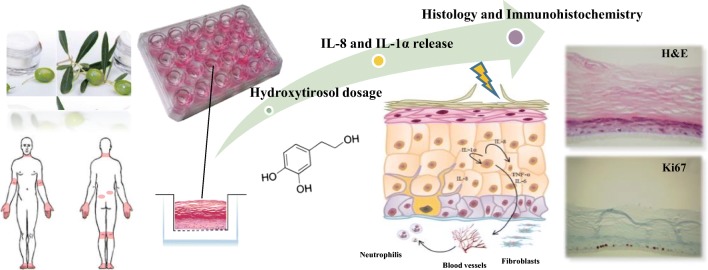
Abstract
Background
Atopic dermatitis is a multifactorial immune-mediated skin disorder characterized by an alteration of epidermal barrier function and onset of skin lesions, which range from mild erythema to severe lichenification. Treatment consists in hydration with possible use of topical or immunomodulatory corticosteroids, which, however sometimes showed side effects. Recently, the interest in natural compounds has grown significantly and among these, hydroxytyrosol (HT) plays a pivotal role due to its strong and well-known anti-inflammatory activity.
Objectives
The aim of this study was to investigate the safety and efficacy of Fenolia® Eudermal Cream 15 (HT-based formulation) on epidermal barrier impaired as consequence of skin injury.
Methods
Whit this purpose, morphologic and structural as well as anti-inflammatory evaluations, after treatment with pro-inflammatory mediators (PBS 1 X and LPS) and HT-based formulation on reconstructed human epidermis (RHE) were carried out by qualitative (hematoxylin/eosin- and immunostaining) and quantitative (MTT assay, IL-1α and IL-8 release by ELISA) techniques. Furthermore, HT absorption through the epidermal barrier was evaluated by RP-LC-DAD analysis.
Results
A rise in the thickness of the epidermis as well as an appropriate maturation and protein expression (Loricrin, Fillagrin, E-Cadherin and Cytokeratins 5&6) were detected in treated RHE samples. In particular, the HT-based formulation was found to stimulate cell proliferation, as evidenced by the significant increase in Ki67 expression, which suggests the involvement of repair mechanisms, increasing epithelial regeneration and differentiation and improving the epidermal barrier effect. Furthermore, HT-based formulation showed a statistically significant anti-inflammatory activity by reducing both IL-1α and IL-8 release by RHE tissues, greater than the reference drug dexamethasone. Finally, excellent transcutaneous absorption values were found for HT, demonstrating how this new formulation increases the availability of the bioactive compound.
Conclusions
In light of these results, Fenolia® Eudermal Cream 15 could be an effective agent to counteract atopic dermatitis.
Graphical abstract.
Safety and efficacy of hydroxytyrosol-based formulation on skin inflammation: in vitro evaluation on reconstructed human epidermis model
Keywords: Atopic dermatitis, Semi-solid formulation, Hydroxytyrosol, In vitro reconstructed human epidermis, Anti-inflammatory effect, Histology and immunohistochemistry
Introduction
Atopic dermatitis (AD) is the most prevalent chronic inflammatory disease in childhood, with an incidence of about 20% [1]. Although it usually reverts spontaneously during adolescence, it sometimes persists in adulthood (1–3%) and in some cases even in the elderly [2–4]. The incidence in industrialized countries is constantly increasing [5]. AD presents a chronic relapsing course and a significant influence on patients’ quality of life [6]. Diagnosis is usually clinical, even though the traditional criteria used for children are in some cases not applicable to adults [4]. Furthermore, up to date, its physiopathology has not been fully clarified [7, 8]. Usually, topical treatment, including corticosteroids and calcineurin inhibitors as well as emollient agents, is sufficient to obtain a good remission [7]. In more severe cases, additional systemic treatment is needed [9]. Cyclosporine A, methotrexate and azathioprine as well as phototherapy are the mainstay for chronic treatment, whereas corticosteroids are prescribed only for acute exacerbations [7].
Recently, the role of some cytokines involved in the onset of AD has been better clarified [10] leading to the introduction of new drugs, in particular biological agents (e.g. dupilumab), which act as mediators of the inflammatory response [7, 9, 11, 12].
In order to avoid treatment with conventional drugs, whose effectiveness is sometimes controversial and may cause side effects, researchers have recently focused their attention on new formulations based on natural substances [9]. In particular, plants and their derived-products, already used in traditional medicine to counteract several skin disorders, have been explored by studying the biological properties of one or more representative molecules [13].
Olea europaea L. is one of the oldest cultivated trees on earth [14]. Its fruit is mostly destined to olive oil production, but it is also an important health-promoting factor in the Mediterranean diet, having a several-century long folk medicine tradition [15]. Since remote times, olive oil has been used for medicinal and cosmetic purposes, e.g. by ancient Egyptians to make creams and perfumes as well as by Romans to keep the skin elastic after bathing [16]. Furthermore, in the Middle ages, physician monks used it to make lotions for the treatment of burns and skin infections [17].
Olive and olive oil are very rich sources of polyphenols with interesting biological properties [18–20]. Medicinal properties include prevention of cardiovascular diseases, metabolic syndrome, cancer, anti-inflammatory effects and wound healing [21, 22]. In addition, both topical and dietary use of olive oil are known to exert preventive action against skin ailments [16]. Beneficial effects for human health are mainly due to the major secondary metabolite oleuropein, a heterosidic ester of β-glycosylated eleanolic acid and hydroxytyrosol (HT), and to other phenolics such as HT itself [18].
Lately, the latter one arousing a lot of interest due to its potent antioxidant and free-radical scavenging properties as well as for its strong anti-inflammatory activity [23]. Furthermore, it possess a strong antimicrobial capacity by inhibiting the growth rate of several bacteria strains in humans [23] which could be useful in fighting bacterial over infections that often occur as a result of serious cutaneous diseases. Several studies have been carried out to co-formulate HT with other substances, with the aim of improving its absorption and effectiveness. For example, good permeation profiles through the human stratum corneum (SC) and viable epidermis have been reported for HT conjugated with fatty acids [24], while in AD its co-administration with hydrocortisone in co-loaded nanoparticles, produces anti-inflammatory and antioxidant effects [25]. Nevertheless, no one has ever thought to formulate HT in its natural vehicle, the extra virgin olive oil (EVOO), for dermatological purpose. The EVOO in fact has in itself multiple health properties especially as regards the skin. A natural combination of honey, olive oil, and beeswax can reduce the complications of diaper dermatitis, one of the most common skin disorders in infants [26], and has been found useful in the treatment of psoriasis [27]. Furthermore, in Italian traditional medicine, in addition to the common use of olive oil as an excellent emollient to restore and maintain skin integrity, other topical uses have been reported such as the treatment of burns, cracking, wounds, sores, acne, eczema, milk crust and insect bites. [28]. Finally, a recent study showed that the delivery of HT in EVOO enhances bioavailability of the bioactive compound [22].
In light of this, the aim of the study was to evaluate the safety and efficacy of a new HT-based topic formulation, conveyed in EVOO, on the epidermal barrier structure as well as keratinocytes-promoted skin inflammation, which is associated with the onset of AD, by using an in vitro Reconstructed Human Epidermis model.
Materials and methods
Chemicals
Lipopolysaccharides from Escherichia coli O55:B5 (LPS), 3-Hydroxytyrosol ≥98%, anti-Loricrin, anti-Filaggrin, Phosphate buffered saline (PBS) 10X concentrate BioPerformance Certified suitable for cell culture were purchased from Sigma Aldrich (Saint Louis, MO, USA). Anti-Cytokeratin 5&6 and anti-Ki67 were purchased from Ventana Medical Systems (Oro Valley, AZ, USA). Anti-E cadherin was purchased from Cell Marque (Merck KGaA, Darmstadt). Thiazoyl blue tetrazolium bromide (MTT) ultrapure grade and dexamethasone were purchased from VWR (Milan, Italy). Fenolia® Eudermal Cream 15 comprising an olive extract titrated in HT (0.025%) and conveyed in organic EVOO (15%) was purchased from P&P Farma (Turin, Italy).
Titration of the bioactive compound HT in the semi-solid formulation
The HT titration was performed by a validated extraction procedure followed by RP-LC-DAD analysis according to Smeriglio et al., [29]. Chromatographic separation was carried out by Ascentis C18 column (150 × 4.6 mm, 5 μm) (Supelco, Milan, Italy) using (A) CH3COOH 0.2% (pH 3.1) and (B) methanol, as mobile phase, according to the following gradient elution: 0–2 min, 95% A; 2–10 min 75% A; 10–13 min, 95% A and equilibrated 2 min for a total run time of 15 min. Injection volume was 20 μL, flow rate was set at 1.5 mL/min and column oven was maintained at 25 °C. The temperature of the auto-sampler was set at 4 °C and burnished glassware was used for sample preparation to avoid any possible degradation of the bioactive compound.
HT was recognized comparing the retention time and relative UV-Vis spectra (range 190–400 nm) with that of the commercially available standard and quantified at 280 nm by an external standard calibration curve (range 20–320 μM).
In vitro model
In vitro Reconstructed Human Epidermis (RHE) of 0.33 cm2, aged 17 days, was purchased by Episkin (Lyon Cedex, France). This standardized model, histologically similar to in vivo human epidermis, is constituted by normal human keratinocytes cultured on an inert polycarbonate filter at the air-liquid interface in a chemically characterised medium. The tissue cultures were maintained in the incubator at 37 °C, 5% CO2 and saturated humidity only for 48 h using SkinEthic Maintenance Medium (SMM). The medium was changed after 24 h and the treatments started after overnight incubation.
Treatments
The following treatments, by using six replicates for each one, were carried out for 24 h: i) Negative control (SMM); ii) Positive control (Fenolia® Eudermal Cream 15); iii) IL-1α inflammation positive control (PBS 1X) [30]; iv) IL-8 inflammation positive control (LPS 100 μg/mL); v) IL-1α anti-inflammatory positive control (PBS 1X + 10 μM Dexamethasone); vi) IL-8 anti-inflammatory positive control (LPS 100 μg/mL + 10 μM Dexamethasone); vii) IL-1α inflammation treatment + HT-based formulation (PBS 1X + Fenolia® Eudermal Cream 15); viii) IL-8 inflammation treatment + HT-based formulation (LPS 100 μg/mL + Fenolia® Eudermal Cream 15). A pre-treatment of 4 h was carried out when inflammation was induced by PBS 1X according to Matabuena-de Yzaguirre et al. [31].
Pro-inflammatory agents (PBS 1X and LPS 100 μg/mL) were diluted in SMM and treatments were performed in basolateral side while HT-based formulation necessary to cover the entire surface (25 mg) was applied via a syringe to standardize the topical application dosage on the apical side of RHE model.
At the end of the experiments, the cell media were collected and stored at −20 °C until analysis, while RHE tissues were suitably processed for histological and immunohistochemical studies as well as for cellular viability assay.
Histological and immunohistochemical studies
Sample preparation
In vitro RHE tissues were fixed in 10% buffered formalin for 72 h at 4 °C, subsequently placed in orienting tissue cassettes (Securesette™ Thermo Scientific, UK) and routinely processed in paraffin on the same day. In consideration of the potential problems in obtaining perfect orientation due to the thin nature of the samples, great care was taken in optimal tissue orientation during paraffin embedding with the help of lens magnification. From each paraffin block, a 4 μm-thick section was cut and stained with hematoxylin and eosin (H&E) as tissue control. Further, five 4 μm-thick sections were mounted on Superfrost Plus (Thermo Scientific, Braunschweig, Germany) microscope slides for immunohistochemistry [32].
Immunohistochemistry
Immunohistochemistry technique standardization was assured by using the automated BenchMark Ultra immunostainer® (Ventana Medical Systems, Arizona, USA). The system uses the Ultraview universal DAB detection kit (Ventana Medical Systems, Arizona, USA) which is a biotin free indirect method. Tissue sections were deparaffinised and rehydrated. Endogenous peroxidase was blocked with 5% H2O2 for 10 min. Heat pre-treatment was carried out either by Thermopad™ (Ventana Medical Systems, Arizona, USA) or microwave with pH 6 citrate buffer as specified for each antibody. Enzymatic pre-treatment was performed with protease 1 with incubation time of 8 min. After immunostaining, slides were counterstained with haematoxylin and cover slipped.
The following antibodies were used:
anti-Filaggrin, polyclonal, dilution 1:100, microwave heat pre-treatment; this is a protein which is expressed during transition from the granular to the cornified layers and is a marker of epidermal differentiation;
anti-Loricrin, polyclonal, dilution 1:500, enzymatic pre-treatment; this is a protein which is expressed mainly in the upper granular layer as it is a precursor of the cornified layer;
anti-Cytokeratin 5&6, ready to use, Thermopad ™ heat pre-treatment 30 min; these are cytoskeleton proteins found in the basal and spinous layers of epidermal epithelium;
anti-E Cadherin, ready to use, Thermopad ™ heat pre-treatment 64 min; this is a cell adhesion molecule expressed on cell membranes in particular in the spinous and granular layers;
anti-Ki67, ready to use, Thermopad ™ heat pre-treatment 30 min; this is a nuclear protein expressed in all cells which enter the proliferative cycle and in the normal epidermis these are located in the basal layer.
Microscopic observations
All sections were evaluated simultaneously on the light microscope Olympus U-MDOB3 with Olympus camera DP70 (Olympus Corporation, Japan).
On the H&E sections, epidermal thickness (both with the SC and without) was measured by using digital analysis with the Leica DMD 108 digital microscope (Leica Microsystems, Germany) which enables direct measurement in microns. The final value was expressed as the mean of three different measurements for each sample.
Immunoreactions were compared with the negative and positive controls for all antibodies.
Ki67, which stains the nuclei of proliferating cells, was counted in 1 linear mm using the Leica DMD 108 digital microscope, in the area of highest expression.
MTT assay
Cellular viability was assessed by MTT assay, based on the activity of mitochondrial dehydrogenases [33]. Stock solution of MTT (5 mg/ml in PBS), was diluted (1 mg/ml) in SMM, added (600 μL) to the basolateral compartment of RHE tissues and incubated at 37 °C, 5% CO2, 95% humidified atmosphere, for 3 h. At the end of the incubation time, the apical compartments move on a new plate containing isopropanol (1.5 ml), extracting for 2 h at room temperature (RT) under constant and gentle agitation, in order to dissolve the resulting formazan crystals. The optical density was read at 570 nm by a Multiskan™ GO reader plate (Thermo Fisher Scientific, Waltham, MA, USA).
Evaluation of the HT permeability
The quantification of HT permeated through the RHE model, after topical application, was evaluated at the end of experiments, by liquid-liquid extraction of the cell medium with methanol (1:1, v/v) followed by RP-LC-DAD analysis according to the method reported in the section 2.2. Results were expressed as apparent permeability coefficient (Papp) by the following equation:
were Q is the amount of hydroxytrosol in cell medium in function of time (t) expressed in μmol/s; A is the cell tissue surface (cm2) and C0 is the concentration of HT applied in the apical compartment (μM).
Immunodetection of IL-1 α and IL-8
The release of interleukins in cell medium was evaluated using the ELISA kit for IL-1α (Vinci-Biochem, Firenze, Italy) and IL-8 (Vinci-Biochem, Firenze, Italy), following the manufacturer’s instructions. The optical density was read at 450 nm by a Multiskan™ GO reader plate (Thermo Fisher Scientific, Waltham, MA, USA) against the reference blank.
Statistical analysis
Results are expressed as average ± standard deviation (S.D.) of six independent experiments in triplicate (n = 3) and analysed by one-way analysis of variance (ANOVA). The significance of the difference from the respective controls for each experimental test condition was carried out using Tukey’s test for each paired experiment considering a P ≤ 0.05 as statistically significant.
Results and discussions
Morphological and immunohistochemical evaluations
Up to date, the role of HT in skin diseases has not been widely investigated. In recent years, two are the most significant studies that considered HT as a promising molecule in the co-treatment of AD. In both studies, a nanoparticles formulation of HT and hydrocortisone (HC) was used. In the first one, this new formulation, which was evaluated both in ex vivo than in vivo models, significantly reduced the flux and permeation coefficient of HC across full-thickness as well as it was able to counteract efficiently the trans-epidermal water loss, intensity of erythema and mild dermatitis [34]. Furthermore, a subsequent study revealed that this formulation was able to decrease the cytokines release in serum and skin biopsies of tested mice as well demonstrated also by histological findings [25].
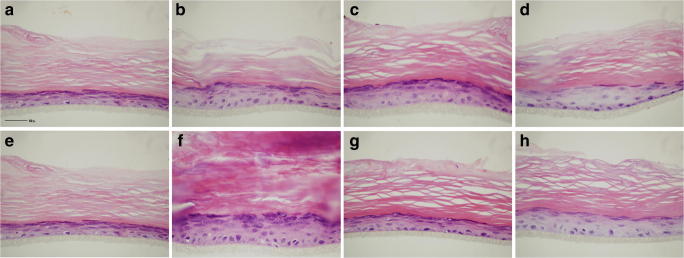
In this study, all RHE samples showed normal stratification with recognizable and well-represented epidermal layers (basal, spinous, granular and cornified) as highlighted by H&E staining (Fig. 1). Dexamethasone (Fig. 1c and g) and, even more, Fenolia® Eudermal Cream 15-treated tissues (Fig. 1d and h) showed an increase of epidermal thickness compared to negative controls (Fig. 1a and e). However, the comparison of the treated-tissues with the inflammation positive controls (Fig. 1b and f), shows that the inflammatory injury causes an abnormal increase of the corneum layer (mostly in LPS-induced injury). This event is relieved by the co-treatment with the reference drug (Fig. c and g) as well as by HT-based formulation (Fig. 1d and h) maintaining the physiological conditions (Fig. 1a and e). This aspect is particularly important to counteract the phenomenon of lichenification, which occurs in chronic inflammatory skin diseases such as AD [35]. As evidence of this, the treatment with the HT-based formulation alone did not show any statistically significant difference with respect to the negative control, both as regards the morphological and immunohistochemical evaluations (data not shown).
Fig. 1.
Histological sections stained with H&E. Negative control, SMM (a and e); positive controls, PBS1X and LPS 100 μg/mL (b and f, respectively); anti-inflammatory reference treatments, PBS 1X and LPS 100 μg/mL + Dexamethasone 10 μM (c and g, respectively); HT-based formulation treatments, PBS 1 X and LPS 100 μg/mL + Fenolia® Eudermal Cream 15 (d and h, respectively) for 24 h. Scale bar: 50 μm
When assessing the epidermal thickness (with and without the corneum layer) through morphological assessment, the results previously described were confirmed (Table 1). Regarding the evaluation of the RHE total thickness, a statistically significant increase versus the negative control (SMM) was found in the following treatments: PBS 1 X, LPS 100 μg/mL, PBS 1X + Dexamethasone 10 μM, and LPS 100 μg/mL + Fenolia® Eudermal Cream 15. Furthermore, a statistically significant decrease was found by comparing the PBS 1X + Fenolia® Eudermal Cream 15 treatment versus its positive control (PBS 1X). The same behavior was also observed between the LPS treatment 100 μg/mL + Dexamethasone 10 μM as well as between LPS 100 μg/mL + Fenolia® Eudermal Cream 15 and the respective positive control (LPS 100 μg/mL). From the evaluation of the RHE thickness without the SC, all treatments remain statistically significant compared to the negative control with the exclusion of PBS treatment 1X + Fenolia® Eudermal Cream 15 (Table 1). Furthermore, both co-treatments with the reference drug (PBS 1X and LPS 100 μg/mL + Dexamethasone 10 μM) are statistically significant compared to positive controls (PBS 1X and LPS 100 μg/mL). The significance of the LPS treatment 100 μg/mL + Fenolia® Eudermal Cream 15 only in the first evaluation (RHE total tickness) supports the hypothesis that the HT-based formulation is particularly effective in preventing the thickness increase of the SC, found in particular in the LPS-induced inflammation.
Table 1.
Assessing of the epidermal thickness (with and without the corneum layer) and Ki67 expression by morphological evaluation
| Treatments | RHE total thickness (micron) | RHE thickness w/o corneum layer (micron) | Ki67/mm |
|---|---|---|---|
| SMM | 92.67 ± 7.23 | 19.40 ± 1.01 | 7 ± 0.47 |
| PBS 1X | 122.00 ± 8.72* | 32.73 ± 1.60# | 15 ± 0.80# |
| LPS 100 μg/mL | 168.00 ± 3.60# | 42.53 ± 1.76# | 12 ± 0.75# |
| PBS 1X + 10 μM Dexamethasone | 119.00 ± 6.00* | 37.97 ± 2.91# | 7 ± 0.50δ |
| LPS 100 μg/mL + 10 μM Dexamethasone | 88.00 ± 1.00φ | 34.30 ± 1.37#§ | 7 ± 0.53φ |
| PBS 1X + Fenolia® Eudermal Cream 15 | 104.67 ± 6.51ψ | 27.50 ± 1.80*ψ | 6 ± 0.20δ |
| LPS 100 μg/mL + Fenolia® Eudermal Cream 15 | 142.33 ± 8.74*§ | 41.03 ± 2.99# | 34 ± 0,80#φ |
Results were expressed as mean ± standard deviation of three independent experiments (n = 3)
*P ≤ 0.05 vs SMM; #P ≤ 0.001 vs SMM; ψP ≤ 0.05 vs PBS 1 X; δP ≤ 0.001 vs PBS 1 X; §P ≤ 0.05 vs LPS 100 μg/mL; φP ≤ 0.001 vs LPS 100 μg/mL
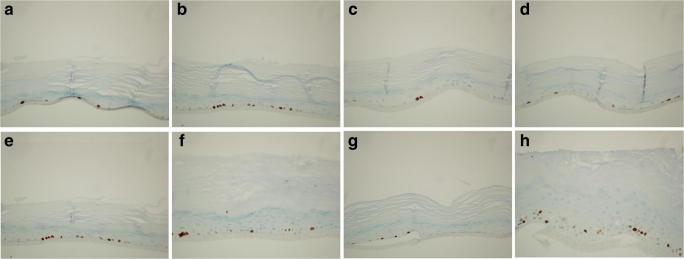
Regarding the expression of essential proteins for the organization and differentiation of epidermis, all RHE samples express Loricrin, Fillagrin, E-Cadherin and Cytokeratins 5&6, detected by immunohistochemistry, showing that epidermis maturation and protein expression in the different layers is maintained and appropriate in location in both treated and untreated samples (data not shown). The various molecules are expressed in the basal or the upper granular and cornified layers with differences in their expression associated with the varying thicknesses of the layers according to treatment. Significant differences in basal layer proliferation were found using the anti-Ki67 antibodies (Fig. 2). In particular, both PBS (Fig. 2b) and LPS (Fig. 2f) stimulate basal layer proliferation when compared to the negative control (Fig. 2a and e), but this effect decrease by the addition of Dexamethasone (Fig. 2c and g). Concerning Fenolia® Eudermal Cream 15, its use in addition to PBS 1X (Fig. 2d) reduces proliferation similarly to the negative control (Fig. 2A) and Dexamethasone treatment (Fig. 2c). On the other hand, LPS 100 μg/mL + Fenolia® Eudermal Cream 15 (Fig. 2h) improves cell proliferation more than twice with respect to the LPS 100 μg/mL alone (Fig. 2f). In light of the MTT results (Fig. 3), which showed that the HT-based formulation alone did not induce any cell proliferation with results superimposable to that of negative control, It is possible to speculate that this effect is due to reparation mechanism in response to LPS-induced inflammation injury.
Fig. 2.
Histological sections immunostained with anti Ki67 antibody. Negative control, SMM (a and e); positive controls, PBS1X and LPS 100 μg/Ml (b and f, respectively); anti-inflammatory reference treatments, PBS 1X and LPS 100 μg/mL + Dexamethasone 10 μM (c and g, respectively); HT-based formulation treatments, PBS 1 X and LPS 100 μg/mL + Fenolia® Eudermal Cream 15 (d and h, respectively) for 24 h. Scale bar: 50 μm
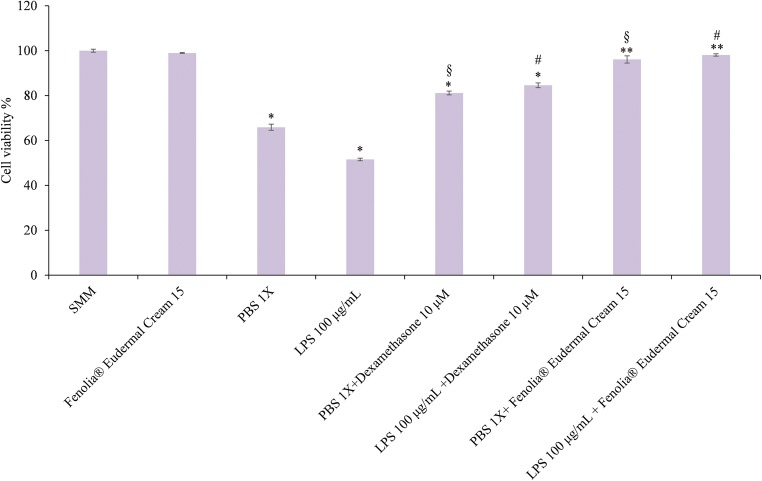
Fig. 3.
Cell viability (%) estimated by the MTT assay: negative control (SMM), positive control (Fenolia® Eudermal Cream 15), pro-inflammatory positive controls (PBS 1X and LPS 100 μg/mL), anti-inflammatory reference treatments (PBS 1X and LPS 100 μg/mL+ Dexamethasone 10 μM) and HT-based formulation treatments (PBS 1 X and LPS 100 μg/mL+ Fenolia® Eudermal Cream 15) for 24 h. *P ≤ 0.001 vs SMM; **P ≤ 0.05 vs SMM; §P ≤ 0.001 vs PBS 1X; #P ≤ 0.05 vs LPS 100 μg/mL
Safety and efficacy evaluation by MTT assay
The positive inflammation controls (PBS 1X and LPS 100 μg/mL) are able to reduce cell viability of 48.44 and 34.11% respectively compared to the negative control, while no statistically significant difference was found for HT-based formulation alone (Fig. 3). As we expected, the co-treatments with the reference drug (PBS 1X and LPS 100 μg/mL + Dexamethasone 10 μM) counteract this effect by reducing it by 44.66 and 68.16%, respectively (Fig. 3). Interestingly, the co-treatments with the HT-based formulation show cell viability values comparable to those of the negative control (96.07 and 98.06%) (Fig. 3) highlighting how the formulation investigated showed a good tolerability and a greater efficacy than the reference drug (Dexamethasone) in counteracting PBS and LPS-induced damage by increasing cell proliferation.
Transdermal absorbtion of HT
It is well known that HT is an amphiphilic molecule and this property could promote the use of this bioactive compound as well as its derivatives as part of topical compositions for pharmaceutical or cosmetic purpose [36]. Permeation through the skin is a central prerequisite for the topical delivery of bioactive compounds. Indeed, the efficacy of a formulation containing a bioactive molecule derives from its ability to penetrate the main skin barrier, the SC [37] which, for its nature, generally enhances the permeation of lipophilic compounds [38].
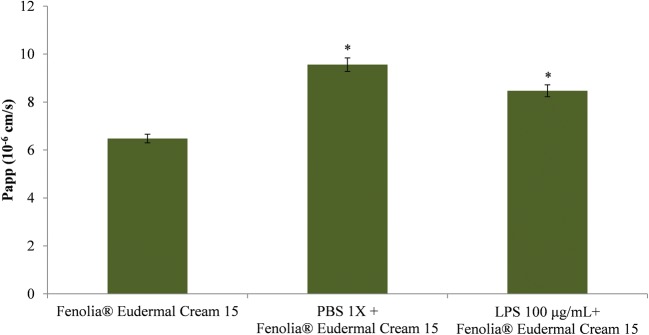
After the titration of the HT in the semi-solid formulation, which was found to comply with the label (0.0252% ± 0.00015), percutaneous absorption experiments were carried out. Results showed how the vehicle in which HT is conveyed, the EVOO, promotes the skin penetration. Indeed, a good transdermal absorption (56%) of the bioactive compound through the un-treated skin in 24 h, with a Papp of 6.48 10−6 cm/s (Fig. 4), was found. This value increases significantly in the presence of an inflammatory stimulus (25.59 and 17.14% for PBS 1X and LPS 100 μg/mL, respectively), enhancing the availability of the bioactive compound and consequently the efficacy of the topic formulation (Fig. 4). It is well known, that the inflammatory stimulus leads to barrier dysfunction and the SC is the cell layer mainly involved. Indeed, when lichenification phenomenon occurs, during inflammation process, leads to an impaired barrier function mainly due to an increased trans-epidermal water loss and decreased water-binding capacity due mainly to altered levels of inter/intracellular components of the SC [39].
Fig. 4.
Evaluation of the HT permeability through the RHE model after topical treatment with Fenolia® Eudermal Cream 15 in absence or in presence of the pro-inflammatory stimuli (PBS 1X or LPS 100 μg/mL). Results were expressed as Papp. *P ≤ 0.001 vs HT-based formulation alone
Anti-inflammatory activity evaluation
Researchers have recently focused their attention on natural compounds and traditional remedies in order to treat skin diseases for which there is no treatment of choice with conventional drugs. The goal is to inhibit the inflammatory event avoiding progression to the chronic stage. These investigations represent the trigger point for cosmetics and dietary supplements development [40, 41]. The olive leaves are esteemed among the best plant complexes containing polyphenols such as HT, tyrosol, caffeic acid and oleuropein with rather strong anti-inflammatory activity [42]. These bioactive compounds are abundant also in the olive fruit and consequently in EVOO. Studies have shown that HT protects red blood cells against oxidative damage [43]. Moreover, beyond its strong antioxidant activity, HT is also known for its antimicrobial efficacy, especially against S. aureus [44], and this plays a pivotal role in all skin diseases, which could exacerbate in a bacterial over-infection.
In this study, the anti-inflammatory activity of the HT-based formulation was evaluated monitoring the IL-1α and IL-8 release by RHE model treated with pro-inflammatory agents.
Keratinocytes, in fact, due to the lesions-induced imbalance, change its inactive state to a migratory, proliferative and pro-inflammatory phenotype releasing pre-formed and stored acute phase proteins [45].
PBS 1 X, being able to induce the IL-1α release, was used to induce an alteration of the epidermal barrier and to investigate the presence of any association between the epithelium integrity and swelling. IL-1α is a pleiotropic cytokine involved in inflammation, immune response, and hematopoiesis; so it increases in response to cell damage, leading to apoptosis [46]. The keratinocyte-derived IL-1α could act as a paracrine and autocrine mediator. In the first case, it induces proliferation of adjacent fibroblasts, synthesis of collagen and release of pro-inflammatory cytokines like IL-6 other than expression of intracellular adhesion molecules on endothelial cells. On the other hand, the autocrine signaling promotes the synthesis and release of IL-6, IL-8 and TNF-α [45].
LPS 100 μg/mL was used, instead, to directly induce the IL-8 release, a chemotactic agent, involved in the attraction and activation of immune cells, synthesized by keratinocytes after an inflammatory stimulus [47]. This cytokine is highly expressed in keratinocytes where plays, with IL-1α, a pivotal role in the initial inflammatory phase triggered by the innate immune system [45].
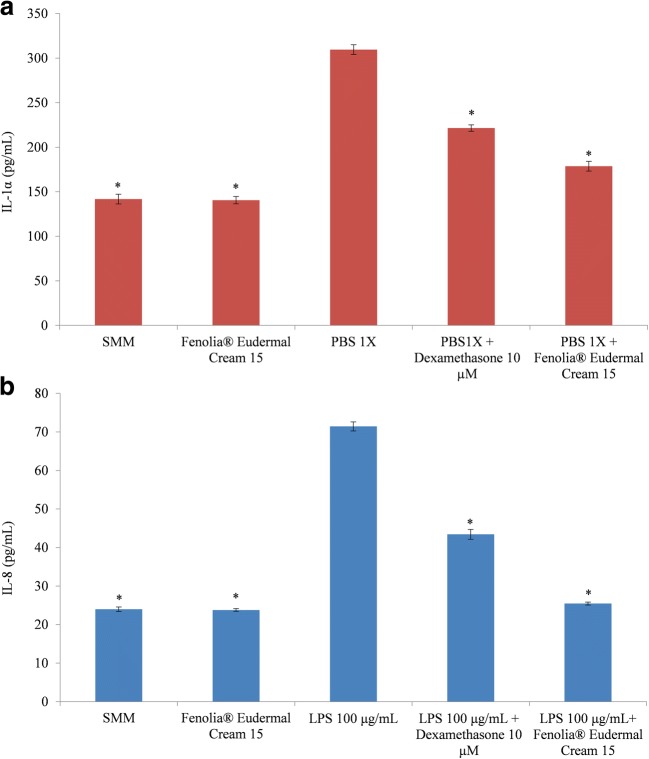
The HT-based formulation alone did not show any pro-inflammatory effect showing an IL-1α and IL-8 release comparable with those of the respective negative controls (Fig. 5). The pro-inflammatory agents (PBS 1X and LPS 100 μg/mL) induce a statistically significant increase (P < 0.001) of the interleukins release by RHE model (Fig. 5) according also to the previous MTT results, which showed a cell viability decrease of 34.11 and 48.44%, respectively (Fig. 3). However, in both cases the co-treatment with the reference drug (Dexamethasone) and HT-based formulation were able to counteract these pro-inflammatory effects in a statistically significant manner (P < 0.001) (Fig. 5). In addition, surprisingly, the HT-based formulation showed, in both cases, the best anti-inflammatory activity against inflammatory damage (IL-1α and IL-8 decrease of 42.30 and 45.97%, respectively) with respect to the reference drug Dexamethasone (IL-1α and IL-8 decrease of 28.45 and 28.01%, respectively) (Fig. 5).
Fig. 5.
IL-1α (a) and IL-8 (b) release by negative control (SMM), positive control (Fenolia® Eudermal Cream 15), pro-inflammatory positive controls (PBS 1X and LPS 100 μg/mL, respectively), anti-inflammatory reference treatments (PBS 1X or LPS 100 μg/mL + Dexamethasone 10 μM) and HT-based formulation treatments (PBS 1X or LPS 100 μg/mL + Fenolia® Eudermal Cream 15) for 24 h. *P ≤ 0.001 vs pro-inflammatory positive control (PBS 1X and LPS 100 μg/mL, respectively)
A similar behavior was already observed in two previous studies carried out on a topical formulation [31, 48]. In that cases, investigated topical formulation exhibited strong anti-inflammatory activity also with respect to the reference drug (Dexamethasone), especially concerning the IL-1α while no statistically significant difference has been observed with respect to IL-8 release [31].
However, studies evaluating the in vitro efficacy of topical formulations on skin diseases are few since they mainly focus on isolated bioactive compounds.
The effectiveness of this new formulation could be ascribed certainly to its active ingredient HT, a molecule with well-known anti-inflammatory activity, as already demonstrated in other in vitro models such as neuroinflammation [49] or human hepatoma in Hep3B and HepG2 cell lines [50].
Furthermore, HT was able to reduce, in a dose-dependent manner, the IL-8 gene expression in human dermal fibroblasts exposed to UVA radiation [51] as well as to decrease the pro-inflammatory IL-1α mRNA levels in LPS-treated murine macrophages [52]. It is well-know that HT exerts its anti-inflammatory effects through attenuating signalling cascades, including NF-kb pathway, involved in the onset and progression of a wide range of chronic diseases [53]. In endothelial cells treated with phorbol myristate acetate, for example, HT was able to reduce the activated levels of NF-kb [53] as well as, in another cellular study, its DNA-binding activity [54]. Furthermore, HT is able to prevent the nuclear translocation of NF-kb in neuronal cells exposed to β-amyloid, a peptide strongly implicated in the pathogenesis of Alzheimer disease [55]. Consequently, the HT inhibits the expression of genes encoding inflammatory mediators and cells stimulated by pro-inflammatory agents do not respond, as it should occur physiologically, because HT prevents the secretion of key inflammatory cytokines [44].
However, despite the proven anti-inflammatory activity of HT, a possible synergistic effect with the EVOO polyphenols, cannot be excluded. Indeed, they could improve the HT absorption, as already demonstrated by a clinical study in which greater bioavailability of the same, so conveyed, was found [22].
Conclusions
This preliminary study demonstrates that Fenolia® Eudermal Cream 15 could be notably effective in mild AD and early stages of a moderate/severe illness improving the epidermal barrier effect and preventing inflammation by activating reparation processes.
RHE model has proven a useful screening tool for evaluating specific features of the epidermal pathophysiology particularly with respect to the ethical limits for the use of animal models in research as well as for the difficulty in translating results from animals to humans [56]. However, the lack of fibroblasts, immune cells and nerve endings remains one of the main limitations of this RHE model and further in vitro co-culture and clinical studies will be necessary to confirm the hypothesis postulated.
Acknowledgements
The authors thank Simona Pigozzi and Ilaria Tosi for technical support.
Abbreviations
- AD
atopic dermatitis
- HT
hydroxytyrosol
- PBS
phosphate buffered saline
- MTT
thiazoyl blue tetrazolium bromide
- RP-LC-DAD
reverse phase-liquid chromatography-diode array detection
- RHE
reconstructed human epidermis
- SMM
skinethic maintenance medium
- LPS
lipopolysaccharides
- H&E
hematoxylin and eosin
- RT
room temperature
- Papp
apparent permeability coefficient
- ANOVA
one-way analysis of variance
- SC
stratum corneum
- EVOO
extra virgin olive oil
Author’s contributions
AS and DT designed this study and wrote the manuscript. AS, MD, LM, FG, performed the experiments. LC, SS, SMN and DT analyzed the data and carried out the statistical analysis. All authors read and approved the final manuscript.
Compliance with ethical standards
Consent for publication
All authors agree to publish the manuscript.
Competing interests
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fabbrocini G, Napolitano M, Megna M, Balato N, Patruno C. Treatment of atopic dermatitis with biologic drugs. Dermatol Ther (Heidelb). 2018. 10.1007/s13555-018-0258-x. [DOI] [PMC free article] [PubMed]
- 2.Napolitano M, Megna M, Patruno C, Gisondi P, Ayala F, Balato N. Adult atopic dermatitis: a review. G Ital Dermatol Venereol. 2016;151:403–411. [PubMed] [Google Scholar]
- 3.Katsarou A, Armenaka MC. Atopic dermatitis in older patients: particular points. J Eur Acad Dermatol Venereol. 2011;25:12–18. doi: 10.1111/j.1468-3083.2010.03737.x. [DOI] [PubMed] [Google Scholar]
- 4.Silvestre Salvador JF, Romero-Pérez D, Encabo-Duràn B. Atopic dermatitis in adults: a diagnostic challenge. J Investig Allergol Clin Immunol. 2017;27:78–88. doi: 10.18176/jiaci.0138. [DOI] [PubMed] [Google Scholar]
- 5.Dinulos JG, Trickett A, Crudele C. New science and treatment paradigms for atopic dermatitis. Curr Opin Pediatr. 2018;30(1):161–168. doi: 10.1097/MOP.0000000000000560. [DOI] [PubMed] [Google Scholar]
- 6.Kim DH, Li K, Seo SJ, Jo SJ, Yim HW, Kim CM, Kim KH, Kim DW, Kim MB, Kim JW, Ro YS, Park YL, Park CW, Lee SC, Cho SH. Quality of life and disease severity are correlated in patients with atopic dermatitis. J Korean Med Sci. 2012;27:1327–1332. doi: 10.3346/jkms.2012.27.11.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Megna M, Napolitano M, Patruno C, Villani A, Balato A, Monfrecola G, Ayala F, Balato N. Systemic treatment of adult atopic dermatitis: a review. Dermatol Ther (Heidelb) 2017;7:1–23. doi: 10.1007/s13555-016-0170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calzavara Pinton P, Cristaudo A, Foti C, Canonica GW, Balato N, Costanzo A, De Pità O, De Simone C, Patruno C, Pellacani G, Peris K, Girolomoni G. Diagnosis and management of moderate to severe adult atopic dermatitis: a consensus by the Italian Society of Dermatology and Venereology (SIDeMaST), the Italian Association of Hospital Dermatologists (ADOI), the Italian Society of Allergy, asthma and clinical immunology (SIAAIC), and the Italian Society of Allergological, environmental and occupational dermatology (SIDAPA) G Ital Dermatol Venereol. 2018;153(2):133–145. doi: 10.23736/S0392-0488.17.05892-8. [DOI] [PubMed] [Google Scholar]
- 9.Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, Gieler U, Girolomoni G, Lau S, Muraro A, Czarnecka-Operacz M, Schäfer T, Schmid-Grendelmeier P, Simon D, Szalai Z, Szepietowski JC, Taïeb A, Torrelo A, Werfel T, Ring J. European dermatology forum (EDF), the European academy of dermatology and venereology (EADV), the European academy of allergy and clinical immunology (EAACI), the European task force on atopic dermatitis (ETFAD), European Federation of Allergy and Airways Diseases Patients’ associations (EFA), the European Society for Dermatology and Psychiatry (ESDaP), the European Society of Pediatric Dermatology (ESPD), global allergy and asthma European network (GA2LEN) and the European Union of medical specialists (UEMS). Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32:850–878. [Google Scholar]
- 10.Rerknimitr P, Otsuka A, Nakashima C, Kabashima K. The etiopathogenesis of atopic dermatitis: barrier disruption, immunological deragement, and pruritus. Inflamm Regen. 2017;37:14. doi: 10.1186/s41232-017-0044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Megna M, Patruno C, Balato A, Rongioletti F, Stingeni L, Balato N. An Italian multicentre study on adult atopic dermatitis: persistent versus adult-onset disease. Arch Dermatol Res. 2017;309:443–452. doi: 10.1007/s00403-017-1739-y. [DOI] [PubMed] [Google Scholar]
- 12.Eshtiaghi P, Gooderham MJ. Dupilumab: an evidence- based review of its potential in the treatment of atopic dermatitis. Core Evid. 2018;13:13–20. doi: 10.2147/CE.S133661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dohil MA. Natural ingredients in atopic dermatitis and other inflammatory skin disease. J Drugs Dermatol. 2013, 12(9):s128–32. [PubMed]
- 14.Liphschitz N, Gophna R, Hartman M, Biger G. The beginning of olive (Olea europaea) cultivation in the old world: a reassessment. J Arch Sci. 1991;18(4):441–453. [Google Scholar]
- 15.Marchetti C, Clericuzio M, Borghesi B, Cornara L, Ribulla S, Gosetti F, Marengo E, Burlando B. Oleuropein-enriched olive leaf extract affects calcium dynamics and impairs viability of malignant mesothelioma cells. Evid Based Complement Alternat Med. 2015;2015:908493. doi: 10.1155/2015/908493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viola P, Viola M. Virgin olive oil as a fundamental nutritional component and skin protector. Clin Dermatol. 2009;27(2):159–165. doi: 10.1016/j.clindermatol.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Caramia G, Gori A, Valli E, Cerretani L. Virgin olive oil in preventive medicine: from legend to epigenetics. Eur J Lipid Sci Technol. 2012;114:375–388. [Google Scholar]
- 18.Omar SH. Oleuropein in olive and its pharmacological effects. Sci Pharm. 2010;78(2):133–154. doi: 10.3797/scipharm.0912-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cicerale S, Lucas L, Keast R. Biological activities of phenolic compounds present in virgin olive oil. Int J Mol Sci. 2010;11(2):458–479. doi: 10.3390/ijms11020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trombetta D, Smeriglio A, Marcoccia D, Giofrè SV, Toscano G, Mazzotti F, et al. Analytical evaluation and antioxidant properties of some secondary metabolites in northern Italian mono- and multi-varietal extra virgin olive oils (EVOOs) from early and late harvested olives. Int J Mol Sci. 2017;18(4). [DOI] [PMC free article] [PubMed]
- 21.Alarcón de la Lastra C, Barranco MD, Motilva V, Herrerías JM. Mediterranean diet and health: biological importance of olive oil. Curr Pharm Des. 2001;7(10):933–950. doi: 10.2174/1381612013397654. [DOI] [PubMed] [Google Scholar]
- 22.Colica C, Di Renzo L, Trombetta D, Smeriglio A, Bernardini S, Cioccoloni G, Costa de Miranda R, Gualtieri P, Sinibaldi Salimei P, De Lorenzo A. Antioxidant effects of a Hydroxytyrosol-based pharmaceutical formulation on body composition, metabolic state, and gene expression: a randomized double-blinded, placebo-controlled crossover trial. Oxidative Med Cell Longev. 2017;2017:2473495. doi: 10.1155/2017/2473495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martínez L, Ros G, Nieto G. Hydroxytyrosol: health benefits and use as functional ingredient in meat. Medicines (Basel) 2018;5(1):pii E13. doi: 10.3390/medicines5010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Procopio A, Celia C, Nardi M, Oliverio M, Paolino D, Sindona G. Lipophilic hydroxytyrosol esters: fatty acid conjugates for potential topical administration. J Nat Prod. 2011;74:2377–2381. doi: 10.1021/np200405s. [DOI] [PubMed] [Google Scholar]
- 25.Hussain Z, Katas H, Mohd Amin MC, Kumolosasi E. Efficient immuno-modulation of TH1/TH2 biomarkers in 2,4-dinitrofluorobenzene-induced atopic dermatitis: nanocarrier-mediated transcutaneous co-delivery of anti-inflammatory and antioxidant drugs. PLoS One. 2014;9(11):e113143. doi: 10.1371/journal.pone.0113143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Waili NS. Clinical and mycological benefits of topical application of honey, olive oil and beeswax in diaper dermatitis. Clin Microbiol Infect. 2005;11(2):160–163. doi: 10.1111/j.1469-0691.2004.01013.x. [DOI] [PubMed] [Google Scholar]
- 27.Al-Waili NS. Topical application of natural honey, beeswax and olive oil mixture for atopic dermatitis or psoriasis: partially controlled, single-blinded study. Compl Ther Med. 2003;11(4):226–234. doi: 10.1016/s0965-2299(03)00120-1. [DOI] [PubMed] [Google Scholar]
- 28.Guarrera PM. Usi e Tradizioni della Flora Italiana. Medicina popolare ed etnobotanica. Aracne Editrice S.r.l., Roma; 2006.
- 29.Smeriglio A, Giovinazzo C, Trombetta D. Development and validation of RP-HPLC-DAD method to quantify hydroxytyrosol content in a semi-solid pharmaceutical formulation. Med Chem. 2015;5:442–446. [Google Scholar]
- 30.Lichtenauer M, Nickl S, Hoetzenecker K, Mangold A, Moser B, Zimmermann M, Hacker S, Niederpold T, Mitterbauer A, Ankersmit HJ. Phosphate buffered saline containing calcium and magnesium elicits increased secretion of Interleukin-1 receptor antagonist. Lab Med. 2009;40(5):290–293. [Google Scholar]
- 31.Matabuena-de Yzaguirre M, Bacchini G, Luna EG, Vila-Martínez E. Anti-inflammatory efficacy of product containing “skin calm complex” in vitro reconstructed epidermis. J Cosmet Dermatol Sci Appl. 2014;4:309–315. [Google Scholar]
- 32.Gambella A, Porro L, Pigozzi S, Fiocca R, Grillo F, Mastracci L. Section detachment in immunohistochemistry: causes, troubleshooting, and problem-solving. Histochem Cell Biol. 2017;148(1):95–101. doi: 10.1007/s00418-017-1558-4. [DOI] [PubMed] [Google Scholar]
- 33.Merlin JL, Azzi S, Lignon D, Ramacci C, Zeghari N. Guillemin F. MTT assays allow quick and reliable measurement of the response of human tumour cells to photodynamic therapy. Eur J Cancer. 1992;28A(8–9):1452–1458. doi: 10.1016/0959-8049(92)90542-a. [DOI] [PubMed] [Google Scholar]
- 34.Hussain Z, Katas H, Mohd Amin MC, Kumolosasi E, Buang F, Sahudin S. Self-assembled polymeric nanoparticles for percutaneous co-delivery of hydrocortisone hydroxytyrosol: an ex vivo and in vivo study using an NC/Nga mouse model. Int J Pharm. 2013;444:109–119. doi: 10.1016/j.ijpharm.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 35.Lias PM, Steinhoff M. “Outside-to-inside” (and now back to “outside”) pathogenic mechanisms in atopic dermatitis. J Invest Dermatol. 2008;128(5):1067–1070. doi: 10.1038/jid.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maldonado OS, Lucas R, Comelles F, González MJ, Parra JL, Medina I, Morales JC. Synthesis and characterization of phenolic antioxidants with surfactant properties: glucosyl- and glucuronosyl alkyl gallates. Tetrahedron. 2011;67:7268–7279. [Google Scholar]
- 37.Wiechers JW, Kelly CL, Blease TG, Dederen JC. Formulating for efficacy. Int J Cosmet Sci. 2004;26:173–182. doi: 10.1111/j.1467-2494.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 38.Alonso C, Lucas R, Barba C, Marti M, Rubio L, Comelles F, Morales JC, Coderch L, Parra JL. Skin delivery of antioxidant surfactants based on gallic acid and hydroxytyrosol. J Pharm Pharmacol. 2015;67(7):900–908. doi: 10.1111/jphp.12382. [DOI] [PubMed] [Google Scholar]
- 39.Lee H-J, Lee S-H. Epidermal permeability barrier defects and barrier repair therapy in atopic dermatitis. Allergy Asthma Immunol Res. 2014;6(4):276–287. doi: 10.4168/aair.2014.6.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tabassum N, Hamdani M. Plants used to treat skin diseases. Pharmacogn Rev. 2014;8(15):52–60. doi: 10.4103/0973-7847.125531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shenefelt PD. Herbal treatment for dermatologic disorders. In: Benzie IFF, Wachtel-Galor S, editors. Herbal medicine, 2nd edition biomolecular and clinical aspects. Boca Raton. FL: CRC Press/Taylor & Francis; 2011. [Google Scholar]
- 42.Choi NY, Lee JH, Shin HS. Antioxidant activity and nitrite scavenging ability of olive leaf (Olea europaea L.) fractions. Korean J Food Sci Technol. 2008;40:257–264. [Google Scholar]
- 43.Fatima PM, Rui C, Susana F, Pedro F, Michael H. Effects of enrichment of refined olive oil with phenolic compounds from olive leaves. J Agric Food Chem. 2007;55:4139–4182. doi: 10.1021/jf063093y. [DOI] [PubMed] [Google Scholar]
- 44.Ziogas V, Tanou G, Molassiotis A, Diamantidis G. Antioxidant and free radical-scavenging activities of phenolic extracts of olive fruits. Food Chem. 2010;120:1097–1110. [Google Scholar]
- 45.Juráňová J, Franková J, Ulrichová J. The role of keratinocytes in inflammation. J Appl Biomed. 2017;15(3):169–179. [Google Scholar]
- 46.Palomo J, Dietrich D, Martin P, Palmer G, Gabay C. The interleukin (IL)-1 cytokine family-balance between agonists and antagonists in inflammatory diseases. Cytokine. 2015;76(1):25–37. doi: 10.1016/j.cyto.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 47.Russo RC, Garcia CC, Teixeira MM, Amaral FA. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev Clin Immunol. 2014;10(5):593–619. doi: 10.1586/1744666X.2014.894886. [DOI] [PubMed] [Google Scholar]
- 48.Matabuena-de Yzaguirre M, Bacchini G, Vila-Martínez E, Juarranz Á. Product containing “skin calm complex” improves barrier effect in vitro. J Cosmet Dermatol Sci Appl. 2014;4:234–243. [Google Scholar]
- 49.Angeloni C, Malaguti M, Barbalace MC, Hrelia S. Bioactivity of olive oil phenols in Neuroprotection. Int J Mol Sci. 2017;18(11):pii E2230. doi: 10.3390/ijms18112230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tutino V, Caruso MG, Messa C, Perri E, Notarnicola M. Antiproliferative, antioxidant and anti-inflammatory effects of hydroxytyrosol on human hepatoma HepG2 and Hep3B cell lines. Anticancer Res. 2012;32(12):5371–5377. [PubMed] [Google Scholar]
- 51.Jeon S, Choi M. Anti-inflammatory and anti-aging effects of hydroxytyrosol on human dermal fibroblasts (HDFs) Biomed Dermatol. 2018;2:21. [Google Scholar]
- 52.Richard N, Arnold S, Hoeller U, Kilpert C, Wertz K, Schwager J. Hydroxytyrosol is the major anti-inflammatory compound in aqueous olive extracts and impairs cytokine and chemokine production in macrophages. Planta Med. 2011;77(17):1890–1897. doi: 10.1055/s-0031-1280022. [DOI] [PubMed] [Google Scholar]
- 53.Killeen MJ, Linder M, Pontoniere P, Crea R. NF-κβ signaling and chronic inflammatory diseases: exploring the potential of natural products to drive new therapeutic opportunities. Drug Discov Today. 2014;19(4):373–378. doi: 10.1016/j.drudis.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 54.Zhang X, Cao J, Jiang L, Zhong L. Suppressive effects of hydroxytyrosol on oxidative stress and nuclear factor-kappaB activation in THP-1 cells. Biol Pharm Bull. 2009;32:578–582. doi: 10.1248/bpb.32.578. [DOI] [PubMed] [Google Scholar]
- 55.St-Laurent-Thibault C, Arseneault M, Longpré F, Ramassamy C. Tyrosol and hydroxytyrosol, two main components of olive oil, protect N2a cells against amyloid-b-induced toxicity. Involvement of the NF-kB signaling. Curr Alzheimer Res. 2011;8:543–551. doi: 10.2174/156720511796391845. [DOI] [PubMed] [Google Scholar]
- 56.Huet F, Severino-Freire M, Chéret J, Gouin O, Praneuf J, Pierre O, Misery L, Le Gall-Ianotto C. Reconstructed human epidermis for in vitro studies on atopic dermatitis: a review. J Dermatol Sci. 2018;89(3):213–218. doi: 10.1016/j.jdermsci.2017.11.015. [DOI] [PubMed] [Google Scholar]