Abstract
Objectives
Despite advances in our understanding of metabolic syndrome (MetS) and the treatment of each of its components separately, currently there is no single therapy approved to manage it as a single condition. Since multi-drug treatment increases drug interactions, decreases patient compliance and increases health costs, it is important to introduce single therapies that improve all of the MetS components.
Evidence acquisition
We conducted a PubMed, Scopus, Google Scholar, Web of Science, US FDA, utdo.ir and clinicaltrial.gov search, gathered the most relevant preclinical and clinical studies that have been published since 2010, and discussed the beneficial effects of dipeptidyl peptidase (DPP)-4 inhibitors to prevent and treat different constituent of the MetS as a single therapy. Furthermore, the pharmacology of DPP-4 inhibitors, focusing on pharmacodynamics, pharmacokinetics, drug interactions and their side effects are also reviewed.
Results
DPP-4 inhibitors or gliptins are a new class of oral anti-diabetic drugs that seem safe drugs with no severe side effects, commonly GI disturbance, infection and inflammatory bowel disease. They increase mass and function of pancreatic β-cells, and insulin sensitivity in liver, muscle and adipose tissue. It has been noted that gliptin therapy decreases dyslipidemia. DPP-4 inhibitors increase fatty oxidation, and cholesterol efflux, and decrease hepatic triglyceride synthase and de novo lipogenesis. They delay gastric emptying time and lead to satiety. Besides, gliptin therapy has anti-inflammatory and anti-atherogenic impacts, and improves endothelial function and reduces vascular stiffness.
Conclusion
The gathered data prove the efficacy of DPP-4 inhibitors in managing MetS in some levels beyond anti-diabetic effects. This review could be a lead for designing new DPP-4 inhibitors with greatest effects on MetS in future. Introducing drugs with polypharmacologic effects could increase the patient’s compliance and decrease the health cost that there is not in multi-drug therapy.
Graphical abstract.
ᅟ
Keywords: Dipeptidyl peptidase-4 inhibitors; Dyslipidemia; Gliptins, GLP-1; Hypertension; Metabolic syndrome
Objectives
Metabolic syndrome (MetS) is a major public health problem globally with a prevalence of over a billion people in the world [1]. It is a clustering of three of the five following clinical features: a waist circumference (≥ 102 cm for men and ≥ 88 cm for women), serum fasted triglycerides (≥ 150 mg/dl), serum HDL-C (˂ 40 mg/dl in men and ˂ 50 mg/dl in women), blood pressure (≥ 130 mmHg systolic blood pressure or ≥ 85 mmHg diastolic blood pressure) and a fasting blood glucose (≥ 100 mg/dl) [2]. Although the exact mechanism leading to MetS is still unclear, but there is accumulating evidence that supports the notion that MetS is due to a combination of genetic factors [3], diet [4] and exposure to endocrine disturbing chemicals [5] that could increase the rate of MetS in special occupations [6].
There is a direct association between central obesity and MetS [7, 8]. Increase in the mass of adipose tissue elevates the rate of lipolysis. It causes in hypertriglyceridemia, hyperglycemia, impaired glucose tolerance, insulin resistance and type 2 diabetes mellitus (T2DM). In obese patients, there is a pro-inflammatory state in some glucose regulatory organs that drive in an activation of prothrombotic signaling pathways and cardiovascular diseases [7]. Furthermore, individuals with MetS are susceptible to fatty liver, polycystic ovary syndrome, cholelithiasis, sleep apnea [2] and malignancies [7].
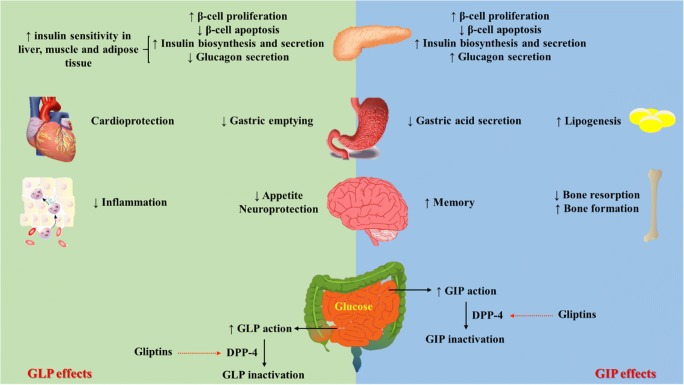
Gastric inhibitory polypeptide (GIP) and glucagon-like peptide (GLP)-1 are incretin hormones and both potentiate glucose-stimulated insulin secretion [9]. Dipeptidyl peptidase (DPP)-4 is a transmembrane glycoprotein amino peptide that is widely expressed by tissues, including the liver, lung, kidney, intestine, lymphocytes and endothelial cells and is measurable in plasma [10]. DPP-4 inactivates GIP and GLP and accounts for their relatively short half-lives in plasma [11]. Figure 1 demonstrates GLP-1 and GIP effects in tissues. DPP-4 inhibitors or gliptins are the new class of antidiabetic drugs that have pleiotropic effects and are capable of producing more than one benefit in addition to their blood glucose lowering properties [14].
Fig. 1.
Glucagon-like peptide-1 (left part) and gastric inhibitory polypeptide (right part) action in peripheral tissues. GIP is secreted from K-cells predominantly located in the proximal small intestine and GLP-1 is secreted from L-cells especially located in the distal ileum [12]. GIP is also expressed in hippocampus and its receptors are detectable in the hippocampus, cerebral cortex and olfactory bulb. It has a role in behavior modification and neural progenitor cell proliferation. Furthermore, the GLP-1 receptors and nerve containing GLP-1 are localized in parts of the brain and regulate appetite, gastric motility, and cardiac function. In the pancreas, GIP and GLP-1 increase glucose-dependent insulin secretion. Besides, GLP-1 inhibits glucagon secretion from α-cells glucose-dependently that reduces the risk of hypoglycemia. GLP-1 also increases insulin sensitivity. It reduces hepatic gluconeogenesis and increases glucose uptake and storage in skeletal muscles. GIP and GLP-1 evoke both lipolytic and lipogenic effects [9, 11]. Surprisingly, GLP-1 attenuates cutaneous inflammation by reducing both lymphocyte migration and macrophage activation, and decreasing cytokine production. It increases regulatory T cells and decreases invariant natural killer T [13]. There is evidence that GIP induces bone formation by stimulating cAMP and increasing intracellular Ca2+ level coupled to increase in alkaline phosphatase activity and collagen type 1 mRNA [9].GLP-1: glucagon-like peptide-1, GIP: gastric inhibitory polypeptide, DPP-4: dipeptidyl peptidase-4
Exercise, dietary poly phenols and herbs [7] including saffron [15], green tea [16], berberine [17] and avocado [18], and use of special diet for modification of gut microbiome containing prebiotics, probiotics or other dietary interventions [19] may partly influence some compartments of MetS. Beyond lifestyle modification, multiple pharmacological interventions are required to resolve MetS both underlying and related risk factors [7, 8].
In this regard, chimeric DPP-4/angiotensin converting enzyme inhibitors are introduced that exhibits polypharmacological effects to treating MetS [20]. However, the other studies showed the interaction between DPP-4 inhibitors and angiotensin converting enzyme inhibitors including angioedema and reducing antihypertensive effects of the later drugs [21], especially when angiotensin converting enzyme is maximally inhibited [22].
There are many studies investigating the effects of DPP-4 inhibitors on the different features of MetS, but there is no comprehensive study on this disorder as a whole. To improve patients’ compliance and reduce the cost and probable drug interactions, medicines with multiple effects may be of value in managing MetS. In this regards, we have tried to determine the effects of DPP-4 inhibitors on the components of MetS according to the preclinical (in vitro, in vivo) and clinical study observations. Herein, we explain DPP-4 inhibitors repositioning in handling MetS risk factors beyond its anti-diabetic properties.
Evidence acquisition
The data were collected by searching the PubMed, Scopus, Google Scholar, Web of Science, US FDA, utdo.ir and clinicaltrial.gov. The keywords used as search terms were dipeptidyl peptidase-4, DPP-4, sitagliptin, evogliptin, gosogliptin, vildagliptin, trelagliptin, omarigliptin, saxagliptin, linagliptin, gemigliptin, anagliptin, teneligliptin, alogliptin, metabolic syndrome, obesity, triglyceride, cholesterol, lipid, hypercholesterolemia hyperlipidemia, atherosclerosis, hypertension, blood pressure, hyperglycemia, hypoglycemia, blood glucose. All kinds of in vitro, in vivo and clinical studies, that have been published since 2010 have been included. Furthermore, the reference lists of key papers for further leads were searched. However, congress abstracts, as well as non-English language studies, were considered ineligible for inclusion.
Based on the aforementioned aims, gathered data were categorized in the following main headings pharmacology of DPP-4 inhibitors and the effects of DPP-4 inhibitors on metabolic syndrome.
Results
Pharmacology of DPP-4 inhibitors
Pharmacodynamics and therapeutic properties
DPP-4 inhibitors are incretin-base anti-diabetic agents. DPP-4 inhibitors increase the concentration of GLP-1 and GIP by inhibiting DPP-4 enzyme activity that in turn increase the insulin secretion from β-cells and reduce blood glucose [14]. Furthermore, DPP-4 inhibitors slow down the progression of T2DM by preserving pancreatic β-cell function. In comparison with GLP-1 receptor agonists, the other group of incretin base anti-diabetic agents, DPP-4 inhibitors are associated with good patient compliance due to their oral administration and low risk of side effects [23]. Because insulinotropic and glucose-lowering effects of GIP is absent in T2DM, it is concluded that the anti-hyperglycemic effects of DPP-4 inhibitors are mediated by increasing GLP-1 concentration [24]. DPP-4 inhibitors may evoke effects in type 1 diabetes (T1D), however, clinical studies have shown some controversial results [25].
Since the launch of sitagliptin in 2006, DPP-4 inhibitors have found an important place in T2DM treatment [23]. Nowadays, they are a suitable class for diabetes therapy as they have a low incidence of hypoglycemia, good reduction in hemoglobin A1c (HbA1c) and lack of weight gain [14]. It has been noted that DPP-4 inhibitors are weight-gain neutral [26] and may actually reduce weight [27]. Alogliptin, anagliptin, evogliptin, gemigliptin, linagliptin, omarigliptin, saxagliptin, sitagliptin, teneligliptin, trelagliptin and vildagliptin are commonly used DPP-4 inhibitors [28]. In comparison with sitagliptin (100 mg, once a day), it seems the newest derivatives of this class including evogliptin (5 mg, once a day), teneligliptin (20 mg, once a day) and omarigliptin (25 mg, once a week) drive greater decline in HbA1c after 12-week monotherapy [29–32]. More details are included in Table 1, where the cost, usage mode and different dosage forms of the related drugs are reported based on http://www.utdo.ir and drugs marketing status information are gathered from https://www.accessdata.fda.gov/scripts/cder/daf/. However, in these aforementioned databases, there is no information about some newly introduced drugs.
Table 1.
Some therapeutic properties of dipeptidyl peptidase-4 inhibitors in monotherapy of type 2 diabetic patients
| Drug | Approval history | Cost | marketing status | Usage mode | Different dosage | Daily dose | Efficacy in HbA1c reduction (%) | Ref. | side effects |
|---|---|---|---|---|---|---|---|---|---|
| Sitagliptin | in 2006, by US FDA, 50–100 mg once a day | 25 mg (per each): $17.18 50 mg (per each): $17.18 100 mg (per each): $17.18 | prescription (JANUVIA®) | Tablet, oral | 25, 50 and 100 mg | 100 mg once a day for over 12 weeks | 0.54 | [29] | gastrointestinal disturbance as abdominal pain, nausea and diarrhea, [29], nasopharyngitis, increase in uric acid, decrease in alkaline phosphatase and increase of white blood cell count [33], (acute pancreatitis and pancreas cancer, controversial data) [34], decrease of renal function [35] |
| 100 mg once a day for 24 weeks | 0.61 | [36] | |||||||
| Saxagliptin | in 2009, by US FDA, 2.5–5 mg once daily | 2.5 mg (per each): $16.33 5 mg (per each): $16.33 | prescription (ONGLYZA ®) | Tablet, oral | 2.5 and 5 mg | 2.5, 5 and 10 mg once a day for 24 weeks | 0.43, 0.46 and 0.54, respectively | [37, 38] | heart failure-controversial data- [27, 34], upper respiratory tract infections, urinary tract infections, headache [37], nasopharyngitis, back [39], abdominal pain [40], (lymphopenia, rash, increase of blood creatinine and creatine phosphokinase- severe side effects) [39]. |
| Linagliptin | in 2011, by US FDA, 5 mg once daily | 5 mg (per each): $16.46 | prescription (TRADJENTA®) | Tablet, oral | 5 mg | 5 mg once a day after 24-week | 0.69 | [41] | hypersensitivity reactions (urticaria, angioedema, localized skin exfoliation, bronchospasm, myalgia and pancreas disorders)3 [34, 40], Headache, hypertension, and back pain [40] |
| Alogliptin | in 2013, by US FDA, 25 mg once daily | Tablets (Alogliptin Benzoate Oral) 6.25 mg (per each): $7.80 12.5 mg (per each): $7.80 25 mg (per each): $7.80 Tablets (Nesina oral) 6.25 mg per each): $14.97 12.5 mg (per each): $14.97 25 mg (per each): $ 14.97 | prescription (NESINA ®) | Tablet, oral | 6.25, 12.5 and 25 mg, | 12.5 and 25 mg once a day after 26-week | 0.56–0.96 | [42] | dizziness, constipation [43], nasopharyngitis and upper respiratory tract infections [44], headache, hypoglycemic episodes [43, 44], pruritic rash [42], hepatic adverse effects [34, 42], acute pancreatitis, hypersensitivity reactions (anaphylaxis, angioedema- controversial data-[34] and severe cutaneous reactions including Stevens-Johnson syndrome- severe side effects) [44] |
| Vildagliptin | 2007 by European Union, 50 mg twice a day | – | – | – | – | 50 mg once a day, 50 mg twice a day and 100 mg once a day after 24-week | 0.5, 0.7 and 0.8 respectively | [34, 45] | pancreas- or hepatic-related adverse effects- controversial data- [34] |
| Gemigliptin | in 2012, by Republic of Korea with 50 mg once daily | – | – | – | – | 50 mg once a day, after 24-week | 0.713 from placebo baseline | [46] | nasopharyngitis, urinary tract infection, diarrhea, headache, cough, arthralgia and hypertension [47]. |
| Anagliptin | in 2012, by Japan with 200 mg daily | – | – | – | – | 100 mg or 200 mg twice a day for 12-week | 0.75–0.82, respectively | [48] | constipation, gastritis and somnolence, hypokalemia and muscle weakness [48] |
| Evogliptin | in 2015, by South Korea with 5 mg once daily | – | – | – | – | 5 mg once daily for 12 weeks | 0.7 | [30] | Nervous system disorders (dizziness and headache), sleep disorder, fatigue, abdominal discomfort, epigastric discomfort, urticarial, oral mucosa erosion, chest discomfort, urinary frequency, hyperglycemia, pruritus [30, 49], nasopharyngitis and arthralgia [50] |
| Omarigliptin | in 2015, by Japan with 25 mg once a week | – | – | – | – | 25 mg once a week for 12 weeks | 0.71 | [32, 51] | nasopharyngitis, bronchitis, influenza, pharyngitis, diarrhea, gastritis [30], dyspepsia, constipation, abdominal discomfort, constipation and abdominal discomfort [52] |
| Trelagliptin | in 2015, by Japan with 100 mg once a week | – | – | – | – | 12.5–200 mg once weekly for 12 weeks | 0.32 to 0.55 | [53, 54] | Nasopharyngitis |
| Teneligliptin | in 2012, by Japan with 20 mg once a day | – | – | – | – | 10, 20 and 40 mg once a day for 12 weeks | 0.8, 0.8 and 0.9, respectively | [31, 55, 56] |
US FDA: United States Food and Drug Administration
Pharmacokinetics
DPP-4 inhibitors differ from each other based on their pharmacokinetic properties (Table 2). Based on half-life and dissociation from the DPP-4 enzyme they are prescribed twice a day, once a day or once a week. Alogliptin [43, 44], evogliptin [30], gemigliptin [46], linagliptin [58, 59], omarigliptin [52], sitagliptin [61, 62], teneligliptin [55] and trelagliptin [53] have long half-lives with once daily dosing. Omarigliptin [52], and trelagliptin [53] are administrated once weekly. Although anagliptin, vildagliptin and saxagliptin have shorter half-lives, they remain bound to the DPP-4 enzyme longer than predicted by their half-lives and dissociate slowly. A twice-daily administration is sufficient for anagliptin and vildagliptin, and saxagliptin may even be used once daily [28, 57, 63, 64]. Evogliptin is normally prescribed as a daily dose [30].
Table 2.
Some pharmacokinetic characteristics of dipeptidyl peptidase-4 inhibitors
| Drug | Tmax (hour) | T1/2 (hour) | Administration interval | Main elimination route | Metabolism | Ref. |
|---|---|---|---|---|---|---|
| Alogliptin | 1 | 12.5–21.1 | Once daily | Renal | Hepatic CYP2D6 and 3A4 | [43, 44] |
| Anagliptin | 1.8 | 4.37 | Twice-daily | Renal | Cyano and amid groups hydrolysis, oxidation of the cyanopyrrolidine moiety | [57] |
| Evogliptin | 3.5–5.5 | 32.9 | Once daily |
34% of the dose is eliminated in urine Over half of the drug dose is metabolized, primarily by hydrolysis |
Hydrolysis, oxidation, dealkylation, sulfation and glucuronidation | [30] |
| Gemigliptin | 4.5 | 30.8 | Once daily | Metabolism, urine and faeces | Hepatic CYP3A4 | [46] |
| Linagliptin | 1.5 |
˃ 100 (terminal) 10 (for accumulation) |
Once daily | Faeces | CYP3A4 and P-gp (weak-to-moderate CYP3A4 inhibitor, P-gp substrate and mild P-gp inhibitor) | [58, 59] |
| Omarigliptin | 1 | 132–159 | Once weekly | Renal | Oxidation | [52] |
| Saxagliptin | 1.5 | 2.5–3 | Once-daily | Renal and fecal | CYP3A4/5 | [28, 60] |
| Sitagliptin | 1–4 | 11.8–14 | Once daily | Renal | CYP3A4 and 2C8 | [61, 62] |
| Teneligliptin | 1.33 | 26.9 | Once daily | Metabolism and renal | CYP3A4 and FMO3 | [55] |
| Trelagliptin | 1.3 | 54.3 | Once weekly | Renal | CYP2D6 | [53] |
| Vildagliptin | 0.5–1.5 | 1.6–2.8 | Twice-daily | Renal | Lack of significant P450 metabolism | [63, 64] |
Tmax: time in which the maximum serum concentration of the drug is seen
T1/2: half-life
DPP-4 inhibitors undergo hepatical-CYP- dependent and independent metabolism. Some of them have active metabolite after metabolism in the liver and hepatic impairment may influence their pharmacokinetics, but clinical studies could not prove it [58]. They are eliminated renal, except linagliptin that is exerted biliary [28]. Totally, the gliptin-dose adjustment is necessary for renal failure [28].
Drug interactions
The risk of hypoglycemia increases in combination therapy of DPP-4 inhibitors with sulfonylureas, insulin or insulin secretagogues, but not with metformin or thiazolidinedione [34, 40, 48].
Some of them are P-glycoprotein (P-gp) substrates. In combination with an inhibitor of P-gp, the absorption of DPP-4 inhibitors increases [65], and with a strong P-gp inducer their exposure and efficacy decreases [40]. About DPP-4 inhibitors that are metabolized with CYP isozymes, caution should be taken in combination therapy of them with strong CYP3A4/5 inhibitors [37] or inducers [40]. Besides, combination therapy with angiotensin-converting enzyme inhibitors may increase the risk of angioedema that might be associated with elevation of bradykinin and substance P concentration [34]. The other related drug interactions are summarized in Table 3.
Table 3.
Dipeptidyl peptidase-4 inhibitors drug interactions
| Drug | Side effects | Refe | |
|---|---|---|---|
| In combination therapy with sulfonylureas, insulin (Except with vildagliptin and alogliptin) or insulin secretagogues, but not with metformin or thiazolidinedione | Hypoglycemia | [34, 40, 48] | |
| Angiotensin-converting enzyme inhibitors | angioedema | [34] | |
| CYP3A4 | inhibitors | increase saxagliptin exposure and efficacy | [37] |
| inducers | reduce linagliptin exposure and efficacy | [40] | |
| Glimepiride | arthralgia, back pain and headache | [40] | |
| Metformin |
cough (linagliptin) arthralgia, back pain and headache |
[40] | |
| P-gp | inhibitors (Cyclosporine) | increases sitagliptin absorption and its plasma concentration | [65] |
| inducers | reduces linagliptin exposure and efficacy | [40] | |
| Pioglitazone |
headache and upper respiratory tract infection (sitagliptin) hyperlipidemia and weight gain |
[29] [40] | |
| Sulfonylureas | nasopharyngitis and lipid abnormalities, cough (linagliptin) | [40] | |
| Thiazolidinedione | peripheral edema (saxagliptin) | [39]. | |
Side effects
Acute pancreatitis and arthralgia have been reported as DPP-4 inhibitors side effects [27]. Another study proposed diabetic medication containing DPP-4 inhibitors might increase the risk of inflammatory bowel disease [66]. Infections, gastrointestinal adverse effects, hypersensitivity and skin-related reactions, malignancies, renal and hepatic toxicity are the other possible gliptin rare and controversial adverse effects [34]. DPP-4, known as CD26, is also expressed on some immune cell subtypes and in the epidermis. Cutaneous reactions may result from inhibition of this enzyme with some DPP-4 inhibitors [13]. The Side effects of dipeptidyl peptidase-4 inhibitors are categorized in Table 1.
These side effects occur with mild-to-moderate severity and some in rare cases. In this regards, DPP-4 inhibitors are considered safe drugs and no dose limiting toxicity has been observed in using them until now. However, since this class of antidiabetic drugs has been launched a little more than one decade, pharmacovigilance monitoring seems necessary for their undefined side effects.
The effects of DPP-4 inhibitors on metabolic syndrome
Effects on lipid profile, body weight and related complications
Non-high-density lipoprotein (HDL)-cholesterol, including low-density lipoprotein cholesterol (LDL-C), intermediate density lipoprotein cholesterol (IDL-C), lipoprotein(a), very-low-density lipoprotein cholesterol (VLDL-C; including VLDL remnants) and chylomicron particles (including chylomicron remnants) are atherogenic lipids and have the ability to increase the risk of atherosclerosis. Atherogenic dyslipidemia, elevation in LDL-C and non-HDL-C and reduction in HDL-C, is seen in MetS and T2DM. Insulin resistance and MetS are related to each other. Usually, insulin resistance is accompanied with abnormal fat distribution, predominant upper body fat, obesity and evidence of inflammatory conditions [2, 67].
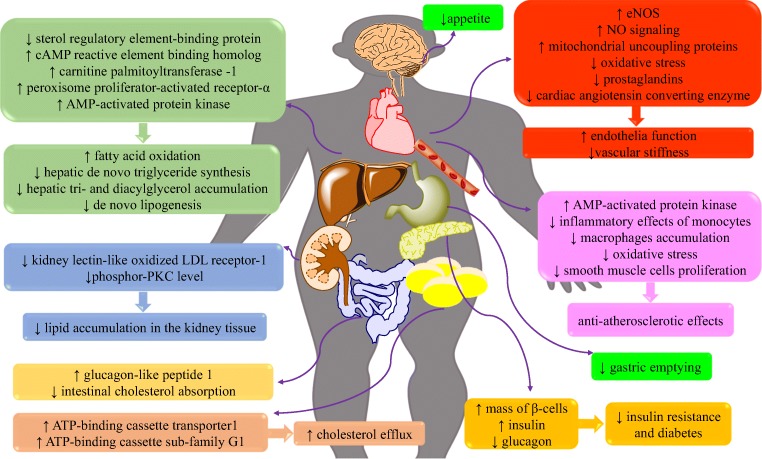
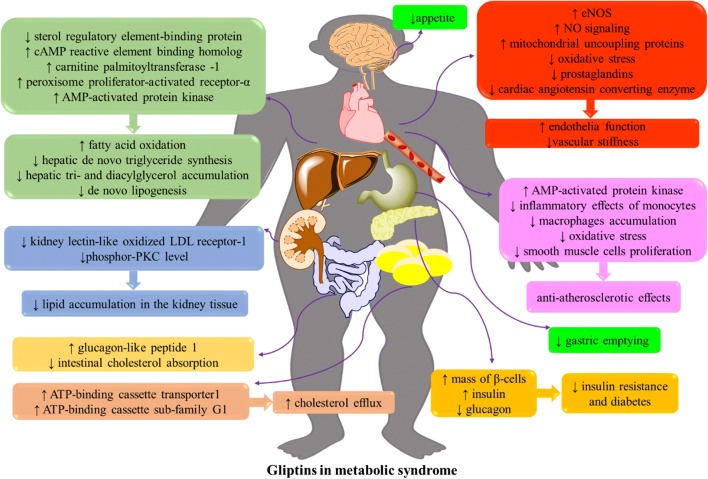
DPP-4 inhibitors may evoke some lipid-lowering and anti-atherogenic outcomes besides their glycemic effects via different mechanisms that are discussed in Fig. 2.
Fig. 2.
Gliptin therapy effects in managing different component of metabolic syndrome (for more detail refer to the main text)
Preclinical studies
The anti-atherosclerotic effects of anagliptin have been attributed to suppression of smooth muscle cell proliferation and blocking inflammatory effects of monocytes [68]. Alogliptin downregulates IL-6 gene expression in mononuclear cells but not in aortic endothelial cells [69]. In HepG2 cells, sterol regulatory element-binding protein activity was suppressed with anagliptin [70]. A model of DPP-4 knockdown in human HepG2 showed that hepatic DPP-4 induces insulin resistance accompanied by a reduction in glycogen storage and the increase of glucose output and lipid accumulation in the liver [71]. The incubation of 3 T3-L1 adipocytes with vildagliptin increased the gene expression and protein level of ATP-binding cassette transporter1 and ATP-binding cassette sub-family G member 1 accompanied with the increase in cholesterol efflux [72]. Besides, its administration in metabolic syndrome is accompanied by a decrease in vascular inflammation and atherosclerosis [73].
So many studies mentioned that administration of DPP-4 inhibitors improves lipid profile and decreases high lipid diet-induced vascular damages and atherosclerosis. These effects are linked to the decrease of intestinal cholesterol absorption [74]. The other mechanism is attributed to an increase in the serum concentration of fibroblast growth factors19 and 21, an elevation in the hepatic level of peroxisome proliferator-activated receptor-α and cyclic adenosine monophosphate (cAMP) reactive element binding homolog, upregulation of liver expression of carnitine palmitoyltransferase −1 and so downregulation of fatty acid synthase [75]. Mice studies showed that DPP-4 inhibitors reduce de novo synthesis of triglycerides in the liver [70]. They suppress hepatic accumulation of triacylglycerol and diacylglycerol, and modulate hepatic mitochondrial function [76]. DPP-4 inhibitors prevent lipid accumulation in the kidney tissue by inhibition of kidney lectin-like oxidized LDL receptor-1 expression and phosphor- protein kinase C level [77]. These drugs may evoke promising therapeutic impacts in MetS by activating 5’ AMP-activated protein kinase, fatty acid oxidation and suppressing both de novo lipogenesis and oxidative stress [78].
Besides, the administration of these drugs decreases atherosclerotic lesions formation and reduces leukocytes adhesion to endothelial cells by activating AMP-activated protein kinase [79]. DPP-4 inhibitors suppress macrophages accumulation and inflammation [80]. They ameliorate atherosclerosis by shifting monocytes phenotype to M2 macrophages via SDF-1/CXCR4 signaling [81]. (Table 4).
Table 4.
Summary of in vitro and animal studies on dipeptidyl peptidase-4 inhibitors in metabolic syndrome
| Type of study | Effect | Study design | Main results | Ref. | |
|---|---|---|---|---|---|
| In vitro studies | Anti-lipidemic/atherogenic activity | Rat aortic smooth muscle cells, pretreated with anagliptin 1.1 or 100 μM for 10 min then exposed to recombinant human (rh) dipeptidyl peptidase-4 (DPP-4) (5–500 ng/ml) for 20 h | ↓ DPP-4-induced smooth muscle cells proliferation | [68] | |
|
Rat aortic smooth muscle cells, pretreated with ZM241385 (an adenosine A2A receptor antagonist) 100 nM, or MRS1754 (an adenosine A2B receptor antagonist) 100 nM for 10 min and then pretreated with anagliptin 100 μM and finally stimulated with soluble DPP-4 (500 ng/ml) for 5 min |
Anagliptin inhibitory effects on smooth muscle cells proliferation is independent of adenosine A2 receptor-signaling pathway | ||||
| Human THP-1 cells, co-treated with LPS 1 μg/ml and anagliptin (1–100 μM) for 24 h | ↓ TNF-α concentration | ||||
| Human THP-1 cells, co-treated with LPS 1 μg/ml and anagliptin (1 or 100 μM) and ZM241385 (100 nM) for 24 h | Anagliptin inhibitory effects on TNF-α production in THP-1 cells is independent of adenosine A2 receptor-signaling pathway | ||||
| Human U937 mononuclear cells, with or without 10 ng/mL of lipopolysaccharide | 0.5, 1, or 5 nM ofalogliptin for 24 h |
↓ IL-6 concentration ↓ IL-6 mRNA expression |
[69] | ||
| Human aortic endothelial cells, with or without 10 ng/mL of lipopolysaccharide | Alogliptin had no effect on LPS-stimulated IL-6 secretion | ||||
| DPP-4 knockdown in HepG2 |
↑ basal glycogen ↑ insulin signaling ↓glucose-6-phosphatase cDNA expression ↓triglyceride (TG) content and sterol regulatory element-binding protein 1 expression ↑Peroxisome proliferator-activated receptor alpha and carnitine palmitoyltransferase I expression |
[71] | |||
| 3 T3-L1 adipocytes, Vildagliptin (10 μM) |
↑ Liver X receptor alpha, ATP-binding cassette transporter A 1 and ATP-binding cassette sub-family G member gene expression and protein level ↑ cholesterol efflux |
[72] | |||
| Human monocytes, Sitagliptin 50 ng/ml for one week |
↓ classically activated M1 macrophages ↑ alternatively activated M2 macrophages ↑ CXC chemokine receptor 4 expression on M2 but not M1 macrophages |
[81] | |||
| Vasodilator effects | Male C57BL/6, isolated aorta method, alogliptinn to tissue bath |
vascular relaxation via NO and endothelium-derived hyperpolarizing factor -mediated mechanisms endothelium-dependent relaxations through Src and PI3-kinase/Akt pathways |
[82]. | ||
| Animal studies | Anti-lipidemic/atherogenic/obesity | Male apoE−/− mice, anagliptin 0.3% in diet for 16 weeks |
↓ plasma DPP-4 activity ↓ total cholesterol ↓ very-low-density lipoprotein cholesterol (VLDL-C) ↓ low-density lipoprotein cholesterol (LDL-C) ↓ monocyte and macrophage accumulation and atherosclerotic lesions of aortic sinus ↓ α-smooth-muscle actin positive area and TNF-α-positive lesions in plaque area of aortic sinus |
[68] | |
| Male apoE−/− mice on high fat diet for 7 weeks, finally received streptozocin (200 mg/kg, intraperitoneal, single dose), after four weeks: alogliptin, 15 mg/kg, gavage, 24 weeks |
↓ fasting blood sugar ↓ cholesterol ↓ TG ↓ atherosclerotic lesions in the aortic origin ↓ IL-6 and IL-1β expression in atherosclerotic plaques |
[69] | |||
| Male LDL- receptor -deficient mice, anagliptin 0.3% of diet for 2 weeks |
↓ total cholesterol, TG, VLDL-C and LDL-C ↓ hepatic sterol-regulatory element-binding protein-2 gene expression at night |
[70] | |||
| Male human cholesteryl ester transfer protein/human apoB100 (CETP-apoB100) transgenic mice on high fat diet for 3 months and continued with high fat diet and sitagliptin 500 mg/kg in drinking water for 4 weeks |
↓ body weight ↓ epididymis fat mass ↓ hemoglobin A1c (HbA1c) ↓ blood glucose ↓ insulin ↓ homeostatic model assessment (HOMA) of β-cell function and insulin resistance (IR) (HOMA-IR) ↑ adiponectin ↓ TG ↓ cholesteryl ester transfer protein ↓ hepatic total cholesterol ↑ fecal cholesterol excretion ↑ macrophage-derived 3H-cholesterol fecal excretion ↓ intestinal cholesterol absorption ↓ apoB100 and apoB48 |
[74] | |||
|
Male C57/BL6 mice on high fat diet for 16 weeks to induce nonalcoholic fatty acid liver disease (NAFLD) after induction of NAFLD: sitagliptin 15 mg/kg/day, gavage, for 16 weeks |
Improved liver disease morphology of NAFLD ↓ blood lipid, alanine transaminase and aspartate aminotransferase ↓ serum fibroblast growth factor-19 and 21 ↓ hepatic gene expression of fatty acid synthase and ↑ carnitine palmitoyltransferase 1 ↑ hepatic level of peroxisome proliferator-activated receptor alpha and cyclic AMP-responsive element-binding protein H |
[75] | |||
| C57Bl/6 mice on Western Diet and with MK0626 10 mg/kg/day via chow |
↓ hepatic steatosis ↓ hepatic insulin resistance ↓ hepatic accumulation of di- and triacylglycerol ↓ mitochondrial incomplete palmitate oxidation ↓ plasma uric acid levels ↑ indices of pyruvate dehydrogenase activity ↑ tricarboxylic acid cycle flux ↑ hepatic TAG secretion ↑ hepatic PGC-1α and CPT-1 mRNA expression and hepatic Sirt1 protein content |
[76] | |||
| Apolipoprotein E knockout (ApoE−/−) mice on high cholesterol diet for 6 weeks, accompanied with tenegliptin (20 mg/kg/day, orally) |
↓ serum total cholesterol, LDL-C and creatinine ↓ renal lipid accumulation ↓ glomerulosclerosis ↓ lectin-like oxidized low-density lipoprotein (LDL) receptor-1 (LOX-1) gene expression in the kidney ↓ phospho-PKC level in the kidney |
[77] | |||
| C57BL/6 J mice on western diet, after 12 weeks: supplemented with gemigliptin 300 mg/kg/day, 4 weeks |
↓ body weight ↓ hypocholesteremia ↓ adipocytrs hyperthrophia ↓ macrophages infiltratioin into adipose tissue ↑ uncoupling protein 1 expression in subcutaneous fat ↑ insulin sensitivity ↓ hepatic TG accumulation ↓ hepatic inflammation and fibrosis |
[78] | |||
| Male Japanese white rabbits on high cholesterol diet, after 2 weeks accompanied with anagliptin, 0.3% w/v, in drinking water, for 10 weeks |
↓ total cholesterol, VLDL ↓ cholesterol absorption ↓ serum and vascular DPP-4 activity ↑ active glucagon like peptide-1 (GLP-1) ↑glucose dependent insulinotropic polypeptide (GIP) ↓ aortic and coronary atherosclerotic lesions ↓ gene expression of TNF-α and IL-6 in the carotid arteries |
[80] | |||
| ApoE−/− mice on high fat diet for 12 weeks, accompanied with sitagliptin 500 mg/kg/day (12 weeks for aorta study and 3 days for blood studies) |
↓ aortic atherosclerotic plaque area ↑ the number of M2 macrophages in the aortic wall ↑ C-X-C chemokine receptor type 4 expression on M2 macrophages in comparison with M1 macrophages in aorta |
[81] | |||
| Anti-hypertension | Male spontaneously hypertensive rats, sitagliptin 10 mg/kg/day, gavage, 2 weeks |
↓ blood pressure ↑ renal blood flow ↑ renal artery endothelial function ↑ endothelium-dependent relaxation in renal arteries ↑ activity of PKA, LKB1, AMPKα and eNOS in renal artery |
[83] | ||
| spontaneously-hypertensive-rat, vildagliptin 10 mg/kg/day, in drinking water, for 12 weeks |
In vivo: ↓ blood pressure In isolated aorta: restored endothelium-dependent relaxation |
[84] | |||
| Male Dahl salt-sensitive hypertensive rats, on high salt regime (NaCl 8%) for 2 weeks, continued with linagliptin 3 mg/kg/day, orally, for 4 weeks |
↓ left ventricular weight/tibia length ↓ liver weight/tibia length ↓ cardiac interstitial fibrosis ↓ ED-1-positive cell numbers ↓ coronary arterial remodeling ↓ cardiac and carotid artery dihydroethidium (DHE) relative fluorescence ↓ cardiac p67phox and angiotensin-converting enzyme protein level ↓ carotid artery p22phox protein level ↑ endothelium-dependent and independent relaxation in carotid artery ↓ serum DPP-4 activity ↑ serum insulin and GLP-1 concentration |
[85] | |||
| Spontaneously hypertensive (SHR)/NDmcr-cp (cp/cp) (SHRcp) rats, tenegliptin 10 mg/kg/day, orally, for 12 weeks |
↓ FBS ↓ fasting insulin ↓ Intercellular adhesion molecule and TNF-α gene expression ↑ eNOS gene expression ↑ vasodilatory responses to acetylcholine |
[86] | |||
| Glucose lowering effects |
Zucker diabetic fatty rats Sitagliptin, 10 mg/kg/day, gavage, 6 weeks |
↓ triglyceride, systoli, diastolic and mean aterail pressure ↓ glycemia, HbA1C and ↑ insulin ↓High-sensitive C-reactive protein, IL-1β, serum total antioxidant status, heart and pancrase MDA ↓ inflammatroy state, fibrosis, intra islet vaculation and congestion in pancratic histology |
[87] | ||
|
spontaneously hypertensive rat – obese strain sitagliptin, 30 mg/kg/day, in drinking water, 6 weeks |
↓ fasting glucagon by 33% In oral glucose (6 g/kg) tolerance test: -↓ plasma glucose -↑ insulin secertion - shiftes peak insulin secretion earlier |
[88] | |||
| Streptozotocin-diabetic mice on des-floor-sitagliptin in chow for 39 days (from 14 days before, during and 20 days after induction of diabetes) |
↓ blood glucose level and HbA1C ↑ glucose-stimulated insulin secretion ↓ fasted plasma glucagon to insulin ratio ↑ pancreatic insulin content ↓ pancreatic glucagon content According to the morfological evaluation: ↓ βcell loss ↓ α cell expasion |
[89] | |||
| high fat diet/streptozotocin mice received alogliptin (5, 15, 45 mg/kg/day) oral, for 10 weeks |
↓ postprandial blood glucose levels, 6-h fasting glucose levels, HbA1C levels and oral glucose tolerance test ↑ pancreas function, its β-cell mass composition, insulin content and islet endocrine secretion ability back to the normal expression: Pfkfb3, Agt, Tgfb1, G6pc, Neurod1, Ins1 |
[90] | |||
Clinical studies
It has been discussed that higher DPP-4 gene expression in visceral adipose tissue is associated with metabolic disturbances and inflammation [91]. A meta-analysis reported that glucose lowering drugs result in varying effects on the lipid profile. It concluded that DPP-4 inhibitors evoke more control on the lipid profile than sulfonylureas [92]. In this regards, some clinical studies mentioned on the diminishing effects of DPP-4 inhibitors on triglyceride-rich lipoprotein remnant [93], remnant-like particle cholesterol [94], and the decrease of the ApoB48 level after a high-fat meal [95].
A meta-analyses review investigated that sitagliptin alone or in combination therapy possesses improving effects on TG and HDL concentration in patients with T2DM [96]. In T2DM, administration of sitagliptin reduced triglyceride-rich ApoB-containing lipoprotein levels by decreasing the synthesis of these particles [97]. A clinical study linked the antiatherogenic effects of sitagliptin to its anti-inflammatory properties [98].
Administration of sitagliptin and vildagliptin in patients with T2DM reduced arterial stiffness, blood pressure, lipid profile and inflammatory parameters beyond glucose control [99].
Administration of vildagliptin to patients with T2DM reduced blood pressure, body weight and fasting lipid profile [100]. Furthermore, administration of alogliptin to T2DM patients evoked improving effects on the level of HbA1c, blood glucose, total cholesterol and LDL-C [101]. Administration of anagliptin to T2DM restored fasting and postprandial hyperlipidemia and hyperglycemia [102]. In T2DM patients, alogliptin treatment in comparison with the other conventional treatments induced better-preventing effects on progression of carotid atherosclerosis [103].
By considering the aforementioned data from pre-clinical and clinical studies in section 4.1, gliptin therapy decreases lipid synthesis. They inhibit sterol regulatory element-binding proteins. Besides, they increase lipid peroxidation by upregulating carnitine palmitoyltransferase-I and leads to a decrease in the intrahepatic lipid content. These drugs increase cholesterol efflux by upregulating ATP-binding cassette transporter1 and ATP-binding cassette sub-family G1. DPP-4 inhibitors decrease appetite and delay gastric emptying. DPP-4 inhibitors evoke suppressing impacts on smooth muscle cells proliferation and inflammatory effects of monocytes. In this regards, they restored dyslipidemia, obesity, atherosclerosis and its related complications independent on GLP-1 increases in some levels (Fig. 2).
Effects on high blood pressure
Hypertension is considered another important cardiovascular risk factor in the MetS [2]. There is a growing body of data has indicated that oral DPP-4 inhibitors play some antihypertensive actions via different mechanisms.
Preclinical studies
In isolated rat aorta, it has been shown that DPP-4 inhibition induces vasodilation via NO release and the activation of vascular potassium channels, independently to the GLP-1 pathway [82]. Isolated aorta from rats with MetS was sensitive to vasodilator effects of sitagliptin. The protective effects of sitagliptin on hyperglycemia-induced vascular damages are linked to NO signaling and epigenetic alteration [104].
In spontaneously hypertensive rats, administration of sitagliptin improved endothelium-dependent relaxation in renal arteries, restored renal blood flow and reduced systolic blood pressure. These effects were linked to the protective effects of sitagliptin on endothelial cell function and NO signaling. Restoration of endothelial NO synthesis (eNOS) activity by sitagliptin was attributed to its effects on cAMP, protein kinase A and glucagon-like peptide 1 receptors [83].
A later study showed that DPP-4 inhibition activates mitochondrial uncoupling proteins that in turn reduces oxidative stress and restores endothelium-dependent contractions in hypertension [105].
In spontaneously-hypertensive-rats, administration of vildagliptin decreased blood pressure and restored endothelium-dependent relaxation of the isolated aorta, via inhibition of prostaglandins [84]. A study showed that linagliptin ameliorated cardiovascular injury in salt-sensitive hypertensive rats via attenuation of cardiac angiotensin-converting enzyme and oxidative stress, independently of blood pressure and blood glucose [85]. Besides, administration of tenegliptin to hypertensive rats restored glycaemia and improved endothelial dysfunction via upregulation of eNOS [86].
Clinical studies
In healthy volunteers, antihypertensive effects of sitagliptin has been shown and discussed that this effect is not directly related to GLP-1 or brain natriuretic peptide concentration [106], it could induce vasodilation by increase in growth hormone secretion [107].
In hypertensive T2DM patients, administration of sitagliptin decreased systolic blood pressure independent of glucose-lowering effects [108]. In patients who suffered from diabetic nephropathy, adding sitagliptin (50 mg/day, 3 months) decreased urine albumin-to-creatinine ratio through the decrease in blood pressure and estimated glomerular filtration rate, especially in patients with micro- and macroalbuminuria. These effects were independent of the reduction in HbA1c concentration [109]. Furthermore, administration of vildagliptin to patients with T2DM and hypertension improved endothelial function and arterial stiffness, independently of glycemic control [110].
However, the other study discussed that although adding sitagliptin to metformin-treated T2DM patients evokes improving impacts on body mass index and TG level but has no beneficial effects on arterial stiffness and blood pressure [111]. The neutral effects of sitagliptin on cardiovascular function have been shown in metformin treated T2DM patients with no history of heart failure or renal insufficiency [112]. Besides, a systematic review and meta-analysis in patients with T2DM revealed there are no significant differences between DPP-4 inhibitors and other antidiabetic drugs on blood pressure changes. DPP-4 inhibitors may exert a modest blood pressure-lowering effects in comparison with placebo [113].
There are controversial clinical data on antihypertensive effects of DPP-4 inhibitors. But totally, DPP-4 inhibitors increase NO bioavailability and signaling and upregulate eNOS. They evoke anti-oxidative effects and inhibit prostaglandins and cardiac angiotensin-converting enzyme. They might decrease the chance of vascular stiffness and hypertension via improvement of endothelial function. Some of these cardioprotective and vasodilatory effects are mediated by specific receptors for DPP-4 or GLP-1 (Fig. 2).
Effects on blood glucose level
Diabetes mellitus is often present in MetS and obesity [1]. DPP-4 inhibitors generally are considered as glucose-lowering agents in T2DM with minimal risk of hypoglycemia. Many preclinical and clinical studies support their effectiveness in T2DM.
Preclinical studies
Chronic administration of sitagliptin favorably improved hyperglycaemia induced dysmetabolism, inflammation and oxidative stress [87]. Furthermore, it favorably decreased fasting glucagon concentration, improved glucose tolerance and restored early phase insulin secretion [88]. Protective effects of des-floor-sitagliptin were linked to alleviation of pancreatic β-cell apoptosis and decrease in pancreatic α-cell proliferation [89].
Alogliptin administration exerts protective effects against development of diabetes linked to its ability in decreasing endoplasmic reticulum and oxidation stress, increasing β-cell differentiation and proliferation, and restoring β-cell function [90]. Furthermore, it has been discussed that linagliptin delays the onset of diabetes by increasing total islet mass and total β-cell mass [114]. In streptozotocin-diabetic rats, vildagliptin favorably improved glucose levels by upregulatin of GLP-1, glucose transport protein 4 and sterol-regulatory-element-binding proteins -1c mRNA levels and increase in β-cell proliferation [115].
Clinical studies
In T2DM patients, whose blood glucose inadequately was controlled with insulin monotherapy adding sitagliptin was a good choice. Sitagliptin suppressed glucagon secretion in response to a meal [116]. Another study reported that adding sitagliptin to metformin in T2DM patients reduces HbA1c without hypoglycemia occurrences and weight gain [117], especially in younger and older patients [118]. In T2DM patients, switching metformin with glimepiride to metformin plus sitagliptin reduced the risk of hypoglycemia. Furthermore, sitagliptin is considered as an optimal treatment to prevent hyperglycemia relapse after remission in obese African American subjects with diabetic ketoacidosis and sever hyperglycemia. This effect is related to improve in β–cell function [119].
Adding acarbose to ongoing alogliptin therapy [120] or sitagliptin to ongoing acarbose therapy [121] decreased blood glucose fluctuations and prevented postprandial insulin secretion. One study introduced sitagliptin as a good choice in insufficiently controlled T2DM. It showed worth effects on decreasing HbA1c and fasting blood sugar levels [103]. Sitagliptin plus basal insulin regimen is as effective and safe as the basal-bolus insulin therapy for the inpatient management of general medicine and surgery patients with T2DM patients [122].
A study in 2010 showed that vildagliptin has a good effect on glycemic control and decrease of hypoglycemic episodes in T2DM patients [123]. In comparison with the low dose of glimepiride (2 mg/day), adding vildagliptin to T2DM patients on metformin monotherapy decreased the risk of hypoglycemic episodes [124]. In patients with T2DM, adding vildagliptin to sulfonylurea [125] or insulin [126] improved glycemic control without increasing the risk of hypoglycemia and weight gain. Besides, administration of vildagliptin to T2DM patients in the end-stage renal disease under hemodialysis decreased blood glucose fluctuation [127].
Switching anti-hyperglycemic drugs from sulfonylurea to sitagliptin reduced episodes of hypoglycemia in T2DM Muslim patients who were fasted during Ramadan [128]. However, a meta-analytical study in comparing DPP-4 inhibitors (vildagliptin and sitagliptin) with gliclazide in T2DM fasted patients in Ramadan revealed that the incidence of hypoglycemia is low with no differences. The authors advised receiving treatment at evening after breaking the fast [129].
A retrospective study on T2DM patients on metformin that received sulfonylurea or linagliptin as the second line-therapy revealed the risk of hypoglycemia is lower in linagliptin treated group and it decreases associated health-care costs [130]. Administration of teneligliptin to T2DM patients evoked a good glycemic control. It decreased 2-h postprandial glucose, 24-h mean glucose and fasting plasma glucose with no adverse effects and hypoglycemia occurrence [131]. Besides, combination therapy of metformin plus DPP-4 inhibitors resulted in lower healthcare costs than with metformin and other oral antidiabetic drugs in relation to better metabolic control, better compliance, and lower incidence of hypoglycemia [132].
DPP-4 inhibitors decrease postprandial hyperglycemia not only in T2DM but also in T1D [133]. In HIV+ patients with impaired glucose tolerance who are on combination antiretroviral therapy, sitagliptin favorably improved inflammation, chronic immune cell activation and reduced glucose area under the curve [134]. In a model of prednisolone-induced diabetes, although sitagliptin improved pancreatic function, it had no improving effects on glucose tolerance [135].
Furthermore, the presence of MetS influences on the response incretin therapy. The efficacy of DPP-4 inhibitors is more than exenatide treatment in T2DM patients without MetS and vice versa [136].
DPP-4 inhibitors are the newest class of drugs for managing T2DM. They inhibit DPP-4 enzyme activity and increase the half-life GLP-1. GLP-1 increases insulin secretion from pancreas β-cells and decrease glucagon secretion from α-cells. Besides, they elevate the mass of β-cells in the pancreas. Totally, they increase insulin sensitivity and have worth effects on lowering postprandial blood sugar and HbA1c (Fig. 2).
Discussion and conclusion
There is a variety of in vitro, in vivo (Table 4), and clinical studies (Table 5) showing that DPP-4 inhibitors attenuate MetS risk factors in some levels (Fig. 2). DPP-4 inhibitors are the newest anti-diabetic drugs with efficient glycemic control and the lowest hypoglycemia fluctuation. They evoke favorable metabolic and vascular effects beyond glucose-control properties. They inhibit inflammatory and atherogenic pathways, the main predisposing factors for cardiovascular diseases. According to the literate data, administration of DPP-4 inhibitors mitigates hypertension, attenuates dyslipidemia and diminishes weight gain. DPP-4 inhibitors improve endothelial function and evoke vasodilation via DPP-4 specific receptors and activating eNOS. They restored dyslipidemia by the reduction in lipids absorption and synthesis and increase of lipids exertion. DPP-4 inhibitors seem safe drugs with no severe side effects.
Table 5.
Summary of clinical studies on dipeptidyl peptidase-4 inhibitors in metabolic syndrome
| Effects | Study design | Results | Ref. |
|---|---|---|---|
| Anti-hyperlipidemia/atherosclerosis/obesity | Patients with diabetes and chronic kidney disease undergoing hemodialysis, teneligliptin 20 mg/day for 12 weeks |
↓ hemoglobin A1c (HbA1c) ↓ fasting plasma glucose (FPG) and remnant-like particle cholesterol |
[93] |
| Patients with type 2 diabetes mellitus (T2DM) gemigliptin 50 mg/day, at the end, subjects underwent a high-fat meal tolerance test |
↓ fasting and post prandial glucose levels ↓ fasting and post prandial triglyceride (TG) levels ↓ fasting, peak and total area under curve levels of ApoB48 |
[95] | |
|
patients with T2DM sitagliptin 100 mg/day for 6 weeks |
↓ plasma triglyceride ↓ apoB-48 ↓ free fatty acid concentrations ↓ HbA1c ↓ plasma glucose levels ↓ production rate and pool size of triglyceride-rich lipoprotein apoB-48 ↓ pool size of VLDL apoB-100 |
[97] | |
| T2DM patients sitagliptin 25–100 mg/day for 12 months |
After 3 months: ↓ HbA1c ↓ pentraxin-3 level ↓ proinsulin level ↓proinsulin/insulin ratio |
[98] | |
| patients with T2DM anagliptin 200 mg/day for 3 months |
↓ waist size ↓ plasma glucose ↓ HOMA-IR ↓ glycoalbumin ↓ HbA1c ↓ total cholesterol ↓ TG ↓ LDL ↓ apoliporpotein B ↓ apoliporpotein B48 ↓ high molecular weight adiponectin |
[102] | |
| T2DM patients with no history of cardiovascular diseases alogliptin 24 months |
↓ thickness of the carotid arteries ↓ serum IL-6 concentration ↓ serum intercellular adhesion molecule-1 concentration ↓ serum vascular cell adhesion molecule-1 concentration |
[103] | |
| Anti-hypertension |
Patients with T2DM and hypertension sitagliptin 50 mg on alternative days for 6 months |
↓ systolic blood pressure ↓ HbA1c |
[108] |
|
Patients with T2DM and hypertension vildagliptin 100 mg/day, twice a day, for 12 weeks |
↑ endothelial function ↓ arterial stiffness |
[110] | |
| glycaemia control | T2DM patients sitagliptin 50 mg/day for 12 weeks |
↓ blood glucose ↑ active glucagone like peptide-1 (GLP-1) ↑ active gastric inhibitory polypeptide (GIP) ↓ total GLP-1 ↓ total GIP ↓ glucagon |
[116] |
| T2DM patients sitagliptin, 100 mg/day + metformin, for 52 weeks |
↓ HbA1c no body weight gain no hypoglycemia events |
[117] | |
|
T2DM patients on glimepiride Vildagliptin was added 50 mg/day, 24 weeks |
↓ HbA1c | [125] | |
|
T2DM patients on insulin therapy ± metformin vildagliptin was added 50 mg/day, twice a day, 24 weeks |
↓ HbA1c ↓ hypoglycemia |
[126] | |
|
T2DM patients on sulfonylurea ± metformin sulfonylurea was switched to sitagliptin 100 mg/day |
↓ hypoglycemia in T2DM patients who was fasted during Ramadan | [128] | |
|
T2DM patients teneligliptin 10 or 20 mg/day for 4 weeks |
↓ 2-h post prandial glucose (2 h-PPG) ↓ fasting blood sugar (FBS) ↓ serum glucagon |
[131] |
In this study, mostly DPP-4 inhibitors influence on T2DM and established cardiovascular disease, hypertension and dyslipidemia were reported and gathered data proved their efficacy in managing them. Though these effects are weak in some levels, but combination therapy of DPP-4 inhibitors with the other appropriate drugs may be relevant and cost-effective. Furthermore, this review presents an idea for introducing new DPP-4 inhibitors with greatest effect on MetS in future. Introducing new DPP-4 inhibitors with pleiotropic effects make it possible to manage MetS as a single disease. In this regard, monotherapy will increase the patient’s compliances, decrease drug-drug interactions and reduce health-cost.”
Future prospective
Since this class of drugs has been introduced for less than a decade, post-marketing evaluation is needed to verify their probable side effects and their safety during pregnancy and lactation.
Collectively, introducing a new design of DPP-4 inhibitors with polypharmacological effects on MetS components engages them as a novel adjutant or main therapies in prevention and managing MetS risk factors. Adding them to therapeutic guidelines increases patient’s compliance, decreases drug-drug interactions and decreases health-costs.
Acknowledgements
The authors thank the Vice Chancellor of the Mashhad University of Medical Sciences, Mashhad, Iran.
Contribution
MR and BMR collected data and drafted the manuscript. HH gave the idea, designed and supervised the study. HH and GAF edited the manuscript. All authors read and approved the final manuscript.
Abbreviations
- cAMP
cyclic adenosine monophosphate
- DPP-4
dipeptidyl peptidase
- eNOS
endothelial NO synthesize
- FBS
fasting blood sugar
- FDA
US Food and Drug Administration
- GIP
gastric inhibitory polypeptide
- GLP-1
glucagon-like peptide
- HbA1c
hemoglobin A1c
- HDL-C
high-density lipoprotein cholesterol
- IDL-C
intermediate density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- P-gp
P-glycoprotein
- t 1/2
half-life
- T1D
type 1 diabetes
- T2DM
type 2 diabetes mellitus
- VLDL-C
very-low-density lipoprotein cholesterol
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Maryam Rameshrad, Email: rameshrm2@mums.ac.ir.
Bibi Marjan Razavi, Email: razavimr@mums.ac.ir.
Hossein Hosseinzadeh, Phone: +985131801193, Email: hosseinzadehh@mums.ac.ir.
References
- 1.Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 3.Masoudi-Kazemabad A, Jamialahmadi K, Moohebati M, Mojarrad M, Manshadi RD, Akhlaghi S, et al. Neuropeptide y Leu7Pro polymorphism associated with the metabolic syndrome and its features in patients with coronary artery disease. Angiology. 2013;64(1):40–45. doi: 10.1177/0003319711435149. [DOI] [PubMed] [Google Scholar]
- 4.Khayyatzadeh SS, Moohebati M, Mazidi M, Avan A, Tayefi M, Parizadeh SMR, et al. Nutrient patterns and their relationship to metabolic syndrome in Iranian adults. Eur J Clin Investig. 2016;46(10):840–852. doi: 10.1111/eci.12666. [DOI] [PubMed] [Google Scholar]
- 5.Stojanoska MM, Milosevic N, Milic N, Abenavoli L. The influence of phthalates and bisphenol a on the obesity development and glucose metabolism disorders. Endocrine. 2017;55(3):666–681. doi: 10.1007/s12020-016-1158-4. [DOI] [PubMed] [Google Scholar]
- 6.Baghshini MR, Nikbakht-Jam I, Mohaddes-Ardabili H, Pasdar A, Avan A, Tayefi M, et al. Higher prevalence of metabolic syndrome among male employees of a gas refinery than in their counterparts in nonindustrial environments. Asian Biomedicine. 2017;11(3):227–234. [Google Scholar]
- 7.Rask Larsen J, Dima L, Correll CU, Manu P. The pharmacological management of metabolic syndrome. Expert Rev Clin Pharmacol. 2018;11(4):397–410. doi: 10.1080/17512433.2018.1429910. [DOI] [PubMed] [Google Scholar]
- 8.Martin KA, Mani MV, Mani A. New targets to treat obesity and the metabolic syndrome. Eur J Pharmacol. 2015;763(Pt A):64–74. doi: 10.1016/j.ejphar.2015.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 10.Yazbeck R, Howarth GS, Abbott CA. Dipeptidyl peptidase inhibitors, an emerging drug class for inflammatory disease? Trends Pharmacol Sci. 2009;30(11):600–607. doi: 10.1016/j.tips.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Seino Y, Fukushima M, Yabe D. GIP and GLP-1, the two incretin hormones: similarities and differences. J Diabetes Investig. 2010;1(1–2):8–23. doi: 10.1111/j.2040-1124.2010.00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barber TM, Begbie H, Levy J. The incretin pathway as a new therapeutic target for obesity. Maturitas. 2010;67(3):197–202. doi: 10.1016/j.maturitas.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 13.Drucker DJ, Rosen CF. Glucagon-like peptide-1 (GLP-1) receptor agonists, obesity and psoriasis: diabetes meets dermatology. Diabetologia. 2011;54(11):2741–2744. doi: 10.1007/s00125-011-2297-z. [DOI] [PubMed] [Google Scholar]
- 14.Nistala R, Savin V. Diabetes, hypertension, and chronic kidney disease progression: role of DPP4. Am J Physiol Renal Physiol. 2017;312(4):F661–FF70. doi: 10.1152/ajprenal.00316.2016. [DOI] [PubMed] [Google Scholar]
- 15.Razavi BM, Hosseinzadeh H. Saffron: a promising natural medicine in the treatment of metabolic syndrome. J Sci Food Agric. 2017;97(6):1679–1685. doi: 10.1002/jsfa.8134. [DOI] [PubMed] [Google Scholar]
- 16.Razavi BM, Lookian F, Hosseinzadeh H. Protective effects of green tea on olanzapine-induced-metabolic syndrome in rats. Biomed Pharmacother. 2017;92:726–731. doi: 10.1016/j.biopha.2017.05.113. [DOI] [PubMed] [Google Scholar]
- 17.Tabeshpour J, Imenshahidi M, Hosseinzadeh H. A review of the effects of berberis vulgaris and its major component, berberine, in metabolic syndrome. Iran J Basic Med Sci. 2017;20(5):557–568. doi: 10.22038/IJBMS.2017.8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabeshpour J, Razavi BM, Hosseinzadeh H. Effects of avocado (Persea americana) on metabolic syndrome: a comprehensive systematic review. Phytother Res. 2017;31(6):819–837. doi: 10.1002/ptr.5805. [DOI] [PubMed] [Google Scholar]
- 19.Mazidi M, Rezaie P, Kengne AP, Mobarhan MG, Ferns GA. Gut microbiome and metabolic syndrome. Diabetes Metab Syndr Clin Res Rev. 2016;10(2):S150–S1S7. doi: 10.1016/j.dsx.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 20.Sattigeri JA, Sethi S, Davis JA, Ahmed S, Rayasam GV, Jadhav BG, et al. Approaches towards the development of chimeric DPP4/ACE inhibitors for treating metabolic syndrome. Bioorg Med Chem Lett. 2017;27(11):2313–2318. doi: 10.1016/j.bmcl.2017.04.036. [DOI] [PubMed] [Google Scholar]
- 21.Jackson EK. Dipeptidyl peptidase IV inhibition alters the hemodynamic response to angiotensin-converting enzyme inhibition in humans with the metabolic syndrome. Hypertension. 2010;56(4):581–583. doi: 10.1161/HYPERTENSIONAHA.110.158527. [DOI] [PubMed] [Google Scholar]
- 22.Marney A, Kunchakarra S, Byrne L, Brown NJ. Interactive hemodynamic effects of dipeptidyl peptidase-IV inhibition and angiotensin-converting enzyme inhibition in humans. Hypertension. 2010;56(4):728–733. doi: 10.1161/HYPERTENSIONAHA.110.156554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baetta R, Corsini A. Pharmacology of dipeptidyl peptidase-4 inhibitors: similarities and differences. Drugs. 2011;71(11):1441–1467. doi: 10.2165/11591400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91(1):301–307. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Q, Long M, Qu H, Shen R, Zhang R, Xu J, et al. DPP-4 inhibitors as treatments for type 1 diabetes mellitus: a systematic review and meta-analysis. J Diabetes Res. 2018;2018:10. doi: 10.1155/2018/5308582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Silva A, Bloom SR. Gut hormones and appetite control: a focus on PYY and GLP-1 as therapeutic targets in obesity. Gut Liver. 2012;6(1):10–20. doi: 10.5009/gnl.2012.6.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheen AJ. The safety of gliptins : updated data in 2018. Expert Opin Drug Saf. 2018;17(4):387–405. doi: 10.1080/14740338.2018.1444027. [DOI] [PubMed] [Google Scholar]
- 28.Deacon CF, Lebovitz HE. Comparative review of dipeptidyl peptidase-4 inhibitors and sulphonylureas. Diabetes Obes Metab. 2016;18(4):333–347. doi: 10.1111/dom.12610. [DOI] [PubMed] [Google Scholar]
- 29.Choy M, Lam S. Sitagliptin: a novel drug for the treatment of type 2 diabetes. Cardiol Rev. 2007;15(5):264–271. doi: 10.1097/CRD.0b013e318123f771. [DOI] [PubMed] [Google Scholar]
- 30.McCormack PL. Evogliptin: first global approval. Drugs. 2015;75(17):2045–2049. doi: 10.1007/s40265-015-0496-5. [DOI] [PubMed] [Google Scholar]
- 31.Kadowaki T, Kondo K. Efficacy, safety and dose-response relationship of teneligliptin, a dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15(9):810–818. doi: 10.1111/dom.12092. [DOI] [PubMed] [Google Scholar]
- 32.Sheu WH, Gantz I, Chen M, Suryawanshi S, Mirza A, Goldstein BJ, et al. Safety and efficacy of Omarigliptin (MK-3102), a novel once-weekly DPP-4 inhibitor for the treatment of patients with type 2 diabetes. Diabetes Care. 2015;38(11):2106–2114. doi: 10.2337/dc15-0109. [DOI] [PubMed] [Google Scholar]
- 33.Deacon CF. Dipeptidyl peptidase 4 inhibition with sitagliptin: a new therapy for type 2 diabetes. Expert Opin Investig Drugs. 2007;16(4):533–545. doi: 10.1517/13543784.16.4.533. [DOI] [PubMed] [Google Scholar]
- 34.Karagiannis T, Boura P, Tsapas A. Safety of dipeptidyl peptidase 4 inhibitors: a perspective review. Ther Adv Drug Saf. 2014;5(3):138–146. doi: 10.1177/2042098614523031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tofovic DS, Bilan VP, Jackson EK. Sitagliptin augments angiotensin II-induced renal vasoconstriction in kidneys from rats with metabolic syndrome. Clin Exp Pharmacol Physiol. 2010;37(7):689–691. doi: 10.1111/j.1440-1681.2010.05389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29(12):2632–2637. doi: 10.2337/dc06-0703. [DOI] [PubMed] [Google Scholar]
- 37.Traynor K. FDA approves saxagliptin for type 2 diabetes. Am J Health Syst Pharm. 2009;66(17):1513. doi: 10.2146/news090069. [DOI] [PubMed] [Google Scholar]
- 38.Rosenstock J, Aguilar-Salinas C, Klein E, Nepal S, List J, Chen R. Effect of saxagliptin monotherapy in treatment-naive patients with type 2 diabetes. Curr Med Res Opin. 2009;25(10):2401–2411. doi: 10.1185/03007990903178735. [DOI] [PubMed] [Google Scholar]
- 39.Lam S, Saad M. Saxagliptin: a new dipeptidyl peptidase-4 inhibitor for type 2 diabetes. Cardiol Rev. 2010;18(4):213–217. doi: 10.1097/CRD.0b013e3181daad5f. [DOI] [PubMed] [Google Scholar]
- 40.Aletti R, Cheng-Lai A. Linagliptin: the newest dipeptidyl peptidase-4 inhibitor for type 2 diabetes mellitus. Cardiol Rev. 2012;20(1):45–51. doi: 10.1097/CRD.0b013e31823a3afc. [DOI] [PubMed] [Google Scholar]
- 41.Del Prato S, Barnett AH, Huisman H, Neubacher D, Woerle HJ, Dugi KA. Effect of linagliptin monotherapy on glycaemic control and markers of beta-cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. 2011;13(3):258–267. doi: 10.1111/j.1463-1326.2010.01350.x. [DOI] [PubMed] [Google Scholar]
- 42.Marino AB, Cole SW. Alogliptin: safety, efficacy, and clinical implications. J Pharm Pract. 2015;28(1):99–106. doi: 10.1177/0897190014522063. [DOI] [PubMed] [Google Scholar]
- 43.Covington P, Christopher R, Davenport M, Fleck P, Mekki QA, Wann ER, et al. Pharmacokinetic, pharmacodynamic, and tolerability profiles of the dipeptidyl peptidase-4 inhibitor alogliptin: a randomized, double-blind, placebo-controlled, multiple-dose study in adult patients with type 2 diabetes. Clin Ther. 2008;30(3):499–512. doi: 10.1016/j.clinthera.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Chen XW, He ZX, Zhou ZW, Yang T, Zhang X, Yang YX, et al. An update on the clinical pharmacology of the dipeptidyl peptidase 4 inhibitor alogliptin used for the treatment of type 2 diabetes mellitus. Clin Exp Pharmacol Physiol. 2015;42(12):1225–1238. doi: 10.1111/1440-1681.12469. [DOI] [PubMed] [Google Scholar]
- 45.Pi-Sunyer FX, Schweizer A, Mills D, Dejager S. Efficacy and tolerability of vildagliptin monotherapy in drug-naive patients with type 2 diabetes. Diabetes Res Clin Pract. 2007;76(1):132–138. doi: 10.1016/j.diabres.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 46.Kim SH, Lee SH, Yim HJ. Gemigliptin, a novel dipeptidyl peptidase 4 inhibitor: first new anti-diabetic drug in the history of Korean pharmaceutical industry. Arch Pharm Res. 2013;36(10):1185–1188. doi: 10.1007/s12272-013-0171-x. [DOI] [PubMed] [Google Scholar]
- 47.Gutch M, Joshi A, Kumar S, Agarwal A, Pahan RK, Razi SM. Gemigliptin: newer promising gliptin for type 2 diabetes mellitus. Indian J Endocr Metab. 2017;21(6):898–902. doi: 10.4103/ijem.IJEM_20_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishio S, Abe M, Ito H. Anagliptin in the treatment of type 2 diabetes: safety, efficacy, and patient acceptability. Diabetes Metab Syndr Obes. 2015;8:163–171. doi: 10.2147/DMSO.S54679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gu N, Park MK, Kim TE, Bahng MY, Lim KS, Cho SH, et al. Multiple-dose pharmacokinetics and pharmacodynamics of evogliptin (DA-1229), a novel dipeptidyl peptidase IV inhibitor, in healthy volunteers. Drug Des Devel Ther. 2014;8:1709–1721. doi: 10.2147/DDDT.S65678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park J, Park SW, Yoon KH, Kim SR, Ahn KJ, Lee JH, et al. Efficacy and safety of evogliptin monotherapy in patients with type 2 diabetes and moderately elevated glycated haemoglobin levels after diet and exercise. Diabetes Obes Metab. 2017;19(12):1681–1687. doi: 10.1111/dom.12987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burness CB. Omarigliptin: first global approval. Drugs. 2015;75(16):1947–1952. doi: 10.1007/s40265-015-0493-8. [DOI] [PubMed] [Google Scholar]
- 52.Xu S, Tatosian D, McIntosh I, Caceres M, Matthews C, Samuel K, et al. Absorption, metabolism and excretion of [(14)C]omarigliptin, a once-weekly DPP-4 inhibitor, in humans. Xenobiotica. 2018;48(6):584–591. doi: 10.1080/00498254.2017.1346333. [DOI] [PubMed] [Google Scholar]
- 53.McKeage K. Trelagliptin: first global approval. Drugs. 2015;75(10):1161–1164. doi: 10.1007/s40265-015-0431-9. [DOI] [PubMed] [Google Scholar]
- 54.Kaku K. First novel once-weekly DPP-4 inhibitor, trelagliptin, for the treatment of type 2 diabetes mellitus. Expert Opin Pharmacother. 2015;16(16):2539–2547. doi: 10.1517/14656566.2015.1099630. [DOI] [PubMed] [Google Scholar]
- 55.Nakamaru Y, Hayashi Y, Ikegawa R, Kinoshita S, Perez Madera B, Gunput D, et al. Metabolism and disposition of the dipeptidyl peptidase IV inhibitor teneligliptin in humans. Xenobiotica. 2014;44(3):242–253. doi: 10.3109/00498254.2013.816891. [DOI] [PubMed] [Google Scholar]
- 56.Morishita R, Nakagami H. Teneligliptin : expectations for its pleiotropic action. Expert Opin Pharmacother. 2015;16(3):417–426. doi: 10.1517/14656566.2015.1000301. [DOI] [PubMed] [Google Scholar]
- 57.Furuta S, Smart C, Hackett A, Benning R, Warrington S. Pharmacokinetics and metabolism of [14C]anagliptin, a novel dipeptidyl peptidase-4 inhibitor, in humans. Xenobiotica. 2013;43(5):432–442. doi: 10.3109/00498254.2012.731618. [DOI] [PubMed] [Google Scholar]
- 58.Graefe-Mody U, Retlich S, Friedrich C. Clinical pharmacokinetics and pharmacodynamics of linagliptin. Clin Pharmacokinet. 2012;51(7):411–427. doi: 10.2165/11630900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 59.Heise T, Graefe-Mody EU, Huttner S, Ring A, Trommeshauser D, Dugi KA. Pharmacokinetics, pharmacodynamics and tolerability of multiple oral doses of linagliptin, a dipeptidyl peptidase-4 inhibitor in male type 2 diabetes patients. Diabetes Obes Metab. 2009;11(8):786–794. doi: 10.1111/j.1463-1326.2009.01046.x. [DOI] [PubMed] [Google Scholar]
- 60.Anderson R, Hayes J, Stephens JW. Pharmacokinetic, pharmacodynamic and clinical evaluation of saxagliptin in type 2 diabetes. Expert Opin Drug Metab Toxicol. 2016;12(4):467–473. doi: 10.1517/17425255.2016.1154044. [DOI] [PubMed] [Google Scholar]
- 61.Vincent SH, Reed JR, Bergman AJ, Elmore CS, Zhu B, Xu S, et al. Metabolism and excretion of the dipeptidyl peptidase 4 inhibitor [14C]sitagliptin in humans. Drug Metab Dispos. 2007;35(4):533–538. doi: 10.1124/dmd.106.013136. [DOI] [PubMed] [Google Scholar]
- 62.Bergman AJ, Stevens C, Zhou Y, Yi B, Laethem M, De Smet M, et al. Pharmacokinetic and pharmacodynamic properties of multiple oral doses of sitagliptin, a dipeptidyl peptidase-IV inhibitor: a double-blind, randomized, placebo-controlled study in healthy male volunteers. Clin Ther. 2006;28(1):55–72. doi: 10.1016/j.clinthera.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 63.He YL, Wang Y, Bullock JM, Deacon CF, Holst JJ, Dunning BE, et al. Pharmacodynamics of vildagliptin in patients with type 2 diabetes during OGTT. J Clin Pharmacol. 2007;47(5):633–641. doi: 10.1177/0091270006299137. [DOI] [PubMed] [Google Scholar]
- 64.He H, Tran P, Yin H, Smith H, Batard Y, Wang L, et al. Absorption, metabolism, and excretion of [14C]vildagliptin, a novel dipeptidyl peptidase 4 inhibitor, in humans. Drug Metab Dispos. 2009;37(3):536–544. doi: 10.1124/dmd.108.023010. [DOI] [PubMed] [Google Scholar]
- 65.Krishna R, Bergman A, Larson P, Cote J, Lasseter K, Dilzer S, et al. Effect of a single cyclosporine dose on the single-dose pharmacokinetics of sitagliptin (MK-0431), a dipeptidyl peptidase-4 inhibitor, in healthy male subjects. J Clin Pharmacol. 2007;47(2):165–174. doi: 10.1177/0091270006296523. [DOI] [PubMed] [Google Scholar]
- 66.Abrahami D, Douros A, Yin H, Yu OHY, Renoux C, Bitton A, et al. Dipeptidyl peptidase-4 inhibitors and incidence of inflammatory bowel disease among patients with type 2 diabetes: population based cohort study. Br Med J. 2018;360:k872. doi: 10.1136/bmj.k872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Srikanth S, Deedwania P. Management of Dyslipidemia in patients with hypertension, diabetes, and metabolic syndrome. Curr Hypertens Rep. 2016;18(10):76. doi: 10.1007/s11906-016-0683-0. [DOI] [PubMed] [Google Scholar]
- 68.Ervinna N, Mita T, Yasunari E, Azuma K, Tanaka R, Fujimura S, et al. Anagliptin, a DPP-4 inhibitor, suppresses proliferation of vascular smooth muscles and monocyte inflammatory reaction and attenuates atherosclerosis in male apo e-deficient mice. Endocrinology. 2013;154(3):1260–1270. doi: 10.1210/en.2012-1855. [DOI] [PubMed] [Google Scholar]
- 69.Ta NN, Schuyler CA, Li Y, Lopes-Virella MF, Huang Y. DPP-4 (CD26) inhibitor alogliptin inhibits atherosclerosis in diabetic apolipoprotein E-deficient mice. J Cardiovasc Pharmacol. 2011;58(2):157–166. doi: 10.1097/FJC.0b013e31821e5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yano W, Inoue N, Ito S, Itou T, Yasumura M, Yoshinaka Y, et al. Mechanism of lipid-lowering action of the dipeptidyl peptidase-4 inhibitor, anagliptin, in low-density lipoprotein receptor-deficient mice. J Diabetes Investig. 2017;8(2):155–160. doi: 10.1111/jdi.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rufinatscha K, Radlinger B, Dobner J, Folie S, Bon C, Profanter E, et al. Dipeptidyl peptidase-4 impairs insulin signaling and promotes lipid accumulation in hepatocytes. Biochem Biophys Res Commun. 2017;485(2):366–371. doi: 10.1016/j.bbrc.2017.02.071. [DOI] [PubMed] [Google Scholar]
- 72.Mostafa AM, Hamdy NM, Abdel-Rahman SZ, El-Mesallamy HO. Effect of vildagliptin and pravastatin combination on cholesterol efflux in adipocytes. IUBMB Life. 2016:535–43. 10.1002/iub.1510. [DOI] [PubMed]
- 73.Renna NF, Diez EA, Miatello RM. Effects of dipeptidyl-peptidase 4 inhibitor about vascular inflammation in a metabolic syndrome model. PloS one. 2014;9(9):e106563-e. doi: 10.1371/journal.pone.0106563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Briand F, Thieblemont Q, Burcelin R, Sulpice T. Sitagliptin promotes macrophage-to-faeces reverse cholesterol transport through reduced intestinal cholesterol absorption in obese insulin resistant CETP-apoB100 transgenic mice. Diabetes Obes Metab. 2012;14(7):662–665. doi: 10.1111/j.1463-1326.2012.01568.x. [DOI] [PubMed] [Google Scholar]
- 75.Xu B, Shen T, Chen L, Xia J, Zhang C, Wang H, et al. The effect of sitagliptin on lipid metabolism of fatty liver mice and related mechanisms. Med Sci Monit. 2017;23:1363–1370. doi: 10.12659/MSM.900033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aroor AR, Habibi J, Ford DA, Nistala R, Lastra G, Manrique C, et al. Dipeptidyl peptidase-4 inhibition ameliorates western diet-induced hepatic steatosis and insulin resistance through hepatic lipid remodeling and modulation of hepatic mitochondrial function. Diabetes. 2015;64(6):1988–2001. doi: 10.2337/db14-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu H, Li N, Liu Y, Xing J, Feng S, Li M, et al. The dipeptidyl peptidase-4 inhibitor teneligliptin reduces kidney damage from hypercholesterolemia in apolipoprotein E-deficient mice. RSC Adv. 2017;7(14):8702–8708. [Google Scholar]
- 78.Choi SH, Leem J, Park S, Lee CK, Park KG, Lee IK. Gemigliptin ameliorates western-diet-induced metabolic syndrome in mice. Can J Physiol Pharmacol. 2017;95(2):129–139. doi: 10.1139/cjpp-2016-0026. [DOI] [PubMed] [Google Scholar]
- 79.Zeng Y, Li C, Guan M, Zheng Z, Li J, Xu W, et al. The DPP-4 inhibitor sitagliptin attenuates the progress of atherosclerosis in apolipoprotein-E-knockout mice via AMPK- and MAPK-dependent mechanisms. Cardiovasc Diabetol. 2014;13(1). 10.1186/1475-2840-13-32. [DOI] [PMC free article] [PubMed]
- 80.Hirano T, Yamashita S, Takahashi M, Hashimoto H, Mori Y, Goto M. Anagliptin, a dipeptidyl peptidase-4 inhibitor, decreases macrophage infiltration and suppresses atherosclerosis in aortic and coronary arteries in cholesterol-fed rabbits. Metabolism. 2016;65(6):893–903. doi: 10.1016/j.metabol.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 81.Brenner C, Franz WM, Kühlenthal S, Kuschnerus K, Remm F, Gross L, et al. DPP-4 inhibition ameliorates atherosclerosis by priming monocytes into M2 macrophages. Int J Cardiol. 2015;199:163–169. doi: 10.1016/j.ijcard.2015.07.044. [DOI] [PubMed] [Google Scholar]
- 82.Shah Z, Pineda C, Kampfrath T, Maiseyeu A, Ying Z, Racoma I, et al. Acute DPP-4 inhibition modulates vascular tone through GLP-1 independent pathways. Vasc Pharmacol. 2011;55(1–3):2–9. doi: 10.1016/j.vph.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu L, Liu J, Wong WT, Tian XY, Lau CW, Wang YX, et al. Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon-like peptide 1-dependent mechanism. Hypertension. 2012;60(3):833–841. doi: 10.1161/HYPERTENSIONAHA.112.195115. [DOI] [PubMed] [Google Scholar]
- 84.Hamidi Shishavan M, Henning RH, Van Buiten A, Goris M, Deelman LE, Buikema H. Metformin improves endothelial function and reduces blood pressure in diabetic spontaneously hypertensive rats independent from glycemia control: comparison to vildagliptin. Sci Rep. 2017;7(1). 10.1038/s41598-017-11430-7. [DOI] [PMC free article] [PubMed]
- 85.Koibuchi N, Hasegawa Y, Katayama T, Toyama K, Uekawa K, Sueta D, et al. DPP-4 inhibitor linagliptin ameliorates cardiovascular injury in salt-sensitive hypertensive rats independently of blood glucose and blood pressure. Cardiovasc Diabetol. 2014;13(1). 10.1186/s12933-014-0157-0. [DOI] [PMC free article] [PubMed]
- 86.Nakagami H, Pang Z, Shimosato T, Moritani T, Kurinami H, Koriyama H, et al. The dipeptidyl peptidase-4 inhibitor teneligliptin improved endothelial dysfunction and insulin resistance in the SHR/NDmcr-cp rat model of metabolic syndrome. Hypertens Res. 2014;37(7):629–635. doi: 10.1038/hr.2014.53. [DOI] [PubMed] [Google Scholar]
- 87.Reis F, Ferreira L, Teixeira-De-Lemos E, Pinto F, Parada B, Mega C, et al. Effects of sitagliptin treatment on dysmetabolism, inflammation, and oxidative stress in an animal model of type 2 diabetes (ZDF rat) Mediat Inflamm. 2010;2010:1–11. doi: 10.1155/2010/592760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen B, Moore A, Escobedo LV, Koletsky MS, Hou D, Koletsky RJ, et al. Sitagliptin lowers glucagon and improves glucose tolerance in prediabetic obese SHROB rats. Exp Biol Med (Maywood, NJ) 2011;236(3):309–314. doi: 10.1258/ebm.2010.010161. [DOI] [PubMed] [Google Scholar]
- 89.Takeda Y, Fujita Y, Honjo J, Yanagimachi T, Sakagami H, Takiyama Y, et al. Reduction of both beta cell death and alpha cell proliferation by dipeptidyl peptidase-4 inhibition in a streptozotocin-induced model of diabetes in mice. Diabetologia. 2012;55(2):404–412. doi: 10.1007/s00125-011-2365-4. [DOI] [PubMed] [Google Scholar]
- 90.Zhang X, Wang Z, Huang Y, Wang J. Effects of chronic administration of alogliptin on the development of diabetes and β-cell function in high fat diet/streptozotocin diabetic mice. Diabetes Obes Metab. 2011;13(4):337–347. doi: 10.1111/j.1463-1326.2010.01354.x. [DOI] [PubMed] [Google Scholar]
- 91.Turcot V, Bouchard L, Faucher G, Tchernof A, Deshaies Y, Pérusse L, et al. DPP4 gene DNA methylation in the omentum is associated with its gene expression and plasma lipid profile in severe obesity. Obesity. 2011;19(2):388–395. doi: 10.1038/oby.2010.198. [DOI] [PubMed] [Google Scholar]
- 92.Monami M, Vitale V, Ambrosio ML, Bartoli N, Toffanello G, Ragghianti B, et al. Effects on lipid profile of dipeptidyl peptidase 4 inhibitors, pioglitazone, acarbose, and sulfonylureas: meta-analysis of placebo-controlled trials. Adv Ther. 2012;29(9):736–746. doi: 10.1007/s12325-012-0045-5. [DOI] [PubMed] [Google Scholar]
- 93.Homma K, Yoshizawa J, Shiina Y, Ozawa H, Igarashi M, Matsuoka T, et al. A dipeptidyl Peptidase-4 inhibitor, teneligliptin, decreases plasma triglyceride-rich lipoprotein remnants in diabetic patients with chronic kidney disease undergoing hemodialysis. Drugs in R and D. 2017;17(3):397–402. doi: 10.1007/s40268-017-0189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tahara N, Yamagishi SI, Bekki M, Kodama N, Nakamura T, Sugiyama Y, et al. Anagliptin, a dipeptidyl peptidase-4 inhibitor ameliorates arterial stiffness in association with reduction of remnant-like particle cholesterol and alanine transaminase levels in type 2 diabetic patients. Curr Vasc Pharmacol. 2016;14(6):552–562. doi: 10.2174/1570161114666160625090212. [DOI] [PubMed] [Google Scholar]
- 95.Ahn CH, Kim EK, Min SH, Oh TJ, Cho YM. Effects of gemigliptin, a dipeptidyl peptidase-4 inhibitor, on lipid metabolism and endotoxemia after a high-fat meal in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19(3):457–462. doi: 10.1111/dom.12831. [DOI] [PubMed] [Google Scholar]
- 96.Fan M, Li Y, Zhang S. Effects of sitagliptin on lipid profiles in patients with type 2 diabetes mellitus: A meta-analysis of randomized clinical trials. Medicine (United States). 2016;95(2). 10.1097/MD.0000000000002386. [DOI] [PMC free article] [PubMed]
- 97.Tremblay AJ, Lamarche B, Kelly I, Charest A, Lépine MC, Droit A, et al. Effect of sitagliptin therapy on triglyceride-rich lipoprotein kinetics in patients with type 2 diabetes. Diabetes Obes Metab. 2014;16(12):1223–1229. doi: 10.1111/dom.12359. [DOI] [PubMed] [Google Scholar]
- 98.Tsurutani Y, Omura M, Matsuzawa Y, Saito J, Higa M, Taniyama M, et al. Efficacy and safety of the dipeptidyl Peptidase-4 inhibitor Sitagliptin on atherosclerosis, β-cell function, and glycemic control in Japanese patients with type 2 diabetes mellitus who are treatment Naïve or poorly responsive to Antidiabetes agents: a multicenter, prospective observational, uncontrolled study. Curr Ther Res Clin Exp. 2017;84:26–31. doi: 10.1016/j.curtheres.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Duvnjak L, Blaslov K. Dipeptidyl peptidase-4 inhibitors improve arterial stiffness, blood pressure, lipid profile and inflammation parameters in patients with type 2 diabetes mellitus. Diabetol Metab Syndr. 2016;8(1). 10.1186/s13098-016-0144-6. [DOI] [PMC free article] [PubMed]
- 100.Evans M, Schweizer A, Foley JE. Blood pressure and fasting lipid changes after 24 weeks’ treatment with vildagliptin: a pooled analysis in >2,000 previously drug-naïve patients with type 2 diabetes mellitus. Vasc Health Risk Manag. 2016;12:337–340. doi: 10.2147/VHRM.S112148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kusunoki M, Sato D, Nakamura T, Oshida Y, Tsutsui H, Natsume Y, et al. The beneficial effects of the DPP-4 inhibitor Alogliptin on hemoglobin A1c and serum lipids in Japanese patients with type 2 diabetes. Drug Res. 2015;66(1):18–22. doi: 10.1055/s-0035-1547254. [DOI] [PubMed] [Google Scholar]
- 102.Kakuda H, Kobayashi J, Kakuda M, Yamakawa J, Takekoshi N. The effect of anagliptin treatment on glucose metabolism and lipid metabolism, and oxidative stress in fasting and postprandial states using a test meal in Japanese men with type 2 diabetes. Endocrine. 2015;48(3):1005–1009. doi: 10.1007/s12020-014-0376-x. [DOI] [PubMed] [Google Scholar]
- 103.Sakura H, Hashimoto N, Sasamoto K, Ohashi H, Hasumi S, Ujihara N, et al. Effect of sitagliptin on blood glucose control in patients with type 2 diabetes mellitus who are treatment naive or poorly responsive to existing antidiabetic drugs: The JAMP study. BMC Endocr Disord. 2016;16(1). 10.1186/s12902-016-0149-z. [DOI] [PMC free article] [PubMed]
- 104.Amber CF, Zeynep TK, Evren O, Yusuf B, Can AK, Belma T. Di-peptidyl peptidase-4 inhibitor sitagliptin protects vascular function in metabolic syndrome: possible role of epigenetic regulation. Mol Biol Rep. 2014;41(8):4853–4863. doi: 10.1007/s11033-014-3392-2. [DOI] [PubMed] [Google Scholar]
- 105.Liu L, Liu J, Tian XY, Wong WT, Lau CW, Xu A, et al. Uncoupling protein-2 mediates DPP-4 inhibitor-induced restoration of endothelial function in hypertension through reducing oxidative stress. Antioxid Redox Signal. 2014;21(11):1571–1581. doi: 10.1089/ars.2013.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Devin JK, Pretorius M, Nian H, Yu C, Billings FT, Brown NJ. Dipeptidyl-peptidase 4 inhibition and the vascular effects of glucagon-like peptide-1 and brain natriuretic peptide in the human forearm. J Am Heart Assoc. 2014;3(4). 10.1161/jaha.114.001075. [DOI] [PMC free article] [PubMed]
- 107.Wilson JR, Brown NJ, Nian H, Yu C, Bidlingmaier M, Devin JK. Dipeptidyl peptidase-4 inhibition potentiates stimulated growth hormone secretion and vasodilation in women. J Am Heart Assoc. 2018;7(5). 10.1161/jaha.117.008000. [DOI] [PMC free article] [PubMed]
- 108.Ogawa S, Ishiki M, Nako K, Okamura M, Senda M, Mori T, et al. Sitagliptin, a dipeptidyl peptidase-4 inhibitor, decreases systolic blood pressure in Japanese hypertensive patients with type 2 diabetes. Tohoku J Exp Med. 2011;223(2):133–135. doi: 10.1620/tjem.223.133. [DOI] [PubMed] [Google Scholar]
- 109.Kawasaki I, Hiura Y, Tamai A, Yoshida Y, Yakusiji Y, Ikuno Y, et al. Sitagliptin reduces the urine albumin-to-creatinine ratio in type 2 diabetes through decreasing both blood pressure and estimated glomerular filtration rate. J Diabetes. 2015;7(1):41–46. doi: 10.1111/1753-0407.12153. [DOI] [PubMed] [Google Scholar]
- 110.Cosenso-Martin LN, Giollo-Júnior LT, Martineli DD, Cesarino CB, Nakazone MA, Cipullo JP, et al. Twelve-week randomized study to compare the effect of vildagliptin vs. glibenclamide both added-on to metformin on endothelium function in patients with type 2 diabetes and hypertension. Diabetol Metab Syndr. 2015;7(1). 10.1186/s13098-015-0062-z. [DOI] [PMC free article] [PubMed]
- 111.Koren S, Shemesh-Bar L, Tirosh A, Peleg RK, Berman S, Hamad RA, et al. The effect of sitagliptin versus glibenclamide on arterial stiffness, blood pressure, lipids, and inflammation in type 2 diabetes mellitus patients. Diabetes Technol Ther. 2012;14(7):561–567. doi: 10.1089/dia.2011.0296. [DOI] [PubMed] [Google Scholar]
- 112.Widlansky ME, Puppala VK, Suboc TM, Malik M, Branum A, Signorelli K, et al. Impact of DPP-4 inhibition on acute and chronic endothelial function in humans with type 2 diabetes on background metformin therapy. Vasc Med (London, England) 2017;22(3):189–196. doi: 10.1177/1358863X16681486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang X, Zhao Q. Effects of dipeptidyl peptidase-4 inhibitors on blood pressure in patients with type 2 diabetes: a systematic review and meta-analysis. J Hypertens. 2016;34(2):167–175. doi: 10.1097/HJH.0000000000000782. [DOI] [PubMed] [Google Scholar]
- 114.Jelsing J, Vrang N, van Witteloostuijn SB, Mark M, Klein T. The DPP4 inhibitor linagliptin delays the onset of diabetes and preserves beta-cell mass in non-obese diabetic mice. J Endocrinol. 2012;214(3):381–387. doi: 10.1530/JOE-11-0479. [DOI] [PubMed] [Google Scholar]
- 115.Akarte AS, Srinivasan BP, Gandhi S. Vildagliptin selectively ameliorates GLP-1, GLUT4, SREBP-1c mRNA levels and stimulates beta-cell proliferation resulting in improved glucose homeostasis in rats with streptozotocin-induced diabetes. J Diabetes Complicat. 2012;26(4):266–274. doi: 10.1016/j.jdiacomp.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 116.Otsuka Y, Yamaguchi S, Furukawa A, Kosuda M, Nakazaki M, Ishihara H. Addition of sitagliptin or metformin to insulin monotherapy improves blood glucose control via different effects on insulin and glucagon secretion in hyperglycemic Japanese patients with type 2 diabetes. Endocr J. 2015;62(2):133–143. doi: 10.1507/endocrj.EJ14-0148. [DOI] [PubMed] [Google Scholar]
- 117.Seck TL, Engel SS, Williams-Herman DE, Sisk CM, Golm GT, Wang H, et al. Sitagliptin more effectively achieves a composite endpoint for A1C reduction, lack of hypoglycemia and no body weight gain compared with glipizide. Diabetes Res Clin Pract. 2011;93(1):e15–ee7. doi: 10.1016/j.diabres.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 118.Krobot KJ, Ferrante SA, Davies MJ, Seck T, Meininger GE, Williams-Herman D, et al. Lower risk of hypoglycemia with sitagliptin compared to glipizide when either is added to metformin therapy: a pre-specified analysis adjusting for the most recently measured HbA1c value. Curr Med Res Opin. 2012;28(8):1281–1287. doi: 10.1185/03007995.2012.703134. [DOI] [PubMed] [Google Scholar]
- 119.Vellanki P, Smiley DD, Stefanovski D, Anzola I, Duan W, Hudson M, et al. Randomized controlled study of metformin and Sitagliptin on Long-term Normoglycemia remission in African American patients with hyperglycemic crises. Diabetes Care. 2016;39(11):1948–1955. doi: 10.2337/dc16-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kusunoki Y, Katsuno T, Myojin M, Miyakoshi K, Ikawa T, Matsuo T, et al. Effect of additional administration of acarbose on blood glucose fluctuations and postprandial hyperglycemia in patients with type 2 diabetes mellitus under treatment with alogliptin. Endocr J. 2013;60(4):431–439. [PubMed] [Google Scholar]
- 121.Osonoi T, Saito M, Tamasawa A, Ishida H, Osonoi Y. Effects of sitagliptin or mitiglinide as an add-on to acarbose on daily blood glucose fluctuations measured by 72 h subcutaneous continuous glucose monitoring in Japanese patients with type 2 diabetes: a prospective randomized study. Expert Opin Pharmacother. 2014;15(10):1325–1335. doi: 10.1517/14656566.2014.920323. [DOI] [PubMed] [Google Scholar]
- 122.Pasquel FJ, Gianchandani R, Rubin DJ, Dungan KM, Anzola I, Gomez PC, et al. Efficacy of sitagliptin for the hospital management of general medicine and surgery patients with type 2 diabetes (Sita-Hospital): a multicentre, prospective, open-label, non-inferiority randomised trial. Lancet Diabetes Endocrinol. 2017;5(2):125–133. doi: 10.1016/S2213-8587(16)30402-8. [DOI] [PubMed] [Google Scholar]
- 123.Dejager S, Schweizer A. Minimizing the risk of hypoglycemia with vildagliptin: clinical experience, mechanistic basis, and importance in type 2 diabetes management. Diabetes Ther. 2011;2(2):51–66. doi: 10.1007/s13300-010-0018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ahrén B, Foley JE, Dejager S, Akacha M, Shao Q, Heimann G, et al. Higher risk of hypoglycemia with glimepiride versus Vildagliptin in patients with type 2 diabetes is not driven by high doses of glimepiride: divergent patient susceptibilities? Diabetes Ther. 2014;5(2):459–469. doi: 10.1007/s13300-014-0082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang W, Xing X, Lv X, Li Y, Ma J, Yuan G, et al. Vildagliptin added to sulfonylurea improves glycemic control without hypoglycemia and weight gain in Chinese patients with type 2 diabetes mellitus. J Diabetes. 2015;7(2):174–181. doi: 10.1111/1753-0407.12169. [DOI] [PubMed] [Google Scholar]
- 126.Ning G, Wang W, Li L, Ma J, Lv X, Yang M, et al. Vildagliptin as add-on therapy to insulin improves glycemic control without increasing risk of hypoglycemia in Asian, predominantly Chinese, patients with type 2 diabetes mellitus. J Diabetes. 2016;8(3):345–353. doi: 10.1111/1753-0407.12303. [DOI] [PubMed] [Google Scholar]
- 127.Qiu Y, Luan X, Chen J, Kong Y, Xu X. Effects of vildagliptin on blood glucose fluctuation in patients with end-stage renal disease under hemodialysis in type 2 diabetes mellitus. Int J Clin Exp Med. 2016;9(6):9557–9562. [Google Scholar]
- 128.Aravind SR, Ismail SB, Balamurugan R, Gupta JB, Wadhwa T, Loh SM, et al. Hypoglycemia in patients with type 2 diabetes from India and Malaysia treated with sitagliptin or a sulfonylurea during Ramadan: a randomized, pragmatic study. Curr Med Res Opin. 2012;28(8):1289–1296. doi: 10.1185/03007995.2012.707119. [DOI] [PubMed] [Google Scholar]
- 129.Mbanya JC, Al-Sifri S, Abdel-Rahim A, Satman I. Incidence of hypoglycemia in patients with type 2 diabetes treated with gliclazide versus DPP-4 inhibitors during Ramadan: a meta-analytical approach. Diabetes Res Clin Pract. 2015;109(2):226–232. doi: 10.1016/j.diabres.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 130.Raju A, Shetty S, Cai B, D'Souza AO. Hypoglycemia incidence rates and associated health care costs in patients with type 2 diabetes mellitus treated with second-line linagliptin or sulfonylurea after metformin monotherapy. J Manag Care Spec Pharm. 2016;22(5):483–492. doi: 10.18553/jmcp.2016.22.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Eto T, Inoue S, Kadowaki T. Effects of once-daily teneligliptin on 24-h blood glucose control and safety in Japanese patients with type 2 diabetes mellitus: a 4-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2012;14(11):1040–1046. doi: 10.1111/j.1463-1326.2012.01662.x. [DOI] [PubMed] [Google Scholar]
- 132.Sicras-Mainar A, Navarro-Artieda R. Healthcare costs of the combination of metformin/dipeptidyl peptidase-4 inhibitors compared with metformin/other oral antidiabetes agents in patients with type 2 diabetes and metabolic syndrome. Diabetes Technol Ther. 2014;16(11):722–727. doi: 10.1089/dia.2014.0091. [DOI] [PubMed] [Google Scholar]
- 133.Underland LJ, Ilkowitz JT, Katikaneni R, Dowd A, Heptulla RA. Use of Sitagliptin with closed-loop technology to decrease postprandial blood glucose in type 1 diabetes. J Diabetes Sci Technol. 2017;11(3):602–610. doi: 10.1177/1932296817699847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Best C, Struthers H, Laciny E, Royal M, Reeds DN, Yarasheski KE. Sitagliptin reduces inflammation and chronic immune cell activation in HIV+ adults with impaired glucose tolerance. J Clin Endocrinol Metab. 2015;100(7):2621–2629. doi: 10.1210/jc.2015-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.van Genugten RE, van Raalte DH, Muskiet MH, Heymans MW, Pouwels PJ, Ouwens DM, et al. Does dipeptidyl peptidase-4 inhibition prevent the diabetogenic effects of glucocorticoids in men with the metabolic syndrome? A randomized controlled trial. Eur J Endocrinol. 2014;170(3):429–439. doi: 10.1530/EJE-13-0610. [DOI] [PubMed] [Google Scholar]
- 136.Fadini GP, de Kreutzenberg SV, Gjini R, Avogaro A. The metabolic syndrome influences the response to incretin-based therapies. Acta Diabetol. 2011;48(3):219–225. doi: 10.1007/s00592-011-0296-7. [DOI] [PubMed] [Google Scholar]