Abstract
β3‐Adrenoceptor agonists have proven useful in the treatment of overactive bladder syndrome, but it is not known whether their efficacy during chronic administration may be limited by receptor‐induced desensitisation. Whereas the β2‐adrenoceptor has phosphorylation sites that are important for desensitisation, the β3‐adrenoceptor lacks these; therefore, it had been assumed that β3‐adrenoceptors are largely resistant to agonist‐induced desensitisation. While all direct comparative studies demonstrate that β3‐adrenoceptors are less susceptible to desensitisation than β2‐adrenoceptors, desensitisation of β3‐adrenoceptors has been observed in many models and treatment settings. Chimeric β2‐ and β3‐adrenoceptors have demonstrated that the C‐terminal tail of the receptor plays an important role in the relative resistance to desensitisation but is not the only relevant factor. While the evidence from some models, such as transfected CHO cells, is inconsistent, it appears that desensitisation is observed more often after long‐term (hours to days) than short‐term (minutes to hours) agonist exposure. When it occurs, desensitisation of β3‐adrenoceptors can involve multiple levels including down‐regulation of its mRNA and the receptor protein and alterations in post‐receptor signalling events. The relative contributions of these mechanistic factors apparently depend on the cell type under investigation. Which if any of these factors is applicable to the human urinary bladder remains to be determined.
Linked Articles
This article is part of a themed section on Adrenoceptors—New Roles for Old Players. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.14/issuetoc
1. INTRODUCTION
In 1967, Lands, Arnold, McAuliff, Luduena, and Brown (1967) proposed a subdivision of β‐adrenoceptors into β1 and β2. Soon thereafter, it emerged that some β‐adrenergic‐like responses were not mediated by either of these two subtypes (Furchgott, 1972). This included lipolytic responses in rat white adipose tissue (Harms, Zaagsma, & van der Wal, 1974) and smooth muscle relaxation responses in rat colon (Bianchetti & Manara, 1990) and human urinary bladder (Nergardh, Boreus, & Naglo, 1977), which looked to be mediated by β‐adrenoceptors based on the order of potency of catecholamines. However, the existence of a third subtype, the β3‐adrenoceptor, only became fully accepted after it was cloned in 1989 (Emorine et al., 1989). β3‐adrenoceptors have a more restricted expression pattern in humans than β1‐ or β2‐adrenoceptors (Michel & Gravas, 2016) and have a unique and species‐dependent ligand recognition profile, including a low affinity for propranolol and several other classic antagonists previously considered to block all β‐adrenoceptor subtypes (Cernecka, Sand, & Michel, 2014). Some agonists including mirabegron and solabegron have higher affinity for the human than the rodent β3‐adrenoceptor, whereas others including BRL 27,344 and ritobegron have higher affinity for the rodent receptor. Similarly, some agonists including CL 316,243 have greater efficacy at the rodent than the human receptor (Baker, 2005). While β3‐adrenoceptors play a key role in lipolysis in rodents, particularly in brown adipose tissue, brown adipose tissue is largely lacking in adult humans, and lipolysis in white adipose tissue involves β3‐adrenoceptor only to a minor extent, if at all, in humans (Lönnqvist et al., 1993). Finally, the mouse β3‐adrenoceptor gene has splice variants, whereas the human orthologue does not (Evans, Papaioannou, Hamilton, & Summers, 1999).
Recently, β3‐adrenoceptor agonists have been introduced clinically for the treatment of the overactive bladder syndrome. While they effectively improve bladder symptoms and are well tolerated (Chapple, Cardozo, Nitti, Siddiqui, & Michel, 2014; Ohlstein, von Keitz, & Michel, 2012; Yoshida, Takeda, Gotoh, Nagai, & Kurose, 2018), they are not curative, implying a need for long‐term treatment. Other β‐adrenoceptor subtypes exhibit desensitisation upon extended exposure to an agonist, for instance, in the use of β2‐adrenoceptor agonists for treatment of preterm labour (Engelhardt et al., 1997; Frambach et al., 2005; Michel, Pingsmann, Nohlen, Siekmann, & Brodde, 1989). This can limit the effectiveness of this treatment for women with preterm labour (The Canadian Preterm Labor Investigators Group, 1992) and animal models thereof (Caritis, Chiao, & Kridgen, 1991; Lye, Dayes, Freitag, Brooks, & Casper, 1992). β2‐adrenoceptors also exhibit desensitisation upon chronic use in patients with asthma (Salpeter, Ormiston, & Salpeter, 2004), although it is debatable whether this is a treatment‐limiting effect. Therefore, the question arises as to whether β3‐adrenoceptors similarly undergo agonist‐induced desensitisation and how this affects their therapeutic efficacy in long‐term use.
The β2‐adrenoceptor is the prototype for studying agonist‐induced regulation of GPCRs (Lefkowitz, 1998). Agonist‐induced β2‐adrenoceptor desensitisation can occur within seconds to minutes, which is often studied by determining the proxy parameter, cAMP production or receptor internalisation (January et al., 1997). It can also occur after more prolonged agonist exposure (hours to days), which often involves down‐regulation of the receptor at the protein level as a result of internalisation followed by lysosomal degradation and/or reduced mRNA abundance, which in turn can be due to decreased transcription and/or mRNA stability (Bouvier et al., 1989). Moreover, it can involve regulation of G protein expression (Adie & Milligan, 1994) and the function of ACs (Feldman, 1989; Medina‐Martinez & Garcia‐Sainz, 1993) and PDEs (Ortiz, Dasi, Cortijo, & Morcillo, 2000). The desensitising stimulus may be endogenous catecholamines, which exhibit elevated levels in disease states such as congestive heart failure (Maisel et al., 1989; Thomas & Marks, 1978), but may also be exogenously administered catecholamines and xenobiotics, for example, for the treatment of preterm labour (Engelhardt et al., 1997; Frambach et al., 2005; Michel et al., 1989) or congestive heart failure (Mauro & Mauro, 1986). While the mechanisms found to underlie agonist‐induced β2‐adrenoceptor desensitisation have been qualitatively confirmed for many other GPCRs, the relative roles of the contributing mechanisms as well as the time courses of desensitisation differ among GPCRs. Even closely related receptors such as β2‐ and β1‐adrenoceptors may exhibit differential regulation (Marullo, Nantel, Strosberg, & Bouvier, 1995; Michel, Feth, Sundermann, Rascher, & Brodde, 1993).
Based on the relative lack of phosphorylation sites in its C‐terminus (Emorine et al., 1989; Lelias et al., 1993; Muzzin et al., 1991; Nahmias et al., 1991), it was originally assumed that β3‐adrenoceptors are resistant to agonist‐induced desensitisation. While this assumption was supported by some early studies (Granneman, 1992; Nantel et al., 1993), later work shows that desensitisation can occur in at least some settings (Nantel, Bouvier, Strosberg, & Marullo, 1995). Therefore, this review will concentrate on tissues and cell types in which β3‐adrenoceptor desensitisation occurs upon prolonged agonist exposure, and what possibly differentiates these from those where desensitization does not occur. We will discuss desensitisation of tissue and cell responses, including those of signal transduction, and the underlying molecular and cellular mechanisms. We will also discuss the implications of β3‐adrenoceptor desensitisation for chronic treatment with corresponding agonists. Of note, some studies reporting on desensitisation of responses apparently occurring via β3‐adrenoceptors have not unequivocally established that this receptor is really mediating the response under investigation, for example, in neonatal rat cardiomyocytes. For ease of reading, descriptions of β3‐adrenoceptor ligands and cell lines mentioned in the manuscript are shown in Table 1.
Table 1.
Characterisation of β3‐adrenoceptor (AR) ligands and cell lines mentioned in this article
| β3‐Adrenoceptor ligands | |
|---|---|
| Ligand | Property |
| BRL 26,830 | β3‐AR‐selective agonist |
| BRL 37,344 | β‐AR with poor selectivity for β3‐AR |
| CGP 12,177 | β1/β2‐adrenoceptor antagonist; partial β3‐AR agonist; β1/β2‐AR radioligand, lower affinity for β3‐AR |
| CL 316,243 | β3‐AR‐selective agonist |
| D7114 | β3‐AR‐selective agonist |
| Fenoterol | β2‐AR‐selective agonist |
| FR 149,175 | β3‐AR‐selective agonist |
| Iodocyanopindolol | β1/β2‐AR radioligand, lower affinity for β3‐AR |
| Isoprenaline | β‐AR antagonist, all subtypes |
| L 748,337 | β3‐AR‐selective antagonist/biased agonist; also radioligand |
| Mirabegron | β3‐AR‐selective agonist |
| Ritobegron | β3‐AR‐selective agonist |
| Ro 16‐8714 | β3‐AR‐selective agonist |
| Salbutamol | β2‐AR‐selective agonist |
| SB 206,606 | β3‐AR radioligand |
| Solabegron | β3‐AR‐selective agonist |
| SR 59,119 | β3‐AR‐selective agonist |
| SR 59,230 | β‐AR antagonist, all subtypes |
| Cell lines | |
|---|---|
| Cell line | Origin |
| 3T3‐F442 | Mouse adipocyte |
| CHO | Hamster ovary |
| CHW | Hamster fibroblast |
| HEK 293 | HEK |
| L | Mouse sarcoma |
| Ltk− | Mouse fibroblast |
| M1 | Mouse kidney cortical duct |
| SK‐N‐MC | Human neuroblastoma |
2. DESENSITISATION AFFECTING CELL/TISSUE FUNCTION
Desensitisation of cell and tissue function mediated by β3‐adrenoceptors has been explored in a small number of studies based on pretreatment of animals in vivo or of isolated tissue ex vitro (Table 2). In an early study, hamsters were treated for 6 days with osmotic minipumps delivering noradrenaline or with daily injections of noradrenaline or adrenaline, followed by assessment of lipolytic responses to isoprenaline, noradrenaline, adrenaline, the β2/3‐adrenoceptor agonist BRL 37,344, or the β1/2‐antagonist and partial β3‐adrenoceptor agonist CGP 12,177 in isolated adipocytes (Carpene et al., 1993). While responses to these agonists were unchanged upon treatment, those to the β1‐adrenoceptor agonist dobutamine and the β2‐adrenoceptor agonist procaterol were reduced, indicating that in contrast to β1‐ and β2‐adrenoceptors, the β3‐adrenoceptor was refractory to desensitisation. Similarly, oxygen consumption in response to the β3‐adrenoceptor agonist FR 149,175 remained stable after 2 weeks of treatment with the same compound in Zucker fatty rats (Hatakeyama et al., 2004).
Table 2.
In vivo and in vitro studies on desensitisation of β3‐adrenoceptor function
| Tissue | Species | Response | Intervention | Outcome | Reference |
|---|---|---|---|---|---|
| In vivo treatments | |||||
| White adipocytes | Hamster | Lipolysis | Noradrenaline 5 μg·min−1·kg−1 infusion, 6 days | No change | Carpene et al., 1993 |
| White adipocytes | Rat | Oxygen consumption | FR 149,175, 0.1–3.2 mg·kg−1 twice daily orally, 2 weeks | No change | Hatakeyama, Sakata, Takakura, Manda, & Mutoh, 2004 |
| Ileum | Mouse | Relaxation | CL 316,243, 1 mg·kg−1 injection, 1 hr | No change | Hutchinson, Evans, & Summers, 2000 |
| CL 316,243, 1 mg·kg−1 injection, 4 or 24 hr | Reduced | ||||
| Pulmonary artery | Rat | Relaxation | Isoprenaline 0.3 mg·kg−1·day−1 injection, 7 days | No change | Davel, Victorio, Delbin, Fukuda, & Rossoni, 2015 |
| In vitro treatments | |||||
| Myometrium | Human | Relaxation | SR 59,119, 10 μM, 5 or 15 hr | No change | Rouget et al., 2004 |
| Urinary bladder | Rat | Relaxation | Isoprenaline, CL 316,243, mirabegron 10 μM each, 6 hr | No change | Michel, 2014 |
Note: For details, see Section 2.
In mouse ileum, a 4‐ or 24‐hr treatment with 1 mg·kg−1 of the β3‐adrenoceptor‐selective agonist CL 316,243 reduced maximum relaxation in response to freshly added CL 316,243 by 52% and 56%, respectively, whereas the potency of the agonist was not affected; a 1 hr pretreatment did not affect the subsequent relaxation response (Hutchinson et al., 2000). These data indicate the occurrence of desensitisation upon long‐term stimulation but not shorter time stimulation. In vivo treatment with the antagonist SR 59,230 (1 mg·kg−1) for 4 hr increased maximum relaxation to freshly added CL 316,243 by 15% with a minor increase in agonist potency (pEC50 from 7.27 to 7.49). In contrast, a 4‐hr in vivo treatment with 3 mg·kg−1 forskolin did not affect relaxation responses to CL 316,243. Of note, the changes that occurred following treatment with CL 316,243 occurred without changes in receptor binding or mRNA, whereas the changes with SR 59,230A were associated with an increase in β3‐adrenoceptor mRNA. These data were interpreted as showing agonist‐induced desensitisation of β3‐adrenoceptor tissue function and sensitisation by an antagonist. The lack of effect of forskolin may indicate that the agonist‐induced desensitisation may not be cAMP‐mediated.
Relaxation of rat isolated pulmonary artery was assessed after pretreatment with vehicle or 0.3‐mg·kg−1·day−1 isoprenaline for 7 days (Davel et al., 2015). As an internal control of the action of isoprenaline, the treatment induced right and left ventricular hypertrophy. The authors used phenylephrine‐contracted artery rings to test responses to various vasodilators including the β‐adrenoceptor agonists, isoprenaline, metaproterenol, and the β3‐adrenoceptor‐selective agonist mirabegron, the endothelium‐dependent ACh, and the receptor‐independent NO donor nitroprusside. Whether this represents a β3‐adrenoceptor‐mediated response has not been fully established. Pretreatment with isoprenaline did not alter relaxation responses to any of the three β‐adrenoceptor agonists or to nitroprusside but caused some degree of sensitisation of the ACh response (Emax from 78.4% to 92.7%, pEC50 7.03 to 7.39). These findings were interpreted to represent a lack of β‐adrenoceptor desensitisation, including that of β3‐adrenoceptors based on the finding that the response to mirabegron was unaffected, but enhanced NO modulation of vascular tone after isoprenaline treatment. In line with this interpretation, NO levels and cGMP content as well as immunoreactivity for endothelial NOS in the pulmonary artery were increased after isoprenaline treatment. Thus, in vivo studies in three different tissues of two species did not yield a consistent pattern with desensitisation being observed in one but not two other studies; moreover, duration of agonist exposure appears to be a critical factor.
Desensitisation of β3‐adrenoceptor‐mediated smooth muscle relaxation following ex vivo pretreatment of organ strips with agonists has been explored in two studies. Pretreatment of human near‐term myometrial strips for 5 or 15 hr with β2‐adrenoceptor agonist salbutamol right‐shifted the concentration–response curve to freshly added salbutamol, indicating desensitisation of the β2 component of the response, but did not affect relaxation by the β3‐adrenoceptor agonist SR 59,119; pretreatment with SR 59,119 for the same times did not affect either response, indicating resistance to desensitisation of the β3‐adrenoceptor component (Rouget et al., 2004). Pretreatment of rat urinary bladder strips with isoprenaline or the β2‐adrenoceptor agonist fenoterol for 6 hr led to a desensitisation of the β2‐adrenoceptor component of the relaxation (response to fenoterol), whereas pretreatments with isoprenaline, fenoterol, CL 316,243, or mirabegron caused less if any desensitisation of the β3‐adrenoceptor component of relaxation (response to CL 316,243 or to mirabegron; Michel, 2014).
Thus, most studies on tissue and cell responses other than signal transduction failed to detect desensitisation of β3‐adrenoceptor responses. However, the total number of studies on cell and tissue responses other than signal transduction is too limited to draw generalisable conclusions in the face of heterogeneity of species, tissues, and treatment regimens that were investigated.
3. β3‐ADRENOCEPTOR‐INDUCED DESENSITISATION OF cAMP SIGNALLING
Stimulation of AC leading to the formation of cAMP is the canonical signalling pathway of β‐adrenoceptors, including the β3‐adrenoceptor subtype (Bylund et al., 1994). While stimulation of AC can be measured directly in studies with cell membrane preparations, for convenience, most investigators have explored desensitisation of β3‐adrenoceptor‐mediated cAMP formation as cAMP accumulation in intact cells. However, use of cAMP accumulation assays has limitations that may affect the interpretation of the resulting data. Most importantly, cAMP is readily degraded by PDEs. Most investigators try to limit the impact by including PDE inhibitors such as IBMX in their assays. However, how well such inhibitors prevent cAMP degradation and whether they may have other effects, for example, by acting on adenosine receptors, is not well understood for most model systems (Michel & Seifert, 2015). Moreover, accumulating cAMP can leave cells, making intracellular concentrations lower than they should be and concomitantly act on adenosine receptors to inhibit cAMP formation (Pacini, Sanders‐Silveira, & Godinho, 2018). Due to a combination of these two factors, cellular cAMP accumulation is not necessarily linear over time. An alternative only rarely used in desensitisation studies are FRET‐ or BRET‐based cAMP probes (Barak et al., 2008), but this has been used in only one study in the field (Milano et al., 2018). Another complication of AC and cAMP accumulation assays is that β3‐adrenoceptors not only couple to Gs proteins to stimulate AC but can also couple to Gi proteins to inhibit it (Germack & Dickenson, 2006; Hadi et al., 2013; Sato et al., 2012; Soeder et al., 1999). Thus, unless Gi proteins are inhibited, for instance, by pretreatment with pertussis toxin, AC and cAMP accumulation assays reflect a net effect of stimulation via Gs and inhibition via Gi; in some models, this net effect may be an inhibition rather than a stimulation of cAMP accumulation (Germack & Dickenson, 2006). Of note, changes in cAMP formation may at least partly result from changes in the expression or function of G proteins, AC, or PDEs (see Section 1), but only a few studies have explored this. These factors must be taken into account in the interpretation of desensitisation data at the AC/cAMP level.
Desensitisation studies at the level of AC/cAMP accumulation have been reported in four models representing endogenously expressed β3‐adrenoceptors, rat adipose tissue, neonatal rat cardiomyocytes, human myometrium, and human SK‐N‐MC neuroblastoma cells (Table 3). In rat white adipose tissue, stimulation for 1 hr with isoprenaline or BRL 37,344 did not desensitise the β3‐adrenoceptor component of AC stimulation but did markedly desensitise the β1‐adrenoceptor component of the response (Granneman, 1992). In a follow‐up study, pretreatment with isoprenaline or the β3‐adrenoceptor agonist BRL 26,830 in vivo, reduced BRL 37,344‐induced AC stimulation ex vivo (Granneman & Lahners, 1992). However, AC responses evoked by the G protein stimulator NaF were not altered, suggesting that desensitisation may have occurred at the receptor level. Cold exposure reduced AC responses to BRL 37,344 in brown adipose tissue of young and senescent rats, although to a smaller extent in the latter (Scarpace et al., 1999), indicating that the ability to undergo desensitisation within a given system may be modulated by additional factors such as ageing. In that model, desensitisation also extended to AC stimulation induced by GTP but not to that mediated by forskolin, suggesting that it may at least partly have occurred at the G protein level. Thus, data obtained in rat adipose tissues are not consistent.
Table 3.
Studies on desensitisation of β3‐adrenoceptor‐mediated cAMP formation.
| Intervention | Outcome | Reference |
|---|---|---|
| In vivo treatments of animals | ||
| Rat white adipose tissue | ||
| Isoprenaline 2 × 200 μg or BRL 26,830, 2‐mg injection, 8 hr | Decrease | Granneman & Lahners, 1992 |
| Rat brown adipose tissue | ||
| Cold exposure, 48 hr | Decrease | Scarpace, Matheny, & Thümer, 1999 |
| In vitro treatments of tissues and cells natively expressing β3‐adrenoceptor | ||
| Rat brown adipocytes | ||
| Isoprenaline 100 μM, 1 hr | No change | Granneman, 1992 |
| Neonatal rat cardiomyocytes | ||
| Noradrenaline 100 μM, 24 hr | No change | Germack & Dickenson, 2006 |
| Human myometrium | ||
| SR 59,119, 10 μM, 5 or 15 hr | No change | Rouget et al., 2004 |
| SK‐N‐MC human neuroblastoma cells | ||
| Isoprenaline 100 μM, 1 hr | Decrease | Chaudhry & Granneman, 1994 |
| Isoprenaline 10 μM, 30 min | No change | Curran & Fishman, 1996 |
| In vitro treatments of cells transfected with β3‐adrenoceptors | ||
| Ltk− cells | ||
| Isoprenaline 10 μM, 30 min | No change | Nantel et al., 1993 |
| Isoprenaline 10 μM, 0–24 hr | No change | Nantel et al., 1995 |
| CHW cells | ||
| Isoprenaline 100 μM, 30 min | No change | Liggett, Freedman, Schwinn, & Lefkowitz, 1993 |
| Isoprenaline 10 μM, 30 min | No change | Nantel et al., 1993 |
| Isoprenaline 10 μM, 0–24 hr | No change | Nantel et al., 1995 |
| CHO cells | ||
| Isoprenaline 100 μM, 24 hr | Decrease | Chambers et al., 1994 |
| Isoprenaline 100 μM, 1 hr | No change | Chaudhry & Granneman, 1994 |
| Isoprenaline 10 μM, 15 min to 6 hr | Decrease | Candelore et al., 1996 |
| Isoprenaline 10 μM, 24 hr | No change | Michel‐Reher & Michel, 2013 |
| Isoprenaline 10 μM, 24 hr | Decrease | Okeke, Michel‐Reher, & Michel, 2018 |
| HEK 293 cells | ||
| Isoprenaline 100 μM, 1 hr | Decrease | Chaudhry & Granneman, 1994 |
| Isoprenaline 10 μM, 24 hr | Decrease | Vrydag, Alewijnse, & Michel, 2009 |
| Isoprenaline 10 nM to 10 μM, 2–24 hr | Decrease | Michel‐Reher & Michel, 2013 |
| Isoprenaline, L 755,507, CL 316,243, solabegron 10 μM, 24 hr | Decrease | Okeke, Michel‐Reher, & Michel, 2017 |
| Mouse renal epithelial cells | ||
| Mirabegron 10 nM, 1–12 hr | No change | Milano et al., 2018 |
| Mirabegron 10 nM, 24 hr | Decrease | |
In neonatal rat cardiomyocytes, treatment with noradrenaline markedly reduced cAMP accumulation in response to noradrenaline, isoprenaline, dobutamine, or procaterol; responses deemed to occur via β1‐ and/or β2‐adrenoceptors (Germack & Dickenson, 2006). When β1‐ and β2‐adrenoceptors had been blocked during pretreatment, stimulation by isoprenaline turned into inhibition, apparently a β3‐adrenoceptor‐mediated and pertussis toxin‐sensitive response. This inhibitory β3‐adrenoceptor response did not exhibit desensitisation. In isolated human myometrial strips, a 15 hr pretreatment with 10 μM salbutamol reduced cAMP accumulation in response to freshly added salbutamol, reflecting desensitisation of the β2‐adrenoceptor in this tissue; however, pretreatment with either salbutamol or SR 59,119 did not affect cAMP responses to freshly added SR 59,119, suggesting that the β3‐adrenoceptor in this tissue did not undergo desensitisation (Rouget et al., 2004). In SK‐N‐MC cells, treatment with 100 μM isoprenaline for 1 hr was reported to desensitise the β3 component of AC stimulation (Chaudhry & Granneman, 1994), whereas treatment with 10 μM isoprenaline for 30 min did not (Curran & Fishman, 1996). However, interpretation of data from SK‐N‐MC cells is complicated by their co‐expression of β1‐adrenoceptors, which also undergo agonist‐induced desensitisation (Michel et al., 1993). Thus, desensitisation and a lack thereof were both reported in tissues and cells with native expression of β3‐adrenoceptors However, the number of models being studied is too small to allow robust conclusions, particularly since each model has been studied only once.
Given the heterogeneous results in models endogenously expressing β3‐adrenoceptors, several investigators have turned to transfected cells as simpler models, potentially allowing more detailed dissection of underlying molecular pathways (Table 3). Upon expression in mouse fibroblast Ltk− cells, two studies from the same group of investigators reported a lack of change in maximum AC stimulation upon pretreatment with isoprenaline for 30 min to 24 hr, but under at least some conditions, agonist potency decreased in pretreated cells (Nantel et al., 1993, 1995). In contrast, a marked reduction of maximum responses was observed in Ltk− cells transfected with β2‐adrenoceptors. A chimeric receptor consisting of a β3‐adrenoceptor with substitution of its third cytoplasmic loop and carboxyl‐terminal tail for the corresponding region of the β2‐adrenoceptors resulted in an intermediate phenotype with a minor reduction in maximum AC stimulation along with a major reduction in agonist potency. Thus, these two regions of the receptor may play a role in desensitisation but apparently do not entirely explain the difference between the two subtypes. Upon expression of β3‐adrenoceptors in Chinese hamster fibroblast CHW cells, a largely similar observation was reported by the same investigators (Nantel et al., 1993, 1995); no change in maximum AC responses upon pretreatment with isoprenaline for 30 min to 24 hr, whereas these responses were markedly reduced in cells transfected with β2‐adrenoceptors. However, an additional experiment provided an interesting insight: if cAMP accumulation in intact cells was studied, a time‐dependent desensitisation of the β3‐adrenoceptor response became detectable; while this was smaller than that of the β2 response, it pointed to the possibility that desensitisation in intact cells may involve changes in cAMP breakdown or extrusion. Overall, these results indicated that the β3‐adrenoceptor was less prone to desensitisation than the β2‐adrenoceptor for stimulation times varying between 30 min and 24 hr and that, in addition to a relative lack of phosphorylation sites present in the third intracellular loop and the carboxyl tail of the β2‐adrenoceptor, other determinants contribute to the relative resistance of the β3‐adrenoceptor to desensitisation. Little agonist‐induced desensitisation of cAMP formation has also been observed in murine renal cortical collecting duct cells transfected with the human β3‐adrenoceptor (Milano et al., 2018).
Data obtained from transfected CHO cells yielded more variable data. Desensitisation of cAMP accumulation was observed in some studies with short‐term (Candelore et al., 1996) and long‐term pretreatment (Chambers et al., 1994; Okeke et al., 2018), whereas no such desensitisation was observed in other studies with short‐term (Chaudhry & Granneman, 1994) or long‐term pretreatment (Michel‐Reher & Michel, 2013; Figure 1). In HEK 293 cells, five studies consistently demonstrated agonist‐induced desensitisation of AC activity and cAMP accumulation with rat (Chaudhry & Granneman, 1994) and human receptors (Chaudhry & Granneman, 1994; Michel‐Reher & Michel, 2013; Okeke et al., 2017; Vrydag et al., 2009; Figure 1). Desensitisation was also observed with a truncated version of the human receptor that lacked the six C‐terminal amino acids encoded by the second exon, which include two serines potentially serving as acceptor sites for phosphorylation. Thus, these and studies with chimeric receptors expressed in Ltk− cells question the role of the C‐terminus of the β3‐adrenoceptor in desensitisation responses.
Figure 1.
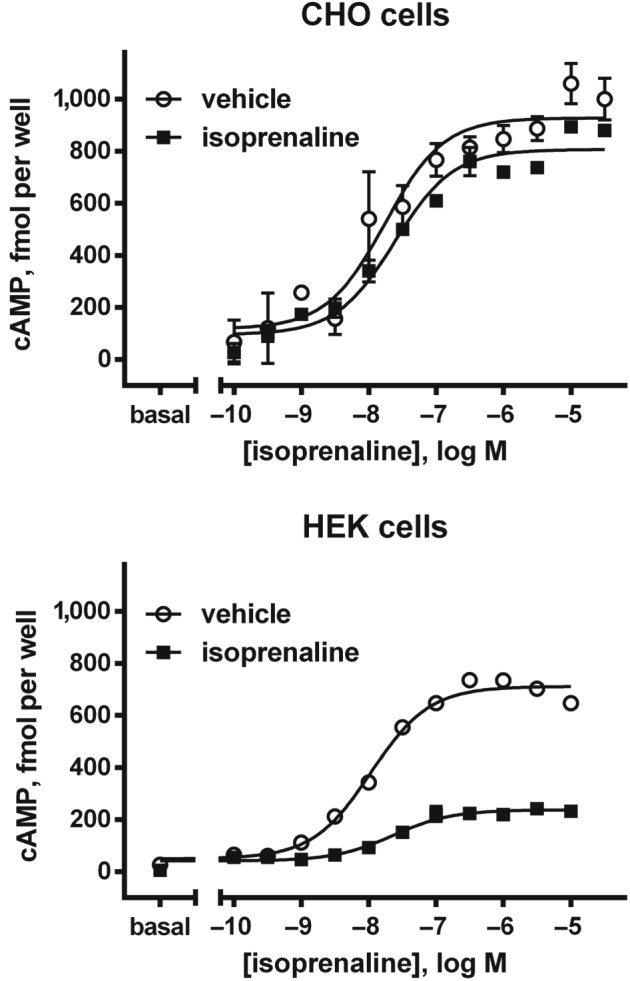
Desensitisation of cAMP accumulation mediated by human β3‐adrenoceptors transfected into CHO and HEK 293 cells. Cells were treated for 24 hr with 10 μM isoprenaline or vehicle, followed by washout and exposure to fresh isoprenaline. Desensitisation was observed in HEK 293 but not in CHO cells. Reproduced with permission from Michel‐Reher and Michel (2013)
In conclusion, agonist‐induced desensitisation of β3‐adrenoceptor‐stimulated cAMP formation is consistently absent in some models, for example, Ltk− cells, and consistently present in others, for example, HEK 293 cells, whereas inconsistent data have been reported in several other models including CHO cells. However, in studies where it was compared with the β2‐adrenoceptor, the β3‐adrenoceptor was generally found to be less prone to desensitisation than its closely related subtype. These findings, as well as limited findings on cell/tissue function other than signal transduction (see Section 2), highlight the probability that the presence of β3‐adrenoceptor desensitisation is largely determined by the cell type in which it is expressed.
3.1. Changes in G proteins, AC, and PDEs
The formation of cAMP upon β‐adrenoceptor stimulation is modulated by the abundance and activity of α‐subunits of Gs and Gi and of AC isoforms, whereas the fate of the cAMP formed is modulated by the abundance and activity of PDEs. Studies with β1‐ and β2‐adrenoceptors have shown that prolonged agonist exposure may cause a down‐regulation of the α‐subunit of Gs and/or an up‐regulation of the α‐subunit of Gi proteins (Adie & Milligan, 1994; Eschenhagen et al., 1992; Kimura, Miyamoto, & Oshika, 1993; Marzo et al., 1991). Therefore, several groups have explored whether a similar regulation also occurs upon β3‐adrenoceptor stimulation. To test this involvement functionally, one group treated rats with isoprenaline or BRL 26,830 and found that AC responses to freshly added BRL 37,344 were reduced in adipose tissue; whereas, responses to the direct G protein activator NaF were not altered, suggesting that the attenuated response to the agonist did not occur at the G protein or AC level (Granneman & Lahners, 1992). Others exposed young rats to cold temperature and found that responses to BRL 37,344 and the direct G protein stimulator GTP were similarly desensitised in white adipose tissue, whereas those to forskolin were not, suggesting that desensitisation may have largely occurred at the G protein level (Scarpace et al., 1999). Similar changes did not occur in senescent rats.
More direct assessments have been based on immunoblotting of G protein α‐subunits. In CHO cells transfected with human β2‐ or β3‐adrenoceptors, a 7‐hr treatment with isoprenaline concentration‐dependently reduced immunoreactivity of Gs by approximately 50% (Chambers et al., 1994). The time course of the down‐regulation of Gs was comparable with both receptor subtypes, but the concentration–response curve exhibited a lower potency for down‐regulation via the β3‐adrenoceptor. This could explain the observed desensitisation of isoprenaline‐stimulated AC activity, as no changes in β3‐adrenoceptor protein expression were observed. A study in HEK 293 cells transfected with human β3‐adrenoceptors reported a reduction of Gs immunoreactivity upon a 24‐hr treatment with isoprenaline, but the extent of this down‐regulation was only about 20% (Michel‐Reher & Michel, 2013). Within the same study, isoprenaline treatment reduced immunoreactivity of Gi1 by about 50% but did not change that of Gi2, Gi3, or Gq/11. As cAMP formation induced by forskolin desensitised to a similar extent as that by isoprenaline upon pretreatment with isoprenaline, the down‐regulation of Gsα was unlikely to be a major contributor to functional desensitisation of the receptor. Of note, the authors reported immunoreactivity of G protein α‐subunits based on loaded protein content, that is, without correcting for a reference protein. In a follow‐up communication on re‐analysis of the same data (Michel‐Reher & Michel, 2015), isoprenaline treatment in these cells was found to reduce GAPDH immunoreactivity by approximately 30%. Had they normalised to GAPDH expression, as many authors do, the down‐regulation of Gs and Gi1 would have been masked, and the lack of significant regulation of Gi3 would have turned into an apparent up‐regulation. A study with in vivo pretreatment of rats with isoprenaline for 7 days reported a lack of change of expression of Gi1/2 and Gi3 protein in pulmonary artery, when normalised for α‐actin expression (Davel et al., 2015). Taken together, two studies in cell lines consistently reported a down‐regulation of Gs upon treatment with isoprenaline, but it is unclear to what extent this may have contributed to the observed desensitisation of cAMP formation. However, in vitro and in vivo studies did not find an up‐regulation of Gi, suggesting that this did not contribute to desensitisation.
In one of the above studies, performed in HEK 293 cells, pretreatment with isoprenaline reduced cAMP responses to forskolin to a similar extent as those to freshly added isoprenaline, suggesting that desensitisation of the cAMP formation could fully be explained by an alteration in the level of AC (Michel‐Reher & Michel, 2013). Reduced AC activity was confirmed to have a major role in desensitisation in a follow‐up study from these investigators (Okeke et al., 2017). However, the same investigators observed no change in forskolin‐stimulated relaxation in rat urinary bladder after 6 hr of agonist treatment (Michel, 2014); similarly, rat ileum relaxation induced by forskolin was also found to be unaltered after 4 hr of agonist treatment (Hutchinson et al., 2000). A study in pulmonary artery demonstrated that a 7 day pretreatment of rats with isoprenaline reduced PDE5 expression (Davel et al., 2015). While this may be relevant to enhanced endothelial NOS activity in the same study, it is unlikely to have contributed to β‐adrenoceptor function because PDE5 is selective for cGMP, not cAMP; accordingly, relaxation responses to agonists including the β3‐adrenoceptor selective mirabegron were unchanged. An in vitro study with human myometrium did not detect changes in PDE4 upon pretreatment with β2‐selective salbutamol or β3‐adrenoceptor selective SR 59,119 (Rouget et al., 2004). Taken together, these studies suggest that prolonged stimulation of β3‐adrenoceptors can change the activity and/or expression of AC or PDEs, but the number of reports available is insufficient for a robust conclusion in the face of the divergent outcomes across models.
The local intracellular concentration of cAMP resulting from the net effect of the above factors leads to activation of more distal signalling responses such as activation of PKA or Epac or modulation of expression of various transcription factors. However, these have been investigated only rarely. One study used CHW and mouse sarcoma L cells, each transfected with either β2‐ or β3‐adrenoceptors and treated for 10 min or 24 hr with isoprenaline (Nantel et al., 1995). In CHW cells, both short and extended treatment increased PKA activity, irrespective of which subtype had been stimulated. However, in L cells, both subtypes increased PKA activity only after short but not after prolonged stimulation, again similarly with both subtypes.
In conclusion, some studies have presented evidence that components of the signal transduction chain downstream of the β3‐adrenoceptor may exhibit desensitisation upon prolonged agonist exposure. However, such changes do not appear consistent across model systems and have been studied in too few instances to allow robust conclusions.
β3‐adrenoceptors couple not only to stimulation of cAMP formation but also to that of other signalling pathways, including phosphorylation of the MAPKs ERK and p38, and to extracellular acidification rates (Gerhardt, Gros, Strosberg, & Issad, 1999; Hadi et al., 2013; Robay, Toumaniantz, Leblais, & Gauthier, 2005; Sato, Horinouchi, Hutchinson, Evans, & Summers, 2007, 2008; Soeder et al., 1999; Steinle, Booz, Meininger, Day, & Granger, 2003). Interestingly, only one report published in abstract form has explored desensitisation of signalling pathways other than cAMP formation (Okeke et al., 2018). While the authors detected desensitisation of ERK phosphorylation in transfected CHO cells, this exhibited a different pattern from that of cAMP accumulation within the same series of experiments. These data suggest that different signalling responses to β3‐adrenoceptor stimulation may undergo differential regulation upon agonist exposure.
4. CHANGES IN β3‐ADRENOCEPTOR PROTEIN EXPRESSION, PHOSPHORYLATION, AND LOCALISATION
Two main tools have been used for the detection of receptor expression at the protein level, radioligand binding, and immunodetection; while the former is easier to quantify, the latter often allows better spatial resolution in histological or cytological studies. However, both approaches can be problematic when applied to β3‐adrenoceptors. Standard β‐adrenoceptor radioligands such as [3H]‐CGP 12,177 or [125I]‐iodocyanopindolol have much lower affinity for β3‐adrenoceptors as compared to β1‐ and β2‐adrenoceptors (Niclauß, Michel‐Reher, Alewijnse, & Michel, 2006). The high concentrations required to use them as labels for β3‐adrenoceptors imply saturation of the other two subtypes, high non‐specific binding and binding to non‐β‐adrenoceptor sites including 5‐HT receptors (Hoyer, Engel, & Kalkman, 1985a,b) and a protein later identified as a member of the TM9SF multi‐spanning membrane protein family (Sugasawa et al., 2001, 1997). Thus, only one group has extensively used [3H]‐CGP 12,177 in intact transfected cells (Baker, 2005, 2010). In transfected cells, not expressing any of the other subtypes, [125I]‐iodocyanopindolol appears more suitable and is routinely used (Emorine et al., 1989; Hoffmann et al., 2004; Niclauß et al., 2006; Vrydag et al., 2009). It has also been used in some tissues with native expression of the receptor, but the considerable non‐specific binding has limited interpretation of the data (Hutchinson, Chernogubova, Sato, Summers, & Bengtsson, 2006; Rathi et al., 2003; Roberts, Molenaar, & Summers, 1993; Rouget et al., 2005; Schneider & Michel, 2010). [3H]‐SB 206,606, a tritiated form of the active RR‐enantiomer of the agonist BRL 37,344, has been introduced as a β3‐adrenoceptor‐selective radioligand (Muzzin et al., 1994) but the reported K d values of 38–93 nM limit its use. Accordingly, [3H]‐SB 206,606 has only rarely been used (Deng et al., 1996) but has been employed in desensitisation studies (Klaus et al., 1995). More recently, a tritiated version of the antagonist L 748,337 has been validated as a β3‐adrenoceptor‐selective radioligand (van Wieringen, Michel‐Reher, Hatanaka, Ueshima, & Michel, 2013). Its affinity of 2–3 nM makes [3H]‐L 748,337 the radioligand of choice for the labelling of human β3‐adrenoceptors but due to a lower affinity in rodents, may be less useful in mice and rats, species that are often used to study β3‐adrenoceptors. [3H]‐L 748,337 has also been used in desensitisation studies of the human β3‐adrenoceptor (Michel‐Reher & Michel, 2013).
While many β3‐adrenoceptor antibodies lack useful target specificity, some have recently been validated based on stringent criteria (Cernecka, Ochodnicky, Lamers, & Michel, 2012; Cernecka, Pradidarcheep, Lamers, Schmidt, & Michel, 2014; Kullmann et al., 2009; Limberg et al., 2010); however, even the usefulness of these antibodies is limited to some species and applications. A reported monoclonal antibody with good validation data (Chamberlain et al., 1999) unfortunately has never become widely distributed and is no longer available even from the original authors. Therefore, antibodies have only played a minor if any role in desensitisation studies. Use of transfected, tagged receptors may be an interesting alternative as they can be detected immunologically in a more robust way (Milano et al., 2018).
GPCRs can be present in a high‐ and low‐affinity state for agonists (van Wieringen, Booij, Shalgunov, Elsinga, & Michel, 2013). The high‐affinity state reflects the receptor‐G protein coupled form and the low affinity, the uncoupled form of the receptor. Accordingly, desensitisation can also be reflected by a shift between these two states. Studies with transfected L cells expressing little if any of other subtypes did not find a shift in high‐ versus low‐affinity state of the β3‐adrenoceptor upon treatment with 10 μM isoprenaline for 30 min, despite the presence of agonist‐induced down‐regulation of β3‐adrenoceptors (Nantel et al., 1993). A lack of agonist‐induced shift of agonist affinity state was also observed with a chimeric receptor consisting of a β3‐adrenoceptor in which the third cytoplasmic loop and the C‐terminus had been replaced with corresponding stretches of the β2‐adrenoceptor. In contrast, β2‐adrenoceptors exhibited a reduction in agonist high‐affinity states upon agonist exposure.
Chronic exposure to agonists can lead to a reduction in total cellular receptor number, which has been widely observed with β1‐ and β2‐adrenoceptors (Engelhardt et al., 1997; Matthews, Falckh, Molenaar, & Summers, 1996). Only few studies have explored this for the β3‐adrenoceptor, mostly using [125I]‐iodocyanopindolol as the radioligand (Table 4). Treatment with the β3‐adrenoceptor agonist Ro 16‐8714 for 24–48 hr decreased receptor protein expression in brown adipose tissue in lean and obese Zucker rats (Revelli et al., 1992). In contrast, treatment with CL 316,243 for up to 24 hr did not reduce β3‐adrenoceptor protein expression in mouse ileum, despite causing desensitisation of relaxation responses (Hutchinson et al., 2000). Several studies in rats (Sun et al., 2011), dogs (Cheng et al., 2001), and patients with congestive heart failure (Moniotte et al., 2001; Napp et al., 2009) reported an increased abundance of β3‐adrenoceptor protein, although only some of them were based on validated antibodies. Results from a study in a rat model of colitis (Vasina et al., 2008) are difficult to interpret for two reasons. First, it is based on immunohistochemistry; while using a validated antibody (Cernecka, Pradidarcheep, et al., 2014), the use of immunohistochemistry resulted in qualitative rather than quantitative conclusions on expression levels. Second, treatment with the β3‐adrenoceptor agonist SR 58,611 for 7 days improved the colitis, and the disease associated down‐regulation of the receptor, making it difficult to determine whether the apparent increase in receptor protein expression was a direct consequence of agonist exposure or reflected improvement in the pathology that had caused the down‐regulation.
Table 4.
Studies on regulation of β3‐adrenoceptor protein and mRNA by agonist exposure
| Intervention | Protein | mRNA | Reference |
|---|---|---|---|
| In vivo treatments of animals | |||
| Rat brown adipose tissue | |||
| Ro 16‐8741, 0.87 mg·kg−1 orally, every 24 hr | Decrease | Decrease | Revelli, Muzzin, & Giacobino, 1992 |
| Noradrenaline 2 × 250 μg injection, 8 hr | n.r. | Decrease | Granneman & Lahners, 1992 |
| Isoprenaline 2 × 200 μg injection, 8 hr | n.r. | Decrease | |
| BRL 26,830, 2 × 2 mg injection, 8 hr | n.r. | Decrease | |
| Mouse brown adipose tissue | |||
| CL 316,243, 1 mg·kg−1 injection, 1–24 hr | n.r. | Decreasea | Hutchinson et al., 2000 |
| Rat white adipose tissue | |||
| Noradrenaline 2 × 250 μg injection, 8 hr | n.r. | Decrease | Granneman & Lahners, 1992 |
| Isoprenaline 2 × 200 μg injection, 8 hr | n.r. | Decrease | |
| BRL 26,830, 2 × 2 mg injection, 8 hr | n.r. | Decrease | |
| BRL 26,830, 10 mg·kg−1 injection, 4 hr | n.r. | Decrease | Granneman & Lahners, 1995 |
| FR 149,175, 0.1–3.2 mg·kg−1 twice daily p.o., 2 weeks | n.r. | No change | Hatakeyama et al., 2004 |
| Mouse white adipose tissue | |||
| CL 316,243, 1 mg·kg−1 injection, 1–24 hr | n.r. | Decrease | Hutchinson et al., 2000 |
| Mouse ileum | |||
| CL 316,243, 1 mg·kg−1 injection, 1–24 hr | No change (63–77) | No change | Hutchinson et al., 2000 |
| In vitro treatments of tissues and cells natively expressing β3‐adrenoceptor | |||
| Neonatal rat cardiomyocytes | |||
| Noradrenaline 100 μM, 24 hr | Increase | Increase | Germack & Dickenson, 2006 |
| Noradrenaline 100 mM, 72 hr | n.r. | Increase | Watts et al., 2013 |
| Hamster brown adipocytes | |||
| Isoprenaline 1 μM, 2–8 hr | n.r. | Decrease | Klaus et al., 1995 |
| Noradrenaline 1 μM, 2–8 hr | n.r. | Decrease | |
| D7114, 1 μM, 2–8 hr | n.r. | Decrease | |
| Mouse brown adipocytes | |||
| Noradrenaline 100 nM, 24 hr | n.r. | Decrease | Bengtsson, Redegren, Strosberg, Nedergaard, & Cannon, 1996 |
| 3T3‐F442 mouse adipocytes | |||
| Isoprenaline 100 μM, 4–30 hr | Increase (39) | Increase | Thomas, Holt, Schwinn, & Liggett, 1992 |
| Isoprenaline 100 μM, 2–8 hr | n.r. | Decrease | Granneman & Lahners, 1994 |
| Isoprenaline 100 μM, 4 hr | n.r. | Decrease | Granneman & Lahners, 1995 |
| Human myometrial strips | |||
| Salbutamol or SR 59,119, 10 μM, 15 hr | n.r. | No change | Rouget et al., 2004 |
| SK‐N‐MC human neuroblastoma cells | |||
| Isoprenaline 100 μM, 2–6 hr | n.r. | No change | Granneman & Lahners, 1994 |
| Isoprenaline 1–100 nM, 1–24 hr | No change (189) | n.r. | Curran & Fishman, 1996 |
| In vitro treatments of cells transfected with β3‐adrenoceptor | |||
| CHW cells | |||
| Isoprenaline 100 μM, 6–24 hr | No change (5,900) | n.r. | Liggett et al., 1993 |
| Isoprenaline 10 μM, 1–24 hr | No change (281) | No change | Nantel, Marullo, Krief, Strosberg, & Bouvier, 1994 |
| Isoprenaline 10 μM, 2–24 hr | No change (200–350) | n.r. | Nantel et al., 1995 |
| CHO cells | |||
| Isoprenaline 100 μM, 0–24 hr | No change (3,000) | n.r. | Chambers et al., 1994 |
| HEK 293 cells | |||
| Isoprenaline 100 μM, 1 hr | No change (750) | n.r. | Chaudhry & Granneman, 1994 |
| Isoprenaline 10 μM, 24 hr | No change (306) | n.r. | Michel‐Reher & Michel, 2013 |
| L cells | |||
| Isoprenaline 10 μM, 1–24 hr | Decrease (122) | Decrease | Nantel et al., 1994 |
| Isoprenaline 10 μM, 3–24 hr | Decrease (200–350) | n.r. | Nantel et al., 1995 |
| M1 cells | |||
| Mirabegron 10 nM, 6–24 hr | Decrease | n.r. | Milano et al., 2018 |
Note: Where available, baseline β3‐adrenoceptor density (fmol·mg−1·protein) is shown in parentheses. Note that some of the mRNA expression changes were transient, that is, not found at all time points investigated. Studies in transfected cells refer to the human receptor. For details, see Sections 4 and 5. n.r.: not reported.
Decrease observed for β3a‐ but not β3b‐adrenoceptor.
Others have used tissue samples or cell lines endogenously expressing β3‐adrenoceptors with ex vivo or in vitro exposure to agonist (Table 4). Surprisingly, treatment of murine adipocyte 3T3‐F442 cells with isoprenaline time‐dependently increased [125I]‐iodocyanopindolol binding (Thomas et al., 1992). A similar increase was observed in neonatal rat cardiomyocytes (Germack & Dickenson, 2006; Watts et al., 2013). Such agonist‐induced increases in β3‐adrenoceptor expression may relate to the cAMP response elements in the receptor gene (Liggett & Schwinn, 1991; Thomas et al., 1992). In contrast, studies in human myometrial strips (Rouget et al., 2004) or in SK‐N‐MC neuroblastoma cells did not show any alterations in β3‐adrenoceptor protein expression upon agonist treatment (Curran & Fishman, 1996). The negative studies showed a down‐regulation of β1‐ or β2‐adrenoceptors. Taken together, studies with endogenously expressed rat, mouse, or human β3‐adrenoceptors showed highly heterogeneous results with up‐regulation, no change, and down‐regulation; however, whenever investigated, findings of corresponding mRNA were concordant with those of protein expression (Hutchinson et al., 2000; Revelli et al., 1992; Thomas et al., 1992).
Apparently because of the better detectability of β3‐adrenoceptor protein (see above), most investigators have explored agonist‐induced regulation at the protein level in transfected cells. In line with the findings in tissues and cells natively expressing β3‐adrenoceptors, the results appear to be cell type‐dependent (Table 4). A lack of agonist‐induced down‐regulation has consistently been reported in transfected CHW cells (Liggett et al., 1993; Nantel et al., 1995, 1994). While most studies have transfected a β2‐adrenoceptor as a positive control for a receptor that undergoes agonist‐promoted down‐regulation, a chimeric receptor that comprised the β3‐adrenoceptor up to proline‐365 of the cytoplasmic tail and the C‐terminus of the β2‐adrenoceptor was used in one study (Liggett et al., 1993). While the β3‐adrenoceptor did not exhibit down‐regulation and the β2‐adrenoceptor did undergo a marked down‐regulation, the chimeric receptor had an intermediate phenotype being closer to that of the β2‐adrenoceptor, providing additional insight into the molecular basis of down‐regulation. A lack of β3‐adrenoceptor protein down‐regulation has also been reported in transfected CHO cells (Chambers et al., 1994) and HEK 293 cells (Chaudhry & Granneman, 1994; Michel‐Reher & Michel, 2013). In contrast, agonist‐induced down‐regulation of β3‐adrenoceptor protein has consistently been observed upon transfection in L cells (Nantel et al., 1995, 1994). This agonist‐promoted down‐regulation of the β3‐adrenoceptor was found to result from a reduction in the mRNA encoding the receptor and not a change in the half‐life of the protein, pointing to a change in RNA stability as the underlying mechanism (Nantel et al., 1994; see below). Using tagged β3‐adrenoceptors, their down‐regulation was observed in transfected murine renal epithelial cells; in a time course experiment, this was absent after 1–3 hr, moderate after 6–12 hr, and marked with the 24‐hr treatment (Milano et al., 2018).
Other than down‐regulation, the number of agonist accessible receptors can also change through an altered subcellular localisation. Thus, receptors may migrate from the plasma membrane to an intracellular compartment where they are no longer accessible to hydrophilic agonists such as catecholamines. This process is termed internalisation, sequestration, or endocytosis. It is believed to mainly play a role in short‐term re‐sensitisation and has extensively been explored for β2‐adrenoceptors (Lefkowitz, 1998). Studies in SK‐N‐MC neuroblastoma cells natively expressing β3‐adrenoceptors (Curran & Fishman, 1996) and in transfected CHW cells (Liggett et al., 1993; Nantel et al., 1993) did not detect agonist‐induced internalisation of the β3‐adrenoceptor, with concomitantly expressed β1‐adrenoceptors (Curran & Fishman, 1996) or transfected β2‐adrenoceptors (Nantel et al., 1993) serving as an internal positive control. A lack of internalisation was also reported upon expression of human β3‐adrenoceptors in mouse renal epithelial cells (Milano et al., 2018). The observed lack of β3‐adrenoceptor sequestration is in‐line with the absence of phosphorylation sites in the C‐terminus of the receptor deemed relevant for desensitisation (Emorine et al., 1989; Lelias et al., 1993; Muzzin et al., 1991; Nahmias et al., 1991), believed to be a molecular prerequisite for internalisation of the other β‐adrenoceptor subtypes (Lefkowitz, 1998).
Phosphorylation of C‐terminal serine and threonine residues by GPCR kinases (GRKs), also known as β‐adrenergic receptor kinases, is considered as an important molecular step in the agonist‐induced desensitisation of β2‐adrenoceptors since removal of these sites by site‐directed mutagenesis resulted in largely diminished desensitisation (Bouvier et al., 1988; Bouvier, Leeb‐Lundberg, Benovic, Caron, & Lefkowitz, 1987). Also, adding extra phosphorylation sites increases receptor–arrestin interaction (Zindel et al., 2015), an interaction believed to initiate the process of desensitisation by sterically interfering with G protein engagement and promoting receptor endocytosis via the association of β‐arrestin with components of the endocytic machineries (Goodman et al., 1996; Laporte, Oakley, Holt, Barak, & Caron, 2000). The human (Emorine et al., 1989; Lelias et al., 1993), rat (Muzzin et al., 1991) and murine β3‐adrenoceptor (Nahmias et al., 1991) contain only few C‐terminal serine or threonine residues, and those few are located in positions considered unfavourable for phosphorylation, leading to the hypothesis that this paucity in phosphorylation sites may underlie the generally observed reduced propensity of the β3‐adrenoceptor to undergo desensitisation when compared with the β2‐adrenoceptor. To test the functional consequence of this lack of phosphorylation sites, CHW cells were transfected with human β2‐adrenoceptor, β3‐adrenoceptor, or chimera thereof (Liggett et al., 1993). In whole‐cell phosphorylation studies with such cells, β2‐adrenoceptor exhibited isoprenaline‐induced phosphorylation whereas β3‐adrenoceptors did not, even if a GRK was overexpressed. A chimeric receptor, consisting of the β3‐adrenoceptor with the cytoplasmic tail of the β2‐adrenoceptor, however, exhibited phosphorylation. To directly test whether the difference in phosphorylation may be responsible for the different desensitisation observed between the two receptor subtypes, a series of β3/β2‐adrenoceptor chimeras were expressed in HEK 293 cells and tested for their ability to undergo internalisation and β‐arrestin recruitment (Angers, 2003). For this study, β‐arrestin recruitment was measured by monitoring agonist‐promoted BRET between β‐arrestin and the receptors (Angers et al., 2000). In contrast to the β2‐adrenoceptor that promoted a robust recruitment of β‐arrestin and was internalised upon agonist stimulation, no such recruitment or internalisation was observed for the wild‐type β3‐adrenoceptor (Figure 2). This is consistent with the observation that the β3‐adrenoceptor does not undergo agonist‐promoted phosphorylation (Liggett et al., 1993), a post‐translational modification needed to favour high‐affinity interaction with β‐arrestin. The introduction of the second intracellular loop and carboxyl tail of the β2‐adrenoceptor into the β3‐adrenoceptor (β3‐3232) restored agonist‐promoted internalisation to some extent (Jockers, Da Silva, Strosberg, Bouvier, & Marullo, 1996; Figure 2) but was insufficient to restore any detectable β‐arrestin recruitment suggesting that a β‐arrestin‐independent mechanism may be involved in the internalisation of the β3‐3232. A similar lack of β‐arrestin recruitment was also observed for the other chimeras tested (β3‐3233, β3‐3323, β3‐3332, β3‐3322, and β3‐3222). These data also show that linear determinants from the β2‐adrenoceptor, including phosphorylation sites, are not sufficient to instill the high‐affinity interaction with β‐arrestin and suggest that a more complex conformational rearrangement must be required. Interestingly, a chimeric receptor in which the carboxyl tail of the β3‐adrenoceptor was inserted in the β2‐adrenoceptor (β2‐2223) showed a reduced but not abolished ability to recruit β‐arrestin. This confirmed a role for the C‐tail phosphorylation sites in the recruitment of β‐arrestin to the β2‐adrenoceptor. However, it also indicated that while being important to stabilise a high‐affinity interaction, it may not be essential for the initial recruitment. These findings are consistent with a recent observation suggesting that β‐arrestin may have a bitopic mode of interaction involving a distinct domain of the receptor (Cahill et al., 2017; Thomsen et al., 2016).
Figure 2.
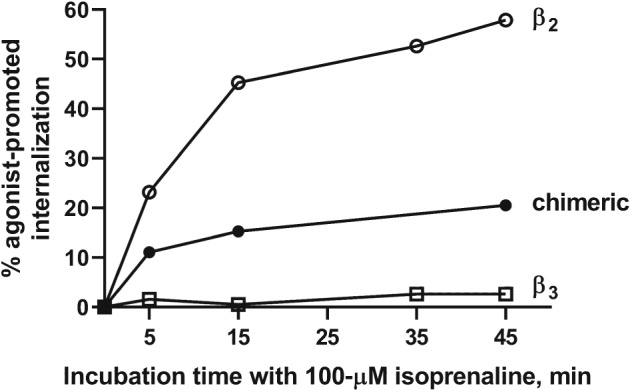
Time course of agonist‐promoted internalisation of the β3‐, β2‐, and a β3‐3232 chimeric adrenoceptor. HEK 293 cells stably expressing the β2‐adrenoceptor, β3‐adrenoceptor, or the β3‐3232 (chimeric) were stimulated with 100 μM isoprenaline for the indicated times. Each of the constructs was tagged at their N‐terminus with a Myc‐tag allowing their immuno‐detection using an anti‐Myc antibody. Internalisation was assessed by flow cytometry and expressed as % loss of cell surface receptor immunoreactivity. Reproduced with permission from Angers (2003). The data show that in contrast to the β2‐adrenoceptor that undergoes a time‐dependent internalisation upon isoprenaline stimulation, the β3‐adrenoceptor does not. However, introducing the second intracellular loop and the carboxyl‐terminus of the β2‐adrenoceptor within the β3‐adrenoceptor backbone (β3‐3232) restored agonist‐promoted internalisation to some extent
Taken together, these data demonstrate that the presence of agonist‐induced down‐regulation of β3‐adrenoceptor protein appears to be a cell type‐dependent phenomenon, with a lack of down‐regulation being the more frequent phenotype. In contrast to β2‐adrenoceptors, β3‐adrenoceptors apparently do not undergo agonist‐induced internalisation. This may be related to their lack of C‐terminal phosphorylation sites and their limited ability to bind β‐arrestin.
5. CHANGES IN β3‐ADENOCEPTOR mRNA EXPRESSION
Investigation of mRNA expression can serve to explore whether changes in protein expression can be explained by those of corresponding mRNA abundance. A small number of studies have applied them in this way in the β3‐adrenoceptor field (Germack & Dickenson, 2006; Revelli et al., 1992; Thomas et al., 1992). However, most investigators have explored alterations in β3‐adrenoceptor mRNA expression as a proxy for those of protein expression, presumably because mRNA levels are technically easier to quantify for β3‐adrenoceptors than those of functional protein (see Section 4). Four considerations apply when interpreting mRNA data with regard to functional receptor desensitisation. First, any reduction in mRNA abundance is only likely to translate into changes in receptor responsiveness after hours or days because the half‐life of β3‐adrenoceptor protein has been estimated at 15–18 hr (Nantel et al., 1994) whereas that of β3‐adrenoceptor mRNA has been estimated at 30–90 min (Bengtsson et al., 1996; Granneman & Lahners, 1995; Klaus et al., 1995). Hence, changes in mRNA expression have mostly been studied in the context of extended (hours to days) exposure to agonists. Second, any change in mRNA abundance may result from those of transcription and/or mRNA stability. While altered mRNA expression upon agonist treatment mainly reflects mRNA stability for β2‐adrenoceptors (Danner, Frank, & Lohse, 1998; Hadcock, Wang, & Malbon, 1989), it appears to reflect transcription for β3‐adrenoceptors (Bengtsson et al., 1996; Granneman & Lahners, 1995). Of note, studies on transfected receptors provide limited insight into possible changes in receptor transcription as this parameter is typically not controlled by the native promoter of the receptor but rather by an artificial one such as that derived from cytomegalovirus. Hence, with few exceptions (Nantel et al., 1994), studies on agonist effects on β3‐adrenoceptor mRNA abundance have been reported in cells and tissues natively expressing the receptor. Third, the promotor region of the β3‐adrenoceptor contains four cAMP response elements (Liggett & Schwinn, 1991; Thomas et al., 1992) and an intron containing a putative enhancer element specific for adipose tissue (Granneman, Lahners, & Rao, 1992). This may at least partly explain tissue‐selective regulation of mRNA expression. However, there are also less well‐defined mechanisms leading to inhibition of transcription upon agonist exposure (Granneman & Lahners, 1995). The net effect of these mechanisms in each cell type is difficult to predict. Finally, the predictive value of mRNA levels for corresponding β3‐adrenoceptor protein expression has been questioned (Nantel et al., 1994).
Several investigators have treated lean and obese Zucker rats (Granneman & Lahners, 1992; Revelli et al., 1992), hamster (Klaus et al., 1995), or mice (Hutchinson et al., 2000) in vivo with agonists including isoprenaline, noradrenaline, BRL 26,830, CL 316,243, D7114, or Ro 16‐8741 for 1–48 hr and mostly observed a down‐regulation of β3‐adrenoceptor mRNA. In some cases, such down‐regulation was transient and in mice only the β3a was affected but not the β3b splice variant of the receptor. Cold exposure, considered to stimulate a release of endogenous noradrenaline, also yielded a transient down‐regulation (at 1–12 hr but not 24 hr) in mice (Bengtsson et al., 1996) and did not exhibit down‐regulation at later time points (48–72 hr) in rats (Granneman & Lahners, 1992; Scarpace et al., 1999). Conversely, surgical denervation increased β3‐adrenoceptor mRNA expression in rats (Granneman & Lahners, 1992). Acute treatment with noradrenaline similarly reduced β3‐adrenoceptor mRNA expression in white and brown adipose tissues in rats (Granneman & Lahners, 1992). A study in mice confirmed a similar reduction in white and brown adipose tissues for the β3a‐adrenoceptor, but a reduction in β3b‐adrenoceptor mRNA was only observed in white adipose tissue (Hutchinson et al., 2000). A similar regulation of both murine splice variants has also been reported by others in vivo and in rat adipocyte 3T3‐F442 cells (Granneman & Lahners, 1995). In contrast, treatment of Zucker fatty rats with the β3‐adrenoceptor agonist FR‐149,175 did not change β3‐adrenoceptor mRNA expression (Hatakeyama et al., 2004).
Investigations with in vitro treatment of tissues and cells natively expressing β3‐adrenoceptors have yielded conflicting results. Increased mRNA expression was reported in 3T3‐F442 mouse adipocytes following treatment with isoprenaline by some (Thomas et al., 1992), but a decreased expression was reported by others (Granneman & Lahners, 1995). The decrease was mimicked by treatment with 8‐Br‐cAMP and found to be due to reduced transcription and not to altered mRNA degradation. In neonatal rat cardiomyocytes, treatment with noradrenaline increased β3‐adrenoceptor mRNA expression (Watts et al., 2013). Similarly, in congestive heart failure, a condition associated with an increased sympathetic tone, an increased cardiac β3‐adrenoceptor mRNA expression is consistently exhibited in rats (Ozakca et al., 2013; Zhao et al., 2013), dogs (Cheng et al., 2001), and humans (Moniotte et al., 2001). In SK‐N‐MC human neuroblastoma cells, treatment with 8‐Br‐cAMP increased β3‐adrenoceptor mRNA expression, whereas treatment with isoprenaline had no effect (Granneman & Lahners, 1994). Similarly, dexamethasone and the PKC activator phorbol myristate acetate did not change β3‐adrenoceptor mRNA levels in SK‐N‐MC cells but reduced it in 3T3‐F442 cells (Granneman & Lahners, 1995). This reduction by phorbol myristate acetate in 3T3‐F442 cells was confirmed by other investigators (Feve, Pietri‐Rouxel, El Hadri, Drumare, & Strosberg, 1995). Treatment of human myometrial strips with 10 μM salbutamol or SR 59,119 also failed to change β3‐adrenoceptor mRNA expression (Rouget et al., 2004). Among the limited studies with transfected β3‐adrenoceptors, reduced mRNA expression was reported upon expression in L cells (Nantel et al., 1995) but no change with expression in CHW cells (Nantel et al., 1994).
In conclusion, data on agonist‐induced regulation of β3‐adrenoceptor mRNA expression are inconclusive when interpreted across tissues (Table 4). However, they appear more consistent within a tissue, further supporting the view that regulation of β3‐adrenoceptor presence and function depends critically on cellular context, in the majority of cases yielding a down‐regulation. Of note, agonist‐induced up‐regulation has also been reported in some cases. The molecular basis for this appears to involve cAMP‐mediated inhibitory and stimulatory effects on β3‐adrenoceptor gene transcription, the balance of which may be cell type‐specific.
6. EVIDENCE OF SYNTHESIS AND CONCLUSIONS
Based upon a lack of consensus phosphorylation sites in its C‐terminal and third intracellular domain, it had been assumed that β3‐adrenoceptor‐mediated functions are resistant to desensitisation. The above data demonstrate that resistance to desensitisation is present in many but not all tissues and cell types (Figure 3). When desensitisation is observed, it often is less pronounced and/or requires longer agonist exposure as compared to that of β1‐ and β2‐adrenoceptors. Moreover, the presence of desensitisation and the molecular level at which it occurs depend strongly on the cell type in which the receptor is expressed.
Figure 3.
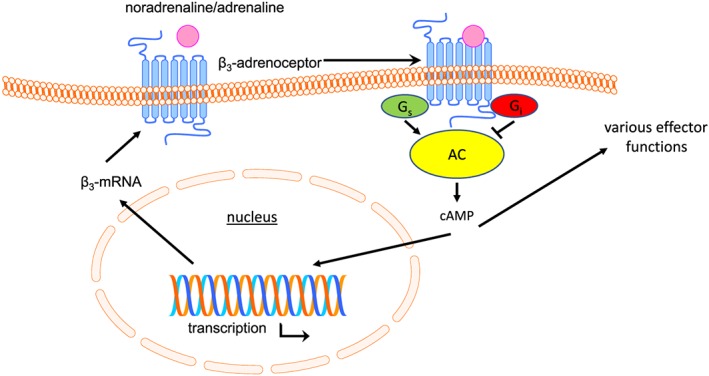
Schematic diagram of key aspects of β3‐adrenoceptor function and regulation. In many if not most tissues and cell types, these do not change upon prolonged agonist exposures, but in some, each of them may decrease (except for Gi, which may increase). For details, see main text
6.1. What are the molecular alterations underlying β3‐adrenoceptor desensitisation?
All available data suggest that β3‐adrenoceptors do not undergo internalisation upon agonist exposure (Curran & Fishman, 1996; Liggett et al., 1993; Milano et al., 2018; Nantel et al., 1993). Given that there is strong evidence that internalisation requires phosphorylation (Lefkowitz, 1998) and that such phosphorylation sites are missing in the β3‐adrenoceptor (Emorine et al., 1989), this is not too surprising.
Down‐regulation of β3‐adrenoceptor protein upon agonist exposure has been reported only in one in vivo and several studies with transfection into Ltk− cells, whereas most studies have found no change or even increases in receptor expression (Table 4). The technical difficulties of detecting β3‐adrenoceptors may explain this scarcity of data (see Section 4). Many more studies have demonstrated a down‐regulation of β3‐adrenoceptor mRNA (Table 4), but in most cases, we do not know whether this translates into changes in expression of functional receptor protein. Apparently, reduced mRNA expression is caused by changes in transcription, not those of mRNA stability (Bengtsson et al., 1996).
Interestingly, desensitisation of β3‐adrenoceptor‐mediated function can occur despite a documented lack of down‐regulation of receptor protein (Chambers et al., 1994; Chaudhry & Granneman, 1994; Hutchinson et al., 2000; Michel‐Reher & Michel, 2013), indicating that changes at the post‐receptor level must contribute to desensitisation. Such changes at the level of G protein expression (Chambers et al., 1994; Michel‐Reher & Michel, 2013), cAMP formation in the presence of direct G protein stimulators (Scarpace et al., 1999), and AC activity (Michel‐Reher & Michel, 2013; Okeke et al., 2017) have been reported, but examples also exist where desensitisation was not accompanied by changes at these levels (Davel et al., 2015; Granneman & Lahners, 1992; Hutchinson et al., 2000; Scarpace et al., 1999).
β3‐adrenoceptor desensitisation can be induced in some models by agents not acting on the receptor, for example, forskolin (Michel‐Reher & Michel, 2013) or agonists of other Gs‐coupled GPCRs. This points to a role for cAMP in mediating desensitisation. However, the opposite can also occur; the β3‐adrenoceptor gene contains cAMP response elements (Liggett & Schwinn, 1991; Thomas et al., 1992), and an elevation of intracellular cAMP can increase receptor expression and/or function (Germack & Dickenson, 2006; Thomas et al., 1992). This raises the possibility that, at least in some cell types, the lack of desensitisation observed does not represent a true resistance but rather the net effect of ongoing sensitisation and desensitisation, but this has not been explored sufficiently for firm conclusions to be reached. Based on limited data, it appears that desensitisation may be independent of β3‐adrenoceptor gene polymorphisms (Vrydag et al., 2009). As β3‐adrenoceptors couple not only to cAMP formation but also to other signalling pathways such as ERK or p38 phosphorylation, it is surprising that with one exception (Okeke et al., 2018), desensitisation of these alternative signalling pathways has not been tested.
6.2. Why is desensitisation tissue/cell type specific?
Both the presence of desensitisation and the level at which it occurs appear to be highly cell type‐dependent. While dependency on cell type has been observed for desensitisation of several GPCR that bind arrestin (Tobin, Butcher, & Kong, 2008), it is not fully clear how this can be explained for β3‐adrenoceptors. The presence of an intron containing a putative enhancer element specific for adipose tissue (Granneman et al., 1992) may at least partly explain tissue‐selective regulation of mRNA expression. Moreover, the presence of desensitisation is modulated within a tissue by additional factors such as age of the animal or innervation status. Understanding the molecular mechanisms underlying such differential desensitisation could help to devise strategies to exploit the presence or absence of desensitisation in a therapeutic manner.
6.3. Desensitisation in disease states
Many GPCRs undergo changes in disease states (Insel, Tang, Hahntow, & Michel, 2007), a phenomenon that has perhaps best been studied for β1‐adrenoceptors, which are desensitised and down‐regulated in patients with congestive heart failure and animal models thereof (Brodde, 1991; Hammond, 1993). However, the regulation of β3‐adrenoceptor expression in disease may differ as it appears to be up‐regulated in heart failure at the mRNA and/or protein level (Cheng et al., 2001; Moniotte, Kobzik, et al., 2001; Napp et al., 2009; Ozakca et al., 2013; Sun et al., 2011; Zhang et al., 2005; Zhao et al., 2013). These data have led to the hypothesis that β3‐adrenoceptor agonists could be useful in patients with heart failure (Rasmussen, Figtree, Krum, & Bundgaard, 2009). An up‐regulation of cardiac β3‐adrenoceptor expression at the mRNA level has also repeatedly (Amour et al., 2007, 2008; Dinçer et al., 2001; Kayki‐Mutlu, Arioglu Inan, Ozakca, Ozcelikay, & Altan, 2014) but not unequivocally (Haley, Thackeray, Kolajova, Thorn, & DaSilva, 2015; Jiang et al., 2015) been observed in animal models of diabetes or of hypothyroidism (Arioglu, Guner, Ozakca, Altan, & Ozcelikay, 2010). Nevertheless, β3‐adrenoceptors were also reported to be down‐regulated in an animal model of colitis, where treatment with an agonist improved symptoms and reversed the down‐regulation (Vasina et al., 2008). However, our overall knowledge on changes in β3‐adrenoceptor expression and function in disease is too limited and needs expansion.
6.4. Conclusions and clinical implications
Based on known β3‐adrenoceptor functions, it is anticipated that potential clinical uses of ligands for this subtype will be limited to agonists (Dehvari, da Silva Junior, Bengtsson, & Hutchinson, 2018; Michel, Ochodnicky, & Summers, 2010). For such uses, a relative resistance to desensitisation is likely to be a desirable property. For instance, desensitisation is a treatment‐limiting factor in the use of β2‐adrenoceptor agonists in women with preterm labour (Engelhardt et al., 1997; Frambach et al., 2005; Michel et al., 1989). Tocolysis has also been proposed as an indication for β3‐adrenoceptor agonists (Bardou et al., 2007; Ursino, Vasina, Raschi, Crema, & De Ponti, 2009); however, whether tocolytic responses to β3‐adrenoceptor agonists are indeed resistant to desensitisation remains to be studied. The only validated clinical use of β3‐adrenoceptor agonists is the treatment of the overactive bladder syndrome (Chapple et al., 2014; Ohlstein et al., 2012). While the value of the reported lack of desensitisation in in vitro animal studies (Michel, 2014) remains to be determined, a 1‐year study in patients presented no evidence of desensitisation for the same agonist (Chapple et al., 2013) but may not have included sufficiently early time points to assess desensitisation during the first 2 weeks of treatment. The key translational challenge of β3‐adrenoceptor desensitisation studies discussed here is the remarkable heterogeneity between species, tissues/cell types, and functional assessments; this makes it difficult to predict what will happen in the target species (humans) and tissue (e.g., urinary bladder or myometrium).
6.5. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Christopoulos et al., 2017; Alexander, Fabbro et al., 2017).
CONFLICT OF INTEREST
M.C.M. is a consultant to Astellas and a consultant and shareholder of Velicept.
ACKNOWLEDGEMENTS
Work in the authors' labs has been supported in part by grants from Deutsche Forschungsgemeinschaft (Mi 294/8‐1), Astellas, and Velicept.
Okeke K, Angers S, Bouvier M, Michel MC. Agonist‐induced desensitisation of β3‐adrenoceptors: Where, when, and how? Br J Pharmacol. 2019;176:2539–2558. 10.1111/bph.14633
REFERENCES
- Adie, J. J. , & Milligan, G. (1994). Agonist regulation of cellular Gs α‐subunit levels in neuroblastoma × glioma hybrid NG108‐15 cells transfected to express different levels of the human β2 adrenoceptor. The Biochemical Journal, 300, 709–715. 10.1042/bj3000709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174, S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … Collaborators, C. G. T. P. (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174(S1), S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amour, J. , Loyer, X. , Le Guen, M. , Mabrouk, N. , David, J.‐S. , Camors, E. , … Riou, B. (2007). Altered contractile response due to increased β3‐adrenoceptor stimulation in diabetic cardiomyopathy. The role of nitric oxide synthase 1‐derived nitric oxide. Anesthesiology, 107, 452–460. 10.1097/01.anes.0000278909.40408.24 [DOI] [PubMed] [Google Scholar]
- Amour, J. , Loyer, X. , Michelet, P. , Birenbaum, A. , Riou, B. , & Heymes, C. (2008). Preservation of the positive lusitropic effect of β‐adrenoceptors stsimulation in diabetic cardiomyopathy. Anesthesia and Analgesia, 107, 1130–1138. 10.1213/ane.0b013e3181806903 [DOI] [PubMed] [Google Scholar]
- Angers, S. (2003). Études in vivo d'interactions protéine‐protéine impliquées dans les voies de signalisation des récepteurs couplés aux protéines G. Montreal: In Dept of Biochemistry. Université de Montreal. [Google Scholar]
- Angers, S. , Salahpour, A. , Joly, E. , Hilairet, S. , Chelsky, D. , Dennis, M. , & Bouvier, M. (2000). Detection of β2‐adrenergic receptor dimerization in living cells using bioluminescence resonance energy transfer (BRET). Proceedings of the National Academy of Sciences of the United States of America, 97, 3684–3689. 10.1073/pnas.060590697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arioglu, E. , Guner, S. , Ozakca, I. , Altan, V. M. , & Ozcelikay, A. T. (2010). The changes in β‐adrenoceptor‐mediated cardiac function in experimental hypothyroidism: The possible contribution of cardiac β3‐adrenoceptors. Molecular and Cellular Biochemistry, 335, 59–66. 10.1007/s11010-009-0241-z [DOI] [PubMed] [Google Scholar]
- Baker, J. G. (2005). The selectivity of β‐adrenoceptor antagonists at the human β1, β2 and β3 adrenoceptors. British Journal of Pharmacology, 144, 317–322. 10.1038/sj.bjp.0706048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, J. G. (2010). The selectivity of β‐adrenoceptor agonists at human β1‐, β2‐ and β3‐adrenoceptors. British Journal of Pharmacology, 160, 1048–1061. 10.1111/j.1476-5381.2010.00754.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak, L. S. , Salahpour, A. , Zhang, X. , Masri, B. , Sotnikova, T. D. , Ramsey, A. J. , … Gainetdinov, R. R. (2008). Pharmacological characterization of membrane‐expressed human trace amine‐associated receptor 1 (TAAR1) by a bioluminescence resonance energy transfer cAMP biosensor. Molecular Pharmacology, 74, 585–594. 10.1124/mol.108.048884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardou, M. , Rouget, C. , Breuiller‐Fouche, M. , Loustalot, C. , Naline, E. , Sagot, P. , … Morrison, J. J. (2007). Is the β3‐adrenoceptor (ADRB3) a potential target for uterorelaxant drugs? BMC Pregnancy Childbirth, 7(Suppl 1), S14 10.1186/1471-2393-7-S1-S14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson, T. , Redegren, K. , Strosberg, A. D. , Nedergaard, J. , & Cannon, B. (1996). Down‐regulation of β3‐adrenoceptor gene expression in brown fat cells is transient and recovery is dependent upon a short‐lived protein factor. The Journal of Biological Chemistry, 271, 33366–33375. 10.1074/jbc.271.52.33366 [DOI] [PubMed] [Google Scholar]
- Bianchetti, A. , & Manara, L. (1990). In vitro inhibition of intestinal motility by phenylethanolaminetetralines: Evidence of atypical β‐adrenoceptors in rat colon. British Journal of Pharmacology, 100, 831–839. 10.1111/j.1476-5381.1990.tb14100.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier, M. , Collins, S. , O'Dowd, B. F. , Campbell, P. T. , de Blasi, A. , Kobilka, B. K. , … Lefkowitz, R. J. (1989). Two distinct pathways for cAMP‐mediated down‐regulation of the beta 2‐adrenergic receptor. Phosphorylation of the receptor and regulation of its mRNA level. Journal of Biological Chemistry, 264, 16786–16792. [PubMed] [Google Scholar]
- Bouvier, M. , Hausdorff, W. P. , DeBlasi, A. , O'Dowd, B. F. , Kobilka, B. K. , Caron, M. G. , & Lefkowitz, R. J. (1988). Removal of phosphorylation sites from the β2‐adrenergic receptor delays onset of agonist‐promoted desensitization. Nature, 333, 370–373. 10.1038/333370a0 [DOI] [PubMed] [Google Scholar]
- Bouvier, M. , Leeb‐Lundberg, L. M. F. , Benovic, J. L. , Caron, M. G. , & Lefkowitz, R. J. (1987). Regulation of adrenergic receptor function by phosphorylation. II. Effects of agonist occupancy on phosphorylation of α1‐ and β2‐adrenergic receptors by protein kinase C and the cyclic AMP‐dependent protein kinase. The Journal of Biological Chemistry, 262, 3106–3113. [PubMed] [Google Scholar]
- Brodde, O. E. (1991). β1‐ and β2‐adrenoceptors in the human heart: Properties, function, and alterations in chronic heart failure. Pharmacological Reviews, 43, 203–241. [PubMed] [Google Scholar]
- Bylund, D. B. , Eikenberg, D. C. , Hieble, J. P. , Langer, S. Z. , Lefkowitz, R. J. , Minneman, K. P. , … Trendelenburg, U. (1994). IV. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacological Reviews, 46, 121–136. [PubMed] [Google Scholar]
- Cahill, T. J. , Thomsen, A. R. B. , Tarrasch, J. T. , Plouffe, B. , Nguyen, A. H. , Yang, F. , … Lefkowitz, R. J. (2017). Distinct conformations of GPCR–β‐arrestin complexes mediate desensitization, signaling, and endocytosis. Proceedings of the National Academy of Sciences of the United States of America, 114, 2562–2567. 10.1073/pnas.1701529114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candelore, M. R. , Deng, L. , Tota, L. M. , Kelly, L. J. , Cascieri, M. A. , & Strader, C. D. (1996). Pharmacological characterization of a recently described human β3‐adrenergic receptor mutant. Endocrinology, 137, 2638–2641. 10.1210/endo.137.6.8641219 [DOI] [PubMed] [Google Scholar]
- Caritis, S. N. , Chiao, J. P. , & Kridgen, P. (1991). Comparison of pulsatile and continuous ritodrine administration: Effects on uterine contractility and β‐adrenergic receptor cascade. American Journal of Obstetrics and Gynecology, 164, 1005–1012. 10.1016/0002-9378(91)90574-B [DOI] [PubMed] [Google Scholar]
- Carpene, C. , Galitzky, J. , Collon, P. , Esclapez, F. , Dauzats, M. , & Lafontan, M. (1993). Desensitization of beta‐1 and beta‐2, but not beta‐3 adrenoceptor‐mediated lipolytic responses of adipocytes after long‐term norepinephrine infusion. The Journal of Pharmacology and Experimental Therapeutics, 265, 237–247. [PubMed] [Google Scholar]
- Cernecka, H. , Ochodnicky, P. , Lamers, W. H. , & Michel, M. C. (2012). Specificity evaluation of antibodies against human β3‐adrenoceptors. Naunyn‐Schmiedeberg's Archives of Pharmacology, 385, 875–882. 10.1007/s00210-012-0767-6 [DOI] [PubMed] [Google Scholar]
- Cernecka, H. , Pradidarcheep, W. , Lamers, W. H. , Schmidt, M. , & Michel, M. C. (2014). Rat β3‐adrenoceptor protein expression: Antibody validation and distribution in rat gastrointestinal and urogenital tissues. Naunyn‐Schmiedeberg's Archives of Pharmacology, 387, 1117–1127. 10.1007/s00210-014-1039-4 [DOI] [PubMed] [Google Scholar]
- Cernecka, H. , Sand, C. , & Michel, M. C. (2014). The odd sibling: Features of β3‐adrenoceptor pharmacology. Molecular Pharmacology, 86, 479–484. 10.1124/mol.114.092817 [DOI] [PubMed] [Google Scholar]
- Chamberlain, P. D. , Jennings, K. H. , Paul, F. , Cordell, J. , Berry, A. , Holmes, S. D. , … Murphy, G. J. (1999). The tissue distribution of the human β3‐adrenoceptor studied using a monoclonal antibody: Direct evidence of the β3‐adrenoceptor in human adipose tissue, atrium and skeletal muscle. International Journal of Obesity and Related Metabolic Disorders, 23, 1057–1065. 10.1038/sj.ijo.0801039 [DOI] [PubMed] [Google Scholar]
- Chambers, J. , Park, J. , Cronk, D. , Chapman, C. , Kennedy, F. R. , Wilson, S. , & Milligan, G. (1994). β3‐Adrenoceptor agonist‐induced down‐regulation of Gsα and functional desensitization in a Chinese hamster ovary cell line expressing a β3‐adrenoceptor refractory to down‐regulation. The Biochemical Journal, 303, 973–978. 10.1042/bj3030973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapple, C. R. , Cardozo, L. , Nitti, V. W. , Siddiqui, E. , & Michel, M. C. (2014). Mirabegron in overactive bladder: A review of efficacy, safety, and tolerability. Neurourology and Urodynamics, 33, 17–30. 10.1002/nau.22505 [DOI] [PubMed] [Google Scholar]
- Chapple, C. R. , Kaplan, S. A. , Mitcheson, D. , Klecka, J. , Cummings, J. , Drogendijk, T. , … Martin, N. (2013). Randomized double‐blind, active‐controlled phase 3 study to assess 12‐month safety and effiaccy of mirabegron, a β3‐adrenoceptor agonist, in overactive bladder. European Urology, 63, 296–305. 10.1016/j.eururo.2012.10.048 [DOI] [PubMed] [Google Scholar]
- Chaudhry, A. , & Granneman, J. G. (1994). Influence of cell type upon the desensitization of the β3‐adrenergic receptor. The Journal of Pharmacology and Experimental Therapeutics, 271, 1253–1258. [PubMed] [Google Scholar]
- Cheng, H. J. , Zhang, Z. S. , Onishi, K. , Ukai, T. , Sane, D. C. , & Cheng, C. P. (2001). Upregulation of functional β3‐adrenergic receptor in the failing canine myocardium. Circulation Research, 89, 599–606. 10.1161/hh1901.098042 [DOI] [PubMed] [Google Scholar]
- Curran, P. K. , & Fishman, P. H. (1996). Endogenous β3‐ but not β1‐adrenergic receptors are resistant to agonist‐mediated regulation in human SK‐N‐MC neurotumor cells. Cellular Signalling, 8, 355–364. 10.1016/0898-6568(96)00068-X [DOI] [PubMed] [Google Scholar]
- Danner, S. , Frank, M. , & Lohse, M. J. (1998). Agonist regulation of human β2‐adrenergic receptor mRNA stability occurs via a specific AU‐rich element. The Journal of Biological Chemistry, 273, 3223–3229. 10.1074/jbc.273.6.3223 [DOI] [PubMed] [Google Scholar]
- Davel, A. P. , Victorio, J. A. , Delbin, M. A. , Fukuda, L. E. , & Rossoni, L. V. (2015). Enhanced endothelium‐dependent relaxation of rat pulmonary artery following β‐adrenergic overstimulation: Involvement of the NO/cGMP/VASP pathway. Life Sciences, 125, 49–56. 10.1016/j.lfs.2015.01.018 [DOI] [PubMed] [Google Scholar]
- Dehvari, N. , da Silva Junior, E. D. , Bengtsson, T. , & Hutchinson, D. S. (2018). Mirabegron: Potential off target effects and uses beyond the bladder. British Journal of Pharmacology, 175(21), 4072–4082. 10.1111/bph.14121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, C. , Paoloni‐Giacobino, A. , Kuehne, F. , Boss, O. , Revelli, J. P. , Moinat, M. , … Giacobino, J. P. (1996). Respective degree of expression of β1‐, β2‐ and β3‐adrenoceptors in human brown and white adipose tissue. British Journal of Pharmacology, 118, 929–934. 10.1111/j.1476-5381.1996.tb15488.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinçer, Ü. D. , Bidasee, K. R. , Güner, Ş. , Tay, A. , Özçelikay, A. T. , & Altan, V. M. (2001). The effect of diabetes on expression of β1‐, β2‐, and β3‐adrenoreceptors in rat hearts. Diabetes, 50, 455–461. 10.2337/diabetes.50.2.455 [DOI] [PubMed] [Google Scholar]
- Emorine, L. J. , Marullo, S. , Briden‐sutren, M. M. , Patey, G. , Tate, K. , Delavier‐Klutchko, C. , & Strosberg, A. D. (1989). Molecular characterization of the human β3‐adrenergic receptor. Science, 245, 1118–1121. 10.1126/science.2570461 [DOI] [PubMed] [Google Scholar]
- Engelhardt, S. , Zieger, W. , Kassubek, J. , Michel, M. C. , Lohse, M. J. , & Brodde, O. E. (1997). Tocolytic therapy with fenoterol induces selective down‐regulation of β‐adrenergic receptors in human myometrium. The Journal of Clinical Endocrinology and Metabolism, 82, 1235–1242. 10.1210/jcem.82.4.3885 [DOI] [PubMed] [Google Scholar]
- Eschenhagen, T. , Mende, U. , Diederich, M. , Nose, M. , Schmitz, W. , Scholz, H. , … Schäfer, H. (1992). Long term β‐adrenoceptor‐mediated up‐regulation of Giα and Goα mRNA levels and pertussis toxin‐sensitive guanine nucleotide‐binding proteins in rat heart. Molecular Pharmacology, 42, 773–783. [PubMed] [Google Scholar]
- Evans, B. A. , Papaioannou, M. , Hamilton, S. , & Summers, R. J. (1999). Alternative splicing generates two isoforms of the β3‐adrenoceptor which are differentially expressed in mouse tissues. British Journal of Pharmacology, 127, 1525–1531. 10.1038/sj.bjp.0702688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman, R. D. (1989). β‐Adrenergic desensitization reduces the sensitivity of adenylate cyclase for magnesium in permeabilized lymphocytes. Molecular Pharmacology, 35, 304–310. [PubMed] [Google Scholar]
- Feve, B. , Pietri‐Rouxel, F. , El Hadri, K. , Drumare, M. F. , & Strosberg, A. D. (1995). Long term phorbol ester treatment down‐regulates the β3‐adrenergic receptor in 3T3‐F4424 adipocytes. The Journal of Biological Chemistry, 270, 10952–10959. 10.1074/jbc.270.18.10952 [DOI] [PubMed] [Google Scholar]
- Frambach, T. , Müller, T. , Freund, S. , Engelhardt, S. , Sutterlin, M. , Lohse, M. J. , & Dietl, J. (2005). Self‐limitation of intravenous tocolysis with β2‐adrenergic agonists is mediated through receptor G protein uncoupling. The Journal of Clinical Endocrinology and Metabolism, 90, 2882–2887. 10.1210/jc.2004-1732 [DOI] [PubMed] [Google Scholar]
- Furchgott, R. F. (1972). The classification of adrenoceptors (adrenergic receptors). An evaluation from the standpoint of receptor theory In Blaschko H., & Muecholl E. (Eds.), Catecholamines (pp. 283–335). New York: Springer Verlag. [Google Scholar]
- Gerhardt, C. C. , Gros, J. , Strosberg, A. D. , & Issad, T. (1999). Stimulation of the extracellular signal‐regulated kinase 1/2 pathway by human beta‐3 adrenergic receptor: New pharmacological profile and mechanism of action. Molecular Pharmacology, 55, 255–262. 10.1124/mol.55.2.255 [DOI] [PubMed] [Google Scholar]
- Germack, R. , & Dickenson, J. M. (2006). Induction of β3‐adrenergic receptor functional expression following chronic stimulation with noradrenaline in neonatal rat cardiomyocytes. The Journal of Pharmacology and Experimental Therapeutics, 316, 392–402. 10.1124/jpet.105.090597 [DOI] [PubMed] [Google Scholar]
- Goodman, O. B. Jr. , Krupnick, J. G. , Santini, F. , Gurevich, V. V. , Penn, R. B. , Gagnon, A. W. , … Benovic, J. L. (1996). β‐Arrestin acts as a clathrin adaptor in endocytosis of the β2‐adrenergic receptor. Nature, 383, 447. [DOI] [PubMed] [Google Scholar]
- Granneman, J. G. (1992). Effects of agonist exposure on the coupling of beta1 and beta3 adrenergic receptors to adenylyl cyclase in isolated adipocytes. The Journal of Pharmacology and Experimental Therapeutics, 261, 638–642. [PubMed] [Google Scholar]
- Granneman, J. G. , & Lahners, K. N. (1992). Differential adrenergic regulation of β1‐ and β3‐adrenoceptor messenger ribonucleic acids in adipose tissues. Endocrinology, 130, 109–114. 10.1210/endo.130.1.1309320 [DOI] [PubMed] [Google Scholar]
- Granneman, J. G. , & Lahners, K. N. (1994). Analysis of human and rodent β3‐adrenergic receptor messenger ribonucleic acids. Endocrinology, 135, 1025–1031. 10.1210/endo.135.3.8070345 [DOI] [PubMed] [Google Scholar]
- Granneman, J. G. , & Lahners, K. N. (1995). Regulation of mouse beta 3‐adrenergic receptor gene expression and mRNA splice variants in adipocytes. American Journal of Physiology ‐ Cell Physiology, 268, C1040–C1044. 10.1152/ajpcell.1995.268.4.C1040 [DOI] [PubMed] [Google Scholar]
- Granneman, J. G. , Lahners, K. N. , & Rao, D. D. (1992). Rodent and human β3‐adrenergic receptor genes contain an intron within the protein‐coding block. Molecular Pharmacology, 42, 964–970. [PubMed] [Google Scholar]
- Hadcock, J. R. , Wang, H. Y. , & Malbon, C. C. (1989). Agonist‐induced destabilization of β‐adrenergic receptor mRNA. Attenuation of glucocorticoid‐induced up‐regulation of β‐adrenergic receptors. Journal of Biological Chemistry, 264, 19928–19933. [PubMed] [Google Scholar]
- Hadi, T. , Barichon, M. , Mourtialon, P. , Wendremaire, M. , Garrido, C. , Sagot, P. , … Lirussi, F. (2013). Biphasic ERK1/2 activation sequentially involving Gs and Gi signaling is required in beta3‐adrenergic receptor‐induced primarny smooth muscle cell proliferation. Biochimica et Biophysica Acta, 1833, 1041–1051. 10.1016/j.bbamcr.2013.01.019 [DOI] [PubMed] [Google Scholar]
- Haley, J. M. , Thackeray, J. T. , Kolajova, M. , Thorn, S. L. , & DaSilva, J. N. (2015). Insulin therapy normalizes reduced myocardial β‐adrenoceptors at both the onset and after sustained hyperglycemia in diabetic rats. Life Sciences, 132, 101–107. 10.1016/j.lfs.2015.03.024 [DOI] [PubMed] [Google Scholar]
- Hammond, H. K. (1993). Mechanisms for myocardial β‐adrenergic receptor desensitization in heart failure. Circulation, 87, 652–654. 10.1161/01.CIR.87.2.652 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms, H. H. , Zaagsma, J. , & van der Wal, B. (1974). β‐Adrenoceptor studies. III. On the β‐adrenoceptors in rat adipose tissue. European Journal of Pharmacology, 25, 87–97. 10.1016/0014-2999(74)90098-3 [DOI] [PubMed] [Google Scholar]
- Hatakeyama, Y. , Sakata, Y. , Takakura, S. , Manda, T. , & Mutoh, S. (2004). Acute and chronic effects of FR‐149175, a β3‐adrenergic receptor agonist, on energy expenditure in Zucker fatty rats. The American Journal of Physiology, 287, R336–R341. [DOI] [PubMed] [Google Scholar]
- Hoffmann, C. , Leitz, M. R. , Oberdorf‐Maass, S. , Lohse, M. J. , & Klotz, K. N. (2004). Comparative pharmacology of human β‐adrenergic receptor subtypes—Characterization of stably transfected receptors in CHO cells. Naunyn‐Schmiedeberg's Archives of Pharmacology, 369, 151–159. 10.1007/s00210-003-0860-y [DOI] [PubMed] [Google Scholar]
- Hoyer, D. , Engel, G. , & Kalkman, H. O. (1985a). Characterization of the 5‐HT1B recognition site in rat brain: Binding studies with (−)[125I]iodocyanopindolol. European Journal of Pharmacology, 118, 1–12. 10.1016/0014-2999(85)90657-0 [DOI] [PubMed] [Google Scholar]
- Hoyer, D. , Engel, G. , & Kalkman, H. O. (1985b). Molecular pharmacology of 5‐HT1 and 5‐HT2 recognition sites in rat and pig brain membranes: Radioligand binding studies with [3H]5‐HT, [3 H]8‐OH‐DPAT, (−)[125I]iococyanopindolol, [3H]mesulergide and [3H]ketanserin. European Journal of Pharmacology, 118, 13–23. 10.1016/0014-2999(85)90658-2 [DOI] [PubMed] [Google Scholar]
- Hutchinson, D. S. , Chernogubova, E. , Sato, M. , Summers, R. J. , & Bengtsson, T. (2006). Agonist effects of zinterol at the mouse and human β3‐adrenoceptor. Naunyn‐Schmiedeberg's Archives of Pharmacology, 373, 158–168. 10.1007/s00210-006-0056-3 [DOI] [PubMed] [Google Scholar]
- Hutchinson, D. S. , Evans, B. A. , & Summers, R. J. (2000). β3‐Adrenoceptor regulation and relaxation responses in mouse ileum. British Journal of Pharmacology, 129, 1251–1259. 10.1038/sj.bjp.0703160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel, P. A. , Tang, C. M. , Hahntow, I. , & Michel, M. C. (2007). Impact of GPCRs in clinical medicine: Genetic variants and drug targets. Biochimica et Biophysica Acta, 1768, 994–1005. 10.1016/j.bbamem.2006.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- January, B. , Seibold, A. , Whaley, B. , Hipkin, R. W. , Lin, D. , Schonbrunn, A. , … Clark, R. B. (1997). β2‐Adrenergic receptor desensitization, internalization, and phosphorylation in response to full and partial agonists. The Journal of Biological Chemistry, 272, 23871–23879. 10.1074/jbc.272.38.23871 [DOI] [PubMed] [Google Scholar]
- Jiang, C. , Carillion, A. , Na, N. , De Jong, A. , Feldman, S. , Lacorte, J. M. , … Amour, J. (2015). Modification of the β‐adrenoceptor pathway in Zucker obese and obese diabetic rat myocardium. Critical Care Medicine, 43, e241–e249. 10.1097/CCM.0000000000000999 [DOI] [PubMed] [Google Scholar]
- Jockers, R. , Da Silva, A. , Strosberg, A. D. , Bouvier, M. , & Marullo, S. (1996). New molecular and structural determinants involved in β2‐adrenergic receptor desensitization and sequestration: Delineation using chimeric β3/β2‐adrenergic receptors. The Journal of Biological Chemistry, 271, 9355–9362. 10.1074/jbc.271.16.9355 [DOI] [PubMed] [Google Scholar]
- Kayki‐Mutlu, G. , Arioglu Inan, E. , Ozakca, I. , Ozcelikay, A. T. , & Altan, V. M. (2014). β3‐Adrenoceptor‐mediated responses in diabetic rat heart. General Physiology and Biophysics, 33, 99–109. 10.4149/gpb_2013065 [DOI] [PubMed] [Google Scholar]
- Kimura, H. , Miyamoto, A. , & Oshika, H. (1993). Down‐regulation of β‐adrenoceptors and loss of Gsα subunit levels in ventricular myocardium of rats treated with isoproterenol. Life Sciences, 53, PL171–PL176. 10.1016/0024-3205(93)90511-Z [DOI] [PubMed] [Google Scholar]
- Klaus, S. , Muzzin, P. , Revelli, J. P. , Cawthorne, M. A. , Giacobino, J. P. , & Ricquier, D. (1995). Control of β3‐adrenergic receptor gene expression in brown adipocytes in culture. Molecular and Cellular Endocrinology, 109, 189–195. 10.1016/0303-7207(95)03502-X [DOI] [PubMed] [Google Scholar]
- Kullmann, F. A. , Limberg, B. J. , Artim, D. E. , Shah, M. , Downs, T. R. , Contract, D. , … de Groat, W. C. (2009). Effects of β3‐adrenergic receptor activation on rat urinary bladder hyperactivity induced by ovariectomy. The Journal of Pharmacology and Experimental Therapeutics, 330, 704–717. 10.1124/jpet.109.155010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lands, A. M. , Arnold, A. , McAuliff, J. P. , Luduena, F. P. , & Brown, T. G. (1967). Differentiation of receptor systems activated by sympathetic amines. Nature, 214, 597–598. 10.1038/214597a0 [DOI] [PubMed] [Google Scholar]
- Laporte, S. A. , Oakley, R. H. , Holt, J. A. , Barak, L. S. , & Caron, M. G. (2000). The interaction of β‐arrestin with the AP‐2 adaptor is required for the clustering of β2‐adrenergic receptor into clathrin‐coated pits. The Journal of Biological Chemistry, 275, 23120–23126. 10.1074/jbc.M002581200 [DOI] [PubMed] [Google Scholar]
- Lefkowitz, R. J. (1998). G protein‐coupled receptors. III. New roles for receptor kinases and β‐arrestins in receptor signaling and desensitization. Journal of Biological Chemistry, 273, 18677–18680. [DOI] [PubMed] [Google Scholar]
- Lelias, J. M. , Kaghad, M. , Rodriguez, M. , Chalon, P. , Bonnin, J. , Dupre, I. , … Caput, D. (1993). Molecular cloning of a human β3‐adrenergic receptor cDNA. FEBS Letters, 324, 127–130. 10.1016/0014-5793(93)81377-C [DOI] [PubMed] [Google Scholar]
- Liggett, S. B. , Freedman, N. J. , Schwinn, D. A. , & Lefkowitz, R. J. (1993). Structural basis for receptor subtype‐specific regulation revealed by a chimeric β3/β2‐adrenergic receptor. Proceedings of the National Academy of Sciences, 90, 3665–3669. 10.1073/pnas.90.8.3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggett, S. B. , & Schwinn, D. A. (1991). Multiple potential regulatory elements in the 5′ flanking region of the β3‐adrenergic receptor. DNA Sequence, 2, 61–63. 10.3109/10425179109008441 [DOI] [PubMed] [Google Scholar]
- Limberg, B. J. , Andersson, K. E. , Kullmann, F. A. , Burmer, G. , De Groat, W. C. , & Rosenbaum, J. S. (2010). β‐Adrenergic receptor subtype expression in myocyte and non‐myocyte cells in human female bladder. Cell and Tissue Research, 342, 295–306. 10.1007/s00441-010-1053-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnqvist, F. , Krief, S. , Strosberg, A. D. , Nyberg, B. , Emorine, L. J. , & Arner, P. (1993). Evidence for a functional β3‐adrenoceptor in man. British Journal of Pharmacology, 110, 929–936. 10.1111/j.1476-5381.1993.tb13902.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lye, S. J. , Dayes, B. A. , Freitag, C. L. , Brooks, J. , & Casper, R. F. (1992). Failure of ritodrine to prevent preterm labor in sheep. American Journal of Obstetrics and Gynecology, 167, 1399–1408. 10.1016/S0002-9378(11)91725-6 [DOI] [PubMed] [Google Scholar]
- Maisel, A. S. , Scott, N. A. , Motulsky, H. J. , Michel, M. C. , Boublik, J. H. , Rivier, J. E. , … Brown, M. R. (1989). Elevation of plasma neuropeptide Y levels in congestive heart failure. The American Journal of Medicine, 86, 43–48. 10.1016/0002-9343(89)90228-3 [DOI] [PubMed] [Google Scholar]
- Marullo, S. , Nantel, F. , Strosberg, A. D. , & Bouvier, M. (1995). Variability in the regulation of β‐adrenoceptor subtypes. Biochemical Society Transactions, 23, 126–129. 10.1042/bst0230126 [DOI] [PubMed] [Google Scholar]
- Marzo, K. P. , Frey, M. J. , Wilson, J. R. , Liang, B. T. , Manning, D. R. , Lanoce, V. , & Molinoff, P. B. (1991). β‐Adrenergic receptor‐G protein‐adenylate cyclase complex in experimental canine congestive heart failure produced by rapid ventricular pacing. Circulation Research, 69, 1546–1556. 10.1161/01.RES.69.6.1546 [DOI] [PubMed] [Google Scholar]
- Matthews, J. M. , Falckh, P. H. J. , Molenaar, P. , & Summers, R. J. (1996). Chronic (−)‐isoprenaline infusion down‐regulates β1‐ and β2‐adrenoceptors but does not transregulate muscarinic cholinoceptors in rat heart. Naunyn‐Schmiedeberg's Archives of Pharmacology, 353, 213–225. [DOI] [PubMed] [Google Scholar]
- Mauro, V. F. , & Mauro, L. S. (1986). Use of intermittent dobutamine infusion in congestive heart failure. Drug Intelligence & Clinical Pharmacy, 20, 919–924. 10.1177/106002808602001201 [DOI] [PubMed] [Google Scholar]
- Medina‐Martinez, O. , & Garcia‐Sainz, J. A. (1993). Hepatocyte homologous β2‐adrenergic desensitization is associated with a decrease in number of plasma membrane β2‐adrenoceptors. European Journal of Pharmacology, 244, 145–151. 10.1016/0922-4106(93)90020-A [DOI] [PubMed] [Google Scholar]
- Michel, M. C. (2014). Do β‐adrenoceptor agonists induce homologous or heterologous desensitization in rat urinary bladder? Naunyn‐Schmiedeberg's Archives of Pharmacology, 387, 215–224. 10.1007/s00210-013-0936-2 [DOI] [PubMed] [Google Scholar]
- Michel, M. C. , Feth, F. , Sundermann, M. , Rascher, W. , & Brodde, O. E. (1993). β‐Adrenoceptor desensitization in SK‐N‐MC cells, a human cell line containing a homogeneous population of β1‐adrenoceptors. Journal of Autonomic Pharmacology, 13, 425–438. 10.1111/j.1474-8673.1993.tb00290.x [DOI] [Google Scholar]
- Michel, M. C. , & Gravas, S. (2016). Safety and tolerability of β3‐adrenoceptor agonists in the treatment of overactive bladder syndrome—Insight from transcriptosome and experimental studies. Expert Opinion on Drug Safety, 15, 647–657. [DOI] [PubMed] [Google Scholar]
- Michel, M. C. , Ochodnicky, P. , & Summers, R. J. (2010). Tissue functions mediated by β3‐adrenoceptors—Findings and challenges. Naunyn‐Schmiedeberg's Archives of Pharmacology, 382, 103–108. 10.1007/s00210-010-0529-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel, M. C. , Pingsmann, A. , Nohlen, M. , Siekmann, U. , & Brodde, O. E. (1989). Decreased myometrial β‐adrenoceptors in women receiving β2‐adrenergic tocolytic therapy: Correlation with lymphocyte β‐adrenoceptors. Clinical Pharmacology and Therapeutics, 45, 1–8. 10.1038/clpt.1989.1 [DOI] [PubMed] [Google Scholar]
- Michel, M. C. , & Seifert, R. (2015). Selectivity of pharmacological tools: Implications for use in cell physiology. The American Journal of Physiology, 308, C505–C520. 10.1152/ajpcell.00389.2014 [DOI] [PubMed] [Google Scholar]
- Michel‐Reher, M. B. , & Michel, M. C. (2013). Agonist‐induced desensitization of human β3‐adrenoceptors expressed in human embryonic kidney cells. Naunyn‐Schmiedeberg's Archives of Pharmacology, 386, 843–851. 10.1007/s00210-013-0891-y [DOI] [PubMed] [Google Scholar]
- Michel‐Reher, M. B. , & Michel, M. C. (2015). Regulation of GAPDH expression by treatment with the β‐adrenoceptor agonist isoprenaline—Is GAPDH a suitable loading control in immunoblot experiments? Naunyn‐Schmiedeberg's Archives of Pharmacology, 388, 1119–1120. 10.1007/s00210-015-1166-6 [DOI] [PubMed] [Google Scholar]
- Milano, S. , Gerbino, A. , Schena, G. , Carmosino, M. , Svelto, M. , & Procino, G. (2018). Human β3‐adrenoreceptor is resistant to agonist‐induced desensitization in renal epithelial cells. Cellular Physiology and Biochemistry, 48, 847–862. 10.1159/000491916 [DOI] [PubMed] [Google Scholar]
- Moniotte, S. , Kobzik, L. , Feron, O. , Trochu, J. N. , Gauthier, C. , & Balligand, J. L. (2001). Upregulation of β3‐adrenoceptors and altered contractile response to inotropic amines in human failing myocardium. Circulation, 103, 1649–1655. 10.1161/01.CIR.103.12.1649 [DOI] [PubMed] [Google Scholar]
- Moniotte, S. , Vaerman, J. L. , Kockx, M. M. , Larrouy, D. , Langin, D. , Noirhomme, P. , & Balligand, J. L. (2001). Real‐time RT‐PCR for the detection of beta‐adrenoceptor messenger RNAs in small human endomyocardial biopsies. Journal of Molecular and Cellular Cardiology, 33, 2121–2133. 10.1006/jmcc.2001.1475 [DOI] [PubMed] [Google Scholar]
- Muzzin, P. , Boss, O. , Mathis, N. , Revelli, J. P. , Giacobino, J. P. , Willcocks, K. , … Cawthorne, M. A. (1994). Characterization of a new, highly specific, β3‐adrenergic receptor radioligand, [3H]SB 206606. Molecular Pharmacology, 46, 357–363. [PubMed] [Google Scholar]
- Muzzin, P. , Revelli, J. P. , Kuhne, F. , Gocayne, J. D. , McCombie, W. R. , Venter, J. C. , … Fraser, C. M. (1991). An adipose tissue‐specific β‐adrenergic receptor. Molecular cloning and down‐regulation in obesity. Journal of Biological Chemistry, 266, 24053–24058. [PubMed] [Google Scholar]
- Nahmias, C. , Blin, N. , Elalouf, J. M. , Mattei, M. G. , Strosberg, A. D. , & Emorine, L. J. (1991). Molecular characterization of the mouse β3‐adrenergic receptor: Relationship with the atypical receptor of adipocytes. The EMBO Journal, 10, 3721–3727. 10.1002/j.1460-2075.1991.tb04940.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nantel, F. , Bonin, H. , Emorine, L. J. , Zelberfarb, V. , Strosberg, A. D. , Bouvier, M. , & Marullo, S. (1993). The human β3‐adrenergic receptor is resistant to short term agonist‐promoted desensitization. Molecular Pharmacology, 43, 548–555. [PubMed] [Google Scholar]
- Nantel, F. , Bouvier, M. , Strosberg, A. D. , & Marullo, S. (1995). Functional effects of long‐term activation of human β2‐ and β3‐adrenoceptor signalling. British Journal of Pharmacology, 114, 1045–1051. 10.1111/j.1476-5381.1995.tb13311.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nantel, F. , Marullo, S. , Krief, S. , Strosberg, A. D. , & Bouvier, M. (1994). Cell‐specific down‐regulation of the β3‐adrenergic receptor. The Journal of Biological Chemistry, 269, 13148–13155. [PubMed] [Google Scholar]
- Napp, A. , Brixius, K. , Pott, C. , Ziskoven, C. , Boelck, B. , Mehlhorn, U. , … Bloch, W. (2009). Effects of the β3‐adrenergic agonist BRL 37344 on endothelial nitric oxide synthase phosphorylation and force of contraction in human failing myocardium. Journal of Cardiac Failure, 15, 57–67. 10.1016/j.cardfail.2008.08.006 [DOI] [PubMed] [Google Scholar]
- Nergardh, A. , Boreus, L. O. , & Naglo, A. S. (1977). Characterization of the adrenergic beta‐receptor in the urinary bladder of man and cat. Acta Pharmacologica et Toxicologica (Copenh), 40, 14–21. [DOI] [PubMed] [Google Scholar]
- Niclauß, N. , Michel‐Reher, M. B. , Alewijnse, A. E. , & Michel, M. C. (2006). Comparison of three radioligands for the labelling of human β‐adrenoceptor subtypes. Naunyn‐Schmiedeberg's Archives of Pharmacology, 374, 99–105. 10.1007/s00210-006-0104-z [DOI] [PubMed] [Google Scholar]
- Ohlstein, E. H. , von Keitz, A. , & Michel, M. C. (2012). A multicenter, double‐blind, randomized, placebo controlled trial of the β3‐adrenoceptor agonist solabegron for overactive bladder. European Urology, 62, 834–840. 10.1016/j.eururo.2012.05.053 [DOI] [PubMed] [Google Scholar]
- Okeke, K. , Michel‐Reher, M. , & Michel, M. C. (2018). β3‐Adrenoceptor desensitisation in CHO cells: Comparison of cAMP and ERK signalling. pA2 online 18: 017.
- Okeke K, Michel‐Reher MB, & Michel MC (2017). β3‐Adrenoceptors desensitisation in HEK cells: A role for biased agonism? PA2 online 6: 058P.
- Ortiz, J. L. , Dasi, F. J. , Cortijo, J. , & Morcillo, E. J. (2000). β‐Adrenoceptor stimulation up‐regulates phosphodiesterase 4 activity and reduces prostglandin E2‐inhibitory effects in human neutrophils. Naunyn‐Schmiedeberg's Archives of Pharmacology, 361, 410–417. 10.1007/s002100000215 [DOI] [PubMed] [Google Scholar]
- Ozakca, I. , Arioglu‐Inan, E. , Esfahani, H. , Altan, V. M. , Balligand, J.‐L. , Kayki‐Mutlu, G. , & Ozcelikay, A. T. (2013). Nebivolol prevents desensitization of β‐adrenoceptor signaling and induction of cardiac hypertrophy in response to isoprenaline beyond β1‐adrenoceptor blockage. American Journal of Physiology ‐ Heart and Circulatory Physiology, 304, H1267–H1276. 10.1152/ajpheart.00352.2012 [DOI] [PubMed] [Google Scholar]
- Pacini, E. S. A. , Sanders‐Silveira, S. , & Godinho, R. O. (2018). The extracellular cAMP‐adenosine pathway in airway smooth muscle. The Journal of Pharmacology and Experimental Therapeutics, 366, 75–83. 10.1124/jpet.118.247734 [DOI] [PubMed] [Google Scholar]
- Rasmussen, H. H. , Figtree, G. A. , Krum, H. , & Bundgaard, H. (2009). Use of β3 adrenergic receptor agonists in the treatment of heart failure. Current Opinion in Investigational Drugs, 10, 955–962. [PubMed] [Google Scholar]
- Rathi, S. , Kazerounian, S. , Banwait, K. , Schulz, S. , Waldman, S. A. , & Rattan, S. (2003). Functional and molecular characterization of β‐adrenoceptors in the internal anal sphincter. The Journal of Pharmacology and Experimental Therapeutics, 305, 615–624. 10.1124/jpet.102.048462 [DOI] [PubMed] [Google Scholar]
- Revelli, J. P. , Muzzin, P. , & Giacobino, J. P. (1992). Modulation in vivo of β‐adrenergic‐receptor subtypes in rat brown adipose tissue by the thermogenic agonist Ro 16‐8714. The Biochemical Journal, 286, 743–746. 10.1042/bj2860743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robay, A. , Toumaniantz, G. , Leblais, V. , & Gauthier, C. (2005). Transfected β3‐ but not β2‐adrenergic receptors regulate cystic fibrosis transmembrane conductance regulator activity via a new pathway involving the mitogen‐activated protein kinases extracellular signal‐regulated kinases. Molecular Pharmacology, 67, 648–654. 10.1124/mol.104.002097 [DOI] [PubMed] [Google Scholar]
- Roberts, S. J. , Molenaar, P. , & Summers, R. J. (1993). Characterization of propranolol‐resistant (−)‐[125I]‐cyanopindolol binding sites in rat soleus muscle. British Journal of Pharmacology, 109, 344–352. 10.1111/j.1476-5381.1993.tb13576.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouget, C. , Bardou, M. , Breuiller‐Foucher, M. , Loustalot, C. , Qi, H. , Naline, E. , … Leroy, M. J. (2005). β3‐Adrenoceptor is the predominant β‐adrenoceptor subtype in human myometrium and its expression is up‐regulated in pregnancy. The Journal of Clinical Endocrinology and Metabolism, 90, 1644–1650. 10.1210/jc.2004-0233 [DOI] [PubMed] [Google Scholar]
- Rouget, C. , Breullier‐Fouche, M. , Mercier, F. J. , Leroy, M. J. , Loustalot, C. , Naline, E. , … Bardou, M. (2004). The human near‐term myometrial β3‐adrenoceptor but not the β2‐adrenoceptor is resistant to desensitisation after sustained agonist stimulation. British Journal of Pharmacology, 141, 831–841. 10.1038/sj.bjp.0705616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter, S. R. , Ormiston, T. M. , & Salpeter, E. E. (2004). Meta‐analysis: Respiratory tolerance to regular β2‐agonist use in patients with asthma. Annals of Internal Medicine, 140, 802–813. 10.7326/0003-4819-140-10-200405180-00010 [DOI] [PubMed] [Google Scholar]
- Sato, M. , Horinouchi, T. , Hutchinson, D. S. , Evans, B. A. , & Summers, R. J. (2007). Ligand‐directed signaling at the β3‐adrenoceptor produced by SR59230A relative to receptor agonists. Molecular Pharmacology, 74, 1359–1368. [DOI] [PubMed] [Google Scholar]
- Sato, M. , Hutchinson, D. S. , Evans, B. A. , & Summers, R. J. (2008). The β3‐adrenoceptor agonist 4‐[[(hexylamino)carbonyl]amino]‐N‐[4‐[2‐[[(2S)‐2‐hydroxy‐3‐(4‐hydroxyphenoxy)propyl]amino]ethyl]‐phenyl]‐benzenesulfonamide (L755507) and antagonist (S)‐N‐[4‐[2‐[[3‐[3‐(acetamidomethyl)phenoxy]‐2‐hydroxypropyl]amino]‐ethyl]phenyl]benzenesulfonamide (L748337) activate different signaling pathways in Chinese hamster ovary‐K1 cells stably expressing the human β3‐adrenoceptor. Molecular Pharmacology, 74, 1417–1428. 10.1124/mol.108.046979 [DOI] [PubMed] [Google Scholar]
- Sato, M. , Hutchinson, D. S. , Halls, M. L. , Furness, S. G. , Bengtsson, T. , Evans, B. A. , & Summers, R. J. (2012). Interaction with caveolin‐1 modulates G protein coupling of mouse β3‐adrenoceptor. The Journal of Biological Chemistry, 287, 20674–20688. 10.1074/jbc.M111.280651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpace, P. J. , Matheny, M. , & Thümer, N. (1999). Differential down‐regulation of β3‐adrenergic receptor mRNA and signal transduction by cold exposure in brown adipose tissue of young and senescent rats. Pflügers Archiv: European Journal of Physiology, 437, 479–483. 10.1007/s004240050804 [DOI] [PubMed] [Google Scholar]
- Schneider, T. , & Michel, M. C. (2010). Can [125I]‐iodocyanopindolol label β3‐adrenoceptors in rat urinary bladder? Frontiers in Pharmacology, 1, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeder, K. J. , Snedder, S. K. , Cao, W. , della Rocca, G. J. , Daniel, K. W. , Luttrell, L. M. , & Collins, S. (1999). The β3‐adrenergic receptor activates mitogen‐activated protein kinase in adipocytes through a Gi‐dependent mechanism. The Journal of Biological Chemistry, 274(17), 12017–12022. 10.1074/jbc.274.17.12017 [DOI] [PubMed] [Google Scholar]
- Steinle, J. J. , Booz, G. W. , Meininger, C. J. , Day, J. N. E. , & Granger, H. J. (2003). β3‐Adrenergic receptors regulate retinal endothelial cell migration and proliferation. The Journal of Biological Chemistry, 278, 20681–20686. 10.1074/jbc.M300368200 [DOI] [PubMed] [Google Scholar]
- Sugasawa, T. , Lenzen, G. , Simon, S. , Hidaka, J. , Cahen, A. , Guillaume, J. L. , … Nahmias, C. (2001). The iodoyanopindolol and SM‐11044 binding protein belongs to the TM9SF multispanning membran protein superfamily. Gene, 273, 227–237. 10.1016/S0378-1119(01)00587-X [DOI] [PubMed] [Google Scholar]
- Sugasawa, T. , Matsuzaki‐Fujita, M. , Guillaume, J. L. , Camoin, L. , Morooka, S. , & Strosberg, A. D. (1997). Characterization of a novel iodocyanopindolol and SM‐11044 binding protein, which may mediate relaxation of depolarized rat colon tonus. The Journal of Biological Chemistry, 272, 21244–21252. 10.1074/jbc.272.34.21244 [DOI] [PubMed] [Google Scholar]
- Sun, J. , Fu, L. , Tang, X. , Han, Y. , Ma, D. , Cao, J. , … Ji, H. (2011). Testosterone modulation of cardiac β‐adrenergic signals in a rat model of heart failure. General and Comparative Endocrinology, 172, 518–525. 10.1016/j.ygcen.2011.04.019 [DOI] [PubMed] [Google Scholar]
- The Canadian Preterm Labor Investigators Group (1992). Treatment of preterm labor with the beta‐adrenergic agonist ritodrine. The New England Journal of Medicine, 327, 308–312. [DOI] [PubMed] [Google Scholar]
- Thomas, J. A. , & Marks, B. H. (1978). Plasma norepinephrine in congestive heart failure. The American Journal of Cardiology, 41, 233–243. 10.1016/0002-9149(78)90162-5 [DOI] [PubMed] [Google Scholar]
- Thomas, R. F. , Holt, B. D. , Schwinn, D. A. , & Liggett, S. B. (1992). Long‐term agonist exposure induces upregulation of β3‐adrenergic receptor expression via multiple cAMP response elements. Proceedings of the National Academy of Sciences of the United States of America, 89, 4490–4494. 10.1073/pnas.89.10.4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen, A. R. B. , Plouffe, B. , Cahill, T. J. , Shukla, A. K. , Tarrasch, J. T. , Dosey, A. M. , … Lefkowitz, R. J. (2016). GPCR‐G protein‐β‐arrestin super‐complex mediates sustained G protein signaling. Cell, 166, 907–919. 10.1016/j.cell.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin, A. B. , Butcher, A. J. , & Kong, K. C. (2008). Location, location, location … site‐specific GPCR phosphorylation offers a mechanism for cell‐type‐specific signalling. Trends in Pharmacological Sciences, 29, 413–420. 10.1016/j.tips.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursino, M. G. , Vasina, V. , Raschi, E. , Crema, F. , & De Ponti, F. (2009). The β3‐adrenoceptor as a therapeutic target: Current perspectives. Pharmacological Research, 59, 221–234. 10.1016/j.phrs.2009.01.002 [DOI] [PubMed] [Google Scholar]
- Vasina, V. , Abu‐Gharbieh, E. , Barbara, G. , De Giorgio, R. , Colucci, R. , Blandizzi, C. , … de Ponti, F. (2008). The β3‐adrenoceptor agonist SR58611A ameliorates experimental colitis in rats. Neurogastroenterology and Motility, 20, 1030–1041. 10.1111/j.1365-2982.2008.01138.x [DOI] [PubMed] [Google Scholar]
- Vrydag, W. , Alewijnse, A. E. , & Michel, M. C. (2009). Do gene polymorphisms alone or in combination affect the function of human β3‐adrenoceptors? British Journal of Pharmacology, 156, 127–134. 10.1111/j.1476-5381.2008.00014.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wieringen, J. P. , Booij, J. , Shalgunov, V. , Elsinga, P. , & Michel, M. C. (2013). Agonist low‐ and high‐affinity states of dopamine D 2 receptors: Methods of detection and clinical implications. Naunyn‐Schmiedeberg's Archives of Pharmacology, 386, 135–154. 10.1007/s00210-012-0817-0 [DOI] [PubMed] [Google Scholar]
- van Wieringen, J. P. , Michel‐Reher, M. B. , Hatanaka, T. , Ueshima, K. , & Michel, M. C. (2013). The new radioligand [3H]‐L 748,337 differentially labels human and rat β3‐adrenoceptors. European Journal of Pharmacology, 720, 124–130. 10.1016/j.ejphar.2013.10.039 [DOI] [PubMed] [Google Scholar]
- Watts, V. L. , Sepulveda, F. M. , Cingolani, O. H. , Ho, A. S. , Niu, X. , Kim, R. , … Barouch, L. A. (2013). Anti‐hypertrophic and anti‐oxidant effect of beta3‐adrenergic stimulation in myocytes requires differential neuronal NOS phosphorylation. Journal of Molecular and Cellular Cardiology, 62, 8–17. 10.1016/j.yjmcc.2013.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, M. , Takeda, M. , Gotoh, M. , Nagai, S. , & Kurose, T. (2018). Vibegron, a novel potent and selective b3‐adrenoreceptor agonist, for the treatment of patients with overactive bladder: A randomized, double‐blind, placebo‐controlled phase 3 study. European Urology, 73(5), 783–790. 10.1016/j.eururo.2017.12.022 [DOI] [PubMed] [Google Scholar]
- Zhang, Z. S. , Cheng, H. J. , Onishi, K. , Ohte, N. , Wannenburg, T. , & Cheng, C. P. (2005). Enhanced inhibition of L‐type Ca2+ current by β3‐adrenergic stimulation in failing rat heart. The Journal of Pharmacology and Experimental Therapeutics, 315, 1203–1211. 10.1124/jpet.105.089672 [DOI] [PubMed] [Google Scholar]
- Zhao, Q. , Zeng, F. , Liu, J.‐B. , He, Y. , Li, B. , Jiang, Z.‐F. , … Wang, L. X. (2013). Upregulation of β3‐adrenergic receptor expression in the atrium of rats with chronic heart failure. Journal of Cardiovascular Pharmacology and Therapeutics, 18, 133–137. 10.1177/1074248412460123 [DOI] [PubMed] [Google Scholar]
- Zindel, D. , Butcher, A. J. , Al‐Sabah, S. , Lanzerstorfer, P. , Weghuber, J. , Tobin, A. B. , … Krasel, C. (2015). Engineered hyperphosphorylation of the β2‐adrenoceptor prolongs arrestin‐3 binding and induces arrestin internalization. Molecular Pharmacology, 87, 349–362. 10.1124/mol.114.095422 [DOI] [PMC free article] [PubMed] [Google Scholar]


