijten, I. H. , Hasegawa, Y. , Hong, H. , Sonne, S. B. , Kim, M. , … Kajimura, S. (2015). Genetic and functional characterization of clonally derived adult human brown adipocytes. Nature Medicine, 21, 389–394. 10.1038/nm.3819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirenko, S. , Maltsev, V. A. , Maltseva, L. A. , Yang, D. , Lukyanenko, Y. , Vinogradova, T. M. , … Lakatta, E. G. (2014). Sarcoplasmic reticulum Ca2+ cycling protein phosphorylation in a physiologic Ca2+ milieu unleashes a high‐power, rhythmic Ca2+ clock in ventricular myocytes: Relevance to arrhythmias and bio‐pacemaker design. Journal of Molecular and Cellular Cardiology, 66, 106–115. 10.1016/j.yjmcc.2013.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skomedal, T. , Borthne, K. , Aass, H. , Geiran, O. , & Osnes, J. B. (1997). Comparison between alpha‐1 adrenoceptor‐mediated and beta adrenoceptor‐mediated inotropic components elicited by norepinephrine in failing human ventricular muscle. The Journal of Pharmacology and Experimental Therapeutics, 280, 721–729. [PubMed] [Google Scholar]
- Sriram, K. , & Insel, P. A. (2018). G protein‐coupled receptors as targets for approved drugs: How many targets and how many drugs? Molecular Pharmacology, 93, 251–258. 10.1124/mol.117.111062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg, S. F. , Goldberg, M. , & Rybin, V. O. (1995). Protein kinase C isoform diversity in the heart. Journal of Molecular and Cellular Cardiology, 27, 141–153. [DOI] [PubMed] [Google Scholar]
- Tao, R. , Xiong, X. , Liangpunsakul, S. , & Dong, X. C. (2015). Sestrin 3 protein enhances hepatic insulin sensitivity by direct activation of the mTORC2‐Akt signaling. Diabetes, 64, 1211–1223. 10.2337/db14-0539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiebe, M. , Lutz, M. , De La Garza, A. , Buechling, T. , Boutros, M. , & Teleman, A. A. (2015). REPTOR and REPTOR‐BP regulate organismal metabolism and transcription downstream of TORC1. Developmental Cell, 33, 272–284. 10.1016/j.devcel.2015.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin, A. B. , Butcher, A. J. , & Kong, K. C. (2008). Location, location, location … site‐specific GPCR phosphorylation offers a mechanism for cell‐type‐specific signalling. Trends in Pharmacological Sciences, 29, 413–420. 10.1016/j.tips.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toullec, D. , Pianetti, P. , Coste, H. , Bellevergue, P. , Grand‐Perret, T. , Ajakane, M. , … Loriolle, F. (1991). The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. The Journal of Biological Chemistry, 266, 15771–15781. [PubMed] [Google Scholar]
- Tran, C. M. , Mukherjee, S. , Ye, L. , Frederick, D. W. , Kissig, M. , Davis, J. G. , … Baur, J. A. (2016). Rapamycin blocks induction of the thermogenic program in white adipose tissue. Diabetes, 65, 927–941. 10.2337/db15-0502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchione, C. , Fratta, L. , Rizzoni, D. , Notte, A. , Poulet, R. , Porteri, E. , … Lembo, G. (2002). Cardiovascular influences of alpha1b‐adrenergic receptor defect in mice. Circulation, 105, 1700–1707. 10.1161/01.CIR.0000012750.08480.55 [DOI] [PubMed] [Google Scholar]
- Volkers, M. , Konstandin, M. H. , Doroudgar, S. , Toko, H. , Quijada, P. , Din, S. , … Sussman, M. A. (2013). Mechanistic target of rapamycin complex 2 protects the heart from ischemic damage. Circulation, 128, 2132–2144. 10.1161/CIRCULATIONAHA.113.003638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, B. H. , Du, X. J. , Autelitano, D. J. , Milano, C. A. , & Woodcock, E. A. (2000). Adverse effects of constitutively active alpha(1B)‐adrenergic receptors after pressure overload in mouse hearts. American Journal of Physiology. Heart and Circulatory Physiology, 279, H1079–H1086. [DOI] [PubMed] [Google Scholar]
- Wang, L. , Gout, I. , & Proud, C. G. (2001). Cross‐talk between the ERK and p70 S6 kinase (S6K) signaling pathways. MEK‐dependent activation of S6K2 in cardiomyocytes. The Journal of Biological Chemistry, 276, 32670–32677. 10.1074/jbc.M102776200 [DOI] [PubMed] [Google Scholar]
- Wang, L. , Hao, H. , Wang, J. , Wang, X. , Zhang, S. , Du, Y. , … Liu, H. (2015). Decreased autophagy: A major factor for cardiomyocyte death induced by beta1‐adrenoceptor autoantibodies. Cell Death & Disease, 6, e1862 10.1038/cddis.2015.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Lu, K. , Hao, H. , Li, X. , Wang, J. , Wang, K. , … Liu, H. (2013). Decreased autophagy in rat heart induced by anti‐beta1‐adrenergic receptor autoantibodies contributes to the decline in mitochondrial membrane potential. PLoS ONE, 8, e81296 10.1371/journal.pone.0081296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , & Proud, C. G. (2002). Ras/Erk signaling is essential for activation of protein synthesis by Gq protein‐coupled receptor agonists in adult cardiomyocytes. Circulation Research, 91, 821–829. 10.1161/01.RES.0000041029.97988.E9 [DOI] [PubMed] [Google Scholar]
- Wang, L. , Rolfe, M. , & Proud, C. G. (2003). Ca(2+)‐independent protein kinase C activity is required for alpha1‐adrenergic‐receptor‐mediated regulation of ribosomal protein S6 kinases in adult cardiomyocytes. The Biochemical Journal, 373, 603–611. 10.1042/bj20030454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, A. Y. , Song, Y. , Xiao, R. P. , & Zhu, W. (2015). Biased beta2‐adrenoceptor signalling in heart failure: Pathophysiology and drug discovery. British Journal of Pharmacology, 172, 5444–5456. 10.1111/bph.12965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J. , Bostrom, P. , Sparks, L. M. , Ye, L. , Choi, J. H. , Giang, A. H. , … Spiegelman, B. M. (2012). Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell, 150, 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, R. P. , Cheng, H. , Zhou, Y. Y. , Kuschel, M. , & Lakatta, E. G. (1999). Recent advances in cardiac beta(2)‐adrenergic signal transduction. Circulation Research, 85, 1092–1100. 10.1161/01.RES.85.11.1092 [DOI] [PubMed] [Google Scholar]
- Xu, L. , & Brink, M. (2016). mTOR, cardiomyocytes and inflammation in cardiac hypertrophy. Biochimica et Biophysica Acta, 1863, 1894–1903. 10.1016/j.bbamcr.2016.01.003 [DOI] [PubMed] [Google Scholar]
- Xu, Y. , Lai, E. , Liu, J. , Lin, J. , Yang, C. , Jia, C. , … Li, M. (2013). IKK interacts with rictor and regulates mTORC2. Cellular Signalling, 25, 2239–2245. 10.1016/j.cellsig.2013.07.008 [DOI] [PubMed] [Google Scholar]
- Yano, T. , Ferlito, M. , Aponte, A. , Kuno, A. , Miura, T. , Murphy, E. , & Steenbergen, C. (2014). Pivotal role of mTORC2 and involvement of ribosomal protein S6 in cardioprotective signaling. Circulation Research, 114, 1268–1280. 10.1161/CIRCRESAHA.114.303562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, M. A. , Vatner, D. E. , & Vatner, S. F. (1990). Alpha‐ and beta‐adrenergic control of large coronary arteries in conscious calves. Basic Research in Cardiology, 85(Suppl 1), 97–109. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Nicholatos, J. , Dreier, J. R. , Ricoult, S. J. , Widenmaier, S. B. , Hotamisligil, G. S. , … Manning, B. D. (2014). Coordinated regulation of protein synthesis and degradation by mTORC1. Nature, 513, 440–443. 10.1038/nature13492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, J. , Zhai, B. , Gygi, S. P. , & Goldberg, A. L. (2015). mTOR inhibition activates overall protein degradation by the ubiquitin proteasome system as well as by autophagy. Proceedings of the National Academy of Sciences of the United States of America, 112, 15790–15797. 10.1073/pnas.1521919112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X. , Balaji, P. , Pachon, R. , Beniamen, D. M. , Vatner, D. E. , Graham, R. M. , & Vatner, S. F. (2015). Overexpression of cardiomyocyte alpha1A‐adrenergic receptors attenuates postinfarct remodeling by inducing angiogenesis through heterocellular signaling. Arteriosclerosis, Thrombosis, and Vascular Biology, 35, 2451–2459. 10.1161/ATVBAHA.115.305919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, P. , & Pu, W. T. (2016). Recounting cardiac cellular composition. Circulation Research, 118, 368–370. 10.1161/CIRCRESAHA.116.308139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, W. Z. , Zheng, M. , Koch, W. J. , Lefkowitz, R. J. , Kobilka, B. K. , & Xiao, R. P. (2001). Dual modulation of cell survival and cell death by beta(2)‐adrenergic signaling in adult mouse cardiac myocytes. Proceedings of the National Academy of Sciences of the United States of America, 98, 1607–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]


