Abstract
An optimized DNA-based bioassay for Leptospira interrogans detection has been developed. Electrochemical studies of the developed biosensor were done using cyclic voltammetry (CV) and differential pulse voltammetry (DPV). Surface characterization of the biosensor was done using scanning electron microscopy (SEM). The biosensor showed specificity to L. interrogans as determined by specificity studies. The sensitivity of the biosensor was 264.5 µA/cm2/ng and lower limit of detection (LOD) was 0.015 ng/6 µl using CV. The biosensor was also validated with serum samples spiked with single-stranded leptospiral DNA. The developed biosensor also showed good stability for a period of 6 months at 4 °C as shown by the DPV analysis.
Keywords: DNA biosensor, Leptospira interrogans, LipL32 gene, Nano-Au/c-MWCNTs, Specificity
Research highlights
The developed DNA-based bioassay is the first report on biosensor-based leptospirosis diagnosis.
The developed bioassay is a DNA-based amperometric biosensor specific to L. interrogans causing leptospirosis.
The biosensor has a sensitivity of 264.5 µA/cm2/ng and a lower limit of detection (LOD) of 0.015 ng/6µl.
The biosensor was stable for 6 months with only 13% loss in DPV peak current.
Introduction
Leptospirosis is the most widespread zoonosis (Sehgal 2000). Due to its infectious nature, it has emerged as a matter of major concern in India and many other countries. A large number of clinical manifestations are associated with leptospirosis. It includes respiratory distress, pulmonary hemorrhage, meningitis and renal failure (Bharti et al. 2003). Efficient laboratory diagnosis of leptospirosis is very essential, because its clinical signs and symptoms mimic those characterized in various other diseases and disorders. Leptospirosis cannot be diagnosed alone on the basis of clinical manifestations it exhibits, but an array of laboratory diagnostic methods is required for its correct diagnosis (Vijayachari et al. 2008).
Dark field microscopic examination of leptospires requires expertise and careful examination (Muso and La Scola 2013). Leptospires are fastidious and may take a long period of time for growth. Serology-based methods for leptospirosis diagnosis are less sensitive and specific, however, microscopic agglutination test (MAT) is still the gold standard method (Picardeau et al. 2014). Polymerase chain reaction (PCR) is expensive as a routinely diagnostic tool for leptospirosis and is prone to contamination (Khaki 2016). The methods based on molecular typing are cumbersome to perform and time consuming. Therefore, newer and improved techniques such as biosensors due to their importance in disease diagnosis and pathogen detection have become prevalent. Nucleic acid-based amperometric biosensors are most suitable for disease diagnosis and pathogen detection due to their high sensitivity and specificity (Huang et al. 2017). Amino labeled single-stranded DNA probes can be immobilized on the surface of carboxylated gold electrodes via covalent bonding. The target pathogen’s complementary DNA can be captured by these probes and the hybridization can be detected in the form of electrochemical changes (Cinti et al. 2017).
There are various virulent markers involved in leptospirosis. Outer membrane proteins of leptospires are important factors in causing the disease (Levett 2001). LipL32, an outer membrane protein of 32 kD is a highly conserved marker expressed in all pathogenic species of leptospires (Haake et al. 2000). Hence, in the present study, an amperometric DNA biosensor based on LipL32 gene as a probe has been developed for the detection of Leptospira interrogans causing leptospirosis using gold nanoparticle-embedded carboxylated multiwalled carbon nanotubes (nano-Au/c-MWCNTs) electrode.
Materials and methods
N-hydroxysuccinimide (NHS), 1-ethyl-3-(3-dimetylaminopropyl)-carbodiimide (EDC) and methylene blue (MB) were obtained from Sigma-Aldrich, USA. Sodium chloride (NaCl), ethanol (C2H5OH), hydrochloric acid (HCl), sodium di-hydrogen orthophosphate (NaH2PO4), di-sodium hydrogen orthophosphate (Na2HPO4), Tris, ethylenediamine-tetraacetic acid (EDTA) and other chemicals were obtained from Qualigens, India. Nano-Au/c-MWCNTs electrodes were purchased from DropSens, Spain. All other chemicals and glassware used in the present study were of analytical grade. 5′-amino labeled single-stranded DNA probe (5′NH2-TGGCTATCTCCGTTGCACTC-3′) specific to LipL32 gene of L. interrogans was obtained from Eurofins, Bangalore. Serum samples used in the present study were procured from Microbiology Department of Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh. The bacteria used in this study were isolated from leptospirosis patient blood samples at PGIMER, Chandigarh.
DNA isolation
The genomic DNA (G-DNA) of L. interrogans was isolated at PGIMER, Chandigarh using an earlier reported method (Pereira et al. 2011). The double stranded G-DNA was denatured (95 °C, 5 min) to make it single stranded for hybridization with the immobilized probe (Kaushal et al. 2016).
Construction of DNA biosensor
Nano-Au/c-MWCNTs electrode was fabricated for the development of LipL32 gene-based DNA biosensor. A new nano-Au/c-MWCNTs electrode washed with autoclaved milli Q water was taken and treated with PBS buffer (pH 7). After washing, the electrode was allowed to dry at room temperature. Nano-Au/c-MWCNTs electrode was then treated with the 6 µl of equimolar mixture (10 mM each) of EDC and NHS (1:1, v/v in PBS, pH 7) and kept for 1.5 h. The EDC: NHS cross-linker was used for carboxyl group activation on the electrode surface (Singh et al. 2014). To remove excess reagents, electrode washing with PBS (pH 7) was done and kept for drying at room temperature. Then, 6 µl of 10 µM amine labeled ssDNA probe was made to immobilize on the surface of activated nano-Au/c-MWCNTs electrode and kept for 5 h at 25 °C. After this, washing with TE buffer was done to eliminate the excess probe and then desiccated at room temperature.
Hybridization and electrochemical characterization
The leptospiral genomic DNA was denatured (95 °C, 5 min) and 6 µl of it was added on the surface of fabricated nano-Au/c-MWCNTs electrode and allowed to hybridize with the probe for 10 min. The same procedure was adopted for hybridization of various concentrations of ssG-DNA of L. interrogans and the corresponding electrochemical changes were recorded using cyclic voltammetry and differential pulse voltammetry with methylene blue (MB) as redox indicator.
Surface morphological studies
The surface morphological characterization studies of the developed nano-Au/c-MWCNT-based DNA biosensor were performed using scanning electron microscopy (SEM).
Specificity and stability studies
To check the specificity of the developed biosensor, ssG-DNA of different pathogens present in patient blood samples was allowed to hybridize with the immobilized ssDNA probe of L. interrogans. The CV peak current (Ip) in each case was compared with the (Ip) recorded with L. interrogans. To check the stability of the developed biosensor (nano-Au/c-MWCNTs electrode with the probe immobilized stored at 4 °C, the DPV was determined regularly at 30 days’ interval for a period of 6 months.
Statistical analysis
The difference in CV peak current was validated using the one-way analysis of variance (ANOVA) followed by a least significant difference test at 95% confidence (Kaushik et al. 2018). For the calculation of means and standard error mean (SEM), Microsoft Excel, 2016 (Microsoft Corp., Redmond, WA) was used.
Biosensor study in serum
The developed biosensor was tested by artificially spiking single-stranded leptospiral DNA in diluted serum samples (Das et al. 2014) and the results were confirmed using PCR. The serum sample was diluted (1:10) in PBS buffer (10 mM, pH 7) and spiked with a known concentration of single-stranded DNA (10 nM) of Leptospira. A desired volume (6 µl) of this mixture was applied on the surface of the developed biosensor and the corresponding electrochemical variations post-hybridization were read in the form of CV.
Results and discussion
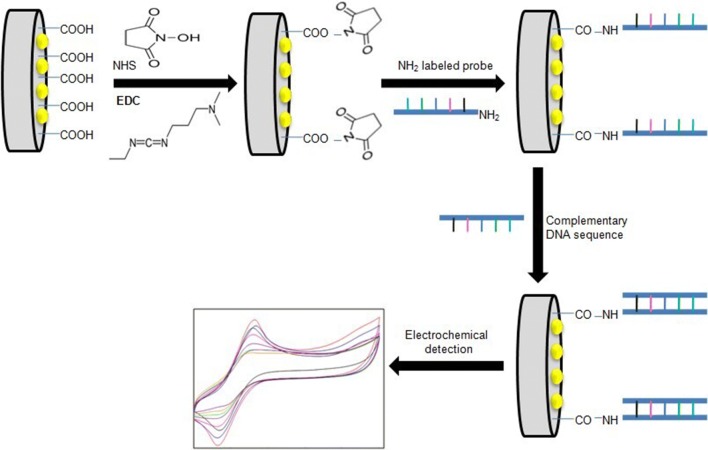
The fabrication of the nano-Au/c-MWCNTs electrode, immobilization with the probe and hybridization with ssG-DNA of L. interrogans are shown in Fig. 1.
Fig. 1.
Schematic representation of immobilization of 5′-amino labeled LipL32 ssDNA probe on nano-Au/c-MWCNT-based electrode and hybridization with ssG-DNA of L. interrogans
CV analysis
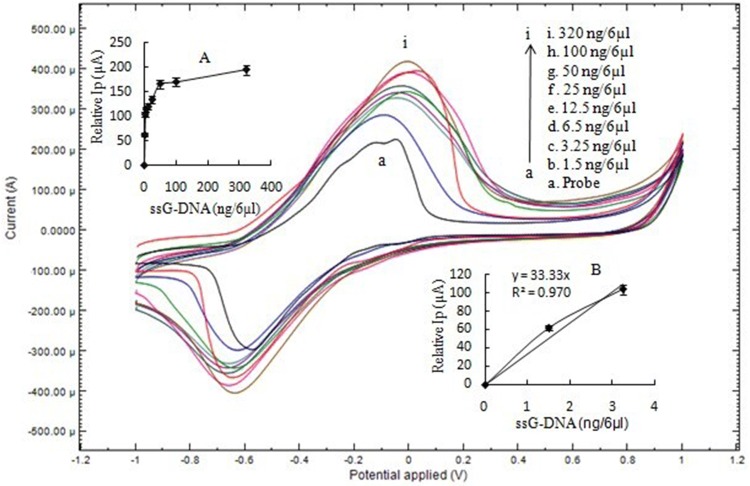
The CV results corresponding to immobilized single-stranded DNA probe and after its hybridization with ssG-DNA concentrations (1.5–320 ng) of L. interrogans are shown in Fig. 2. The results showed a rise in oxidation peak current with an increase in G-DNA concentration of L. interrogans. This may be due to the interaction between methylene blue and DNA molecules. Methylene blue (MB) interacts with guanine bases of DNA molecules. As the concentration of DNA is increased, methylene blue has a higher number of guanine residues to interact as compared to lesser DNA concentration having lesser guanine residues and hence shows more conductance (Singh et al. 2014). Therefore, there is an increase in the peak current.
Fig. 2.
CV of (a) ssDNA-NH2/nano-Au/c-MWCNTs electrode and (b–i) hybridization with of L. interrogans ssG-DNA at 50 mV/s using 1 mM MB in 50 mM PBS, pH 7. The inset a shows hyperbolic curve from 0 to 320 ng/6 µl with linear peak current (Ip) up to 3.25 ng/6 µl of ssG-DNA of L. interrogans. Inset b shows the linear plot from 0 to 3.25 ng/6 µl ssG-DNA for the calculation of sensitivity and LOD
A hyperbolic graph showing relative peak current (Ip) with respect to probe (as zero) plotted against different ssG-DNA concentrations as shown in Fig. 1. The graph showed an increase in Ip up to 320 ng/6 µl ssG-DNA (Fig. 2 inset a). The concentration of ssG-DNA increased further (above 320 ng), but the Ip showed no further increase. This is because of the saturation of the immobilized probe on the working electrode and no more availability of probe for hybridization with ssG-DNA. To calculate sensitivity and LOD value, the concentration of ssG-DNA of L. interrogans was varied to zero (0), 1.5 and 3.25 ng/6 µl to obtain the best value of regression coefficient. The plot from 0 to 3.25 ng/6 µl ssG-DNA was linear with a regression coefficient value (R2) of 0.97 (Fig. 2 inset b). The sensitivity of the developed biosensor was 264.5 µA/cm2/ng with a LOD value of 0.015 ng/6 µl.
DPV analysis
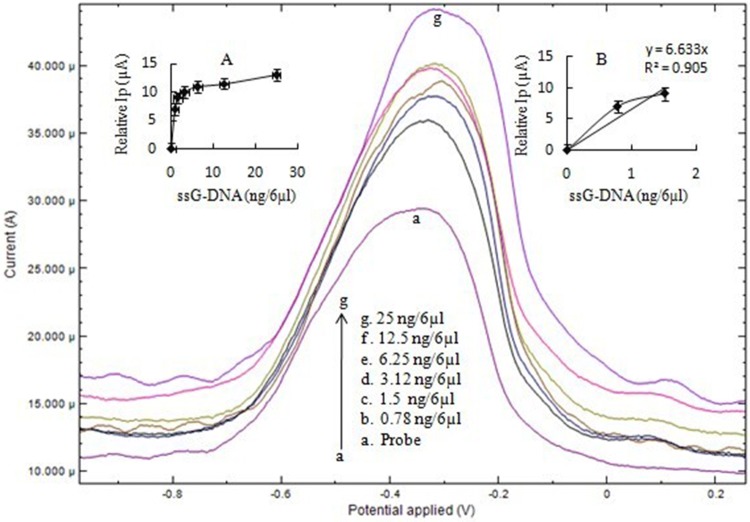
The DPV of ssDNA probe after immobilization on the electrode and after hybridization with leptospiral DNA (single stranded) is exhibited in the Fig. 3. DPV peak current (Ip) varied with different concentrations of ssG-DNA as reported with CV analysis. The Ip for immobilized ssDNA probe was 29 µA which after hybridization with ssG-DNA increased up to 42 µA. Subsequently, Ip does not show a further increase after hybridization on increasing the concentration of ssG-DNA to 25 ng/6 µl. The plot from 0 to 1.5 ng/6 µl ssG-DNA was linear with a regression coefficient (R2) of 0.90 (Fig. 3). The sensitivity of biosensor was 52 µA/cm2/ng with a LOD value of 0.035 ng/6 µl.
Fig. 3.
DPV of (a) ssDNA-NH2/nano-Au/c-MWCNTs electrode and (b–g) hybridization with L. interrogans ssG-DNA using 1 mM MB in 50 mM PBS, pH 7. The inset a shows hyperbolic curve from 0 to 25 ng/6 µl with linear peak current (Ip) up to 1.5 ng/6 µl of ssG-DNA of L. interrogans. Inset b shows the linear plot from 0 to 1.5 ng/6 µl ssG-DNA for calculation of sensitivity and LOD
Scanning electron microscopy (SEM) analysis of nano-Au/c-MWCNT-based biosensor
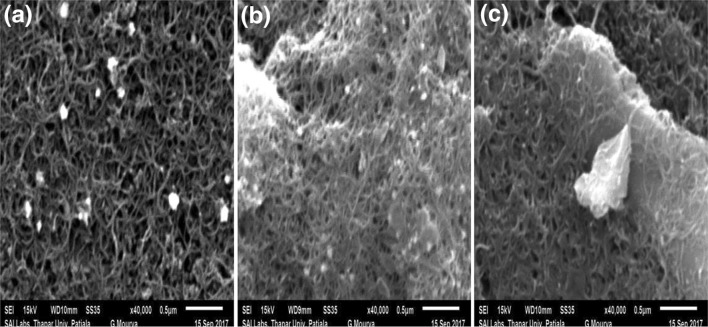
The developed nano-Au/c-MWCNTs electrode-based biosensor was analyzed for its surface characterization by scanning electron microscopy (SEM). The results of SEM studies showing different surface modifications on nano-Au/c-MWCNTs electrode are exhibited in Fig. 4. The surface of the nano-Au/c-MWCNTs electrode showed continuous fibrous net-like tubular crossed structure of c-MWCNTs as shown in Fig. 4a. The immobilization of the probe on the electrode surface (nano-Au/c-MWCNTs/ssDNA complex) resulted in changed surface morphology of nano-Au/c-MWCNTs as shown in Fig. 4b. Further, nano-Au/c-MWCNTs/dsDNA electrode (ssDNA hybridized with immobilized probe) exhibited dense surface morphology of the electrode confirming the hybridization of probe and leptospiral DNA (ssG-DNA) (Fig. 4 c).
Fig. 4.
SEM images of a bare electrode, b nano-Au/c-MWCNTs/ssDNA probe and c nano-Au/c-MWCNTs/dsDNA after hybridization with 100 ng/6 µl ssG-DNA of L. interrogans
Specificity and stability studies of developed nano-Au/c-MWCNT-based biosensor
The specificity of the developed nano-Au/c-MWCNT-based DNA biosensor was checked by hybridization with ssG-DNA of L. interrogans and three selected pathogens, viz., Staphylococcus aureus, Escherichia coli and Enterobacter aerogenes, generally found in humans with LipL32 gene-specific ssDNA probe immobilized on nano-Au/c- MWCNTs working electrode surface. The CV peak current (Ip) value of ssDNA probe after hybridization with 10 ng of ssG-DNA of L. interrogans, E. coli, S. aureus and E. aerogenes is shown in Fig. 5. There was no change in Ip (relative to probe) of the biosensor with S. aureus, E. aerogenes, and E. coli which was observed almost the same as with immobilized probe. However, on the other hand, the Ip increased with L. interrogans confirming the specificity of the probe to L. interrogans. Statistically, the CV peak current values ranged from 22 to 53 µA. CV peak current with L. interrogans showed a significant (p < 0.05) increase, whereas a non-significant increase in CV was observed with other pathogens.
Fig. 5.
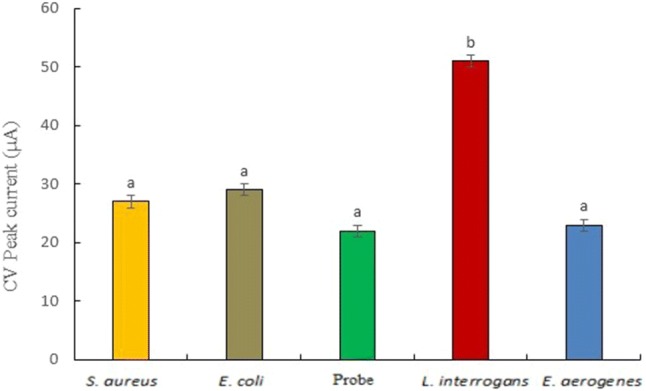
Specificity of LipL32-based biosensor with L. interrogans and other bacteria. The Ip value of CV (with respect to immobilized probe) after hybridization with 10 ng/6 µl ssG-DNA of L. interrogans and other pathogens
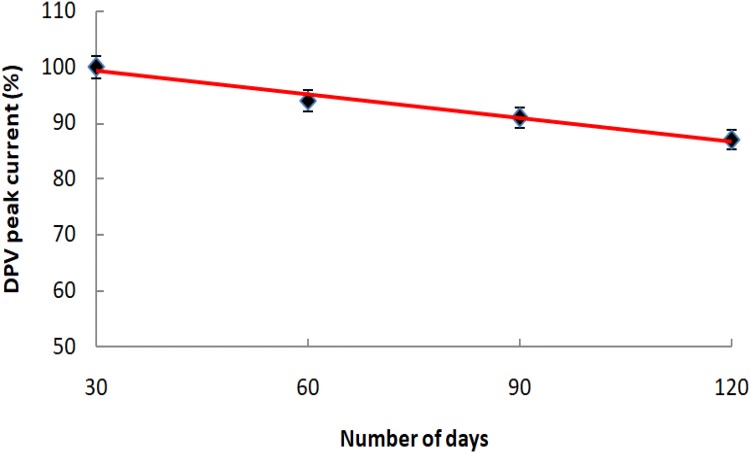
The stability of the developed nano-Au/c-MWCNT-based biosensor (at 4 °C storage conditions) was studied by DPV analysis done regularly at an interval of 30 days for a period of 6 months. The results of the stability study are shown in Fig. 6. The developed nano-Au/c-MWCNT-based biosensor was found stable for 6 months with approx. 13% loss in the original DPV current.
Fig. 6.
DPV (% peak current) of ssDNA-NH2/nano-Au/c-MWCNTs electrode observed at a regular interval of 30 days for 6 months on storage at 4 °C. Each value is the average of three readings under similar conditions
Biosensor validation in serum
The CV peak current in artificially spiked serum samples was observed almost equal as recorded in earlier CV analysis with equivalent ssDNA concentration with slight decrease in peak current (data not shown). This may be due to the various other factors such as proteins present in serum. The developed biosensor results were also compared with PCR using the same serum sample and showed amplification of specific gene (LipL 32) in Leptospira using specific primers (Fig. 7). However, when the concentration of spiked ssDNA was decreased to 25 ng, the developed biosensor was able to resolve it in the form of CV peak current (data not shown), but PCR could not amplify the same sample confirming better sensitivity of the developed biosensor in comparison to PCR.
Fig. 7.
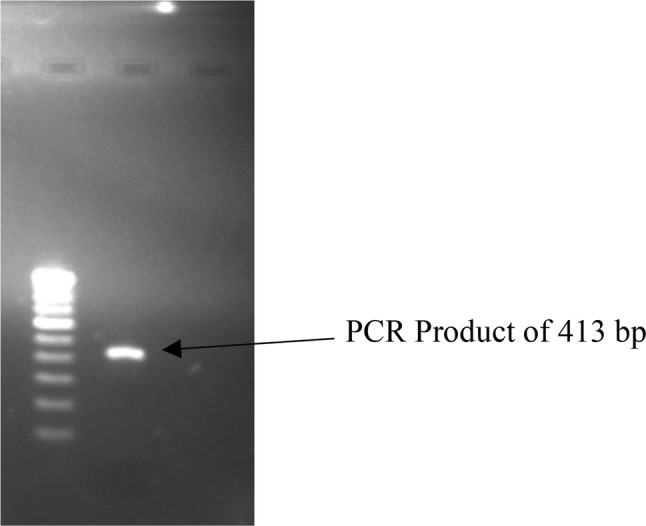
Gel electrophoresis image showing amplification product (413 bp) of LipL32 gene of L. interrogans
Conclusion
The developed biosensor is a novel method for the detection of L. interrogans. It is highly specific and rapid method that can be used for the detection of L. interrogans and showed 264.5 µA/cm2/ng for sensitivity and 0.015 ng/6 µl for LOD using CV. The developed biosensor also showed good stability for 6 months when stored at 4 °C as shown by the DPV analysis. The developed biosensor can be used for routine detection of L. interrogans causing leptospirosis and therefore help in timely management of the disease.
Acknowledgements
The authors thank Shoolini University for providing necessary facilities to carry out the research work.
Compliances with ethical standards
Conflict of interest
There is no conflict of interest for authorship or related to any other context between authors.
Ethical standards
The authors have complied and worked within standard ethical norms.
Contributor Information
Rupak Nagraik, Phone: 7018858252, Email: rupak.nagraik@gmail.com.
Ankur Kaushal, Phone: 9882546292, Email: ankur.biotech85@gmail.com.
Shagun Gupta, Phone: 8629878797, Email: shagun22_88@yahoo.com.
Prasenjit Dhar, Phone: 8628956097, Email: prasen727779@hotmail.com.
Sunil Sethi, Phone: 9872882609, Email: sunilsethi10@hotmail.com.
Dinesh Kumar, Phone: 9816321855, Email: dkchatanta@gmail.com.
References
- Bharti AR, Nally JE, Ricaldi JN, Matthias MA, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757–771. doi: 10.1016/S1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- Cinti S, Volpe G, Piermarini S, Delibato E, Pallesschi G. Electrochemical biosensors for rapid detection of food borne Salmonella: a critical overview. Sensors. 2017;17:1910. doi: 10.3390/s17081910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R, Sharma MK, Rao VK, Bhattacharya BK, Garg I, Venkatesh V, Upadhyay S. An electrochemical genosensor for Salmonella typhi on gold nanoparticles-mercaptosilane modified screen printed electrode. J Biotechnol. 2014;188:9–16. doi: 10.1016/j.jbiotec.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Haake DA, Chao G, Zuerner RL, Barnett JK, Barnett D, Mazel M. The leptospiral outer membrane protein LipL32 is a lipoprotein expressed during the mammalian infection. Infect Immun. 2000;68:2276–2285. doi: 10.1128/IAI.68.4.2276-2285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YL, Mo S, Gao ZF, Chen JR, Lei JL, Luo HQ, Li NB. Amperometric biosensor for microRNA based on the use of tetrahedral DNA nanostructure probes and guanine nanowire amplification. Microchim Acta. 2017;184:2597–2604. doi: 10.1007/s00604-017-2246-8. [DOI] [Google Scholar]
- Kaushal A, Singh S, Kala D, Kumar D, Kumar A. speB genosensor for rapid detection of Streptococcus pyogenes causing damage of heart valves in human. Cell Mol Biol. 2016;62:140. [Google Scholar]
- Kaushik R, Chawla P, Kumar N, Janghu S, Lohan A. Effect of premilling treatments on wheat gluten extraction and noodle quality. Food Sci Technol Int. 2018;24:627–636. doi: 10.1177/1082013218782368. [DOI] [PubMed] [Google Scholar]
- Khaki P. Clinical laboratory diagnosis of human leptospirosis. Int J Enteric Pathog. 2016;4:1–7. doi: 10.17795/ijep31859. [DOI] [Google Scholar]
- Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muso D, La Scola B. Laboratory diagnosis of leptospirosis: a challenge. J Microbiol Immunol Infect. 2013;46:245–252. doi: 10.1016/j.jmii.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Pereira JC, Chaves R, Bastos E, Leitao A. An efficient method for genomic DNA extraction. 2011;12:8086–8095. doi: 10.3390/ijms12118086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picardeau M, Bertherat E, Jancloes M, Skouloudis AN, Dursi K, Hartskeerl RA. Rapid tests for diagnosis of leptospirosis: current tools and emerging technologies. Diagnost Microbiol Infect Dis. 2014;78:1–8. doi: 10.1016/j.diagmicrobio.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Sehgal SC. Leptospirosis in the horizon. Natl Med J India. 2000;13:228–230. [PubMed] [Google Scholar]
- Singh S, Kaushal A, Khare S, Kumar A. Mga genosensor for early detection of human rheumatic heart disease. Appl Biochem Biotechnol. 2014;173:228–238. doi: 10.1007/s12010-014-0836-z. [DOI] [PubMed] [Google Scholar]
- Vijayachari P, Sugunan AP, Shriram AN. Leptospirosis: an emerging global public health problem. J Biosci. 2008;33:557–569. doi: 10.1007/s12038-008-0074-z. [DOI] [PubMed] [Google Scholar]