Abstract
Clubroot, caused by Plasmodiophora Brassicae, is a serious soil-borne disease in worldwide. In recent years, progression of clubroot is rapid and serious in Shanghai, China. In this study, The inheritance of clubroot resistance (CR) were determined in pakchoi using F2 segregation population that were developed by crossing highly resistant line ‘CR38’ and susceptible line ‘CS22’. Two novel QTLs, qBrCR38-1 and qBrCR38-2, was identified by BSA-seq (Bulked Segregant Sequencing) resistant to P. brassicae physiological race 7. Two significant peak qBrCR38-1 and qBrCR38-2 were observed by three statistical methods between interval of 19.7–20.6 Mb in chromosome A07 and 20.0–20.6 Mb in chromosome A08, respectively. In addition, Polymorphic SNPs identified within target regions were converted to kompetitive allele-specific PCR (KASP) assays. In target regions of qBrCR38-1 and qBrCR38-2, there were twenty SNP sites identified, eleven KASP markers of which are significantly associated to CR (P < 0.05). Seven candidate genes were identified and found to be involved in disease resistance (TIR-NBS-LRR proteins), defense responses of bacterium and fungi and biotic/abiotic stress response in the target regions harboring the two QTLs. Two novel QTLs and candidate genes identified from the present study provide insights into the genetic mechanism of CR in B.rapa, and the associated SNPs can be effectively used for marker-assisted breeding.
Subject terms: Genetic markers, DNA sequencing, Genome duplication
Introduction
Clubroot, caused by the obligate endoparasite Plasmodiophora Brassicae, is recognized as a serious soil-borne disease. It infects cruciferous Brassica oil crops and vegetables and is specially associated with considerable yield losses1–3. The pathogen causes abnormal growth of the plant and eventually leads to a massive gall formation on the root4. Clubroot prevents the transportation of water and nutrients, causing the plant to wilt and finally dies off. In recent years, the incidence of clubroot in the suburbs of Shanghai, China, has gradually become increasingly more serious. Until 2017, 39 counties and 9 towns of Shanghai had a breakout of clubroot disease and the affected area had reached 2500 hm2. Pathotypes of P. brassicae was physiological race 7 in Qingpu distract of Shanghai. Clubroot disease is difficult to completely prevent and control by cultural practices or chemical treatments such as anti-microbial compounds or crop rotations5,6. Therefore, development of resistant cultivars is the most effective approach to control clubroot disease.
In Brassica Rapa, genetic analysis and quantitative trait locus (QTL) mapping studies have identified at least eight race-specific clubroot resistance(CR) loci. Crr1, Crr2, and Crr4 CR genes from European turnip cv ‘Siloga’, which were mapped onto chromosomes A08, A01, and A06, respectively7,8. The resistance genes CRa from turnip line ‘ECD02’9, CRb from turnip line ‘Gelria R’10,11 and Crr3 from turnip cv. “Milan White”12,13 were both located on the chromosome A03. CRk and CRc were identified from turnip cv. “Debra” line ECD01 and ECD02, mapped onto A03 and A02, respectively14,15. In B. rapa canola, Rcr4, Rcr8, and Rcr9 breeding line T19 were mapped to chromosomes A03, A02, and A08, respectively through genotyping by sequencing16. The resistance gene Rcr1 from pak choy cv. “Flower Nabana” was also mapped to A03 by transcriptome analysis, and two candidate genes Bra019409 and Bra019410 were screened for correlation with clubroot-resistance17. Meanwhile, clubroot-resistant gene Rcr2 was also mapped on chromosome 3 in chinese cabbage ‘Jazz’. Five SNP markers co-segregated with Rcr2 were developed between 22 to 26 Mb, and Bra019410 and Bra019413 are most likely candidates of Rcr2, with conserved domain of TIR-NBS-LRR resistance protein18.
Bulked segregant analysis by sequencing (BSA-seq), is an effective technique used to identify quantitative trait loci (QTLs)19,20. It is a genomics tool used for genetic mapping which takes advantage of bulked-segregant analysis and high-throughput genotyping using next-generation sequencing (NGS)21,22. BSA-Seq has been applied to mapping agronomically important loci in Arabidopsis, rice and wheat23–25. Candidate genes for disease resistance have been identified successfully by this approach, such as a broad-spectrum resistance gene Pi65 (t) in rice26, downy mildew and powdery mildew resistance QTLs in cucumber27,28. BSA-seq technologies, have proven successful for rapidly establishing the association of agronomic traits with molecular markers and had a major impact on crop breeding29. Meanwile, different statistical methods for poolsd QTL mapping have been proposed, including MutMap23, G’ value30 and ED31, that would have noise reduction and highlighting effect of QTLs. Moreover, it requires an efficient platform for applying molecular markers to marker-assisted selection (MAS) in breeding. Kompetitive Allele Specific PCR (KASP) is one of the high-throughput SNP genotyping technologies is a cost-effective, low genotyping error rates and flexible system which is widely used for genetic mapping, trait-specific markers development, germplasm characterization (genetic diversity, genetic relationship, and population structure), and quality control (QC) analysis (genetic identity, genetic purity, and parentage verification)32–34.
The Pakchoi (Brassica campestris ssp. chinensis Makino), also called non-heading Chinese cabbage, is one of the most important Brassica vegetable crop in China and East Asia35. Most Pakchoi cultivars are highly susceptible to the P. brassicae. In this study, BSA-seq was applied to determine the inheritance of clubroot resistance (CR) in Pakchoi inbred line ‘CR38’ and F2 population. Then, the underlying QTLs were mapped by three statistical methods and examined sequence variation in the target region to identify the most probable candidate gene associated with CR.
Results
Clubroot Resistance evaluations in CR38 × CS22
‘CR38’ were highly resistant (DSI = 3.33 in 2016, DSI = 4.76 in 2017) to the 7 physiology race of P. brassicae in contrast to the ‘CS22’ which was more susceptible (DSI = 100 in 2016, DSI = 94.44 in 2017). ‘CR38’ and ‘CS22’ (Fig. 1a), were crossed to develop segregating populations for QTL analysis of CR. Clubroot symptoms of 294 F2 individuals were identified in autumn 2017. F2 population exhibited a continuous frequency distribution with a range of 0–3 and DSI of 34.24, suggesting polygenic control of CR in this population (Table 1).
Figure 1.

phenotype of the parents: CR38 (Clubroot resistance) and CS22 (Clubroot susceptible). Plants were inoculated with 7th physiology race of P. brassicae.
Table 1.
Disease evaluations of CR in CR38 × CS22.
| Parents | Type | Year | Disease rating | DSI | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Total | ||||
| CR38 | R | 2016 | 27 | 3 | 0 | 0 | 30 | 3.33 |
| 2017 | 18 | 2 | 0 | 0 | 20 | 4.76 | ||
| CS22 | S | 2016 | 0 | 0 | 0 | 15 | 15 | 100.00 |
| 2017 | 0 | 0 | 4 | 18 | 22 | 94.44 | ||
| CR38 × CS22 | F2 | 2017 | 81 | 145 | 43 | 23 | 292 | 34.24 |
.
Whole genome sequencing analysis
BSA-seq were used to identify the loci controlling CR in our F2 population, from which 22 highly resistant and 22 highly susceptible individuals were selected and two DNA pool were created. Illumina high-throughput sequencing generated 51,292,360 and 59,493,747 short reads (150 bp in length) from the CR-pool and the CS-pool, with a coverage of 98.11% (39-fold genome coverage) and 98.46% (45-fold genome coverage), respectively. Over 97% of the reads in both pools were mapped to the Chiifu-401 reference genome. The Q20% was 96.12% and 95.91% of the CR -pool and CS-pool. The effective sequencing depths for CR38 and CS22 were 20-fold and 25-fold genome coverage, respectively, which guaranteed accuracy of the subsequent analysis. The results from sequencing are presented in Table 2.
Table 2.
Summary of BSA sequencing data for each sample.
| Sample Name | Clean Reads | Clean Base(G) | Clean Q20 (%) | Clean GC Content (%) | coverage rate (%) | Map reads rate (%) | Effective Rate (%) | Sequencing depth |
|---|---|---|---|---|---|---|---|---|
| CR-pool | 51,292,360 | 15.39 | 96.12 | 39.62 | 98.11% | 97.03% | 99.35 | 39× |
| CS-pool | 59,493,747 | 17.85 | 95.91 | 39.16 | 98.46% | 97.07% | 99.56 | 45× |
| CR38 | 27,262,388 | 8.18 | 97.75 | 39.91 | 91.89% | 97.11% | 98.99 | 20× |
| CS22 | 32,499,790 | 9.75 | 96.08 | 39.8 | 94.09% | 97.08% | 99.26 | 25× |
Mapping of Clubroot resistance
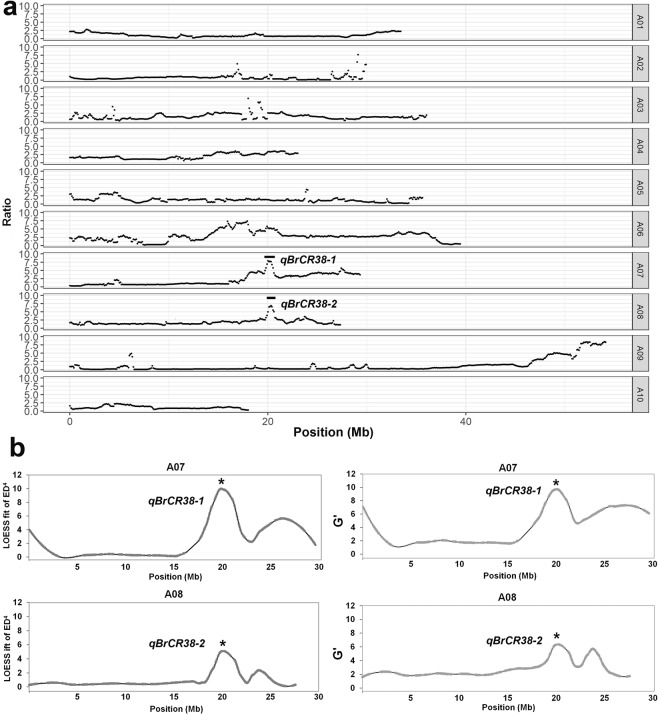
Sequence data were trimmed and filtered prior to analysis. Compared to the reference genome, 1,489,940 SNPs were identified between the CR38 and CS22 parents. Based on the uniquely mapped reads, association analysis between the two bulks was performed on 1,079,828 SNPs. To identify genomic regions associated with CR, three methods were used to mapping the QTLs. Firstly, Scanning each SNP in the whole genome, the Ratio of SNP-ratioin CR-pool and CS-pool was calculated36 (Fig. 2A). Two significant peak qBrCR38-1 and qBrCR38-2 was observed between 19.7–20.6 Mb of chromosome A07 and 20.0–20.6 Mb of chromosome A08 (Fig. 2a). Next, Allelic segregation between CR and CS pools by Euclidean Distance (ED)31 were measured. ED4 was raising ED to the fourth power to decrease noise. Local polynomial regression methods (LOESS fit) of ED4 calculated shown in Fig. 2b. The identified peaks that have an ED above a threshold and a high allele frequency in the CR-pool. G value at each SNP were calculated, and G is a smooth version of G30. The statistics result demonstrated that there were two QTLs at the same peaks position about 20.1 Mb in chromosome A07 and 20.2 Mb in chromosome A08 (Fig. 2b). We found 5441 and 1887 SNPs between the two parents in the target regions of chromosome A07 and chromosome A08.
Figure 2.
Genome-wide scan for clubroot resistance QTLs using BSA-seq. Two significant QTL of qBrCR38-1 and qBrCR38-2 detected in chromosomes A07 and A08 using three statistical approach. (a) Ratio (CR-pool/CS-pool) of the SNP-ratios (Resistant alleles/sensitive alleles) is presented. The significance peak is indicated by the horizontal dotted line. (b) Distribution of G’value and Loess fit curve calculated using ED4 data on chromosome A07 and A08
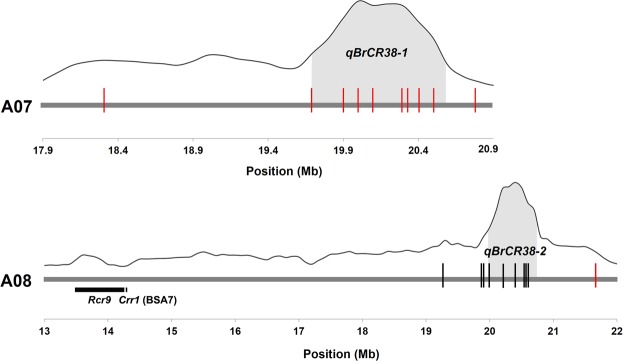
Corresponding sequences in flanking 50 bp region were used to develop allele-specific KASP primers which were genotype of the parental lines and F2 population. There were twenty SNP sites identified at physical positon 18.4 Mb – 20.8 Mb and 19.4 Mb – 21.8 Mb around regions of qBrCR38-1 and qBrCR38-2 (Table 3). qBrCR38-1 was mapped in the interval of 0.35 cM flanked by Br_K_07106 and Br_K_07107. Br_K_080103 and Br_K_080107 are linkage to qBrCR38-2 within the genetic distance of 0.33 cM. To evaluate the effect of qBrCR38-1 and qBrCR38-2, F2 population were classified into two groups (AA and aa) based on the genotype of the most tightly linked markers. Lines carrying the CR38 alleles (AA) are significantly (P < 0.05) clubroot resistant than those carrying the CS22 alleles (aa) in eleven SNP markers (Table 3). Close-up view of QTLs and KASP markers link to the target regions was shown in Fig. 3.
Table 3.
KASP marker in target region.
| Marker Name | QTL | Chr | Target region (Mb) | Position (bp) | P value |
|---|---|---|---|---|---|
| Br_K_070101 | qBrCR38-1 | A07 | 19.7–20.6 | 18391107 | 0.027* |
| Br_K_070113 | qBrCR38-1 | A07 | 19.7–20.6 | 19759488 | 0.026* |
| Br_K_070105 | qBrCR38-1 | A07 | 19.7–20.6 | 19995501 | 0.008** |
| Br_K_070106 | qBrCR38-1 | A07 | 19.7–20.6 | 20107291 | 0.003** |
| Br_K_070107 | qBrCR38-1 | A07 | 19.7–20.6 | 20209491 | 0.002** |
| Br_K_070115 | qBrCR38-1 | A07 | 19.7–20.6 | 20333647 | 0.002** |
| Br_K_070109 | qBrCR38-1 | A07 | 19.7–20.6 | 20389706 | 0.002** |
| Br_K_070110 | qBrCR38-1 | A07 | 19.7–20.6 | 20447844 | 0.003** |
| Br_K_070116 | qBrCR38-1 | A07 | 19.7–20.6 | 20501616 | 0.009** |
| Br_K_070103 | qBrCR38-1 | A07 | 19.7–20.6 | 20813113 | 0.008** |
| Br_K_080101 | qBrCR38-2 | A08 | 20.0–20.6 | 19389881 | 0.345 |
| Br_K_080112 | qBrCR38-2 | A08 | 20.0–20.6 | 19918702 | 0.240 |
| Br_K_080115 | qBrCR38-2 | A08 | 20.0–20.6 | 19936667 | 0.328 |
| Br_K_080118 | qBrCR38-2 | A08 | 20.0–20.6 | 20067617 | 0.356 |
| Br_K_080107 | qBrCR38-2 | A08 | 20.0–20.6 | 20254038 | 0.484 |
| Br_K_080109 | qBrCR38-2 | A08 | 20.0–20.6 | 20444233 | 0.531 |
| Br_K_080111 | qBrCR38-2 | A08 | 20.0–20.6 | 20608800 | 0.652 |
| Br_K_080120 | qBrCR38-2 | A08 | 20.0–20.6 | 20628063 | 0.536 |
| Br_K_080121 | qBrCR38-2 | A08 | 20.0–20.6 | 20638194 | 0.652 |
| Br_K_080103 | qBrCR38-2 | A08 | 20.0–20.6 | 21748451 | 0.048* |
*P < 0.05, **P < 0.01.
Figure 3.
Close-up view of qBrCR38-1 and qBrCR38-2 and KASP markers link to the target regions of CR. Vertical lines represent the position of KASP markers, where red lines were significant (P < 0.05) associated with CR. Gray areas is the target regions.
Candidate gene identification
A total of 188 and 116 annotated genes encoded sequences encompassed the target regions of qBrCR38-1 and qBrCR38-2, respectively. The functional annotation of genes in the target region indicates their potential involvement in fungal disease resistance assessed for further analysis. In chromosome A07, candidate gene BraA07002412, homologous to AT1G80460, encodes protein NHO1 (nonhost resistant gene 1) similar to glycerol kinase and performs a rate-limiting step in glycerol metabolism. In addition, candidate gene BraA08002455 in chromosome A08, homologous to AT1G65850, encodes Toll-Interleukin-1 receptor/nucleotide binding site/leucine-rich repeat (TIR-NBS-LRR class) disease resistance protein. Moreover, six genes with F-box domain (BraA07002249, and BraA07002494 in A07, BraA08002451 and BraA08002452in A08) and a WRKY transcription factor (BraA08002471) were considered as the functional genes associated with CR (Table 4).
Table 4.
Candidate gene annotation.
| Gene ID | Chr | Position | A. thaliana homologs | Annotations |
|---|---|---|---|---|
| BraA07002249 | A07 | 19177733–19181091 | AT4G02310 | F-box family protein |
| BraA07002412 | A07 | 19975263–19977367 | AT1G80460 | NHO1 (nonhost resistance gene.) |
| BraA07002494 | A07 | 20387426–20389115 | AT1G78750 | F-box family protein |
| BraA08002451 | A08 | 19715762–19716810 | AT4G39756 | F-box family protein |
| BraA08002452 | A08 | 19719445–19720378 | AT4G39753 | F-box family protein |
| BraA08002455 | A08 | 19759513–19760286 | AT1G65850 | disease resistance protein (TIR-NBS-LRR class) |
| BraA08002471 | A08 | 19872938–19875271 | AT4G39410 | WRKY transcription factor |
Discussion
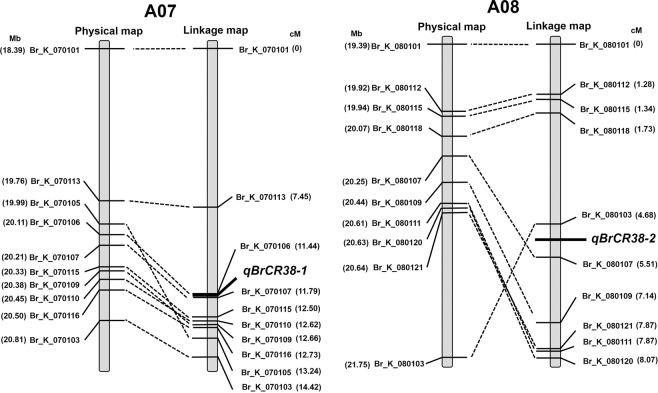
The traditional fine mapping method is time-consuming and is rather laborious to require the identification of genome-wide polymorphic DNA markers for linkage analysis. Furthermore, it needs to use the genetic mapping and physical mapping to locate the target gene in the specific location of chromosomes. With the rapid development of sequencing technology and genomics, using BSA-seq of extreme mixed pools, a large number of traits can be located quickly and effectively. Compared to traditional QTL mapping, BAS-seq has the advantages of simple operation and short test period, and does not require specific near-isogenic lines to be constructed. Through high-throughput sequencing, thousands of SNPs can be captured at one time, and this variation information can be used to identify trait-related genes or QTLs as well as to develop molecular markers. The accuracy of BSA-seq results is very important to identify the resistance loci of CR, and there are many influencing factors. With improvement of reference genome in Chinese cabbage using NGS and Pacbio SMRT sequencing, it is possible to map genes for different traits in Chinese cabbage crops effectively and get more complete gene sequences. In order to highlight the significance of the positioning interval for better noise reduction, Three appropriate statistical approach were used to observed same two significant peak of qBrCR38-1 and qBrCR38-2. SNP-ratio and ED4 increasing CR sensiticity near the high allele frequency locus in the CR-pool. G’ is expected to decrease much more rapidly around the causal site, implying narrower intervals of support around QTLs30. BSA-Seq combination with KASP analysis is a powerful approach for fine mapping of causal genes. KASP is a cost-effective single-step genotyping technology, cheaper than SSRs and more flexible than genotyping by sequencing (GBS) or array-based genotyping. In our research, KASP markers linkaged CR were obtained in the target region of QTLs. Alignments of the physical map corresponds to the genetic map around target regions were showed good collinearity (Fig. 4). The inconsistencies such as Br_K_080103 might be caused by chromosomal rearrangement in Pakchoi. Eleven SNP sites were significantly associated to CR, but nine SNP sites have week effective to CR in chromosome A08. Thus it is speculated that qBrCR38-1 is a major effective locus. In general, our research provide an important way to molecular marker assisted selection for CR on backcross breeding and gene pyramiding in B.rapa.
Figure 4.
The physical map corresponds to the genetic map around region of qBrCR38-1 and qBrCR38-2. Broken lines drawn regions defined by KASP markers on chromosome A07 and A08. Physical locations are on the left, and the genetic distance is shown on the right.
Several recent studies reported that there were at least eight pathotypes of P. brassica pathogen. Pathotypes 2, 3, 4, 5, 6, 7, 10, 11 represent the main races of P. brassicae pathogen in China as classified by Williams identification system37,38. The clubtoot-resistant genes Crr1, Crr2 and CRb have resistance to physiological race 47,8. Rcr1-Rcr9 have high resistance to pathotypes 2, 3, 5, 6 and 8 in Canada17,18,39. In the present study, the resistant parent “CR38” has high resistance to P. brassicae of physiological race 7 which is widespread in Shanghai, China. There were no clubroot resistant genes, even no resistant cultivars. Therefore, the development of cultivars and the mapping genes resistant to physiological race 7 in Pakchoi will provide a basis for disease resistance breeding of Chinese cabbage.
Previous researches have applied several strategies to identify and map genes and QTL involved in CR in B.rapa. Crr1 and Rcr916 were both located in chromosomes A08, while no genes or QTLs were found in chromosomes A07. In the present study, clubroot resistance in ‘CR38’ was identified as quantitative inheritance and was regulated by multiple genes. In addition, qBrCR38-1 and qBrCR38-2 in chromosomes A07 and A08 associated with CR in Pakchoi were detected by BSA-seq. We blasted the sequence from linkage marker (BSA7) of Crr1 and Rcr9 target region to B.rapa reference genome V2.5. Crr1 and Rcr9 were located in 14.27 Mb and 13.5–14.2 Mb respectively, thus showing that these two gene were not in the same chromosomal position as qBrCR38-2 (Fig. 3).
Annotation information and homologous sequence alignment of candidate genes revealed new disease-resistant genes involved in CR and further studies are required. NOH1 was reported to be related to both general and specific resistance against bacterium and fungi40. TIR-NBS-LRR resistant protein plays an important role in regulating the activity of plant disease resistance proteins41. Recent studies suggested that F-box proteins may up-regulate defense-related gene expression in rice42, and play a role in cell death and defense responses activated during pathogen recognition in tobacco and tomato43. WRKY transcription factors family is known to be involved in biotic/abiotic stress response44. Overall, Genomic intervals of QTL loci still need to be narrowed down to determine candidate genes. Candidate genes identified from the present study provide insights into the genetic mechanism of clubroot resistant in B.rapa.
In conclusion, BSA-seq Combine with KASP analysis was carried out to conduct the mapping of CR in Pakchoi. As a result, two novel QTLs regions on chromosome A07 and A08 respectively were detected, and seven genes in target chromosome region associated with the disease resistance were considered as candidates for CR. These genes need to be further studied, and the associated SNPs should be used for marker-assisted breeding of CR in B.rapa
Materials and Methods
Plant materials and phenotypic data collection
Two Pakchoi inbred line, CR38 (Clubroot resistance) and CS22 (Clubroot susceptible), were used as parents to generate 292 F2 individuals for studies on inheritance and genetic mapping. CR38 is cold tolerant type and highly resistant to the 7th physiology race of Plasmodiophora Brassicae by using the inoculation method of Williams (1966)45. The pathogen was propagated on Pakchoi, and the clubs in infected roots were stored at −20 °C until required. All CR test plants were sown in a pot containing 5 × 106 spores per gram of dry soil, and cultivation was carried out in a growth chamber at 25/20 °C (day/night) with a photo-period of 14 h. At 6 weeks after sowing, the root symptoms of each plant were evaluated. A disease severity index (DSI) of two parents was calculated as according to Suwabe (2003)8. Each test included control-resistant and susceptible cultivars. All plant materials examined in this study were obtained from Shanghai academy of agricultural science.
Library construction and Whole genome sequencing
Total genomic DNA from the two parental lines, CR38 and CS22, was isolated from young leaves according to the CTAB method46. Equal amounts of genomic DNA were sampled from individuals with leaves (22 DNA samples for each extreme trait) and bulked to generate the Clubroot Resistance pool (CR-pool) and Clubroot Sensitive pool (CS-pool). Pair-end sequencing libraries with a read length of 150 bp and insert sizes of approximately 350 bp were subjected to whole-genome re-sequencing with Illumina HiSeq 2500. Short reads obtained from both parents and two DNA-bulks were aligned against the B. rapa Chiifu-401 reference genome sequence v2.5 (http://brassicadb.org/brad/) to obtain the consensus sequence using BWA software47. Reads of CR-pool and CS-pool were separately aligned to CR38 and CS22 consensus sequence reads to call SNPs with GATK tools software48. Heterozygote alleles in two parents were filtered out during the process. Raw read data are archived at the NCBI Sequence Read Archive (SRA) under Accession PRJNA497673.
QTL mapping and target region annotation
Assuming that “A” is a SNP identified between CR38 and CS22, the CR38 and CS22 genotypes at this site are “AA” and “aa”, respectively. For the CR-pool, nRA and nRa are the numbers of reads containing “A” and “a”, Respectively. Three methods were used to mapping QTLs. Firstly, SNP-ratio (Resistant alleles/sensitive alleles) of CR-pool and CS-pool were calculated. Then, CR-pool SNP-ratio was divided by CS-pool SNP-ratio and plotted across the genomic regions that showed ratio peaks, which indicate the possible existence of a QTL36
Read depth for each allele at segregating allelic SNPs in 500 kb sliding windows was summed using a 100 kb step increment.
The ED for each SNP was calculated according to a previous report31. ED is the sum of 100 ED-SNP values within a window of 100 consecutive SNPs. ED4 was then calculated by raising ED to the fourth power. Loess fit curves for SNP allele frequency ED4. G’, the G value averaged across neighboring SNPs, was calculated as described by Magwene et al. (2011)30. There methods for mapping common target regions were considered as significant loci.
A list of genes within the target regions was generated using the B.rapa genome annotation data (http://brassicadb.org/brad/), and these genes were used to query the GO annotation on the website of gene ontology to identify putative resistance related genes for further analysis.
KASP Marker development and genotyping in target region
Polymorphic SNPs identified within the target regions for clubroot resistance were converted into KASP markers (Supplementary Table S1). Each SNP site were parsed to retrieve the flanking sequences 50 bp for KASP markers in the target region. The criteria for selection were that the flanking sequences (a) did not contain any other SNP and InDels, (b) had no more than four consecutive repeats, (c) was close to the ratio peaks on the physical location and (d) Primer sequences were relatively unique in the genome.
For each SNP, two allele-specific forward primers and one common reverse primer were designed using the Primer3 software. Target region SNPs were converted into KASP primers and were used to test the entire F2 population. Reactions were performed in 384 well plates, with a final reaction volume of 3 μl, which contained 1.48 μl of KASP 2X reaction mix, 50 ng of template DNA, 0.17 μM Hex forward primer, 0.17 μM FAM forward primer and 0.42 μM universal reverse primer. The following cycling conditions were used: 15 min at 94 °C followed by 10 touchdown cycles of 20 s at 95 °C and 60 s at 65 °C (dropping 0.8 °C per cycle); after the final annealing temperature of 56 °C was achieved, there were 26 cycles of 20 s at 94 °C and 60 s at 57 °C. Thermocycling and fluorescence readings were performed on Hydrocycler and PHERAstar of LGC SNPline platform. Genotyping data viewed as a cluster plot by SNPviewer software supported from LGC Genomics (http://www.lgcgenomics.com). The significance of the correlation coefficients between genotype and phenotype was determined with t-tests. Linkage groups were performed with JoinMap 4.1 (Van Ooijenand Voorrips, 2001).
Supplementary information
Acknowledgements
This work was supported by the Shanghai Municipal Agriculture Commission 7-8-1 (2014) and National Key R&D Program of China 2017YFD0101803. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We also thank Huazhi Rice Bio-tech co., LTD for providing the LGC SNPline platform.
Author Contributions
H.Z. and W.Z. conceived and conducted the major part of the research including preparation of the samples, conducted bio-informatics analysis and wrote the manuscript. X.L. carried out part of the experiments. Y.Z. designed and guided the research and was involved in manuscript preparation. All authors read and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hongfang Zhu and Wen Zhai contributed equally.
Contributor Information
Wen Zhai, Email: zhaiwen1105@163.com.
Yuying Zhu, Email: yy5@saas.sh.cn.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-44724-z.
References
- 1.Wallenhammar AC. Observations On Yield Loss From Plasmodiophora Brassicae Infections in Spring Oilseed Rape. Journal of Plant Diseases & Protection. 1998;105:1–7. [Google Scholar]
- 2.Dixon GR. The Occurrence and Economic Impact of Plasmodiophora Brassicae and Clubroot Disease. J Plant Growth Regul. 2009;28:194–202. doi: 10.1007/s00344-009-9090-y. [DOI] [Google Scholar]
- 3.Strelkov SE, Hwang S. Clubroot in the Canadian Canola Crop: 10 Years Into the Outbreak. Can J Plant Pathol. 2014;36:27–36. doi: 10.1080/07060661.2013.863807. [DOI] [Google Scholar]
- 4.Ingram DS, Tommerup IC. The Life History of Plasmodiophora Brassicae Woron. Roy Soc London Proc Ser B Biol Sci. 1972;180:103–112. doi: 10.1098/rspb.1972.0008. [DOI] [Google Scholar]
- 5.Tsushima S. Perspective of Integrated Pest Management: A Case Study: Clubroot Disease of Crucifers(Pesticide Science in the 21St Century) J PESTIC SCI. 2000;25:296–299. doi: 10.1584/jpestics.25.296. [DOI] [Google Scholar]
- 6.Voorrips RE. Plasmodiophora Brassicae: Aspects of Pathogenesis and Resistance in Brassica Oleracea. Euphytica. 1995;83:139–146. doi: 10.1007/BF01678041. [DOI] [Google Scholar]
- 7.Suwabe K, et al. Simple Sequence Repeat-Based Comparative Genomics Between Brassica Rapa and Arabidopsis Thaliana: The Genetic Origin of Clubroot Resistance. Genetics. 2006;173:309–319. doi: 10.1534/genetics.104.038968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suwabe K, et al. Identification of Two Loci for Resistance to Clubroot (Plasmodiophora Brassicae Woronin) in Brassica Rapa L. Theoretical & Applied Genetics. 2003;107:997–1002. doi: 10.1007/s00122-003-1309-x. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto E, Yasui C, Ohi M, Tsukada M. Linkage Analysis of Rflp Markers for Clubroot Resistance and Pigmentation in Chinese Cabbage (Brassica Rapa Ssp. Pekinensis) Euphytica. 1998;104:79. doi: 10.1023/A:1018370418201. [DOI] [Google Scholar]
- 10.Piao ZY, Deng YQ, Choi SR, Park YJ, Lim YP. Scar and Caps Mapping of Crb, a Gene Conferring Resistance to Plasmodiophora Brassicae in Chinese Cabbage (Brassica Rapa Ssp. Pekinensis) Tag.theoretical & Applied Genetics.theoretische Und Angewandte Genetik. 2004;108:1458–1465. doi: 10.1007/s00122-003-1577-5. [DOI] [PubMed] [Google Scholar]
- 11.Kato T, Hatakeyama K, Fukino N, Matsumoto S. Fine Mapping of the Clubroot Resistance Gene Crb and Development of a Useful Selectable Marker in Brassica Rapa. Breed Sci. 2013;63:116–124. doi: 10.1270/jsbbs.63.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito M, et al. Fine Mapping of the Clubroot Resistance Gene, Crr3, in Brassica Rapa. Tag.theoretical & Applied Genetics.theoretische Und Angewandte Genetik. 2006;114:81–91. doi: 10.1007/s00122-006-0412-1. [DOI] [PubMed] [Google Scholar]
- 13.Hirai M, et al. A Novel Locus for Clubroot Resistance in Brassica Rapa and its Linkage Markers. Theoretical & Applied Genetics. 2004;108:639–643. doi: 10.1007/s00122-003-1475-x. [DOI] [PubMed] [Google Scholar]
- 14.Sakamoto K, Saito A, Hayashida N, Taguchi G, Matsumoto E. Mapping of Isolate-Specific Qtls for Clubroot Resistance in Chinese Cabbage (Brassica Rapa L. Ssp. Pekinensis) Theor Appl Genet. 2008;117:759–767. doi: 10.1007/s00122-008-0817-0. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto E, Ueno H, Aruga D. Accumulation of Three Clubroot Resistance Genes through Marker-Assisted Selection in Chinese Cabbage (Brassica Rapa Ssp. Pekinensis) Journal of the Japanese Society for Horticultural Science. 2012;81:184–190. doi: 10.2503/jjshs1.81.184. [DOI] [Google Scholar]
- 16.Yu, F. et al. Genotyping-by-Sequencing Reveals Three Qtl for Clubroot Resistance to Six Pathotypes of Plasmodiophora Brassicae in Brassica Rapa. Sci Rep. 7 (2017). [DOI] [PMC free article] [PubMed]
- 17.Yu F, et al. Identification of Genome-Wide Variants and Discovery of Variants Associated with Brassica Rapa Clubroot Resistance Gene Rcr1 through Bulked Segregant Rna Sequencing. Plos One. 2016;11:e153218. doi: 10.1371/journal.pone.0153218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Z, et al. Fine Mapping of a Clubroot Resistance Gene in Chinese Cabbage Using Snp Markers Identified From Bulked Segregant Rna Sequencing. Front Plant Sci. 2017;8:1448. doi: 10.3389/fpls.2017.01448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michelmore RW, Paran I, Kesseli RV. Identification of Markers Linked to Disease-Resistance Genes by Bulked Segregant Analysis: A Rapid Method to Detect Markers in Specific Genomic Regions by Using Segregating Populations. P Natl Acad Sci USA. 1991;88:9828–9832. doi: 10.1073/pnas.88.21.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe S, et al. A Map-Based Cloning Strategy Employing a Residual Heterozygous Line Reveals that the Gigantea Gene is Involved in Soybean Maturity and Flowering. Genetics. 2011;188:395–407. doi: 10.1534/genetics.110.125062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolger ME, et al. Plant Genome Sequencing — Applications for Crop Improvement. Plant Biotechnol J. 2014;26:31–37. doi: 10.1016/j.copbio.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Takagi H, et al. Qtl-Seq: Rapid Mapping of Quantitative Trait Loci in Rice by Whole Genome Resequencing of Dna From Two Bulked Populations. The Plant Journal. 2013;74:174–183. doi: 10.1111/tpj.12105. [DOI] [PubMed] [Google Scholar]
- 23.Abe A, et al. Genome Sequencing Reveals Agronomically Important Loci in Rice Using Mutmap. Nat Biotechnol. 2012;30:174–178. doi: 10.1038/nbt.2095. [DOI] [PubMed] [Google Scholar]
- 24.Trick M, et al. Combining Snp Discovery From Next-Generation Sequencing Data with Bulked Segregant Analysis (Bsa) to Fine-Map Genes in Polyploid Wheat. Bmc Plant Biol. 2012;12:14. doi: 10.1186/1471-2229-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin RS, et al. Next-Generation Mapping of Arabidopsis Genes. Plant Journal for Cell & Molecular Biology. 2011;67:715. doi: 10.1111/j.1365-313X.2011.04619.x. [DOI] [PubMed] [Google Scholar]
- 26.Zheng W, et al. Genetic Mapping and Molecular Marker Development for Pi65(T), a Novel Broad-Spectrum Resistance Gene to Rice Blast Using Next-Generation Sequencing. Theoretical & Applied Genetics. 2016;129:1035–1044. doi: 10.1007/s00122-016-2681-7. [DOI] [PubMed] [Google Scholar]
- 27.KT W, J V, C Z, K S, S L. Qtl Mapping for Downy Mildew Resistance in Cucumber Via Bulked Segregant Analysis Using Next-Generation Sequencing and Conventional Methods. Theor Appl Genet. 2017;130:199–211. doi: 10.1007/s00122-016-2806-z. [DOI] [PubMed] [Google Scholar]
- 28.Zhang P, Zhu Y, Wang L, Chen L, Zhou S. Mining Candidate Genes Associated with Powdery Mildew Resistance in Cucumber Via Super-Bsa by Specific Length Amplified Fragment (Slaf) Sequencing. Bmc Genomics. 2015;16:1058. doi: 10.1186/s12864-015-2041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song, J., Li, Z., Liu, Z., Guo, Y. & Qiu, L. J. Next-Generation Sequencing From Bulked-Segregant Analysis Accelerates the Simultaneous Identification of Two Qualitative Genes in Soybean. Front Plant Sci. 8 (2017). [DOI] [PMC free article] [PubMed]
- 30.Magwene PM, Willis JH, Kelly JK. The Statistics of Bulk Segregant Analysis Using Next Generation Sequencing. Plos Comput Biol. 2011;7:e1002255. doi: 10.1371/journal.pcbi.1002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill JT, et al. Mmappr: Mutation Mapping Analysis Pipeline for Pooled Rna-Seq. Genome Res. 2013;23:687–697. doi: 10.1101/gr.146936.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semagn K, Babu R, Hearne S, Olsen M. Single Nucleotide Polymorphism Genotyping Using Kompetitive Allele Specific Pcr (Kasp): Overview of the Technology and its Application in Crop Improvement. Mol Breeding. 2014;33:1–14. doi: 10.1007/s11032-013-9917-x. [DOI] [Google Scholar]
- 33.B T Ertiro, V. O. M. W. & Semagn, M. L. A. K. Comparison of Kompetitive Allele Specific Pcr (Kasp) and Genotyping by Sequencing (Gbs) for Quality Control Analysis in Maize. Bmc Genomics (2015). [DOI] [PMC free article] [PubMed]
- 34.Bourras S, et al. Multiple Avirulence Loci and Allele-Specific Effector Recognition Control the Pm3 Race-Specific Resistance of Wheat to Powdery Mildew. Plant Cell. 2015;27:2991. doi: 10.1105/tpc.15.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xi-lin, H. Advances in Breeding of Non-Heading Chinese Cabbage. Journal of Nanjing Agricultural University (2003).
- 36.Soyk S, et al. Bypassing Negative Epistasis On Yield in Tomato Imposed by a Domestication Gene. CELL. 2017;169:1142–1155. doi: 10.1016/j.cell.2017.04.032. [DOI] [PubMed] [Google Scholar]
- 37.Shen, X. Q., Nie, K., Wu, Q., Zhang, Y. G. & Meng, X. H. Initial Research Report On Differentiation Identification of Chinese Cabbage Clubroot Main Physiological Races. China Vegetables (2009).
- 38.Zhang H, et al. Resistance to Plasmodiophora Brassicae in Brassica Rapa and Brassica Juncea Genotypes From China. Plant Dis. 2015;99:8–14. doi: 10.1094/PDIS-08-14-0863-RE. [DOI] [PubMed] [Google Scholar]
- 39.Feng J, Jiang J, Feindel D, Strelkov SE, Hwang SF. The Gene Cr811 is Present Exclusively in Pathotype 5 and New Emerged Pathotypes of the Clubroot Pathogen Plasmodiophora Brassicae. Eur J Plant Pathol. 2016;145:615–620. doi: 10.1007/s10658-016-0903-0. [DOI] [Google Scholar]
- 40.Kang L, et al. Interplay of the Arabidopsis Nonhost Resistance Gene Nho1 with Bacterial Virulence. P Natl Acad Sci USA. 2003;100:3519–3524. doi: 10.1073/pnas.0637377100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van OG, et al. Structure-Function Analysis of the Nb-Arc Domain of Plant Disease Resistance Proteins. J Exp Bot. 2008;59:1383. doi: 10.1093/jxb/ern045. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y, Zhang LY, Zhu K, Liu YL. Overexpression of a Rice Defense‐Related F‐Box Protein Gene Osdrf1 in Tobacco Improves Disease Resistance through Potentiation of Defense Gene Expression&Dagger. Physiol Plantarum. 2008;134:440. doi: 10.1111/j.1399-3054.2008.01149.x. [DOI] [PubMed] [Google Scholar]
- 43.Ha VDB, et al. The F-Box Protein Acre189/Acif1 Regulates Cell Death and Defense Responses Activated During Pathogen Recognition in Tobacco and Tomato. Plant Cell. 2008;20:697–719. doi: 10.1105/tpc.107.056978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phukan UJ, Jeena GS, Shukla RK. Wrky Transcription Factors: Molecular Regulation and Stress Responses in Plants. Front Plant Sci. 2016;7:760. doi: 10.3389/fpls.2016.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams PH. A System for the Determination of Races of Plasmodiophora Brassicae that Infect Cabbage and Rutabaga. Phytopathology. 1966;56:624–626. [Google Scholar]
- 46.Doyle JJ. A Rapid Dna Isolation Procedure for Small Amounts of Fresh Leaf Tissue. Phytochem Bull. 1987;19:11–15. [Google Scholar]
- 47.Li H, Durbin R. Fast and Accurate Short Read Alignment with Burrows-Wheeler Transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McKenna A, et al. The Genome Analysis Toolkit: A Mapreduce Framework for Analyzing Next-Generation Dna Sequencing Data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.