Abstract
Background
Medicinal and aromatic plants are natural raw materials. Since ancient times these herbal materials are being commonly used as herbal drugs, food products, and cosmetics. The phytomolecules isolated from the medicinal and aromatic plants (MAPs) are in high demand specifically in drug industries. However, these phytomolecules have certain limitations of low absorption, high toxicity, and other side effects, bioavailability and efficacy. These limitations may be overcome by using nanotechnological tools. The plant extract or essential oil of MAPs are also useful in the synthesis of nanoparticles. In future this combinatorial application of MAPs and nanotechnology would be advantageous in the healthcare area.
Methods
Literature search was performed using databases like Pubmed, Scopus and Google Scholar with the keywords “nanoparticles,” “phytomolecules,” “medicinal and aromatic plants” and “green synthesis of nanoparticles” in the text.
Result
Phytomolecules of medicinal and aromatic plants like curcumin, camptothecin, thymol, and eugenol have certain limitations of bioavailability, efficacy, and solubility. It limits its biological activity and therefore application in the biomedical area. The increment in the biological activity and sustained delivery was observed after the encapsulation of these potent phytomolecules encapsulated in the nanocarriers. Besides, MAPs and/or their molecules/oils mediate the synthesis of metal nanocarriers with less toxicity.
Conclusion
This review highlights the impact of the combination of the MAPs with the nanotechnology along with the challenges. It would be an effective technique for the efficient delivery of different phytomolecules and also in the synthesis of novel nano-materials, which escalates the opportunity of exploration of potential molecules of MAPs.
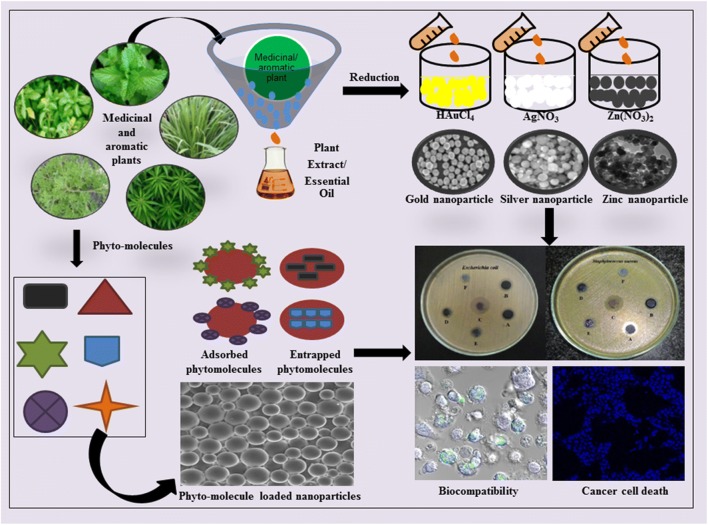
Graphical abstract.
Graphical representation of the combinatorial approach of MAPs and nanotechnology.
Keywords: Medicinal, Aromatic, Plants, Nanoparticles, Biological activity
Introduction
The active constituent, extract, and oils derived from Medicinal and Aromatic Plants (MAPs) play a vital role in herbal remedies to cure and manage various diseases and disorders since antiquity. According to the World Health Organisation (WHO), about 80% of the global population is dependent on traditional plant-based medicines. It is estimated that about 60% medicinal formulations which are available in the market or tested are based on natural products of MAPs. Presently, about 80% of the anticancer, antimicrobial, immunosuppressive and cardiovascular drugs are primarily based on plant sources. Among 177 anticancer drugs, about 70% moieties are based on medicinal plants [1]. Similar to the phytomolecules, aromatic oils/aroma-molecules, have also been used for centuries in healthcare on a global scale. Currently, there are almost 3000 well-known essential oils. Among those, 10% essential oils are known for their commercial importance in various sectors of agronomy, cosmetic, pharmaceutical and perfume industries. Mostly, the chemical constituents present in the aromatic oil are responsible for their biological activity. The major component types are terpenoids, phenylpropanoids, and short-chain aliphatic hydrocarbon derivatives, with characteristics of low molecular weight. There are several approaches to the application of aromatic oils because of its therapeutic effects. In the case of oral administration, the oils are usually diluted in milk or olive oil. Also, for direct application of oils on the skin, the oils are diluted by mixing with other solvents, as pure oil cause skin irritation or darkening of the skin, such as citrus oil are UV sensitive [2]. The external application of pure oil on the injured skin may lead to severe side effects as they are permeation enhancers. [3].
The long-term toxicity, side-effects, low solubility and low stability of the pharmaceutically active compounds of MAPs has become a significant problem which leads to a demand for novel drug delivery system which may reduce or fully overcome the effect associated with the active compounds. In this context, the union of nanotechnology and MAPs could be a useful approach for improving the treatment, diagnosis, monitoring tools in the healthcare system. The technology aims to design and develop new tools and dosage forms in the range of 1 to 100 nm. For this purpose, several nanocarriers have been prepared which include synthetic biodegradable polymers like polysaccharides and lipids. These nanocarriers are useful in the formation of nano dosage forms which enhanced the solubility and bioavailability, reduced toxicity; increase the pharmacological activity, etc.
Every year many nano-drugs enter the clinical investigation and around 56 clinical trials including the term ‘nano’ were listed as active on ClinicalTrials.gov [4]. Among nano-drugs, two of the top 10 bestselling drugs in the United States in 2013 were polymeric drugs- Copaxone (glatiramer acetate injection, Teva Pharmaceuticals), approved in 1996 for the treatment of relapsing-remitting multiple sclerosis, and Neulasta (Pegfilgrastim, Amgen), recommended in 2002 for chemotherapy-induced neutropenia [5]. Many nano-drugs are being investigated in clinical trials. For example, CRLX101 (drug conjugate formulation of camptothecin and cyclodextran-polyethylene glycol polymer) is investigated alone and also by mixing with different drugs in various phase one and two clinical trials for the treatment of solid tumors, and lung cancers. It was found successful for gastrointestinal cancer and renal cell carcinoma in clinical studies [6].
Not only deliveries of phytomolecules, but MAPs are also useful in the synthesis of various metallic nanoparticles. It is known that metallic nanoparticles possess unique properties, which make them suitable for industrial, agricultural, biomedical and household applications. As a result, the global production of metallic nanoparticles for 2010 was 260,000–309,000 metric tons [7]. In the first three decades of the twenty-first century, several nano-based products are exposed to society. Metallic nanoparticles are synthesized not only by chemical methods, but it can also be produced by using MAPs, which is known as a green method. A literature search using ScienceDirect.com and keywords ‘green synthesis of metal nanoparticles’ showed 13,457 results while PubMed showed 1761 results as on July 26, 2018. This current review focuses on how does the combination of MAPs with nanotechnology could improve or overcome the issues associated with plant-based herbal medicines. Additionally, it also showed the importance of MAPs in the green synthesis of nanoparticles with specific biological activity, which might be useful in the healthcare area.
Effectiveness and limitations of MAPs in healthcare
The chemical constituents in the plant’s extract act as an active ingredient in the medicinal formulation. The active constituents of plant extracts like tannins, flavonoids, alkaloids, phenylpropanoids, and terpenoids are water soluble but shows poor absorption, which then results in decreased bioavailability and efficacy. The efficacy of any medicine is mainly dependent on the delivery of therapeutic active compounds to its site of action. Studies have shown that herbal formulations shows bioactivity under in-vitro conditions but fail to reproduce the same effect in in-vivo. The most common and well-studied example of plant-based molecules is Curcumin (diferuloylmethane) which is the most studied chemopreventive agent. It is a polyphenol which is assimilated in the rhizome part of Curcuma longa [8]. It has also been applied for the treatment of neurodegenerative diseases, diabetes, bronchitis, cardiovascular diseases, rheumatoid arthritis, inflammatory bowel diseases, psoriasis, renal ischemia, scleroderma, acquired immunodeficiency disease, cancer, anti-aging, and scar formation agent [9]. Regardless of showing unique medicinal properties, curcumin has limitations like low solubility, rapid metabolism and poor bioavailability [10].
Another example is Cannabis sativa which is generally used for the treatment of malaria, constipation, rheumatic pains and during childbirth. The plant mainly consists of 60 terpenophenolic compounds named as phytocannabinoids [11]. The first reported active component found in Cannabis sativa was ∆9 -tetrahydrocannabinol (∆9 -THC). The cannabinoids have typical symptoms and side effects like nausea, vomiting, loss of appetite and cachexia [12]. Camptothecin, is a potential drug-like molecule, exhibiting antitumor activity against prostate, lung, breast, stomach, bladder and ovarian cancers. It is a pentacyclic alkaloid isolated from Chinese tree camptotheca acuminate. However, its efficacy was significantly restricted by its poor solubility, stability, and toxic side effects like, nausea and myelosuppression, which restricts its application in targeted therapies [13].
The aromatic oils are also known for their excellent medicinal properties like antimicrobial, anti-oxidant, but the chemical constituents can readily undergo oxidation reactions which result in allergenic products with decreased biological activity and increased side effect. These problems make these drug a poor candidate for medicinal and therapeutic use [14]. For example, Thymol, which is a natural monoterpenoid phenol derivative and antimicrobial agent isolated from thyme. According to the United States Food and Drug Administration (FDA), thymol is generally recognized as a safe food additive. However, due to its poor water solubility, a strong result in flavoring food led to interaction with the food components like fat and protein, it is difficult to disperse it [15]. It has been reported that the antimicrobial activity of thymol is related to depolarization of the bacterial cytoplasmic membrane and high concentration of thymol was required in altering the structure of the membrane [16]. In aroma molecules, the complexity mainly starts with poor membrane permeability [17].
Similarly, Menthol is a broadly used flavoring agent used in several products like toothpaste, chewing gum, balms, etc. It is monocyclic monoterpene alcohol, naturally isolated from the peppermint oil. Many previous studies showed the antibacterial and antifungal activity of menthol. However, its high volatility, insolubility, instability and high crystallization in aqueous medium becomes a critical problem in its application and shelf life [18]. So, there are still requirements of carrier systems which can be useful in herbal drug delivery system.
Importance of combinatorial approaches of nanotechnology and MAPs
The idea of ‘nanotechnology’ was first initiated by Richard P. Feynman in 1959, followed by the discovery of carbon nanotubes in 1991. Nanotechnology is an interdisciplinary approach which includes production and implementation of materials, equipment’s or systems in the range of nanometer. Nanotechnology is expected to play a critical role in biomedical applications, not only in drug delivery but also in molecular imaging, biomarkers, and biosensors. At present, several biocompatible polymers, liposomes, and micelles are being researched as carriers system for vaccines, drugs, and genes. The application of nanocarriers/nanomaterial is very beneficial in the formulation of herbal drugs or administration of pure phytomolecule or aroma molecule. It does so by acting as a drug delivery carrier to deliver optimum drugs doses to its site of action. There is an increasing hope that nanotechnology, in combination with MAPs , will bring significant advances in the field of biomedical science.
The MAPs contain several drugs like molecules which shows biological activity but consists of various limitations. The nanotechnological strategies attempted to remove the various limitations and helpful in enhancing the properties and behavior of a drug like a molecule in the biological environment. In nano-formulation research, nano-sized drug delivery system can help in improveing the solubility, bioavailability, stability, toxicity, pharmacological activity, and tissue macrophages distribution, sustained delivery, increase target specificity, etc. The incorporation of nanocarriers and medicinal drugs can efficiently combat many diseases like diabetes, tuberculosis, cancer and many others [19]. The nano-carrier based drug delivery tool not only enhances drugs effectiveness but also helps in reducing toxicity and side effects, which makes this approach attractive. Not only drug delivery but these plants also have a significant role in the production of nanoparticles which are non-toxic. In this review, the impact of a combination approach of MAPs with nanotechnology along with their impacts are discussed.
Combinatorial approach of nanotechnology with phytomolecules of MAPs
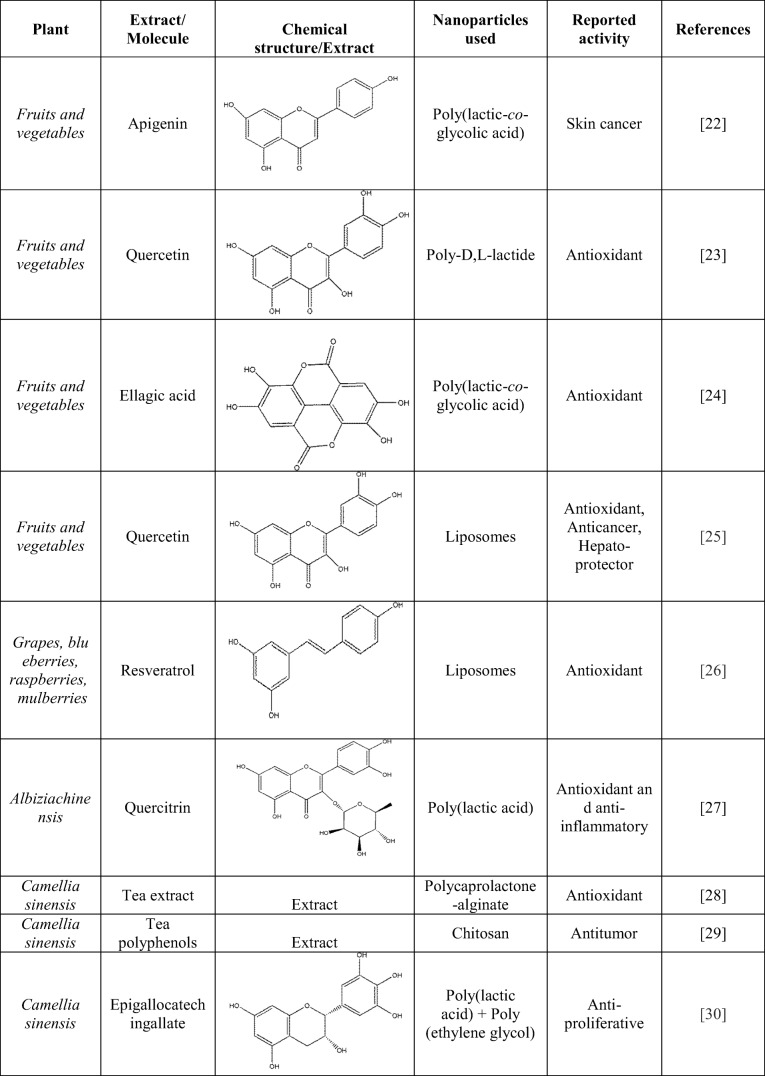
An enhancement in the bioavailability of berberine was observed when encapsulated in chitosan nanoparticles without affecting the structure and function of berberine. Additionally, it also enhanced cytotoxic behavior against the cancer cells by directly targeting its nuclei without affecting the normal cells. In-vivo studies demonstrated an enhancement in the bioavailability when administered orally, and encapsulation of berberine reduced the dosages up to three times. Several plant-based drugs have been used for cervical cancer [20]. Cisplatin loaded folic acid-conjugated gelatin nanoparticles have shown cellular uptake of 81% in comparison to cisplatin and cisplatin-gelatin nanoparticles, which shows 51% cellular uptake [21]. Based on the nanostructured herbal formulations, several examples are presented in Table 1. Paclitaxel was encapsulated in stearyl amine-based positively charged multi-layered liposomes. Then, the nanoformulation was coated with ‘anionic polyacrylic acid’ and further by ‘cationic chitosan.’ The chitosan-polyacrylic acid-paclitaxel liposomes showed sustained release and increased cytotoxicity in HeLa cell lines as compared to paclitaxel liposomal formulation [48]. Similarly, lipid-based nanomaterial encapsulated green tea, and ginseng extracts were prepared in different formulations for enhancing the intake of active ingredients. Liposomes based nanoparticle using Artemisia arborescens L. are used for the entry of active constituents through the cytoplasmic boundary [49].
Table 1.
Nanostructured herbal formulations from medicinal plants and their activity
The enhanced antimicrobial activity against Escherichia coli, Bacillus subtilis, Pseudomonas aeruginosa, and Staphylococcus aureus was observed in methanolic extract of Ocimum sanctum L. encapsulated nanoparticles [50]. Honokiol, also known as 3′,5-di(2-propenyl)-1,1′-biphenyl-2,4′-diol is an active component of Magnolia officinalis. It has multiple pharmacological properties but due to certain limitations like high hydrophobicity prevents its vascular administration. However, honokiol encapsulated polymeric nanoparticles increased the possibility of vascular administration and showed better results compared to free honokiol [51]. Like honokiol, quercetin is one of the many flavonoids consisting various pharmacological activities. Quercetin loaded nanoparticles were prepared using polyvinyl alcohol and eudragit®E, to estimate the antioxidant of pure quercetin and quercetin loaded nanoparticles. The system was found to be more effective relative to di (phenyl)-(2,4,6-trinitrophenyl) iminoazanium scavenging, superoxide anion scavenging, anti-superoxide formation, and anti-lipid peroxidation activities, as compared to pure quercetin with encapsulation efficiency higher than 99% [52]. The camptothecin is a potent anticancer molecule but due to low solubility, unstable lactone ring, it was unsuitable for clinical purposes. When camptothecin was loaded in hydrophobically modified glycol chitosan nanoparticle, it showed loading efficiency greater than 80%. It helps in protecting the lactone ring from physiological conditions. It was also demonstrated to have strong antitumoral activity along with higher penetration in tumors, as proved by the near-infrared study [53]. Similarly, when curcumin was loaded in poly(lactic-co-glycolic acid) nanoparticle, it showed 90.88% ± 0.14% encapsulation efficiency with biological activity against prostate cancer [54].
Das et al. (2012) demonstrated that the root extract of Phytolacca decandra in the free form and poly(lactic-co-glycolic acid) loaded form in the animal model. It was found that nanoparticles increased the drug’s bioavailability and showed better chemopreventive action against lung cancer in both in vivo and in vitro experiments [55]. Celastrol nanoparticles composed of poly (ethylene glycol)-block poly (ε-caprolactone) nano polymeric micelles and helps in improving hydrophilicity [56]. It was reported that Panax notoginsenoside, when encapsulated in core-shell hybrid liposomal vesicles helps in enhancing its bioavailability [57]. When ginkgolides A, B, C and bilobalide from Ginkgo biloba extract, was loaded in monomethoxy (polyethylene glycol)-poly (lactide-co-glycolide)-monomethoxy (polyethyleneglycol) by co-encapsulation method, it showed sustained and synchronized release in both in vitro and in vivo [58].
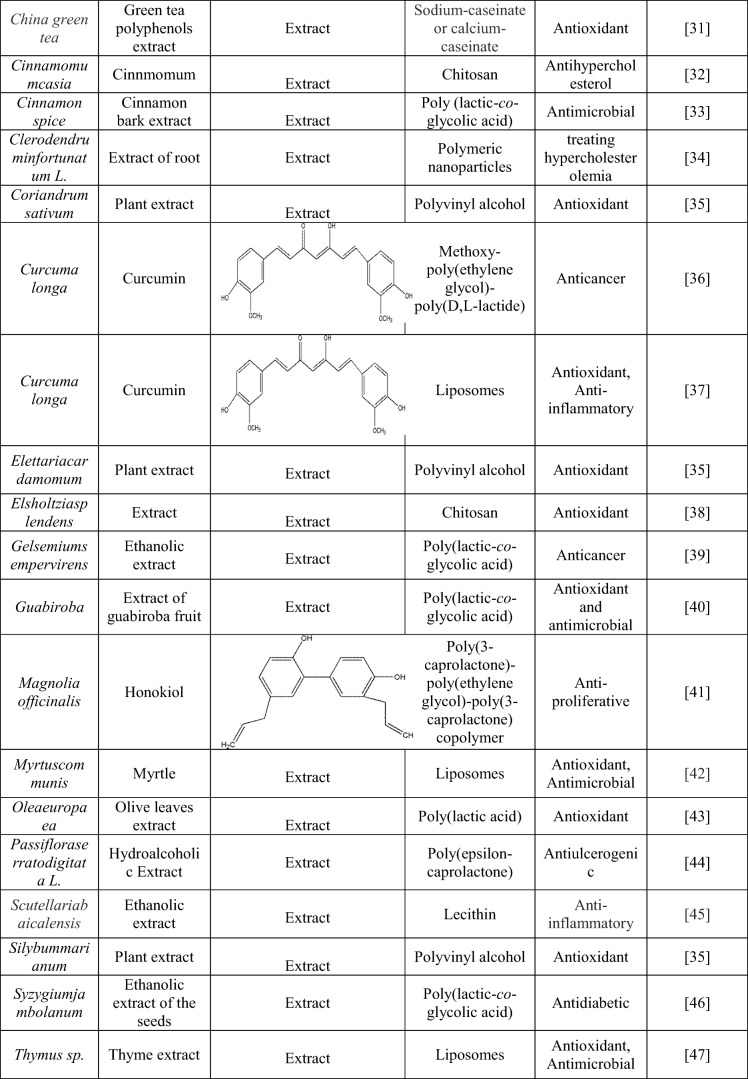
In addition, Apocynin, a bioactive organic phytochemical, which is known as a specific NADPH oxidase inhibitor, with potent antioxidant and anti-inflammatory activities. The bioactivity of apocynin was revealed in various diseases like cancer, atherosclerosis, asthma, vascular and neurodegenerative diseases, collagen-induced arthritis and inflammatory bowel disease pharmacotherapy [59]. However, due to its rapid elimination and poor bioavailability properties, it has becomes a challenge for pharmaceutical scientists. The encapsulation of the apocynin in the chitosan-based solid lipid nanoparticle showed potential in enhancing the oral and intravenous bioavailability in rats [60]. These examples help in better understanding of the combinatorial approach of MAPs and nanotechnology.
Combinatorial approach of nanotechnology with aromatic molecules
The nano-encapsulation of the aromatic molecules in modified nanocarriers improves their solubility, stability, and efficacy by maintaining therapeutic drug to their specific target of action [19]. They also possess desirable properties like the controlled and sustained release of the drug molecule, deep penetration through tissue due to nanometer size, helps in protecting its therapeutics potential at both extracellular and intracellular levels [2]. The nanoparticles prepared using polysaccharides are considered to be promising carriers of a hydrophilic drug-like molecule, due to their unique properties. The releasing of essential oil takes place through different processes like dissolution, desorption, diffusion through the matrix; of the surface-bound/adsorbed functional ingredient, matrix erosion including enzyme degradation, and a combination of these processes [61]. Different examples of aromatic component encapsulated nanoparticles are listed in Table 2.
Table 2.
Nanostructured herbal formulations from aromatic plants and their activity
| Plant | Aroma constituents | Nanoparticles | Reported activity | References |
|---|---|---|---|---|
| Anise myrtle, Mentha spicata | Anethole, Carvone | Poly(lactic-co-glycolic acid) | Antimicrobial | [62] |
| Artemisia arborescens | Essential oil | Liposome | Antiviral | [49] |
| Cinnamomum verum | Cinnamon oil | Polylactide | Antibacterial | [63] |
| Cinnamomum verum | trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extract | Beta-cyclodextrin complex | Antimicrobial | [64] |
| Cinnamomum verum | Essential oil | Polylactide + Bimaetallic Ag-Cu nanoparticle | Antibacterial | [65] |
| Citrus aurantifolia | Lime oil | Chitosan | Antibacterial | [66] |
| Citrus aurantium | Essential oil | Chitosan | Antioxidant | [67] |
| Cuminum cyminum | Essential oil | Chitosan | Antioxidant | [68] |
| Curcuma longa | Turmeric oil | Chitosan and alginate | Antiproliferative | [69] |
| Cymbopogon citrates | Lemongrass oil | Chitosan and alginate | Antiproliferative | [69] |
| Cymbopogon citrates | Citral | Nanoemulsion | Antimicrobial | [70] |
| Eucalyptus staigeriana | Eucalyptus oil | Cashew gum | Antimicrobial | [71] |
| Eucalyptus staigeriana | Essential oil | Lipid nanoparticle | Antimicrobial | [72] |
| Eugenia caryophyllata | Essential oil | Solid lipid nanoparticles | Antimicrobial | [73] |
| Melaleuca alternifolia | Tea Tree Oil | Nanoemulsions | Antifungal | [74] |
| Melaleuca alternifolia | Tea tree oil | Liposome | Antimicrobial | [75] |
| Mentha piperita oil | Peppermint oil | Chitosan-cinnamic acid nanogels | Antimicrobial | [76] |
| Ocimum species | Eugenol | Nanoemulsion | Antimicrobial | [77] |
| Origanum dictamnus L. | Carvacrol, Thymol | Phosphatidyl choline-based liposomes | Antimicrobial | [78] |
| Origanum dictamnus L. | Thymol | Liposome | Antimicrobial | [79] |
| Origanum dictamnus L. | Carvacrol | Liposome | Antimicrobial | [79] |
| Origanum dictamnus L. | Carvacrol | Chitosan | Antimicrobial | [80] |
| Origanum dictamnus L. | Thymol | Methyl and Ethylcellulose | Antimicrobial | [81] |
| Origanum dictamnus L. | Carvacrol, thymol, p-cymene, and c-terpinene | Phosphatidyl choline based liposomes | Antimicrobial | [78] |
| Pogostemon cablin | Wild patchouli oil | Nanoemulsion | Antibacterial and anti-candida | [82] |
| Punica granatum | Pomegranate oil | Hydrogel containing silibinin | Anti-inflammatory | [83] |
| Salvia multicaulis | Essential oil | Nanoemulsion | Antimicrobial & antioxidant | [84] |
| Saturejamontana | Savory oil | Chitosan | Antimicrobial & antioxidant | [85] |
| Syzygium aromaticum | Clove oil | Polylactide | Antibacterial | [63] |
| Syzygium aromaticum | Clove oil | Nanoemulsion | Antibacterial | [86] |
| Syzygium aromaticum | trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extract | Beta-cyclodextrin complex | Antimicrobial | [64] |
| Thymus daenensis | Essential oil | Nanoemulsion | Antibacterial | [87] |
| Thymus vulgaris | Thyme oil | Chitosan-benzoic acid nanogel | Antifungal, antiviral and antibacterial | [88] |
| Thymus vulgaris | Thyme oil | Phospholipon G90, cholesterol and calcium. | Antioxidant | [89] |
| Zanthoxylum tingoassuiba | Essential oil | Liposome | Antimicrobial | [90] |
| Zataria multiflora | Essential oil | Chitosan | Antifungal | [91] |
An aromatic molecule, eugenol is one of the main components from the diverse group of essential oil. However, due to its instability, high volatility, sensitivity from oxygen, light, heat, and storage problems, it cannot be used in therapeutic applications. When eugenol was loaded with chitosan nanoparticle, it was found thermally stable, with the average size less than 100 nm [92]. The in vitro release study of solid lipid nanoparticles containing essential oil of Zataria multiflora showed 93.2% release of the essential oil after 24 h. Carvacrol, thymol, p-cymene, and c-terpinene were examined as the major components and prepared by using essential oil from Origanum dictamnus L. These molecules were entrapped in phosphatidyl choline-based liposomes, led to improvement in its antioxidant activity. Along with this, its antimicrobial activity was investigated against different Gram-positive and Gram-negative bacteria, fungi, and food-borne pathogens showing increment in antimicrobial activities [78].
The insecticidal activity against Tribolium castaneum was observed using polyethylene glycol nanoparticle encapsulated garlic essential oil, which helps control the store-product pest [93]. The antimicrobial activity against E. coli and Salmonella and DPPH radical scavenging activity has been reported using thymol loaded zein coated sodium casinate nanoparticle [94]. The polyvinyl alcohol/cinnamon essential oil/β-cyclodextrin antimicrobial nanofibrous film was developed, which can enhance the shelf life of strawberry, demonstrating its application in food packaging [95]. The peppermint essential oil encapsulated in the bio-nanocomposite film was prepared by filling a pectin matrix with modified halloysite nanotubes, exhibiting antibacterial activity against Escherichia coli and Staphylococcus aureus [96]. It was reported that the inhibitory activity of thyme essential oil loaded chitosan nanoparticle was higher against Staphylococcus aureus, and thyme essential oil loaded chitosan nanocapsules show inhibitory activity against Bacillus cereus [66]. It was demonstrated that when frankincense and myrrh essential oil was loaded in solid lipid nanoparticles using high-pressure homogenization method, enhance its bioavailability and improves hydrophilicity [97]. The combinatorial approach proves to be effective for the delivery of essential molecules.
Combinatorial approach of nanotechnology with MAPs in the synthesis of nanoparticles
Biological synthesis or green synthesis of nanoparticles is an eco-friendly method. There are various biologically active components found in the plants which possess reducing capability in producing metallic nanoparticles with effective therapeutic potential. Plant extracts and essential oils are considered as the best-reducing agents to overcome the problem of toxicity during chemical synthesis. The plant-based molecules like flavonoids, alkaloids, polyphenols, vitamins, tannins, plant pigments polysaccharides, and aroma molecules are responsible for many reduction reactions of metal salts to metal nanoparticles [98]. The application of nanoparticles isolated by employing green synthesis method has played a significant role as antimicrobial agents or potential drug carrier in the treatment of cancer [99]. Several examples of synthesis of nanoparticles using plant MAPs as reducing agents along with their biological activities are listed in Table 3. Gold nanoparticles isolated using flowers of Couroupita guianensis tree showed anticancer activity [138]. The highly stabilized gold and silver nanoparticles were synthesized using glucoxylans which were isolated from seeds of Mimosa pudica [139]. Silver nanoparticles were developed using Taraxacum officinale leaf extract and also showed significant antioxidant and anticancer activity [140]. The silver nanoparticles produced using stem bark extract of Helicteres isora, showed antioxidant effects determined using DPPH, hydrogen peroxide and nitric oxide radical scavenging and a reducing power assays. It also exhibits antibacterial effect against test strains, showing significant inhibition. Along with antioxidant and antibacterial, it showed antiproliferative activity which was demonstrated using oral carcinoma (KB) cells with MTT assay [141].
Table 3.
Summary of the green synthesis of nanoparticles using medicinal and aromatic plants
| Plant | Reducing agent | Material precursor | Nano-particle | Application | References |
|---|---|---|---|---|---|
| Phaseolus vulgaris | Black bean extract | Copper sulphate | Copper oxide | Anticancer | [100] |
| Acalypha indica | Leaf extract | Chloroauric acid, silver nitrate | Gold, Silver | Antibacterial | [101] |
| Allium sativum | Garlic extract | Silver nitrate | Silver | Antibacterial | [102] |
| Aloysia triphylla | Aqueous extract | Silver nitrate | Silver | Antifungal | [103] |
| Alternanthe rasessilis | Whole plant | Silver nitrate | Silver | Antioxidant, antimicrobial | [104] |
| Anisochilus carnosus | Leaf extract | Zinc nitrate | Zinc oxide | Antibacterial and photocatalytic | [105] |
| Artocarpus heterophyllus | Seed extract | Silver nitrate | Silver | Antibacterial | [106] |
| Azadirachta indica | Leaf extract | Zinc acetate dihydrate | Zinc oxide | Antibacterial activity, photocatalytic applications | [107] |
| Caesalpinia coriaria | Leaf extract | Silver nitrate | Silver | Antibacterial | [108] |
| Calotropis gigantea | leaf extract | Silver nitrate | Silver | Antibacterial | [109] |
| Calotropis gigantea | flowers extract | Titanium dioxide hydrate | Titanium oxide | Antiparasitic | [110] |
| Cassia auriculata | propanoic acid 2-(3-acetoxy- 4,4,14-trimethylandrost-8-en-17-yl) | Chloroauric acid | Gold | Antidiabetic | [111] |
| Cassia fistula | plant extract | Zinc nitrate hexahydrate | Zinc oxide | photodegradative, antioxidant and antibacterial | [112] |
| Cassia tora | leaf extract | Silver nitrate | Silver | Antibacterial | [113] |
| Catharanthus roseus | leaf extract | Silver nitrate | Silver | Antiplasmodial | [114] |
| Cauler papeltata, Hypnea valencia, S. myriocystum | leaf extract | Zinc nitrate | Zinc oxide | Antimicrobial | [115] |
| Chelidonium majus L. | Plant extract | Silver nitrate | Silver | Antioxidant, antimicrobial | [116] |
| Cinnamomum zeylanicum | leaf extract | Chloroauric acid | Gold | Antibacterial and antifungal | [117] |
| Citrullus colocynthis | Calli | Silver nitrate | Silver | Antioxidant, anticancer | [118] |
| Cocous nucifera | methanol and ethyl acetate extracts of the inflorescence | Silver nitrate | Silver | Antibacterial | [119] |
| Coptidis rhizome | plant extract | Zinc nitrate | Zinc oxide | Antibacterial, antioxidant, and cytotoxic | [120] |
| Dendropanaxmorbifera | leaf extract | Silver nitrate | Silver Gold | Anticancer | [121] |
| Dysosma pleiantha | aqueous rhizome extract | Chloroauric acid | Gold | Anti-metastatic | [122] |
| Gloriosa superba L. | leaf extract | Cerium(III) chloride | Cerium oxide | Antibacterial | [123] |
| Hibiscus subdariffa | flower extract | Copper nitrate | Copper | Antibacterial | [124] |
| Hibiscus subdariffa | leaf extract | Zinc acetate | Zinc oxide | Anti-bacterial activity, anti-diabetic | [125] |
| Laurelia sempervirens | aqueous extract | Silver nitrate | Silver | Antifungal | [103] |
| Lemon (Citrus sp.) | fruit extract | Copper chloride | Copper | Antibacterial | [126] |
| Magnolia kobus | leaf extract | Copper sulfatepentahydratesulfate | Copper | Antibacterial | [127] |
| Mentha piperita | plant extract | Chloroauric acid | Gold | Antibacterial | [128] |
| Mimusop selengi, Linn. | leaf extract | Silver nitrate | Silver | Antibacterial | [129] |
| Musa paradisiaca | peel extract | Chloroauric acid | Gold | Antimicrobial | [130] |
| Musa paradisiaca | Peel extract | Chloroauric acid | Gold | Anticancer | [131] |
| Nerium oleander | leaf extract | Copper sulphate | Copper | Antibacterial | [132] |
| Nyctanthes arbortristis | ethanolic flower extract | Silver nitrate | Silver | Antibacterial and cytotoxic | [133] |
| Ocimum sanctum | leaf extract | Silver nitrate | Silver | Antibacterial | [134] |
| Parthenium hysterophorus | leaf extract | Zinc nitrate | Zinc | Antifungal | [135] |
| Phyllanthus Embilica | fruit extract | Copper sulphate | Copper | Antibacterial | [136] |
| Pleurotus florida | edible mushroom | Chloroauric acid | Gold | Anticancer | [137] |
The rod-shaped copper oxide nanoparticle was synthesized using Carica papaya leaf extract [142]. The copper nanoparticle synthesized using the aqueous extract of the latex of Calotropis procera L. showed long-term stability along with excellent cell viability at 120 μM concentrations [143]. Similar to copper oxide nanoparticles, zinc oxide nanoparticles were also prepared by using flower extract of Nyctanthes arbortristis, and exhibit significant antifungal potential [144]. The gold nanoparticles were synthesized by using aromatic plant extract like Ocimum, and Eucalyptus in size range of 3–16 nm [145]. Silver nanoparticles were prepared by using a solution of Pimpinella anisum seeds and showed effective antibacterial potential against S. pyogenes, A. baumannii, K. pneumoniae, S. typhi, and P. aeruginosa [146]. The aromatic plants are found to be useful in the production of nanoparticles. For example, gold nanoparticles synthesized using the essential oil of Mentha piperita showed high activity against Aspergillus flavus [147]. Fluorescent-based peppermint oil nanoparticles exhibited high antibacterial activity and fluorescence in E. coli testing and proves to be bifunctional agents as antibacterial and bioimaging applications [148].
The silver nanoparticles isolated using leaf extract of Erythrina suberosa showed excellent anticancer activity against A-431 osteosarcoma cell line along with antimicrobial and wound healing activity [149]. The free radical scavenging and antimicrobial activity of silver nanoparticles isolated using Carica papaya peel extract were reported [150]. The antibacterial activity was reported from silver nanoparticles isolated using seed extract of Pongamia pinnata [151].
New approaches and challenges
The incorporation of the nanotechnology and MAPs could be a successful approach in healthcare. The nanoparticles help in the increment of biological activity, overcoming the issues associated with plant-based medicines and also in the formation of novel materials. There are several MAPs which are useful in the synthesis of nanoparticles along with potential biological activities. With the advancement, there are several challenges remain for applying them in clinically feasible therapies. The trials of novel nanoparticles interaction with the biological system and transforming them to therapies depicted current limitations. Some other challenges related to nanoparticles include examining their potential of targeting, fulfilling international standards of toxicity and biocompatibility. Many reports suggest the formation of reactive oxygen species from different metal nanoparticles like zinc oxide, and titanium oxide [152]. Gold can be used as potential carriers for the drug delivery, imaging molecules, and formation of novel cancer therapy formulations [153]. However, the presence of residues from stabilizer CTAB resulted in cytotoxicity. However, toxicity can be reduced by using a green synthesis method or by coating with the prominent polymers.
The toxicity can be affected by the interaction between nanomaterial and in vivo system. Recently, studies were conducted for assessing the nanoparticle toxicity. It was found that when 15–20 nm nanosilver given to rat for 28 days, it gets accumulated in all organs with the highest concentration in the liver and spleen [154]. The cytotoxic effect of 20 nm nanosilver was observed using human liver HepG2 and colon Caco2 cells as in vitro models. They found concentration-dependent cytotoxic effects in both HepG2 and colon Caco2 cells, along with mitochondrial injury and loss of double-stranded DNA in both cells. No cellular oxidative stress was found in dichlorofluorescein assay implying that cellular oxidative stress does not show significant role in the cytotoxicity profile of the nanosilver in both cells [155]. The silver nanoparticles synthesized using aloe vera extract were non-toxic to human peripheral blood mononuclear cells, which demonstrates the application for biomedical applications [156].
The toxicity assessment is the primary concern where different cells and organs are treated with different doses of chemicals, and their response was taken at different time points. These experiments are essential in determining the exact amount of the drug-like molecules, i.e., lethal dose (LD50) and inhibition concentration (IC50). In traditional cytotoxic assays, the main concern is upon the chemicals that exhibit the cellular toxicity, whereas, in the case of the nanoparticle, the main focus is on the sizes and their shapes. This is resulting in the agglomeration of the nanoparticles at the target site, which leads to the misstatement of the toxicity data [157]. Some reports showed the problem of DNA damage in the cells due to exposure of nanomaterials, which causes cancer and developmental toxicity. Some other reasons behind toxicity are the surface functionalization of the nanoparticles and the presence of the gaps in the cells allowing the transmission of molecules into the cells. So, alternative methods should be opted to overcome the issue. Green synthesis is one such method which is helpful in the production of safe and effective nanoparticles along with beneficial effects of cost and non-toxic behavior [158].
Future possibilities
Nanoparticles improve pharmacokinetics profile and help in the appropriate diffusion of the drug in the body system. The production of nanoparticles using green methods holds endless opportunities in the field of drug delivery. It also serves as the best tool for limiting the dosage while increasing bioavailability, bioactivities, and cost-effectiveness. Therefore, nanomaterials require effective analysis for detection of long-term toxicity. It is necessary for the drug-loaded nanomaterials as well as nanomaterials to go through the mechanism involved in the injured cells/tissue before using in therapies. Presently, there are various nanoparticles used for the delivery of plant-based molecules. As these experimental assays are time-consuming and require huge resources, scientists have developed computational analysis for, eg. Quantitative Structure-Activity Relationship (QSAR). These models are developed by taking parameters like biochemical chemical or structural descriptors from the published literature, which help in determine the cytotoxicity of nanoparticles. A conceptual understanding is required to develop safe nanoparticles.
For future possibilities, the scientific background for the reactivity of nanoparticles should be a major concern. The interaction between the nanoparticles and the biological system should be under check. Proper clinical trials should be followed before a final decision. There is a need for further extensive research in the field of nano therapy and efforts are on worldwide to exploit the technology for the better healthcare of humanity. If the plant extract, molecules, or oils replace the chemicals/solvents, it may also reduce the chances of toxicity. The combinatorial approach could be a successful technique which will provide opportunities in the diverse application in the area of healthcare.
Conclusion
Overall, MAPs are a beneficial source of natural agents with excellent biological activities. Current pieces of evidence showed combinational approaches of MAPs with the nanotechnology could better support its application in the area of healthcare. However, future mechanistic studies, toxicity assessment, and well-prepared clinical trials are necessary to estimate the safety and efficacy of the obtained products.
Acknowledgments
We are grateful to the Director, CSIR-Central Institute of Medicinal and Aromatic Plants, Lucknow for rendering essential facilities required for the experimental work. We are also thankful to Council of Scientific and Industrial Research, New Delhi for funding support under CSIR-Aroma Mission (HCP007) and CSIR-Phytopharma Mission (HCP010). PK aquiesce University Grants Commission, New Delhi for Rajeev Gandhi National Fellowship.
Compliance with ethical standards
Conflict of interest
The author(s) confirm that this article content has no conflict of interest.
Footnotes
Highlights
• Importance of medicinal and aromatic plants along with certain limitations
• The combinatorial approach of nanotechnology with MAPs
• Effectiveness of the approach in phytomolecule delivery and green synthesis of nanoparticles.
• Challenges of the combinatorial approaches
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sen S, Chakraborty R. Revival, modernization and integration of Indian traditional herbal medicine in clinical practice: importance, challenges and future. J Tradit Complement Med. 2017;7(2):234–244. doi: 10.1016/j.jtcme.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilia AR, Guccione C, Isacchi B, Righeschi C, Firenzuoli F, Bergonzi MC. Essential oils loaded in nanosystems: a developing strategy for a successful therapeutic approach. Evid Based Complement Alternat Med. 2014;2014. [DOI] [PMC free article] [PubMed] [Retracted]
- 3.Adorjan B, Buchbauer G. Biological properties of essential oils: an updated review. Flavour Fragr J. 2010;25(6):407–426. [Google Scholar]
- 4.Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33(10):2373–2387. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- 5.Havel HA. Where are the Nanodrugs? An industry perspective on development of drug products containing nanomaterials. AAPS J. 2016;18(6):1351–1353. doi: 10.1208/s12248-016-9970-6. [DOI] [PubMed] [Google Scholar]
- 6.Ventola CL. Progress in nanomedicine: approved and investigational Nanodrugs. PT. 2017;42(12):742. [PMC free article] [PubMed] [Google Scholar]
- 7.Keller AA, McFerran S, Lazareva A, Suh S. Global life cycle releases of engineered nanomaterials. J Nanopart Res. 2013;15(6):1692. [Google Scholar]
- 8.Jackson SJT, Murphy LL, Venema RC, Singletary KW, Young AJ. Curcumin binds tubulin, induces mitotic catastrophe, and impedes normal endothelial cell proliferation. Food Chem Toxicol. 2013;60:431–438. doi: 10.1016/j.fct.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thangapazham RL, Sharad S, Maheshwari RK. Skin regenerative potentials of curcumin. BioFactors. 2013;39(1):141–149. doi: 10.1002/biof.1078. [DOI] [PubMed] [Google Scholar]
- 10.Gao M, Chen C, Fan A, Zhang J, Kong D, Wang Z, et al. Covalent and non-covalent curcumin loading in acid-responsive polymeric micellar nanocarriers. Nanotechnology. 2015;26(27):275101. [DOI] [PubMed]
- 11.Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(S2):S1–1. [DOI] [PMC free article] [PubMed]
- 12.Grotenhermen F, Müller-Vahl K. The therapeutic potential of cannabis and cannabinoids. Dtsch Arztebl Int. 2012;109(29–30):495. doi: 10.3238/arztebl.2012.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du F, Meng H, Xu K, Xu Y, Luo P, Luo Y, et al. CPT loaded nanoparticles based on beta-cyclodextrin-grafted poly(ethylene glycol)/poly (l-glutamic acid) diblock copolymer and their inclusion complexes with CPT. Colloids Surf B: Biointerfaces. 2014;113:230–6. [DOI] [PubMed]
- 14.Kesarwani K, Gupta R, Mukerjee A. Bioavailability enhancers of herbal origin: an overview. Asian Pac J Trop Biomed. 2013;3(4):253–266. doi: 10.1016/S2221-1691(13)60060-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marques HMC. A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Fragr J. 2010;25(5):313–326. [Google Scholar]
- 16.Xu J, Zhou F, Ji BP, Pei RS, Xu N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett Appl Microbiol. 2008;47(3):174–179. doi: 10.1111/j.1472-765X.2008.02407.x. [DOI] [PubMed] [Google Scholar]
- 17.Rahman MA, Harwansh R, Mirza MA, Hussain S, Hussain A. Oral lipid based drug delivery system (LBDDS): formulation, characterization and application: a review. Curr Drug Deliv. 2011;8(4):330–345. doi: 10.2174/156720111795767906. [DOI] [PubMed] [Google Scholar]
- 18.Piran P, Kafil HS, Ghanbarzadeh S, Safdari R, Hamishehkar H. Formulation of menthol-loaded nanostructured lipid carriers to enhance its antimicrobial activity for food preservation. Adv Pharm Bull. 2017;7(2):261. doi: 10.15171/apb.2017.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ansari S, Sameem M, Islam F. Influence of nanotechnology on herbal drugs: a review. J Adv Pharm Technol Res. 2012;3(3):142. doi: 10.4103/2231-4040.101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou CW, Batnyam O, Hung HS, Harn HJ, Lee WF, Lin HR, et al. Highly bioavailable anticancer herbal-loaded nanocarriers for use against breast and colon cancer in vitro and in vivo systems. Polym Chem. 2013;4(6):2040–52.
- 21.Dixit N, Vaibhav K, Pandey RS, Jain UK, Katare OP, Katyal A, et al. Improved cisplatin delivery in cervical cancer cells by utilizing folate-grafted non-aggregated gelatin nanoparticles. Biomed Pharmacother. 2015;69:1–10. [DOI] [PubMed]
- 22.Das S, Das J, Samadder A, Paul A, Khuda-Bukhsh AR. Efficacy of PLGA-loaded apigenin nanoparticles in benzo[a]pyrene and ultraviolet-B induced skin cancer of mice: mitochondria mediated apoptotic signalling cascades. Food Chem Toxicol. 2013;62:670–680. doi: 10.1016/j.fct.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 23.Kumari A, Yadav SK, Pakade YB, Singh B, Yadav SC. Development of biodegradable nanoparticles for delivery of quercetin. Colloids Surf B: Biointerfaces. 2010;80(2):184–192. doi: 10.1016/j.colsurfb.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Bala I, Bhardwaj V, Hariharan S, Kharade SV, Roy N, Ravi Kumar MN. Sustained release nanoparticulate formulation containing antioxidant-ellagic acid as potential prophylaxis system for oral administration. J Drug Target. 2006;14(1):27–34. doi: 10.1080/10611860600565987. [DOI] [PubMed] [Google Scholar]
- 25.Tong-Un T, Wannanon P, Wattanatho J, Phachonpai W. Quercetin liposomes via nasal administration reduce anxiety and depression-like behaviors and enhance cognitive performances in rats. Am J Pharmacol Toxicol. 2010;5(2):80–88. [Google Scholar]
- 26.Kristl J, Teskač K, Caddeo C, Abramović Z, Šentjurc M. Improvements of cellular stress response on resveratrol in liposomes. Eur J Pharm Biopharm. 2009;73(2):253–259. doi: 10.1016/j.ejpb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Kumari A, Yadav SK, Pakade YB, Kumar V, Singh B, Chaudhary A, et al. Nanoencapsulation and characterization of Albizia chinensis isolated antioxidant quercitrin on PLA nanoparticles. Colloids Surf B: Biointerfaces. 2011;82(1):224–32. [DOI] [PubMed]
- 28.Sanna V, Lubinu G, Madau P, Pala N, Nurra S, Mariani A, et al. Polymeric nanoparticles encapsulating white tea extract for nutraceutical application. J Agric Food Chem. 2015;63(7):2026–32. [DOI] [PubMed]
- 29.Liang J, Li F, Fang Y, Yang W, An X, Zhao L, et al. Synthesis, characterization and cytotoxicity studies of chitosan-coated tea polyphenols nanoparticles. Colloids Surf B: Biointerfaces. 2011;82(2):297–301. [DOI] [PubMed]
- 30.Siddiqui IA, Adhami VM, Bharali DJ, Hafeez BB, Asim M, Khwaja SI, et al. Introducing Nanochemoprevention as a novel approach for cancer control: proof of principle with green tea polyphenol Epigallocatechin-3-Gallate. Cancer Res. 2009;69(5):1712–6. [DOI] [PMC free article] [PubMed]
- 31.Dehkharghanian M, Lacroix M, Vijayalakshmi MA. Antioxidant properties of green tea polyphenols encapsulated in caseinate beads. Dairy Sci Technol. 2009;89(5):485–499. [Google Scholar]
- 32.Fachriyah E. Cinnamomum casia extract encapsulated Nanochitosan as Antihypercholesterol. IOP Conf Ser Mater Sci Eng. 2017;172:012035. [Google Scholar]
- 33.Hill LE, Taylor TM, Gomes C. Antimicrobial efficacy of poly (DL-lactide-co-glycolide) (PLGA) nanoparticles with entrapped cinnamon bark extract against Listeria monocytogenes and Salmonella typhimurium. J Food Sci. 2013;78(4):N626–N632. doi: 10.1111/1750-3841.12069. [DOI] [PubMed] [Google Scholar]
- 34.Tiwari S, Gupta R. Development of herbal biodegradable polymeric nanoparticle from Clerodendrum infortunatum L. J Bionanoscience. 2013;7(4):341–347. [Google Scholar]
- 35.Jahan N, Aslam S, Rahman KU, Fazal T, Anwar F, Saher R. Formulation and characterisation of nanosuspension of herbal extracts for enhanced antiradical potential. J Exp Nanosci. 2016;11(1):72–80. [Google Scholar]
- 36.Kumari P, Swami MO, Nadipalli SK, Myneni S, Ghosh B, Biswas S. Curcumin delivery by poly(Lactide)-based co-polymeric micelles: An in vitro anticancer study. Pharm Res. 2016;33(4):826–841. doi: 10.1007/s11095-015-1830-z. [DOI] [PubMed] [Google Scholar]
- 37.Agrawal R, Kaur IP. Inhibitory effect of encapsulated curcumin on ultraviolet-induced Photoaging in mice. Rejuvenation Res. 2010;13(4):397–410. doi: 10.1089/rej.2009.0906. [DOI] [PubMed] [Google Scholar]
- 38.Lee JS, Kim GH, Lee HG. Characteristics and antioxidant activity of Elsholtzia splendens extract-loaded nanoparticles. J Agric Food Chem. 2010;58(6):3316–3321. doi: 10.1021/jf904091d. [DOI] [PubMed] [Google Scholar]
- 39.Bhattacharyya SS, Paul S, Khuda-Bukhsh AR. Encapsulated plant extract ( Gelsemium sempervirens ) poly (lactide-co-glycolide) nanoparticles enhance cellular uptake and increase bioactivity in vitro. Exp Biol Med. 2010;235(6):678–688. doi: 10.1258/ebm.2010.009338. [DOI] [PubMed] [Google Scholar]
- 40.Pereira MC, Hill LE, Zambiazi RC, Mertens-Talcott S, Talcott S, Gomes CL. Nanoencapsulation of hydrophobic phytochemicals using poly (dl-lactide-co-glycolide) (PLGA) for antioxidant and antimicrobial delivery applications: Guabiroba fruit (Campomanesia xanthocarpa O. berg) study. LWT Food Sci Technol. 2015;63(1):100–107. [Google Scholar]
- 41.Dong P, Wang X, Gu Y, Wang Y, Wang Y, Gong C, et al. Self-assembled biodegradable micelles based on star-shaped PCL-b-PEG copolymers for chemotherapeutic drug delivery. Colloids Surfaces A Physicochem Eng Asp. 2010;358(1–3):128–34.
- 42.Gortzi O, Lalas S, Chinou I, Tsaknis J. Reevaluation of bioactivity and antioxidant activity of Myrtus communis extract before and after encapsulation in liposomes. Eur Food Res Technol. 2008;226(3):583–590. [Google Scholar]
- 43.Kesente M, Kavetsou E, Roussaki M, Blidi S, Loupassaki S, Chanioti S, et al. Encapsulation of olive leaves extracts in biodegradable PLA nanoparticles for use in cosmetic formulation. Bioeng. 2017;4(3):75. [DOI] [PMC free article] [PubMed]
- 44.Strasser M, Noriega P, Löbenberg R, Bou-Chacra N, Bacchi EM. Antiulcerogenic potential activity of free and nanoencapsulated Passiflora serratodigitata L. extracts. Biomed Res Int. 2014;2014. [DOI] [PMC free article] [PubMed]
- 45.Choi W, No RH, Kwon HS, Lee HY. Enhancement of skin anti-inflammatory activities of Scutellaria baicalensis extract using a nanoencapsulation process. J Cosmet Laser Ther. 2014;16(6):271–278. doi: 10.3109/14764172.2014.946051. [DOI] [PubMed] [Google Scholar]
- 46.Samadder A, Das S, Das J, Paul A, Khuda-Bukhsh A. Ameliorative effects of Syzygium jambolanum extract and its poly (lactic-co-glycolic) acid Nano-encapsulated form on arsenic-induced hyperglycemic stress: a multi-parametric evaluation. J Acupunct Meridian Stud. 2012;5(6):310–318. doi: 10.1016/j.jams.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Gortzi O, Lalas S, Chinou I, Tsaknis J. Reevaluation of antimicrobial and antioxidant activity of thymus spp. extracts before and after encapsulation in liposomes. J Food Prot. 2006;69(12):2998–3005. doi: 10.4315/0362-028x-69.12.2998. [DOI] [PubMed] [Google Scholar]
- 48.Chen MX, Li BK, Yin DK, Liang J, Li SS, Peng DY. Layer-by-layer assembly of chitosan stabilized multilayered liposomes for paclitaxel delivery. Carbohydr Polym. 2014;111:298–304. doi: 10.1016/j.carbpol.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 49.Bhattacharya S, Ghosh A. Phytosomes: the emerging technology for enhancement of bioavailability of botanicals and nutraceuticals. Int J Health Res. 2009;2(1):141–153. [Google Scholar]
- 50.Rajendran R, Radhai R, Kotresh TM, Csiszar E. Development of antimicrobial cotton fabrics using herb loaded nanoparticles. Carbohydr Polym. 2013;91(2):613–617. doi: 10.1016/j.carbpol.2012.08.064. [DOI] [PubMed] [Google Scholar]
- 51.Zheng X, Kan B, Gou M, Fu S, Zhang J, Men K, et al. Preparation of MPEG–PLA nanoparticle for honokiol delivery in vitro. Int J Pharm. 2010;386(1–2):262–7. [DOI] [PubMed]
- 52.Wu TH, Yen FL, Lin LT, Tsai TR, Lin CC, Cham TM. Preparation, physicochemical characterization, and antioxidant effects of quercetin nanoparticles. Int J Pharm. 2008;346(1–2):160–168. doi: 10.1016/j.ijpharm.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 53.Min KH, Park K, Kim YS, Bae SM, Lee S, Jo HG, et al. Hydrophobically modified glycol chitosan nanoparticles-encapsulated camptothecin enhance the drug stability and tumor targeting in cancer therapy. J Control Release. 2008;127(3):208–18. [DOI] [PubMed]
- 54.Mukerjee A, Vishwanatha JK. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res. 2009;29(10):3867–3875. [PubMed] [Google Scholar]
- 55.Das J, Das S, Samadder A, Bhadra K, Khuda-Bukhsh AR. Poly (lactide-co-glycolide) encapsulated extract of Phytolacca decandra demonstrates better intervention against induced lung adenocarcinoma in mice and on A549 cells. Eur J Pharm Sci. 2012;47(2):313–324. doi: 10.1016/j.ejps.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 56.Li Z, Wu X, Li J, Yao L, Sun L, Shi Y, et al. Antitumor activity of celastrol nanoparticles in a xenograft retinoblastoma tumor model. Int J Nanomedicine. 2012;7:2389. [DOI] [PMC free article] [PubMed]
- 57.Zhang J, Han X, Li X, Luo Y, Zhao H, Yang M, et al. Core-shell hybrid liposomal vesicles loaded with panax notoginsenoside: preparation, characterization and protective effects on global cerebral ischemia/reperfusion injury and acute myocardial ischemia in rats. Int J Nanomedicine. 2012;7:4299. [DOI] [PMC free article] [PubMed]
- 58.Han L, Fu Y, Cole AJ, Liu J, Wang J. Co-encapsulation and sustained-release of four components in ginkgo terpenes from injectable PELGE nanoparticles. Fitoterapia. 2012;83(4):721–731. doi: 10.1016/j.fitote.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 59.de Oliveira JK, Ronik DF, Ascari J, Mainardes RM, Khalil NM. A stability-indicating high performance liquid chromatography method to determine apocynin in nanoparticles. J Pharm Anal. 2017;7(2):129–133. doi: 10.1016/j.jpha.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aman RM, Abu Hashim II, Meshali MM. Novel chitosan-based solid-lipid nanoparticles to enhance the bio-residence of the miraculous phytochemical “Apocynin”. Eur J Pharm Sci. 2018;124:304–318. doi: 10.1016/j.ejps.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 61.Liu Z, Jiao Y, Wang Y, Zhou C, Zhang Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv Drug Deliv Rev. 2008;60(15):1650–1662. doi: 10.1016/j.addr.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 62.Esfandyari-Manesh M, Ghaedi Z, Asemi M, Khanavi M, Manayi A, Jamalifar H, et al. Study of antimicrobial activity of anethole and carvone loaded PLGA nanoparticles. J Pharm Res. 2013;7(4):290–5.
- 63.Sinico C, De Logu A, Lai F, Valenti D, Manconi M, Loy G, et al. Liposomal incorporation of Artemisia arborescens L. essential oil and in vitro antiviral activity. Eur J Pharm Biopharm. 2005;59(1):161–8. [DOI] [PubMed]
- 64.Ahmed J, Hiremath N, Jacob H. Antimicrobial efficacies of essential oils/nanoparticles incorporated polylactide films against L. monocytogenes and S. typhimurium on contaminated cheese. Int J Food Prop. 2017;20(1):53–67. [Google Scholar]
- 65.Hill LE, Gomes C, Taylor TM. Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. LWT Food Sci Technol. 2013;51(1):86–93. [Google Scholar]
- 66.Sotelo-Boyás M, Correa-Pacheco Z, Bautista-Baños S. Gómez y Gómez Y. release study and inhibitory activity of thyme essential oil-loaded chitosan nanoparticles and nanocapsules against foodborne bacteria. Int J Biol Macromol. 2017;103:409–414. doi: 10.1016/j.ijbiomac.2017.05.063. [DOI] [PubMed] [Google Scholar]
- 67.Ahmed J, Arfat YA, Bher A, Mulla M, Jacob H, Auras R. Active chicken meat packaging based on Polylactide films and bimetallic ag-cu nanoparticles and essential oil. J Food Sci. 2018;83(5):1299–1310. doi: 10.1111/1750-3841.14121. [DOI] [PubMed] [Google Scholar]
- 68.Karimirad R, Behnamian M, Dezhsetan S, Sonnenberg A. Chitosan nanoparticles loaded Citrus aurantium essential oil: a novel delivery system for preserving the postharvest quality of Agaricus bisporus. J Sci Food Agric. 2018;98(13):5112–5119. doi: 10.1002/jsfa.9050. [DOI] [PubMed] [Google Scholar]
- 69.Karimirad R, Behnamian M, Dezhsetan S. Development and characterization of nano biopolymer containing cumin oil as a new approach to enhance antioxidant properties of button mushroom. Int J Biol Macromol. 2018;113:662–668. doi: 10.1016/j.ijbiomac.2018.02.043. [DOI] [PubMed] [Google Scholar]
- 70.Natrajan D, Srinivasan S, Sundar K, Ravindran A. Formulation of essential oil-loaded chitosan–alginate nanocapsules. J Food Drug Anal. 2015;23(3):560–568. doi: 10.1016/j.jfda.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu WC, Huang DW, Wang CC, Yeh CH, Tsai JC, Huang YT, et al. Preparation, characterization, and antimicrobial activity of nanoemulsions incorporating citral essential oil. J Food Drug Anal. 2018;26(1):82–9. [DOI] [PMC free article] [PubMed]
- 72.Herculano ED, de Paula HC, de Figueiredo EA, Dias FG, Pereira VD. Physicochemical and antimicrobial properties of nanoencapsulated eucalyptus staigeriana essential oil. LWT Food Sci Technol. 2015;61(2):484–491. [Google Scholar]
- 73.Saporito F, Sandri G, Bonferoni MC, Rossi S, Boselli C, Cornaglia AI, et al. Essential oil-loaded lipid nanoparticles for wound healing. Int J Nanomedicine. 2017;13:175. [DOI] [PMC free article] [PubMed]
- 74.Fazly Bazzaz BS, Khameneh B, Namazi N, Iranshahi M, Davoodi D, Golmohammadzadeh S. Solid lipid nanoparticles carrying Eugenia caryophyllata essential oil: the novel nanoparticulate systems with broad-spectrum antimicrobial activity. Lett Appl Microbiol. 2018;66(6):506–513. doi: 10.1111/lam.12886. [DOI] [PubMed] [Google Scholar]
- 75.Flores FC, De Lima JA, Ribeiro RF, Alves SH, Rolim CM, Beck RC, et al. Antifungal activity of Nanocapsule suspensions containing tea tree oil on the growth of Trichophyton rubrum. Mycopathologia. 2013;175(3–4):281–6. [DOI] [PubMed]
- 76.Ge Y, Ge M. Development of tea tree oil-loaded liposomal formulation using response surface methodology. J Liposome Res. 2015;25(3):222–231. doi: 10.3109/08982104.2014.987786. [DOI] [PubMed] [Google Scholar]
- 77.Beyki M, Zhaveh S, Khalili ST, Rahmani-Cherati T, Abollahi A, Bayat M, et al. Encapsulation of Mentha piperita essential oils in chitosan–cinnamic acid nanogel with enhanced antimicrobial activity against aspergillus flavus. Ind Crop Prod. 2014;54:310–9.
- 78.Liolios CC, Gortzi O, Lalas S, Tsaknis J, Chinou I. Liposomal incorporation of carvacrol and thymol isolated from the essential oil of Origanum dictamnus L. and in vitro antimicrobial activity. Food Chem. 2009;112(1):77–83. [Google Scholar]
- 79.Ghosh V, Mukherjee A, Chandrasekaran N. Eugenol-loaded antimicrobial nanoemulsion preserves fruit juice against, microbial spoilage. Colloids Surf B: Biointerfaces. 2014;114:392–397. doi: 10.1016/j.colsurfb.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 80.Engel JB, Heckler C, Tondo EC, Daroit DJ, da Silva Malheiros P. Antimicrobial activity of free and liposome-encapsulated thymol and carvacrol against salmonella and Staphylococcus aureus adhered to stainless steel. Int J Food Microbiol. 2017;252:18–23. doi: 10.1016/j.ijfoodmicro.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 81.Keawchaoon L, Yoksan R. Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf B: Biointerfaces. 2011;84(1):163–171. doi: 10.1016/j.colsurfb.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 82.Wattanasatcha A, Rengpipat S, Wanichwecharungruang S. Thymol nanospheres as an effective anti-bacterial agent. Int J Pharm. 2012;434(1–2):360–365. doi: 10.1016/j.ijpharm.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 83.Adhavan P, Kaur G, Princy A, Murugan R. Essential oil nanoemulsions of wild patchouli attenuate multi-drug resistant gram-positive, gram-negative and Candida albicans. Ind Crop Prod. 2017;100:106–116. [Google Scholar]
- 84.Marchiori MCL, Rigon C, Camponogara C, Oliveira SM, Cruz L. Hydrogel containing silibinin-loaded pomegranate oil based nanocapsules exhibits anti-inflammatory effects on skin damage UVB radiation-induced in mice. J Photochem Photobiol B Biol. 2017;170:25–32. doi: 10.1016/j.jphotobiol.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 85.Gharenaghadeh S, Karimi N, Forghani S, Nourazarian M, Gharehnaghadeh S, Kafil HS. Application of Salvia multicaulis essential oil-containing nanoemulsion against food-borne pathogens. Food Biosci. 2017;19:128–133. [Google Scholar]
- 86.Feyzioglu GC, Tornuk F. Development of chitosan nanoparticles loaded with summer savory (Satureja hortensis L.) essential oil for antimicrobial and antioxidant delivery applications. LWT Food Sci Technol. 2016;70:104–110. [Google Scholar]
- 87.Majeed H, Liu F, Hategekimana J, Sharif HR, Qi J, Ali B, et al. Bactericidal action mechanism of negatively charged food grade clove oil nanoemulsions. Food Chem. 2016;197:75–83. [DOI] [PubMed]
- 88.Moghimi R, Ghaderi L, Rafati H, Aliahmadi A, McClements DJ. Superior antibacterial activity of nanoemulsion of thymus daenensis essential oil against E. coli. Food Chem. 2016;194:410–415. doi: 10.1016/j.foodchem.2015.07.139. [DOI] [PubMed] [Google Scholar]
- 89.Khalili ST, Mohsenifar A, Beyki M, Zhaveh S, Rahmani-Cherati T, Abdollahi A, et al. Encapsulation of thyme essential oils in chitosan-benzoic acid nanogel with enhanced antimicrobial activity against aspergillus flavus. LWT Food Sci Technol. 2015;60(1):502–8.
- 90.Asprea M, Leto I, Bergonzi MC, Bilia AR. Thyme essential oil loaded in nanocochleates: encapsulation efficiency, in vitro release study and antioxidant activity. LWT. 2017;77:497–502. [Google Scholar]
- 91.Detoni CB, Cabral-Albuquerque ECM, Hohlemweger SVA, Sampaio C, Barros TF, Velozo ES. Essential oil from Zanthoxylum tingoassuiba loaded into multilamellar liposomes useful as antimicrobial agents. J Microencapsul. 2009;26(8):684–691. doi: 10.1080/02652040802661887. [DOI] [PubMed] [Google Scholar]
- 92.Woranuch S, Yoksan R. Eugenol-loaded chitosan nanoparticles: I. thermal stability improvement of eugenol through encapsulation. Carbohydr Polym. 2013;96(2):578–585. doi: 10.1016/j.carbpol.2012.08.117. [DOI] [PubMed] [Google Scholar]
- 93.Yang FL, Li XG, Zhu F, Lei CL. Structural characterization of nanoparticles loaded with garlic essential oil and their insecticidal activity against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) J Agric Food Chem. 2009;57(21):10156–10162. doi: 10.1021/jf9023118. [DOI] [PubMed] [Google Scholar]
- 94.Li KK, Yin SW, Yang XQ, Tang CH, Wei ZH. Fabrication and characterization of novel antimicrobial films derived from thymol-loaded Zein–sodium Caseinate (SC) nanoparticles. J Agric Food Chem. 2012;60(46):11592–11600. doi: 10.1021/jf302752v. [DOI] [PubMed] [Google Scholar]
- 95.Wen P, Zhu DH, Wu H, Zong MH, Jing YR, Han SY. Encapsulation of cinnamon essential oil in electrospun nanofibrous film for active food packaging. Food Control. 2016;59:366–376. [Google Scholar]
- 96.Biddeci G, Cavallaro G, Di Blasi F, Lazzara G, Massaro M, Milioto S, et al. Halloysite nanotubes loaded with peppermint essential oil as filler for functional biopolymer film. Carbohydr Polym. 2016;152:548–57. [DOI] [PubMed]
- 97.Feng N, Zhao JH, Liu Y, Wang Z, Zhang YT, Feng NP. Preparation and characterization of solid lipid nanoparticles loaded with frankincense and myrrh oil. Int J Nanomedicine. 2012;7:2033. doi: 10.2147/IJN.S30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mohammadi A, Hashemi M, Hosseini SM. Nanoencapsulation of Zataria multiflora essential oil preparation and characterization with enhanced antifungal activity for controlling Botrytis cinerea, the causal agent of gray mould disease. Innov Food Sci Emerg Technol. 2015;28:73–80. [Google Scholar]
- 99.Mukunthan KS, Balaji S. Cashew apple juice ( Anacardium occidentale L.) speeds up the synthesis of silver nanoparticles. Int J Green Nanotechnol. 2012;4(2):71–79. [Google Scholar]
- 100.Beg M, Maji A, Mandal AK, Das S, Aktara MN, Jha PK, et al. Green synthesis of silver nanoparticles using Pongamia pinnata seed: characterization, antibacterial property, and spectroscopic investigation of interaction with human serum albumin. J Mol Recognit. 2017;30(1):e2565. [DOI] [PubMed]
- 101.Nagajyothi PC, Muthuraman P, Sreekanth TV, Kim DH, Shim J. Green synthesis: in-vitro anticancer activity of copper oxide nanoparticles against human cervical carcinoma cells. Arab J Chem. 2017;10(2):215–225. [Google Scholar]
- 102.Krishnaraj C, Jagan EG, Rajasekar S, Selvakumar P, Kalaichelvan PT, Mohan N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf B: Biointerfaces. 2010;76(1):50–56. doi: 10.1016/j.colsurfb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 103.Rastogi L, Arunachalam J. Sunlight based irradiation strategy for rapid green synthesis of highly stable silver nanoparticles using aqueous garlic (Allium sativum) extract and their antibacterial potential. Mater Chem Phys. 2011;129(1–2):558–563. [Google Scholar]
- 104.Deyá C, Bellotti N. Biosynthesized silver nanoparticles to control fungal infections in indoor environments. Adv Nat Sci Nanosci Nanotechnol. 2017;8(2):025005. [Google Scholar]
- 105.Niraimathi KL, Sudha V, Lavanya R, Brindha P. Biosynthesis of silver nanoparticles using Alternanthera sessilis (Linn.) extract and their antimicrobial, antioxidant activities. Colloids Surf B: Biointerfaces. 2013;102:288–291. doi: 10.1016/j.colsurfb.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 106.Anbuvannan M, Ramesh M, Viruthagiri G, Shanmugam N, Kannadasan N. Anisochilus carnosus leaf extract mediated synthesis of zinc oxide nanoparticles for antibacterial and photocatalytic activities. Mater Sci Semicond Process. 2015;39:621–628. [Google Scholar]
- 107.Jagtap UB, Bapat VA. Green synthesis of silver nanoparticles using Artocarpus heterophyllus lam. Seed extract and its antibacterial activity. Ind Crop Prod. 2013;46:132–137. [Google Scholar]
- 108.Bhuyan T, Mishra K, Khanuja M, Prasad R, Varma A. Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater Sci Semicond Process. 2015;32:55–61. [Google Scholar]
- 109.Jeeva K, Thiyagarajan M, Elangovan V, Geetha N, Venkatachalam P. Caesalpinia coriaria leaf extracts mediated biosynthesis of metallic silver nanoparticles and their antibacterial activity against clinically isolated pathogens. Ind Crop Prod. 2014;52:714–720. [Google Scholar]
- 110.Baskaralingam V, Sargunar CG, Lin YC, Chen JC. Green synthesis of silver nanoparticles through Calotropis gigantea leaf extracts and evaluation of antibacterial activity against vibrio alginolyticus. Nanotechnol Dev. 2012;2(1):e3–3.
- 111.Marimuthu S, Rahuman AA, Jayaseelan C, Kirthi AV, Santhoshkumar T, Velayutham K, et al. Acaricidal activity of synthesized titanium dioxide nanoparticles using Calotropis gigantea against Rhipicephalus microplus and Haemaphysalis bispinosa. Asian Pac J Trop Med. 2013;6(9):682–8. [DOI] [PubMed]
- 112.Venkatachalam M, Govindaraju K, Mohamed Sadiq A, Tamilselvan S, Ganesh Kumar V, Singaravelu G. Functionalization of gold nanoparticles as antidiabetic nanomaterial. Spectrochim Acta Part A Mol Biomol Spectrosc. 2013;116:331–8. [DOI] [PubMed]
- 113.Suresh D, Nethravathi PC, Udayabhanu RH, Nagabhushana H, Sharma SC. Green synthesis of multifunctional zinc oxide (ZnO) nanoparticles using Cassia fistula plant extract and their photodegradative, antioxidant and antibacterial activities. Mater Sci Semicond Process. 2015;31:446–454. [Google Scholar]
- 114.Saravanakumar A, Ganesh M, Jayaprakash J, Jang HT. Biosynthesis of silver nanoparticles using Cassia tora leaf extract and its antioxidant and antibacterial activities. J Ind Eng Chem. 2015;28:277–281. [Google Scholar]
- 115.Ponarulselvam S, Panneerselvam C, Murugan K, Aarthi N, Kalimuthu K, Thangamani S. Synthesis of silver nanoparticles using leaves of Catharanthus roseus Linn. G. Don and their antiplasmodial activities. Asian Pac J Trop Biomed. 2012;2(7):574–580. doi: 10.1016/S2221-1691(12)60100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nagarajan S, Arumugam Kuppusamy K. Extracellular synthesis of zinc oxide nanoparticle using seaweeds of gulf of Mannar, India. J Nanobiotechnology. 2013;11(1):39. doi: 10.1186/1477-3155-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Barbinta-Patrascu ME, Badea N, Ungureanu C, Constantin M, Pirvu C, Rau I. Silver-based biohybrids “green” synthesized from Chelidonium majus L. Opt Mater. 2016;56:94–99. [Google Scholar]
- 118.Smitha SL, Gopchandran KG. Surface enhanced Raman scattering, antibacterial and antifungal active triangular gold nanoparticles. Spectrochim Acta Part A Mol Biomol Spectrosc. 2013;102:114–119. doi: 10.1016/j.saa.2012.09.055. [DOI] [PubMed] [Google Scholar]
- 119.K S, S G, T R, T B. Biomedical potential of silver nanoparticles synthesized from calli cells of Citrullus colocynthis (L.) Schrad. J Nanobiotechnology. 2011;9(1):43. doi: 10.1186/1477-3155-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mariselvam R, Ranjitsingh AJ, Usha Raja Nanthini A, Kalirajan K, Padmalatha C, Mosae Selvakumar P. Green synthesis of silver nanoparticles from the extract of the inflorescence of Cocos nucifera (family: Arecaceae) for enhanced antibacterial activity. Spectrochim Acta Part A Mol Biomol Spectrosc. 2014;129:537–541. doi: 10.1016/j.saa.2014.03.066. [DOI] [PubMed] [Google Scholar]
- 121.Nagajyothi PC, Sreekanth TV, Tettey CO, Jun YI, Mook SH. Characterization, antibacterial, antioxidant, and cytotoxic activities of ZnO nanoparticles using Coptidis Rhizoma. Bioorg Med Chem Lett. 2014;24(17):4298–4303. doi: 10.1016/j.bmcl.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 122.Wang C, Mathiyalagan R, Kim YJ, Castro-Aceituno V, Singh P, Ahn S, et al. Rapid green synthesis of silver and gold nanoparticles using Dendropanax morbifera leaf extract and their anticancer activities. Int J Nanomedicine. 2016;11:3691. [DOI] [PMC free article] [PubMed]
- 123.Karuppaiya P, Satheeshkumar E, Chao WT, Kao LY, Chen EC, Tsay HS. Anti-metastatic activity of biologically synthesized gold nanoparticles on human fibrosarcoma cell line HT-1080. Colloids Surf B: Biointerfaces. 2013;110:163–170. doi: 10.1016/j.colsurfb.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 124.Arumugam A, Karthikeyan C, Haja Hameed AS, Gopinath K, Gowri S, Karthika V. Synthesis of cerium oxide nanoparticles using Gloriosa superba L. leaf extract and their structural, optical and antibacterial properties. Mater Sci Eng C. 2015;49:408–415. doi: 10.1016/j.msec.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 125.Rajendran A, Siva E, Dhanraj C, Senthilkumar S. A green and facile approach for the synthesis copper oxide nanoparticles using Hibiscus rosa-sinensis flower extracts and It’s antibacterial activities. J Bioprocess Biotech. 2018;08(3):324. [Google Scholar]
- 126.Bala N, Saha S, Chakraborty M, Maiti M, Das S, Basu R, et al. Green synthesis of zinc oxide nanoparticles using hibiscus subdariffa leaf extract: effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 2015;5(7):4993–5003.
- 127.Jayandran M, Haneefa MM, Balasubramanian V. Green synthesis of copper nanoparticles using natural reducer and stabilizerand an evaluation of antimicrobial activity. J Chem Pharm Res. 2015;7(2):251–259. [Google Scholar]
- 128.Lee HJ, Song JY, Kim BS. Biological synthesis of copper nanoparticles using Magnolia kobus leaf extract and their antibacterial activity. J Chem Technol Biotechnol. 2013;88(11):1971–1977. [Google Scholar]
- 129.MubarakAli D, Thajuddin N, Jeganathan K, Gunasekaran M. Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf B: Biointerfaces. 2011;85(2):360–365. doi: 10.1016/j.colsurfb.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 130.Prakash P, Gnanaprakasam P, Emmanuel R, Arokiyaraj S, Saravanan M. Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. For enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf B: Biointerfaces. 2013;108:255–259. doi: 10.1016/j.colsurfb.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 131.Bankar A, Joshi B, Ravi Kumar A, Zinjarde S. Banana peel extract mediated synthesis of gold nanoparticles. Colloids Surf B: Biointerfaces. 2010;80(1):45–50. doi: 10.1016/j.colsurfb.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 132.Vijayakumar S, Vaseeharan B, Malaikozhundan B, Gopi N, Ekambaram P, Pachaiappan R, et al. Therapeutic effects of gold nanoparticles synthesized using Musa paradisiaca peel extract against multiple antibiotic resistant enterococcus faecalis biofilms and human lung cancer cells (A549). Microb Pathog. 2017;102:173–83. [DOI] [PubMed]
- 133.Gopinath M, Subbaiya R, Selvam MM, Suresh D. Synthesis of copper nanoparticles from Nerium oleander leaf aqueous extract and its antibacterial activity. Int J Curr Microbiol App Sci. 2014;3(9):814–818. [Google Scholar]
- 134.Gogoi N, Babu PJ, Mahanta C, Bora U. Green synthesis and characterization of silver nanoparticles using alcoholic flower extract of Nyctanthes arbortristis and in vitro investigation of their antibacterial and cytotoxic activities. Mater Sci Eng C. 2015;46:463–469. doi: 10.1016/j.msec.2014.10.069. [DOI] [PubMed] [Google Scholar]
- 135.Jain S, Mehata MS. Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci Rep. 2017;7(1):15867. doi: 10.1038/s41598-017-15724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rajiv P, Rajeshwari S, Venckatesh R. Bio-fabrication of zinc oxide nanoparticles using leaf extract of Parthenium hysterophorus L. and its size-dependent antifungal activity against plant fungal pathogens. Spectrochim Acta Part A Mol Biomol Spectrosc. 2013;112:384–387. doi: 10.1016/j.saa.2013.04.072. [DOI] [PubMed] [Google Scholar]
- 137.Caroling G, Vinodhini E, Ranjitham AM, Shanthi P. Biosynthesis of copper nanoparticles using aqueous Phyllanthus Embilica (gooseberry) extract-characterisation and study of antimicrobial effects. Int J Nanomater Chem. 2015;1(2):53–63. [Google Scholar]
- 138.Nayak D, Ashe S, Rauta PR, Kumari M, Nayak B. Bark extract mediated green synthesis of silver nanoparticles: evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Mater Sci Eng C. 2016;58:44–52. doi: 10.1016/j.msec.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 139.Geetha R, Ashokkumar T, Tamilselvan S, Govindaraju K, Sadiq M, Singaravelu G. Green synthesis of gold nanoparticles and their anticancer activity. Cancer Nanotechnol. 2013;4(4):91–98. doi: 10.1007/s12645-013-0040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Iram F, Iqbal MS, Athar MM, Saeed MZ, Yasmeen A, Ahmad R. Glucoxylan-mediated green synthesis of gold and silver nanoparticles and their phyto-toxicity study. Carbohydr Polym. 2014;104:29–33. doi: 10.1016/j.carbpol.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 141.Saratale RG, Benelli G, Kumar G, Kim DS, Saratale GD. Bio-fabrication of silver nanoparticles using the leaf extract of an ancient herbal medicine, dandelion (Taraxacum officinale), evaluation of their antioxidant, anticancer potential, and antimicrobial activity against phytopathogens. Environ Sci Pollut Res. 2018;25(11):10392–10406. doi: 10.1007/s11356-017-9581-5. [DOI] [PubMed] [Google Scholar]
- 142.Bhakya S, Muthukrishnan S, Sukumaran M, Grijalva M, Cumbal L, Benjamin JF, et al. Antimicrobial, antioxidant and anticancer activity of biogenic silver nanoparticles – an experimental report. RSC Adv. 2016;6(84):81436–46.
- 143.Padil VV, Černík M. Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int J Nanomedicine. 2013;8:889. doi: 10.2147/IJN.S40599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Harne S, Sharma A, Dhaygude M, Joglekar S, Kodam K, Hudlikar M. Novel route for rapid biosynthesis of copper nanoparticles using aqueous extract of Calotropis procera L. latex and their cytotoxicity on tumor cells. Colloids Surf B: Biointerfaces. 2012;95:284–288. doi: 10.1016/j.colsurfb.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 145.Jamdagni P, Khatri P, Rana JS. Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J King Saud Univ - Sci. 2018;30(2):168–175. [Google Scholar]
- 146.Jha AK, Prasad K. Biosynthesis of gold nanoparticles using common aromatic plants. Int J Green Nanotechnol. 2012;4(3):219–224. [Google Scholar]
- 147.Alsalhi MS, Devanesan S, Alfuraydi AA, Vishnubalaji R, Munusamy MA, Murugan K, et al. Green synthesis of silver nanoparticles using Pimpinella anisum seeds: antimicrobial activity and cytotoxicity on human neonatal skin stromal cells and colon cancer cells. Int J Nanomedicine. 2016;11:4439. [DOI] [PMC free article] [PubMed]
- 148.Thanighaiarassu RR, Sivamai P, Devika R, Nambikkairaj B. Green synthesis of gold nanoparticles characterization by using plant essential oil Menthapiperita and their antifungal activity against human pathogenic fungi. J Nanomed Nanotechnol. 2014;5(5):1. [Google Scholar]
- 149.Kung ML, Lin PY, Hsieh CW, Tai MH, Wu DC, Kuo CH, et al. Bifunctional peppermint oil nanoparticles for antibacterial activity and fluorescence imaging. ACS Sustain Chem Eng. 2014;2(7):1769–75.
- 150.Mohanta YK, Panda SK, Jayabalan R, Sharma N, Bastia AK, Mohanta TK. Antimicrobial, antioxidant and cytotoxic activity of silver nanoparticles synthesized by leaf extract of Erythrina suberosa (Roxb.) Front Mol Biosci. 2017;4:14. doi: 10.3389/fmolb.2017.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kokila T, Ramesh PS, Geetha D. Biosynthesis of AgNPs using Carica Papaya peel extract and evaluation of its antioxidant and antimicrobial activities. Ecotoxicol Environ Saf. 2016;134:467–473. doi: 10.1016/j.ecoenv.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 152.Bhat R, Sharanabasava VG, Deshpande R, Shetti U, Sanjeev G, Venkataraman A. Photo-bio-synthesis of irregular shaped functionalized gold nanoparticles using edible mushroom Pleurotus Florida and its anticancer evaluation. J Photochem Photobiol B Biol. 2013;125:63–69. doi: 10.1016/j.jphotobiol.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 153.He W, Cai J, Jiang X, Yin JJ, Meng Q. Generation of reactive oxygen species and charge carriers in plasmonic photocatalytic au@TiO 2 nanostructures with enhanced activity. Phys Chem Chem Phys. 2018;20(23):16117–16125. doi: 10.1039/c8cp01978a. [DOI] [PubMed] [Google Scholar]
- 154.Farooq MU, Novosad V, Rozhkova EA, Wali H, Ali A, Fateh AA, et al. Gold nanoparticles-enabled efficient dual delivery of anticancer therapeutics to HeLa cells. Sci Rep. 2018;8(1):2907. [DOI] [PMC free article] [PubMed] [Retracted]
- 155.van der Zande M, Vandebriel RJ, Van Doren E, Kramer E, Herrera Rivera Z, Serrano-Rojero CS, et al. Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day Oral exposure. ACS Nano. 2012;6(8):7427–42. [DOI] [PubMed]
- 156.Sahu SC, Zheng J, Graham L, Chen L, Ihrie J, Yourick JJ, et al. Comparative cytotoxicity of nanosilver in human liver HepG2 and colon Caco2 cells in culture. J Appl Toxicol. 2014;34:1155–66. [DOI] [PubMed]
- 157.Tippayawat P, Phromviyo N, Boueroy P, Chompoosor A. Green synthesis of silver nanoparticles in aloe vera plant extract prepared by a hydrothermal method and their synergistic antibacterial activity. PeerJ. 2016;4:e2589. doi: 10.7717/peerj.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Roy N, Gaur A, Jain A, Bhattacharya S, Rani V. Green synthesis of silver nanoparticles: An approach to overcome toxicity. Environ Toxicol Pharmacol. 2013;36(3):807–812. doi: 10.1016/j.etap.2013.07.005. [DOI] [PubMed] [Google Scholar]