Abstract
Background
Trachyspermum ammi (L.) Sprague is used for treating gastrointestinal disorders. Several studies indicated gastric antiulcer activity of T. ammi extract, yet the effect of its essential oil has not been studied on.
Objectives
The present study evaluates chemical composition of T. ammi essential oil and anti-peptic ulcer effect of the essential oil as well as its three major components in ethanol induced-gastric ulcers in rats.
Methods
Primarily chemical composition of the essential oil was analyzed by gas chromatography-mass spectrometry (GC/MS). Rats received the essential oil (500, 250, 125, 62.5, 31.25 mg/kg), thymol (30, 100 mg/kg), para-cymene (100, 150 mg/kg) and gamma-terpinene (100, 150 mg/kg) using gavage tube along with ethanol 80%. Finally, dissected stomachs were assessed both macroscopically and microscopically to evaluate anti-ulcerative effect of the essential oil and the pure compounds. Moreover, molecular docking was utilized to explore the interactive behavior of the main components with active site residues of H+/K+ ATPase.
Results
Analysis of the essential oil indicated that para-cymene (37.18%), gamma-terpinene (35.36%) and thymol (20.51%) are the main components. Administration of different doses of the essential oil noticeably diminished the number of peptic ulcers in a dose-dependent manner. Among the main components, thymol was more potent than para-cymene and gamma-terpinene. Administration of the essential oil (500 mg/kg) and thymol (100 mg/kg) observed maximum inhibition percentage (98.58% and 79.37%, respectively). Molecular docking study provides the evidence of thymol ability to inhibit H+/K+ ATPase.
Conclusions
The findings revealed that T. ammi essential oil can be applied to treat gastric ulcer as a natural agent.
Graphical abstract
Keywords: Pharmacology, Thymol, Molecular docking, H+/K+ ATPase, Peptic ulcers
Introduction
Persian medicine, as a holistic paradigm based on humoral theory, dates back to ancient time [1]. From the perspective of Persian medicine, gastrointestinal system including stomach plays an essential role in general health [2]. As an ancient valuable resource, Persian medicine suggests using many plants in gastrointestinal disorders [3]. A notable example is Trachyspermum ammi (L.) Sprague is an annual plant in Apiaceae family, mostly distributing in the temperate regions of the world [4]. T. ammi is known as “Nankhah or Zenian” in Persian medicine, of which the fruit has been applied in the treatment of a broad spectrum of disorders such as neural, respiratory, cardiovascular, urogenital and gastrointestinal diseases since ancient times [5]. Traditionally, this herb has been used as a carminative and antispasmodic as well as remedial effect for indigestion, colic and stomachache [5].
There are some reports on biological effects of T. ammi essential oil including antioxidant, antibacterial, anti-inflammatory and analgesic effects [6, 7]. Myers et al. (2009) revealed that the essential oil of T. ammi has a significant effect on intestinal dysbiosis [8]. Previous researches depicted that the extract of T. ammi possesses anti-Helicobacter pylori and gastric antiulcer activities [9–11].
Peptic ulcer is one of the most prevalent gastrointestinal disorders worldwide with unclear etiologies thus far [12]. Several conventional medicine such as PPIs (Proton Pomp Inhibitors) and H2 Receptor Antagonists are commonly used for treating dyspepsia and gastric ulcers [13]. Side effects of the medicines and recurrence of the disease after discontinuing of the drugs, have caused remarkable attempts to discover appropriate and safe medicines such as natural remedies [14, 15]. There are many researches done on anti-ulcer activity of plants and natural compounds [16–18]. Previously, the ethanolic extract of T. ammi showed gastroprotective activity [19]. Alqasoumi et al. (2011) exhibited that T. ammi hydroalcholic extract has significant effect on the treatment of peptic ulcer induced by ibuprofen in rats [20]. Moreover, there are several reports on the gastroprotective activity of thymol and carvacrol, as the main chemical constitutes of T. ammi essential oil [21, 22], yet there is no previous report on the effect of T. ammi total essential oil on gastric ulcer.
Hence, the purpose of this study is to evaluate the gastroprotective activity of T. ammi volatile oil in ethanol induced-gastric ulcers in rats conducted for the first time. In addition, to understand the interaction between the H+/K+ ATPase, as the primary gastric proton pump, and the main components of T. ammi essential oil and the selectivity of the best component, as PPI through controlling gastric pH, molecular docking studies were performed.
Methods
Plant material
To prepare essential oil of T. ammi, dried fruits were obtained from herbal traditional market and the sample, identified by Prof. Gholamreza Amin, was deposited at the herbarium of Faculty of Pharmacy, Tehran University of Medical Sciences (PMP-663).
Isolation of essential oil
One hundred grams of dried T. ammi fruits powder was subjected to hydrodistillation, using a Clevenger type apparatus. After 4 h, the volatile oil was isolated and dried over anhydrous sodium sulfate to be kept in a sealed amber vial in refrigerator for analysis afterward [23].
Gas chromatography-mass spectroscopy
The analysis of the essential oil was performed using gas chromatography (Agilent 7000, Triple Quad, GC 7890A) equipped with flame ionization detector (FID). One μL of the sample was injected into a DB-5 column capillary column (30 m length). Injector temperature was 230 °C. Helium was used as carrier gas (flow rate: 1 ml/min). Detector temperature was 300 °C. GC-MS with similar condition of GC was applied. 70 eV ionization energy was used for Mass spectra. Series of n-alkanes were utilized to calculate retention indices of compositions. Chemical composition of the essential oil was identified through comparing their retention indices and mass spectra with previously reported compounds [24] .
Pharmacological examination
Preparation of samples concentrations
Different concentrations of the essential oil (500, 250, 125, 62.5, 31.25 mg/kg), thymol (30, 100 mg/kg), pare-cymen (100, 150 mg/kg) and gama-terpinene (100, 150 mg/kg) were prepared in saline (NaCl 0.9%) containing 10% Tween 80.
Animals
Wistar male rats (200–240 g) were purchased from Pasture Institute of Iran, Tehran, Iran. Animals were treated based on Tehran University of Medical Sciences ethical guidelines for the care and use of laboratory animals. Ethical committee approval was obtained from the Ethics Committee of Tehran University of Medical Sciences with code number: 90–11-26-6032.
The animals were kept in laboratory (25°c, 12 h light-dark cycle) for 4 days for adaptation in experimental conditions. The rats were fed with standard pellet diets and water. 24 h before carrying out the experiment, animals were fasted without concurrent water deprivation. To avoid coprophagy, the animals were kept in special cages and then were categorized into thirteen groups, five different doses of the essential oil, two different doses of each of the main components, positive control and negative control groups. Six rats were examined in each group. Rats were treated orally (p.o) using gavage tube with different concentrations of the test materials (essential oil, thymol, para-cymene and gamma-terpinene). Saline 0.9% containing 10% Tween 80 was used as a vehicle in negative control group rats. It should be mentioned that the animals received only a single dose of each test materials. Positive control group received cimetidine (100 mg/kg) [25].
Peptic ulcer induced by ethanol
Ethanol (80%) was given by gavage tube to induce acute ulcer in stomach after 15 min orally administration of the test materials, cimetidine and vehicle. One hour later implementation of ethanol; rats were killed. Then the rats were fixed on the table for stomach removal. Each stomach was cut through its major curvature, washed by normal saline and fixed on dissection tray. In the final step, to examine the lesions on stomach tissue and calculate ulcer index, the following formula was applied:
Gastric ulcerations were scored according to an arbitrary system:
Score 1: each fifth petechiae was calculated as 1 mm
Score 2: lesion length between 1 and 2 mm
Score 3: lesion length between 2 and 4 mm
Score 4: lesion length between 4 and 6 mm
Score 5: lesion length more than 6 mm
The inhibition percentage was calculated via the following equation:
- UI
Ulcer Index [26].
Histological effects of the tested materials on the inhibition of ethanol-induced peptic ulcer
The gastric tissues were fixed in fresh formalin solution (10%) for 4–6 h and prepared sections. Hematoxylin and eosin were used for staining the sections. Finally, the sections were analyzed using a light microscope [25].
Molecular docking of the main components of T. ammi essential oil into binding site of H+/K+ ATPase
AutoDock software version 4.2 was used to investigate the interaction between three main components of the essential oil as a ligand and the binding site of H+/K+ ATPase [27]. A three-dimensional (3D) structure of the Pig Gastric H+/K+ ATPase receptor with 2XZB PDB code was obtained at 7 Å resolution from http://www.pdb.org [28]. The 2D structures of cimetidine and three main components including para-cymene, gamma-terpinene and thymol were drawn and optimized using HyperChem 7.0 software (version 7.0; Hypercube, Inc., Gainesville, FL, USA; http://www.hyper.com) as described previously [29]. Using the MM+ molecular mechanical force field, 3D geometry optimization calculations for each ligand performed. The ultimate conformations were calculated with the Semi-empirical AM1 method using Polak-Ribiere algorithm in Hyperchem software until the root mean square gradient was 0.01 kcal/mol/Å [29]. Each compound was docked into the enzyme-binding pocket applying the routine procedure and default parameters of the software. Grid map can be prepared using information about the binding sites of desired receptor in Site-directed Mutagenesis [30, 31]. Thus, the center of the grid box was positioned on the active site of the cysteine residue (Cys813) of chain A of the 2XZB [30, 32]. The dimensions of the grid box with default grid point spacing of 0.375 Å were 40x40x40. A Lamarckian genetic algorithm (LGA) program, as the most effective method in the Autodock, was utilized to calculate the 200 ligand conformers of the type of bonds and functional groups involved in the binding of the ligands against the H+/K+ ATPase [33, 34]. Analyzing the calculated energy values and logic of the results together determined the best outcome. Among the various conformations of these compounds obtained from the docking procedure, the conformation with the best scored pose with the lowest binding energy was selected for these components. The docking results of the Ligplot software were used for a 2D schematic design [35].
Statistical analysis
The result of experiments were declared as mean ± standard error of mean (SEM) for six animals in each group. Statistical analysis was performed with GraphPad Prism software version 7, one –way ANOVA test. P value<0.05 was expressed as a significant difference.
Results
Chemical composition of the essential oil
The essential oil of T. ammi, dried fruits yielded 4% v/w essential oil using hydro-distillation method. The result of GC-MS analysis of the essential oil is shown in Table 1.
Table 1.
Chemical composition of Trachyspermum ammi essential oil
| No | Compound | Percentage | LRI* |
|---|---|---|---|
| 1 | α-Thujene | 0.41 | 929 |
| 2 | α- Pinene | 0.39 | 938 |
| 3 | β- Pinene | 2.11 | 980 |
| 4 | β- Myrcene | 0.47 | 989 |
| 5 | α- Phellandrene | 0.07 | 1004 |
| 6 | α- Terpinene | 0.24 | 1015 |
| 7 | p- Cymene | 37.18 | 1023 |
| 8 | β- Phellandrene | 0.61 | 1030 |
| 9 | γ- Terpinene | 35.36 | 1058 |
| 10 | Terpinolen | 0.13 | 1088 |
| 11 | Unknown | 0.05 | 1116 |
| 12 | Unknown | 0.52 | 1140 |
| 13 | 4-Terpineol | 0.25 | 1176 |
| 14 | α –Terpineol | 0.08 | 1187 |
| 15 | Cuminaldehyde | 0.14 | 1237 |
| 16 | Carvacrol methyl ether | 0.12 | 1248 |
| 17 | Thymol | 20.51 | 1287 |
| 18 | Carvacrol | 0.29 | 1296 |
| 19 | Unknown | 0.09 | 1366 |
| 20 | 1-Nonadecene | 0.05 | 1879 |
| 21 | Nonadecane | 0.06 | 1900 |
| 22 | Unknown | 0.05 | 1927 |
| 23 | Hexadecanoic acid | 0.12 | 1978 |
| 24 | Methyl linoleate | 0.24 | 2099 |
| 25 | Linoleic acid | 0.46 | 2152 |
*Linear Retention Index (LRI) on DB-5
Twenty-five components were identified in the volatile oil with 76.97% monoterpene hydrocarbons, 21.39% oxygenated monoterpenes and 1.64 other constituents.
Effects of T. ammi essential oil and its major components on gastric ulcer parameters
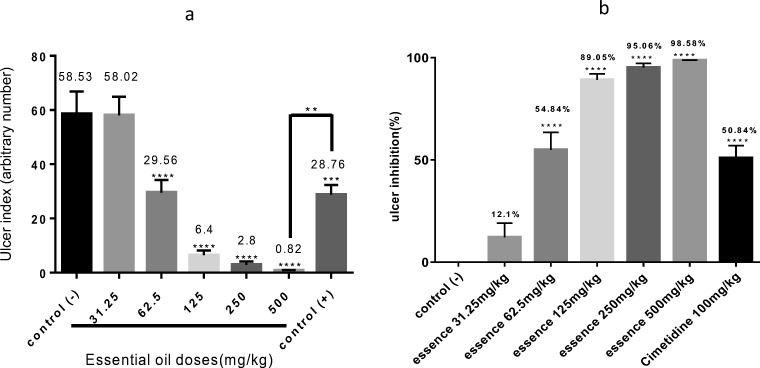
As seen in Fig. 1, gastric ulcer measurements showed that oral administration of different doses of the essential oil (31.25, 62.5, 125, 250, 500 mg/kg) noticeably diminished the number of ethanol-induced peptic ulcers in a dose-dependent manner. The findings revealed that the administration of the essential oil with doses more than 62.5 mg/kg showed significant inhibition of peptic ulcer compared to negative control (p < 0.0001). Implementation high dose of the essential oil (500 mg/kg) inhibited ethanol-induced peptic ulcer more potently than cimetidine 100 mg/kg (p < 0.01). Maximum inhibition (98.58%) was observed by administrating of 500 mg/kg of the essential oil compared to cimetidine-treated group (50.84%).
Fig. 1.
Oral administration of Trachyspermum ammi essential oil inhibited peptic ulcers induced by ethanol 80% in a dose-dependent response. There was a significant difference between the essential oil-treated groups and the control group in ulcer index (a) and ulcer inhibition (b) parameters. Significant differences **p < 0.01 ***p < 0.001 and ****p < 0.0001 in comparison with negative control and other groups
Peptic ulcer assessments were used for thymol, para-cymene and gamma-terpinene, the major three components of the essential oil, as indicated from the essential oil analysis.
According to the findings, thymol with dose of 100 mg/kg showed highest inhibitory effect of peptic ulcer compared to control group (p < 0.0001). As seen in Fig. 2, maximum inhibitory effect (79.37%) was observed by administrating thymol (100 mg/kg) compared to cimetidine-treated group (50.84%)), thymol shows comparable ulcer inhibitory effect at lower dose (30 mg/kg). Thymol was more potent than para-cymene and gamma-terpinene for inhibition of gastric ulcer induced by ethanol at comparable dose (100 mg/kg) or even higher dose (150 mg/kg) as seen in Fig. 2b. Administration of the 150 mg/kg gamma-terpinene and para-cymene inhibited peptic ulcer 56.82% and 58.77%, respectively. In neither of their doses for inhibition of gastric ulcer was there significant difference between gamma-terpinene and para-cymene.
Fig. 2.
Oral administration of thymol, para-cymene, gama-terpinene inhibited peptic ulcers induced by ethanol 80% in a dose-dependent manner. There was a significant difference between thymol, gamma-terpinene and para-cymene-treated groups and the control group in ulcer index (a) and ulcer inhibition (b) parameters. Significant differences **p < 0.01, ***p < 0.001 and ****p < 0.0001 in comparison with negative control group
Histological effects of T. ammi essential oil and its major components on the inhibition of ethanol-induced peptic ulcer
Histological damage emerged as necrosis of the mucosa and parietal cells with scratch of mucosal layer and the presence of bleeding in the gastric mucosa. In addition, the density of polymorphonuclear leukocytes and lymphocytes was observed in the submucosa. Severe lesions were found in stomach section of rats- treated with ethanol 80% that were used as negative control (Fig. 3). Gavage administration of the volatile oil (500 mg/kg) and thymol (100 mg/kg) reduced significantly peptic ulcer lesions compared to the positive group (cimetidine 100 mg/kg). As seen in Fig. 3, all images showed a degree of density of polymorphonuclear leukocytes except essential oil (500 mg/kg) and thymol (100 mg/kg).
Fig. 3.
Microscopic images of hematoxylin and eosin-stained dissected stomachs showing antiulcer effect of Trachyspermum ammi essential oil and its major components. (100X) Histological assessment of gastric mucosal tissue. aTrachyspermum ammi essential oil (500 mg/kg), b thymol (100 mg/kg), c para-cymene (150 mg/kg), d gamma-terpinene (150 mg/kg), e The negative group (ethanol 80%), f The positive group (cimetidine 100 mg/kg). Microscopic images of dissected stomachs showing a protective effect of essential oil of T. ammi (500 mg/kg) and thymol (100 mg/kg) on ethanol induced peptic ulcer compared to positive and negative group. Administration of the essential oil (500 mg/kg) and thymol cause significant reduction in the infiltration of polymorphonuclear leukocytes and hemorrhage, while hemorrhage was observed in other groups (para-cymene, gamma-terpinene, positive and negative groups). Leukocyte infiltration and hemorrhage were indicated by arrows in blue and white, respectively
Molecular docking results
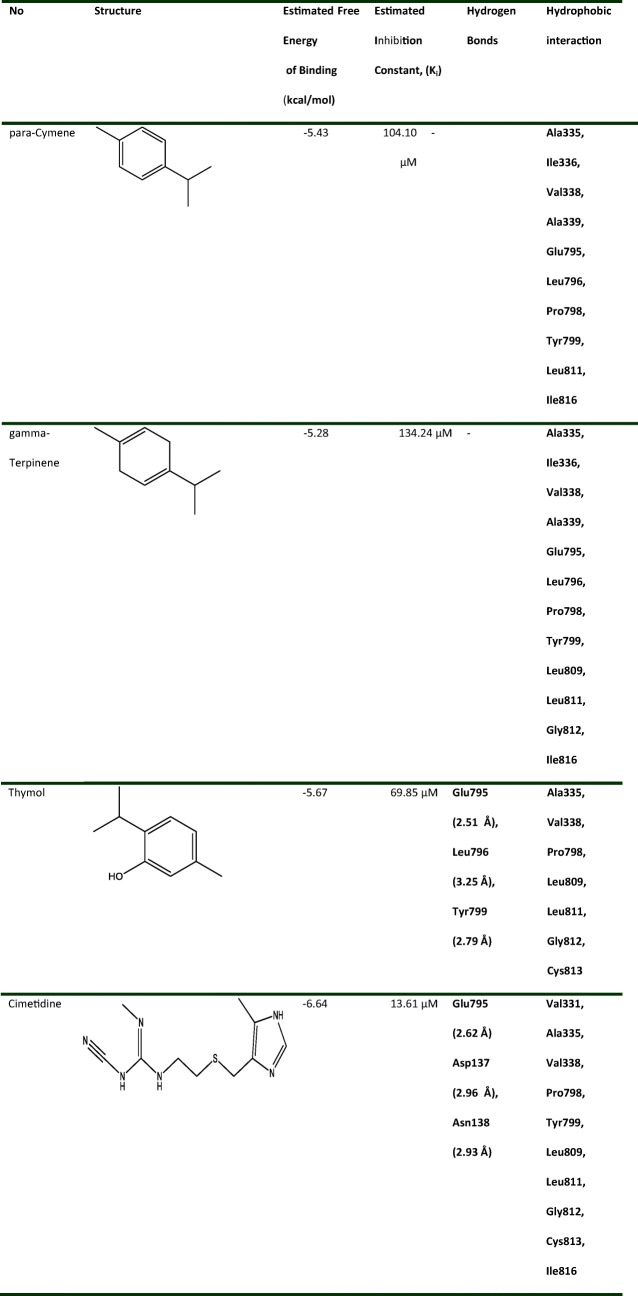
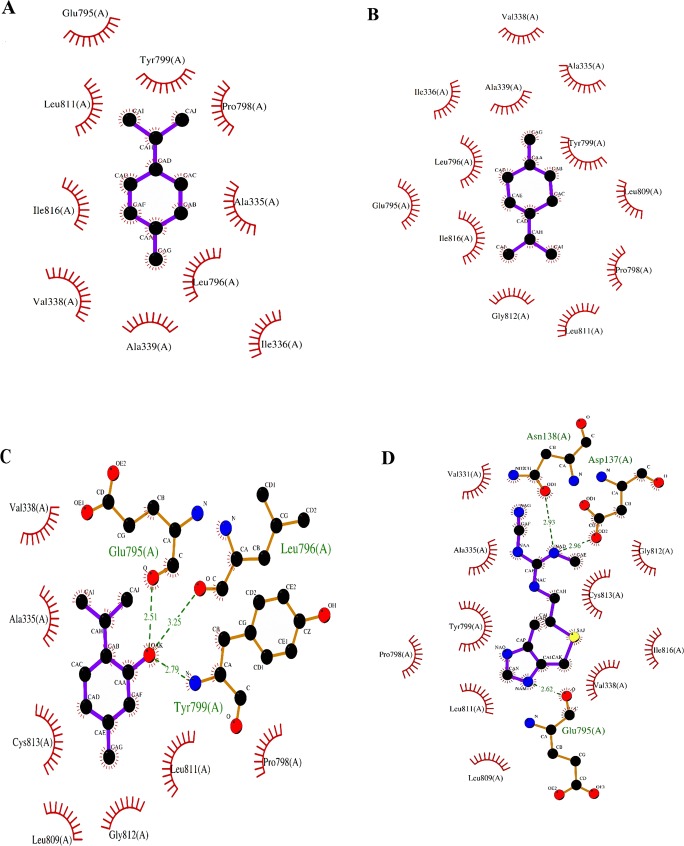
Docking scores of the best docked possess are given in Table 2. Figure 4 A-D shows 2D schematic of three components as well as cimetidine while docked into the H+/K+ ATPase binding site, supporting the idea that the compounds are well incorporated in the binding pocket. As shown in Fig. 4 and Table 2, three compounds extend deeply into the binding sites of H+/K+ ATPase, but only thymol and cimetidine were established three hydrogen bonds with H+/K+ ATPase. Meanwhile, the hydroxyl group of thymol can form two strong hydrogen bonds with the oxygen atoms of Glu795 and Leu796 at distances 2.51 Å and 3.25 Å, respectively, which is consistent with decomposition analysis of the electrostatic interaction. Interestingly, more complex stabilization might result from another hydrogen bond between thymol as PPI and enzyme via the nitrogen atom of Tyr799 (2.79 Å). It could be suggested that cimetidine interact with the enzyme via three hydrogen bonds. NH group attached to imidazole established one hydrogen bond with the Glu795 core (2.62 Å). In addition, cimetidine can form two strong hydrogen bonds with the oxygen atoms of residues Asp137 and Asn138 at distances 2.93 Å, and 2.96 Å, respectively. Thymol and cimetidine are sandwiched between Cys813 and Tyr799. Hence, both compounds cover the Cys813 in the binding pocket of H+/K+ ATPase binding site. Since the closest distance between Cys813 and thymol and cimetidine are 2.43 Å and 2.32 Å, respectively, there is possible formation of a covalent bond [30, 31]. This is more agreeable with a covalent binding mechanism. Based on recent studies, there is reversible binding of such interactions within the active site [30–32]. These results are consistent with the X-ray crystal structure and previously studies, indicating the important roles of those residues [28, 30–32, 36]. As previous mutagenesis studies proposed, some key amino acids are likely to interact directly with SCH28080, as a K+-competitive inhibitor [36, 37]. For example, the double mutant Ala335Cys /Cys813Ala has almost no affinity for SCH28080 [38]. Tyr799Ala has a 100-fold reduced apparent affinity for SCH28080, while Tyr799Phe mutant has negligible effects on SCH28080 affinity, suggesting the importance of the hydroxyl group on tyrosine residue [39]. Mutation of Leu809Phe led to a more than 90-fold reduced affinity [36].
Table 2.
Energy-based interactions for three main components as the H+/K+ ATPase potential inhibitors and cimetidine
Fig. 4.
Schematic interaction of the best docking resulting from AutoDock software presented by LigPlot software. In this figure, the ligand exposure is highlighted in blue for (a) para-Cymene, (b) gamma-Terpinene, (c) Thymol and (d) Cimetidine. Hydrogen bonding is in green and van der Waals interactions are in red circulars
No hydrogen bond interaction between para-cymene or gamma-terpinene and enzyme was predicted. Lack of hydrogen bond interaction has reduced the dock score of both compounds and increased inhibition constant compared to thymol and cimetidine (Table 2). Due to lack of electronegative atom, para-cymene or gamma-terpinene failed to form a hydrogen bonding interaction, yet as an alternative, ring of compounds enables hydrophobic/van der Waals interactions with enzyme (Fig. 4 A, B). These hydrophobic sites of the ligands are conserved in the majority of the structures. The residue that interacts through a hydrogen bonding with the acceptor/donor site of the ligand is different, depending on the ligand. In addition, molecular docking results are in accordance with in vivo results in the current study.
Discussion
Ethanol induces peptic ulcer by several mechanisms such as decreasing of gastric mucosa secretion, increasing oxidative stress and inflammatory mediators activation [40, 41]. Leukotrienes, as important inflammatory mediators, induce gastric vasoconstriction [42]. Gastric vasoconstrictor actions of leukotrienes cause gastric injury [43]. Furthermore, previous researches have highlighted the role of prostaglandins, matrix metalloproteinase-9 (MMP-9) and TNF-α in ethanol induced-peptic ulcer [21, 44]. Agents with antioxidant, anti-inflammatory and cytoprotective activities can play crucial role for treating peptic ulcer [45]. A growing body information declare phytochemicals such as phenolic compounds have an important role in peptic ulcer by multifaceted mechanisms [18, 46]. Gastro protective effect of these compounds are due to their antioxidant, anti-secretary of gastric acid, anti-inflammatory and cytoprotective activities [47]. Previous studies showed that ethanolic extract of T. ammi fruit had cytoprotective and anti-secretary effects [20]. Moreover, enhancement of the mucus secretion a highly effective factor of gastro protective of the T. ammi extract. Antiulcerogenic activity of the extract may be due to the presence of phenolic compounds such as tannins and flavonoids in the extract [19]. Consistent with the present study, a previously published revealed that thymol, as the major compounds of T. ammi essential oil, diminishes the acute and chronic gastric ulcer by raising the amount of prostaglandins, mucus secretion and ATP-sensitive K+ channels [21]. Souza et al. (2011) showed that α-terpineol, as a minor compounds of the essential oil, had gastroprotective effect against ethanol-induced ulcers which do not associate with the role of gastric acid secretion and prostaglandin synthesis [48]. Chauhan et al. (2015) depicted thymol through modulating the antioxidant enzymes secretion and reducing the expression of the MMP-9 protein had noticeable antiulcerogenic activity [49]. On the other hand, gama terpinene, as other main component of the volatile oil, has anti-inflammatory effect by reducing TNF-α, IL-1β and neutrophils level [50]. Hence, antioxidant, anti-inflammatory and vasorelaxant effects of these compounds may be responsible for their antiulcer activity [51, 52]. The findings of this research suggest that T. ammi essential oil have considerable antiulcer activity in a dose dependent manner that could be justified with synergistic activity of its main compounds and its antioxidant activity [53]. The results indicated that thymol (100 mg/kg) had more potent antiulcer effect than para-cymene (150 mg/kg) and gamma-terpinene (150 mg/kg).
In addition, docking studies confirm that these compounds block H+/K+ ATPase binding site. In consistent with in vivo experiment, thymol showed higher binding affinity as compared to other components. The careful analysis of the investigation revealed that thymol compound against H+/K+ ATPase as the most promising compound based on the docking score energies and number of hydrogen bonds. This suggests that thymol might be acting through the same mechanism as PPIs, but involvement of other mechanisms cannot be ignored especially for cimetidine. The best possible interactions of the compounds are simulated for stability using molecular modeling. The results of this investigation provide valuable information on the mechanism of selective compounds against H+/K+ ATPase. These results could be a sign of confirming the positive role of thymol as an anti-ulcerative by means of H+/K+ ATPase inhibition. A research research on acute and sub-chronic toxicity of T. ammi essential oil on rats exhibited that the LD50 value of the gavage administration of the essential oil was 2294 mg/kg and no significant differences were found in behavioral, histopathological and biochemical biochemical parameters during sub-chronic toxicity [54]. Although all the doses of the essential oil used in the present study were lower than the identified LD50 values but further studies are required before clinical trials regarding the efficacy and safety of the volatile oil in human application for peptic ulcer.
Conclusion
This study, in confirmation with reference to Persian Medicine manuscripts, suggests that the essential oil of Trachyspermum ammi fruits can be used as a natural remedy for treatment of gastric ulcer. The present study confirmed that thymol compound could present a new class of lead molecules for drug discovery as H+/K+ ATPase inhibitors. However, further studies are necessary to evaluate the exact anti ulcerative mechanism and the toxicity of the essential oil and its main compounds.
Acknowledgements
The authors are thankful to Professor Gholamreza Amin, head of the Herbarium of Faculty of Pharmacy, Tehran University of Medical Science, for identifying the plant.
Funding
The study was a part of thesis of Afsaneh Hoseinsalari and has been supported by Tehran University of Medical Sciences, Tehran, Iran.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kordafshari G, Kenari HM, Esfahani MM, Ardakani MRS, Keshavarz M, Nazem E, Moghimi M, Zargaran A. Nutritional aspects to prevent heart diseases in traditional Persian medicine. J Evid Based Complementary Altern Med. 2015;20(1):57–64. doi: 10.1177/2156587214553939. [DOI] [PubMed] [Google Scholar]
- 2.Jahromi MM, Pasalar M, Afsharypuor S, Choopani R, Mosaddegh M, Kamalinejad M, et al. Preventive care for gastrointestinal disorders; role of herbal medicines in traditional persian medicine. Jundishapur J Nat Pharm Prod. 2015;10(4):e21029. [Google Scholar]
- 3.Farzaei MH, Rahimi R, Abbasabadi Z, Abdollahi M. An evidence-based review on medicinal plants used for the treatment of peptic ulcer in traditional Iranian medicine. Int J Pharmacol. 2013;9(2):108–124. [Google Scholar]
- 4.Chauhan B, Kumar G, Ali M. A review on phytochemical constituents and activities of Trachyspermum ammi (l.) Sprague fruits. Am J Pharmtech Res. 2012;2(4):329–340. [Google Scholar]
- 5.Aghili M. Makhzan-Al-Advia. Tehran University of medical sciences. Tehran: Iran; 2009. [Google Scholar]
- 6.Umar S, Asif M, Sajad M, Ansari MM, Hussain U, Ahmad W, et al. Anti-inflammatory and antioxidant activity of Trachyspermum ammi seeds in collagen induced arthritis in rats. Int J Drug Dev Res. 2012;4(1):210–219. [Google Scholar]
- 7.Mahboubi M, Kazempour N. Chemical composition and antimicrobial activity of Satureja hortensis and Trachyspermum copticum essential oil. Iran J Microbiol. 2011;3(4):194–200. [PMC free article] [PubMed] [Google Scholar]
- 8.Myers SR, Hawrelak J, Cattley T. Essential oils in the treatment of intestinal dysbiosis: a preliminary in vitro study. Altern Med Rev. 2009;14(4):380–384. [PubMed] [Google Scholar]
- 9.Zaidi SFH, Yamada K, Kadowaki M, Usmanghani K, Sugiyama T. Bactericidal activity of medicinal plants, employed for the treatment of gastrointestinal ailments, against helicobacter pylori. J Ethnopharmacol. 2009;121(2):286–291. doi: 10.1016/j.jep.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Komeili G, Sargazi M, Soluki S, Maleki S-a-D. The therapeutic effect of Carum copticum seed aqueous extract on peptic ulcers induced by ibuprofen in rat. Zahedan J Res Med Sci. 2012;14(5):21–24. [Google Scholar]
- 11.Nariman F, Eftekhar F, Habibi Z, Falsafi T. Anti-helicobacter pylori activities of six Iranian plants. Helicobacter. 2004;9(2):146–151. doi: 10.1111/j.1083-4389.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 12.Bak-Romaniszyn L, Wojtuń S, Gil J. Płaneta-Małecka I. peptic ulcer disease etiology, diagnosis and treatment. Pol Merkur Lekarski. 2004;17(Suppl 1):128–132. [PubMed] [Google Scholar]
- 13.Ramakrishnan K, Salinas RC. Peptic ulcer disease. Am Fam Physician. 2007;(7):76. [PubMed]
- 14.Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci. 2011;56(4):931–950. doi: 10.1007/s10620-010-1560-3. [DOI] [PubMed] [Google Scholar]
- 15.Kuna L, Jakab J, Smolic R, Raguz-Lucic N, Vcev A, Smolic M. Peptic ulcer disease: a brief review of conventional therapy and herbal treatment options. J Clin Med. 2019;8(2):179. doi: 10.3390/jcm8020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zayachkivska O, Konturek S, Drozdowicz D, Konturek P, Brzozowski T, Ghegotsky M. Gastroprotective effects of flavonoids in plant extracts. J Physiol Pharmacol. 2005;56(Suppl 1):219–231. [PubMed] [Google Scholar]
- 17.Borrelli F, Izzo AA. The plant kingdom as a source of anti-ulcer remedies. Phytother Res. 2000;14(8):581–591. doi: 10.1002/1099-1573(200012)14:8<581::aid-ptr776>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 18.Sumbul S, Ahmad MA, Mohd A, Mohd A. Role of phenolic compounds in peptic ulcer: an overview. J Pharm Bioall Sci. 2011;3(3):361. doi: 10.4103/0975-7406.84437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramaswamy S, Sengottuvelu S, Sherief SH, Jaikumar S, Saravanan R, Prasadkumar C, et al. Gastroprotective activity of ethanolic extract of Trachyspermum ammi fruit. Int J Pharma Bio Sci. 2010;1(1):1–15. [Google Scholar]
- 20.Alqasoumi S. Gastric antisecretory and antiulcer effects of ajowan Carum copticum in rats. Afr J Pharm Pharmacol. 2011;5(5):572–576. [Google Scholar]
- 21.Ribeiro ARS, Diniz PB, Pinheiro MS, Albuquerque-Júnior RL, Thomazzi SM. Gastroprotective effects of thymol on acute and chronic ulcers in rats: the role of prostaglandins, ATP-sensitive K+ channels, and gastric mucus secretion. Chem Biol Interact. 2016;244:121–128. doi: 10.1016/j.cbi.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira IS, da Silva FV, Viana AFS, dos Santos MR, Quintans-Júnior LJ, Maria do Carmo CM, et al. Gastroprotective activity of carvacrol on experimentally induced gastric lesions in rodents. Naunyn Schmiedeberg's Arch Pharmacol. 2012;385(9):899–908. doi: 10.1007/s00210-012-0771-x. [DOI] [PubMed] [Google Scholar]
- 23.Eftekhari M, Ardekani MRS, Amin M, Attar F, Akbarzadeh T, Safavi M, et al. Oliveria decumbens, a bioactive essential oil: chemical composition and biological activities. Iran J Pharm Res. 2019;18(1). [PMC free article] [PubMed]
- 24.Adams RP. Identification of essential oils by gas chromatography quadrupole mass spectroscopy. IL: Allured Publ. Corp., Carol Stream; 2001. [Google Scholar]
- 25.Khanavi M, Ahmadi R, Rajabi A, Arfaee SJ, Hassanzadeh G, Khademi R, et al. Pharmacological and histological effects of Centaurea bruguierana ssp. belangerana on indomethacin-induced peptic ulcer in rats. J Nat Med. 2012;66(2):343–349. doi: 10.1007/s11418-011-0598-7. [DOI] [PubMed] [Google Scholar]
- 26.Nabavizadeh F, Alizadeh AM, Adeli S, Golestan M, Moloudian H, Kamalinejad M. Gastroprotective effects of Stachys Lavandulifolia extract on experimental gastric ulcer. Afr J Pharm Pharmacol. 2011;5(2):155–159. [Google Scholar]
- 27.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30(16):2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abe K, Tani K, Fujiyoshi Y. Conformational rearrangement of gastric H+, K+-ATPase induced by an acid suppressant. Nat Commun. 2011;2:155. doi: 10.1038/ncomms1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mansourian M, Saghaie L, Fassihi A, Madadkar-Sobhani A, Mahnam K. Linear and nonlinear QSAR modeling of 1, 3, 8-substituted-9-deazaxanthines as potential selective A2BAR antagonists. Med Chem Res. 2013;22(10):4549–4567. [Google Scholar]
- 30.Lambrecht N, Munson K, Vagin O, Sachs G. Comparison of covalent with reversible inhibitor binding sites of the gastric H, K-ATPase by site-directed mutagenesis. J Biol Chem. 2000;275(6):4041–4048. doi: 10.1074/jbc.275.6.4041. [DOI] [PubMed] [Google Scholar]
- 31.Munson K, Garcia R, Sachs G. Inhibitor and ion binding sites on the gastric H, K-ATPase. Biochemistry. 2005;44(14):5267–5284. doi: 10.1021/bi047761p. [DOI] [PubMed] [Google Scholar]
- 32.Noor A, Qazi NG, Nadeem H, Khan A-u, Paracha RZ, Ali F, et al. Synthesis, characterization, anti-ulcer action and molecular docking evaluation of novel benzimidazole-pyrazole hybrids. Chem Cent J. 2017;11(1):85. doi: 10.1186/s13065-017-0314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem. 1998;19(14):1639–1662. [Google Scholar]
- 34.Mansourian M, Mahnam K, Madadkar-Sobhani A, Fassihi A, Saghaie L. Insights into the human A1 adenosine receptor from molecular dynamics simulation: structural study in the presence of lipid membrane. Med Chem Res. 2015;24(10):3645–3659. [Google Scholar]
- 35.Wallace AC, Laskowski RA, Thornton JM. LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein Eng Des Sel. 1995;8(2):127–134. doi: 10.1093/protein/8.2.127. [DOI] [PubMed] [Google Scholar]
- 36.Abe K, Shimokawa J, Naito M, Munson K, Vagin O, Sachs G, Suzuki H, Tani K, Fujiyoshi Y. The cryo-EM structure of gastric H+, K+-ATPase with bound BYK99, a high-affinity member of K+-competitive, imidazo [1, 2-a] pyridine inhibitors. Sci Rep. 2017;7(1):6632. doi: 10.1038/s41598-017-06698-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vagin O, Munson K, Lambrecht N, Karlish SJ, Sachs G. Mutational analysis of the K+-competitive inhibitor site of gastric H, K-ATPase. Biochemistry. 2001;40(25):7480–7490. doi: 10.1021/bi0105328. [DOI] [PubMed] [Google Scholar]
- 38.Vagin O, Denevich S, Munson K, Sachs G. SCH28080, a K+-competitive inhibitor of the gastric H, K-ATPase, binds near the M5− 6 luminal loop, preventing K+ access to the ion binding domain. Biochemistry. 2002;41(42):12755–12762. doi: 10.1021/bi025921w. [DOI] [PubMed] [Google Scholar]
- 39.Asano S, Yoshida A, Yashiro H, Kobayashi Y, Morisato A, Ogawa H, Takeguchi N, Morii M. The cavity structure for docking the K+-competitive inhibitors in the gastric proton pump. J Biol Chem. 2004;279(14):13968–13975. doi: 10.1074/jbc.M308934200. [DOI] [PubMed] [Google Scholar]
- 40.Lee J-S, Oh T-Y, Kim Y-K, Baik J-H, So S, Hahm K-B, Surh YJ. Protective effects of green tea polyphenol extracts against ethanol-induced gastric mucosal damages in rats: stress-responsive transcription factors and MAP kinases as potential targets. Mutat Res Fundam Mol M. 2005;579(1):214–224. doi: 10.1016/j.mrfmmm.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 41.Liu ES, Cho CH. Relationship between ethanol-induced gastritis and gastric ulcer formation in rats. Digestion. 2000;62(4):232–239. doi: 10.1159/000007821. [DOI] [PubMed] [Google Scholar]
- 42.Wallace JL, Ma L. Inflammatory mediators in gastrointestinal defense and injury. Exp Biol Med. 2001;226(11):1003–1015. doi: 10.1177/153537020122601107. [DOI] [PubMed] [Google Scholar]
- 43.Peskar B, Lange K, Hoppe U, Peskar B. Ethanol stimulates formation of leukotriene C4 in rat gastric mucosa. Prostaglandins. 1986;31(2):283–293. doi: 10.1016/0090-6980(86)90054-7. [DOI] [PubMed] [Google Scholar]
- 44.Sugimoto M, Furuta T, Shirai N, Nakamura A, Xiao F, Kajimura M, Sugimura H, Hishida A. Different effects of polymorphisms of tumor necrosis factor-alpha and interleukin-1 beta on development of peptic ulcer and gastric cancer. J Gastroenterol Hepatol. 2007;22(1):51–59. doi: 10.1111/j.1440-1746.2006.04442.x. [DOI] [PubMed] [Google Scholar]
- 45.Repetto M, Llesuy S. Antioxidant properties of natural compounds used in popular medicine for gastric ulcers. Braz J Med Biol Res. 2002;35(5):523–534. doi: 10.1590/s0100-879x2002000500003. [DOI] [PubMed] [Google Scholar]
- 46.Al Asmari A, Al Shahrani H, Al Masri N, Al Faraidi A, Elfaki I, Arshaduddin M. Vanillin abrogates ethanol induced gastric injury in rats via modulation of gastric secretion, oxidative stress and inflammation. Toxicol Rep. 2016;3:105–113. doi: 10.1016/j.toxrep.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Venkateswararao C, Venkataramana K. A pharmacological review on natural antiulcer agents. J Global Trends Pharmaceut Sci. 2013;4:1118–1131. [Google Scholar]
- 48.Souza R, Cardoso M, Menezes C, Silva J, De Sousa D, Batista J. Gastroprotective activity of α-terpineol in two experimental models of gastric ulcer in rats. DARU J Pharm Sci. 2011;19(4):277. [PMC free article] [PubMed] [Google Scholar]
- 49.Chauhan AK, Kang SC. Therapeutic potential and mechanism of thymol action against ethanol-induced gastric mucosal injury in rat model. Alcohol. 2015;49(7):739–745. doi: 10.1016/j.alcohol.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 50.de Oliveira Ramalho TR, de Oliveira MTP, de Araujo Lima AL, Bezerra-Santos CR, Piuvezam MR. Gamma-terpinene modulates acute inflammatory response in mice. Planta Med. 2015;81(14):1248–1254. doi: 10.1055/s-0035-1546169. [DOI] [PubMed] [Google Scholar]
- 51.Peixoto-Neves D, Silva-Alves K, Gomes M, Lima F, Lahlou S, Magalhães P, Ceccatto VM, Coelho-de-Souza AN, Leal-Cardoso JH. Vasorelaxant effects of the monoterpenic phenol isomers, carvacrol and thymol, on rat isolated aorta. Fundam Clin Pharmacol. 2010;24(3):341–350. doi: 10.1111/j.1472-8206.2009.00768.x. [DOI] [PubMed] [Google Scholar]
- 52.Barbosa RL, Santos JH, Cunha PS, Quintans-Júnior LJ, Bonjardim LR, de Souza Araújo AA. Vasorelaxant activity of p-cymene in superior mesenteric artery of rats. FASEB J. 2013;27(1):lb599–lb5lb. [Google Scholar]
- 53.Gandomi H, Abbaszadeh S, JebelliJavan A, Sharifzadeh A. Chemical constituents, antimicrobial and antioxidative effects of Trachyspermum ammi essential oil. J Food Process Preserv. 2014;38(4):1690–1695. [Google Scholar]
- 54.Vazirian M, Hekmati D, Ostad S, Manayi A. Toxicity evaluation of essential oil of Trachyspermum ammi in acute and sub-chronic toxicity experiments. Journal of Medicinal Plants. 2018;1(69):70–77. [Google Scholar]